
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 23 June 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.917498
This article is part of the Research Topic Thyroid Endocrine Disruptors View all 10 articles
Background: With the rapid advance in percutaneous coronary intervention (PCI) technology, patients absorb large volume of iodinated contrast media (ICM). Recent studies suggested that ICM may lead to hyperthyroidism, but the association between ICM volume and thyroid is still unclear. We sought to evaluate the long-term influence of ICM on thyroid dysfunction and disease in patients received PCI.
Methods: This single-center retrospective study included consecutive coronary artery disease (CAD) patients. A covariance (ANCOVA) model was performed to evaluate the change of serum TSH, FT3 and FT4 before and one-year after the PCI procedure. Restricted cubic splines and logistic regression were performed to evaluate the association between ICM volume and thyroid disease.
Results: 2062 patients met inclusion criteria (1381 patients in the low-volume group and 681 patients in the high-volume group). The high-volume group was 0.238 ± 0.092 pmol/L higher than the low-volume group (P = 0.010) in the serum FT4. Restricted cubic splines show that there were linear dose-response relationships for ICM volume and composite endpoint and hyperthyroidism. In all models, there were significant differences in composite endpoint between the two groups. (OR 1.75, 95% CI (1.05, 2.92), P = 0.032, OR 1.73, 95% CI (1.01-2.96), P= 0.032 and OR 1.83, 95% CI (1.09-3.06), P= 0.022, respectively). The positive results were also showed for hyperthyroidism in all models (OR 2.35, 95% CI (1.14-4.84), P = 0.021, OR 10.36, 95% CI (1.20-89.00), P = 0.033 and OR 2.35, 95% CI (1.13-4.87), P = 0.022, respectively).
Conclusion: The present analysis gives an overview that ICM volume is associated with an increased risk of thyroid dysfunction and thyroid disease.
The practice of coronary angiography and percutaneous coronary intervention (PCI) procedures has heavily relied on iodinated contrast media (ICM). The rapid advance in PCI technology has greatly improved the diagnosis and treatment for consecutive coronary artery disease (CAD) patients. Unfortunately, because of the aging population and burden of complications, the overall quantity and complexity of PCI have increased (1). In a routine PCI procedure, the patient may absorb around 200 times the daily requirement for iodine. The nephrotoxicity effect of ICM has been widely and deeply explored (2, 3). However, a few studies have evaluated the effects of ICM on the normal thyroid gland of patients received PCI (4–7), and no well-defined protocols exist to guide the administration for this population.
The thyroid gland utilizes free iodide in serum to synthesize thyroid hormone for metabolic functions. The normal thyroid gland possesses the ability to adapt to the increasement of iodine. But rapid increase of serum iodine may prevent normal adaptation and either hypothyroidism or hyperthyroidism may occur. Overload iodine can lead to suppression of synthesis of thyroid hormone (Wolff-Chaikoff effect) (8). Iodine may also inhibit thyroid hormone release and either of these phenomena can lead to the development of hypothyroidism. Inversely, high iodine levels may cause hyperthyroidism (or Jod-Basedow disease) (9). Worse, the prolonged hyperthyroid or hypothyroid status may pose negative effects on cardiovascular disease and survival (10).
Until now, the risk of ICM has not been clarified in patients with euthyroidism received PCI. Hence, we conducted this retrospective cohort study to determine whether ICM exposure is associated with thyroid dysfunction and thyroid disease in the PCI population.
This retrospectively designed observational study included all consecutive patients who were diagnosed with CAD from January 2015 to December 2017 in Fujian Medical University Union Hospital. According to the American College of Cardiology/American Heart Association 2014 criteria, CAD was defined as follows: defined as ≥ 50% vessel stenosis or having undergone interventional therapy according to coronary angiography results (11). Inclusion criteria were: (A) patients with age equal to or over 18 years; (B) patients received primary PCI as a management strategy; (C) patients completed an one-year follow-up. Exclusion criteria were: (A) were suffering or had suffered thyroid or metabolic disease or thyroid dysfunction; (B) were suffering or had suffered hypothalamic or pituitary diseases; (C) had exposed to ICM, including prior PCI; (D) were with a current prescription for levothyroxine, antithyroid drugs, amiodarone or lithium; (E) were STEMI (ST-segment elevation myocardial infarction); (F) with heart failure or reduced ejection fraction (< 50%); (G) with hepatic or renal dysfunction. Eligible patients were divided into two groups according to volume of ICM: low-volume group (≤ 200ml) and high-volume group (> 200ml). The flow diagram for identifying the study population was shown in Figure 1.
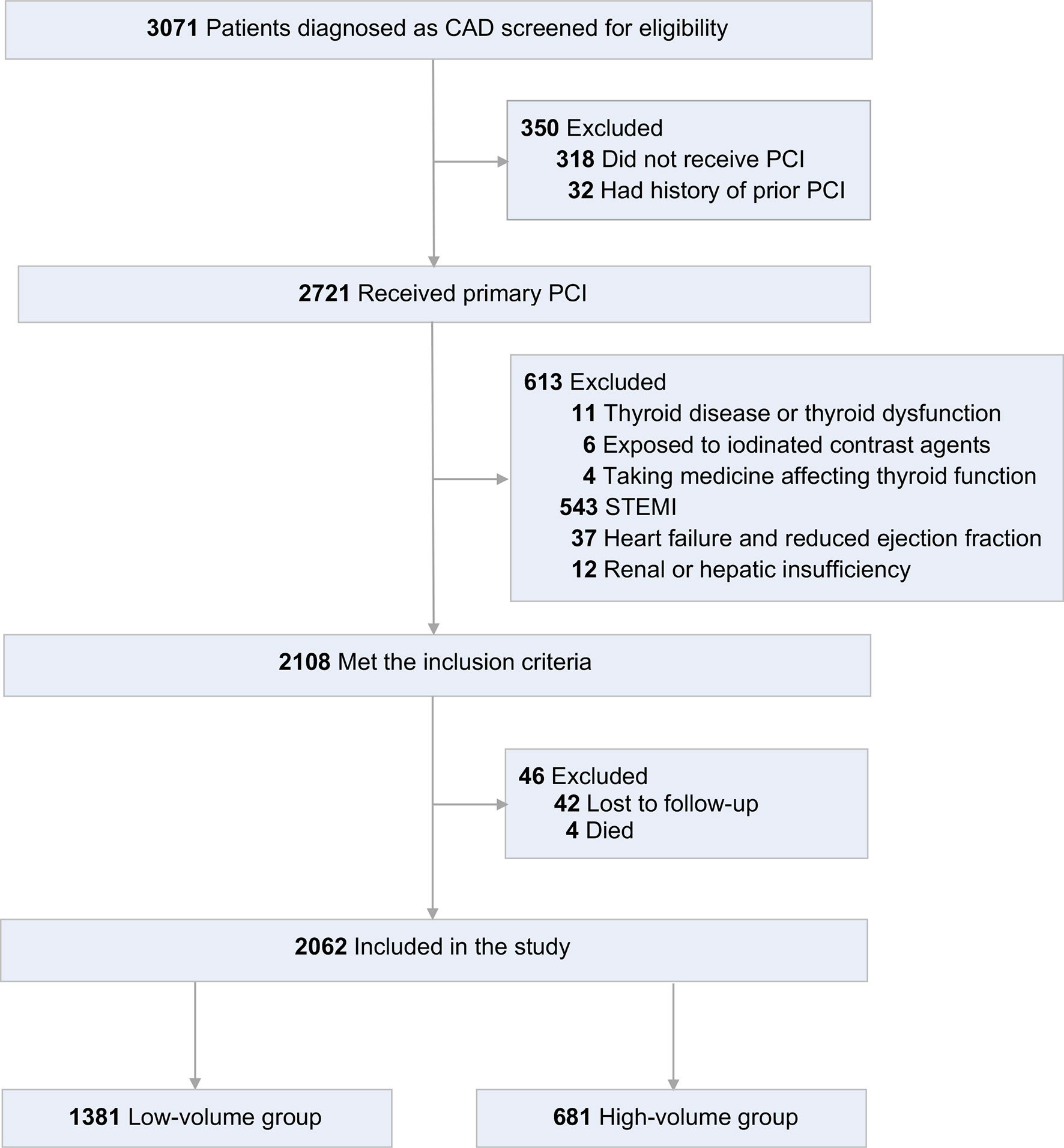
Figure 1 Flow diagram for identifying study population. CAD, coronary artery disease; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Fujian Medical University Union Hospital (No. 2019JYKY010). According to local policy, informed consent was waived due to the retrospective nature of the study.
All data was extracted from the hospital’s electronic database. The following demographic, clinical and laboratory data were exported from the medical hospital electronic database within one day of admission independently by 2 investigators: demographic characteristics (age, gender, weight and height at admission), behavioral habit (current smoker), medical history (hypertension, diabetes, hyperlipidemia, peripheral vascular disease and stroke), clinical diagnosis (unstable angina pectoris, stable angina pectoris and non-ST-segment elevation myocardial (NSTEMI), PCI procedure characteristics (number of implanted stents and ICM volume) and medications at baseline (aspirin, clopidogrel or ticagrelor and statins). Patients were asked to bring all their medication to the interview before the start of treatment.
Thyroid function tests were conducted in all patients 24 to 48 hours before coronary angiography. Serum TSH, free triiodothyronine (FT3) and free thyroxine (FT4) were measured at the Fujian Medical University Union Hospital Immuno-Serology Laboratory. Blood samples were obtained during fasting. Serum was separated and stored at −80°C till the end of the study for measurement of thyroid hormones. TSH was measured using the immune enzymatic Access TSH (3rd IS) assay (Beckman Coulter, Brea, CA) in a DXI 800 analyzer (Beckman Coulter); TSH reference range: 0.27-4.20 mIU/L. FT3 and FT4 were measured using the FT3 and FT4 ADVIA Centaur immunoassay (Siemens Healthcare Diagnostics) in an ADVIA Centaur analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY); FT3 reference range: 3.10-4.80 pmol/L. FT4 reference range: 12.00-22.00 pmol/L. Both the laboratory and the specific assays are certified for clinical use.
Patient treatment was performed according to the current standard practice of coronary heart disease by qualified cardiologists (12, 13). All patients were required to take aspirin plus clopidogrel or potent P2Y12 inhibitor for 1 year in the absence of contraindications. Coronary angiography was performed using the Judkins technique by two experienced interventional cardiologists. Significant coronary artery stenosis was diagnosed visually if accumulating plaque has narrowed diameter of coronary arteries equal to or over 50%. Complex PCI was defined as any of the following: 3 vessels treated, equal to or over 3 lesions treated, total stent length over 60 mm, bifurcation with 2 stents implanted, atherectomy device use, left main PCI, surgical bypass graft or chronic total occlusion as target lesions (14–17). All patients underwent coronary stent implantation with pre-dilatation by balloon. The type of implant-stent and intervention strategy was determined by interventionists.
Technical success was defined as the achievement of less than 20% final diameter stenosis after stent implantation and less than 50% after coronary angioplasty without stent implantation in visual assessment associated with Thrombolysis In Myocardial Infarction (TIMI) flow grade equal to or more than 3 (18). Procedure details including time of contrast administration, contrast volume, contrast administration route was recorded during the procedure. The contrast volume was measured in a 1-mL increment, with the total established from the manual injection used during the procedure. All procedures were done with the same injector type (Angiodyn angiographic syringes for manual contrast medium injection). The contrast volume was measured precisely with manual iodinated contrast delivery. All patients received Iohexol (Omnipaque, 300 mg iodine/mL; GE Healthcare, Chicago, IL, USA), a low osmolar and nonionic contrast medium, for coronary angiography and PCI.
Face-to-face clinically follow-up was scheduled on the 1st month, 3rd month, 6th month and 12th month after PCI. Visit windows are ±3 days for Month 1, ± 7 days for Month 3 and 6, and ±10 days for Month 12. At each study visit, thyroid function including TSH, T3 and T4 were measured and thyroid dysfunction-related symptom, including palpitations, weight loss, tremor, insomnia, anxiety, diarrhea, fatigue, cold intolerance, constipation, weight gain, delayed relaxation of deep tendon reflexes, bradycardia, were evaluated. If patients were suspected of thyroid related disease according to above evaluation, they would be further diagnosed with thyroid ultrasound and treated in the endocrinology department.
This study was powered for a 1-year endpoint: thyroid disease. The composite endpoint was a combination of overt hyperthyroidism, subclinical hyperthyroidism, overt hypothyroidism and subclinical hypothyroidism. Overt hyperthyroidism was defined as low serum TSH concentrations and raised serum concentrations of thyroid hormones: FT4, FT3, or both (19). Subclinical hyperthyroidism was defined as a subnormal serum TSH level along with serum FT4 and FT3 concentrations within the normal reference ranges (20). Hyperthyroidism included overt hyperthyroidism and subclinical hyperthyroidism. Overt hypothyroidism was defined as TSH concentrations above the reference range and free thyroxine concentrations below the reference range (21). Subclinical hypothyroidism was defined as an elevated serum thyrotropin level and a serum-free thyroxine level within the reference range (20). Hypothyroidism included overt hypothyroidism and subclinical hypothyroidism.
Before further analysis, categorical variables were expressed as a percentage (number) and analyzed by the chi-square test or Fisher exact test. For continuous variables, the mean or median in each group, depending on the distribution, was calculated with measures of dispersion (SD or IQR). Normally distributed variables were compared using independent sample t test, and non-normally distributed variables using the Mann-Whitney test. Counting variables were compared using the chi-squared test. For the evaluation of change in serum TSH, FT3 and FT4, parallelism test was conducted to make sure the homogeneity variances were compared. And between-group comparisons were performed using an analysis of covariance (ANCOVA) model adjusted for baseline value with baseline, group, and baseline by group interaction as factors. Restricted cubic splines (RCS) were applied to visualize the dose-response association between ICM volume and clinical endpoint based on odds ratios with logistic regression.
Binary logistic regression analysis using enter method was performed to assess the endpoints between two groups. The initial model was adjusted for age, gender and obesity. The second model was additionally adjusted for the current smoker, hypertension, hyperlipemia and previous stroke based on Model 1. Results were expressed as OR (OR less than 1.0 favored low-volume group, OR equal to or over 1.0 favored high-volume group) with 95% CI. The goodness of fit was evaluated using the Hosmer-Lemeshow test. Further analyses were performed based on the non-complex PCI group and complex PCI group for the 1-year clinical endpoints. All probability values were 2-tailed, and a P value less than 0.05 was considered statistically significant. The statistical analyses were done with software IBM SPSS. 23.0 (SPSS Inc, Chicago, IL, USA) and R (version 3.6.3).
From January 2015 to December 2017, 3071 patients with CAD were assessed for eligibility. 350 patients were excluded for did not receive PCI or had a history of prior PCI. Of the 2721 patients received primary PCI, 2108 patients met the inclusion criteria. Then, for lost to follow-up or died, 46 patients were excluded. Finally, 2062 patients were included in the study (Figure 1). Patients had a mean age of 62.28 ± 9.8 years; 81.0% were men; 42.4% were current smokers; 42.8% had a history of hypertension, 31.4% diabetes, 35.5% hyperlipidemia, and 9.7% stroke.
Among all patients, the distribution ICM volume was left-skewed, ranging from 80 to 530 mL with 25th, 50th and 75th percentile being 120, 150 and 220mL, respectively. To illustrate the association between ICM volume and clinical outcomes, we modeled ICM volume as RCS to provide a smooth and flexible description of their linear dose-response relationship. As displayed in Figure 2, ICM volume is associated with the risk of the composite endpoint (P value for non-linear spline terms < 0.0001), hyperthyroidism (P < 0.0001), overt hyperthyroidism (P < 0.0044) and subclinical hyperthyroidism (P < 0.0028) with a non-linear dose–response relationship (Figure 2).
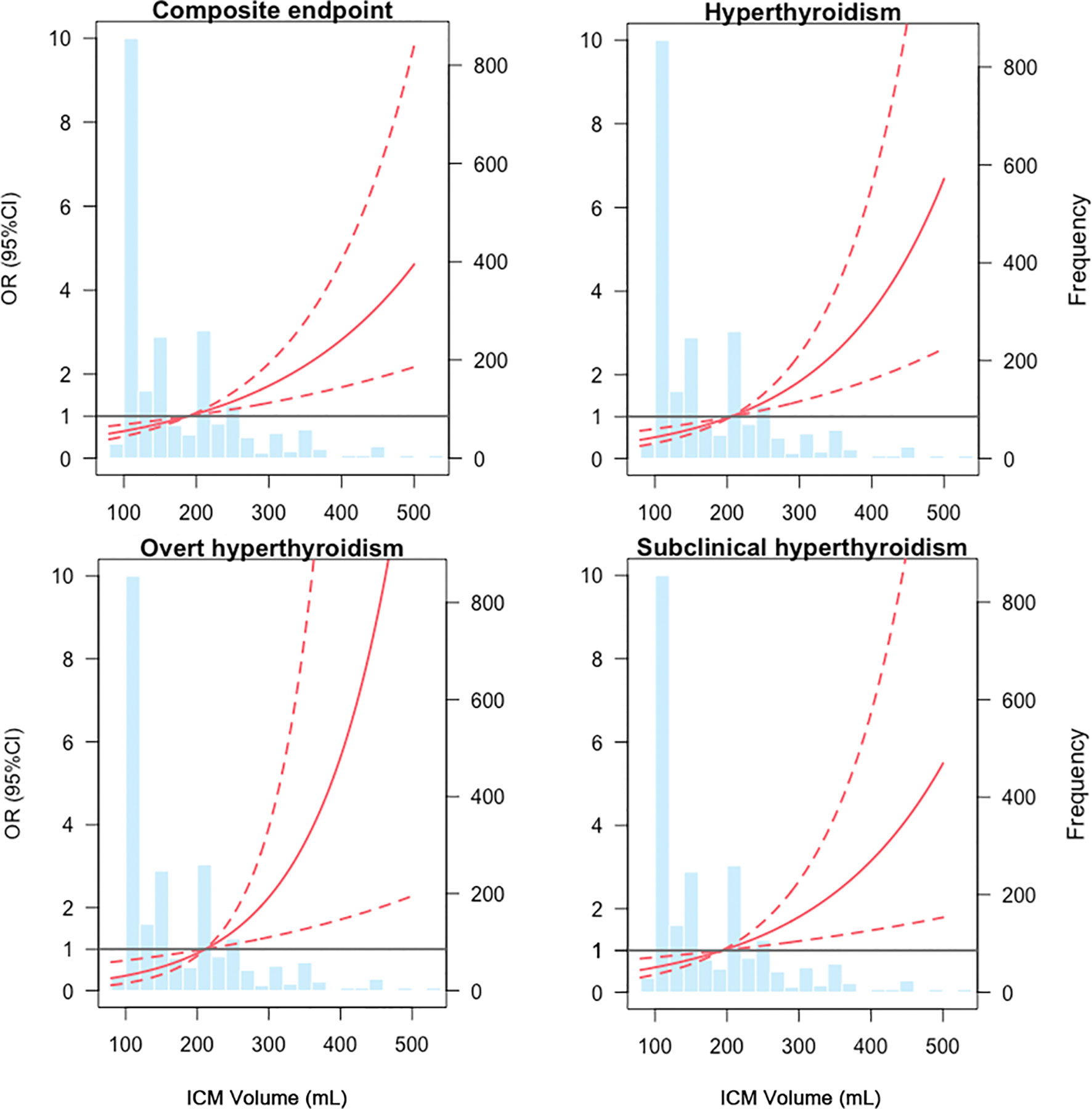
Figure 2 Dose-response relationships between ICM volume and composite endpoints from restricted cubic splines model. The solid red lines are the estimate of odds ratios, with dashed red lines showing 95% confidence intervals derived from restricted cubic spline regressions. Reference lines for no association are indicated by the grey bold lines at an odds ratio of 1.0. Vertical blue bars represent the frequency of ICM volume in a histogram. ICM, iodinated contrast media, OR, odds ratio, CI, confidence interval.
From restricted cubic splines, a cut-off value of 189.23ml was derived for composite endpoint (OR = 1), 205.11ml for hyperthyroidism (OR = 1), 211.34ml for overt hyperthyroidism (OR = 1) and 193.28ml for subclinical hyperthyroidism (OR = 1). To provide an ICM volume value for clinical outcomes, a threshold of 200ml was determined according to the combined consideration of RCS results (Figure 2). Base on it, the included patients were divided into low-volume group (ICM volume <= 200ml) and high-volume group (ICM volume > 200ml).
The analysis population included 1381 patients in the low-volume group and 681 patients in the high-volume group. Between the two groups, the baseline demographic characteristics, medical history, diagnosis, medications and thyroid function were comparable. Compared to low-volume group, patients in high-volume group receive more stents (1.29 ± 0.50 vs. 1.59 ± 0.77, P < 0.0001). And the complexity of the PCI procedure has a significant difference between the two groups (109 ± 7.9 vs. 423 ± 62.1, P < 0.0001) (Table 1).
During the study period, 33 patients of the low-volume group had events——1 overt hyperthyroidism, 13 subclinical hyperthyroidism, 8 overt hypothyroidism, and 11 subclinical hypothyroidism, while 28 patients of the high-volume group had events——5 overt hyperthyroidism, 11 subclinical hyperthyroidism, 8 overt hypothyroidism, and 4 subclinical hypothyroidism.
When comparing thyroid function between groups, with baseline values as a covariable, there was a significant increase in serum FT4 at the one-year PCI intervention. Specifically, the high-volume group was 0.238 ± 0.092 pmol/L higher than the low-volume group (P = 0.010) in the serum FT4. In contrast, no significant differences were observed between groups in serum TSH (95% CI, -0.307-0.001, P = 0.048) and serum FT3 (95% CI, -0.030-0.085, P = 0.348) after one year (Table 2).
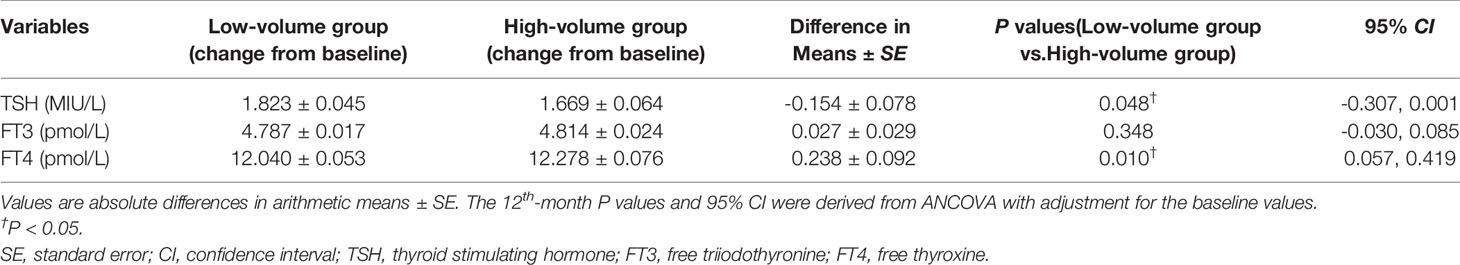
Table 2 Comparison of thyroid function between the low-volume group and high-volume group at the 12th month.
Calibration for all models was adequate with non-significant statistical result (eTable 1). The binary regression analysis results are shown in Table 3. In the crude model, there was a significant difference in composite between the two groups. (OR 1.75, 95% CI (1.05, 2.92), P = 0.032). After adjusted confounding factors, the same result was demonstrated in model1 and model2(OR 1.73, 95% CI (1.01-2.96), P = 0.032 and OR 1.83, 95% CI (1.09-3.06), P = 0.022, respectively). The results also showed a positive correlation for hyperthyroidism in all models (OR 2.35, 95% CI (1.14-4.84), P = 0.021, OR 10.36, 95% CI (1.20-89.00), P = 0.033 and OR 2.35, 95% CI (1.13-4.87), P = 0.022, respectively). Of note, similar observations were acquired in crude model and adjusted model II when and overt hyperthyroidism as the endpoint (OR 10.21, 95% CI (1.19, 87.54), P = 0.034 and OR 10.73, 95% CI (1.24-92.72), P = 0.031, respectively). Yet null results were found in subclinical hyperthyroidism, hypothyroidism, overt hypothyroidism and subclinical hypothyroidism.
The median (quartile1, quartile3) of ICM volume for non-complex PCI patients were 50% higher than non-complex PCI patients (250 vs.120 mL, P < 0.0001). (eFigure). Specifically, the serum TSH of complex PCI patients was 0.210 ± 0.084 (95% CI, 0.046-0.374, P = 0.012) MIU/L higher, and the serum FT4 was 0.242 ± 0.099 (95% CI, 0.047-0.437, P = 0.015) pmol/L higher than non-complex PCI patients (eTable 2).
In the crude model, there was no significant difference for outcome between the non-complex PCI group and the complex PCI group. However, in adjusted model I and adjusted model II, patients received complexed PCI had an increased risk of composite endpoint (OR 1.73, 95% CI (1.01-2.96), P = 0.045 and OR 1.86, 95% CI (1.06-3.28), P = 0.032, respectively). Of note, a positive correlation was acquired in adjusted model II when hyperthyroidism was endpoint (OR 2.28, 95% CI (1.03, 5.01), P = 0.041). Yet null results were found in hypothyroidism. (Table 4).
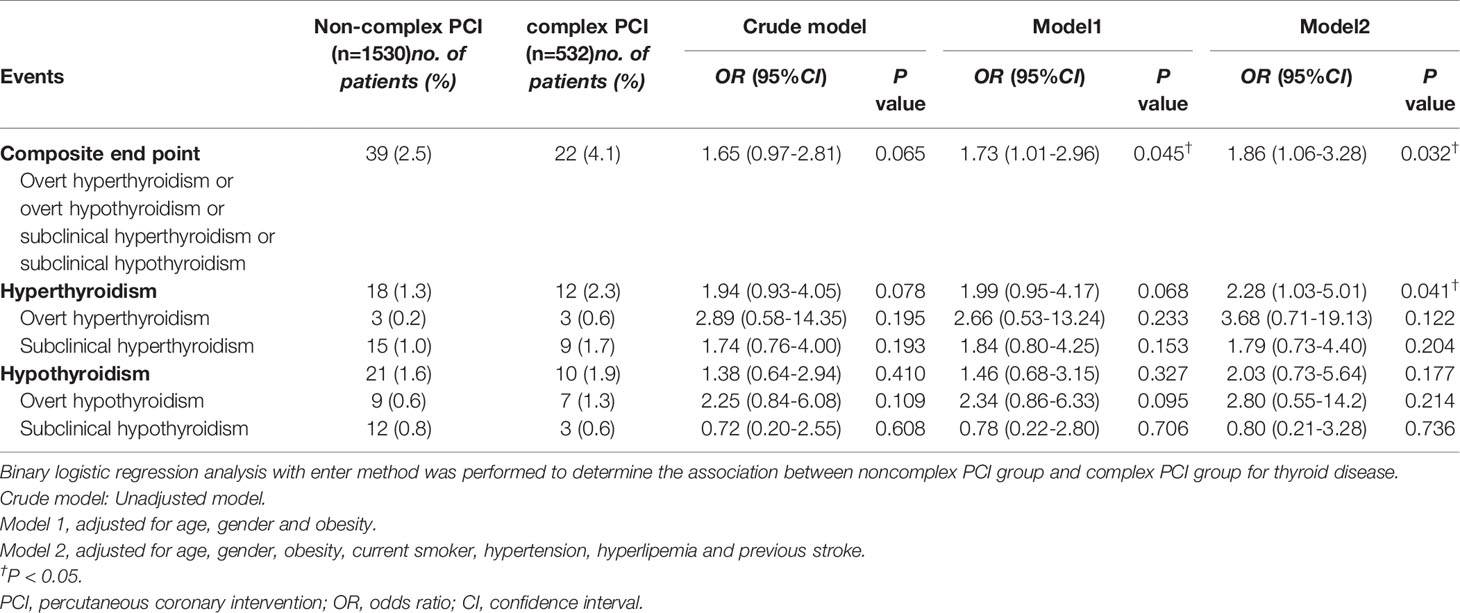
Table 4 Subgroup analysis based on non-complex PCI and complex PCI population for 1-year clinical outcomes.
This is the largest retrospective study performed to date to assess the impact of ICM exposure on thyroid disease for patients received PCI. First, we demonstrated that after 1-year PCI, the serum level of FT4 was significantly increased. Second, the volume of ICM correlated with composite endpoint and hyperthyroidism with a nonlinear dose-response relationship. Third, the risk of hyperthyroidism increased in high-volume ICM group patients.
ICM is routinely used in coronary angiography and PCI procedures. The general procedure might use an average 200ml dose of ICM containing 35µg/ml provides 7,000 µg free iodide, far higher than the recommended daily allowance of 150μg for adults (22). Sudden exposure to high iodine levels may cause thyroid dysfunction, especially in patients with nodular goiter and thyroid dysfunction (6, 23, 24). But currently, for the general population, there are no guidelines for routinely screening thyroid function before and after PCI (25). Monitoring for thyroid dysfunction after ICM exposure is only recommended in high-risk patients (26). However, the last decade has witnessed a significant shift in characteristics of the CAD patient population received PCI, including an increase in mean age and prevalence of diabetes, hypercholesterolemia, hypertension and chronic kidney disease, among others. Consequently, the frequency of myocardial infarction and the amount of PCI procedures also increased. Due to the patient-related and angiographic factors, the overall quantity and complexity of PCI have increased (1). That gives a result to a higher dose of ICM patients suffered. But the association between ICM volume and long-term risk of thyroid disease and necessity of thyroid function monitoring still unclear.
The observed association between ICM exposure and hyperthyroidism is likely explained by the iodine or iodide load conveyed by ICM. Iodine is actively transported into the thyroid gland by the Na+/I- symporter(NIS) (27). NIS gene expression and membrane localization are stimulated by TSH (28). Once in thyroid follicles, iodine is used for the synthesis of the thyroid hormones, T4 and T3. That could explain why serum level of FT4 has a significant difference before and after PCI in our study. The mechanism of ICM-induced hyperthyroidism involves impaired autoregulation following an iodine load. The occurrence of thyrotoxicosis arising from excess iodine exposure is termed the Jöd-Basedow phenomenon (9). Excess iodine intake will result in transient or permanent hyperthyroidism (29, 30). The association between hyperthyroidism and ICM-exposure in our study may be explained based on this mechanism. Still, in certain study, thyroid dysfunction of ICM exposure occurred especially in patients had a history of thyroid disease, or living in an iodine-deficient area, or had renal insufficiency (4, 29, 31). And patients with certain prescriptions, such as amiodarone, lithium, and α-interferon, were also considered having more risk of thyroid dysfunction (32). But our study was based on thyroid normal patients. We speculated that the increased risk of hyperthyroidism may due to much higher dose of ICM explored in complex PCI procedure.
With respect to the mechanism of ICM-induced hypothyroidism, the disorder probably occurs when the thyroid gland fails to adapt to the acute Wolff-Chaikoff effect after excess iodine intake. But the symptoms are usually mild and transient, and the vulnerable populations tend to have Hashimoto’s thyroiditis, previously treated Graves’ disease, thyroid operation, and postpartum thyroiditis (8). The observative population was patients with euthyroidism. That may explain the null result in hypothyroidism by above mechanism.
Several studies indicated that ICM exposure can cause thyroid disease (4–7, 23, 33, 34). Rhee, C. M.et al performed a nested case-control study in the euthyroid population. They demonstrated that ICM exposure was a significant associate with hyperthyroidism and overt hypothyroidism. But the baseline data lacked contrast volume and osmolarity (23). A prospective observational cohort study in 2013 investigated long-term effects for thyroid function in patients with euthyroidism. undergoing coronary angiography using nonionic ICM. They found out that ICM may cause subclinical hyperthyroidism after 8 weeks (5). In Taiwan, a retrospective cohort study randomly selected 1 million people, recruiting 19 642 ICM-exposure cases and 78 568 matched non-ICM-exposure controls. After 6-year observation, patients with ICM exposure had a significantly higher risk of thyroid dysfunction [HR, 1.46, 95% CI (1.29-1.66)]. The same result was confirmed in hyperthyroidism [HR, 1.22, 95% CI (1.04-1.44)] and hypothyroidism [HR, 2.00, 95% CI (1.65-2.44)] (6). Bonelli, N.et al. performed a cross-sectional study in 2018. After 1 year follow up for 810 consecutive ischemic heart disease patients with unknown thyroid diseases or treatment with thyroid-related drugs received coronary angiography. It is investigated that the prevalence of spontaneous subclinical hyperthyroidism in ischemic heart disease is surprisingly elevated and is further increased by iodine load, particularly in patients with thyroid nodules and familial history of thyroid diseases, persisting in a not negligible number of them even after one year (7).
Compared to the above studies, the strengths are obvious in our study. First, the sample size is larger than previous studies, which improved the statistical power. Second, patients in our study are strictly chosen from an iodine-sufficient area in the southeast coastal region of China. And our study was carefully designed to record exact dose and detailed ICM osmolarity. Those were not considered in above study. Third, dose-response relationships between ICM volume and composite end point and hyperthyroidism were explored using restricted cubic splines, which made the associations more visual and clearer. Finally, we conducted further analysis based on complexity of PCI procedure. The positive result of that is of great importance to the daily clinical practice of PCI. However, limitations do exist in our study. First, our study was limited by its retrospective nature. Second, patients in this study were predominantly men. Therefore, the results may not be extrapolated to women. Third, it is fairly clear that radiation may associated with a risk of thyroid disorder (35). But due to the lack of data on exposure duration, we cannot adjust the bias of radiation-related outcome. That may lead to deviation for endpoints. Fourth, iodine status is a key determinant of thyroid disorders in adults. Severe iodine deficiency causes goiter and hypothyroidism (36). Though the patients in our study are reside in coastal region, an iodine-sufficient area, the difference in individual diet may lead to bias in our conclusion. Fifth, the laboratory measurements for 1, 3, 6 months was sometimes conducted in community hospitals and the data were inadequate. Unfortunately, further exploration that at which month when thyroid function and thyroid disease began to show significant differences cannot be performed. Finally, due to the difficulty in diagnosis for asymptomatic autoimmune disease and goiter, we may mistakenly include this population. That may lead to bias for clinical outcomes.
The present analysis gives an overview that ICM volume is associated with an increased risk of thyroid dysfunction and thyroid disease. Given the pervasive use of ICM in contemporary practice and the known sequelae of thyroid functional disorders, regular examinations of thyroid function before and after PCI are needed, especially for patients received high-volume ICM. Physicians and patients should be aware of the potential thyroidal complications associated with ICM exposure and novel effective strategies should be investigated to decrease the risk of this complication.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Fujian Medical University Union Hospital. The ethics committee waived the requirement of written informed consent for participation.
YC contributed to data analysis and editing the manuscript. XZ and NL contributed to data analysis and conceptualization. WN and BH contributed revising the manuscript. XY contributed to data collection and conceptualization. CL and YL contributed to project administration and supervision. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China grants (82120108004) to CL.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.917498/full#supplementary-material
1. Bortnick AE, Epps KC, Selzer F, Anwaruddin S, Marroquin OC, Srinivas V, et al. Five-Year Follow-Up of Patients Treated for Coronary Artery Disease in the Face of an Increasing Burden of Co-Morbidity and Disease Complexity (From the NHLBI Dynamic Registry). Am J Cardiol (2014) 113(4):573–9. doi: 10.1016/j.amjcard.2013.10.039
2. Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, Heretis I, Wilks MF, Spandidos DA, et al. Contrast-Induced Nephropathy: Basic Concepts, Pathophysiological Implications and Prevention Strategies. Pharmacol Ther (2017) 180:99–112. doi: 10.1016/j.pharmthera.2017.06.009
3. Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, et al. Prophylactic Hydration to Protect Renal Function From Intravascular Iodinated Contrast Material in Patients at High Risk of Contrast-Induced Nephropathy (AMACING): A Prospective, Randomised, Phase 3, Controlled, Open-Label, Non-Inferiority Trial. Lancet (2017) 389(10076):1312–22. doi: 10.1016/s0140-6736(17)30057-0
4. Hintze G, Blombach O, Fink H, Burkhardt U, Köbberling J. Risk of Iodine-Induced Thyrotoxicosis After Coronary Angiography: An Investigation in 788 Unselected Subjects. Eur J Endocrinol (1999) 140(3):264–7. doi: 10.1530/eje.0.1400264
5. Özkan S, Oysu AS, Kayataş K, Demirtunç R, Eren M, Uslu H, et al. Thyroid Functions After Contrast Agent Administration for Coronary Angiography: A Prospective Observational Study in Euthyroid Patients. Anadolu Kardiyol Derg (2013) 13(4):363–9. doi: 10.5152/akd.2013.134
6. Kornelius E, Chiou JY, Yang YS, Peng CH, Lai YR, Huang CN. Iodinated Contrast Media Increased the Risk of Thyroid Dysfunction: A 6-Year Retrospective Cohort Study. J Clin Endocrinol Metab (2015) 100(9):3372–9. doi: 10.1210/jc.2015-2329
7. Bonelli N, Rossetto R, Castagno D, Anselmino M, Vignolo F, Parasiliti Caprino M, et al. Hyperthyroidism in Patients With Ischaemic Heart Disease After Iodine Load Induced by Coronary Angiography: Long-Term Follow-Up and Influence of Baseline Thyroid Functional Status. Clin Endocrinol (Oxf) (2018) 88(2):272–8. doi: 10.1111/cen.13494
8. Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-Induced Hypothyroidism. Thyroid (2001) 11(5):501–10. doi: 10.1089/105072501300176462
9. Yamada T. [Jod-Basedow (Iodine-Induced Hyperthyroidism)]. Ryoikibetsu Shokogun Shirizu (1993) 1):367–9.
10. Klein I, Ojamaa K. Thyroid Hormone and the Cardiovascular System. N Engl J Med (2001) 344(7):501–9. doi: 10.1056/nejm200102153440707
11. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol (2014) 64(18):1929–49. doi: 10.1016/j.jacc.2014.07.017
12. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., et al. 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol (2014) 64(24):e139–228. doi: 10.1016/j.jacc.2014.09.017
13. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
14. Mauri L, O'Malley AJ, Cutlip DE, Ho KK, Popma JJ, Chauhan MS, et al. Effects of Stent Length and Lesion Length on Coronary Restenosis. Am J Cardiol (2004) 93(11):1340–1346, a1345. doi: 10.1016/j.amjcard.2004.02.027
15. van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, et al. Predictors of Coronary Stent Thrombosis: The Dutch Stent Thrombosis Registry. J Am Coll Cardiol (2009) 53(16):1399–409. doi: 10.1016/j.jacc.2008.12.055
16. Holmes DR Jr., Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, et al. Stent Thrombosis. J Am Coll Cardiol (2010) 56(17):1357–65. doi: 10.1016/j.jacc.2010.07.016
17. Suh J, Park DW, Lee JY, Jung IH, Lee SW, Kim YH, et al. The Relationship and Threshold of Stent Length With Regard to Risk of Stent Thrombosis After Drug-Eluting Stent Implantation. JACC Cardiovasc Interv (2010) 3(4):383–9. doi: 10.1016/j.jcin.2009.10.033
18. Smith SC Jr., Feldman TE, Hirshfeld JW Jr., Jacobs AK, Kern MJ, King SB 3rd, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol (2006) 47(1):e1–121. doi: 10.1016/j.jacc.2005.12.001
19. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet (2016) 388(10047):906–18. doi: 10.1016/s0140-6736(16)00278-6
20. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical Thyroid Disease: Scientific Review and Guidelines for Diagnosis and Management. Jama (2004) 291(2):228–38. doi: 10.1001/jama.291.2.228
21. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet (2017) 390(10101):1550–62. doi: 10.1016/s0140-6736(17)30703-1
22. Trumbo P, Yates AA, Schlicker S, Poos M. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J Am Diet Assoc (2001) 101(3):294–301. doi: 10.1016/s0002-8223(01)00078-5
23. Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association Between Iodinated Contrast Media Exposure and Incident Hyperthyroidism and Hypothyroidism. Arch Intern Med (2012) 172(2):153–9. doi: 10.1001/archinternmed.2011.677
24. Kornelius E, Chiou JY, Yang YS, Lo SC, Peng CH, Lai YR, et al. Iodinated Contrast Media-Induced Thyroid Dysfunction in Euthyroid Nodular Goiter Patients. Thyroid (2016) 26(8):1030–8. doi: 10.1089/thy.2016.0051
26. van der Molen AJ, Thomsen HS, Morcos SK. Effect of Iodinated Contrast Media on Thyroid Function in Adults. Eur Radiol (2004) 14(5):902–7. doi: 10.1007/s00330-004-2238-z
27. Dai G, Levy O, Carrasco N. Cloning and Characterization of the Thyroid Iodide Transporter. Nature (1996) 379(6564):458–60. doi: 10.1038/379458a0
28. Bizhanova A, Kopp P. Minireview: The Sodium-Iodide Symporter NIS and Pendrin in Iodide Homeostasis of the Thyroid. Endocrinology (2009) 150(3):1084–90. doi: 10.1210/en.2008-1437
29. Braverman LE. Iodine and the Thyroid: 33 Years of Study. Thyroid (1994) 4(3):351–6. doi: 10.1089/thy.1994.4.351
30. Leung AM, Braverman LE. Iodine-Induced Thyroid Dysfunction. Curr Opin Endocrinol Diabetes Obes (2012) 19(5):414–9. doi: 10.1097/MED.0b013e3283565bb2
31. Martin FI, Tress BW, Colman PG, Deam DR. Iodine-Induced Hyperthyroidism Due to Nonionic Contrast Radiography in the Elderly. Am J Med (1993) 95(1):78–82. doi: 10.1016/0002-9343(93)90235-h
32. Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A Review: Radiographic Iodinated Contrast Media-Induced Thyroid Dysfunction. J Clin Endocrinol Metab (2015) 100(2):376–83. doi: 10.1210/jc.2014-3292
33. Conn JJ, Sebastian MJ, Deam D, Tam M, Martin FI. A Prospective Study of the Effect of Nonionic Contrast Media on Thyroid Function. Thyroid (1996) 6(2):107–10. doi: 10.1089/thy.1996.6.107
34. Üreyen Ç M, Coşansu K, Vural MG, Şahin SE, Çakar MA, Kılıç H, et al. Percutaneous Coronary Intervention for Chronic Total Occlusion Versus Percutaneous Coronary Intervention for Non-Complex Coronary Lesions: Is There a Different Impact on Thyroid Function? Med Princ Pract (2020) 29(2):188–94. doi: 10.1159/000503553
35. Jereczek-Fossa BA, Alterio D, Jassem J, Gibelli B, Tradati N, Orecchia R. Radiotherapy-Induced Thyroid Disorders. Cancer Treat Rev (2004) 30(4):369–84. doi: 10.1016/j.ctrv.2003.12.003
Keywords: iodinated contrast media, coronary artery disease, percutaneous coronary intervention, thyroid disease, hyperthyroidism
Citation: Chen Y, Zheng X, Li N, Niu W, Hu B, Yuan X, Liang C and Lin Y (2022) Impact of Iodinated Contrast Media in Patients Received Percutaneous Coronary Intervention: Focus on Thyroid Disease. Front. Endocrinol. 13:917498. doi: 10.3389/fendo.2022.917498
Received: 11 April 2022; Accepted: 19 May 2022;
Published: 23 June 2022.
Edited by:
Leonidas H. Duntas, National University Of Athens, GreeceReviewed by:
Leila Warszawski, Instituto Estadual de Diabetes e Endocrinologia Luiz Capriglione, BrazilCopyright © 2022 Chen, Zheng, Li, Niu, Hu, Yuan, Liang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Liang, Y2h1bmxpYW5nbGlhbmcxOTg1QDE2My5jb20=; Yunling Lin, eXVubGluZ2xpbkAxMjYuY29t
†These authors have contributed equally to this work and share the first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.