
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 15 July 2022
Sec. Experimental Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.917258
This article is part of the Research Topic Neuroendocrine Regulation of Feeding and Reproduction in Fish View all 14 articles
Kisspeptin has an important role in the regulation of reproduction by directly stimulating the secretion of gonadotropin-releasing hormone (GnRH) in mammals. In non-mammalian vertebrates, there are multiple kisspeptins (Kiss1 and Kiss2) and kisspeptin receptor types, and the two kisspeptins in teleosts have different effects depending on fish species and reproductive stages, serving reproductive and non-reproductive functions. In the grass puffer, Takifugu alboplumbeus, which has only a single pair of kiss2 and kissr2, both genes display seasonal, diurnal, and circadian oscillations in expression in association with the periodic changes in reproductive functions. To elucidate the role of kisspeptin in this species, homologous kisspeptin peptide (gpKiss2) was administered at different reproductive stages (immature, mature and regressed) and the expression levels of the genes that constitute hypothalamo-pituitary-gonadal axis were examined in male grass puffer. gpKiss2 significantly elevated the expression levels of kissr2 and gnrh1 in the brain and kissr2, fshb and lhb in the pituitary of the immature and mature fish. No noticeable effect was observed for kiss2, gnih, gnihr, gnrh2 and gnrh3 in the brain and gpa in the pituitary. In the regressed fish, gpKiss2 was ineffective in stimulating the expression of the gnrh1 and GTH subunit genes, while it stimulated and downregulated the kissr2 expression in the brain and pituitary, respectively. The present results indicate that Kiss2 has a stimulatory role in the expression of GnRH1/GTH subunit genes by upregulating the kissr2 expression in the brain and pituitary at both immature and mature stages, but this role is mostly ineffective at regressed stage in the grass puffer.
The reproduction in vertebrates is regulated by the complex interaction among multiple environmental factors and the reproductive neuroendocrine system, which is composed of kisspeptin, gonadotropin-inhibitory hormone (GnIH) and gonadotropin-releasing hormone (GnRH) in the hypothalamus and two pituitary gonadotropins (GTHs), namely follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (1–5). Kisspeptin, a member of the RFamide peptide family, is encoded by the KISS1/Kiss1 gene in mammals. KISS1 was originally identified as a metastasis suppressor gene (6) and its product was found to be the ligand for an orphan G-protein coupled receptor, GPR54, later named Kiss1r (7). Since mutations in GPR54 were found to be responsible for idiopathic hypogonadotropic hypogonadism (8, 9), kisspeptin has received considerable attention as a potential key player in the neuroendocrine regulation of reproduction. It is well established in mammals that kisspeptin regulates reproductive events including puberty and ovulation through stimulating GnRH secretion (10).
Unlike most mammals that possess a single kisspeptin gene (Kiss1), most teleosts possess two paralogous genes for kisspeptin (kiss1 and kiss2) and kisspeptin receptor (kissr1, kissr2, kissr3 and kissr4) and this increases the complexity of the kisspeptin system in this group (11, 12). It has been shown that the role of the two kisspeptins in the regulation of reproduction varies among fish species. The administration of Kiss1 increased the plasma LH levels in goldfish (13) and stimulated gonadal development in chub mackerel (14). In striped bass, Kiss1 showed stimulatory effects on the expression of fshb and ovarian development, whereas Kiss2 exhibited no effect (5). In contrast, Kiss2 has been shown to have a stimulatory role in reproduction with higher potency when compared to Kiss1 in zebrafish, medaka, chub mackerel, European seabass, Nile tilapia and largemouth seabass (16–21). In addition, the actions of the two kisspeptins are different depending on the stage of gonadal development. In yellowtail kingfish, although only Kiss1 but not Kiss2 stimulated the expression of fshb and lhb during the breeding and non-breeding seasons, Kiss2 was more effective than Kiss1 in stimulating gonadal development during the non-breeding period (22). In hybrid bass, only Kiss2 was effective in stimulating LH secretion at puberty, whereas both Kiss1 and Kiss2 induced LH release at recrudescence stage (23). Moreover, Kiss2 upregulated gnrh1 and kiss2r expressions in the brain at prepuberty, while it downregulated the expression of gnrh1 and kiss2r at the recrudescence stage (23).
In contrast to this stimulatory role in most species depending on reproductive stage, it has recently been shown that the kisspeptin system is dispensable for reproduction in zebrafish (24–26) and medaka (27) using gene knockout models. Further studies suggest that this is because of physiological compensation that takes place in mutant fish to maintain reproductive processes (26, 28, 29). Moreover, co-expression of kisspeptin receptor in GnRH neurons has been controversial: co-expression has been demonstrated in Nile tilapia (30), African cichlid (31), striped bass (23), chub mackerel (32) and zebrafish (33), whereas the lack of co-expression has been shown in medaka (27, 34) and European sea bass (35). Taken together, the functional role and mode of action of kisspeptins in the control of reproduction in fish are controversial and varies depending on fish species and also reproductive stages.
The grass puffer, Takifugu alboplumbeus, shows unique reproductive physiology that is synchronized with the seasonal, lunar, and daily cycles (36, 37). During the spawning season from spring to early summer, spawning occurs on seashore only for several days around the new and full moon days every two weeks (36, 38). Mature fish usually aggregate for spawning at certain seashore locations 2–3.5 hours before high tide in the evening, and spawning occurs for 1.5–2 hours during the rising tidal phase. Therefore, the timing of spawning is tightly connected with seasonal, lunar, and tidal cycles as well as daily rhythm. Since the time and place of the spawning are known, spawning fish can be easily caught by dip net at the spawning bed. Thus, the grass puffer provides a unique animal model for studying the neuroendocrine mechanisms underlying the seasonal, lunar, and circadian controls of reproduction in wild animals.
Grass puffer has only a single pair of genes for kisspeptin (kiss2) and kisspeptin receptor (kissr2) and previous studies on their expression patterns with respect to seasonal, daily and circadian changes have indicated the possible importance of the kisspeptin system in the semilunar-synchronized spawning. The expression levels of both kiss2 and kissr2 show distinct changes during reproductive cycle with a significant increase from the early stage of gametogenesis to the pre-spawning and spawning stages (36, 39). This seasonal variations of kiss2 and kissr2 expressions are certainly important for the spawning in early summer and have recently been found to be regulated by water temperature: high water temperature conditions in summer (over 28°C) suppress the kiss2 and kissr2 expressions, leading to the termination of spawning period (40). Furthermore, kiss2 and kissr2 exhibit diurnal and circadian variations in expression during the spawning period (41). Therefore, the kisspeptin system is considered to be important in the stimulation, maintenance, and cyclicity of the reproductive function in the grass puffer.
In the present study, the effects of Kiss2 administration on the expressions of the genes for various hormones and receptors that are comprised in the hypothalamus-pituitary-gonadal (HPG) axis (kiss2 and kissr2; gnih and gnihr; three GnRH genes, namely gnrh1, gnrh2 and gnrh3; three GTH subunit genes, namely gpa, fshb and lhb) were examined to elucidate the functional significance of Kiss2 in the male grass puffer. For the possible different roles of Kiss2 during gonadal development, the fish were treated with Kiss2 at three reproductive stages, namely immature, mature and regressed stages.
Male fish with fully matured testes were collected from the spawning ground in Kawana, Shizuoka, Japan in June. Male fish with regressed testes were collected from the spawning ground in Minamiise, Mie, Japan at the end of July. The mature and regressed fish were transferred to the Marine Biological Station, Niigata University, Niigata, Japan, and reared in indoor tanks (500 L) with the flow of seawater under natural photoperiod (LD 14:10) for two weeks. The water temperature during the acclimatization period was similar to that of sampling ground, which was 20°C for the matured fish and 25°C for the regressed fish. The fish were fed daily with commercial pellets equivalent to 1% body weight (BW) until the experiment was conducted. Since immature grass puffer is unavailable from wild source, juvenile fish were artificially reared at the Fisheries Laboratory, University of Tokyo, Shizuoka, Japan, and they were transferred to the Marine Biological Station, Niigata University and reared in indoor conditions for one year. They were reared in indoor tanks (500 L) under natural photoperiod. Water temperature ranged from 16°C to 24°C depending on seasons. The experiment using the 1-year-old fish with immature testes was conducted in May.
Grass puffer Kiss2 (gpKiss2, SKFNLNPFGLRFamide) (AB548304) was synthesized and dissolved in 0.9% NaCl and stored at -80°C until use. The fish were anesthetized in 0.008% tricaine methanesulfonate (MS222, Sigma-Aldrich, Tokyo, Japan) for 30 sec. and were immobilized with their ventral side upward. The immature and mature fish were intraperitoneally (ip) injected with gpKiss2 (0.1 and 1.0 μg/4 μl/g BW, n = 6−8) using a fine needle (25G, Terumo Corporation, Tokyo, Japan). Control groups of fish were injected with 0.9% NaCl. For the regressed fish, a preliminary experiment was conducted to examine the effect of gpKiss2 administration (0.01 and 0.1 μg/4 μl/g BW, n = 4) because there had been few reports on the effect of kisspeptin on animals at recrudescence stage. Since there was a trend toward increased kiss2 and gnih expressions in the forebrain sample (telencephalon and diencephalon) at 0.01 μg/g BW in this preliminary experiment, the regressed fish were ip injected with gpKiss2 at 0.01 μg/g BW (n = 6−7) and the control fish were injected with 0.9% NaCl. In all experiments, the fish were injected at Zeitgeber time 2:00 (7:00 a.m.) and left in indoor tanks (100 L, n = 7−9 per tank) for 12 hrs.
The fish were anesthetized in 0.03% MS222 and total length and BW were recorded. Brains and pituitaries were removed after decapitation and soaked in RNAlater (Ambion, Austin, TX) at 4°C overnight. Gonads were removed and weighed for the calculation of gonadosomatic index (GSI = gonad weight/BW × 100). In the next day, brains were trimmed to prepare the forebrain sample that contained the telencephalon and diencephalon. The forebrain and pituitary samples were then stored at -80°C until RNA extraction. All the experimental procedures were carried out following the approved guidance by the Institutional Animal Care and Use Committee of the Niigata University, Niigata, Japan. Total length, BW, and GSI of the fish are shown in Table 1.
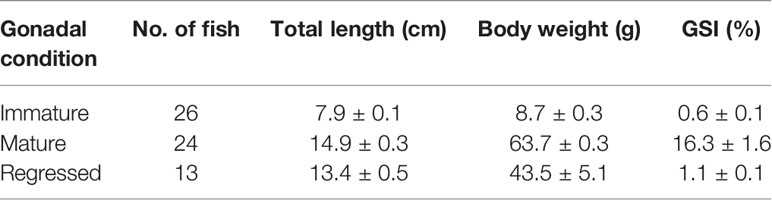
Table 1 Total length, body weight and gonadosomatic index (GSI) of fish samples. Values are presented as mean ± SEM.
Real-time PCR assays for kiss2, kissr2, gnih, gnihr, gnrh1, gnrh2, gnrh3, gpa, fshb and lhb were carried out as described previously (39, 42, 43). Briefly, total RNA was extracted from the forebrain and pituitary samples and treated with DNase I (Takara, Ohtsu, Japan). Total RNA (200 or 500 ng) was used for synthesis of first strand cDNA using MultiScribe Reverse Transcriptase (Applied Biosystem, USA) and an oligo d(T)12-18 primer (2.5 μM) as per manufacturer’s instructions. The profile for reverse transcription reaction was 25°C for 10 min, 48°C for 30 min and 95°C for 5 min. The absolute amount of mRNA was determined using sense reference RNA, which was synthesized in vitro by a MAXIscript kit (Ambion) according to the manufacturer’s instruction and were serially diluted to 1 x 103 − 1 x 108 copies/μl. The standard sense RNAs were reverse transcribed and used as standard cDNAs to establish a standard curve. Real-time PCR was carried out with a Thermal Cycler Dice Real Time System III (TP970, TaKaRa Bio, Japan). PCR reaction mixture (10 μl) contained 1 μl of standard sample cDNA, 0.4 μl of forward and reverse primers (Table 2) and 5 μl of TB Green Premix DimerEraser (TaKaRa, Ohtsu, Japan). Amplification was carried out at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec, 60°C for 30 sec and 72°C for 30 sec. Specific amplification of each cDNA was verified by melting curve analysis and gel electrophoresis of the product.
To compare the effects of gpKiss2 administration on gene expression among various genes at three gonadal stages, the relative mRNA values with respect to control (0 μg/g BW) are expressed as mean ± standard error of the mean (SEM). Data were analyzed by ANOVA followed by Tukey’s HSD post hoc test to assess the statistically significant difference among different groups of immature and mature fish. Student t-test were performed to compare significant difference between the gpKiss2-injected and control groups in the regressed fish. Statistical significance was set at p < 0.05 unless described anywhere in the text. All statistical analyses were performed using SPSS Version 23.0 for windows (SPSS Inc., Chicago, IL).
The administration of gpKiss2 did not alter the expression levels of kiss2 in the immature and mature fish (Figure 1A). However, the expression of kissr2 was significantly stimulated in the gpKiss2-injected fish at both immature and mature stages when compared to the control (Figure 1B). In contrast, gpKiss2 did not show any noticeable effect on the expression of gnih and gnihr in the brain of both immature and mature fish (Figures 2A, B). gpKiss2 significantly elevated the expression of gnrh1 in the brain of immature and mature fish (Figure 3A). In the case of gnrh2 and gnrh3, gpKiss2 did not show any effect at both immature and mature stages at any doses (Figures 3B, C).
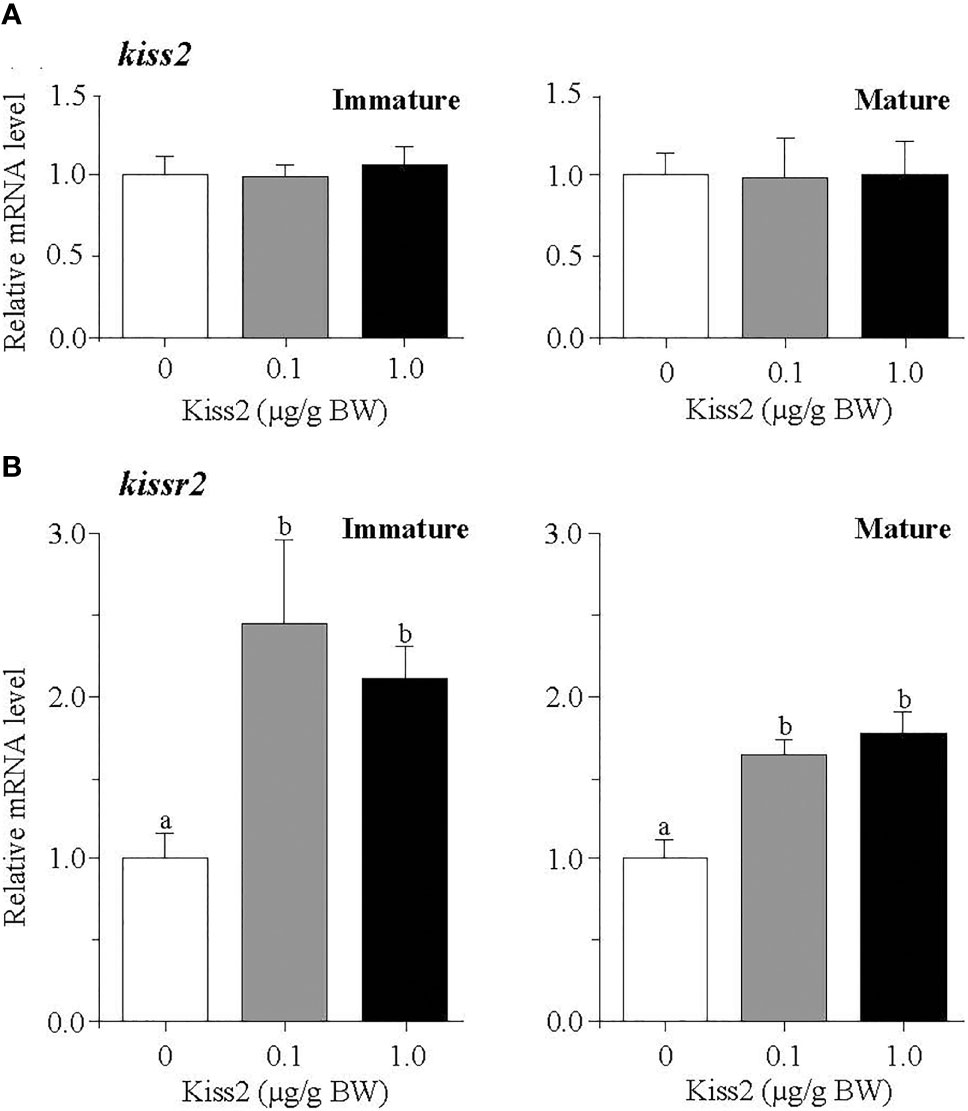
Figure 1 Changes in the relative mRNA levels of kiss2 (A) and kissr2 (B) in the brain of grass puffer at immature and mature stages after intraperitoneal injection of gpKiss2 for the period of 12hrs. Values are presented as mean ± SEM (n = 6−8). Values accompanied by different letters are statistically significantly different (p < 0.05).
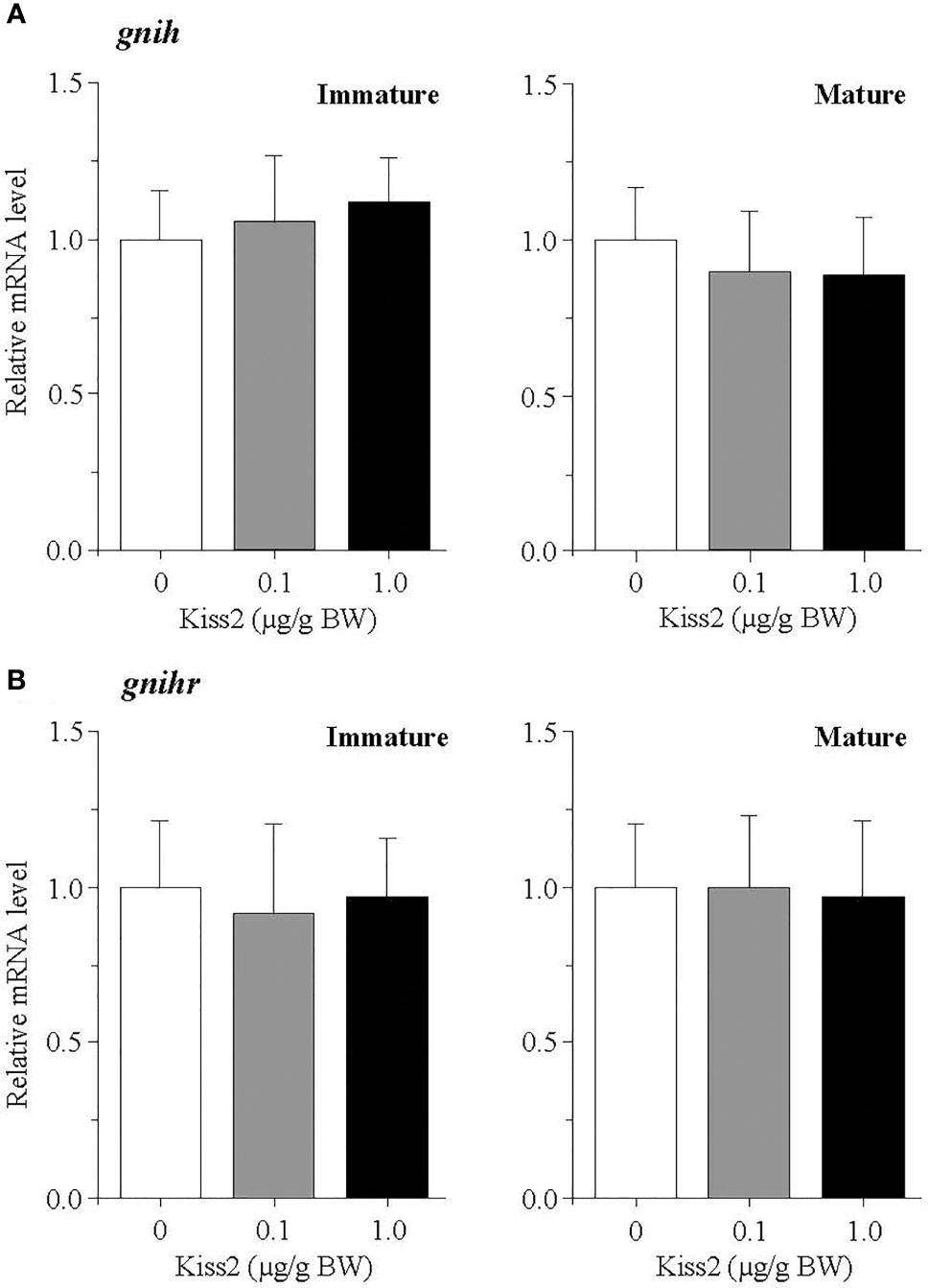
Figure 2 Changes in the relative mRNA levels of gnih (A) and gnihr (B) in the brain of grass puffer at immature and mature stages after intraperitoneal injection of gpKiss2 for the period of 12hrs. Values are presented as mean ± SEM (n = 6−8).
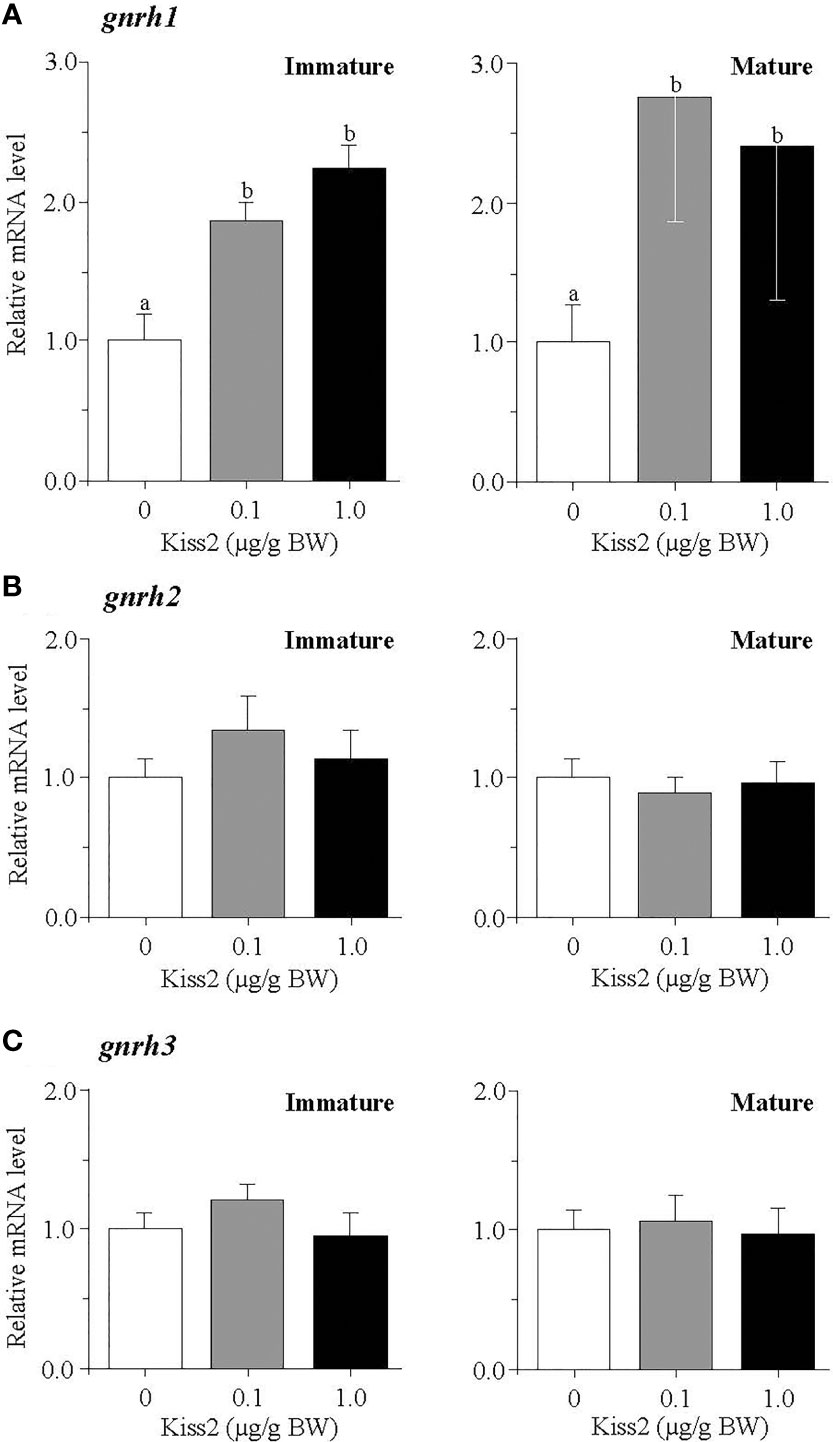
Figure 3 Changes in the relative mRNA levels of gnrh1 (A), gnrh2 (B) and gnrh3 (C) in the brain of grass puffer at immature and mature stages after intraperitoneal injection of gpKiss2 for the period of 12hrs. Values are presented as mean ± SEM (n = 6−8). Values accompanied by different letters are statistically significantly different (p < 0.05).
In the pituitary, the mRNA levels of kissr2 were significantly increased by the gpKiss2 administration in both immature and mature fish and gpKiss2 showed higher potency to stimulate the kissr2 expression in the immature fish compared to the mature fish (fold stimulation: immature 2.54 vs mature 1.14, p = 0.047 by t-test, Figure 4). Similarly, gpKiss2 significantly stimulated the expression of fshb and lhb in the immature and mature fish (Figures 5B, C), whereas no noticeable changes were observed for gpa (Figure 5A).
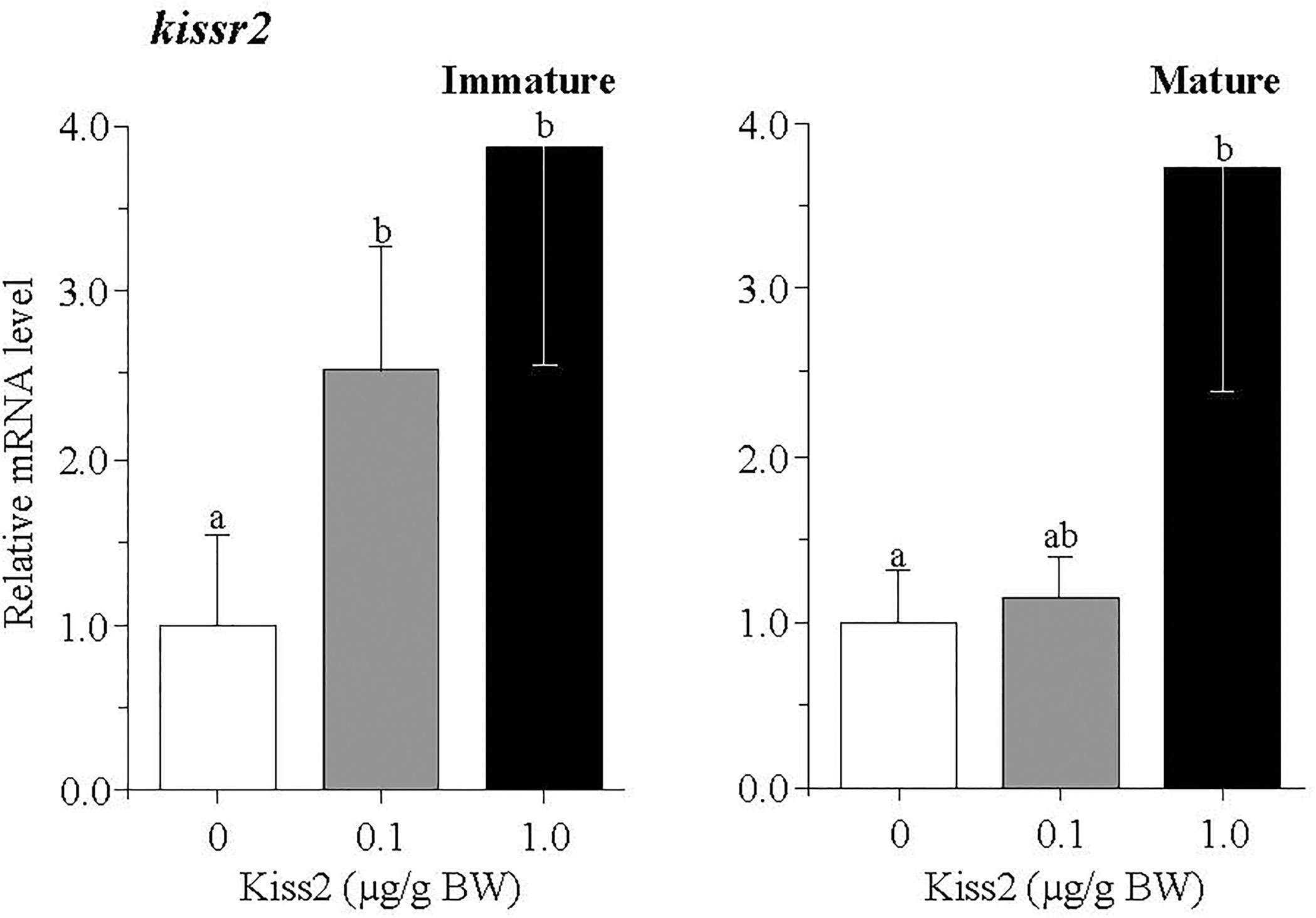
Figure 4 Changes in the relative mRNA levels of kissr2 in the pituitary of grass puffer at immature and mature stages after intraperitoneal injection of gpKiss2 for the period of 12hrs. Values are presented as mean ± SEM (n = 6−8). Values accompanied by different letters are statistically significantly different (p < 0.05).
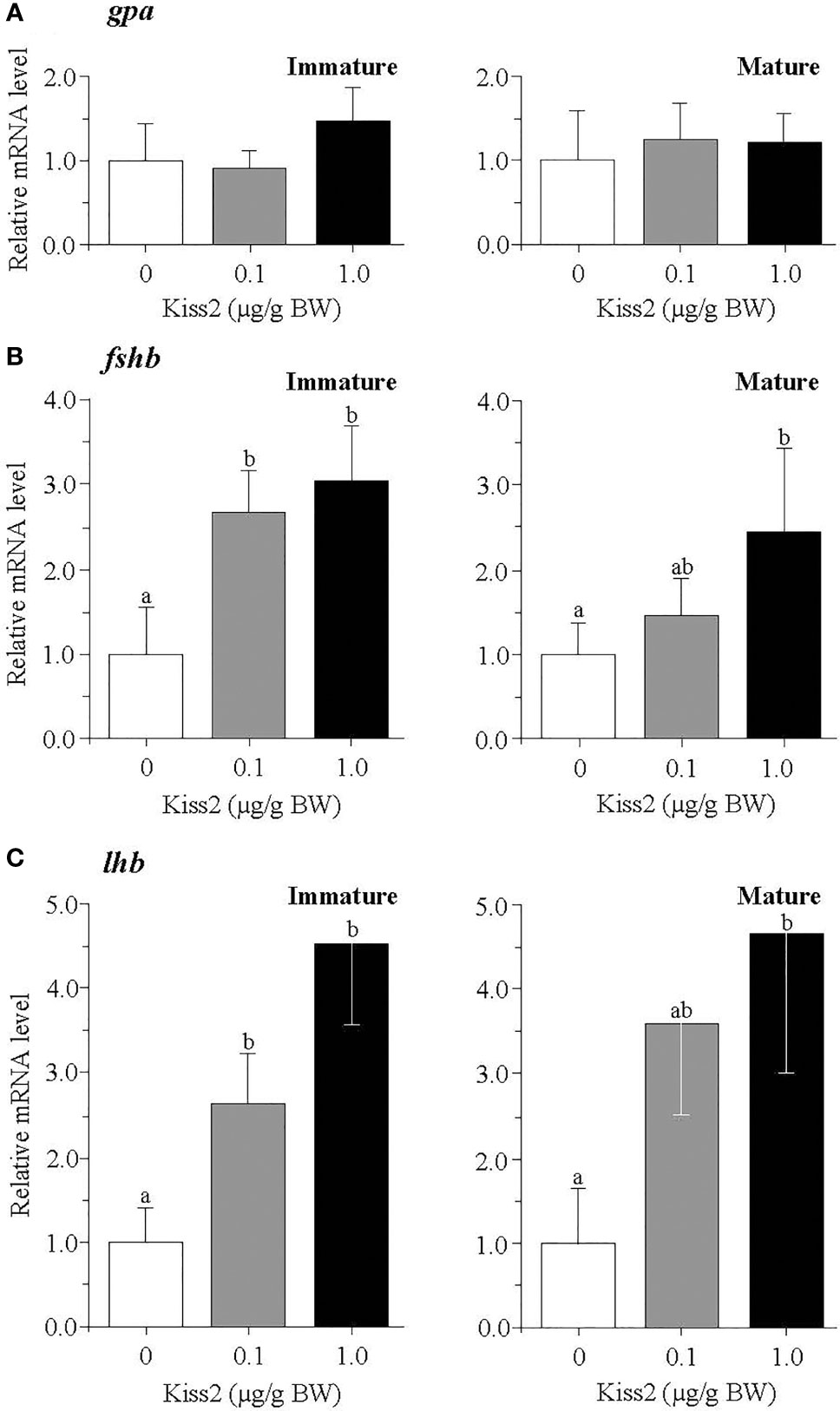
Figure 5 Changes in the relative mRNA levels of gpa (A), fshb (B) and lhb (C) in the pituitary of grass puffer at immature and mature stages after intraperitoneal injection of gpKiss2 for the period of 12hrs. Values are presented as mean ± SEM (n = 6−8). Values accompanied by different letters are statistically significantly different (p < 0.05).
In the brain of regressed fish, gpKiss2 did not show any change in the kiss2 expression but significantly stimulated the expression of kissr2 (Figure 6A). The expression levels of gnih and gnihr tended to be increased by the gpKiss2 administration, though these changes were not statistically significant (Figure 6B). There were no significant changes in the expression levels of three gnrhs after gpKiss2 administration in the regressed fish (Figures 6C–E).
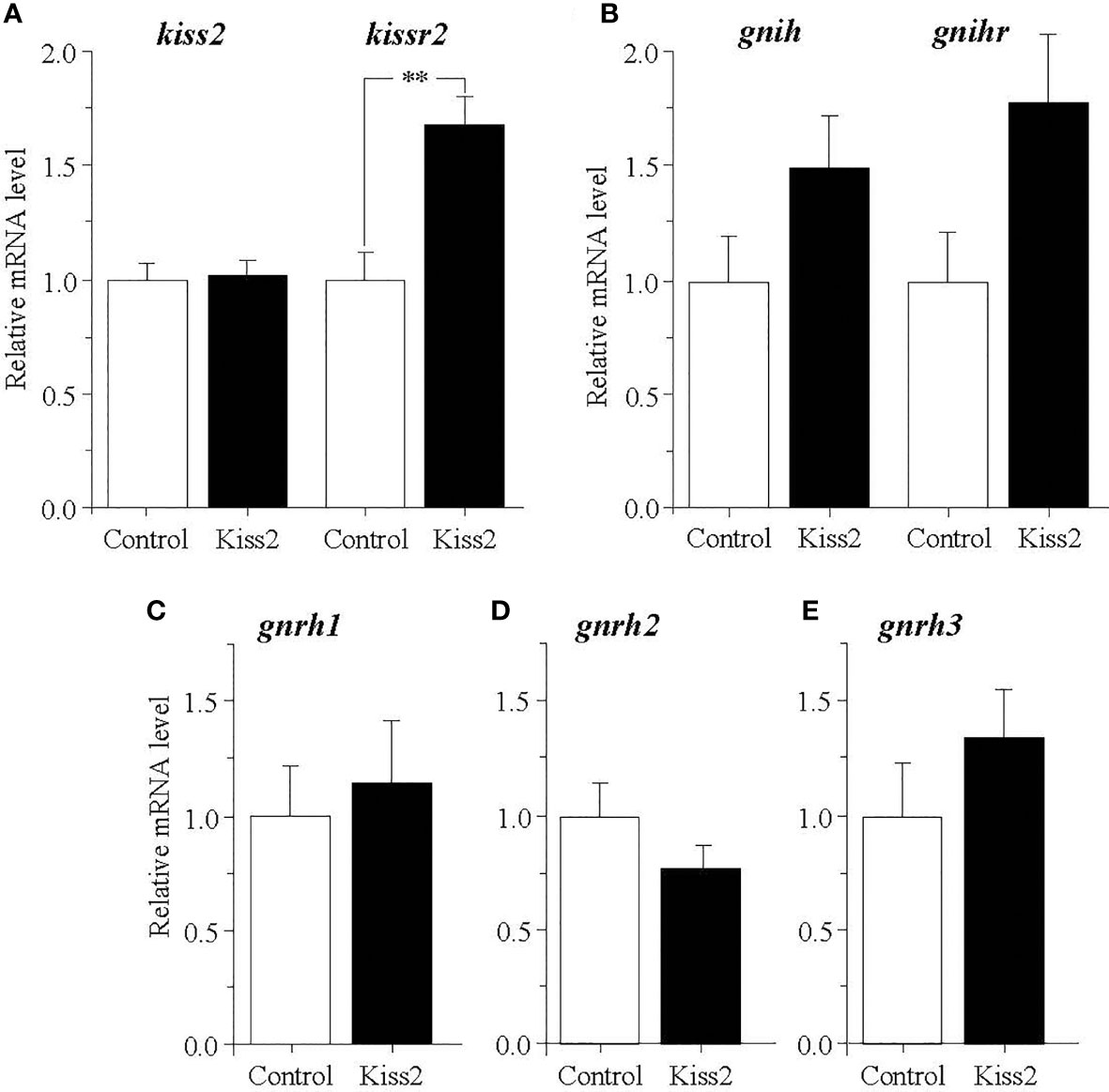
Figure 6 Changes in the relative mRNA levels of kiss2 and kissr2 (A), gnih and gnihr (B), gnrh1 (C), gnrh2 (D) and gnrh3 (E) in the brain of grass puffer at regressed stage after intraperitoneal injection of gpKiss2 for the period of 12hrs. Values are presented as mean ± SEM (n = 6−7). Asterisks denotes a significant difference between the control and gpKiss2 injected fish (**, p < 0.01).
In the pituitary of regressed fish, gpKiss2 significantly decreased the kissr2 expression (Figure 7A). There was a trend toward increased gpa and fshb expression by the gpKiss2 administration (Figures 7B, C) and no noticeable changes were observed in the lhb expression (Figure 7D).
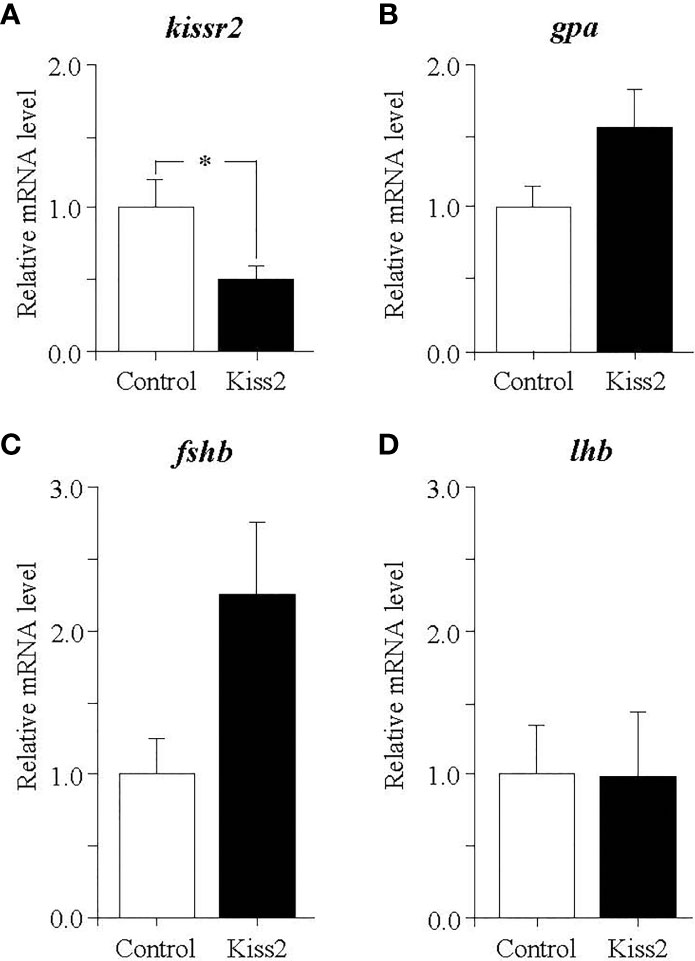
Figure 7 Changes in the relative mRNA levels of kissr2 (A), gpa (B), fshb (C) and lhb (D) in the pituitary of grass puffer at regressed stage after intraperitoneal injection of gpKiss2 for the period of 12hrs. Values are presented as mean ± SEM (n = 6−7). Asterisks denotes a significant difference between the control and gpKiss2 injected fish (*, p < 0.05).
The effect of gpKiss2 administration on the expression of the genes for the HPG axis was examined at three gonadal stages to elucidate the functional importance of the kisspeptin system in the male grass puffer. gpKiss2 significantly stimulated the expression of kissr2 and gnrh1 in the brain and kissr2, fshb and lhb in the pituitary of the immature and mature fish, showing a stimulatory role of gpKiss2 in reproduction by activating the kissr2 expression in the brain and pituitary so as to stimulate the expression of GTHs. In the regressed fish, however, gpKiss2 was ineffective in stimulating the GnRH1 and GTH subunit gene expression, suggesting that the stimulatory role of gpKiss2 is dependent on the gonadal stage. Moreover, gpKiss2 did not alter the expression of gnih, gnihr, gnrh2 and gnrh3 as well as its own gene, kiss2.
In mammals, kisspeptin has a strong stimulatory effect on GTH secretion from the pituitary and this is mainly mediated through the stimulatory action on GnRH secretion (10, 29). Kiss1r is co-localized with GnRH neurons in the hypothalamus and the direct interaction between kisspeptin and GnRH is primarily important in the control of ovulatory cycle in mammals. In the present study, the effect of gpKiss2 was evaluated on the expression of three GnRH genes, gnrh1, gnrh2 and gnrh3 at three gonadal stages. In some teleost fish including the grass puffer, three GnRH neuronal groups are diversified in regard to localization and function (1, 5). GnRH1 neurons are mainly localized in the preoptic area (POA) and have a hypophysiotropic role through stimulating the GTH secretion from the pituitary. GnRH2 neurons are localized in the midbrain tegmentum and involved in appetite-related reproductive function (44–46). GnRH3 neurons are localized in the terminal nerve ganglion-POA region and have neuromodulatory action related to sexual behavior (47, 48). In the present study, gpKiss2 significantly stimulated the expression of gnrh1 but not gnrh2 nor gnrh3, with a concomitant increase in kissr2 expression in the brain (Figures 1B, 3A–C). Stimulatory action of kisspeptin on kisspeptin receptor gene expression has been reported in largemouth bass (21), yellowtail kingfish (22), and hybrid bass (23). In contrast, inhibitory action of Kiss2 on kisspeptin receptor gene expression was reported in the tongue sole (49). Although the co-localization of kisspeptin receptor with GnRH1 neurons remain to be determined in the grass puffer, kissr2 mRNA was previously localized in the magnocellular preoptic nucleus pars magnocellularis (PMm) in the POA (41), which is one of the major hypothalamic nuclei that consist of hypophysiotropic neurons including GnRH1 neurons in many teleost species [for reviews (1) and (5)]. Co-expression of kisspeptin receptor in GnRH1 neurons has been reported in many fishes such as Nile tilapia (30), African cichlid (31), striped bass (23), chub mackerel (32) and zebrafish (33). Moreover, Kiss2 was shown to stimulate gnrh1 expression via kisspeptin receptor using brain slices in striped bass (50). These and the present results suggest that gpKiss2 may directly activate GnRH1 neurons and stimulate the secretion of FSH and LH from the pituitary. Nevertheless, it is also possible that gpKiss2 could act on GnRH1 neurons indirectly via kissr2-expressing cells that are adjacent to GnRH1 neurons. In zebrafish, Kiss2 neurons project widely in the brain including the POA, where GnRH3 neurons are contacted by Kiss2 fibers (51). The immunolocalization of Kiss2 receptor (Kiss2-R) further showed in this species that, in addition to a subset of preoptic GnRH3 neurons were Kiss2-R-immunoreactive (ir), Kiss2-R-ir processes were observed in proximity to some GnRH3 neurons that were negative to Kiss2-R, suggesting that there is a possibility of direct and indirect actions of Kiss2 (33). Such close apposition of kisspeptin receptor and preoptic GnRH neurons has been reported in striped bass (23), European sea bass (35) and medaka (34). Taken as a whole, it is of considerable interest and importance to determine the neuroanatomical structure of Kiss2-R and GnRH1 neurons in grass puffer, which is currently under investigation.
In the pituitary, the augmented expressions of fshb, lhb as well as kissr2 by gpKiss2 administration in the immature and mature fish (Figures 4, 5B, C) suggest that gpKiss2 could have direct action on the regulation of pituitary. Stimulatory action of peripherally administered Kiss2 on GTH synthesis and release in the pituitary has been shown in goldfish (13), zebrafish (16), sea bass (17), hybrid bass (23), Nile tilapia (20), orange-spotted grouper (52) and seahorse (53). In addition, stimulatory effects of centrally administered Kiss2 on the fshb and lhb expressions were reported in chub mackerel (18). On the other hand, direct effect of Kiss2 has been examined using primary pituitary cultures, and the stimulatory effects were observed in sea bass (19) and zebrafish (54), whereas an inhibitory effect on lhb expression was reported in the primary culture of eel pituitary cells (55). The expression of kisspeptin receptor gene in the pituitary has been demonstrated in goldfish (13), European seabass (19), largemouth bass (21), yellowtail kingfish (22), zebrafish (54), eel (55), tongue sole (49), including grass puffer (39). In zebrafish, however, Kiss2-R (Kiss1Ra)-immunoreactivity was seen in corticotropes and melanotropes but not in gonadotropes (33), and the innervation of Kiss2 fibers to the pars distalis and also pars nervosa has been shown in striped bass (5), European sea bass (19, 35) and zebrafish (33, 54). These results suggest that Kiss2 may indirectly act on gonadotropes. Moreover, colocalization of Kiss2 cells with gonadotropes was observed in European sea bass (19). Taken together, these results indicate that Kiss2 has a local action on the pituitary in addition to the neuroendocrine action through preoptic GnRH neurons, and its action is most probably stimulatory in GTH secretion in many fishes including the grass puffer. Nevertheless, it should not be excluded in the present study that gonadal steroids are involved in the augmented expressions of fshb and lhb in response to the peripherally injected Kiss2 since kisspeptin and kisspeptin receptor are expressed in the gonads in many fishes including the grass puffer. The steroid feedback action on Kiss2 neurons and the pituitary gonadotropes needs be further examined.
The effect of gpKiss2 on the expression of gnih and gnihr was also examined in the present study. gpKiss2 did not show any significant changes in the expression of gnih and gnihr in the immature and mature fish (Figure 2). In the grass puffer, gnih and gnihr showed seasonal, daily and circadian variations like kiss2 and kissr2 (36, 37, 41, 42). GnIH administration experiments of grass puffer using a heterologous peptide (goldfish LPXRFamide) on the primary pituitary culture in vitro showed that goldfish LPXRFamide stimulated the expressions of fshb and lhb (42) as well as the genes for growth hormone and prolactin (56). These results suggest that GnIH is a multifunctional hypophysiotropic neurohormone, playing a stimulatory role in the control of reproduction in this species. Stimulatory and inhibitory effects of GnIH on reproduction have been reported in fish and the effects depend on the species and gonadal stages [for reviews (3) and (4)]. Although no changes in the gnih and gnihr expression in response to gpKiss2 administration were observed in the immature and mature fish, the gnih and gnihr mRNA levels tended to be higher in the gpKiss2-injected fish than the control fish at the regressed stage, suggesting that there may be a functional interaction between gpKiss2 and GnIH (Figure 6B). In the tongue sole, Kiss2 upregulated the expression of gnih and downregulated the expression of gnihr in the hypothalami in culture (49) and GnIH was shown to inhibit Kiss2-induced cAMP signaling in COS-7 cells transfected with both Kiss2-R and GnIHR expression vectors (57). It is tempting to speculate that Kiss2 and GnIH may have some functional interaction to stimulate GTH secretion in the grass puffer, in which GnIH positively regulates reproduction (37, 42, 56), and this needs to be further investigated.
In the regressed fish, there was no significant changes in the expression of gnrh1, fshb and lhb in the gpKiss2-injected fish (Figures 6C, 7C, D), while kissr2 expression was increased in the brain by the gpKiss2 injection but decreased in the pituitary (Figures 6A, 7A). Although it is unclear why different response of kissr2 expression was obtained in the brain and pituitary, the present results show that gpKiss2 is mostly ineffective in stimulating the expression of GnRH1/GTH subunit genes at the regressed stage. In yellowtail kingfish, Kiss1 administration significantly augmented the expression of pituitary kiss2r in the non-breeding season, whereas it was ineffective in increasing the kiss2r mRNA levels in the breeding season but was still effective in stimulating the fshb and lhb expressions (22). In hybrid bass, Kiss2 upregulated gnrh1 and kiss2r expressions in the brain at prepuberty, while it downregulated the expression of gnrh1 and kiss2r at the recrudescence stage (23). These results show that Kiss2 has a stimulatory role during gonadal maturation from prepubertal to mature stage in fish. In the grass puffer, the expression profiles of kiss2 and kissr2 during reproductive cycle show that both genes are activated at the early stage of gametogenesis and their expression levels are increased to the maximum levels at the breeding stages (36, 39). In chub mackerel, both kiss1 and kiss2 expressions were temporally increased at the onset of puberty and also during the breeding season (8). Increased expression of kiss2 during the breeding season has also been reported in Senegalese sole (59) and European sea bass (60). Moreover, it has been reported that substantial increase in kisspeptin receptor gene expression occur before onset of puberty or during early puberty in zebrafish and medaka (16), Nile tilapia (20), cobia (61), grey mullet (62), flathead minnow (63), Atlantic halibut (64) and lined seahorse (53). Taken as a whole, these data show that the kisspeptin system is activated at the onset of puberty and also during the breeding stage in fish [see also review (2)]. The stimulatory effects of gpKiss2 on the expression of GnRH1/GTH subunit genes observed in the immature and mature fish in the present study are consistent with this notion.
In conclusion, gpKiss2 significantly stimulated the expression of kissr2 and gnrh1 in the brain and kissr2, fshb and lhb in the pituitary at immature and mature stages. The present results suggest that kisspeptin functions as a stimulatory neurohormone in the control of reproduction indirectly through preoptic GnRH1 neurons and directly by local action in the pituitary via the upregulation of kissr2 expression. The stimulatory action of gpKiss2 on the expression of GnRH1/GTH genes depends on the reproductive stage, being mostly ineffective at the regressed stage in the grass puffer.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of the Niigata University.
MMZ: design, experimentation, statistics, visualization, and writing. MS: design, experimentation, and statistics. HA: conception, design, writing, and supervision. All authors contributed to the article and approved the submitted version.
This study was supported by MEXT/JSPS KAKENHI grants (26.04068, 16H04812 and 20H03288 to HA).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Mr. Tomonobu Uryu for his help in collecting wild grass puffer. We thank Dr. Kiyoshi Kikuchi and Mr. Naoki Mizuno (University of Tokyo) for providing juvenile grass puffer.
1. Zohar Y, Muñoz-Cueto JA, Elizur A, Kah O. Neuroendocrinology of Reproduction in Teleost Fish. Gen Comp Endocrinol (2009) 165:438–55. doi: 10.1016/j.ygcen.2009.04.017
2. Mechaly AS, Viñas J, Piferrer F. The Kisspeptin System Genes in Teleost Fish, Their Structure and Regulation, With Particular Attention to the Situation in Pleuronectiformes. Gen Comp Endocrinol (2013) 188:258–68. doi: 10.1016/j.ygcen.2013.04.010
3. Muñoz-Cueto JA, Paullada- Salmerón JA, Aliaga-Guerrero M, Cowan ME, Parhar IS, Ubuka T. A Journey Through the Gonadotropin-Inhibitory Hormone System of Fish. Front Endocrinol (2017) 8:285. doi: 10.3389/fendo.2017.00285
4. Di Yorio MP, Muñoz-Cueto JA, Paullada-Salmerón JA, Somoza GM, Tsutsui K, Vissio PG. The Gonadotropin-Inhibitory Hormone: What We Know and What We Still Have to Learn From Fish. Front Endocrinol (2019) 10:78. doi: 10.3389/fendo.2019.00078
5. Muñoz-Cueto JA, Zmora N, Paullada-Salmeron JA, Marvel M, Mananos E, Zohar Y. The Gonadotropin-Releasing Hormones: Lessons From Fish. Gen Comp Endocrinol (2020) 291:113422. doi: 10.1016/j.ygcen.2020.113422
6. Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a Novel Human Malignant Melanoma Metastasis-Suppressor Gene. J Natl Cancer Inst (1996) 88:1731–7. doi: 10.1093/jnci/88.23.1731
7. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The Metastasis Suppressor Gene KiSS-1 Encodes Kisspeptins, the Natural Ligands of the Orphan G Protein-Coupled Receptor GPR54. J Biol Chem (2001) 276:34631–6. doi: 10.1074/jbc.M104847200
8. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic Hypogonadism Due to Loss of Function of the KiSS1-Derived Peptide Receptor GPR54. Proc Natl Acad Sci USA (2003) 100:10972–6. doi: 10.1073/pnas.1834399100
9. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, et al. The GPR54 Gene as a Regulator of Puberty. N Engl J Med (2003) 349:1614–27. doi: 10.1056/NEJMoa035322
10. Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocr Rev (2009) 30:713–43. doi: 10.1210/er.2009-0005
11. Pasquier J, Kamech N, Lafont AG, Vaudry H, Rousseau K, Dufour S. Molecular Evolution of GPCRs: Kisspeptin/Kisspeptin Receptors. J Mol Endocrinol (2014) 52:T101–17. doi: 10.1530/JME-13-0224
12. Ogawa S, Parhar IS. Biological Significance of Kisspeptin–Kiss 1 Receptor Signaling in the Habenula of Teleost Species. Front Endocrinol (2018) 9:222. doi: 10.3389/fendo.2018.00222
13. Li S, Zhang Y, Liu Y, Huang X, Huang W, Lu D, et al. Structural and Functional Multiplicity of the Kisspeptin/GPR54 System in Goldfish (Carassius Auratus). J Endocrinol (2009) 201:407–18. doi: 10.1677/JOE-09-0016
14. Selvaraj S, Ohga H, Kitano H, Nyuji M, Yamaguchi A, Matsuyama M. Peripheral Administration of Kiss1 Pentadecapeptide Induces Gonadal Development in Sexually Immature Adult Scombroid Fish. Zool Sci (2013) 30:446–54. doi: 10.2108/zsj.30.446
15. Zmora N, Stubblefield J, Golan M, Servili A, Levavi-Sivan B, Zohar Y. The Medio-Basal Hypothalamus as a Dynamic and Plastic Reproduction-Related Kisspeptin-Gnrh-Pituitary Center in Fish. Endocrinology (2014) 155:1874–86. doi: 10.1210/en.2013-1894
16. Kitahashi T, Ogawa S, Parhar IS. Cloning and Expression of Kiss2 in the Zebrafish and Medaka. Endocrinology (2009) 150:821–31. doi: 10.1210/en.2008-0940
17. Felip A, Zanuy S, Pineda R, Pinilla L, Carrillo M, Tena-Sempere M, et al. Evidence for Two Distinct KiSS Genes in non-Placental Vertebrates That Encode Kisspeptins With Different Gonadotropin-Releasing Activities in Fish and Mammals. Mol Cell Endocrinol (2009) 312:61–71. doi: 10.1016/j.mce.2008.11.017
18. Ohga H, Selvaraj S, Adachi H, Imanaga Y, Nyuji M, Yamaguchi A, et al. Functional Analysis of Kisspeptin Peptides in Adult Immature Chub Mackerel (Scomber Japonicus) Using an Intracerebroventricular Administration Method. Neurosci Lett (2014) 561:203–7. doi: 10.1016/j.neulet.2013.12.072
19. Espigares F, Zanuy S, Gómez A. Kiss2 as a Regulator of Lh and Fsh Secretion via Paracrine/Autocrine Signaling in the Teleost Fish European Sea Bass (Dicentrarchus Labrax). Biol Reprod (2015) 93:1–12. doi: 10.1095/biolreprod.115.131029
20. Park JW, Jin YH, Oh S, Kwon JY. Kisspeptin2 Stimulates the HPG Axis in Immature Nile Tilapia (Oreochromis Niloticus). Comp Biochem Physiol B (2016) 202:31–8. doi: 10.1016/j.cbpb.2016.07.009
21. Li W, Hu J, Sun C, Dong J, Liu Z, Yuan J, et al. Characterization of Kiss2/Kissr2 System in Largemouth Bass (Micropterus Salmoides) and Kiss2–10 Peptide Regulation of the Hypothalamic-Pituitary-Gonadal Axis. Comp Biochem Physiol B (2022) 257:110671. doi: 10.1016/j.cbpb.2021.110671
22. Nocillado JN,Zohar Y,Biran J, Levavi-Sivan B,Elizur A. Chronic Kisspeptin Administration Stimulated Gonadal Development in Pre-Pubertal Male Yellowtail Kingfish (Seriola Lalandi; Perciformes) During the Breeding and non-Breeding Season. Gen Comp Endocrinol (2013) 191:168–76. doi: 10.1016/j.ygcen.2013.06.005
23. Zmora N, Stubblefield J, Zulperi Z, Biran J, Levavi-Sivan B, Muñoz-Cueto JA, et al. Differential and Gonad Stage-Dependent Roles of Kisspeptin1 and Kisspeptin2 in Reproduction in the Modern Teleosts, Morone Species. Biol Reprod (2012) 86:177. doi: 10.1095/biolreprod.111.097667
24. Tang H, Liu Y, Luo D, Ogawa S, Yin Y, Li S, et al. The Kiss/Kissr Systems are Dispensable for Zebrafish Reproduction: Evidence From Gene Knockout Studies. Endocrinology (2015) 156:589–99. doi: 10.1210/en.2014-1204
25. Liu Y, Tang H, Xie R, Li S, Liu X, Lin H, et al. Genetic Evidence for Multifactorial Control of the Reproductive Axis in Zebrafish. Endocrinology (2017) 158:604–11. doi: 10.1210/en.2016-1540
26. Etzion T, Zmora N, Zohar Y, Levavi-Sivan B, Golan M, Gothilf Y. Ectopic Over Expression of Kiss1 may Compensate for The Loss of Kiss2. Gen Comp Endocrinol (2020) 295:113523. doi: 10.1016/j.ygcen.2020.113523
27. Nakajo M, Kanda S, Karigo T, Takahashi A, Akazome Y, Uenoyama Y, et al. Evolutionally Conserved Function of Kisspeptin Neuronal System is non-Reproductive Regulation as Revealed by non-Mammalian Study. Endocrinology (2017) 159:163–83. doi: 10.1210/en.2017-00808
28. Somoza GM, Mechaly AS, Trudeau VL. Kisspeptin and GnRH Interactions in the Reproductive Brain of Teleosts. Gen Comp Endocrinol (2020) 298:113568. doi: 10.1016/j.ygcen.2020.113568
29. Sivalingam M, Parhar IS. Hypothalamic Kisspeptin and Kisspeptin Receptors: Species Variation in Reproduction and Reproductive Behaviours. Front Neuroendocrinol (2022) 64:100951. doi: 10.1016/j.yfrne.2021.100951
30. Parhar IS, Ogawa S, Sakuma Y. Laser-Captured Single Digoxigenin-Labeled Neurons of Gonadotropin-Releasing Hormone Types Reveal a Novel G Protein-Coupled Receptor (Gpr54) During Maturation in Cichlid Fish. Endocrinology (2004) 145:3613–8. doi: 10.1210/en.2004-0395
31. Grone BP, Maruska KP, Korzan WJ, Fernald RD. Social Status Regulates Kisspeptin Receptor mRNA in the Brain of Astatotilapia Burtoni. Gen Comp Endocrinol (2006) 169:98–107. doi: 10.1016/j.ygcen.2010.07.018
32. Ohga H, Adachi H, Kitano H, Yamaguchi A, Matsuyama M. Kiss1 Hexadecapeptide Directly Regulates Gonadotropin-Releasing Hormone 1 in the Scombroid Fish, Chub Mackerel. Biol Reprod (2017) 96:376–88. doi: 10.1095/biolreprod.116.142083
33. Ogawa S, Sivalingam M, Anthonysamy R, Parhar IS. Distribution of Kiss2 Receptor in the Brain and its Localization in Neuroendocrine Cells in the Zebrafish. Cell Tissue Res (2020) 379:349–72. doi: 10.1007/s00441-019-03089-5
34. Kanda S, Akazome Y, Mitani Y, Okubo K, Oka Y. Neuroanatomical Evidence That Kisspeptin Directly Regulates Isotocin and Vasotocin Neurons. PloS One (2013) 8:e62776. doi: 10.1371/journal.pone.0062776
35. Escobar S, Servili A, Espigares F, Gueguen MM, Brocal I, Felip A, et al. Expression of Kisspeptins and Kiss Receptors Suggests a Large Range of Functions for Kisspeptin Systems in the Brain of the European Sea Bass. PloS One (2013) 8:e70177. doi: 10.1371/journal.pone.0070177
36. Ando H, Shahjahan M, Hattori A. Molecular Neuroendocrine Basis of Lunar-Related Spawning in Grass Puffer. Gen Comp Endocrinol (2013) 181:211–4. doi: 10.1016/j.ygcen.2012.07.027
37. Ando H, Shahjahan M, Kitahashi T. Periodic Regulation of Expression of Genes for Kisspeptin, Gonadotropin-Inhibitory Hormone and Their Receptors in the Grass Puffer: Implications in Seasonal, Daily and Lunar Rhythms of Reproduction. Gen Comp Endocrinol (2018) 265:149–53. doi: 10.1016/j.ygcen.2018.04.006
38. Motohashi E, Yoshihara T, Doi H, Ando H. Aggregating Behavior of Grass Puffer, Takifugu Niphobles, Observed in Aquarium During the Spawning Period. Zool Sci (2010) 27:559–64. doi: 10.1016/j.ygcen.2007.07.009
39. Shahjahan M, Motohashi E, Doi H, Ando H. Elevation of Kiss2 and its Receptor Gene Expression in the Brain and Pituitary of Grass Puffer During the Spawning Season. Gen Comp Endocrinol (2010) 169:48–57. doi: 10.1016/j.ygcen.2010.07.008
40. Shahjahan M, Kitahashi T, Ando H. Temperature Affects Sexual Maturation Through the Control of Kisspeptin, Kisspeptin Receptor, GnRH and GTH Subunit Gene Expression in the Grass Puffer During the Spawning Season. Gen Comp Endocrinol (2017) 243:138–45. doi: 10.1016/j.ygcen.2016.11.012
41. Ando H, Ogawa S, Shahjahan M, Ikegami T, Doi H, Hattori A, et al. Diurnal and Circadian Oscillations in Expression of Kisspeptin, Kisspeptin Receptor and Gonadotrophin-Releasing Hormone 2 Genes in the Grass Puffer, a Semilunar-Synchronised Spawner. J Neuroendocrinol (2014) 26:459–67. doi: 10.1111/jne.12165
42. Shahjahan M, Ikegami T, Osugi T, Ukena K, Doi H, Hattori A, et al. Synchronised Expressions of LPXRFamide Peptide and its Receptor Genes: Seasonal, Diurnal and Circadian Changes During Spawning Period in Grass Puffer. J Neuroendocrinol (2011) 23:39–51. doi: 10.1111/j.1365-2826.2010.02081.x
43. Shahjahan Md, Hamabata T, Motohashi E, Doi H, Ando H. Differential Expression of Three Types of Gonadotropin-Releasing Hormone Genes During the Spawning Season in Grass Puffer, Takifugu Niphobles. Gen Comp Endocrinol (2010) 167:153–63. doi: 10.1016/j.ygcen.2010.01.018
44. Matsuda K, Nakamura K, Shimakura S, Miura T, Kageyama H, Uchiyama M, et al. Inhibitory Effect of Chicken Gonadotropin-Releasing Hormone II on Food Intake in the Goldfish, Carassius Auratus. Horm Behav (2008) 54:83–9. doi: 10.1016/j.yhbeh.2008.01.011
45. Nishiguchi R, Azuma M, Yokobori E, Uchiyama M, Matsuda K. Gonadotropin-Releasing Hormone 2 Suppresses Food Intake in the Zebrafish, Danio Rerio. Front Endocrinol (2012) 3:122. doi: 10.3389/fendo.2012.00122
46. Marvel MM, Spicer OS, Wong TT, Zmora N, Zohar Y. Knockout of Gnrh2 in Zebrafish (Danio Rerio) Reveals its Roles in Regulating Feeding Behavior and Oocyte Quality. Gen Comp Endocrinol (2019) 280:15–23. doi: 10.1016/j.ygcen.2019.04.002
47. Okuyama T, Yokoi S, Abe H, Isoe Y, Suehiro Y, Imada H, et al. A Neural Mechanism Underlying Mating Preferences for Familiar Individuals in Medaka Fish. Science (2014) 343:91–4. doi: 10.1126/science.1244724
48. Li L, Wojtowicz JL, Malin JH, Huang T, Lee EB, Chen Z. GnRH-Mediated Olfactory and Visual Inputs Promote Mating-Like Behaviors in Male Zebrafish. PloS One (2017) 12:e0174143. doi: 10.1371/journal.pone.0174143
49. Wang B, Liu Q, Liu X, Xu Y, Shi B. Molecular Characterization of Kiss2 Receptor and In Vitro Effects of Kiss2 on Reproduction-Related Gene Expression in the Hypothalamus of Half Smooth Tongue Sole (Cynoglossus Semilaevis). Gen Comp Endocrinol (2017) 249:55–63. doi: 10.1016/j.ygcen.2017.04.006
50. Zmora N, Stubblefield JD, Wong T-T, Levavi-Sivan B, Millar RP, Zohar Y. Kisspeptin Antagonists Reveal Kisspeptin 1 and Kisspeptin 2 Differential Regulation of Reproduction in the Teleost. Morone saxatilis Biol Reprod (2015) 93:76. doi: 10.1095/biolreprod.115.131870
51. Servili A, Le Page Y, Leprince J, Caraty A, Escobar S, Parhar IS, et al. Organization of Two Independent Kisspeptin Systems Derived From Evolutionary-Ancient Kiss Genes in the Brain of Zebrafish. Endocrinology (2011) 152:1527–40. doi: 10.1210/en.2010-0948
52. Shi Y, Zhang Y, Li S, Liu Q, Lu D, Liu M, et al. Molecular Identification of the Kiss2/Kiss1ra System and its Potential Function During 17-Alpha-Methyltestosterone-Induced Sex Reversal in the Orange-Spotted Grouper. Epinephelus coioides Biol Reprod (2010) 83:63–74. doi: 10.1095/biolreprod.109.080044
53. Zhang H, Zhang B, Qin G, Li S, Lin Q. The Roles of the Kisspeptin System in the Reproductive Physiology of the Lined Seahorse (Hippocampus Erectus), an Ovoviviparous Fish With Male Pregnancy. Front Neurosci (20182018) 12:. doi: 10.3389/fnins
54. Song Y, Chen J, Tao B, Luo D, Zhu Z, Hu W. Kisspeptin2 Regulates Hormone Expression in Female Zebrafish (Danio Rerio) Pituitary. Mol Cell Endocrinol (2020) 513:110858. doi: 10.1016/j.mce.2020.110858
55. Pasquier J, Lafont AG, Leprince J, Vaudry H, Rousseau K, Dufour S. First Evidence for a Direct Inhibitory Effect of Kisspeptins on LH Expression in the Eel, Anguilla Anguilla. Gen Comp Endocrinol (2011) 173:216–25. doi: 10.1016/j.ygcen.2011.05.019
56. Shahjahan M, Doi H, Ando H. LPXRFamide Peptide Stimulates Growth Hormone and Prolactin Gene Expression During the Spawning Period in the Grass Puffer, a Semi-Lunar Synchronized Spawner. Gen Comp Endocrinol (2016) 227:77–83. doi: 10.1016/j.ygcen.2015.09.008
57. Wang B, Yang G, Liu Q, Qin J, Xu Y, Li W, et al. Inhibitory Action of Tongue Sole LPXRFa, the Piscine Ortholog of Gonadotropin-Inhibitory Hormone, on the Signaling Pathway Induced by Tongue Sole Kisspeptin in COS-7 Cells Transfected With Their Cognate Receptors. Peptides (2017) 95:62–7. doi: 10.1016/j.peptides.2017.07.014
58. Ohga H, Adachi H, Matsumori K, Kodama R, Nyuji M, Selvaraj S, et al. mRNA Levels of Kisspeptins, Kisspeptin Receptors, and GnRH1 in the Brain of Chub Mackerel During Puberty. Comp Biochem Physiol Part A: Mol Integr Physiol (2015) 179:104–12. doi: 10.1016/j.cbpa.2014.09.012
59. Mechaly AS, Viñas J, Piferrer F. Sex-Specific Changes in the Expression of Kisspeptin, Kisspeptin Receptor, Gonadotropins and Gonadotropin Receptors in the Senegalese Sole (Solea Senegalensis) During a Full Reproductive Cycle. Comp Biochem Physiol Part A: Mol Integr Physiol (2012) 162:364–71. doi: 10.1016/j.cbpa.2012.04.003
60. Migaud H, Ismail R, Cowan M, Davie A. Kisspeptin and Seasonal Control of Reproduction in Male European Sea Bass (Dicentrarchus Labrax). Gen Comp Endocrinol (2012) 179:384–99. doi: 10.1016/j.ygcen.2012.07.033
61. Mohammed JS, Benninghoff AD, Holt GJ, Khan IA. Developmental Expression of the G Protein-Coupled Receptor 54 and Three GnRH mRNAs in the Teleost Fish Cobia. J Mol Endocrinol (2007) 38:235–44. doi: 10.1677/jme.1.02182
62. Nocillado JN, Levavi-Sivan B, Carrick F, Elizur A. Temporal Expression of G-Protein-Coupled Receptor 54 (GPR54), Gonadotropin-Releasing Hormones (GnRH), and Dopamine Receptor D2 (Drd2) in Pubertal Female Grey Mullet, Mugil Cephalus. Gen Comp Endocrinol (2007) 150:278–87. doi: 10.1016/j.ygcen.2006.09.008
63. Filby AL, Van Aerle R, Duitman J, Tyler CR. The Kisspeptin/Gonadotropin-Releasing Hormone Pathway and Molecular Signaling of Puberty in Fish. Biol Reprod (2008) 78:278–89. doi: 10.1095/biolreprod.107.063420
Keywords: GnIH, GnRH, GPR54, hypothalamus, gonadotropin, kisspeptin, puffer fish, reproduction
Citation: Zahangir MM, Shahjahan M and Ando H (2022) Kisspeptin Exhibits Stimulatory Effects on Expression of the Genes for Kisspeptin Receptor, GnRH1 and GTH Subunits in a Gonadal Stage-Dependent Manner in the Grass Puffer, a Semilunar-Synchronized Spawner. Front. Endocrinol. 13:917258. doi: 10.3389/fendo.2022.917258
Received: 11 April 2022; Accepted: 22 June 2022;
Published: 15 July 2022.
Edited by:
Bin Wang, Chinese Academy of Fishery Sciences (CAFS), ChinaReviewed by:
Karine Rousseau, Muséum National d’Histoire Naturelle, FranceCopyright © 2022 Zahangir, Shahjahan and Ando. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hironori Ando, aGFuZG8zMTFAY2MubmlpZ2F0YS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.