- 1Department of General Surgery, HwaMei Hospital, University of Chinese Academy of Sciences, Ningbo, China
- 2Ningbo Institute of Life and Health Industry, University of Chinese Academy of Sciences, Ningbo, China
- 3The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 4The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 5The Department of Endocrinology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 6School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
Background: This meta-analysis was conducted to explore the association between sodium-glucose cotransporter 2 inhibitors (SGLT-2is) and ocular diseases in type 2 diabetes mellitus (T2DM) patients.
Methods: PubMed, Cochrane Central Registry of Controlled Trials, Web of Science and Springer were searched for articles on randomized controlled trials (RCTs) involving T2DM patients treated with SGLT-2i versus placebo or other hypoglycemic agents published prior to August 2021. The primary outcome of this meta-analysis was incidence of ocular diseases, which was assessed using risk ratios (RR) and 95% confidence intervals (CI). We reviewed 47 papers and compared the effect of SGLT-2i with the effect of the control groups (placebo and other hypoglycemic drugs) on the incidence of ocular diseases.
Results: Compared with controls, overall SGLT-2i use in T2DM patients was not associated with incidences of cataract, glaucoma, retinal disease and vitreous disease. Ertugliflozin (RR=0.47, P=0.01) reduced the risk for retinal disease, while empagliflozin (RR=0.44, P=0.05) reduced the risk for diabetic retinopathy (DR) compared with controls. SGLT-2i (RR=0.50, P=0.02), perhaps empagliflozin (RR=0.47, P=0.06), reduced the risk of retinal disease compared with active hypoglycemic agents. Canagliflozin (RR=4.50, P=0.03) increased the risk for vitreous disease compared with placebo.
Conclusions: There was no significant correlation between overall SGLT-2i and ocular diseases (cataract, glaucoma, retinal disease, vitreous disease, corneal disease, conjunctival disease, uveal disease, eye haemorrhage and vision problems) in T2DM patients. Ertugliflozin and empagliflozin may protect against ocular diseases, but canagliflozin may promote ocular diseases.
Introduction
Currently, diabetes mellitus (DM) and its complications are a global epidemic that pose a serious threat to human health. The prevalence of diabetes for people aged 20-79 years worldwide was 10.5% in 2021 and is expected to reach 12.2% in 2045 (1). Long-term poor control of blood glucose in DM patients affects the large and small arteries and nervous system, leading to serious complications (2), such as cardiovascular disease, diabetic nephropathy, diabetic peripheral neuropathy, diabetic retinopathy (DR) and other diseases (3). DR is the most common microvascular complication of DM involving the eyes, affecting about one-third of adults with DM and is the main cause of blindness (4, 5). DM is also closely related to glaucoma, cataract and other eye diseases, which tend to appear earlier in patients with DM and have a higher probability of impact (6). Studies have shown that intensive glycemic control has an important effect on long-term microvascular complications in patients with type 2 diabetes mellitus (T2DM) and can delay the occurrence and progression of ocular events (7).
A variety of hypoglycemic drugs, including sodium-glucose cotransporter 2 inhibitors (SGLT-2is), are being used either alone or in combination in the clinic to control blood glucose levels. SGLT-2i lower blood glucose levels by inhibiting the reabsorption of glucose by the kidney to produce glycosuria (8). It can also lower body weight and blood pressure (BP), and has significant protective effects on the kidney and cardiovascular system, although it increases the risk of genitourinary infections (8). However, the effect of SGLT-2i on incidences of ocular events compared with other hypoglycemic drugs and/or placebo is unknown. Due to the low incidence of ocular events observed in clinical trials, it is difficult for a single study to have statistical significance. The aim of this study was to carry out a comprehensive and systematic meta-analysis of randomized controlled trials (RCTs) on the effects of SGLT-2i on ocular events in patients with T2DM. We hope that findings from this study can guide future treatment of patients with T2DM.
Methods
Literature Search Strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (9). The RCTs included in this study compared the incidences of ocoular diseases between SGLT-2i and placebo or other active hypoglycemic agents in patients with T2DM. Two researchers conducted a systematic and comprehensive search of PubMed, Cochrane Central Registry of Controlled Trials, Web of Science, and Springer databases to seek relevant papers published in English prior to August 2021. The search terms were (Type 2 diabetes OR type 2 diabetes mellitus OR T2DM OR T2D) AND (sodium-glucose cotransporter 2 inhibitor OR SGLT-2i OR sotagliflozin OR janagliflozin OR dapagliflozin OR canagliflozin OR empagliflozin OR ipragliflozin OR tofogliflozin OR ertugliflozin OR luseogliflozin OR sergliflozin OR licogliflozin OR remogliflozin OR bexagliflozin). The two researchers selected the papers to be included in the study by independently searching the articles, scanning the titles and abstracts of the papers, and viewing the full text and supplementary materials. There were no restrictions on the words related to ocular events during retrieval of the articles, to avoid omission of relevant articles.
Study Selection and Quality Assessment
Studies were included in the meta-analysis based on the following criteria: (1) participants were T2DM patients; (2) RCTs compared the efficacy of SGLT-2i with placebo or other active hypoglycemic drugs; (3) RCTs recorded detailed information about the occurrence of ocular events; (4) follow-up time was ≥12 weeks. Exclusion criteria: (1) duplicate reports; (2) non-English language; (3) data unavailable. Where studies had been updated, the most complete or the most recent paper was included in the analysis. The selected studies were evaluated separately by two researchers, and any differences were discussed and resolved with a third researcher.
The Cochrane Collaboration’s tool was used to evaluate the quality and risk of bias of included RCTs, and to judge from the aspects of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and free of other bias.
Data Extraction
Two researchers independently extracted the data and a third researcher reviewed it. Data extracted included: (1) basic characteristics of study, for example author, publication year, country, Clinical Trials.gov identifier, inclusion criteria, follow-up time and therapeutic regimen; (2) detailed data of ocular events in the experimental and control groups including the types and number; (3) classification of the lesions according to their occurrence sites, such as retinal diseases (including retinal, macular, optic papillae related diseases), vitreous diseases, corneal diseases, conjunctival diseases, uveal disease (including iris, ciliary body, choroid related diseases), eye haemorrhage and vision problems.
Statistical Analysis
This meta-analysis was performed using Review Manager 5.3 statistical analysis software. Risk ratios (RR) and 95% confidence intervals (CI) were used to evaluate the count data. I2 statistics were used to evaluate the heterogeneity of the included studies. I2<30% represented low and I2<60% represented moderate heterogeneity. Random effects model was used in this analysis. P ≤ 0.05 was considered to be statistically significant. When more than 10 studies were included, publication bias was evaluated using funnel plots (10) and sensitivity analysis was conducted to evaluate the stability of the results.
Result
Study Selection and Study Characteristics
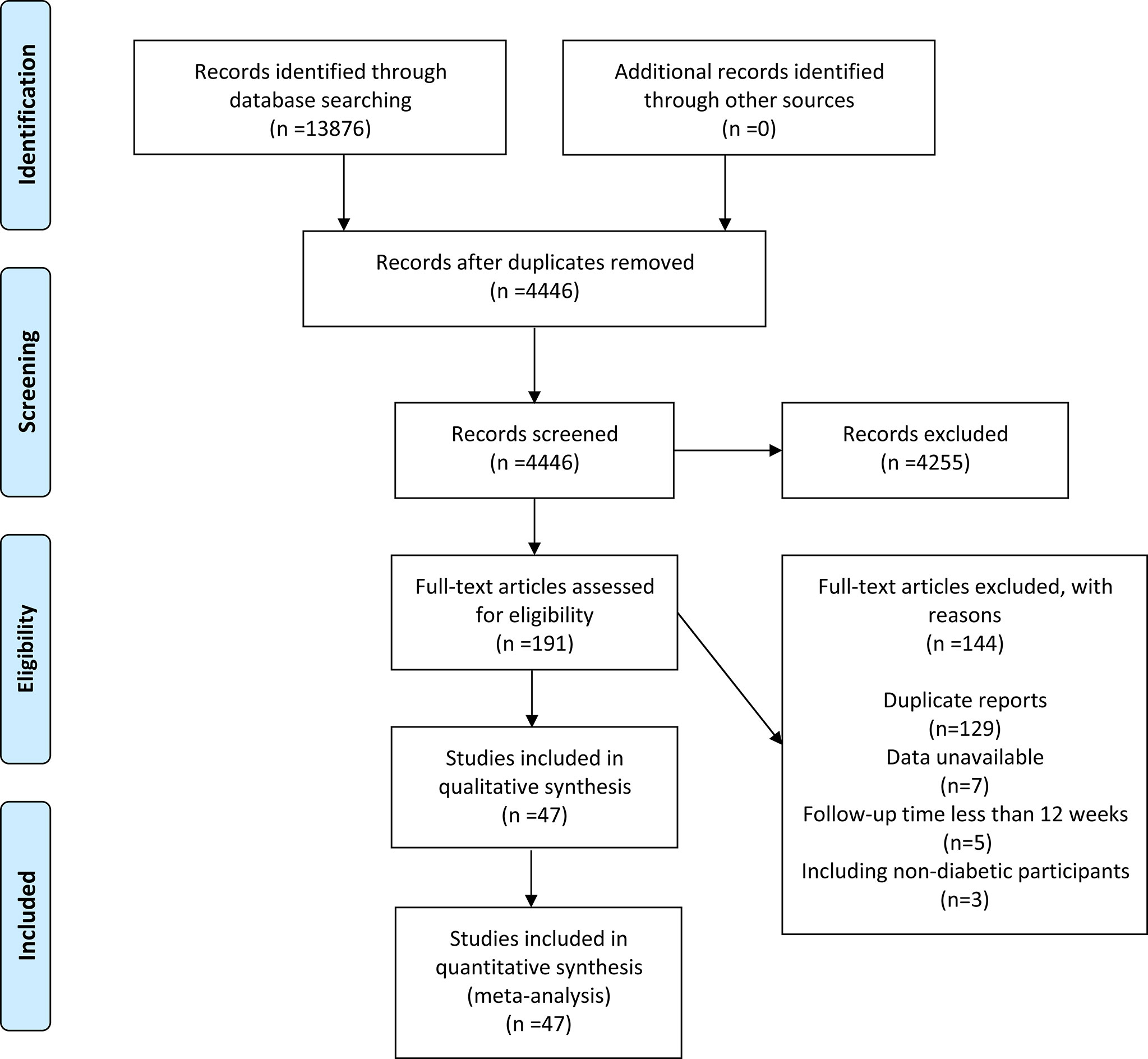
A total of 13876 papers were retrieved, but only 4446 were retained after duplicates were excluded. A total of 4255 articles were removed after reviewing the titles and abstracts. After full-text evaluation of 191 papers, 144 were deleted (129 duplicate reports, 7 data unavailable, 5 follow-up time less than 12 weeks, and 3 included non-diabetic populations). Finally, 47 articles (11–57) (48 RCTs in total) published between 2011 and 2021 were considered (Figure 1).
All the studies included in this analysis involved T2DM patients. The experimental group was treated with SGLT-2i intervention (including dapagliflozin, empagliflozin, ertugliflozin, canagliflozin, sotagliflozin, bexagliflozin, tofogliflozin, ipragliflozin), and the control group was treated with placebo or active hypoglycemic drugs (including metformin, sitagliptin, glimepiride, gemigliptin, semaglutide, glipizide, saxagliptin). Follow-up time ranged from 12 to 416 weeks. Most RCTs were multi-country, multi-center studies, with 12 studies conducted in Japan and 1 in Korea (Table 1).
Risk of Bias Assessment
A total of 41 trials had relatively complete records of sequence generation and allocation concealment, so they were considered to have low risk of bias. Only 7 studies had incomplete information, which made it difficult to clarify the risk of bias in sequence generation and allocation concealment. There were 5 studies with high risk in blinding, and the remaining 43 RCTs were all low risk. There was low risk of bias due to incomplete outcome data and selective outcome reporting in all studies. With respect to free of other bias, all studies were difficult to obtain accurate evaluation, so judgment them unclear (Supplementary Table 1).
Effects of SGLT-2i on Incidences of Ocular Events
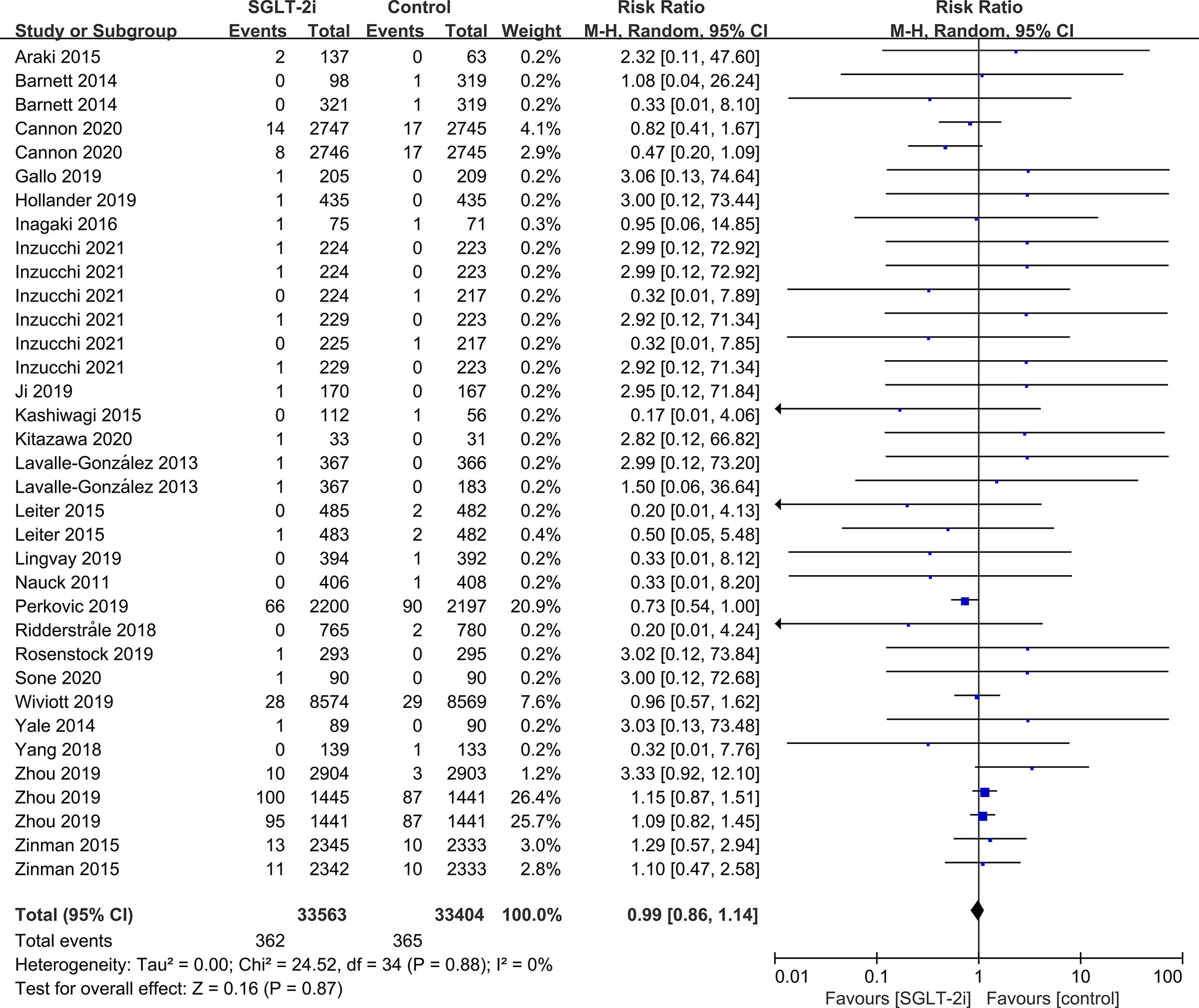
SGLT-2i had no effect on cataract risk in T2DM patients compared with the control group (including placebo and/or other active hypoglycemic agents) (RR=0.99, P=0.87) (Figure 2). Data were collected from 24 RCTs, with cases of cataracts being reported in 359 cases out of 32743 and in 247 cases out of 25421 patients in the SGLT-2i and control group, respectively. A total of 18 RCTs recorded glaucoma data, including 23 cases out of 30102 patients in the experimental group and 15 cases out of 23123 patients in the control group. The results showed that SGLT-2i had no effect on the risk of glaucoma compared with the control group in T2DM patients (RR=0.91, P=0.72) (Figure 3). Analysis of data from 25 RCTs showed that SGLT-2i had no effect on the risk of retinal disease (including retinal, macular, optic papillae related diseases) (RR=0.99, P=0.89), with 254 cases out of 33106 patients and 180 cases out of 25130 patients being reported in the SGLT-2i and control groups, respectively (Figure 4). Analysis of data from 11 RCTs showed no difference in incidences of vitreous diseases between the SGLT-2i intervention group and the control group (RR=0.99, P=0.99), with 25 cases out of 26514 patients and 16 cases out of 19546 patients being reported, respectively (Figure 5).
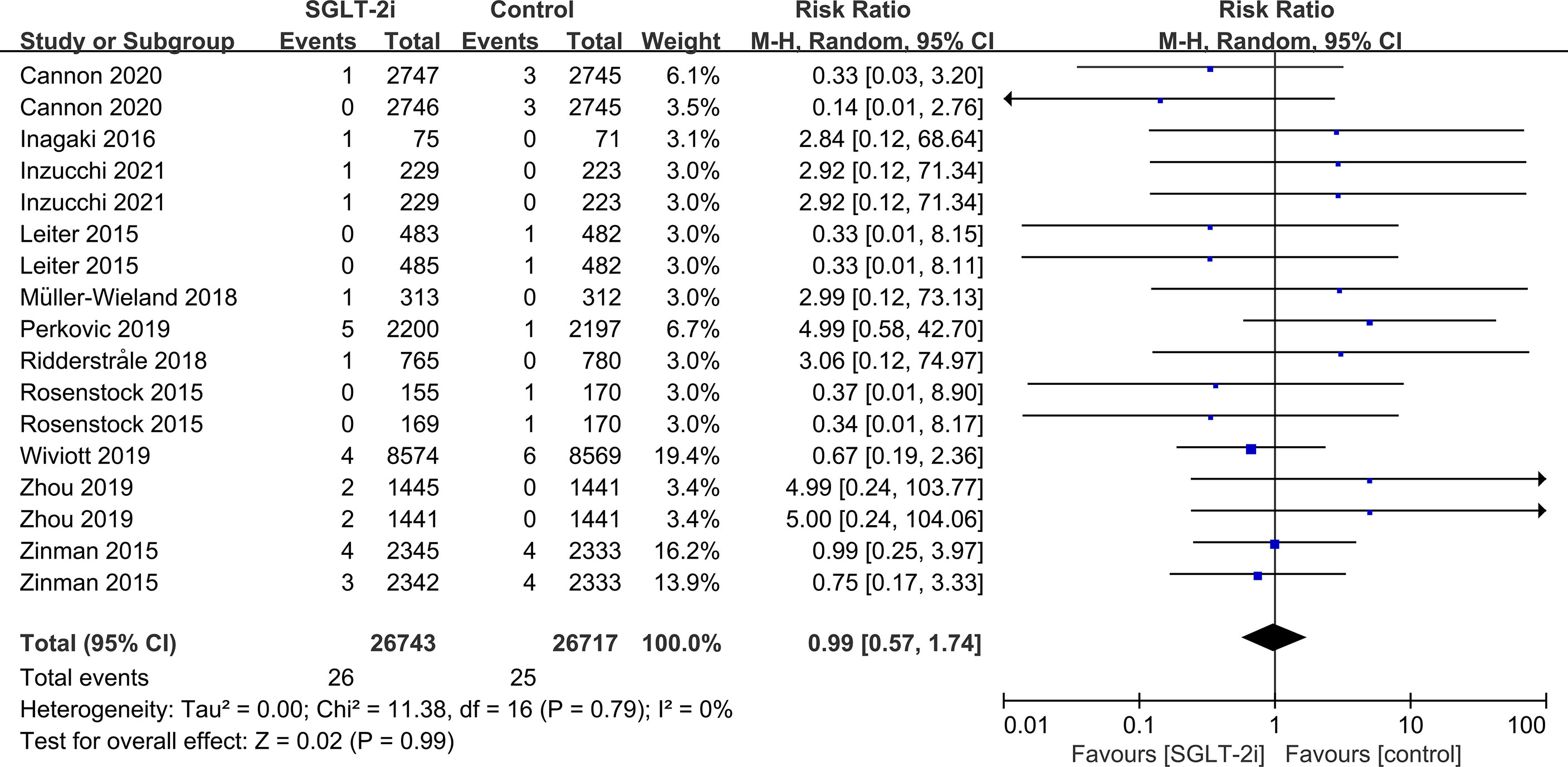
Figure 5 Effect of SGLT-2i on incidences of vitreous disease compared with control in T2DM patients.
Subgroup analysis based on the type of SGLT-2i showed that SGLT-2i had no effect on cataract or glaucoma compared with placebo or active drugs (data not displayed). The risk of cataracts did not change with the type of SGLT-2i (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin) nor the length of follow-up (12-51 weeks, 52-103 weeks, 104-207 weeks, 208 weeks or more) (data not displayed). Similarly, the risk of glaucoma did not change with type of SGLT-2i (empagliflozin, canagliflozin) or length of follow-up (12-51 weeks, 52-207 weeks, 208 weeks or more) (data not shown).
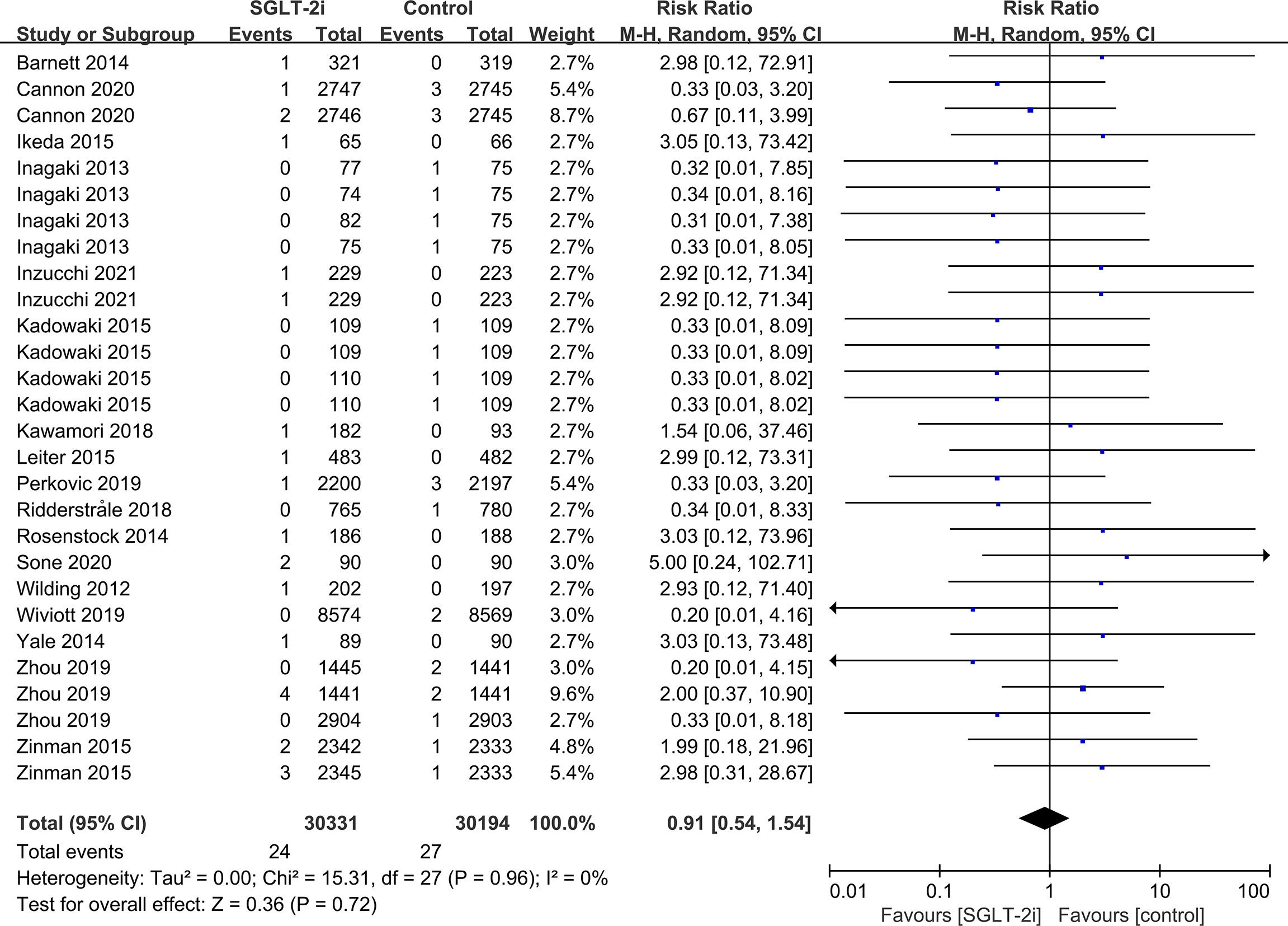
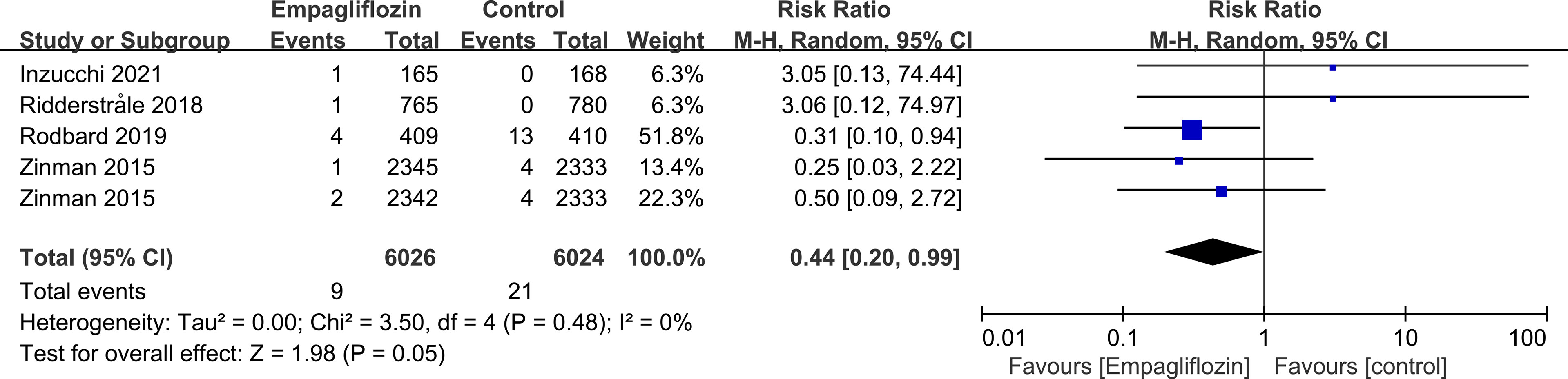
Subgroup analyses of retinal disease based on types of intervention drugs (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin), control drugs (placebo, sulfonylurea) and duration of follow-up (12-51 weeks, 52-103 weeks, 104-207 weeks, 208 weeks or more) showed no effect for the most part (data not shown here), but ertugliflozin was found to significantly reduce the risk of retinal diseases compared with the control group (RR=0.47, 95%CI=0.26 to 0.86, P=0.01) (Figure 6). A more targeted analysis of DR found that empagliflozin significantly reduced the risk of DR compared to the control group. This involved 4 studies, with 9 cases out of 6026 people being reported in the empagliflozin group and 17 cases out of 3691 people being reported in the control group (RR=0.44, 95%CI=0.20 to 0.99, P=0.05) (Figure 7). SGLT-2i had greater protective effects against retinal diseases compared with active hypoglycemic agents (RR=0.50, 95%CI=0.27 to 0.91, P=0.02) (Figure 8), and 4 studies showed that empagliflozin may have a protective effect but not a statistically difference (RR=0.47, 95%CI=0.22 to 1.03, P=0.06) (Figure 9).
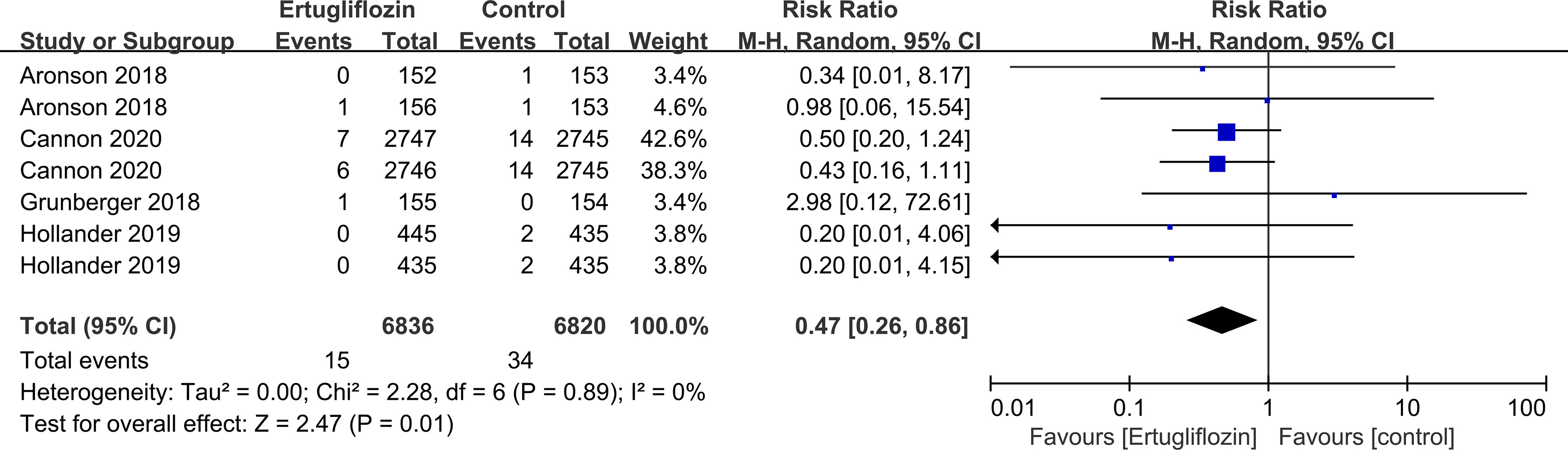
Figure 6 Effect of ertugliflozin on incidences of retinal disease compared with control in T2DM patients.
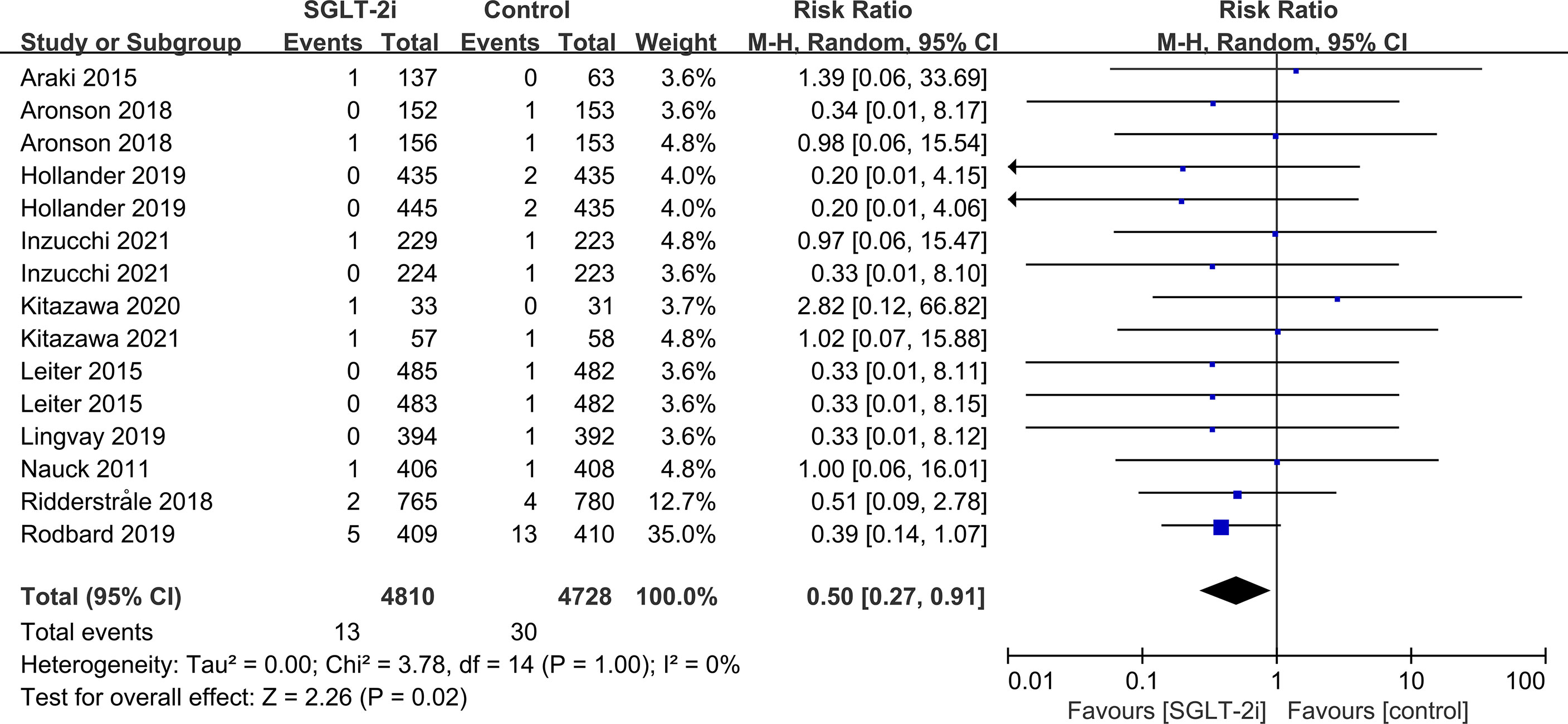
Figure 8 Effect of SGLT-2i on incidences of retinal disease compared with active hypoglycemic agents in T2DM patients.
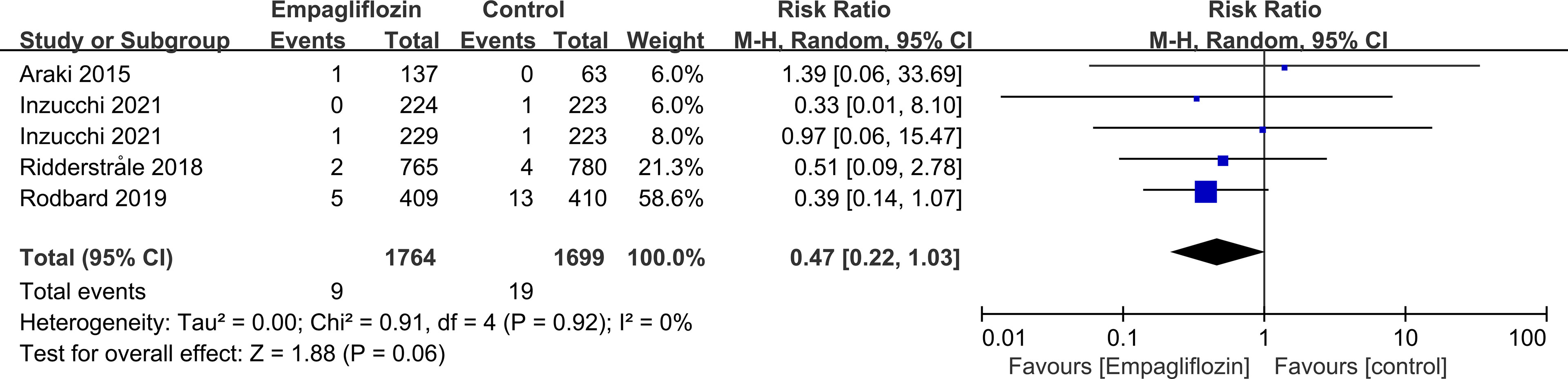
Figure 9 Effect of empagliflozin on incidences of retinal disease compared with active hypoglycemic agents in T2DM patients.
Canagliflozin increased the risk of vitreous disease compared with placebo (RR=4.50, 95%CI=1.14 to 17.70, P=0.03) (Figure 10). Another SGLT-2i type (empagliflozin) was not statistically significant compared with placebo (data not displayed).
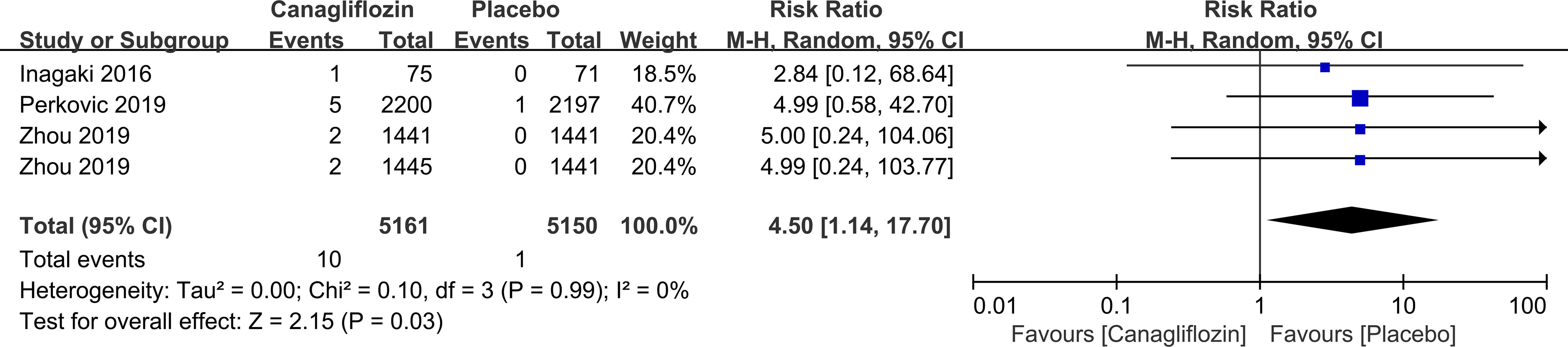
Figure 10 Effect of canagliflozin on incidences of vitreous disease compared with placebo in T2DM patients.
The use of SGLT-2i in patients with T2DM had no significant effect on corneal disease, conjunctival disease, uveal disease, eye haemorrhage and vision problems compared with the controls (Figures S1–5). The types of SGLT-2i, control drugs and the length of follow-up time were not associated with the onset (data not shown here).
Publish Bias and Sensitivity Analyses
Publication bias and sensitivity tests were performed for the effects of SGLT-2i on various ocular events. The funnel plot was roughly symmetrical indicating that there was no significant publication bias. The results were excluded one by one and the sensitivity analysis showed that they were stable.
Discussion
DM is one of the most serious and common chronic diseases in modern times (1), and is closely related to ocular diseases. Findings from our study showed that SGLT-2i was not associated with incidences of cataracts, glaucoma, retinal disease (including retinal, macular, optic papillae related diseases), vitreous disease, corneal disease, conjunctival disease, uveal disease, eye haemorrhage and vision problems compared with controls in T2DM patients.
Cataracts occur more frequently in DM than in non-diabetics patients, with the prevalence increasing by three- to four-fold in DM patients under 65 years of age and by two-fold in those over 65 years of age (58). Diabetes-induced cataracts develop due to conversion of glucose to polyols by aldose reductase, which leads to an increase in osmotic stress in the lens fibers, causing them to swell and rupture (58). Animal studies have shown that ipragliflozin can delay the progression of cataracts (59). Chen et al. proposed that dapagliflozin might by inhibiting the expression of sodium-glucose cotransporter 2 (SGLT2) and glucose transporters to down-regulate receptor for advanced glycation end products (RAGE) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, prevent reactive oxygen species (ROS) accumulation and protect the lens epithelial cells to prevent cataracts in rats with fructose-induced DM (60). In addition, Chin et al. found that T2DM patients treated with metformin had a lower incidence of cataracts than patients not treated with metformin, but it may have something to do with the course of diabetes (61). In our study, we did not observe any correlation between treatment with SGLT-2i and incidences of cataracts, which may be due to the complex mechanisms underlying the development of human cataracts that do not only involve blood glucose levels.
Diabetes may increase the risk of glaucoma in high-risk groups (62), although the underlying mechanism is not completely clear. Some proposed mechanisms include hyperglycemia-induced increase in intraocular pressure by increasing in aqueous humor in anterior chamber and changing trabecular meshwork function, and diabetes-induced microvascular injury and abnormal vascular regulation of optic nerve head and retina which increase the susceptibility to glaucoma injury (63). A retrospective cohort study showed a reduced risk of glaucoma in T2DM patients treated with SGLT-2i compared with patients treated with glucagon-like peptide-1 receptor agonist (GLP-1RA) (64). Due to the limited sample size of this meta-analysis, the data were not enough to compare the incidences of glaucoma between patients treated with SGLT-2i and patients treated with other specific types of hypoglycemic drugs.
In our study, we found that ertugliflozin reduced the risk of retinal disease, and empagliflozin reduced the risk of DR compared to control group. A retrospective cohort study conducted by Su et al. showed that SGLT-2i reduced the risk of diabetic macular edema (DME) in T2DM patients compared with GLP-1RA, through non-hypoglycemic mechanisms (65). Similarly, a study on 3 T2DM patients with chronic DME who were refractory to anti-vascular endothelial growth factor (VEGF) therapy and other treatments found that chronic DME improved after using empagliflozin, dapagliflozin, or luseogliflozin. The exact mechanism of action of SGLT-2i is not clear, but it may be through regulating systemic fluid to improve DME (66). In addition, DME-related symptoms were improved in a T2DM patient with steroid-resistant DME after treatment with ipragliflozin, which is speculated to be related to SGLT-2i’s protection of pericytes from high glucose-induced damage and its direct attenuation of DME by inhibiting VEGF production (67). SGLT-2i is considered to be an alternative for retinal protection in metformin intolerant patients with T2DM (68). A retrospective study showed that treatment with SGLT-2i slowed the progression of DR in patients with T2DM compared with sulfonylurea, independent of its effect on glycemic control (69). However, another study found that SGLT-2i was associated with an increased risk of retinal vein occlusion (RVO), especially in elderly patients and those with chronic kidney disease compared with other hypoglycemic agents (70). Notably, empagliflozin was not linked with retinopathy risk when compared with placebo in the EMPA-REG OUTCOME trial involving T2DM and cardiovascular disease patients (71).
Current studies on the relationship between SGLT-2i and incidences of DR have yielded inconclusive results (72). Long-term hyperglycemia is the basis of the pathogenesis of DR. The initial pathophysiology of DR includes vascular endothelial cell injury and pericytes loss, resulting in hypoxia responses that activate the expression of VEGF and other pro-angiogenic factors, leading to inflammation and tissue dysfunction (73). Hyperglycemia is also associated with defects in red blood cells, which aggravate hypoxia (72). SGLT-2i can reduce glucotoxicity, oxidative stress, inflammation and vascular endothelial dysfunction by reducing glucose in the retinal microcirculation (66). Studies have shown that SGLT2 in retinal pericells may alter cellular tone in response to extracellular glucose concentration. During hyperglycemia, excessive SGLT2 mediates the entry of glucose and sodium in retinal pericytes resulting in change in function and morphology, but this effect can be attenuated by the non-selective sodium-glucose cotransporter (SGLT) inhibitor, phlorizin (74). Herat et al. showed that SGLT-2i can reduce the damage to nerve fibers in the outer layer of the retina by counteracting the overactivation of the sympathetic nervous system (75). A randomized study showed that dapagliflozin has a positive effect on retinal vascular remodeling (76).
Enhanced glycemic control in patients with T2DM has been found to reduce ocular events by 13% (77). In addition, some studies have shown that BP has effect on DR. Analysis of 15 RCTs involving patients with type 1 or 2 diabetes showed that BP control prevented DR for up to 4-5 years (78). Dyslipidemia is also considered a potential risk factor for the progression of DR. Studies have shown that changes in plasma levels of high-density and low-density lipoproteins are closely associated with the severity of DR, and DM patients with dyslipidemia have a higher frequency of retinal abnormalities (73). According to previous studies, SGLT-2i may have a beneficial effect on DR by improving blood glucose, BP and blood lipid in patients with T2DM (79).
In this study, SGLT-2i was found to have a greater protective effect against retinal diseases compared with active hypoglycemic agents. A review by Matuszewski et al. showed that rapid decline in glycaemia would lead to the occurrence and development of DR. The risk of DR was the highest when DM patients were treated with sulfonylurea and insulin, and the lowest when the patients were treated using SGLT-2i, GLP-1RA and dipeptidyl-peptidase-4 inhibitor (DPP-4i) (80). The different mechanisms employed by different drugs to lower blood glucose levels may account for their different effects on retinal diseases. SGLT-2i acts directly on SGLT2 in retinal pericytes, and since SGLT2 is at the beginning of the catastrophic signaling cascade, this pattern may give SGLT-2i unique properties from other antidiabetic agents (79). However, due to the limited sample size, we were unable to conduct a comprehensive statistical analysis of the effects of each class of active hypoglycemic drugs.
Our study found that canagliflozin increased the risk of vitreous disease compared with placebo. Among the studies of T2DM patients using canagliflozin or placebo, only 3 studies recorded the specific data of vitreous diseases (10 cases out of 5161 patients in canagliflozin group and 1 cases out of 3709 patients in placebo group). Vitreous diseases mainly occurred as vitreous haemorrhage. In the canagliflozin group, 8 out of 10 vitreous diseases were vitreous haemorrhage, while in the placebo group, 1 case was vitreous haemorrhage, with no statistical difference between them. And a previous trial showed no statistical difference in vitreous hemorrhage between patients receiving empagliflozin or placebo (81). Retinal ischemia and hypoxia in DM patients have been shown to enhance the expression of angiogenic factors, resulting in the proliferation of retinal neovascular tissue, causing retinal and vitreous adhesion. Hyperplastic traction and constriction of surrounding fibrous components result in vitreous hemorrhage (82). At present, the effect of SGLT-2i on vitreous diseases has not been determined, and the mechanism of action is not entirely clear.
The strength of our study was the inclusion of more trials in the meta-analysis, compared with a similar meta-analysis by Li et al. (6) which included only 9 randomized placebo-controlled trials. In addition, we divided the control group into placebo and other active hypoglycemic agents, which has more diverse directions, and carried out comprehensive subgroup analysis based on the type of hypoglycemic drugs and eye disease. However, there were limitations to our study. Most data on ocular events were derived from reports on adverse event, and there may have been differences in the diagnosis of ocular diseases between studies. Although the subgroup analysis of different types of drugs was comprehensive, the sample size was very limited due to the low incidence of eye diseases. In addition, the use of different medications for varying durations may affect the reliability of statistics. Anyway, this study comprehensively and systematically summarized the relationship between SGLT-2i and eye diseases in RCTs of T2DM patients. More studies on the effect of SGLT-2i on eye prognosis are needed to validate our results.
Conclusion
SGLT-2i was not associated with incidences of cataracts, glaucoma, retinal disease (including retinal, macular, optic papillae related diseases), vitreous disease, corneal disease, conjunctival disease, uveal disease (including iris, ciliary body, choroid related diseases), eye haemorrhage and vision problems compared with controls in T2DM patients. Ertugliflozin may reduce the risk of retinal disease, while empagliflozin may reduce the risk of DR compared with the control drugs. SGLT-2i has greater protective effects against retinal diseases compared to other hypoglycemic agents, suggesting that empagliflozin may also have protective effects. However, canagliflozin may increase the risk of vitreous disease compared with placebo.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
XS and BZ designed the research process. YTS, KJ and LHG searched the database forcorresponding articles. RRF, HXN and JYS extracted useful information from the articles above. YXS, MTZ and XYL used statistical software for analysis. BZ and YTS drafted the meta-analysis. LHG polished this article. All authors had read and approved the manuscript and ensured that this was the case.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.907340/full#supplementary-material
Supplementary Figure 1 | Effect of SGLT-2i on incidences of corneal disease compared with control in T2DM patients.
Supplementary Figure 2 | Effect of SGLT-2i on incidences of conjuctival disease compared with control in T2DM patients.
Supplementary Figure 3 | Effect of SGLT-2i on incidences of uveal disease compared with control in T2DM patients.
Supplementary Figure 4 | Effect of SGLT-2i on incidences of eye haemorrhage compared with control in T2DM patients.
Supplementary Figure 5 | Effect of SGLT-2i on incidences of vision problems compared with control in T2DM patients.
Abbreviations
SGLT-2is, sodium-glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; RCTs, randomized controlled trials; RR, risk ratios; CI, confidence intervals; DR, diabetic retinopathy; DM, diabetes mellitus; SGLT2, sodium-glucose cotransporter 2; RAGE, receptor for advanced glycation end products; NADPH, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; GLP-1RA, glucagon-like peptide-1 receptor agonist; DME, diabetic macular edema; VEGF, vascular endothelial growth factor; RVO, retinal vein occlusion; SGLT, sodium-glucose cotransporter; BP, blood pressure; DPP-4i, dipeptidyl-peptidase-4 inhibitor.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Standl E, Khunti K, Hansen TB, Schnell O. The Global Epidemics of Diabetes in the 21st Century: Current Situation and Perspectives. Eur J Prev Cardiol (2019) 26:7–14. doi: 10.1177/2047487319881021
3. Gilbert MP. Screening and Treatment by the Primary Care Provider of Common Diabetes Complications. Med Clin North Am (2015) 99:201–19. doi: 10.1016/j.mcna.2014.09.002
4. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global Trends in Diabetes Complications: A Review of Current Evidence. Diabetologia (2019) 62:3–16. doi: 10.1007/s00125-018-4711-2
5. Szymanska M, Mahmood D, Yap TE, Cordeiro MF. Recent Advancements in the Medical Treatment of Diabetic Retinal Disease. Int J Mol Sci (2021) 22:9441. doi: 10.3390/ijms22179441
6. Li C, Zhou Z, Neuen BL, Yu J, Huang Y, Young T, et al. Sodium-Glucose Co-Transporter-2 Inhibition and Ocular Outcomes in Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Obes Metab (2021) 23:252–7. doi: 10.1111/dom.14197
7. Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, et al. Effects of Intensive Glucose Control on Microvascular Outcomes in Patients With Type 2 Diabetes: A Meta-Analysis of Individual Participant Data From Randomised Controlled Trials. Lancet Diabetes Endocrinol (2017) 5:431–7. doi: 10.1016/S2213-8587(17)30104-3
8. Lupsa BC, Inzucchi SE. Use of SGLT2 Inhibitors in Type 2 Diabetes: Weighing the Risks and Benefits. Diabetologia (2018) 61:2118–25. doi: 10.1007/s00125-018-4663-6
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann Intern Med (2009) 151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136
10. Fan X, Shen W, Wang L, Zhang Y. Efficacy and Safety of DL-3-N-Butylphthalide in the Treatment of Poststroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Front Pharmacol (2021) 12:810297. doi: 10.3389/fphar.2021.810297
11. Araki E, Onishi Y, Asano M, Kim H, Ekholm E, Johnsson E, et al. Efficacy and Safety of Dapagliflozin in Addition to Insulin Therapy in Japanese Patients With Type 2 Diabetes: Results of the Interim Analysis of 16-Week Double-Blind Treatment Period. J Diabetes Investig (2016) 7:555–64. doi: 10.1111/jdi.12453
12. Araki E, Tanizawa Y, Tanaka Y, Taniguchi A, Koiwai K, Kim G, et al. Long-Term Treatment With Empagliflozin as Add-on to Oral Antidiabetes Therapy in Japanese Patients With Type 2 Diabetes Mellitus. Diabetes Obes Metab (2015) 17:665–74. doi: 10.1111/dom.12464
13. Aronson R, Frias J, Goldman A, Darekar A, Lauring B, Terra SG. Long-Term Efficacy and Safety of Ertugliflozin Monotherapy in Patients With Inadequately Controlled T2DM Despite Diet and Exercise: VERTIS MONO Extension Study. Diabetes Obes Metab (2018) 20:1453–60. doi: 10.1111/dom.13251
14. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and Safety of Empagliflozin Added to Existing Antidiabetes Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol (2014) 2:369–84. doi: 10.1016/S2213-8587(13)70208-0
15. Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, et al. Long-Term Efficacy and Safety of Canagliflozin Over 104 Weeks in Patients Aged 55-80 Years With Type 2 Diabetes. Diabetes Obes Metab (2015) 17:294–303. doi: 10.1111/dom.12428
16. Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of Dapagliflozin on Body Weight, Total Fat Mass, and Regional Adipose Tissue Distribution in Patients With Type 2 Diabetes Mellitus With Inadequate Glycemic Control on Metformin. J Clin Endocrinol Metab (2012) 97:1020–31. doi: 10.1210/jc.2011-2260
17. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular Outcomes With Ertugliflozin in Type 2 Diabetes. N Engl J Med (2020) 383:1425–35. doi: 10.1056/NEJMoa2004967
18. Cherney DZI, Ferrannini E, Umpierrez GE, Peters AL, Rosenstock J, Carroll AK, et al. Efficacy and Safety of Sotagliflozin in Patients With Type 2 Diabetes and Severe Renal Impairment. Diabetes Obes Metab (2021) 23:2632–42. doi: 10.1111/dom.14513
19. Ferrannini E, Berk A, Hantel S, Pinnetti S, Hach T, Woerle HJ, et al. Long-Term Safety and Efficacy of Empagliflozin, Sitagliptin, and Metformin: An Active-Controlled, Parallel-Group, Randomized, 78-Week Open-Label Extension Study in Patients With Type 2 Diabetes. Diabetes Care (2013) 36:4015–21. doi: 10.2337/dc13-0663
20. Gallo S, Charbonnel B, Goldman A, Shi H, Huyck S, Darekar A, et al. Long-Term Efficacy and Safety of Ertugliflozin in Patients With Type 2 Diabetes Mellitus Inadequately Controlled With Metformin Monotherapy: 104-Week VERTIS MET Trial. Diabetes Obes Metab (2019) 21:1027–36. doi: 10.1111/dom.13631
21. Grunberger G, Camp S, Johnson J, Huyck S, Terra SG, Mancuso JP, et al. Ertugliflozin in Patients With Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus: The VERTIS RENAL Randomized Study. Diabetes Ther (2018) 9:49–66. doi: 10.1007/s13300-017-0337-5
22. Halvorsen YD, Walford G, Thurber T, Russell H, Massaro M. Freeman MwA 12-Week, Randomized, Double-Blind, Placebo-Controlled, Four-Arm Dose-Finding Phase 2 Study Evaluating Bexagliflozin as Monotherapy for Adults With Type 2 Diabetes. Diabetes Obes Metab (2020) 22:566–73. doi: 10.1111/dom.13928
23. Hollander P, Hill J, Johnson J, Wei Jiang Z, Golm G, Huyck S, et al. Results of VERTIS SU Extension Study: Safety and Efficacy of Ertugliflozin Treatment Over 104 Weeks Compared to Glimepiride in Patients With Type 2 Diabetes Mellitus Inadequately Controlled on Metformin. Curr Med Res Opin (2019) 35:1335–43. doi: 10.1080/03007995.2019.1583450
24. Ikeda S, Takano Y, Cynshi O, Tanaka R, Christ AD, Boerlin V, et al. A Novel and Selective Sodium-Glucose Cotransporter-2 Inhibitor, Tofogliflozin, Improves Glycaemic Control and Lowers Body Weight in Patients With Type 2 Diabetes Mellitus. Diabetes Obes Metab (2015) 17:984–93. doi: 10.1111/dom.12538
25. Inagaki N, Harashima S, Maruyama N, Kawaguchi Y, Goda M, Iijima H. Efficacy and Safety of Canagliflozin in Combination With Insulin: A Double-Blind, Randomized, Placebo-Controlled Study in Japanese Patients With Type 2 Diabetes Mellitus. Cardiovasc Diabetol (2016) 15:89. doi: 10.1186/s12933-016-0407-4
26. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and Safety of Canagliflozin in Japanese Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, 12-Week Study. Diabetes Obes Metab (2013) 15:1136–45. doi: 10.1111/dom.12149
27. Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and Safety of Canagliflozin Monotherapy in Japanese Patients With Type 2 Diabetes Inadequately Controlled With Diet and Exercise: A 24-Week, Randomized, Double-Blind, Placebo-Controlled, Phase III Study. Expert Opin Pharmacother (2014) 15:1501–15. doi: 10.1517/14656566.2014.935764
28. Inzucchi SE, Davies MJ, Khunti K, Trivedi P, George JT, Zwiener I, et al. Empagliflozin Treatment Effects Across Categories of Baseline HbA1c, Body Weight and Blood Pressure as an Add-on to Metformin in Patients With Type 2 Diabetes. Diabetes Obes Metab (2021) 23:425–33. doi: 10.1111/dom.14234
29. Ji L, Liu Y, Miao H, Xie Y, Yang M, Wang W, et al. Safety and Efficacy of Ertugliflozin in Asian Patients With Type 2 Diabetes Mellitus Inadequately Controlled With Metformin Monotherapy: VERTIS Asia. Diabetes Obes Metab (2019) 21:1474–82. doi: 10.1111/dom.13681
30. Kadowaki T, Haneda M, Inagaki N, Terauchi Y, Taniguchi A, Koiwai K, et al. Efficacy and Safety of Empagliflozin Monotherapy for 52 Weeks in Japanese Patients With Type 2 Diabetes: A Randomized, Double-Blind, Parallel-Group Study. Adv Ther (2015) 32:306–18. doi: 10.1007/s12325-015-0198-0
31. Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in Combination With Metformin for the Treatment of Japanese Patients With Type 2 Diabetes: ILLUMINATE, a Randomized, Double-Blind, Placebo-Controlled Study. Diabetes Obes Metab (2015) 17:304–8. doi: 10.1111/dom.12331
32. Kawamori R, Haneda M, Suzaki K, Cheng G, Shiki K, Miyamoto Y, et al. Empagliflozin as Add-on to Linagliptin in a Fixed-Dose Combination in Japanese Patients With Type 2 Diabetes: Glycaemic Efficacy and Safety Profile in a 52-Week, Randomized, Placebo-Controlled Trial. Diabetes Obes Metab (2018) 20:2200–9. doi: 10.1111/dom.13352
33. Kitazawa M, Katagiri T, Suzuki H, Matsunaga S, HY M, Ikarashi T, et al. A 52-Week Randomized Controlled Trial of Ipragliflozin or Sitagliptin in Type 2 Diabetes Combined With Metformin: The N-ISM Study. Diabetes Obes Metab (2021) 23:811–21. doi: 10.1111/dom.14288
34. Kitazawa T, Seino H, Ohashi H, Inazawa T, Inoue M, Ai M, et al. Comparison of Tofogliflozin Versus Glimepiride as the Third Oral Agent Added to Metformin Plus a Dipeptidyl Peptidase-4 Inhibitor in Japanese Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Controlled Trial (STOP-Ob). Diabetes Obes Metab (2020) 22:1659–63. doi: 10.1111/dom.14059
35. Kohan DE, Fioretto P, Tang W, List JF. Long-Term Study of Patients With Type 2 Diabetes and Moderate Renal Impairment Shows That Dapagliflozin Reduces Weight and Blood Pressure but Does Not Improve Glycemic Control. Kidney Int (2014) 85:962–71. doi: 10.1038/ki.2013.356
36. Kwak SH, Hwang YC, Won JC, Bae JC, Kim HJ, Suh S, et al. Comparison of the Effects of Gemigliptin and Dapagliflozin on Glycaemic Variability in Type 2 Diabetes: A Randomized, Open-Label, Active-Controlled, 12-Week Study (STABLE II Study). Diabetes Obes Metab (2020) 22:173–81. doi: 10.1111/dom.13882
37. Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and Safety of Canagliflozin Compared With Placebo and Sitagliptin in Patients With Type 2 Diabetes on Background Metformin Monotherapy: A Randomised Trial. Diabetologia (2013) 56:2582–92. doi: 10.1007/s00125-013-3039-1
38. Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, et al. Canagliflozin Provides Durable Glycemic Improvements and Body Weight Reduction Over 104 Weeks Versus Glimepiride in Patients With Type 2 Diabetes on Metformin: A Randomized, Double-Blind, Phase 3 Study. Diabetes Care (2015) 38:355–64. doi: 10.2337/dc13-2762
39. Lingvay I, Catarig AM, Frias JP, Kumar H, Lausvig NL, le Roux CW, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Daily Canagliflozin as Add-on to Metformin in Patients With Type 2 Diabetes (SUSTAIN 8): A Double-Blind, Phase 3b, Randomised Controlled Trial. Lancet Diabetes Endocrinol (2019) 7:834–44. doi: 10.1016/S2213-8587(19)30311-0
40. Müller-Wieland D, Kellerer M, Cypryk K, Skripova D, Rohwedder K, Johnsson E, et al. Efficacy and Safety of Dapagliflozin or Dapagliflozin Plus Saxagliptin Versus Glimepiride as Add-on to Metformin in Patients With Type 2 Diabetes. Diabetes Obes Metab (2018) 20:2598–607. doi: 10.1111/dom.13437
41. Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, et al. Dapagliflozin Versus Glipizide as Add-on Therapy in Patients With Type 2 Diabetes Who Have Inadequate Glycemic Control With Metformin: A Randomized, 52-Week, Double-Blind, Active-Controlled Noninferiority Trial. Diabetes Care (2011) 34:2015–22. doi: 10.2337/dc11-0606
42. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
43. Ridderstråle M, Rosenstock J, Andersen KR, Woerle HJ, Salsali A. Empagliflozin Compared With Glimepiride in Metformin-Treated Patients With Type 2 Diabetes: 208-Week Data From a Masked Randomized Controlled Trial. Diabetes Obes Metab (2018) 20:2768–77. doi: 10.1111/dom.13457
44. Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg S, et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care (2019) 42:2272–81. doi: 10.2337/dc19-0883
45. Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved Glucose Control With Weight Loss, Lower Insulin Doses, and No Increased Hypoglycemia With Empagliflozin Added to Titrated Multiple Daily Injections of Insulin in Obese Inadequately Controlled Type 2 Diabetes. Diabetes Care (2014) 37:1815–23. doi: 10.2337/dc13-3055
46. Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. Impact of Empagliflozin Added on to Basal Insulin in Type 2 Diabetes Inadequately Controlled on Basal Insulin: A 78-Week Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Obes Metab (2015) 17:936–48. doi: 10.1111/dom.12503
47. Rosenstock J, Perl S, Johnsson E, García-Sánchez R, Jacob S. Triple Therapy With Low-Dose Dapagliflozin Plus Saxagliptin Versus Dual Therapy With Each Monocomponent, All Added to Metformin, in Uncontrolled Type 2 Diabetes. Diabetes Obes Metab (2019) 21:2152–62. doi: 10.1111/dom.13795
48. Ross S, Thamer C, Cescutti J, Meinicke T, Woerle HJ, Broedl UC. Efficacy and Safety of Empagliflozin Twice Daily Versus Once Daily in Patients With Type 2 Diabetes Inadequately Controlled on Metformin: A 16-Week, Randomized, Placebo-Controlled Trial. Diabetes Obes Metab (2015) 17:699–702. doi: 10.1111/dom.12469
49. Sone H, Kaneko T, Shiki K, Tachibana Y, Pfarr E, Lee J, et al. Efficacy and Safety of Empagliflozin as Add-on to Insulin in Japanese Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Obes Metab (2020) 22:417–26. doi: 10.1111/dom.13909
50. Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, et al. Secondary Analyses to Assess the Profound Effects of Empagliflozin on Endothelial Function in Patients With Type 2 Diabetes and Established Cardiovascular Diseases: The Placebo-Controlled Double-Blind Randomized Effect of Empagliflozin on Endothelial Function in Cardiovascular High Risk Diabetes Mellitus: Multi-Center Placebo-Controlled Double-Blind Randomized Trial. J Diabetes Investig (2020) 11:1551–63. doi: 10.1111/jdi.13289
51. Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Long-Term Efficacy of Dapagliflozin in Patients With Type 2 Diabetes Mellitus Receiving High Doses of Insulin: A Randomized Trial. Ann Intern Med (2012) 156:405–15. doi: 10.7326/0003-4819-156-6-201203200-00003
52. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
53. Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and Safety of Canagliflozin Over 52 Weeks in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Diabetes Obes Metab (2014) 16:1016–27. doi: 10.1111/dom.12348
54. Yang W, Han P, Min KW, Wang B, Mansfield T, T'Joen C, et al. Efficacy and Safety of Dapagliflozin in Asian Patients With Type 2 Diabetes After Metformin Failure: A Randomized Controlled Trial. J Diabetes (2016) 8:796–808. doi: 10.1111/1753-0407.12357
55. Yang W, Ma J, Li Y, Li Y, Zhou Z, Kim JH, et al. Dapagliflozin as Add-on Therapy in Asian Patients With Type 2 Diabetes Inadequately Controlled on Insulin With or Without Oral Antihyperglycemic Drugs: A Randomized Controlled Trial. J Diabetes (2018) 10:589–99. doi: 10.1111/1753-0407.12634
56. Zhou Z, Jardine M, Perkovic V, Matthews DR, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and Fracture Risk in Individuals With Type 2 Diabetes: Results From the CANVAS Program. Diabetologia (2019) 62:1854–67. doi: 10.1007/s00125-019-4955-5
57. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
58. Pollreisz A, Schmidt-Erfurth U. Diabetic Cataract-Pathogenesis, Epidemiology and Treatment. J Ophthalmol (2010) 2010:608751. doi: 10.1155/2010/608751
59. Takakura S, Toyoshi T, Hayashizaki Y, Takasu T. Effect of Ipragliflozin, an SGLT2 Inhibitor, on Progression of Diabetic Microvascular Complications in Spontaneously Diabetic Torii Fatty Rats. Life Sci (2016) 147:125–31. doi: 10.1016/j.lfs.2016.01.042
60. Chen YY, Wu TT, Ho CY, Yeh TC, Sun GC, Kung YH, et al. Dapagliflozin Prevents NOX- and SGLT2-Dependent Oxidative Stress in Lens Cells Exposed to Fructose-Induced Diabetes Mellitus. Int J Mol Sci (2019) 20:4357. doi: 10.3390/ijms20184357
61. Chin SO, Ha IG, Rhee SY, Jeong SJ, Chon S, Kim SH, et al. Clinical Characteristics and Prevalence of Comorbidities According to Metformin Use in Korean Patients With Type 2 Diabetes. Int J Endocrinol (2020) 2020:9879517. doi: 10.1155/2020/9879517
62. Graw J, Welzl G, Ahmad N, Klopp N, Heier M, Wulff A, et al. The KORA Eye Study: A Population-Based Study on Eye Diseases in Southern Germany (KORA F4). Invest Ophthalmol Vis Sci (2011) 52:7778–86. doi: 10.1167/iovs.10-7113
63. Jung Y, Han K, Ohn K, Kim DR, Moon JI. Association Between Diabetes Status and Subsequent Onset of Glaucoma in Postmenopausal Women. Sci Rep (2021) 11:18272. doi: 10.1038/s41598-021-97740-3
64. Shao SC, Su YC, Lai EC, Chang KC, Lee CN, Hung MJ, et al. Association Between Sodium Glucose Co-Transporter 2 Inhibitors and Incident Glaucoma in Patients With Type 2 Diabetes: A Multi-Institutional Cohort Study in Taiwan. Diabetes Metab (2022) 48:101318. doi: 10.1016/j.diabet.2022.101318
65. Su YC, Shao SC, Lai EC, Lee CN, Hung MJ, Lai CC, et al. Risk of Diabetic Macular Oedema With Sodium-Glucose Cotransporter-2 Inhibitors in Type 2 Diabetes Patients: A Multi-Institutional Cohort Study in Taiwan. Diabetes Obes Metab (2021) 23:2067–76. doi: 10.1111/dom.14445
66. Takatsuna Y, Ishibashi R, Tatsumi T, Koshizaka M, Baba T, Yamamoto S, et al. Sodium-Glucose Cotransporter 2 Inhibitors Improve Chronic Diabetic Macular Edema. Case Rep Ophthalmol Med (2020) 2020:8867079. doi: 10.1155/2020/8867079
67. Yoshizumi H, Ejima T, Nagao T, Wakisaka M. Recovery From Diabetic Macular Edema in a Diabetic Patient After Minimal Dose of a Sodium Glucose Co-Transporter 2 Inhibitor. Am J Case Rep (2018) 19:462–6. doi: 10.12659/AJCR.909708
68. Sabaner MC, Duman R, Dogan M, Akdogan M, Vurmaz A, Bozkurt E, et al. Do SGLT2 Inhibitors Prevent Preclinical Diabetic Retinopathy? A Prospective Pilot Optical Coherence Tomography Angiography Study. J Fr Ophtalmol (2021) 44:1159–67. doi: 10.1016/j.jfo.2021.01.005
69. Cho EH, Park SJ, Han S, Song JH, Lee K, Chung YR. Potent Oral Hypoglycemic Agents for Microvascular Complication: Sodium-Glucose Cotransporter 2 Inhibitors for Diabetic Retinopathy. J Diabetes Res (2018) 2018:6807219. doi: 10.1155/2018/6807219
70. Lee MK, Kim B, Han K, Lee JH, Kim M, Kim MK, et al. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Retinal Vein Occlusion Among Patients With Type 2 Diabetes: A Propensity Score-Matched Cohort Study. Diabetes Care (2021) 7:dc203133. doi: 10.2337/figshare.14824089
71. Inzucchi SE, Wanner C, Hehnke U, Zwiener I, Kaspers S, Clark D, et al. Retinopathy Outcomes With Empagliflozin Versus Placebo in the EMPA-REG OUTCOME Trial. Diabetes Care (2019) 42:e53–5. doi: 10.2337/dc18-1355
72. Lahoti S, Nashawi M, Sheikh O, Massop D, Mir M, Chilton R. Sodium-Glucose Co-Transporter 2 Inhibitors and Diabetic Retinopathy: Insights Into Preservation of Sight and Looking Beyond. Cardiovasc Endocrinol Metab (2021) 10:3–13. doi: 10.1097/XCE.0000000000000209
73. Tomita Y, Lee D, Tsubota K, Negishi K, Kurihara T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J Clin Med (2021) 10:4666. doi: 10.3390/jcm10204666
74. Klen J, Goričar K, Dolžan V. Genetic Variability in Sodium-Glucose Cotransporter 2 Influences Glycemic Control and Risk for Diabetic Retinopathy in Type 2 Diabetes Patients. J Med Biochem (2020) 39:276–82. doi: 10.2478/jomb-2019-0040
75. Herat LY, Matthews VB, Rakoczy PE, Carnagarin R, Schlaich M. Focusing on Sodium Glucose Cotransporter-2 and the Sympathetic Nervous System: Potential Impact in Diabetic Retinopathy. Int J Endocrinol (2018) 2018:9254126. doi: 10.1155/2018/9254126
76. Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, et al. A Randomised Study of the Impact of the SGLT2 Inhibitor Dapagliflozin on Microvascular and Macrovascular Circulation. Cardiovasc Diabetol (2017) 16:26. doi: 10.1186/s12933-017-0510-1
77. Giugliano D, Maiorino MI, Bellastella G, Esposito K. Type 2 Diabetes and Cardiovascular Prevention: The Dogmas Disputed. Endocrine (2018) 60:224–8. doi: 10.1007/s12020-017-1418-y
78. Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, et al. Blood Pressure Control for Diabetic Retinopathy. Cochrane Database Syst Rev (2015) 1:Cd006127. doi: 10.1002/14651858.CD006127.pub2
79. Wakisaka M, Nagao T. Sodium Glucose Cotransporter 2 in Mesangial Cells and Retinal Pericytes and its Implications for Diabetic Nephropathy and Retinopathy. Glycobiology (2017) 27:691–5. doi: 10.1093/glycob/cwx047
80. Matuszewski W, Baranowska-Jurkun A, Stefanowicz-Rutkowska MM, Gontarz-Nowak K, Gątarska E, Bandurska-Stankiewicz E. The Safety of Pharmacological and Surgical Treatment of Diabetes in Patients With Diabetic Retinopathy-A Review. J Clin Med (2021) 10:705. doi: 10.3390/jcm10040705
81. Dorsey-Treviño EG, González-González JG, Alvarez-Villalobos N, González-Nava V, Contreras-Garza BM, Díaz González-Colmenero A, et al. Sodium-Glucose Cotransporter 2 (SGLT-2) Inhibitors and Microvascular Outcomes in Patients With Type 2 Diabetes: Systematic Review and Meta-Analysis. J Endocrinol Invest (2020) 43:289–304. doi: 10.1007/s40618-019-01103-9
Keywords: SGLT-2i, T2DM, ocular diseases, meta-analysis, RCTs
Citation: Zhou B, Shi Y, Fu R, Ni H, Gu L, Si Y, Zhang M, Jiang K, Shen J, Li X and Sun X (2022) Relationship Between SGLT-2i and Ocular Diseases in Patients With Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 13:907340. doi: 10.3389/fendo.2022.907340
Received: 29 March 2022; Accepted: 25 April 2022;
Published: 26 May 2022.
Edited by:
Konstantinos Tziomalos, Aristotle University of Thessaloniki, GreeceReviewed by:
Yao Hao Teo, National University of Singapore, SingaporeAnu Grover, Ipca Laboratories, India
Copyright © 2022 Zhou, Shi, Fu, Ni, Gu, Si, Zhang, Jiang, Shen, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing Sun, Y3JhZ2U5MjNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Bin Zhou1,2†
Bin Zhou1,2† Yetan Shi
Yetan Shi Lihu Gu
Lihu Gu Mengting Zhang
Mengting Zhang Ke Jiang
Ke Jiang