- 1School of Second Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Endocrinology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Department of Endocrinology, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
Background: Obesity is becoming a global epidemic. Flavonoids, with anti-inflammatory and antioxidative functions, are proposed to treat insulin resistance (IR) in obese subjects. We aimed to evaluate the effectiveness and safety of flavonoids-containing supplements on IR and associated metabolic risk factors in overweight and obese participants.
Methods: Randomized controlled trials (RCTs) involving flavonoids-containing supplements used to treat overweight and obese subjects with results of IR, other associated metabolic risk factors, and adverse effects published were retrieved from 5 electronic databases from the year of inception to January 2, 2022.
Results: Twenty-five RCTs (n = 1950) were included. Pooled results demonstrated that HOMA-IR in the group receiving flavonoids-containing supplements significantly decreased versus the control group (WMD = -0.132, 95% CI: -0.236 to -0.027, p = 0.013). Subgroup analyses showed that HOMA-IR in the subgroup receiving flavonoid-containing mixtures significantly decreased (WMD = -0.25, 95% CI: -0.43 to -0.06, p = 0.008), whereas such result was not found in the singly-used flavonoids subgroup (WMD = -0.08, 95% CI: -0.20 to 0.05, p = 0.240). In addition, QUICKI in the experimental group had an increasing trend compared to that in the control group (WMD = 0.01, 95% CI: -0.00 to 0.02, p = 0.065). For secondary outcomes, FBG, FBI, TC, TG, SBP, weight, BMI, and WHR in the group receiving flavonoids-containing supplements dropped significantly compared to those in the controls (WMD = -0.05, 95% CI: -0.08 to -0.02, p = 0.002; WMD = -0.58, 95% CI: -1.04 to -0.12, p = 0.014; WMD = -0.04, 95% CI: -0.06 to -0.03, p < 0.001; WMD = -0.04, 95% CI: -0.05 to -0.03, p < 0.001; WMD = -2.01, 95% CI: -3.17 to -0.86, p = 0.001; WMD = -0.29, 95% CI: -0.49 to -0.09, p = 0.004; WMD = -0.10 95% CI: -0.17 to -0.04, p = 0.003; WMD = -0.10, 95% CI: -0.01 to -0.00, p = 0.015; respectively). Adverse reactions did not differ between the group receiving flavonoids-containing supplements and the control group (RR = 0.97, 95% CI: 0.62 to 1.52, p = 0.905).
Conclusion: This study showed that flavonoids-containing supplements may be efficacious and safe in improving IR and associated metabolic risk factors in overweight and obese participants. Nevertheless, doubt over the findings remains because limited RCTs per type of flavonoids-containing supplement were investigated, and many of the RCTs had a small sample size. Therefore, the findings must be validated in future research.
Systematic Review Registration: https://inplasy.com/inplasy-2022-2-0011/, identifier INPLASY202220011.
1 Introduction
Obesity is becoming a global epidemic, which perplexes people worldwide. Obesity has nearly tripled worldwide since the 1970s (1). In 2016, more than 1.9 billion adults (39% of the global adult population) were overweight, of which over 650 million were obese (2). Obesity is one of the greatest health hazards, posing a significant burden on affected individuals, healthcare systems, and the entire society (3). Obesity is a major risk factor for the onset and progression of insulin resistance (IR). Obese and overweight subjects commonly develop IR, which is caused in part by the development of lipotoxicity in non-adipose tissues (4). Furthermore, obesity escalates the pathogenesis of various metabolic diseases (such as metabolic syndrome, type 2 diabetes, and cardiovascular disease) through the stimulation of IR.
Current treatment strategies for IR and obesity are primarily focused on lifestyle, pharmacologic, or surgical interventions. However, these interventions demonstrated significant interindividual variability in response, which further requires additional strategies to optimize the treatment of obesity and its associated metabolic disorders as mentioned above (5). Clinicians are currently treating obese and overweight patients with natural compounds isolated from the plant kingdom, and flavonoids appear to be a promising option. Flavonoids, a type of dietary polyphenol, are found in herbs, plant-based food, and beverages. Flavonoids are classified into flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, and isoflavones based on their chemical structures (6–8). Flavonoids are proposed to treat IR in obese subjects because they have a range of physiologic effects including anti-inflammatory and antioxidative functions (9), making them a current focus in treating human metabolic diseases. To date, some randomized controlled trials (RCTs) were conducted concerning the efficacy of flavonoid-containing supplements in treating overweight and obese subjects. However, these RCTs showed inconsistent results, and the evidence remains decentralized. Therefore, this study made a systematic review and meta-analysis of the available evidence on the efficacy of flavonoid-containing supplements on IR and associated metabolic risk factors in overweight and obese participants.
2 Materials and methods
The current systematic review and meta-analysis were conducted following PRISMA 2020 statement (10). INPLASY registration number is INPLASY202220011, which is available from https://inplasy.com/inplasy-2022-2-0011/
2.1 Literature searches
From the inception to January 2, 2022, databases such as Pubmed, Embase, Web of Science, and the Cochrane Library were searched. Unpublished trials were also searched on the ClinicalTrials.gov registry, and the authors were contacted for further information as necessary. Search keywords, developed with the help of an expert medical librarian, included “flavonoid“, “flavonol”, “flavone”, “flavanone”, “flavan-3-ols”, “anthocyanidin”, “isoflavone”, “insulin resistance”, “overweight”, “obesity”, and “trial”. Only human subjects and RCTs were included in the search approach. The Supplementary Appendix listed the search syntaxes utilized. References for the included studies were also gathered to find any additional research that was not found during the original electronic search. There were no restrictions on language, publishing year, or type of publication.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows :(1) RCTs with any length of follow-up and sample size; (2) subjects who are overweight or obese, regardless of gender, age, or ethnicity; (3) flavonoid-containing supplements were used as interventions in the experimental group; (4) primary measures: homeostasis model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI); secondary outcomes: fasting blood glucose (FBG), fasting blood insulin (FBI), blood lipids, blood pressure, weight, body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR), and adverse effects.
The exclusion criteria were as follows: (1) RCTs in which outcome measures were inappropriate or relevant information could not be acquired from the authors; (2) non-RCTs, animal experiments, or reviews; (3) published literature that had already been reported.
2.3 Data extraction
After training and calibrating exercises, a pair of reviewers (J.Y. and Y.Z.) extracted data individually for each qualified trial using a predefined, pilot-tested data extraction form. They gathered data on trial features (author, year of publication, design, and sample size), patient characteristics (age, sex, and BMI), interventions in the experimental and control groups, dosage, route of administration, duration, study population, and desired outcomes. The differences of opinion were solved through negotiation and, when the help of a third party was enlisted if necessary (J.Z.). When the important details of a study were missing, we emailed the associated author(s) and searched the ClinicalTrials.gov database for more information.
2.4 Quality assessment
Reviewers assessed the risk of bias for each eligible trial using a revised Cochrane tool (RoB 2.0) (11). Bias risks were classified into four levels: low risk, some concerns—probably low risk, some concerns—probably high risk, and high risk in the following areas: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The evaluation consisted of a series of signaling questions within each area; a judgment about the danger of bias was made by an algorithm that maps responses to signaling questions to the suggested judgment. If a study was judged to be of low risk of bias in all areas, we graded it as low risk of bias overall. If a trial was considered to have some concerns of bias in at least one area but did not show a high risk of bias in any area, we graded it as having some concerns of bias overall. If a trial was deemed to have a high risk of bias in at least one area or have some concerns of bias in many areas that significantly reduced confidence in the result, we graded it as a high risk of bias overall. When disputes could not be addressed via negotiation, the reviewers turned to a third party for settlement.
2.5 Statistical analysis
For statistical analysis, Stata (version 16.0, StataCorp LLC) was utilized. After standardizing the units, we generated the weighted mean difference (WMD) with a 95% confidence interval (CI) for continuous data. We estimated the relative risk (RR) with 95% CIs for dichotomous data. The mean differences and standard deviations of the group receiving flavonoid-containing supplements and the control group were extracted to calculate the effect size (12). The χ2-based Cochran Q statistic and the I2 statistic were used to assess heterogeneity. When I2 < 50%, a fixed-effects model was employed to pool the estimates from different trials. After clinical heterogeneity between trials was removed, the random-effects model was utilized when I2 >= 50%. Wherever possible, quantitative data were pooled for meta-analysis. Where pooling was not available, the findings were presented narratively. To investigate the potential sources of heterogeneity, subgroup analyses and sensitivity analyses were performed. Subgroup analyses were carried out depending on treatments (the use of singly-used flavonoids or flavonoid-containing mixtures), flavonoid subclasses, duration, and route of administration. The leave-one-out method was used for sensitivity analyses to assess the impact of each research on the overall effect size. The funnel plot approach was used to investigate publication bias. To quantify the publication bias, the Egger’s test and Begg’s test were used. The trim and fill approach was also used to rectify the funnel asymmetry induced by publication bias. A statistically significant difference was established by p < 0.05.
3 Results
3.1 Search results
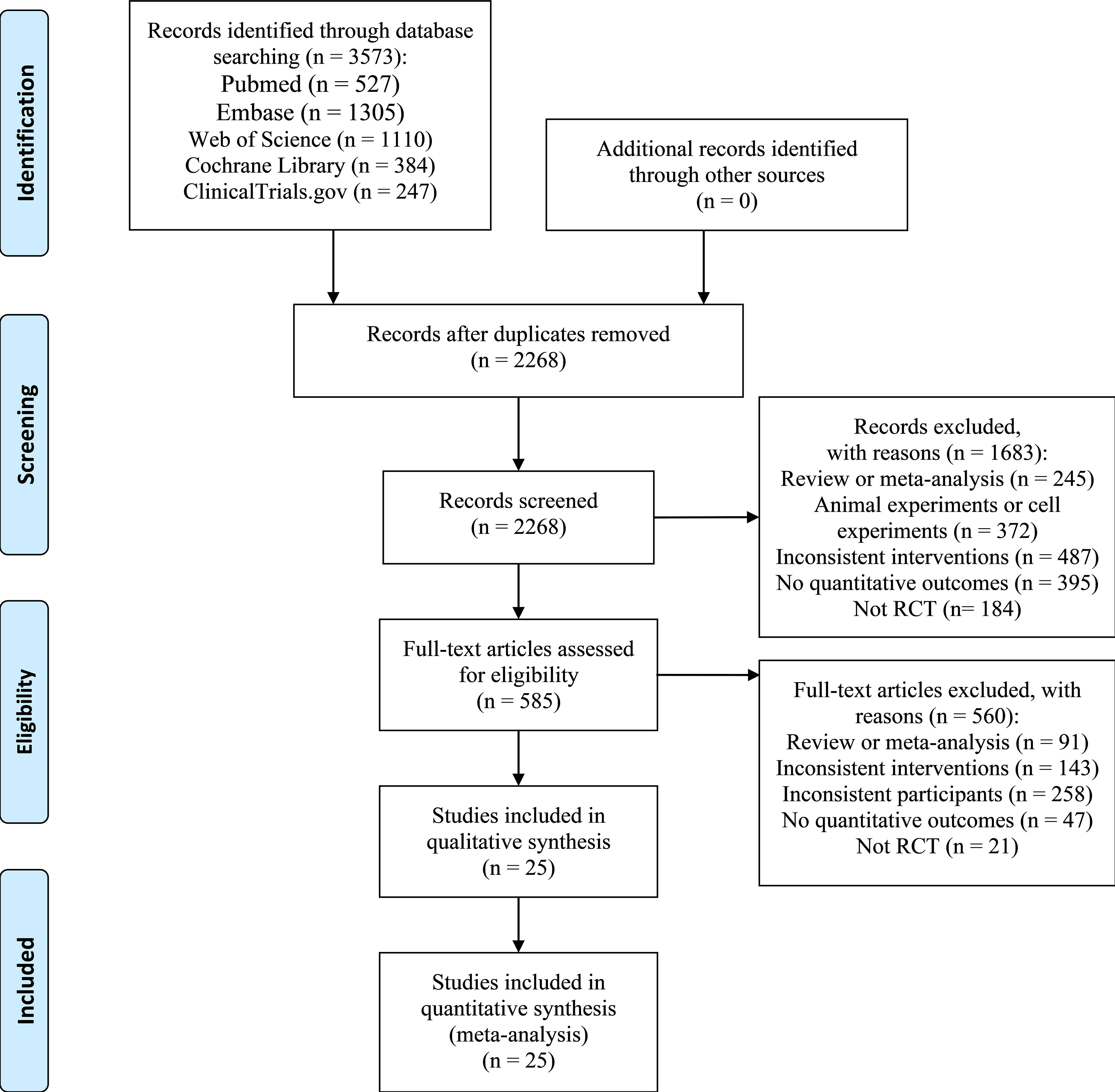
We retrieved 3,573 citations with 1,305 duplicates (Figure 1). After a preliminary screening of the titles and abstracts, we selected 585 studies for further full-text review. We then removed 560 studies, of which 47 did not supply quantitative outcome indicators, 21 were non-RCTs, 91 were reviews or meta-analyses, and the rest studies had unwanted interventions or subjects. We corresponded with the authors of studies without specific data on outcomes by e-mail to obtain relevant information. Unfortunately, we did not obtain replies until the completion of this writing. Ultimately, 25 RCTs (13–37) were included.
3.2 Study characteristics
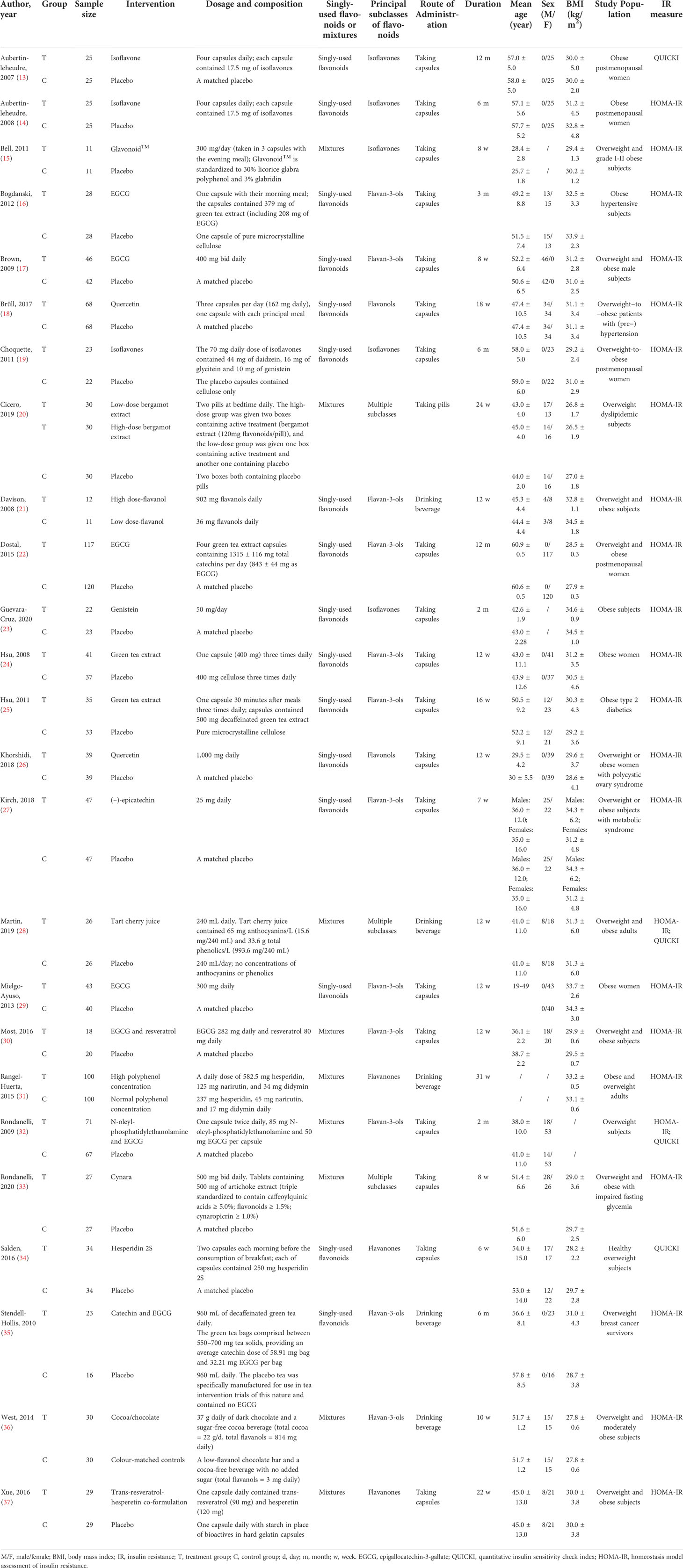
Table 1 presented the baseline characteristics of the RCTs included in the present study. Twenty-five trials (13–37) comprising 1950 people were examined (1000 in the experimental group and 950 in the control group). Flavonoids-containing supplements received by the experimental group included isoflavone, Glavonoid™, epigallocatechin gallate (EGCG), quercetin, bergamot extract, flavanol cocoa, genistein, green tea extract, (-)-epicatechin, tart cherry juice, the co-formulation of EGCG and resveratrol, polyphenol, the co-formulation of N-oleyl-phosphatidylethanolamine and EGCG, cynara, hesperidin 2S, the co-formulation of catechin and EGCG, cocoa/chocolate, and trans-resveratrol-hesperetin co-formulation. In 16 RCTs, singly-used flavonoids were used for interventions. In the other 9 RCTs, flavonoid-containing mixtures were used for interventions. Principal subclasses of flavonoids in the treatment group were isoflavones in 5 RCTs, flavan-3-ols in 12 RCTs, flavonols in 2 RCTs, flavanones in 3 RCTs, and multiple subclasses in 3 RCTs. The routes of administration included taking capsules and drinking beverages. The sample size varied from 22 to 237 individuals, the duration was from 7 to 12 months, and the average age was from 25.7 to 60.9 years. Thirteen RCTs (15, 17, 21, 23, 24, 28–32, 34, 36, 37) included overweight and obese subjects, 4 RCTs (13, 14, 19, 22) included overweight and obese postmenopausal women, 2 RCTs (16, 18) included overweight and obese subjects with hypertension, 1 RCT (20) included overweight dyslipidemic subjects, 1 RCT (25) included obese subjects with type 2 diabetes, 1 study (26) included overweight and obese women with polycystic ovary syndrome, 1 RCT (27) included overweight and obese subjects with metabolic syndrome, 1 study (33) included overweight and obese subjects with impaired fasting glycemia, and 1 RCT (35) included overweight breast cancer survivors.
3.3 Quality assessment
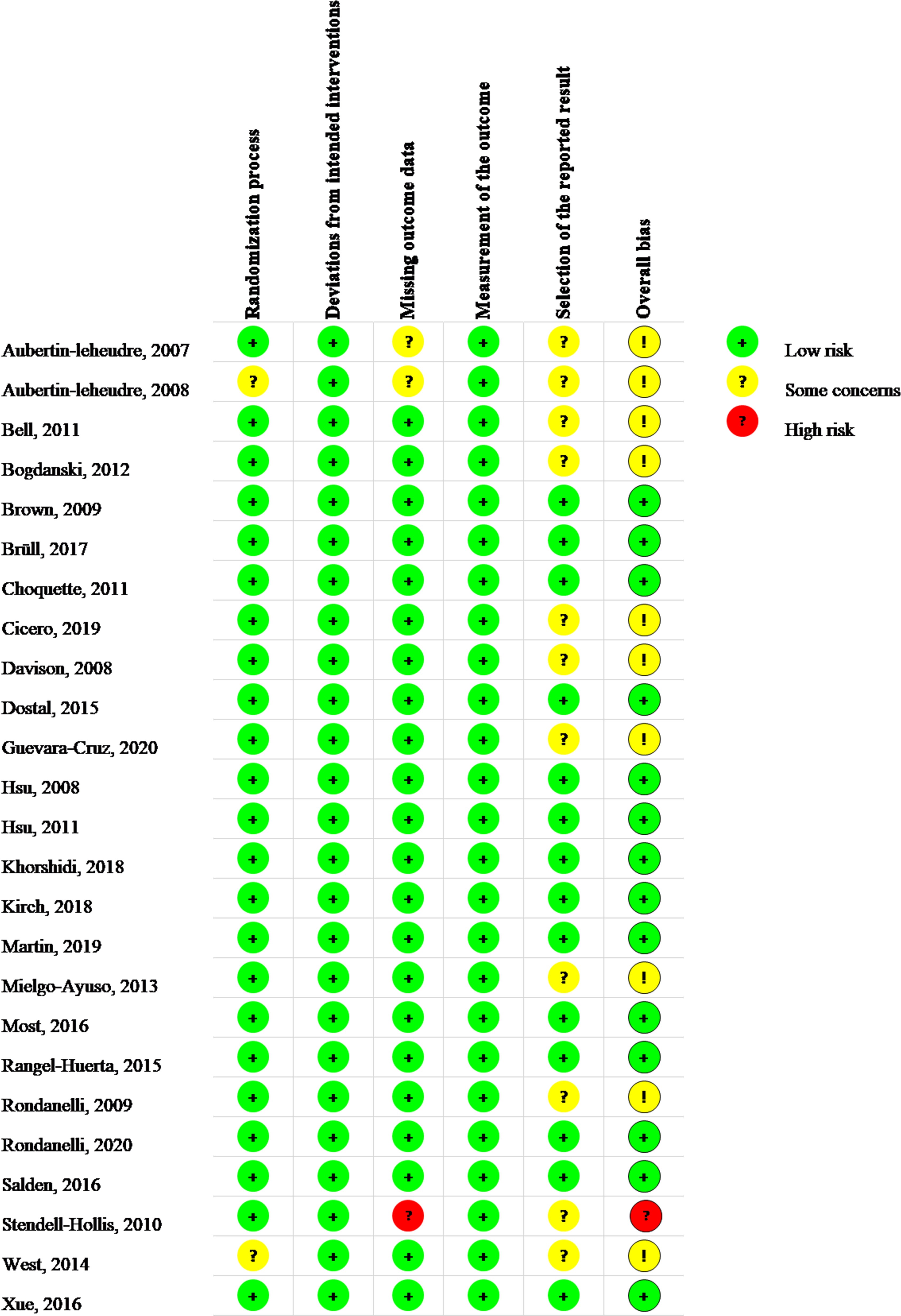
Figure 2 illustrated the data of the risk of bias graded for the 25 RCTs that were included (13–37). Two trials (14, 36) (8%) had some concerns about the randomization procedure due to the lack of details about the concealment method and baseline data; the remaining 23 (92%) RCTs were graded as low risk. Low risk was assigned to the 25 trials based on deviations from the intended interventions. In terms of missing outcome data, one (4%) trial (35) was graded as high risk for its missing outcome data were more than 5%, which may have a detrimental influence on the intervention’s estimated effect. Two RCTs (8%) (13, 14) were flagged as having missing outcome data due to adverse events, but the missing data did not differ between the treatment and control groups. In the 25 RCTs, the measurement bias of the outcome was graded as low risk (100%). Regarding the selection bias of the reported results, for those without protocols or registrations, 11 (44%) RCTs (13–16, 20–23, 29, 32, 35, 36) were graded as having some concerns; the remaining 14 (56%) RCTs were graded as low risk. In terms of the overall risk of bias, 14 (56%) RCTs were graded as low risk, 10 (40%) RCTs were rated as having some issues, and one (4%) RCT was rated as high risk.
3.4 Pooled results
3.4.1 Primary outcomes
(1) HOMA-IR
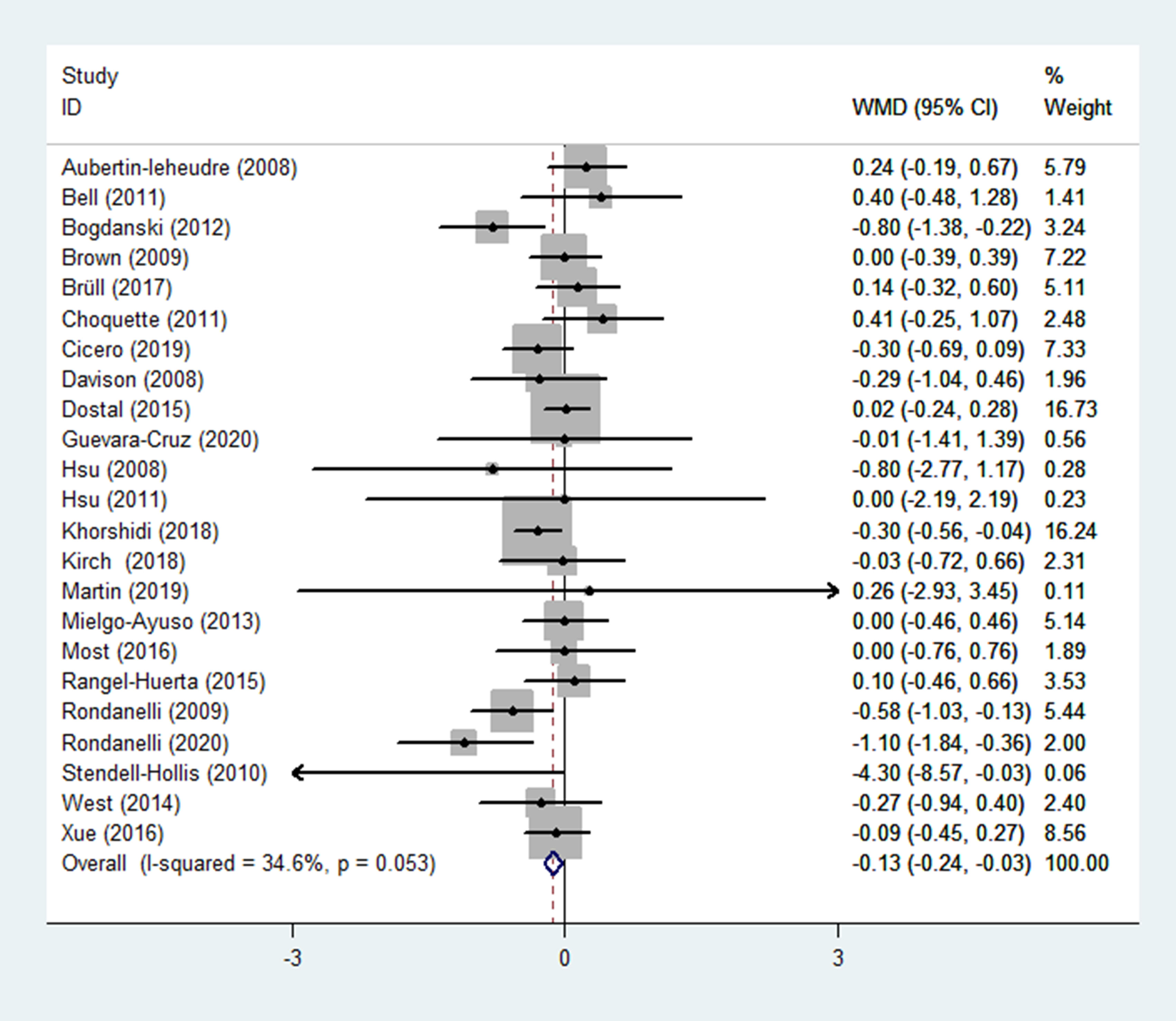
The HOMA-IR was reported as an outcome in 23 RCTs (14–33, 35–37) (n = 1670), with little heterogeneity (p = 0.053; I2 = 34.6%). The findings of a fixed-effects model demonstrated that HOMA-IR was significantly lower in the experimental group (receiving flavonoids-containing supplements) compared to that in the control group (WMD = -0.13, 95% CI: -0.24 to -0.03, p = 0.013) (Figure 3).
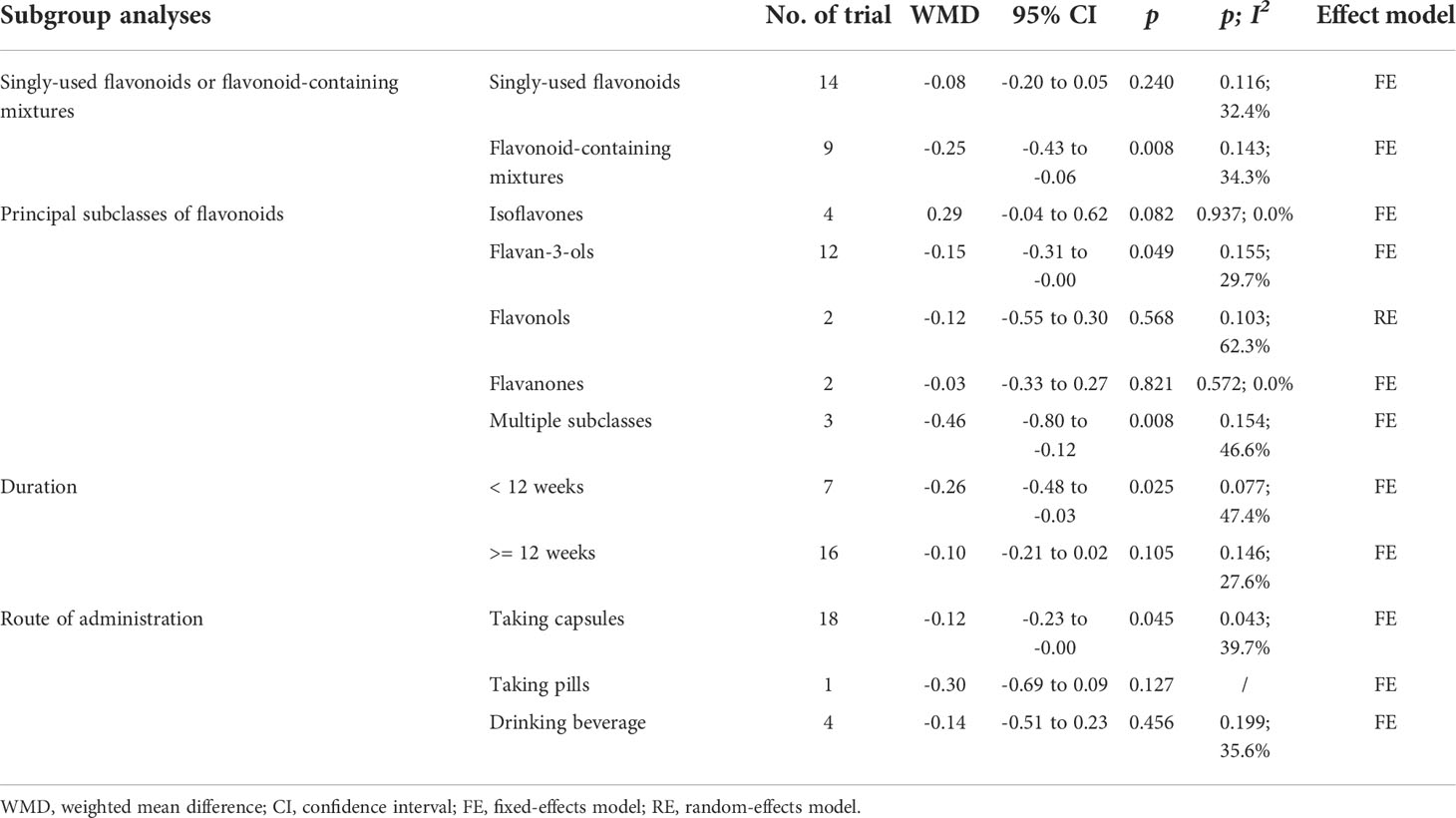
Subgroup analyses were performed based on the interventions (whether the application of singly-used flavonoids or flavonoid-containing mixtures). In 14 RCTs (14, 16–19, 21–27, 29, 35) (n = 1106), flavonoids were singly used for interventions, results of subgroup analysis obtained with a fixed-effects model revealed that the HOMA-IR in the experimental group and the control group did not differ (WMD = -0.08, 95% CI: -0.20 to 0.05, p = 0.240) with low heterogeneity (p = 0.116, I2 = 32.4%) (Table 2). In 9 RCTs (15, 20, 28, 30–33, 36, 37) (n = 564), flavonoid-containing mixtures were used for interventions, subgroup analyses by a fixed-effects model showed that in the experimental group, HOMA-IR was significantly decreased compared to that in the control group (WMD = -0.25, 95% CI: -0.43 to -0.06, p = 0.008) with low heterogeneity (p = 0.143, I2 = 34.3%) (Table 2).
Furthermore, subgroup analyses were performed according to principal subtypes of flavonoids. The results showed that the HOMA-IR of the experimental group significantly decreased compared to that of the control group in the subgroup receiving flavan-3-ols and the subgroup receiving multiple subclasses of flavonoids (WMD = -0.15, 95% CI: -0.31 to -0.00, p = 0.049; WMD = -0.46, 95% CI: -0.80 to -0.12, p = 0.008; respectively); however, in other subgroups, the HOMA-IR in the experimental group and the control group showed no difference (Table 2). The subgroup analyses were also performed based on duration, and results showed that the HOMA-IR significantly decreased in the short-duration subgroup (< 12 weeks) rather than in the long-duration subgroup (>= 12 weeks) (WMD = -0.26, 95% CI: -0.48 to -0.03, p = 0.025; WMD = -0.10, 95% CI: -0.21 to 0.02, p = 0.105; respectively) (Table 2).
Subgroup analyses of the routes of administration showed that, for the subgroup of taking capsules, the HOMA-IR in the experimental group significantly decreased compared to that of the control group (WMD = -0.12, 95% CI: -0.23 to -0.00, p = 0.045); however, for the subgroups of taking pills and drinking beverage, HOMA-IR showed no difference in the experimental group and the control group (WMD = -0.30, 95% CI: -0.69 to 0.09, p = 0.127; WMD = -0.14, 95% CI: -0.51 to 0.23, p = 0.456; respectively) (Table 2).
(2) QUICKI
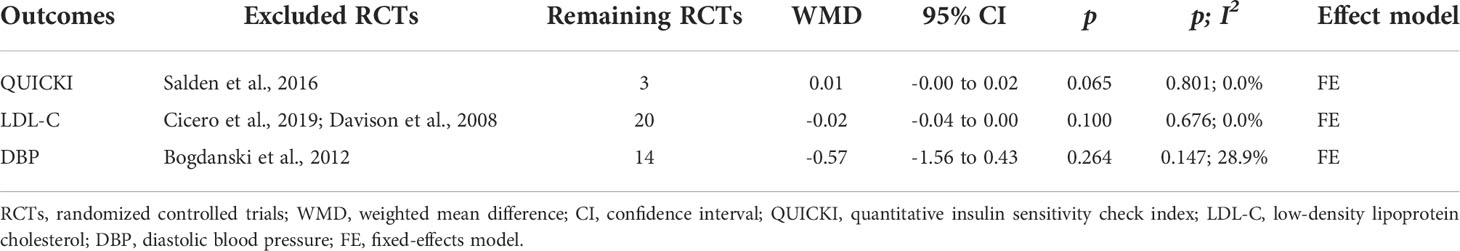
QUICKI was reported as an outcome in 4 RCTs (13, 28, 32, 34) (n = 227), with no difference found between the experimental and control groups, according to pooled data from a random-effects model (WMD = 0.07, 95% CI: -0.05 to 0.19, p = 0.236) with high heterogeneity (p < 0.001; I2 = 99.0%) (Figure 4). The heterogeneity statistically decreased (p = 0.801, I2 = 0.0%) after excluding Salden et al. (34), and pooled results showed that the QUICKI in the experimental group had an increasing trend compared to the control group, and the data were not statistically different (WMD = 0.01, 95% CI: -0.00 to 0.02, p = 0.065) (Table 3).
3.4.2 Secondary outcomes
(1) FBG
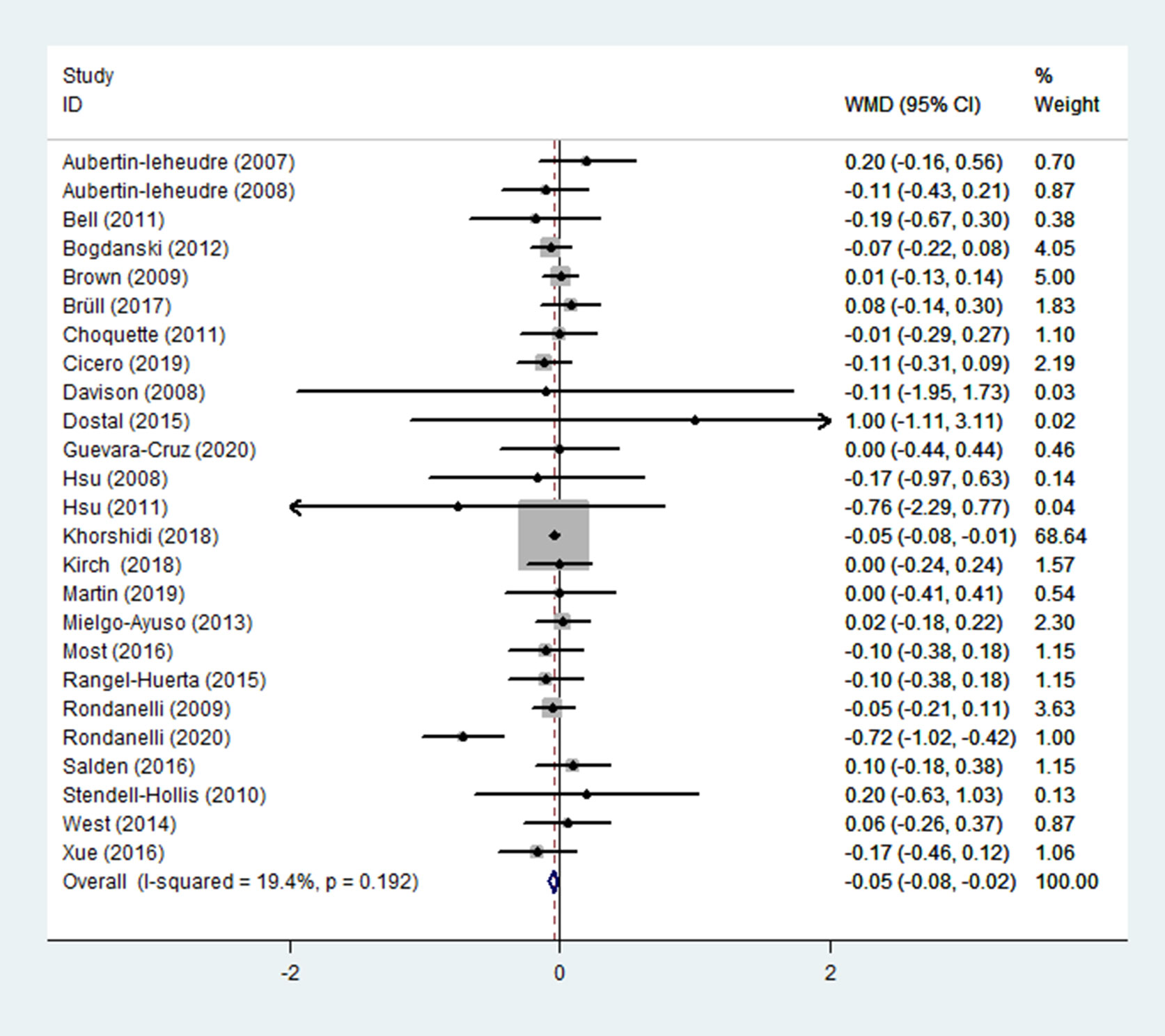
FBG was reported as an outcome in 25 trials (13–37) (n = 1755), in which low heterogeneity was found (p = 0.192; I2 = 19.4%). FBG levels in the experimental group (receiving flavonoid-containing supplements) were significantly lower than in the control group, according to pooled data from a fixed-effects model (WMD = -0.05, 95% CI: -0.08 to -0.02, p = 0.002) (Figure 5).
(2) FBI
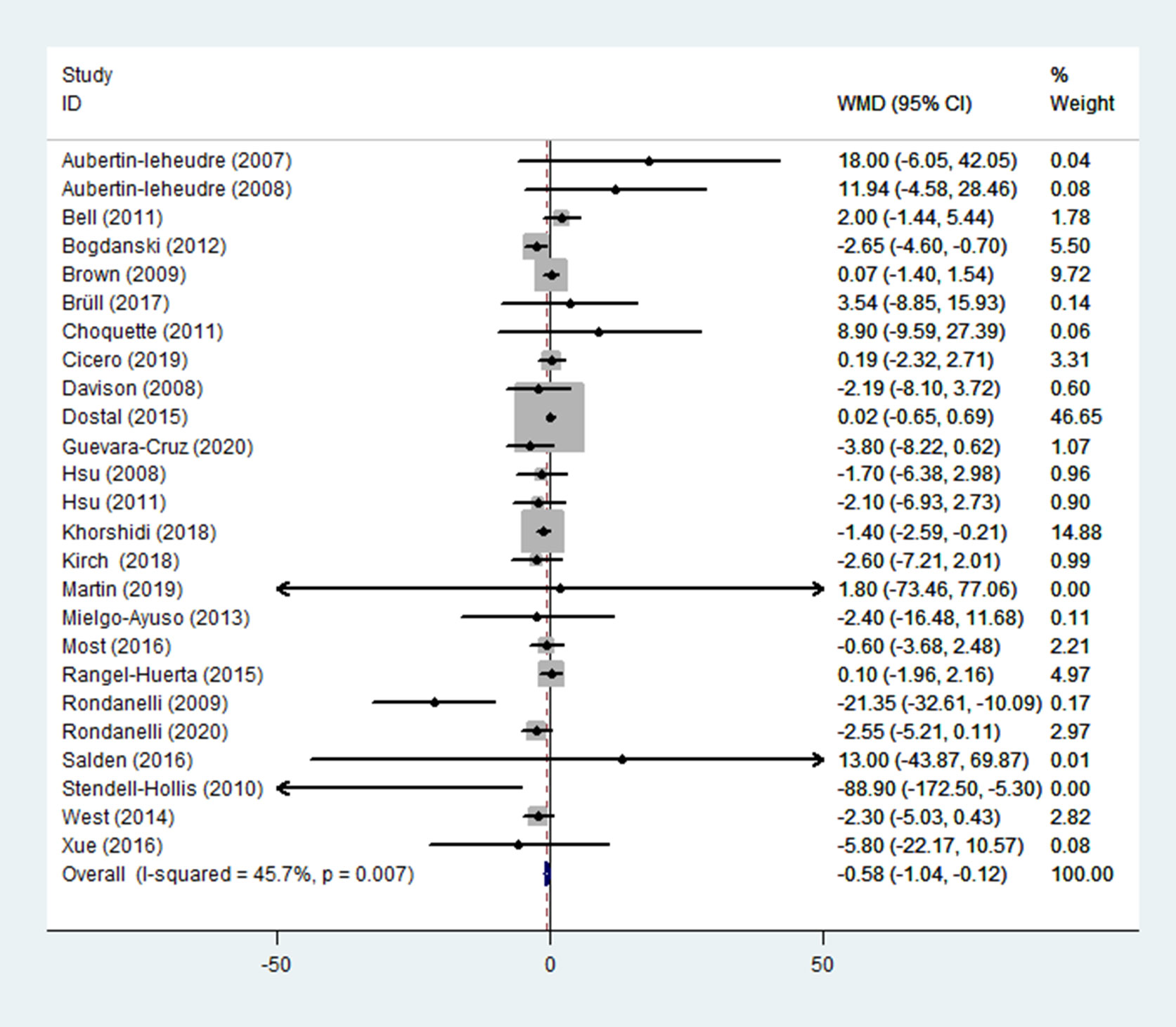
FBI was reported in 25 RCTs (13–37) (n = 1747), in which moderate heterogeneity was observed (p = 0.007; I2 = 45.7%). Results from a fixed-effects model demonstrated that in the experimental group (receiving flavonoids-containing supplements), the FBI significantly reduced compared to that in the control group (WMD = -0.58, 95% CI: -1.04 to -0.12, p = 0.014) (Figure 6).
(3) Blood lipids
Blood lipids including plasma total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were reported in 22 RCTs (13–17, 19–21, 23–25, 27–37) (n = 1305). Results of meta-analysis obtained with a fixed-effects model demonstrated that in the group receiving flavonoids-containing supplements, TC and TG significantly decreased compared to that in the controls (WMD = -0.04, 95% CI: -0.06 to -0.03, p < 0.001; WMD = -0.04, 95% CI: -0.05 to -0.03, p < 0.001; respectively) with moderate heterogeneity (p = 0.039, I2 = 37.8%; p = 0.019, I2 = 42.5%; respectively) (Table 4). However, HDL-C did not differ between the two groups (WMD = 0.01, 95% CI: -0.00 to 0.02, p = 0.143) with moderate heterogeneity (p = 0.025, I2 = 40.9%) (Table 4). Furthermore, LDL-C did not differ between the two groups (WMD = -0.26, 95% CI: -0.54 to 0.03, p = 0.078) with obvious heterogeneity (p < 0.001, I2 = 95.6%) (Table 4). Results of sensitivity analyses revealed that after excluding Cicero et al. (20) and Davison et al. (21), heterogeneity was significantly reduced (p = 0.676, I2 = 0.0%), and results still showed that the LDL-C in the experimental group and the control group did not differ (WMD = -0.02, 95% CI: -0.04 to 0.00, p = 0.100) (Table 3).
(4) Blood pressure
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were presented in 15 RCTs (14–17, 20, 21, 23–25, 27, 28, 31, 33, 34, 37) (n = 909). Pooled results of a fixed-effects model revealed that in the group receiving flavonoids-containing supplements, SBP significantly decreased compared to that in the control group (WMD = -2.01, 95% CI: -3.17 to -0.86, p = 0.001) with low heterogeneity (p = 0.410, I2 = 3.8%) (Table 4). However, DBP in the experimental group and the control group did not differ (WMD = -0.82, 95% CI: -2.23 to 0.60, p = 0.257) with obvious heterogeneity (p = 0.010, I2 = 52.1%) (Table 4). Results of sensitivity analyses showed that after excluding Bogdanski et al. (16), heterogeneity significantly dropped (p = 0.147, I2 = 28.9%), and pooled results still showed that DBP in the experimental group and the control group did not differ (WMD = -0.57, 95% CI: -1.56 to 0.43, p = 0.264) (Table 3).
(5) Weight
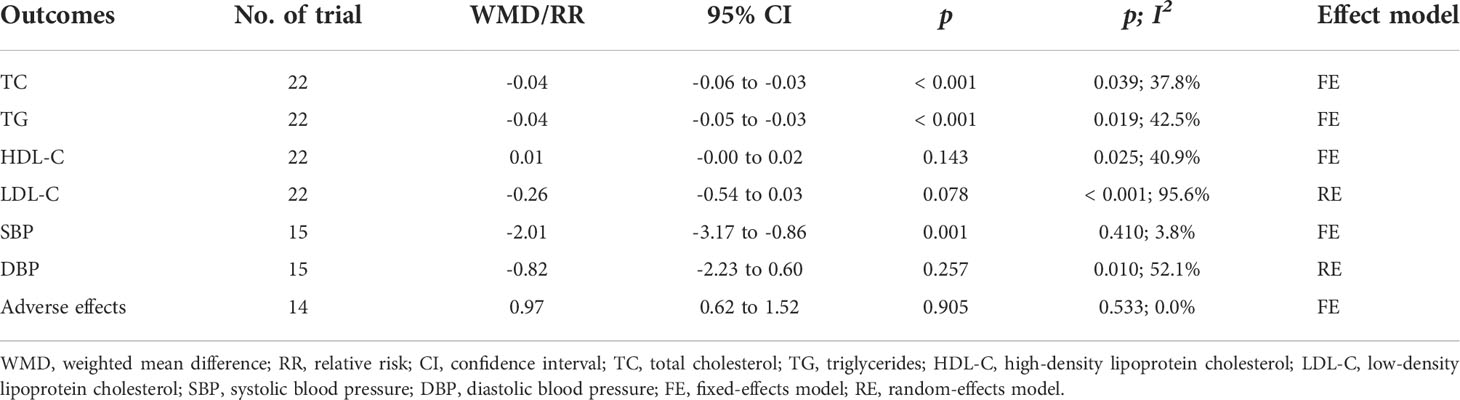
Weight was reported as an outcome in 16 RCTs (14, 15, 19, 22–27, 29–32, 35–37) (n = 1166). Pooled results from a fixed-effects model demonstrated that in the group receiving flavonoids-containing supplements, weight significantly decreased compared to that in the control group (WMD = -0.29, 95% CI: -0.49 to -0.09, p = 0.004) with low heterogeneity (p = 1.000, I2 = 0.0%) (Figure 7).
(6) BMI
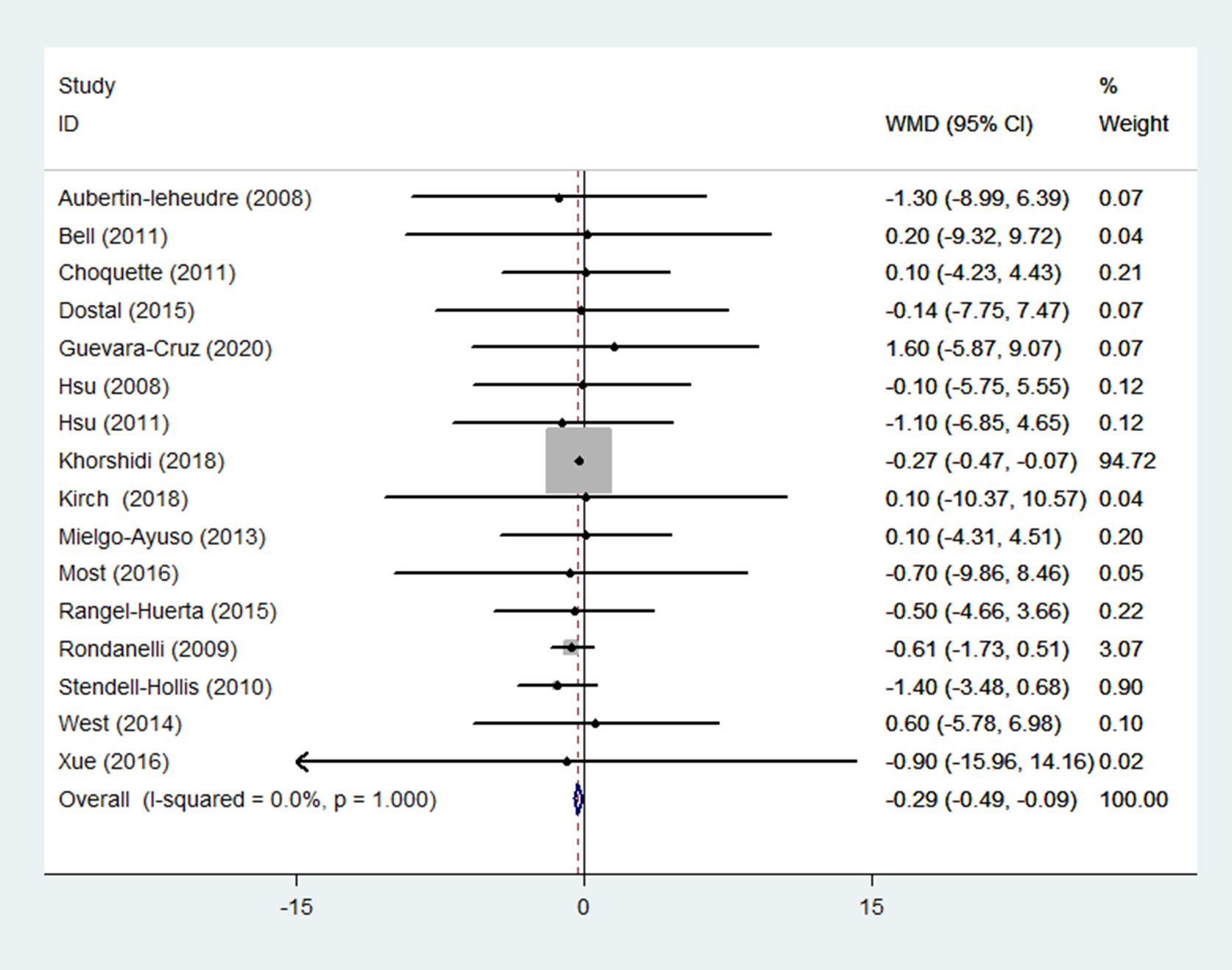
BMI was reported in 20 RCTs (14–17, 19–29, 31, 32, 35–37) (n = 1411). Pooled results obtained from a fixed-effects model indicated that in the group receiving flavonoids-containing supplements, BMI significantly decreased compared to that in the control group (WMD = -0.10 95% CI: -0.17 to -0.04, p = 0.003) with low heterogeneity (p = 0.999, I2 = 0.0%) (Figure 8).
(7) WC
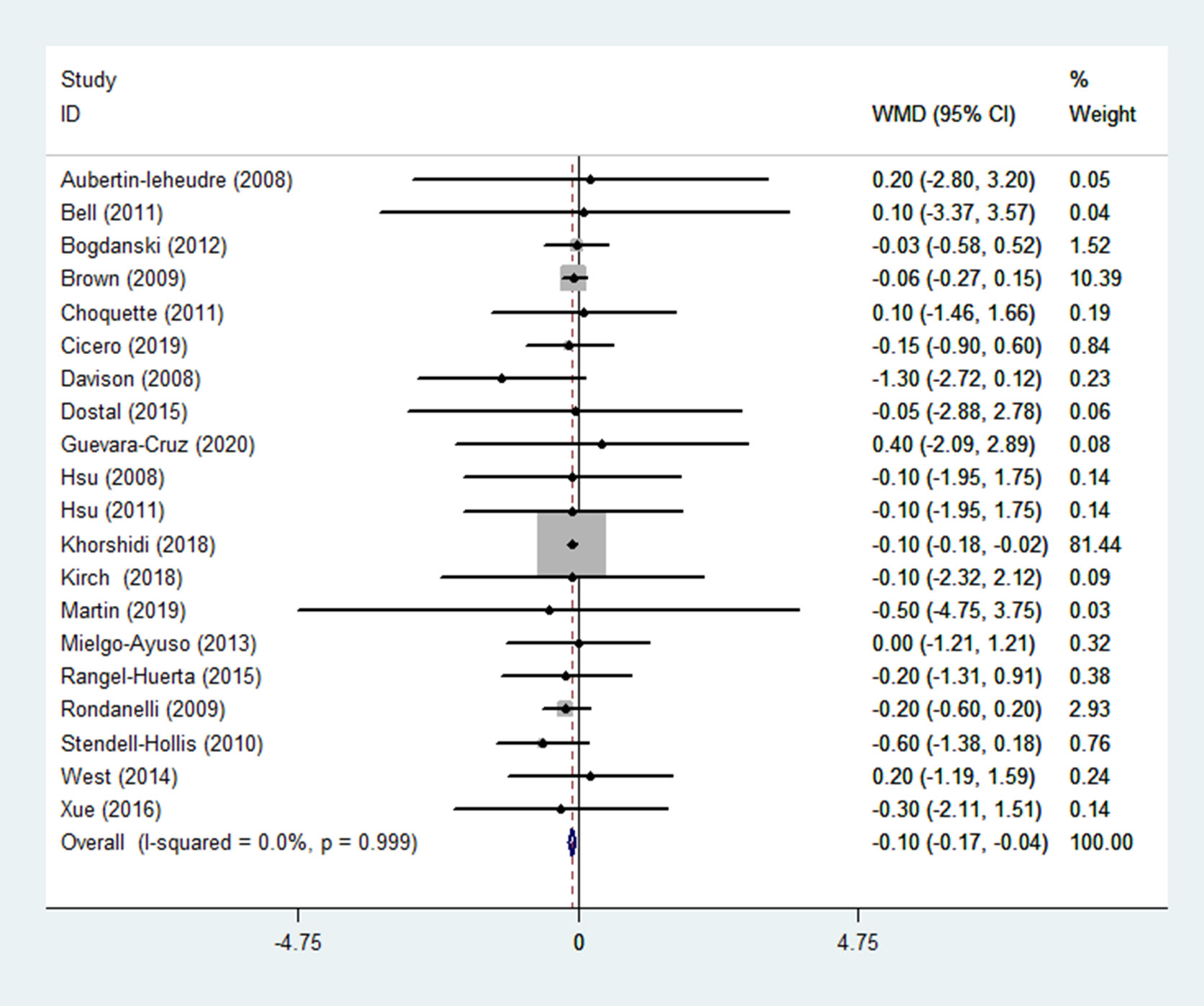
WC was reported in 18 RCTs (14–17, 19, 21–29, 31, 32, 35, 36) (n = 1299). Pooled results obtained with a fixed-effects model showed that WC in the group receiving flavonoids-containing supplements and the control group did not differ (WMD = -0.01, 95% CI: -0.09 to 0.08, p = 0.883) with low heterogeneity (p = 0.528, I2 = 0.0%) (Figure 9).
(8) WHR
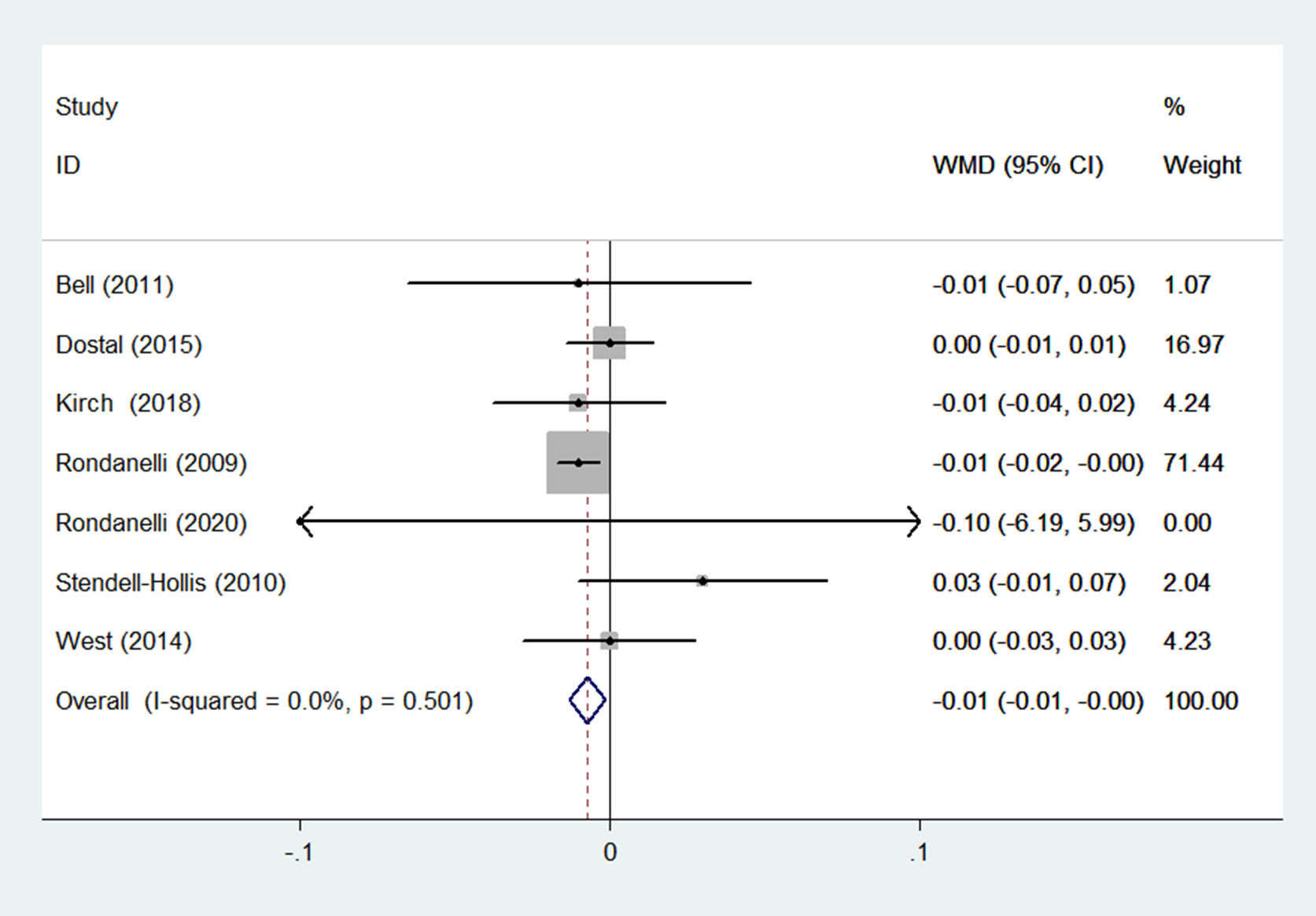
WHR was reported in 7 RCTs (15, 22, 27, 32, 33, 35, 36) (n = 624). Results obtained from a fixed-effects model demonstrated that in the group receiving flavonoids-containing supplements, WHR was significantly lower compared to that in the control group (WMD = -0.01, 95% CI: -0.01 to -0.00, p = 0.015) with small heterogeneity (p = 0.501, I2 = 0.0%) (Figure 10).
(9) Adverse effects
In 14 RCTs (13, 15, 18–20, 22, 24–26, 29, 30, 32, 33, 37) (n = 1130), adverse effects were reported as an outcome. Among 10 of them, no adverse effects were found in the group receiving flavonoid-containing supplements or the control group. Pooled results demonstrated that in the group receiving flavonoid-containing supplements, adverse reactions did not increase compared to that in the control group (RR = 0.97, 95% CI: 0.62 to 1.52, p = 0.905) with no heterogeneity (p = 0.533; I2 = 0.0%) (Table 4).
3.5 Publication bias
The outcomes of HOMA-IR, FBG, FBI, TC, TG, HDL-C, LDL-C, SBP, DBP, weight, BMI, and WC were evaluated based on a publication bias analysis. Most scatter points fell within the confidence limit, and the funnel plots were symmetrical. The p-value of Begg’s tests were 0.342, 0.591, 0.657, 0.535, 0.236, 0.535, 0.693, 0.488, 0.488, 0.893, 0.922, and 0.198, respectively; that of Egger’s tests were 0.483, 0.802, 0.234, 0.261, 0.677, 0.833, 0.175, 0.133, 0.120, 0.552, 0.422, and 0.106, respectively (Figure 11). The results of the publication bias analysis revealed that none of the included trials in the aforementioned outcome indicators had any potential of publication bias.
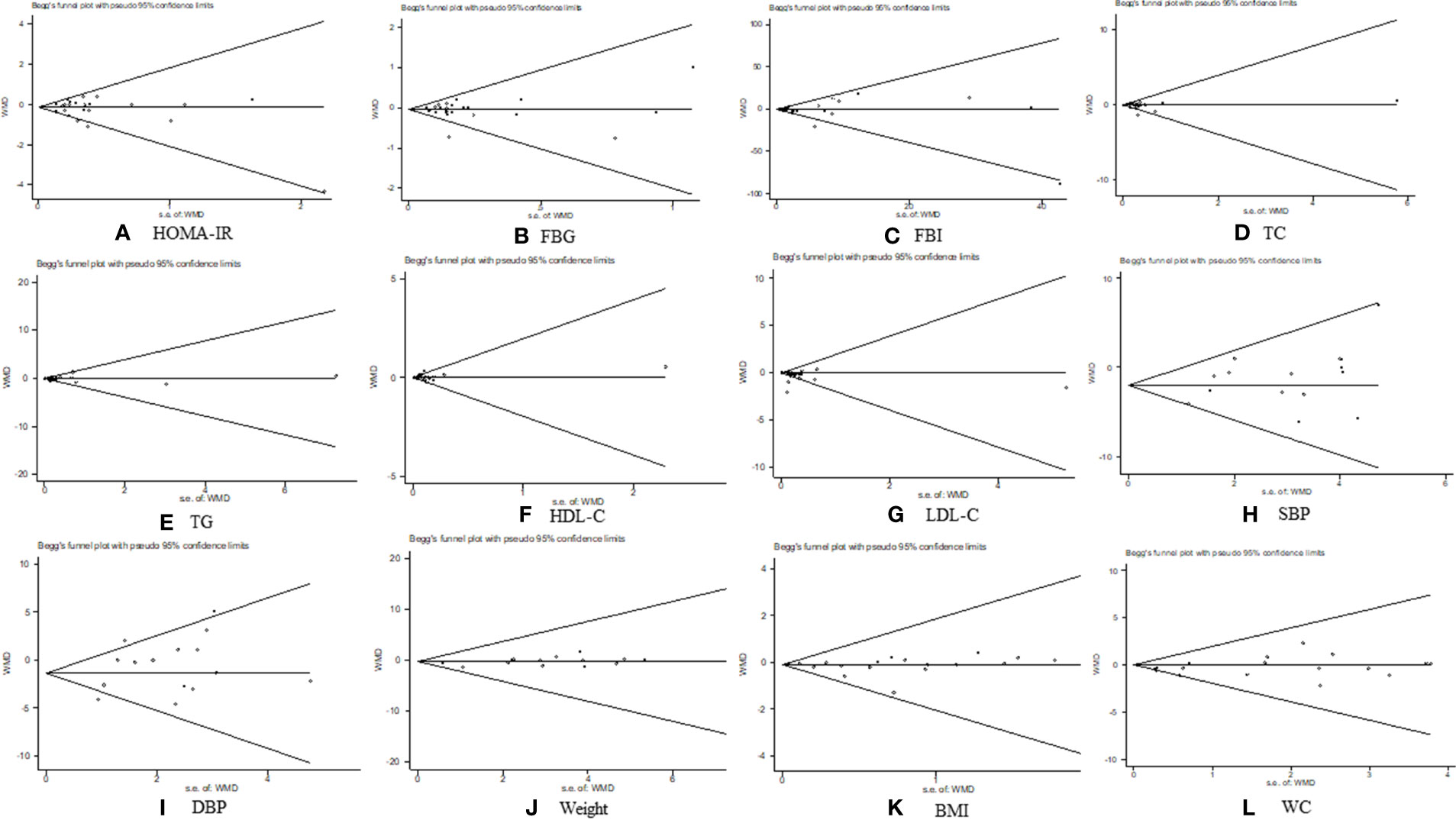
Figure 11 Publication bias analysis. (A) homeostasis model assessment of insulin resistance, (B) fasting blood glucose (C) fasting blood insulin, (D) total cholesterol, (E) triglycerides, (F) high-density lipoprotein cholesterol, (G) low-density lipoprotein cholesterol, (H) systolic blood pressure, (I) diastolic blood pressure, (J) weight, (K) body mass index, and (L) waist circumference.
4 Discussion
The current study firstly analyzed extant data and found that flavonoids-containing supplements might be effective and safe in treating IR and related metabolic risk factors (such as FBG, FBI, TC, TG, SBP, weight, BMI, and WHR) in overweight and obese subjects, based on 25 RCTs. This study provided preliminary evidence for the use of flavonoid-containing supplements as a treatment option for overweight and obesity.
For HOMA-IR, results demonstrated that HOMA-IR in the group receiving flavonoid-containing supplements significantly decreased versus the control group. Subgroup analyses showed that the effect of flavonoid-containing supplements on reducing HOMA-IR might be related to the interventions (singly-used flavonoids or flavonoid-containing mixtures). In the present study, flavonoids were singly used for interventions (isoflavone, EGCG, quercetin, flavanol, genistein, green tea extract, (–)-epicatechin, hesperidin 2S, and catechin) in 14 RCTs. Furthermore, flavonoid-containing mixtures (with flavonoids as the dominant compositions) were used for interventions in 9 RCTs. The RCT conducted by Bell et al. (15) used Glavonoid™ as an intervention, with the main ingredients composed of 30% licorice glabra polyphenol and 3% glabridin, both of which belong to glavonoid. Cicero et al. (20) used bergamot extract (120 mg flavonoids per pill) as intervention. Martin et al. (28) used tart cherry juice with anthocyanins and phenolics as main ingredients. Most et al. (30) used the co-formulation of EGCG and resveratrol, with EGCG (282 mg daily) as the main ingredient. Rangel-Huerta et al. (31) used polyphenol, with hesperidin and narirutin as the main ingredients. Rondanelli et al. (32) used the co-formulation of N-oleyl-phosphatidylethanolamine and EGCG. The amelioration of IR could be attributed to EGCG (100 mg daily), and the effect of N-oleyl-phosphatidylethanolamine mainly included reduction of food intake and amelioration in vivo plasma availability of EGCG (38). Rondanelli et al. (33) used Cynara, of which flavonoids (>= 1.5%) were the main component, and it was only lower than that of caffeoylquinic acids. West et al. (36) used cocoa/chocolate containing flavanols (814 mg daily). Xue et al. (37) used trans-resveratrol-hesperetin co-formulation, and the main ingredient was hesperetin (120 mg daily). In the singly-used flavonoids subgroup, HOMA-IR in the experimental group and the control group did not differ. However, in the subgroup receiving flavonoid-containing mixtures, HOMA-IR significantly decreased in the experimental group compared to that in the control group. Pooled results showed that QUICKI between the experimental group and the control group did not differ. However, the results may be influenced by the obvious heterogeneity (I2 = 99.0%). Salden et al. (34) used hesperidin 2S as an intervention for the experimental group, the sensitivity analyses showed that after excluding Salden et al. (34), the heterogeneity statistically decreased (I2 = 0.0%), pooled results indicated that QUICKI in the experimental group had an increasing trend compared to that in the control group. Similar to our findings, a series of comprehensive reviews also reported the effects of flavonoids on alleviating IR (39–41). Furthermore, according to a meta-analysis conducted of type 2 diabetes mellitus subjects conducted by Liu et al. (42), flavonoids brought significant benefits to glucose metabolism and insulin sensitivity, especially significantly lowing FBG, HOMA-IR, and HbA1c. Another meta-analysis reported the beneficial effects of flavan-3-ol intake on cardiometabolic outcomes including HOMA-IR (43).
Flavonoids have a variety of physiologic properties, including anti-inflammatory and antioxidative activities. They may help to reduce IR (9) by blocking the formation and expression of proinflammatory mediators and/or enzymes, such as suppressing inflammatory cytokines via the TLR4/NF-B signaling pathway, stimulating AMPK, activating autophagy, and protecting against the atrophy of obesity-related skeletal muscle by repressing inflammatory cytokines and macrophage infiltration (44). Additionally, flavonoids may regulate whole-body glucose homeostasis by interacting with a variety of molecular targets in the small intestine, pancreas, skeletal muscle, adipose tissue, and liver. Flavonoids also exhibit pleiotropic properties such as decreasing intestinal glucose absorption, improving insulin secretory and insulin-sensitizing actions, and increasing glucose consumption in peripheral tissues, all of which contribute to improving IR (9). However, in the present study, HOMA-IR did not show a difference in the singly-used flavonoids group compared to that in the control group and the QUICKI in the experimental group had an increasing trend compared to that in the control group, probably because of different subtypes of flavonoids and the limited RCTs included. In addition, apart from flavonoids, the above flavonoid-containing mixtures may contain other ingredients (such as phenolics and caffeoylquinic acids) that may be beneficial to reduce IR.
Furthermore, subgroup analyses based on principal subclasses of flavonoids showed that HOMA-IR significantly decreased in the experimental group versus the control group when using flavan-3-ols and multiple subclasses of flavonoids. Thus, we speculated that different subclasses of flavonoids might have different potencies in reducing HOMA-IR. Flavan-3ols are the most frequently used flavonoids in the diet, and they are found in drinks, fruits, vegetables, grains, herbal medicines, nutritional supplements, and dairy products. Catechin, epicatechin, catechin gallate, gallocatechin, epigallocatechin, epicatechin gallate, gallocatechin gallate, and EGCG are the major components of flavan-3-ols. Flavan-3-ols, particularly EGCG, have been linked to hypoglycemic, anti-inflammatory, antioxidant, and thermogenic activities (45). Various in vivo or in vitro experimental studies on catechins and their chemical derivatives have reported that they can improve IR. There are four hypothesized mechanisms: 1) suppressing the inflammatory pathway mediated by NF-kappa B (46, 47); 2) reducing free radicals via inhibiting lipid peroxidation, stimulating antioxidant enzymes (48), suppressing redox-sensitive transcription factors, and decreasing pro-oxidant enzyme mechanisms (49); 3) stimulating pancreatic beta cells to improve postprandial insulin, thereby improving pancreas function (50); 4) reducing adipocyte proliferation and differentiation while improving glucose receipt by the cells via protein kinase activation, a mechanism similar to that utilized by hypoglycemic medicines such as metformin (24, 51). Our findings suggest that various flavonoid subclasses may have varying potencies in enhancing IR; nevertheless, results remain uncertain because by far no direct comparative studies have been conducted.
As for secondary outcomes, pooled results demonstrated that in the group receiving flavonoid-containing supplements, other metabolic markers including FBG, FBI, TC, TG, SBP, weight, BMI, and WHR significantly decreased compared to those in the controls. Other studies also showed that flavonoids can prevent and/or ameliorate obesity and obesity-associated diseases (52, 53). IR is thought to be the common core pathogenic foundation of metabolic diseases such as metabolic syndrome, hypertension, and diabetes mellitus, all of which endanger human health (54, 55). As mentioned above, flavonoids have the effect of improving insulin resistance, which is beneficial to preventing and/or ameliorating obesity and obesity-associated diseases (such as diabetes mellitus, hypertension, dyslipidemia, and metabolic syndrome). Some other mechanisms by which flavonoids improve obesity and obesity-associated diseases have also been found (52). Studies indicated that many flavonoids can reduce oxidative stress, enhance glucose tolerance, alter lipid metabolism and adipocyte differentiation, inhibit inflammation and apoptosis, and ameliorate endothelial dysfunction (56–60), showing that they may have an anti-diabetic effect. Furthermore, the effects of flavonoids on hypertension are well established and appear to be mechanistically connected to NO bioavailability, which is controlled by NOS activation and/or NOX inhibition. Additionally, flavonoids may reduce lipid absorption in the gastrointestinal tract, as well as help regulate the activity of various enzymes included in lipid metabolism and the expression of transcription factors included in TG and cholesterol synthesis, such as the sterol regulatory element-binding proteins SREBP-1 and SREBP-2 (61), indicating their potential anti-hyperlipidemic effect. Furthermore, aggregated data revealed that the incidence of adverse reactions did not differ between the group receiving flavonoids-containing supplements and the control group, showing that flavonoids-containing supplements were safe and well-tolerated in clinical practice under general usage settings.
Nevertheless, the present study has certain limitations which should be considered. Firstly, this study encompassed all flavonoids-containing supplements, including solely flavonoids and mixtures, but it included only a few RCTs for each type of flavonoids-containing supplement. Therefore, more RCTs are needed to identify the efficacy of each type of flavonoids-containing supplement in overweight and obese subjects. Secondly, due to the small number of included RCTs, publication bias was not assessed in the QUCIKI and WHR outcomes; more RCTs with the outcomes of QUCIKI and WHR reported are needed to further verify our conclusion. Thirdly, several of the included RCTs were of poor quality. For example, they were a single-centered study with a small number of participants. Fourthly, further study is required to quantify the optimal type and amounts of flavonoids for determining the appropriate prescription of flavonoid consumption to treat overweight and obese patients. Fifthly, given that the included RCTs were conducted in different countries and regions, more high-quality studies using uniformly sourced flavonoids-containing supplements are needed to further verify our conclusions. Furthermore, further studies on the pharmacological processes, long-term toxicity, and bioavailability of flavonoids in the treatment of overweight and obese people are needed. Given the limitations of this study, we suggest that the findings should be validated in future research.
5 Conclusion
This systematic review and meta-analysis evaluated the current data and demonstrated that flavonoids-containing supplements might be efficacious and safe in treating IR and related metabolic risk factors (such as FBG, FBI, TC, TG, SBP, weight, BMI, and WHR) in overweight and obese subjects. This study presented preliminary evidence to guide the use of flavonoids-containing supplements as a therapy option for overweight and obese subjects. However, there is still doubt over the findings because limited RCTs for per kind of flavonoids-containing supplement were investigated, and many of the RCTs had a small sample size and short duration. Given the limitations, we propose that the results should be established or confirmed on a larger scale with more precise instructions in future investigations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: JY. Data curation: JY, YZ, JZ, X-ZW. Formal analysis: JZ. Project administration: Y-PL, G-JF, LS. Supervision: G-JF. Validation: JY, Q-YL. Writing – original draft: JY, JZ, YZ. Writing – review & editing: G-JF, LS. All the authors have read and approved the manuscript.
Funding
This study was supported by the Chinese Government, Ministry of Science, and Technology of the People’s Republic of China through the National Science and Technology Support Program (Grant No. 2015BAI04B09) and Guangdong Provincial Hospital of Traditional Chinese Medicine (Grant No. 2021DB02).
Acknowledgments
We thank Zhaojun Yang for preparing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.917692/full#supplementary-material
Abbreviations
IR, insulin resistance; RCTs, randomized controlled trials; HOMA-IR, homeostasis model assessment of insulin resistance; WMD, weighted mean difference; CI, confidence interval; QUICKI, quantitative insulin sensitivity check index; FBG, fasting blood glucose; FBI, fasting blood insulin; TC, total cholesterol; TG, triglycerides; SBP, systolic blood pressure; BMI, body mass index; WHR, waist-to-hip ratio; RR, relative risk. Main text section: IR, insulin resistance; RCTs, randomized controlled trials; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; FBG, fasting blood glucose; FBI, fasting blood insulin; BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; WMD, weighted mean difference; CI, confidence interval; RR, relative risk; EGCG, epigallocatechin gallate; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure.
References
1. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res (2020) 126:1549–64. doi: 10.1161/CIRCRESAHA.119.315896
2. World Health Organization. Obesity and overweight (2019). Available at: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (Accessed 25 November 2019).
3. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev (2012) 70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x
4. Unger RH. Lipotoxic diseases. Annu Rev Med (2002) 53:319–36. doi: 10.1146/annurev.med.53.082901.104057
5. Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabetes Med (2008) 25:597–605. doi: 10.1111/j.1464-5491.2008.02417.x
6. D'Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita (2007) 43:348–61.
7. Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem (2010) 58:4959–69. doi: 10.1021/jf100128b
8. Sebastian RS, Wilkinson Enns C, Goldman JD, Moshfegh AJ. Dietary flavonoid intake is inversely associated with cardiovascular disease risk as assessed by body mass index and waist circumference among adults in the united states. Nutrients (2017) 9:827. doi: 10.3390/nu9080827
9. Eid HM, Haddad PS. The antidiabetic potential of quercetin: Underlying mechanisms. Curr Med Chem (2017) 24:355–64. doi: 10.2174/0929867323666160909153707
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement, an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
11. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2, a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
12. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol (1992) 45:769–73. doi: 10.1016/0895-4356(92)90054-q
13. Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double-blind study. Menopause (2007) 14:624–9. doi: 10.1097/gme.0b013e31802e426b
14. Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Isoflavones and clinical cardiovascular risk factors in obese postmenopausal women: a randomized double-blind placebo-controlled trial. J Womens Health (Larchmt) (2008) 17:1363–9. doi: 10.1089/jwh.2008.0836
15. Bell ZW, Canale RE, Bloomer RJ. A dual investigation of the effect of dietary supplementation with licorice flavonoid oil on anthropometric and biochemical markers of health and adiposity. Lipids Health Dis (2011) 10:29. doi: 10.1186/1476-511X-10-29
16. Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res (2012) 32:421–7. doi: 10.1016/j.nutres.2012.05.007
17. Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr (2009) 101:886–94. doi: 10.1017/S0007114508047727
18. Brüll V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Müller C, et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: a randomized double-blinded, placebo-controlled crossover trial. Eur J Nutr (2017) 56:2265–75. doi: 10.1007/s00394-016-1267-0
19. Choquette S, Riesco É, Cormier É, Dion T, Aubertin-Leheudre M, Dionne IJ. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr (2011) 105:1199–209. doi: 10.1017/S0007114510004897
20. Cicero AFG, Fogacci F, Bove M, Giovannini M, Borghi C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother Res (2019) 33:2094–101. doi: 10.1002/ptr.6402
21. Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) (2008) 32:1289–96. doi: 10.1038/ijo.2008.66
22. Dostal AM, Samavat H, Espejo L, Arikawa AY, Stendell-Hollis NR, Kurzer MS. Green tea extract and catechol-O-Methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J Nutr (2016) 146:38–45. doi: 10.3945/jn.115.222414
23. Guevara-Cruz M, Godinez-Salas ET, Sanchez-Tapia M, Torres-Villalobos G, Pichardo-Ontiveros E, Guizar-Heredia R, et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res Care (2020) 8:e000948. doi: 10.1136/bmjdrc-2019-000948
24. Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr (2008) 27:363–70. doi: 10.1016/j.clnu.2008.03.007
25. Hsu CH, Liao YL, Lin SC, Tsai TH, Huang CJ, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? a randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev (2011) 16:157–63.
26. Khorshidi M, Moini A, Alipoor E, Rezvan N, Gorgani-Firuzjaee S, Yaseri M, et al. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother Res (2018) 32:2282–89. doi: 10.1002/ptr.6166
27. Kirch N, Berk L, Liegl Y, Adelsbach M, Zimmermann BF, Stehle P, et al. A nutritive dose of pure (-)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults-a randomized, placebo-controlled, double-blind crossover study. Am J Clin Nutr (2018) 107:948–56. doi: 10.1093/ajcn/nqy066
28. Martin KR, Coles KM. Consumption of 100% tart cherry juice reduces serum urate in overweight and obese adults. Curr Dev Nutr (2019) 3:nzz011. doi: 10.1093/cdn/nzz011
29. Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, Margareto J, Labayen I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr (2014) 111:1263–71. doi: 10.1017/S0007114513003784
30. Most J, Timmers S, Warnke I, Jocken JW, van Boekschoten M, de Groot P, et al. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: a randomized controlled trial. Am J Clin Nutr (2016) 104:215–27. doi: 10.3945/ajcn.115.122937
31. Rangel-Huerta OD, Aguilera CM, Martin MV, Soto MJ, Rico MC, Vallejo F, et al. Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr (2015) 145:1808–16. doi: 10.3945/jn.115.213660
32. Rondanelli M, Opizzi A, Solerte SB, Trotti R, Klersy C, Cazzola R. Administration of a dietary supplement (N-oleyl-phosphatidylethanolamine and epigallocatechin-3-gallate formula) enhances compliance with diet in healthy overweight subjects: a randomized controlled trial. Br J Nutr (2009) 101:457–64. doi: 10.1017/S0007114508024008
33. Rondanelli M, Riva A, Petrangolini G, Allegrini P, Bernardinelli L, Fazia T, et al. The metabolic effects of cynara supplementation in overweight and obese class I subjects with newly detected impaired fasting glycemia: A double-blind, placebo-controlled, randomized clinical trial. Nutrients (2020) 12:3298. doi: 10.3390/nu12113298
34. Salden BN, Troost FJ, de Groot E, Stevens YR, Garcés-Rimón M, Possemiers S, et al. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am J Clin Nutr (2016) 104:1523–33. doi: 10.3945/ajcn.116.136960
35. Stendell-Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet (2010) 23:590–600. doi: 10.1111/j.1365-277X.2010.01078.x
36. West SG, McIntyre MD, Piotrowski MJ, Poupin N, Miller DL, Preston AG, et al. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br J Nutr (2014) 111:653–61. doi: 10.1017/S0007114513002912
37. Xue M, Weickert MO, Qureshi S, Kandala NB, Anwar A, Waldron M, et al. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes (2016) 65:2282–94. doi: 10.2337/db16-0153
38. Broccali G, Berti M, Pistolesi E, Cestaro B. N-oleoyl-phosphatidylethanolamine reduces food intake and body weight of dietary obese rats ameliorating their antioxidant status. Gazz Med Ital Arch Sci Med (2005) 164:101–7.
39. Ren N, Kim E, Li B, Pan H, Tong T, Yang CS, et al. Flavonoids alleviating insulin resistance through inhibition of inflammatory signaling. J Agric Food Chem (2019) 67:5361–73. doi: 10.1021/acs.jafc.8b05348
40. Dinda B, Dinda M, Roy A, Dinda S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv Protein Chem Struct Biol (2020) 120:159–235. doi: 10.1016/bs.apcsb.2019.08.006
41. Russo B, Picconi F, Malandrucco I, Frontoni S. Flavonoids and insulin-resistance: From molecular evidences to clinical trials. Int J Mol Sci (2019) 20:2061. doi: 10.3390/ijms20092061
42. Liu F, Sirisena S, Ng K. Efficacy of flavonoids on biomarkers of type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr (2021) 29:1–27. doi: 10.1080/10408398.2021.2009761
43. Raman G, Avendano EE, Chen S, Wang J, Matson J, Gayer B, et al. Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies. Am J Clin Nutr (2019) 110:1067–78. doi: 10.1093/ajcn/nqz178
44. Sato S, Mukai Y. Modulation of chronic inflammation by quercetin: The beneficial effects on obesity. J Inflammation Res (2020) 13:421–31. doi: 10.2147/JIR.S228361
45. Ferreira MA, Silva DM, de Morais AC Jr, Mota JF, Botelho PB. Therapeutic potential of green tea on risk factors for type 2 diabetes in obese adults - a review. Obes Rev (2016) 17:1316–28. doi: 10.1111/obr.12452
46. Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-kappa b activation by inhibiting I kappa b kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol (2001) 60:528–33.
47. Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem (2006) 6:945–51. doi: 10.2174/138955706777934937
48. Tsai CF, Hsu YW, Ting HC, Huang CF, Yen CC. The in vivo antioxidant and antifibrotic properties of green tea (Camellia sinensis, theaceae). Food Chem (2013) 136:1337–44. doi: 10.1016/j.foodchem.2012.09.063
49. Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem (2008) 15:1840–50. doi: 10.2174/092986708785132979
50. Sundaram R, Naresh R, Shanthi P, Sachdanandam P. Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine (2013) 20:577–84. doi: 10.1016/j.phymed.2013.01.006
51. Banerjee S, Ghoshal S, Porter TD. Phosphorylation of hepatic AMP-activated protein kinase and liver kinase B1 is increased after a single oral dose of green tea extract to mice. Nutr Res (2012) 32:985–90. doi: 10.1016/j.nutres.2012.10.005
52. Galleano M, Calabro V, Prince PD, Litterio MC, Piotrkowski B, Vazquez-Prieto MA, et al. Flavonoids and metabolic syndrome. Ann N Y Acad Sci (2012) 1259:87–94. doi: 10.1111/j.1749-6632.2012.06511.x
53. Zhang S, Xu M, Zhang W, Liu C, Chen S. Natural polyphenols in metabolic syndrome: Protective mechanisms and clinical applications. Int J Mol Sci (2021) 22:6110. doi: 10.3390/ijms22116110
54. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015. a systematic analysis for the global burden of disease study. Lancet (2015) 2016:1545–602. doi: 10.1016/S0140-6736(16)31678-6
55. Prabhakar SS. Inhibition of renin-angiotensin system: implications for diabetes control and prevention. J Investig Med (2013) 61:551–7. doi: 10.2310/JIM.0b013e31828298ce
56. Mahmoud AM, Hernández Bautista RJ, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev (2019) 2019:5484138. doi: 10.1155/2019/5484138
57. Millar CL, Duclos Q, Blesso CN. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv Nutr (2017) 8:226–39. doi: 10.3945/an.116.014050
58. Rees A, Dodd GF, Spencer JPE. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients (2018) 10:1852. doi: 10.3390/nu10121852
59. Zaidun NH, Thent ZC, Latiff AA. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci (2018) 208:111–22. doi: 10.1016/j.lfs.2018.07.017
60. Zhang X, Li X, Fang H, Guo F, Li F, Chen A, et al. Flavonoids as inducers of white adipose tissue browning and thermogenesis: signalling pathways and molecular triggers. Nutr Metab (Lond) (2019) 16:47. doi: 10.1186/s12986-019-0370-7
Keywords: Flavonoids, insulin resistance, overweight, obesity, systematic review, meta-analysis
Citation: Yao J, Zhang Y, Zhao J, Wang X-Z, Lin Y-P, Sun L, Lu Q-Y and Fan G-J (2022) Efficacy of flavonoids-containing supplements on insulin resistance and associated metabolic risk factors in overweight and obese subjects: a systematic review and meta-analysis of 25 randomized controlled trials. Front. Endocrinol. 13:917692. doi: 10.3389/fendo.2022.917692
Received: 11 April 2022; Accepted: 04 July 2022;
Published: 22 July 2022.
Edited by:
Katherine Samaras, St Vincent’s Hospital Sydney, AustraliaReviewed by:
Siyu Chen, China Pharmaceutical University, ChinaAR SRINIVASAN, Sri Balaji Vidyapeeth University, India
Copyright © 2022 Yao, Zhang, Zhao, Wang, Lin, Sun, Lu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guan-Jie Fan, ZmFuZ3VhbmppZWd6QDE2My5jb20=
†These authors have contributed equally to this work
 Jia Yao1,2,3†
Jia Yao1,2,3† Guan-Jie Fan
Guan-Jie Fan