- 1Department of Neuroscience, Biomedicine and Movement, University of Verona, Verona, Italy
- 2Department of Medicine, University of Verona, Verona, Italy
- 3Geriatrics Division, Department of Medicine, Ospedale Cà Foncello ULSS2, Treviso, Italy
- 4Healthy Aging Center, Department of Medicine, Division of Geriatrics, University of Verona, Verona, Italy
Background: Sarcopenic obesity is characterized by low muscle mass and high body fat; prevalence increases with age, particularly after age 65 years. For this systematic literature review we searched scientific databases for studies on exercise interventions for improving physical performance in adults with sarcopenic obesity; also, we identified potential gaps in clinical practice guidelines that need to be addressed.
Methods: We followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The databases were searched for studies published through November 2021 that measured physical performance in adults with sarcopenic obesity.
Results: Most of the studies applied a strength training protocol in which improvement was noted post-treatment on the Time Chair Rise (TCR), 30-s Chair Stand, and Single Leg Stance (SLS) tests. Discrepancies between the studies were observed when resistance training was combined with or without elastic bands or electromyostimulation, as measured with the Short Physical Performance Battery (SPPB), Physical Performance Test (PPT), Gait Speed, and Timed Up & Go (TUG) test. Post-intervention SPPB, PPT, and gait speed scores showed an increase or maintenance of performance, while TUG test scores were higher according to one study but lower according to another.
Conclusions: Engagement in physical exercise, and resistance training in particular, can improve or maintain physical performance in adults with sarcopenic obesity. Study samples should include more men. A future area of focus should be the impact of different types of training (aerobic, power training, combined modalities). Finally, studies with longer intervention periods and follow-up periods are needed to gain a better understanding of the effectiveness of exercise on physical function in adults with sarcopenic obesity.
Introduction
One of the major public health challenges of the 21st century is obesity. In simple terms, obesity is an excessive increase in fat mass (1). Since the 1980s, its prevalence has tripled in many European countries and the population with weight excess continues to grow at an alarming rate (2). Obesity impacts on morbidity, disability, activities of daily living and increases the risk of developing cardiovascular disease (e.g., heart disease and stroke), type 2 diabetes, musculoskeletal disorders (osteoarthritis), some cancers, and other health-related issues (3).
Musculoskeletal disorders in the aging population are a growing public health concern (4). A prominent change associated with human aging is the progressive decline in skeletal muscle mass and strength. Several studies have suggested that muscle mass declines by nearly 6% per decade after mid-life (4). The percentage of loss of muscle strength per year is 50 to 100% greater than the loss of muscle mass. In the population of the Health ABC Study, the annual rate of decline in leg strength was approximately three times greater than the rate of loss of lean leg mass (~1% per year) (5).
Sarcopenia, derived from the Greek sarx (flesh) and penia (loss), was introduced by I.H. Rosenberg in 1989 (6) to describe the loss of skeletal muscle mass. The term refers to an age-related, progressive, generalized skeletal muscle disorder (7) associated with physical disability, metabolic dysfunction, and increased mortality (8).
Research groups in Europe and Asia have developed consensus on the definition and diagnostic criteria for sarcopenia. In 2010, the European Working Group on Sarcopenia in Older People EWGSOP (9) defined sarcopenia as the presence of both low muscle mass and low muscle strength or performance. In 2019, the EWGSOP updated the definition of sarcopenia diagnosis (EWGSOP2). In its revised definition, the EWGSOP2 recommends the use of low muscle strength (evaluated with the handgrip strength or the chair stand test) as the primary parameter for screening, subsequently confirmed by low appendicular skeletal muscle mass adjusted by height in meters squared.
Two other research groups, the International Working Group on Sarcopenia (IWGS) (10) and the Asian Working Group for Sarcopenia (AWGS) (11) have adopted similar approaches to defining sarcopenia as the presence of low appendicular skeletal muscle mass and poor muscle function.
A related disorder is sarcopenic obesity, a term introduced by Baumgartner (12) in reference to the co-presence of sarcopenia and obesity in a specific phenotype of low muscle mass and high body fat. As the population ages, the prevalence of sarcopenic obesity increases, as the prevalence of obesity and sarcopenia also increases, particularly among adults aged 65 years or older (13). It is associated with a reduction in physical activity and energy expenditure and an increase in body weight. Sarcopenia and obesity result in reduced physical performance (14). A hallmark of sarcopenia is slower gait speed. Besides the higher risk of falls (15), older people with obesity have reduced physical function, as assessed via self-assessment questionnaires or tests such as the Short Physical Performance Battery (SPPB) (14). Sarcopenic obesity is thought to have a synergistic effect on health deterioration compared to sarcopenia or obesity alone. It is responsible for more health problems than either sarcopenia or obesity (16) and is a leading cause of metabolic disorders, disability, cardiovascular disease (4), and mortality. A shared definition is currently lacking, making it difficult to establish standardized diagnosis and management. While progress has been made in defining sarcopenic obesity according to the recent Consensus of the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) (17), on treatment of the condition the discussion is still open.
Prevention and treatment hold importance for public health and individual healthy aging. Exercise strategies have been developed to improve cardiovascular and metabolic function (18), cancers survival (19), and to increase muscle mass, muscle strength, and physical performance in adults with sarcopenic obesity (20). The mechanisms by which physical exercise can induce beneficial effects in sarcopenia and obesity are multifactorial. For example, exercise plays an essential role in regulating the energy balance which, when combined with a low-calorie diet, can set a lower energy balance. Also, exercise can enhance physical functioning parameters, such as handgrip strength, gait speed, and balance capacity in adults with sarcopenia and those with obesity (21). Notably, improvement is closely linked to exercise intensity, volume, frequency, and workout progression. Since exercise is an effective strategy for improving body composition in individuals with sarcopenia and those with obesity, regular exercise can play a central role in treating sarcopenic obesity (8, 18).
Resistance exercise is recognized as an effective strategy for increasing muscle hypertrophy and improving muscle function and strength in older adults (22). Most studies involve healthy older populations, while some reviews or meta-analyses evaluate studies on exercise in combination with amino acid or protein supplementation (18). Limitations that explain the low impact of exercise interventions include lack of standardization of exercise protocols, short duration of interventions, and differences in eligibility criteria (23).
For this systematic review we searched the scientific literature on types of exercise designed to improve physical performance in adults with sarcopenic obesity; also, we identified and analyzed potential gaps in clinical practice guidelines that merit attention in future studies.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 statement and our review methods were established prior to data extraction and were pre-registered with PROSPERO (ID: CRD42022314354).
Identification guidelines
One researcher (LG) carried out the literature search to identify studies of exercise treatment and physical performance in older adults with sarcopenic obesity. The PubMed, Scopus, EBSCO and Cochrane Library databases were searched up to November 2021, without language or publication date restrictions.
Search terms
The search was performed using the keywords: OLDER ADULTS: “elder” OR “elderly” OR “elders” OR “aged” OR “seniors” OR “senior” OR “older” OR “old people” OR “older people” OR “aging”. SARCOPENIC OBESITY: “sarcopenic obese” OR “sarcopenic obesity” OR “sarcopenia obese” OR “sarcopenia obesity” OR “obese sarcopenic” OR “obesity sarcopenic” OR “obese sarcopenia” OR “obesity sarcopenia”. EXERCISE: “training” OR “exercise” OR “resistance training” OR “strength training” OR “resistance exercise” OR “strength exercise” OR “aerobic training” OR “aerobic exercise” OR “high speed circuit training” OR “power training”. PHYSICAL TEST: “short physical performance battery” OR “physical performance test” OR “gait speed” OR “walking speed” OR “chair stand” OR “time chair rise” OR “single leg-stance” OR “one leg balance” OR “time up and go”.
Inclusion and exclusion criteria
● Original research findings (reviews, meta-analyses, editorials, conference abstracts, research protocols were excluded)
● Observational and experimental studies (reference data only, abstracts excluded if no data could be extracted)
● Study sample involving women and men of any race, age ≥60 years with a detailed diagnosis of sarcopenic obesity, ability to undertake bipedal locomotion
● All exercise interventions included in the analysis
Non-human studies and studies not reported pre-post intervention change in outcome (e.g., cross-sectional studies) were excluded.
Data extraction
The records were processed using Rayyan-Intelligent Systematic Review software, which detected duplicates (Systematic Reviews (2016) 5: 210, DOI: 10.1186/s13643-016-0384-4.). Each duplicate was manually checked before removal. The records were identified and then screened; the abstracts were reviewed, and the full text then analyzed when the abstracts were unclear. Finally, the records were selected for analysis.
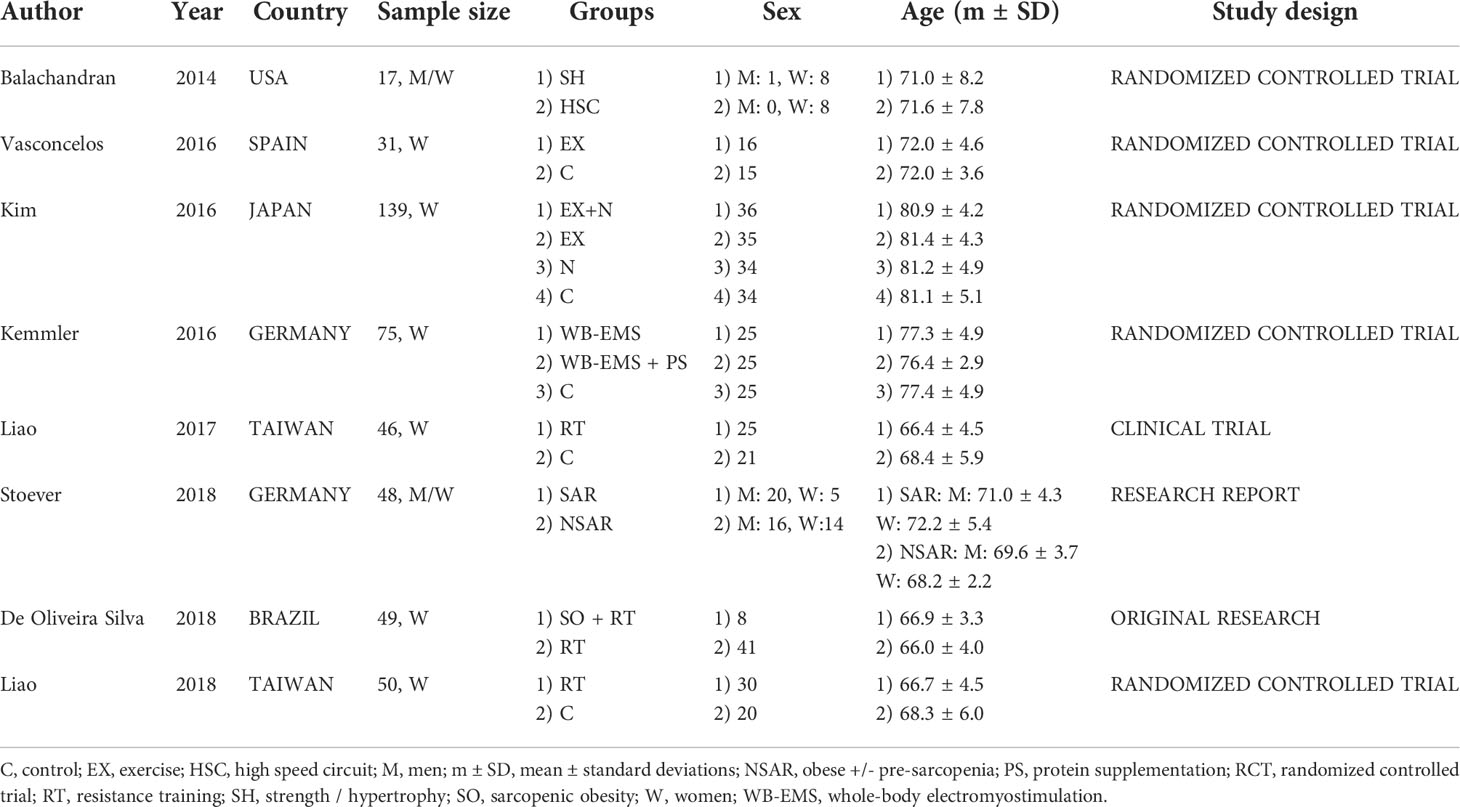
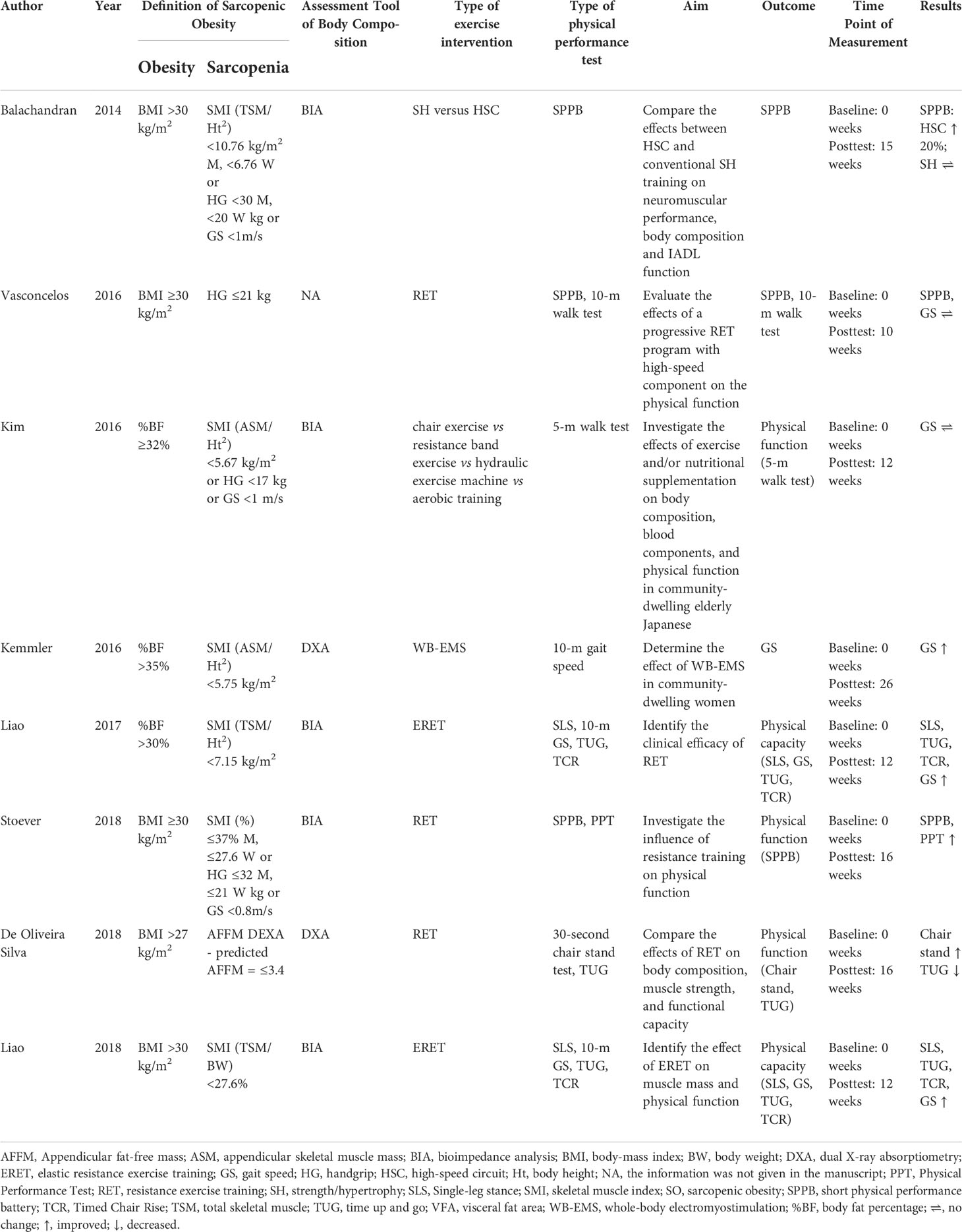
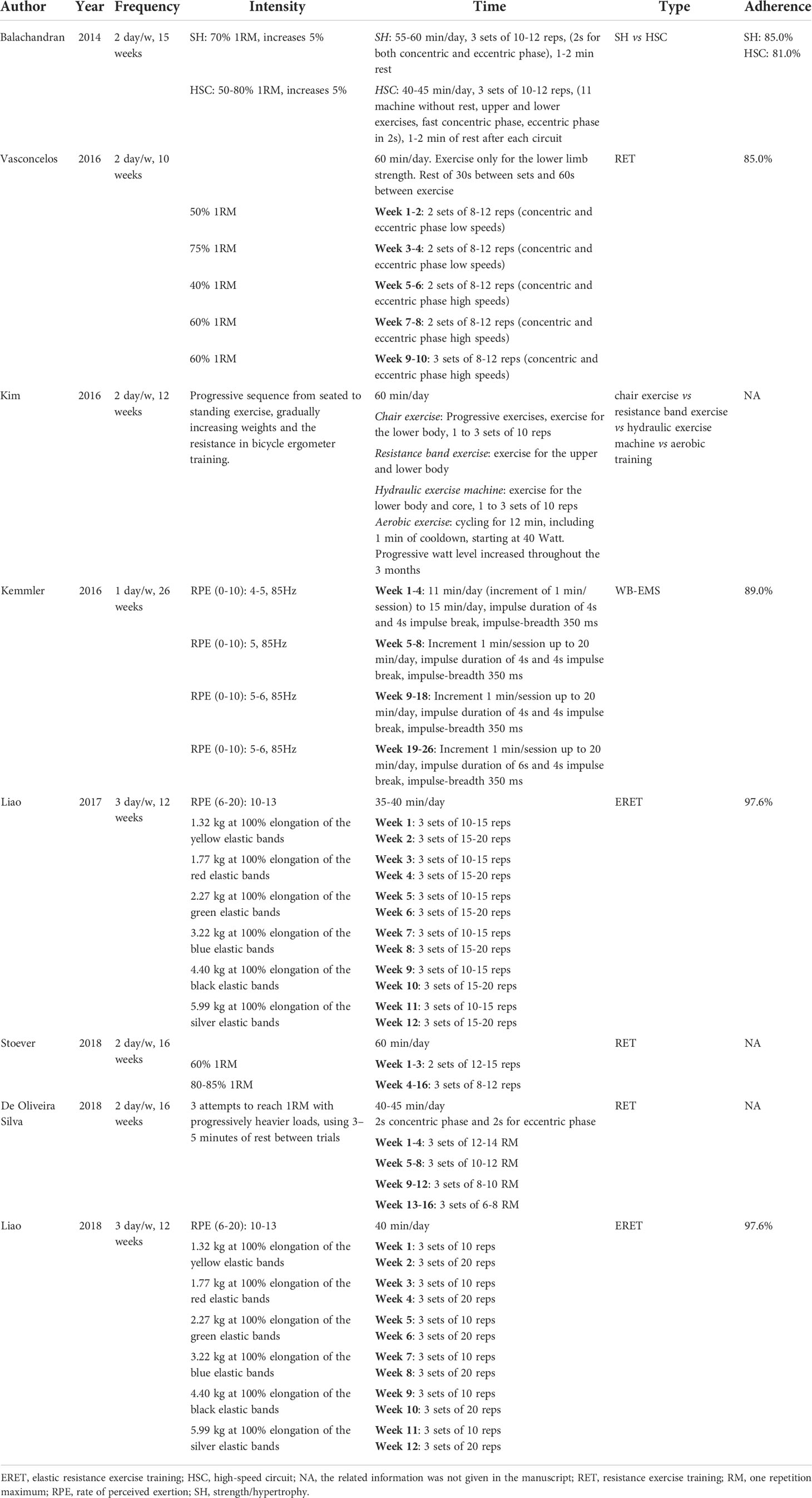
Two authors (LG; TT) independently extracted the data from the studies and entered them into a Microsoft Excel spreadsheet; disagreement was resolved by consensus. Table 1 presents a summary of the study characteristics: 1) authors, 2) publication year, 3) country, 4) sample size and characteristics of the study population, 5) sex of the study population, 6) age of the population, and 7) study design. One author extracted the data, and another checked the extracted data. Table 2 presents the characteristics of the studies 1) definition of sarcopenic obesity, 2) types of intervention and physical performance tests, 3) aim of the study and outcome for physical performance, 4) duration of the intervention, and 5) outcome for physical function.
Quality assessment and Risk of Bias
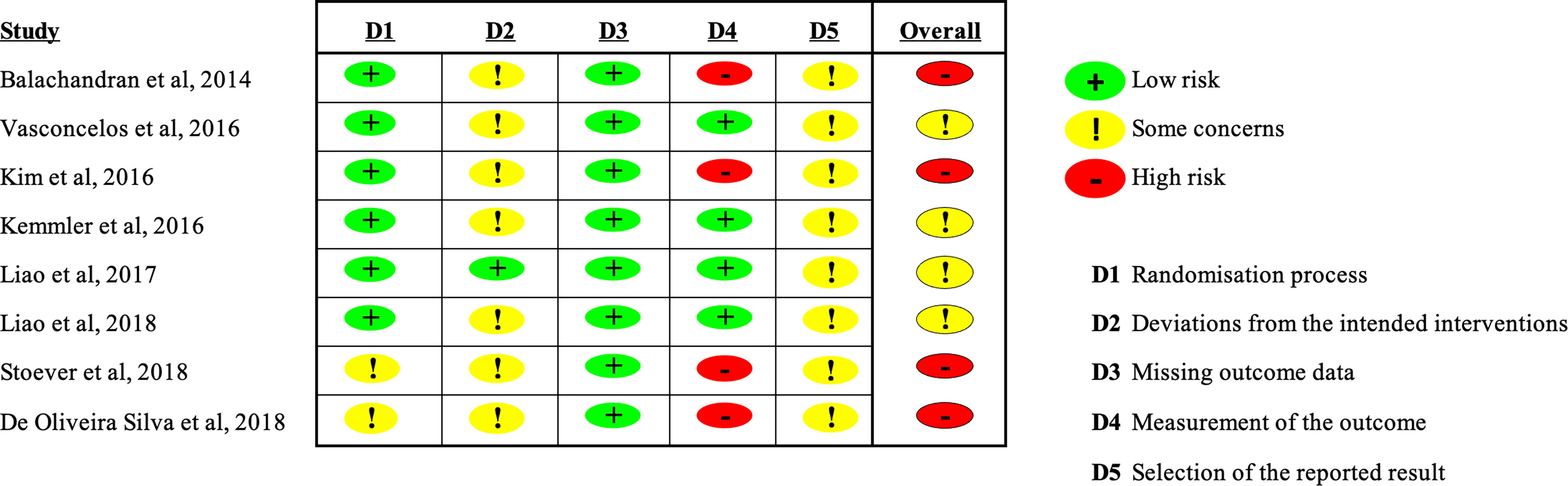
Two authors independently assessed the methodological quality of the studies using the Risk of Bias Assessment Tool for Randomized trials (RoB2) (24) (Figure 1). Study quality was rated high, moderate, or low based on study design and risk of bias. Two authors (LG and TT) independently evaluated the studies; disagreement was resolved by discussion and consensus. A third reviewer (VM) was consulted if needed.
Results
Study selection
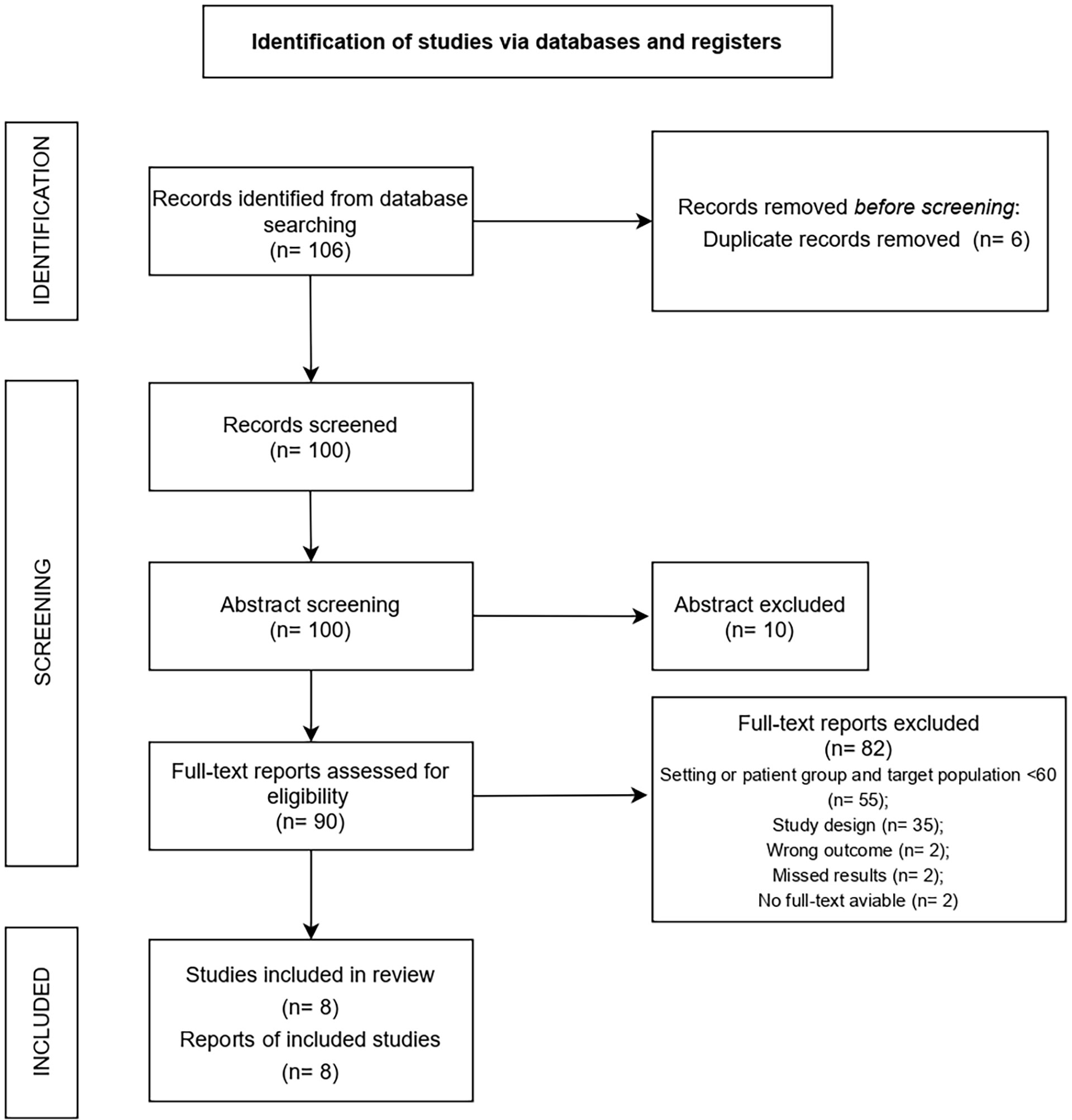
A total of 106 studies were retrieved. After removing duplicates, 100 studies were screened based on title and abstract. After screening, 90 full texts were evaluated. Records were excluded because: study population not conform with inclusion criteria (n=55), study design not conform with inclusion criteria (n=35), outcome did not meet inclusion criteria (n=2), no results were reported (n=2), no full text was available (n=2). The final analysis included 8 studies, 6 of which were randomized controlled trials (RCT) (25–30), 1 was a research report (31), and 1 was an original research article (32) (Figure 2).
Study characteristics
The study population was composed of women in 6 studies (26–30, 32) and of both men and women in 2 (25, 31). Participant age ranged from 60 to 80 years. In detail, the interventions involved resistance training (25, 26, 29, 30, 32) in the patient samples aged from 68 to 72 years (Table 1). While other types of exercise, such as chair exercise vs resistance band exercise vs hydraulic exercise machine vs aerobic training intervention (27) or muscle stimulation (28), where used in samples between 77 and 81 years of age. Other kinds of exercises were not employed (Table 1).
Type of interventions
Obesity was categorised by body-mass index (BMI, weight in kg divided by height in meters squared) in 5 studies (cut off 27 kg/m2 in (32) and 30 kg/m2 in (25, 26, 30, 31)) and by body fat percentage (%BF) in 3 studies (cut off point 30% in (29), 32% in (27) and 35% in (28)). Sarcopenia was defined according to skeletal muscle mass (total skeletal mass (TSM) divided by body height in meters squared (Ht2), appendicular skeletal mass (ASM)/(Ht2), skeletal muscle index percentage (SMI%), ideal appendicular fat-free mass (AFFM) or TSM/BW in (25, 27–32), and handgrip strength in (25–27, 31) (Table 2).
Physical performance was measured with the SPPB in (25, 26, 31) and with the Physical Performance Test (PPT) in (31), the walk test (10-m walk test or 5-m walk test or 10-m GS) in (26–30), the Single Leg-Stance (SLS) in (29, 30), and the Time Up & Go test (TUG) or the Timed chair rise (TCR) or the 30-s chair stand test in (29, 30, 32) (Table 2).
Effects of exercise on physical performance
Six studies investigated the effects of resistance exercise on physical performance (26, 27, 29–32); one evaluated the effects of strength exercise vs high-speed circuit on physical performance (25), and one with electromyostimulation (28) (Table 2). Overall, there was a statistically significant increase in physical performance scores on the SPPB, PPT, SLS, TUG, TCR, GS, and 30-s chair stand (29–32) after resistance exercise intervention One study reported a statistically significant decreases in TUG scores (32) and 2 studies found no statistically pre/post change in SPPB and 10-m GS (26, 27). One study (25) comparing the effects of a strength exercise vs a high-speed circuit noted an increase in SPPB scores for the high-speed circuit group but no change in scores for strength/hypertrophy group. The one study (28) that used electromyostimulation found a post-treatment increase in the 10-m GS (Table 2).
FITT table and adherence to the intervention
Table 3 presents the training protocols following the FITT principle: frequency, intensity, time, and type of training. Furthermore, adherence was added since it is a key component in exercise interventions (33–36). Training frequency differed between studies: 26 weeks of training in (28), and 10-16 weeks in (25–27, 29–32). Exercise intensity during training sessions also varied between studies: one repetition maximum (1RM) in (25, 26, 31, 32), rate of perceive exertion (RPE) in (28–30), and a combination of exercise and weight progression in (27) although the method was not specified. The duration of training sessions ranged between 60 min/day in (25–27, 31) and 15 to 45 minutes in (28–30, 32). Five studies (25, 26, 28–30) reported high adherence (≥81%), while the others 3 studies did not report adherence rates.
Discussion
This systematic review outlines the current landscape of scientific research on the impact of exercise on physical performance outcomes in older adults with sarcopenic obesity; the review also reveals several gaps that merit further investigation.
Physical activity and exercise are often used interchangeably but the terms are not synonymous. Physical activity refers to any body movement produced by skeletal muscles contraction, which then results in a substantial increase in caloric requirements compared to resting energy expenditure (37). Differently, exercise is a type of physical activity consisting of planned, structured, and repetitive body movements performed to improve and/or maintain one or more components of physical fitness (37). Exercise following American College of Sport Medicine guidelines is highly recommended for people with sarcopenic obesity (38) and is key for enhancing physical function.
The majority of the studies in this review were of heterogeneous quality and poor methodology. Despite the paucity of studies, the literature highlights that exercise, and strength training in particular, with or without elastic bands or electromyostimulation, can enhance or at least maintain physical function in older adults with sarcopenic obesity. Higher scores on physical performance have been correlated with lower risk of aging-related diseases (4). These results are corroborated by findings from a recent review (8) that underlined the importance of resistance exercise in improving physical performance in individuals with sarcopenic obesity.
In the present review, the four most common tests used to assess physical performance were SPPB (39), PPT (40), SLS (41) and TUG (42).
While there are other equally validated battery tests for people with frailty (e.g., American Alliance for Health, Physical Education, Recreation and Dance (AAPHERD) (43), Rikli and Jones test (44)), they were not examined in the literature. A possible explanations might be that the tests (SPPB, PPT, SLS,TUG) are valuable tools for standard clinical assessment in older adults because they provide fast, affordable, and reliable measures of functional capacity (45). Furthermore, these tests are among those most commonly used to assess frailty in the older population, especially in individuals with sarcopenic obesity which have a higher risk of functional disability and frailty.
SPPB and PPT outcomes
Good physical performance mirrors the muscle capacity that older adults need to maintain independence in carrying out tasks of daily living (31). The SPPB test is often used to predict the risk of loss of independence and is a standard measure in research and clinical practice (46). Since mobility is impaired in older adults with sarcopenic obesity, one aim of this systematic review was to identify the type of exercise that could improve physical function. Previous studies (39, 47) showed that older adults with the low SPPB scores were more likely to experience disability in daily living than those with high scores. Other studies have also shown that physical exercise can enhance physical performance (48). For instance, Perera et al. (49) reported that an increase of even 1.0 point on the SPPB test signals a significant change in research, as such criteria are useful for assessing the clinical significance of an intervention. Performance measures can help to determine health and physical funtion in older adults and provide a yardstick for understanding and acting on their health needs.
Two (25, 31) of three studies that employed strength training or resistance exercise training reported post-intervention improvements in SPPB and PPT scores, while one study (26) found no significant change. A plausible explanation may be sought in the total duration of training, as a lack of improvement on these tests may stem from shorter duration of an intervention (10 weeks (26) vs 15/16 weeks (25, 31)). Moreover, differences in sex distribution could partially explain the dicrepant results. Balachandran (25) and Stoever (31) observed an improvement in SPPB in a sample of both women and men, whereas Vasconcelos et al. (26) found no improvement in a sample composed solely of women. It should be noted, however, that the study population in the Vasconcelos study (26) had started with high baseline SPPB scores, for which no significant additional improvement could be achieved. The study population in the studies by Balachandran (25) and Stoever (31) were composed of both sexes; though the results were not stratified by sex, there was a statistically significant improvement between pre- and post-training. It would be helpful to have data on the the effects of differences exercise (strength, aerobic, power training, etc.) separately for men and women.
Gait speed test
Gait speed (50), also measured by the 5-meter walk test or the 10-meter walk test, is an easy to administer and reliable tool for assessing physical performance in older adults (31). Three studies (28–30) showed an increase in gait speed; two of them (29, 30) found a marked increase when exercise was combined with elastic resistance training and one (28) reported an increase after electromyostimulation training. The remaining two studies (26, 27) found no change in gait speed. Abellan Van Kan et al. (15) reported that gait speed at usual pace is a consistent risk factor for disability, cognitive impairment, institutionalization, falls, and/or mortality. The authors went on to state that older adults who walk faster than 1.0 m/s generally have a lower risk of disease and a better survival rate. In addition, they suggested a cut off of 0.8 m/s for identifying risk of adverse outcomes when using a 4-m test course and 0.6 m/s as a threshold to predict further functional decline in older adults with impaired mobility. Finally, Peel et al. (51), found gait speed to be an important measure in evaluating comprehensive geriatric syndrome because it is a quick, inexpensive, and reliable measure of functional capacity with a well-documented predictive capability for major health-related outcomes.
A recent review by Hsu et al. (8) reported that gait speed test scores were higher after resistance exercise training than after combined exercise in adults with sarcopenic obesity. The authors reasoned that since obesity reduces the physical capacity of individuals with sarcopenia and since resistance exercise is an optimal way to increase muscle strength, physical performance (such as gait speed) will improve in adults with sarcopenic obesity submitted to a resistance exercise protocol. Considerable improvement in gait speed was noted after administration of a structured and progressive resistance exercise protocol with the use of elastic bands three times a week for a total of 12 sessions, as done in two studies (29, 30). Differently, no significant improvements were observed in the study (27) that employed a bodyweight resistance exercise or in another (26) in which training frequency was twice a week for a total of 10 sessions. A Canadian study (52) involving both men and women reported that the group with sarcopenic obesity and the obese non-sarcopenic group had similar but lower fitness levels (as measured with the gait speed, chair stand, and TUG tests) than the normal weight non-sarcopenic subjects. Obesity rather than sarcopenia seems the predominant factor in reduced physical fitness in such a population. Similarly, a Korean study (53) showed that, although not all results reached statistical significance, the men who engaged in resistance exercise or gait speed training were less likely to develop sarcopenic obesity than those who did not engage in any type of physical exercise. A similar albeit slightly weaker association were found for the women. In their systematic review (54), Graham et al. focused on gait speed and noted that the wide variety of protocols for measuring gait speed left physical performance scores open to different interpretations. Another review (55) reported that gait speed tests may differ according to the pace strategy (normal or maximum speed) and depending whether the subject starts to walk from a static position or when already in motion. Other variables are the distance covered (range, 4 to 500 meters) or the sample population characteristics. As a consequence, the lack of standardization of the walk test protocol can limit comparisons between groups. The response to different types of exercise (strength, aerobics, power training, etc.) as measured on physical performance tests needs to be categorised based on universally agreed cut offs in order to compare different types of populations and training protocols.
Chair stand test and timed chair rise
Measuring lower body strength is critical for assessing functional performance in older adults. The 30-s chair stand test, also known as the TCR test, is a commonly used tool (56). Three studies showed an improvement on this task with the use of elastic resistance training (29, 30) or traditional resistance training (32). A recent study (57) found the 30-s chair stand test useful for evaluating lower muscle strength in community-dwelling older adults and concluded that by establishing an optimal cut off test could be a useful diagnostic tool for assessing sarcopenia risk in older Japanese participants. Liao et al. (29, 30) found that elastic resistance training led to improvement in muscle mass and physical capacity as measured with the TCR test in a sample of women with sarcopenic obesity. Cesari et al. (58) proposed a cut off ≥17 s on the 5 times sit-to-stand chair stand test in the workup of diagnostic sarcopenic obesity and a range of 15 to 9 s and 17 to 9 s on the 30-s chair stand test for older women and men, respectively (44).
One limitation of some of these studies is that the structural features of the chair (height, length, presence or absence of armrests) are either not reported or that chair height differs, making comparison between studies difficult. One study (59) showed that chair characteristics can affect test performance. For example, a lower seat height may make it more difficult to perform the task, while a higher seat height may decrease the amount of work required at the hip and knee and make the task easier. In addition, a recent study (60) involving a population with sarcopenia used a so-called “sit-to-stand” test that proved useful for measuring physical performance and muscle power in particular. This inexpensive test could yield information on muscle quality and contractile muscle capacity in adults with sarcopenic obesity. It would be helpful to agree on the use of a single test depending on the type of physical exercise carried out and the type of population under study.
Single leg stance and timed up and go test
Low muscle mass and strength are associated with impaired balance and a concomitant increase in the risk of falls (61). Two studies (29, 30) of the three studies testing balance reported an improvement and both included elastic resistance training, while one (32) reported a decrease in TUG test scores. In their recent review (62), Barri et al. observed that shorter time is a better indicator of functional performance and that a TUG test score ≥13.5 s is a benchmark to identify people at greater risk of falls in a community setting. However, a wide range of cut offs from 10 to 33 seconds are reported in the literature (63). Springer et al. (64) stated that the SLS test is a valid method to quantify static balance ability. They found that the different in timing is not sex-specific but rather age-related, with the eyes open condition resulting in much longer time on the task than the eyes closed condition. In addition, the study set performance criteria based on age group for eyes closed or open in a healthy older population. Nevertheless, there are no studies that provide an indicative cut off for adults with sarcopenic obesity. The role of these tests needs to be explored according to the type of training, with the cut offs adjusted for age and disease in adults with sarcopenic obesity.
Strengths and limitations
This systematic review has several strengths: the importance of using validated and objective tools to evaluate the effect of training programmes on physical performance in adults with sarcopenic obesity; maintaining or increasing physical function is key to counteract aging-related decline and preserve autonomy; the details of training protocols were reported according to the FITT principle (training frequency, intensity, time, type) which constitute essential features for comparison among studies.
The limitations are: the study authors used heterogeneous criteria for defining sarcopenic obesity and consensus on the definition of sarcopenic obesity is lacking; also lacking is standardization of training program duration (range, 10-26 weeks) and of physical performance in response to the type exercise prescribed (strength, aerobics, power training, etc.); the study samples were composed mainly of women, with moderate to high risk of bias.
Future directions
The success of a training intervention depends on the barriers that affect an individual’s willingness to engage in physical exercise: personal and health aspects (e.g., physical, and psychological well-being), the surrounding environment, behavioral aspects (e.g., motivation, social support, goal setting, positive affect, self-efficacy), and parameters of physical exercise (e.g., frequency, duration, type, goal attainment, enjoyment) (65). Our review shows poor involvement of older men perhaps because they are less willing than women to participate. A previous study involving men over 65 years found that health aspects and enjoyment are two essential parameters for taking up daily exercise; in contrast, lack of interest/motivation, lack of time, and feeling awkward are common barriers to participation in exercise interventions (66). On this note, administering questionnaires to better understand exercise modality preferences and to maximize adherence to prescription in this population and in men especially is warranted. Other promising approaches to improve engagement in long-term exercise might be: i) to increase awareness about the beneficial effects of exercise; ii) to set achievable and measurable goals in an enjoyable and sociable environment.
Standardization of training and common guidelines designed on the FITT principle for enhancing physical performance is within reach. However, the difference in type of exercise (physical capacity and strength components to train, intensity, and volume), spaces, and exercise equipment, time availability (number of sessions and duration per week) make standardization problematic. Given these circumstances, collaboration between clinicians and kinesiologists is fundamental for the design, evaluation, and prescription of tailored exercise training programs.
Despite the lack of a standardization in the sarcopenia screening, future works should include all the most current and updated definitions (e.g., the European (9), the Asian (11), the International (10) working groups and the ESPEN and EASO groups (17)) in order to have a clear picture of the best approach useful in identifying the prevalence of sarcopenia in older adults with obesity.
Conclusion
Physical capacity decreases with age and the decline is steeper in sedentary adults with sarcopenic obesity. Physical exercise, and progressive resistance training in particular, is the most used training modality in adults aged 60-80 years. Of note, none of the previous trials explored differences in exercise prescription by classifying participants in subgroups based on age-level. This is a key aspect to develop in the future.
Outcomes show that physical performance is improved or at least maintained as assessed with SPPB, PPT, Gait Speed, TCR, Chair Stand, and SLS tests. Nonetheless, whether better results can be achieved with other types of training remains to be elucidated. It follows then that other types of functional tests for evaluating muscle function (i.e., muscle mechanical power) should be applied.
Although most of the studies only involved women, the study sample should include more older men in order to comprehensively investigate different types of training (aerobic, power training, combination of these modalities) and better understand whether different protocols could yield greater and faster benefits for physical performance outcomes. In addition, the most recent definition for sarcopenia screening should be considered. Finally, interventions of longer duration with follow-up assessment after the training period could demonstrate the actual effectiveness of exercise in improving physical function in adults with sarcopenic obesity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LG reviewed the literature, collected the data, and drafted the manuscript, VM, TT, and AR revised and edited the manuscripts critically, VM, TT, FS, and AR supervised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgements
The authors wish to thank Kenneth Adolf Britsch for his assistance in reading the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Physical Performance Test (PPT), Short Physical Performance Battery (SPPB), Single Leg-Stance (SLS), Time Up & Go test (TUG), Timed chair rise (TCR).
References
1. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European Guidelines for obesity management in adults. Obes Facts (2015) 8(6):402–24. doi: 10.1159/000442721
2. Gallus S, Lugo A, Murisic B, Bosetti C, Boffetta P, La Vecchia C. Overweight and obesity in 16 European countries. Eur J Nutr (2015) 54(5):679–89.
3. Carmona-Torres JM, Rodríguez-Borrego MA, Laredo-Aguilera JA, López-Soto PJ, Santacruz-Salas E, Cobo-Cuenca AI. Disability for basic and instrumental activities of daily living in older individuals. PloS One (2019) 14(7):1–13. doi: 10.1371/journal.pone.0220157
4. Choi KM. Sarcopenia and sarcopenic obesity. Korean J Internal Med Korean Assoc Internal Med (2016) 31:1054–60. doi: 10.3904/kjim.2016.193
5. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol Ser A Biol Sci Med Sci (2006) 61(10):1059–64. doi: 10.1093/gerona/61.10.1059
6. Rosenberg IH. Symposium: Sarcopenia: Diagnosis and mechanisms sarcopenia: Origins and clinical relevance 1. J Nutr (1997) 127:990–1. doi: 10.1093/jn/127.5.990S
7. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos Int (2010) 21(4):543–59. doi: 10.1007/s00198-009-1059-y
8. Hsu KJ, Liao C, Tsai MW, Chen CN. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: A meta-analysis. Nutrients (2019) 11(9):2163. doi: 10.3390/nu11092163
9. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
10. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: An undiagnosed condition in older adults. current consensus definition: Prevalence, etiology, and consequences. international working group on sarcopenia. J Am Med Dir Assoc (2011) 12(4):249–56. doi: 10.1016/j.jamda.2011.01.003
11. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc (2014) 15(2):95–101. doi: 10.1016/j.jamda.2013.11.025
12. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in new Mexico. Am J Epidemiol (1998) 147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520
13. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol (2018) 14(9):513–37. doi: 10.1038/s41574-018-0062-9
14. Kong HH, Won CW, Kim W. Effect of sarcopenic obesity on deterioration of physical function in the elderly. Arch Gerontol Geriatr (2020) 89:104065. doi: 10.1016/j.archger.2020.104065
15. Abellan Van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an international academy on nutrition and aging (IANA) task force. J Nutr Heal Aging (2009) 13(10):881–9. doi: 10.1007/s12603-009-0246-z
16. Wannamethee SG, Atkins JL. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc (2015) 74(4):405–12. doi: 10.1017/S002966511500169X
17. Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr (2020) 39(8):2368–88. doi: 10.1016/j.clnu.2019.11.024
18. Hita-Contreras F, Bueno-Notivol J, Martínez-Amat A, Cruz-Díaz D, Hernandez AV, Pérez-López FR. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: A meta-analysis of randomized controlled trials. Maturitas (2018) 116:24–35. doi: 10.1016/j.maturitas.2018.07.007
19. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity - a systematic review of longitudinal studies. BMC Public Health (2013) 13(1):1–9. doi: 10.1186/1471-2458-13-813
21. Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, Idoate F, Millor N, Gómez M, et al. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Omaha) (2014) 36(2):773–85. doi: 10.1007/s11357-013-9586-z
22. Trouwborst I, Verreijen A, Memelink R, Massanet P, Boirie Y, Weijs P, et al. Exercise and nutrition strategies to counteract sarcopenic obesity. Nutrients (2018) 10(605):1–21. doi: 10.3390/nu10050605
23. De Mello RGB, Dalla Corte RR, Gioscia J, Moriguchi EH. Effects of physical exercise programs on sarcopenia management, dynapenia, and physical performance in the elderly: A systematic review of randomized clinical trials. J Aging Res (2019) 2019:1–7. doi: 10.1155/2019/1959486
24. JP Higgins, S Green. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Cochrane Database Syst Rev (2016) 10(Suppl 1):674.
25. Balachandran A, Krawczyk SN, Potiaumpai M, Signorile JF. High-speed circuit training vs hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp Gerontol (2014) 60:64–71. doi: 10.1016/j.exger.2014.09.016
26. Vasconcelos KSS, Dias JMD, Araújo MC, Pinheiro AC, Moreira BS, Dias RC. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: A randomized controlled trial. Braz J Phys Ther (2016) 20(5):432–40. doi: 10.1590/bjpt-rbf.2014.0174
27. Kim H, Kim M, Kojima N, Fujino K, Hosoi E, Kobayashi H, et al. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: A randomized controlled trial. J Am Med Dir Assoc (2016) 17(11):1011–9. doi: 10.1016/j.jamda.2016.06.016
28. Kemmler W, Teschler M, Weissenfels A, Bebenek M, von Stengel S, Kohl M, et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. results of the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos Int (2016) 27(11):3261–70. doi: 10.1007/s00198-016-3662-z
29. Liao CD, Tsauo JY, Lin LF, Huang SW, Ku JW, Chou LC, et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity. a CONSORT-compliant prospective randomized controlled trial. Med (Baltimore) (2017) 96(23):e7115. doi: 10.1097/MD.0000000000007115
30. Liao CD, Tsauo JY, Lin LF, Huang SW, Ku JW, Chou LC, et al. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: A randomized controlled trial. Sci Rep (2018) 8(1):1–13. doi: 10.1038/s41598-018-20677-7
31. Stoever K, Heber A, Eichberg S, Brixius K. Influences of resistance training on physical function in older, obese men and women with sarcopenia. J Geriatr Phys Ther (2018) 41(1):20–7. doi: 10.1519/JPT.0000000000000105
32. de Oliveira Silva A, Dutra MT, de Moraes WMAM, Funghetto SS, Lopes de Farias D, Fernandes dos Santos PH, et al. Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity. Clin Interv Aging (2018) 13:411–7. doi: 10.2147/CIA.S156174
33. Castro-Coronado J, Yasima-Vásquez G, Zapata-Lamana R, Toloza-Ramírez D, Cigarroa I. Characteristics of resistance training-based programs in older adults with sarcopenia: Scoping review. Rev Esp Geriatr Gerontol (2021) 56(5):279–88. doi: 10.1016/j.regg.2021.05.004
34. Collado-Mateo D, Lavín-Pérez AM, Peñacoba C, Del Coso J, Leyton-Román M, Luque-Casado A, et al. Key factors associated with adherence to physical exercise in patients with chronic diseases and older adults: An umbrella review. Int J Environ Res Public Health (2021) 18(4):1–24. doi: 10.3390/ijerph18042023
35. Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LHT, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: A systematic review and meta-analysis. Sport Med (2015) 45(2):245–55. doi: 10.1007/s40279-014-0269-4
36. Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain - a systematic review. Obes Rev (2000) 1(2):95–111. doi: 10.1046/j.1467-789x.2000.00016.x
37. Caspersen CJ, Powell KE, CGM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep (1985) 100(2):126–31. doi: 10.1093/nq/s9-IX.228.365-f
38. American College of Sports Medicine, Riebe D, Ehrman JK, Liguori G, Magal M. curatori. ACSM’s guidelines for exercise testing and prescription. Tenth edition. Philadelphia: Wolters Kluwer (2018), p. 651.
39. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol (1994) 49(2):M85–94. doi: 10.1093/geronj/49.2.M85
40. Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. J Am Geriatr Soc (1990) 38(10):1105–12. doi: 10.1111/j.1532-5415.1990.tb01373.x
41. Omaña H, Bezaire K, Brady K, Davies J, Louwagie N, Power S, et al. Functional reach test, single-leg stance test, and tinetti performance-oriented mobility assessment for the prediction of falls in older adults: A systematic review. Phys Ther (2021) 101(10):1–18. doi: 10.1093/ptj/pzab173
42. Browne W, Nair BKR. The timed up and go test. Med J Aust (2019) 210(1):13–5. doi: 10.5694/mja2.12045
43. Yaguchi K, Furutani M. An applicability study of the AAHPERD’s functional fitness test for elderly American adults to elderly Japanese adults. Environ Health Prev Med (1998) 3(3):130–40. doi: 10.1007/BF02931703
44. Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist (2013) 53(2):255–67. doi: 10.1093/geront/gns071
45. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol Ser A Biol Sci Med Sci (2000) 55(4):221–31. doi: 10.1093/gerona/55.4.M221
46. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing (2010) 39(4):412–23. doi: 10.1093/ageing/afq034
47. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med (1995) 332(9):556–62. doi: 10.1056/NEJM199503023320902
48. Buchner DM, Cress ME, De Lateur BJ, Esselman PC, Margherita AJ, Price R, et al. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. J Gerontol Ser A Biol Sci Med Sci (1997) 52(4):218–24. doi: 10.1093/gerona/52A.4.M218
49. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc (2006) 54(5):743–9. doi: 10.1111/j.1532-5415.2006.00701.x
50. Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, et al. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: Analysis from the InCHIANTI study. J Gerontol Ser A Biol Sci Med Sci (2009) 64(2):223–9. doi: 10.1093/gerona/gln022
51. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J Gerontol Ser A Biol Sci Med Sci (2013) 68(1):39–46. doi: 10.1093/gerona/gls174
52. Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: Data from the nutrition as a determinant of successful aging (nuage)the quebec longitudinal study. Obesity (2009) 17(11):2082–8. doi: 10.1038/oby.2009.109
53. Hwang B, Lim JY, Lee J, Choi NK, Ahn YO, Park BJ. Prevalence rate and associated factors of sarcopenic obesity in Korean elderly population. J Korean Med Sci (2012) 27(7):748–55. doi: 10.3346/jkms.2012.27.7.748
54. Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract (2008) 14(4):552–62. doi: 10.1111/j.1365-2753.2007.00917.x
55. Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: A review. Arch Phys Med Rehabil (2008) 89(5):865–72. doi: 10.1016/j.apmr.2007.11.029
56. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport (1999) 70(2):113–9. doi: 10.1080/02701367.1999.10608028
57. Sawada S, Ozaki H, Natsume T, Deng P, Yoshihara T, Nakagata T, et al. The 30-s chair stand test can be a useful tool for screening sarcopenia in elderly Japanese participants. BMC Musculoskelet Disord (2021) 22(1):1–6. doi: 10.1186/s12891-021-04524-x
58. Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, et al. Added value of physical performance measures in predicting adverse health-related events: Results from the health, aging and body composition study. J Am Geriatr Soc (2009) 57(2):251–9. doi: 10.1111/j.1532-5415.2008.02126.x
59. Shamay SM, Cheung SY, Lai LSW, Liu ASL, Ieong SHI, Fong SSM. Five times sit-To-Stand test completion times among older women: Influence of seat height and arm position. J Rehabil Med (2015) 47(3):262–6. doi: 10.2340/16501977-1915
60. Alcazar J, Aagaard P, Haddock B, Kamper RS, Hansen SK, Prescott E, et al. Assessment of functional sit-to-stand muscle power: Cross-sectional trajectories across the lifespan. Exp Gerontol (2021) 152:111448. doi: 10.1016/j.exger.2021.111448
61. Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men - the MINOS study. J Bone Miner Res (2005) 20(5):721–9. doi: 10.1359/JBMR.041230
62. Barry E, Galvin R, Keogh C, Horgan F, Fahey T. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta- analysis. BMC Geriatr (2014) 14(1):1–14. doi: 10.1186/1471-2318-14-14
63. Arnold CM, Faulkner RA. The history of falls and the association of the timed up and go test to falls and near-falls in older adults with hip osteoarthritis. BMC Geriatr (2007) 7:1–9. doi: 10.1186/1471-2318-7-17
64. Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther (2007) 30(1):8–15. doi: 10.1519/00139143-200704000-00003
65. Lachman ME, Lipsitz L, Lubben J, Castaneda-Sceppa C, Jette AM. When adults don’t exercise: Behavioral strategies to increase physical activity in sedentary middle-aged and older adults. Innov Aging (2018) 2(1):1–12. doi: 10.1093/geroni/igy007
Keywords: physical performance, physical function, muscle mass, exercise, elderly, sarcopenic obesity, systematic literature review
Citation: Ghiotto L, Muollo V, Tatangelo T, Schena F and Rossi AP (2022) Exercise and physical performance in older adults with sarcopenic obesity: A systematic review. Front. Endocrinol. 13:913953. doi: 10.3389/fendo.2022.913953
Received: 06 April 2022; Accepted: 30 June 2022;
Published: 28 July 2022.
Edited by:
Carla Lubrano, Sapienza University of Rome, ItalyReviewed by:
Silvia Migliaccio, Foro Italico University of Rome, ItalyLuis Alberto Gobbo, São Paulo State University, Brazil
Luis V. F. Oliveira, University Center of Anápolis, Brazil
Copyright © 2022 Ghiotto, Muollo, Tatangelo, Schena and Rossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea P. Rossi, YW5kcmVhLnJvc3NpQGhvdG1haWwuaXQ=
†These authors share last authorship
 Laura Ghiotto
Laura Ghiotto Valentina Muollo
Valentina Muollo Toni Tatangelo
Toni Tatangelo Federico Schena
Federico Schena Andrea P. Rossi
Andrea P. Rossi