- Department of Ultrasonography, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, China
Aim: The study aimed to systematically evaluate the safety and efficacy of ultrasonography-guided percutaneous thermal ablation in the treatment of cervical lymph node metastasis (LNM) of recurrent papillary thyroid carcinoma (PTC).
Methods: PubMed, PubMed Central (PMC), Embase, and Cochrane were examined. The inclusion and exclusion criteria were determined and the relevant data were extracted from the library and other databases for LNM thermal ablation of recurrent PTC. The data were analyzed using Stata15.1, Revman5.3 software, and the standard errors of 95% confidence intervals were estimated using fixed or random effects models. Volume reduction rate (VRR), Serum thyroglobulin (Tg) level before and after thermal ablation, the total complications and major complications incidence were analyzed.
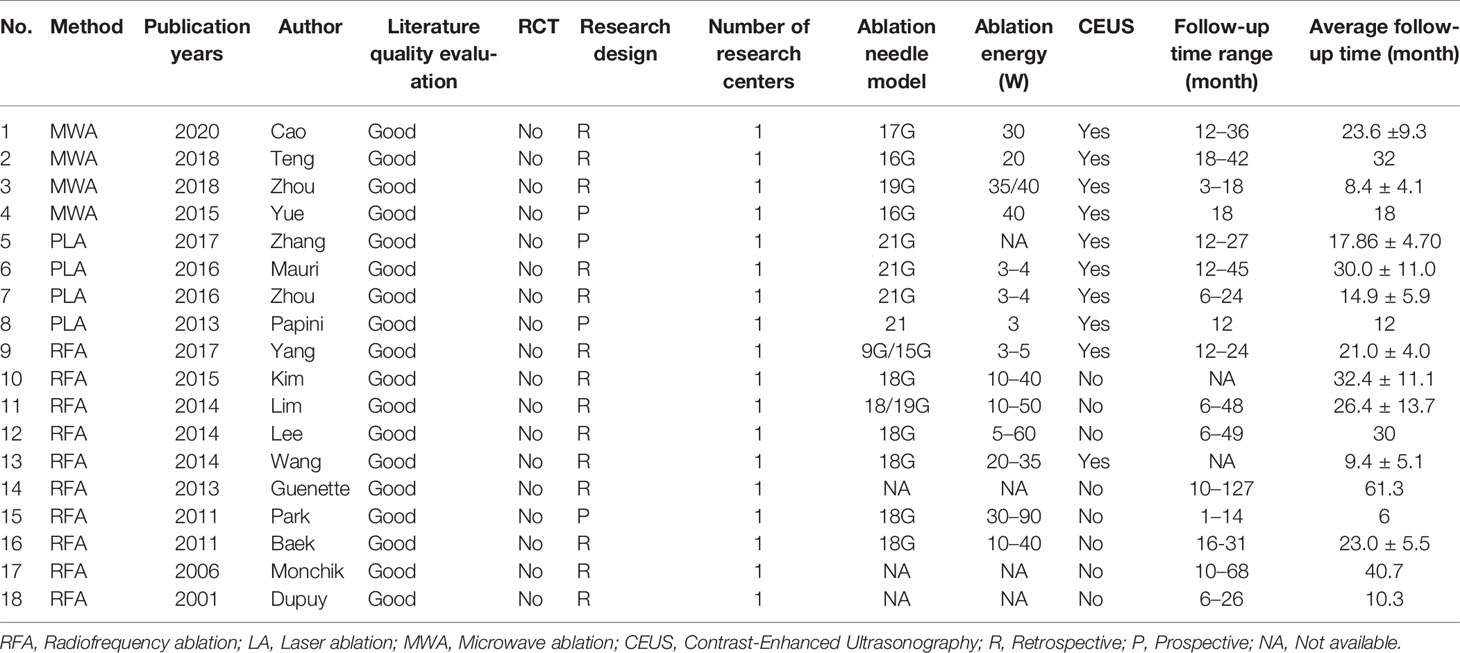
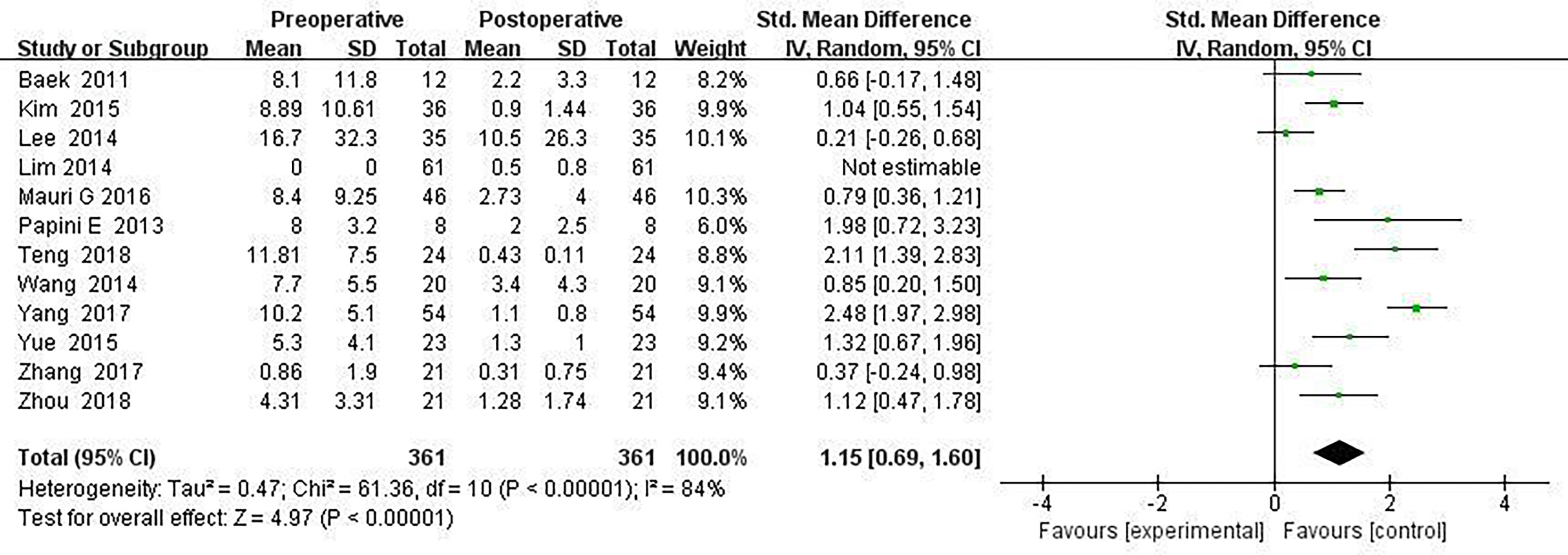
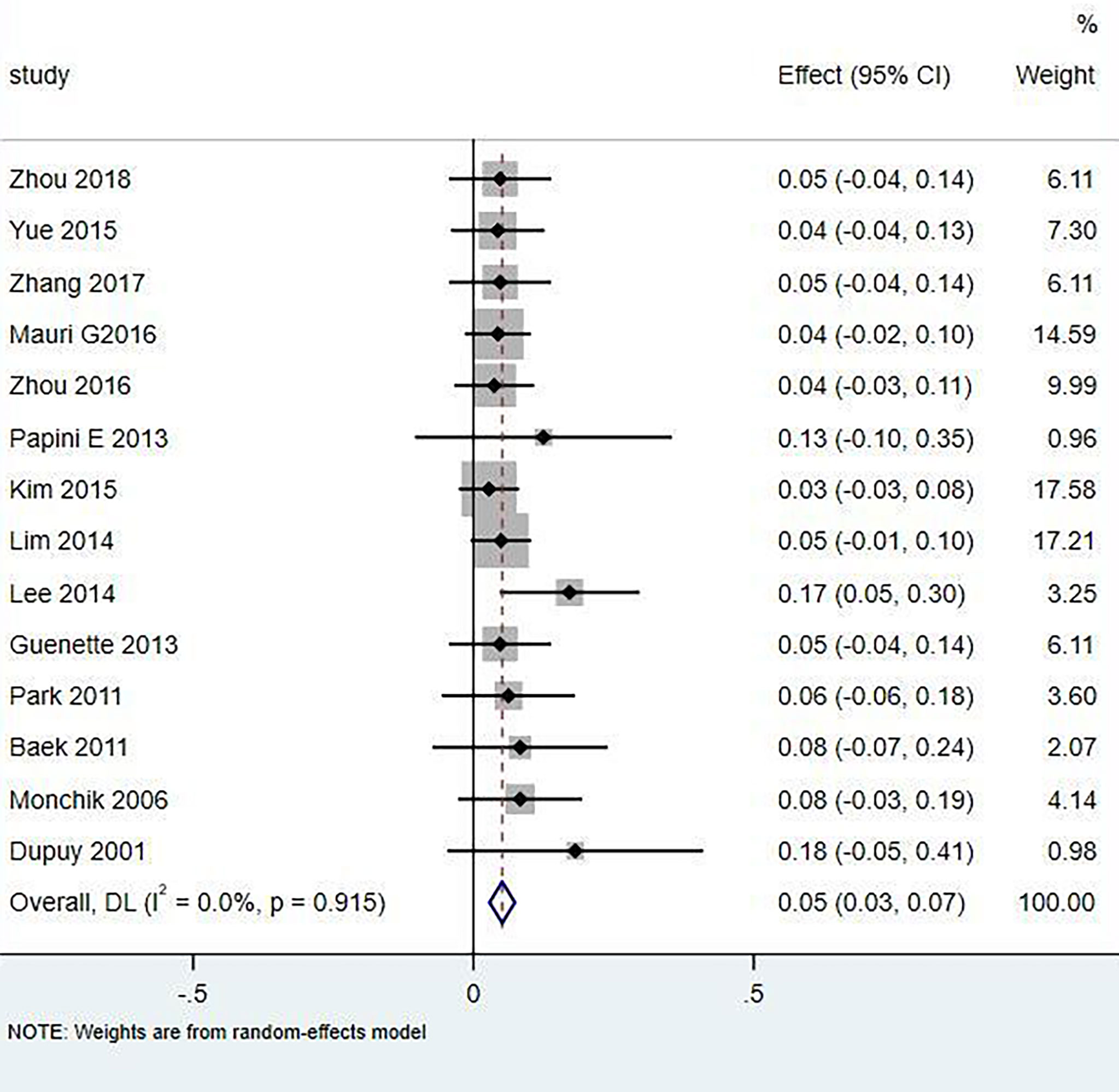
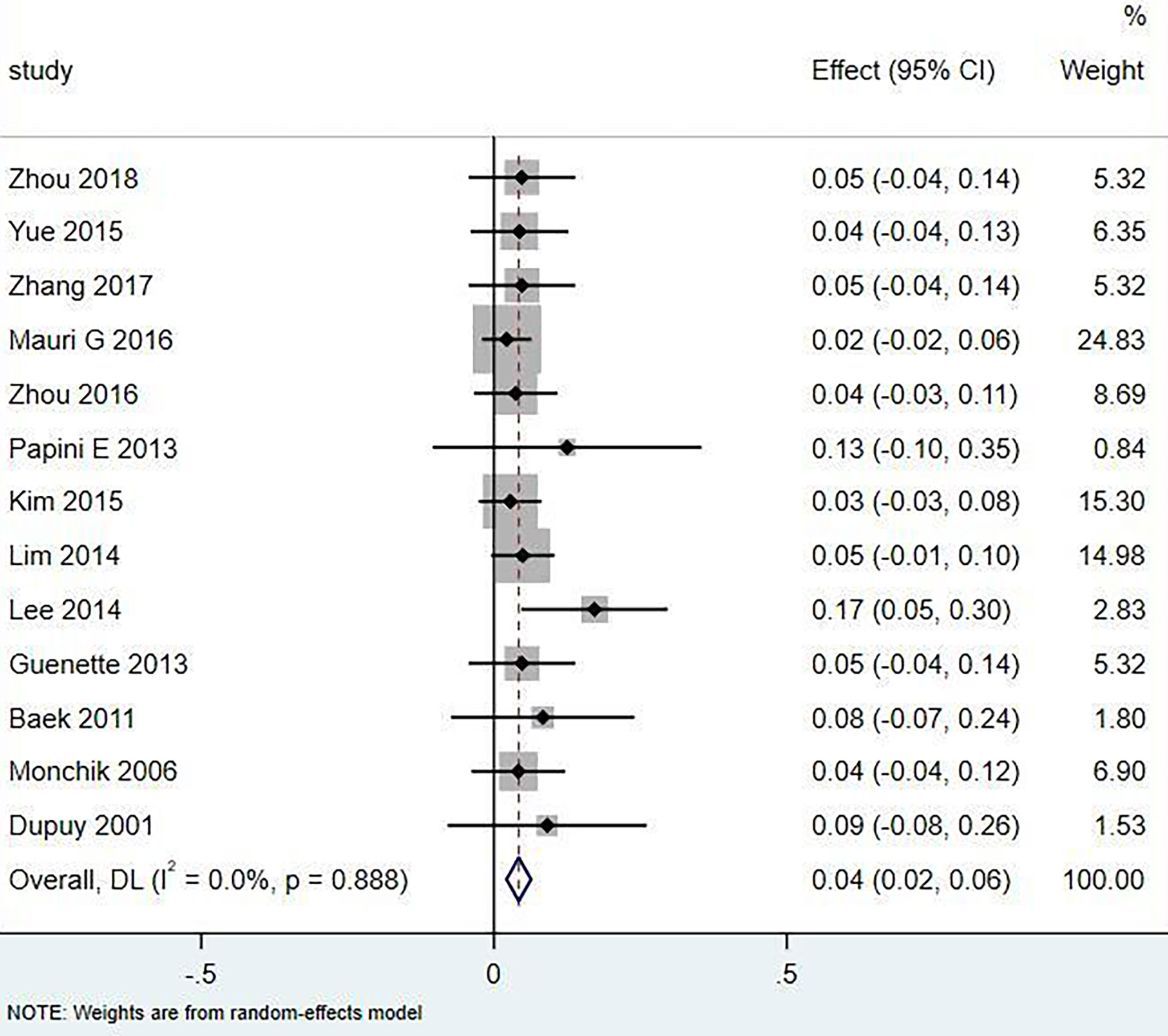
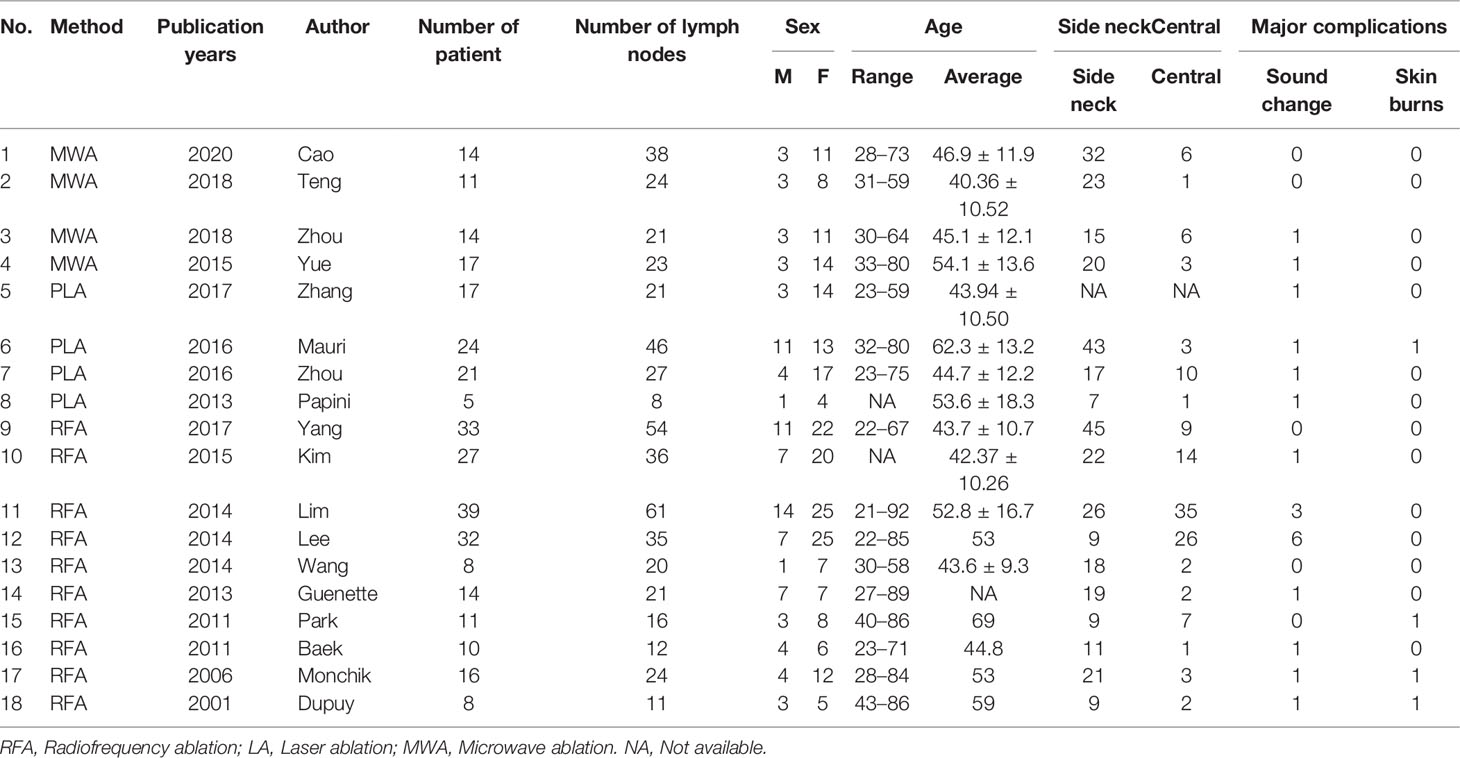
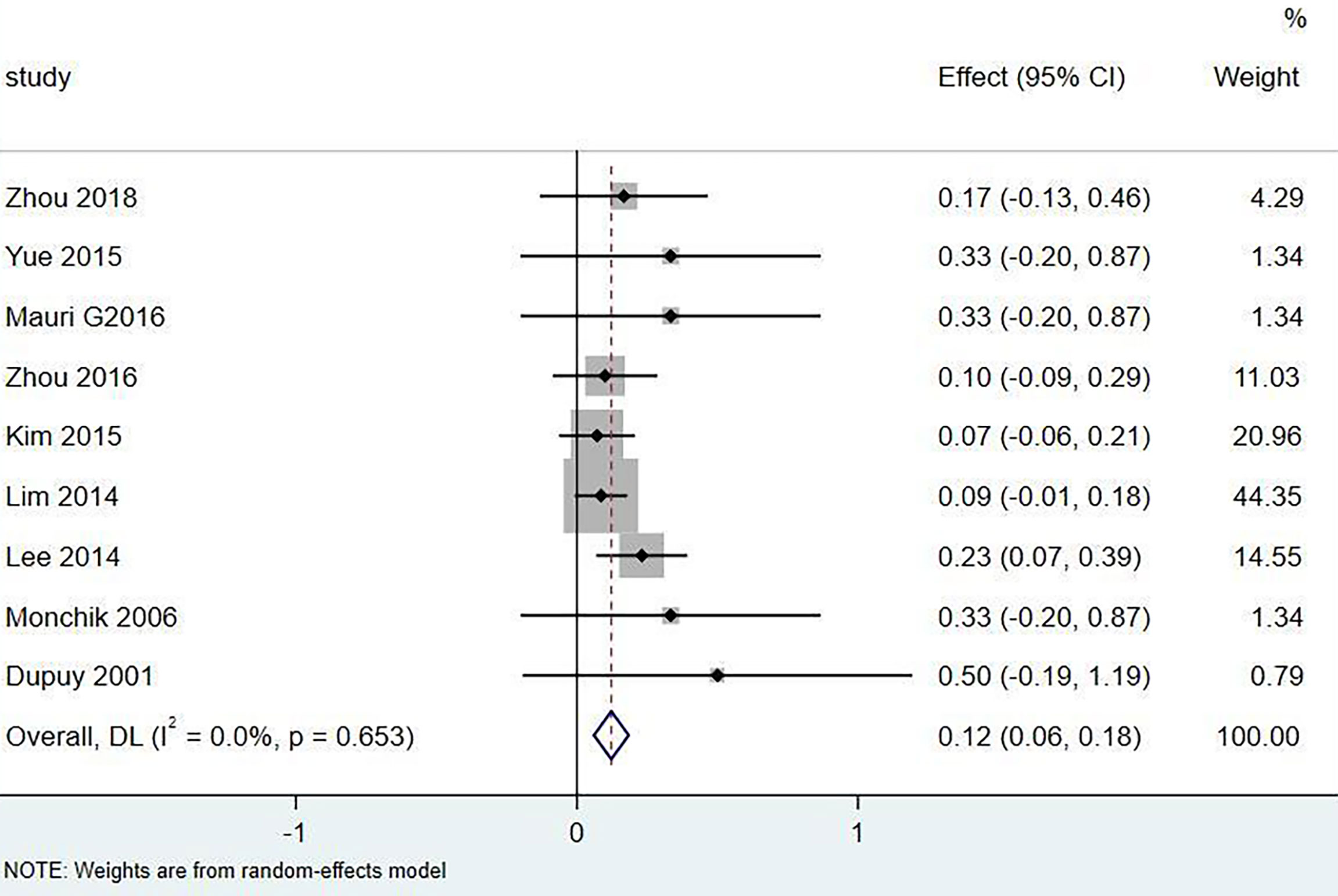
Results: A total of 18 literature articles were included, namely, 10 radiofrequency ablation (RFA), 4 laser ablation (LA), and 4 microwave ablation (MWA). A total of 321 patients had 498 LNM. LNM volume changes before and at the last follow-up of thermal ablation (SMD = 1.04, I2 = 8%, 95% CI 0.86–1.21, P <0.0001). The postoperative lymph node VRR was 88.4% (95% CI 77.8–97.3%, I2 = 34%, P = 0.14). Tg measurements before and after thermal ablation (SMD = 1.15, 95% CI 0.69–1.60, I2 = 84%, P <0.0001). The incidence of total complications was 5.0% (95% CI 3.0–7.0%, I2 = 0.0%, P = 0.915), and the incidence of major complications was 4.0% (95% CI 2.0–6.0%, I2 = 0.0%, P = 0.888). A total of 131 LNM were located in the central region, and the major complication rate was 12.0% (95% CI 6.0–18.0%, I2 = 0.0%, P = 0.653).
Conclusion: Ultrasonography-guided thermal ablation is safe and effective in the treatment of LNM of recurrent PTC. The ablation strategy of central LNM needs to be further explored and improved. It can be used as an alternative to surgery for patients with high surgical risk or who refuse resurgery.
Systematic Review Registration: 10.37766/inplasy2022.6.0004, identifier INPLASY202260004.
Introduction
Papillary thyroid carcinoma (PTC) is the most common form of thyroid cancer. Most patients can be cured by almost all thyroidectomy and neck lymph node dissection, and 131I is used for postoperative ablation of occult microlesions. However, there are still 15–30% of patients with local recurrence and cervical lymph node metastasis (LNM) (1–3). More than 96% of potential driver mutations in PTC have been identified, which can lead to special histological features or biological behavior (4). For example, the BRAFV660E mutation is present in about half of the PTC (5). The American Thyroid Association guidelines recommend PTC treatment as the surgical removal of choice for the neck LNM, but given the risk of reoperation (6). However, postoperative fibrosis changes tissue adhesion and anatomical structure. Even with the rich experience of the surgeon, reoperation also has technical challenges, and the operation process of small and medium-sized LNM is difficult to find. Repeated operations increase the risk of complications, and can even cause temporary or irreversible parathyroid function and nerve damage. Moreover, some patients have surgical difficulties and their own conditions cannot tolerate the surgical operation or their subjective intention to refuse the surgical treatment (7, 8). In addition, long-term follow-up can cause unnecessary anxiety, reduce the quality of life, and harm mental health (9). Therefore, it is of great significance to find a minimally invasive technique to replace surgical resection for LNM.
Recently, studies have shown that ultrasonography-guided percutaneous thermal ablation is a minimally invasive treatment technology, such as laser ablation (LA), radiofrequency ablation (RF), and microwave ablation (MWA), which is considered a new alternative to surgical treatment (6, 9–11). These thermal ablation methods are used in patients with benign and malignant tumors because of their advantages, such as low trauma, low complication rate, local anesthesia, and repeatable treatment (12). For example, ultrasonal-guided thermal ablation for treating benign thyroid nodules and thyroid micropapillary carcinoma has good safety and efficacy (13–15).
Thermal ablation for LNM research is still rare. In this paper, the therapy of cervical LNM from recurrent PTC under ultrasonography-guided percutaneous thermal ablation explores its feasibility, efficacy and safety, and possible directions for future research and treatment.
Methods
Search Strategy
Appropriate articles were retrieved before February 2022 through comprehensive literature retrieval through PubMed, PubMed Central (PMC), Embase, and Cochrane Library (Central). Search terms are based on a combination of medical subjects and free words (synonyms), as well as restrictive language in English. Search terms included (“lymph node metastasis” or “LNM” or “recurrence”) and (“papillary thyroid carcinoma” or “PTC”) and (“ablation technology” or “ablation” or “ultrasonography therapy” or “radiofrequency ablation” or “RFA” or “percutaneous laser ablation” or “PLA” or “laser ablation” or “LA” or “microwave ablation” or “microwave” or “MWA”). Two researchers (TN and XZ) independently completed the selection process and resolved the differences through discussion.
Selection Criteria
The literature inclusion criteria included: (a) patients previously treated with thyroidectomy or neck dissection. After operation, the patient found cervical lymph nodes, and the subsequent fine needle aspiration (FNA) examination confirmed as LNM for PTC by cytopathology, and has received thermal ablation treatment; (b) patients were older than 18 years; and (c) study data include lymph node volume and serum Tg levels before and after thermal ablation, and complications record data.
The exclusion criteria included: (a) patients with bone or lung metastasis; (b) follow-up time of less than 6 months; (c) conference lectures, literature reviews, case reports, and other related articles; and (d) wrong, missing clinical data and incomplete records for patients.
According to the above inclusion and exclusion criteria, the relevant literature in the above database was searched, screened by reading titles and abstracts, and then excluded again by reading the full text. This process was completed independently by two researchers (TN and XZ), and two researchers with different opinions agreed to decide whether to include it.
Literature Quality Evaluation
After reading through the full text, the non-randomized study risk assessment method (Newcastle-Ottawa Scale, NOS) was applied for the quality evaluation (16). The evaluation index included 9 points, including (a) study object selection, 4 points; (b) comparability among groups, 2 points; and (c) exposure factor selection, 3 points. Four points indicate good quality; <4 points indicate poor quality.
Data Extraction and Quality Evaluation
The extracted data information included the general information of the study, including the authors, publication year, study type, follow-up time, number and location of the neck LNM, the mean age and sex of the patients, ablation method, evaluation method, etc. The initial volume of LNM and percentage volume and nodule volume reduction at the last follow-up (Volume Reduction Ratio, VRR), VRR = (initial nodule volume-postoperative nodule volume) ∗ 100/initial nodule volume. Thyroglobulin (Tg) values before and after ablation. The total complications include both major and minor complications. Major complications include transient or permanent voice changes, tracheal or esophageal damage, skin burns, and hematoma formation. Vomiting, mild, transient postoperative pain, burning sensations, neck swelling, and discomfort are minor complications.
Statistical Analysis
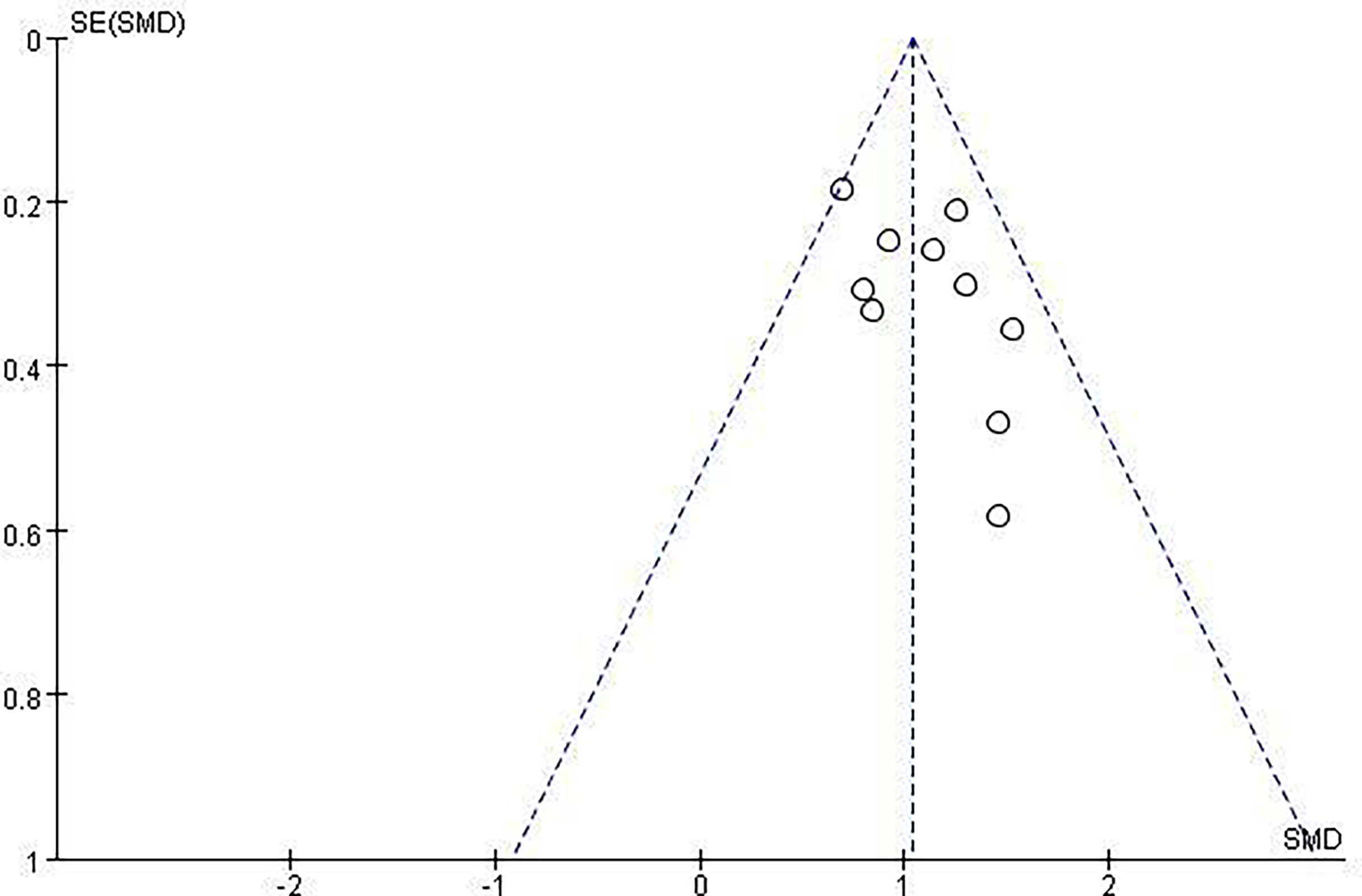
Revman5.3 and Stata15.1 software were used to compare LNM volume changes, VRR, and Tg levels before and after last follow-up of thermal ablation and the overall incidence of postoperative and major complications. Continuity data were compared with means (SMD), count data were compared with the odds ratio (OR), statistical results were expressed with 95% CI, heterogeneity included in I2, I2 >50% indicates heterogeneity, random effect model, and fixed effect model with P <0.0001. The bias assessment was analyzed on the basis of the funnel plot, and the sensitivity analysis was conducted on the effect of the combined effect values was conducted by excluding either of the documents.
Results
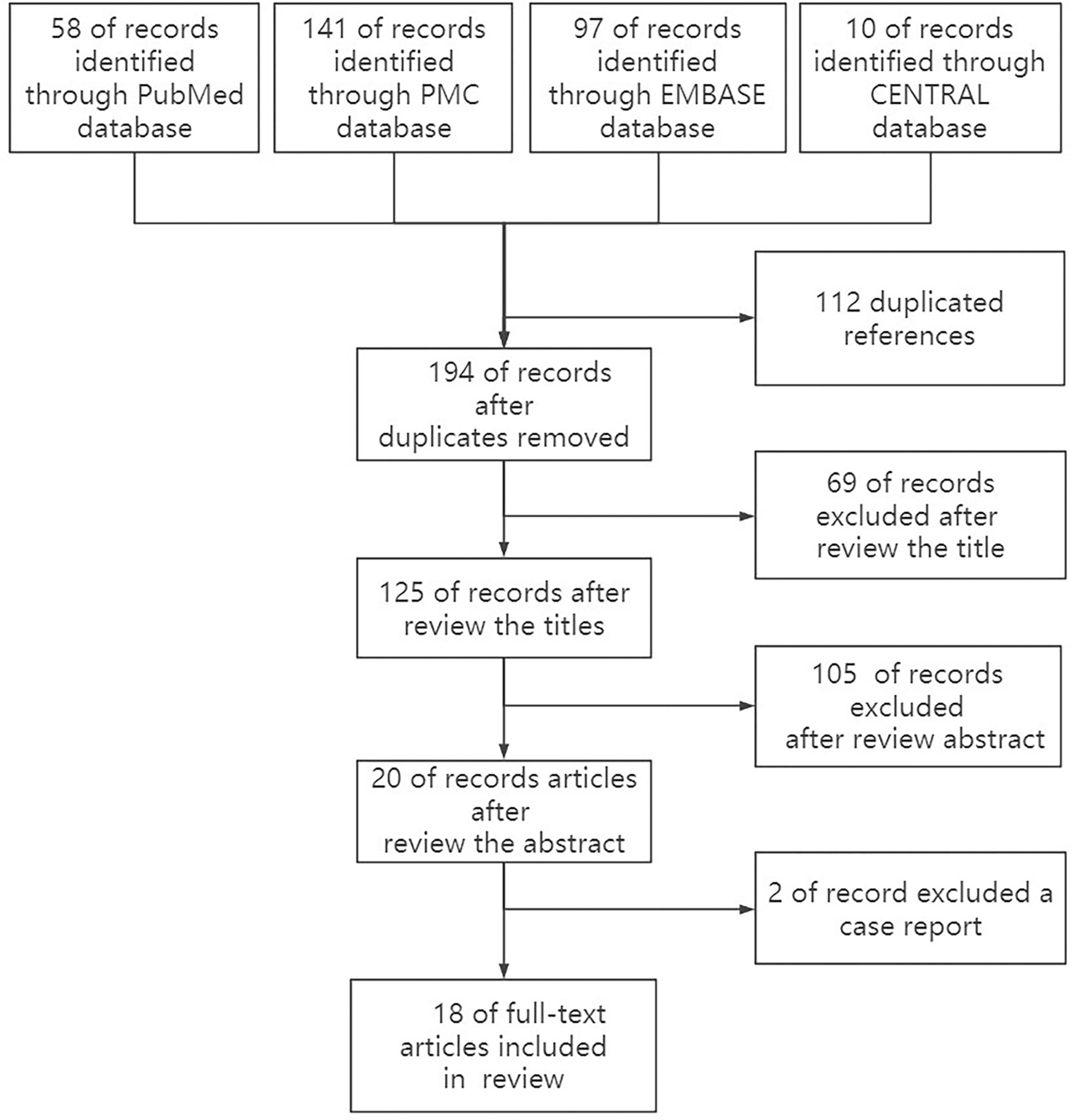
After a careful search of medical databases including PubMed, PMC, Embase, and Cochrane Library, 306 references were found, of which 58 were from PubMed, 141 from PMC, 97 from Embase, and 10 from CENTRAL. Out of 306 references, 112 duplicate references were deleted, and 175 references were deleted by two independent reviewers (TN and XZ) due to their low relevance after careful reading of titles (69) and abstracts (106). Two case reports were deleted after reading the full text. Finally, 18 original articles were included in this review (Figure 1, Table 1). Due to the relatively small number of included documents, the funnel plot showed a relatively concentrated distribution and good symmetry, indicating a small publication bias.
In this study, 8 articles are from China, 5 are from South Korea, 3 are from the United States, and 2 are from Italy. Of the 18 articles, 10 were RFA, 4 were LA, and 4 were MWA. Lymph nodes were evaluated preoperatively, intraoperatively, and postoperatively by conventional ultrasonography or Contrast-Enhanced Ultrasonography (CEUS), and CEUS was used to evaluate and guide ablation in 10 articles (Table 1).
LNM Volume Change and VRR
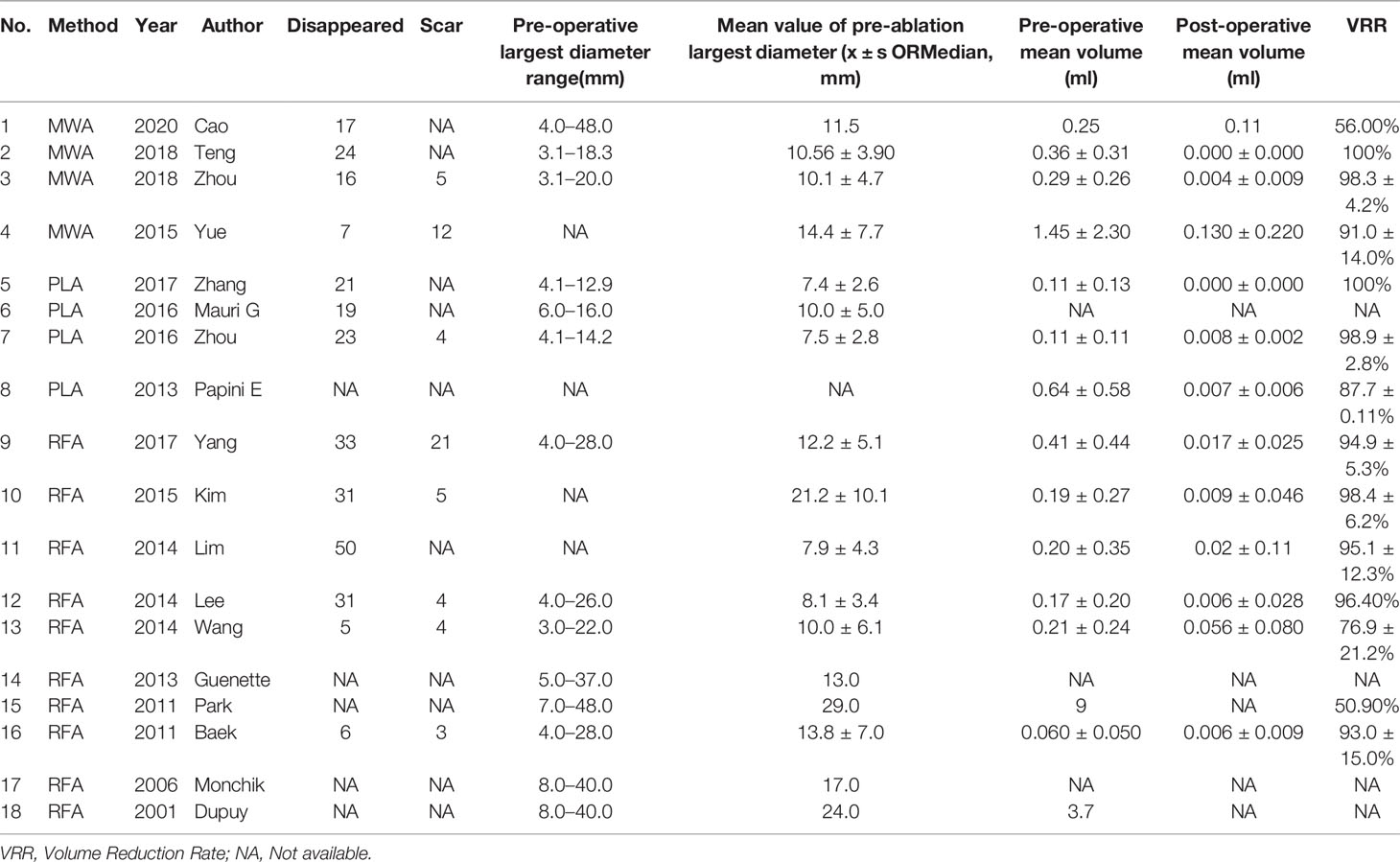
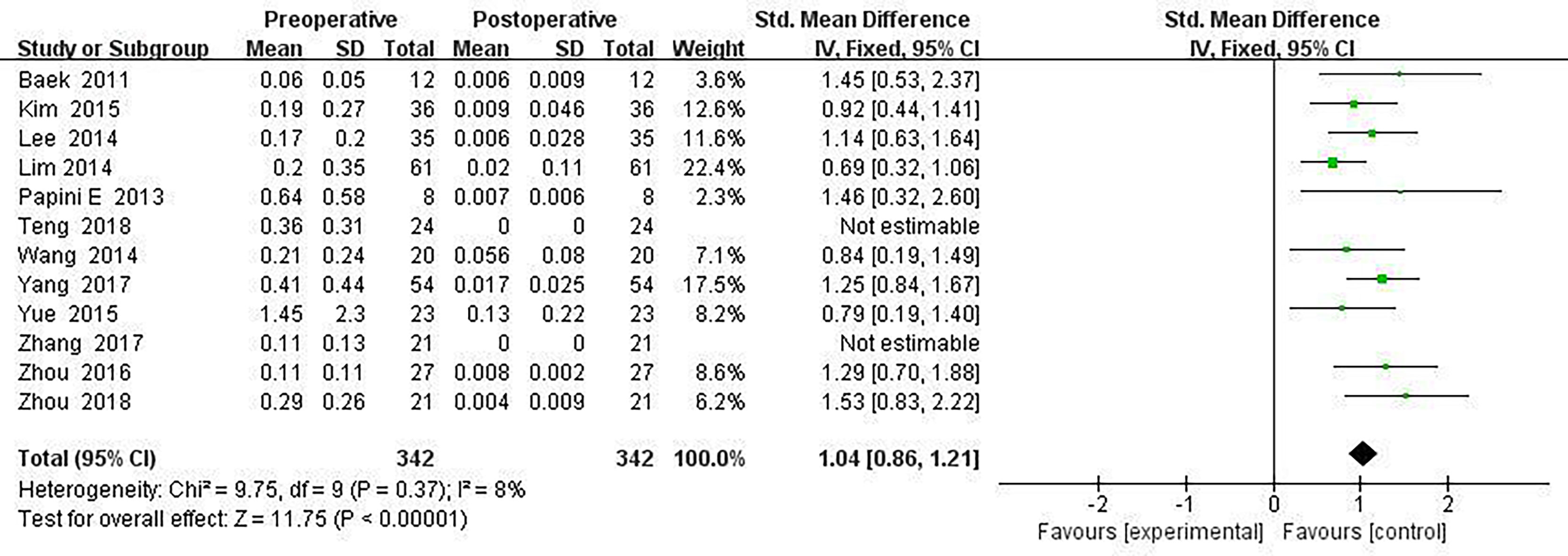
All included ablated lymph nodes, whose pre-operative largest diameter ranged from 3.0 to 48.0 mm, had a minimum volume of 0.060 ± 0.05 ml and the largest volume of 1.45 ± 2.30 ml, in Table 2. Fourteen articles recorded the volume change before and the last follow-up nodules after thermal ablation, with a statistical difference (SMD = 1.04, I2 = 8%, 95% CI = 0.86–1.21, P <0.0001), see Figure 2. Twelve articles analyzed the postoperative lymph node VRR, and the postoperative summary node VRR was 88.4% (95% CI was 77.8–97.3%, I2 was 34%, P = 0.14), in Figure 3.
Tg Change and Low Level of Tg as Efficacy Indicators
Twelve articles mentioned Tg measurements before and after thermal ablation, and Tg level at the end of the last follow-up compared with before surgery (SMD = 1.15, 95% CI was 0.69–1.60, I2 = 84%, P <0.0001) in Figure 4.
Complication Rate
Eighteen articles mentioned both the ablation process and postoperative complications. The total complication rate in all patients was 5.0% (95% CI 3.0–7.0%, I2 = 0.0%, P = 0.915) in Figure 5. The major complication rate was 4.0% (95% CI 2.0–6.0%, I2 = 0.0%, P = 0.888) in Figure 6. Seventeen articles documented the site of the LNM, with 131 located in the central area (Table 3). The major complication rate after LNM ablation in the central region was 12.0% (95% CI 6.0–18.0%, I2 = 0.0%, P = 0.653) in Figure 7.
Discussion
In view of the difficulty of reoperation, the minimally invasive thermal ablation technique can locally control these small lesions and become a new method for treating of LNM of recurrent PTC. The results of this study indicate that thermal ablation is safe and effective for papillary thyroid cancer LNM. The LNM volume decreased significantly after thermal ablation, the Tg levels were relatively low, and the total complication rate was controlled at a lower level. However, the incidence of major complications after LNM ablation in central areas is still at a high level, and thermal ablation of LNM in central areas requires a skilled team and multidisciplinary cooperation.
Thermal ablation of LNM can be used as a safe and effective alternative treatment for high-risk patients or patients who reject surgery. VRR is often used as the efficacy index of ablation treatment. The definition of treatment success is that the tumor volume is reduced by more than 50% (17, 18). Through long-term follow-up, the volume reduction rate of the whole study sequence was greater than 50%, and some studies even reached 98.4% ± 6.2. Among them, 283 (283/498) lymph nodes disappeared completely, and the remaining lymph nodes showed a small scar like tissue. The overall volume reduction rate was between 50 and 80%, and there was a significant difference in the volume change (SMD = 1.04, I2 = 8%, 95% CI 0.86–1.21, P <0.0001). No new recurrent lesions were found in most cases of residual tissue puncture. It can be seen that thermal ablation is an effective method for LNM of PTC (19–21). Simultaneously, thermal ablation can also be used for palliative treatment, which can reduce the compression of LNM on the surrounding tissues (22–24).
In patients with PTC, serum Tg levels are an effective predictor of metastasis and recurrence. In the study of thermal ablation treatment, the serum Tg level has been proven to be an effective index to evaluate the ablation effect (11, 25, 26). The serum thyroid hormone protein level of patients with effective treatment will decrease and be controlled at a lower level. However, this test is less effective in patients who take levothyroxine sodium and have suppressed thyroid stimulating hormone levels, in patients with partial thyroidectomy, and in patients with bone or lung metastases (21). In this study, 12 papers measured the level of Tg as the curative effect index, and there was a significant difference between preoperative and postoperative (SMD = 1.15, 95% CI 0.69–1.60, I2 = 84%, P <0.0001). Stimulated Tg measurement can improve the sensitivity of local recurrence and metastasis. T4 withdrawal is an effective method to obtain a stimulated Tg measurement, but patients often cannot tolerate it because of hypothyroidism. Therefore, postoperative thyroglobulin monitoring and ultrasonography are necessary means to detect potential lymph node metastasis (10, 27).
In the current study, compared with surgery, thermal ablation has proven to be a safe method for the treatment of LNM of PTC. Nerve injury and skin burns are serious complications. A total of 321 patients with 498 lymph nodes were included in this study, of which 131 were located in the central area or thyroid bed, and 20 had hoarseness caused by nerve injury. The incidence of injury was 6.23% (20/321), of which 16 recovered within 3 months and 4 injuries were irreversible. Rocke et al. (28) studied 2,743 patients with total thyroidectomy accompanied by unilateral cervical lymph node dissection and found that 8.3% of the patients had vocal cord paralysis, 1.2% of the patients needed tracheotomy, and 30.5% had parathyroidism. The total complication rate of thermal ablation patients was 5.0% (95% CI 3.0–7.0%, I2 = 0.0%, P = 0.915), which was lower than that caused by surgery. Compared with surgical thermal ablation, it was reliable.
Nevertheless, the incidence of complications caused by thermal ablation of central LNM was 12.0% (95% CI 6.0–18.0%, I2 = 0.0%, P = 0.653). Among the 20 patients with nerve injury, 19 cases were located in the central area, 1 case was located in the superior lateral area of the clavicle, the other 4 cases had skin burns, and 1 case had hematoma. Almost all the serious complications came from the central area. In the study by Lee et al. (27), ablation was performed on 35 lymph nodes of 32 patients, including 26 in the central area. After the operation, 6 cases had voice changes, 5 cases recovered within 3 months, and 1 case did not recover. These cases are located in the central region. Therefore, the ablation of lymph nodes in the central region should be handled with caution. Guenette et al. (22) ablated a lymph node located in the superior lateral chamber of the clavicle, resulting in permanent vocal cord paralysis. Water isolation technology may not be effective for all patients. Some patients even find it difficult to implement water isolation technology due to changes in anatomical structure. A team with extensive ablation experience is a key factor in reducing postoperative complications (29, 30). How to control the complications caused by central LNM ablation is still the direction of future research.
The main method to avoid nerve thermal injury is to inject normal saline or mixed solution between the ablation target lymph nodes, nerves, and surrounding important organs before ablation. Especially for patients with a safe distance of less than 0.5 cm, inject isolation fluid to make the distance between the target lymph node and the key structure of the neck reach a safe distance of at least 1 cm, which can effectively separate the lymph node from the surrounding important tissues to protect the surrounding organs and tissues from thermal damage. However, when the lesion adheres to the surrounding tissue, the risk of thermal ablation injury is also much higher. If the adhesion is serious, it is even impossible to implement water isolation technology, especially when ablating the central lymph nodes (21). Repeated ablation with low power and short time ensures a high local temperature and a low ambient temperature, further ensuring safety. The deployment of energy can be better controlled by low power or by prolonging the ablation time to reduce the risk of burns (26, 27, 31). Park et al. (32) believe that sedatives are not used even for unbearable pain because during ablation, patients can prevent nerve damage by keeping awake and communicating orally.
Skin burns can be cured by local medication in a short time. Dupuy et al. (33) stated in the study that the location of the target lymph node was superficial, and the thermal action range of the ablation needle was not accurately calculated. The hot part of the electrode contacted the skin, and the wound healed after 2 weeks of treatment with local antibiotics (26, 29). The pain and neck swelling during and after ablation can basically disappear within a few hours or a week after the operation by reducing the ablation power or stopping for a few seconds and waiting for a period of time (29). Surgically induced hypoparathyroidism did not occur in ablation patients. It can be seen that thermal ablation technology is relatively safe in the treatment of cervical lymph node metastasis of thyroid papillary carcinoma.
CEUS can show the changes of microvessels after thermal ablation and has become an effective method to evaluate local control of the tumor (34, 35). It is of great significance to evaluate the local treatment effect as soon as possible after thermal ablation treatment, especially when the LNM is large or close to important tissues, so as to find the residual tumor tissue in time and carry out re-ablation treatment (11, 25, 31, 36). The enhancement of residual areas in lymph nodes is considered to be incomplete ablation. The edge of some burned areas is not obvious during routine ultrasonography detection, but the edge can be clearly displayed with the help of CEUS (37–39). Therefore, CEUS plays an important role in monitoring ablation efficacy and postoperative follow-up (40–41). However, Zhang et al. (11) found that 47% of the ablated lymph nodes had larger CEUS perfusion defect volume on the 7th day after ablation than on the 1st day after ablation, indicating that the defect volume assessed by CEUS 1 h after ablation may not represent the final necrosis range. This difference may be the further expansion of the necrotic area because the outermost layer of ablated tissue has hematoma and edema after thermal ablation, which may be followed by the aggregation of inflammatory cells and inflammatory secretory mediators, promoting platelet aggregation and accelerating the release of inflammatory cytokines and tumor necrosis factors. These effects initiate the coagulation cascade and promote micro-thrombosis, which may aggravate tissue ischemia and further expand the necrotic area (42, 43). CEUS is still an effective method for immediate evaluation of ablation range and follow-up of ablation efficacy.
There are still some limitations to this study: (a) 8 of the 18 pieces of literature included are from China, which may be biased by the number of regional publications. (b) The current study is mainly a prospective cohort study, which is lower than that of randomized controlled trials. This requires further research and demonstration in the future. (c) All ablation treated lymph nodes are highly differentiated, and the size, distribution, and proportion of internal calcification are not described in detail. (d) The occult recurrent tumors that cannot be seen on the ultrasonography image cannot be cured by ablation, and the parts that are difficult or cannot be displayed on the ultrasonography image, such as the recurrent tumors in the pharynx and upper mediastinum, cannot be ablated. (e) The experience of operators and the evaluation of the ablation range during treatment are subjective, and there is no unified standard for the consistency of thermal ablation technical parameters. (f) In this study, some smaller lymph nodes were ablated, but these lymph nodes may be inert, and the impact on survival is not known. Consequently, a longer follow-up time and a larger sample size are required to verify their effectiveness.
Conclusion
In summary, ultrasonography-guided thermal ablation for LNM is deemed a safe, viable, and effective minimally invasive treatment. At present, the research sample size is still restricted by the limitations of research methods. Further research is needed on the control of ablation complications of LNM in the central region, but ultrasonography-guided thermal ablation is still a promising treatment method, especially for patients with high surgical risk or refusing cooperation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
XZ was responsible for project administration, methodology, data creation, writing—review, and editing. TN, WZ: data curation and paper revision. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by funding from the Hangzhou Medical and Health Science and Technology Plan Project (A20220044), the Hangzhou Agriculture and Social Development Research Project (20190101A09), and the Hangzhou Science and Technology Plan Guidance Project (20201231Y033).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author thanked many additional members of the Affiliated Hangzhou Chest Hospital of Zhejiang University School of Medicine Department of ultrasound teams who helped with enrollment and evaluation of participants, and finally, the participants themselves.
Abbreviations
PTC, Papillary thyroid carcinoma; LNM, Lymph node metastasis; VRR, Volume reduction rate;, Tg, Thyroglobulin; RFA, Radiofrequency ablation; LA, Laser ablation; MWA, Microwave ablation; CEUS, Contrast-Enhanced Ultrasonography; PMC, PubMed Central.
References
1. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol (2022) 33:27–63. doi: 10.1007/s12022-022-09707-3
2. Shi Y, Yang Z, Heng Y, Ju H, Pan Y, Zhang Y. Clinicopathological Findings Associated With Cervical Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Retrospective Study in China. Cancer Control (2022) 29:1389464322. doi: 10.1177/10732748221084926
3. Xue T, Liu C, Liu JJ, Hao YH, Shi YP, Zhang XX, et al. Analysis of the Relevance of the Ultrasonographic Features of Papillary Thyroid Carcinoma and Cervical Lymph Node Metastasis on Conventional and Contrast-Enhanced Ultrasonography. Front Oncol (2021) 11:794399. doi: 10.3389/fonc.2021.794399
4. Baldini E, Tuccilli C, Pironi D, Catania A, Tartaglia F, Di Matteo FM, et al. Expression and Clinical Utility of Transcription Factors Involved in Epithelial–Mesenchymal Transition During Thyroid Cancer Progression. J Clin Med (2021) 10:4076. doi: 10.3390/jcm10184076
5. Agrawal N, Akbani R, Aksoy BA, Ally A, Arachchi H, Asa SL, et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
7. Shin JE, Baek JH, Lee JH. Radiofrequency and Ethanol Ablation for the Treatment of Recurrent Thyroid Cancers: Current Status and Challenges. Curr Opin Oncol (2013) 25:14–9. doi: 10.1097/CCO.0b013e32835a583d
8. Yoo R, Kim J, Paeng JC, Park YJ. Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancer Presenting as a Metastatic Lymph Node With Dense Macrocalcification: A Case Report and Literature Review. Medicine (2018) 97:e3. doi: 10.1097/MD.0000000000010003
9. Guang Y, Luo YK, Zhang Y, Zhang MB, Li N, Zhang Y, et al. Ultrasound-Guided Radiofrequency Ablation for Cervical Lymph Nodes Metastasis From Papillary Thyroid Carcinoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao (2018) 40:67–71. doi: 10.3881/j.issn.1000-503X.2018.01.010
10. Cao X, Wei Y, Zhao Z, Peng L, Li Y, Yu M. Efficacy and Safety of Microwave Ablation for Cervical Metastatic Lymph Nodes Arising Post Resection of Papillary Thyroid Carcinoma: A Retrospective Study. Int J Hyperther (2020) 37:450–5. doi: 10.1080/02656736.2020.1759829
11. Zhang L, Zhou W, Zhan W. Role of Ultrasound in the Assessment of Percutaneous Laser Ablation of Cervical Metastatic Lymph Nodes From Thyroid Carcinoma. Acta Radiol (2017) 59:434–40. doi: 10.1177/0284185117721261
12. Chu KF, Dupuy DE. Thermal Ablation of Tumours: Biological Mechanisms and Advances in Therapy. Nat Rev Cancer (2014) 14:199–208. doi: 10.1038/nrc3672
13. Chen J, Cao J, Qiu F, Huang P. The Efficacy and the Safety of Ultrasound-Guided Ablation Therapy for Treating Papillary Thyroid Microcarcinoma. J Cancer (2019) 10:5272–82. doi: 10.7150/jca.36289
14. Cabanillas ME, McFadden DG, Durante C. Thyroid Cancer. Lancet (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
15. Ferrara V, Buonomenna C, Mauri G. Image-Guided Ablations in Patients With Thyroid Tumors. J Cancer Res Clin Oncol (2017) 143:2637–9. doi: 10.1007/s00432-017-2503-6
16. Cook DA, Reed DA. Appraising the Quality of Medical Education Research Methods: The Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med (2015) 90:1067–76. doi: 10.1097/ACM.0000000000000786
17. Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional Control of Metastatic Well-Differentiated Thyroid Cancer by Ultrasound-Guided Radiofrequency Ablation. Am J roentgenology (1976) 2011(197):W331.
18. Yue W, Chen L, Wang S, Yu S. Locoregional Control of Recurrent Papillary Thyroid Carcinoma by Ultrasound-Guided Percutaneous Microwave Ablation: A Prospective Study. Int J Hyperther (2015) 31:403–8. doi: 10.3109/02656736.2015.1014433
19. Papini E, Bizzarri G, Bianchini A, Valle D, Misischi I, Guglielmi R, et al. Percutaneous Ultrasound-Guided Laser Ablation Is Effective for Treating Selected Nodal Metastases in Papillary Thyroid Cancer. J Clin Endocrinol Metab (2013) 98:E92–7. doi: 10.1210/jc.2012-2991
20. Kim JH, Yoo WS, Park YJ, Park DJ, Yun TJ, Choi SH, et al. Efficacy and Safety of Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancers Smaller than 2 cm. Radiology (2015) 276:909–18. doi: 10.1148/radiol.15140079
21. Zhou W, Chen Y, Zhang L, Ni X, Xu S, Zhan W. Percutaneous Microwave Ablation of Metastatic Lymph Nodes from Papillary Thyroid Carcinoma: Preliminary results. World J Surg (2019) 43:1029–37. doi: 10.1007/s00268-018-04879-8
22. Guenette JP, Monchik JM, Dupuy DE. Image-Guided Ablation of Postsurgical Locoregional Recurrence of Biopsy-Proven Well-Differentiated Thyroid carcinoma. J Vasc Interv Radiol (2013) 24:672–9. doi: 10.1016/j.jvir.2013.02.001
23. Lim HK, Baek JH, Lee JH, Kim WB, Kim TY, Shong YK, et al. Efficacy and Safety of Radiofrequency Ablation for Treating Locoregional Recurrence from Papillary Thyroid Cancer. Eur Radiol (2015) 25:163–70. doi: 10.1007/s00330-014-3405-5
24. Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. Radiofrequency Ablation and Percutaneous Ethanol Injection Treatment for Recurrent Local and Distant Well-Differentiated Thyroid Carcinoma. Ann Surg (2006) 244:296–304. doi: 10.1097/01.sla.0000217685.85467.2d
25. Mauri G, Cova L, Ierace T, Baroli A, Di Mauro E, Pacella CM, et al. Treatment of Metastatic Lymph Nodes in the Neck from Papillary Thyroid Carcinoma With Percutaneous Laser Ablation. Cardiovasc Inter Rad (2016) 39:1023–30. doi: 10.1007/s00270-016-1313-6
26. Teng D, Ding L, Wang Y, Liu C, Xia Y, Wang H. Safety and Efficiency of Ultrasound-Guided Low Power Microwave Ablation In the Treatment of Cervical Metastatic Lymph Node from Papillary Thyroid Carcinoma: A Mean of 32 Months Follow-Up Study. Endocrine (2018) 62:648–54. doi: 10.1007/s12020-018-1711-4
27. Lee SJ, Jung SL, Kim BS, Ahn KJ, Choi HS, Lim DJ, et al. Radiofrequency Ablation to Treat Loco-Regional Recurrence of Well-Differentiated Thyroid Carcinoma. Korean J Radiol (2014) 15:817. doi: 10.3348/kjr.2014.15.6.817
28. Rocke DJ, Mulder H, Cyr D, Kahmke R, Lee WT, Puscas L, et al. The Effect of Lateral Neck Dissection on Complication Rate for Total Thyroidectomy. Am J Otolaryngol (2020) 41:102421. doi: 10.1016/j.amjoto.2020.102421
29. Wang L, Ge M, Xu D, Chen L, Qian C, Shi K, et al. Ultrasonography-Guided Percutaneous Radiofrequency Ablation for Cervical Lymph Node Metastasis from Thyroid Carcinoma. J Cancer Res Ther (2014) 10 Suppl:C144–9. doi: 10.4103/0973-1482.145844
30. Radzina M, Cantisani V, Rauda M, Nielsen MB, Ewertsen C, D'Ambrosio F, et al. Update on the Role of Ultrasound Guided Radiofrequency Ablation for Thyroid Nodule Treatment. Int J Surg (2017) 41:S82–93. doi: 10.1016/j.ijsu.2017.02.010
31. Guang Y, Luo Y, Zhang Y, Zhang M, Li N, Zhang Y, et al. Efficacy and Safety of Percutaneous Ultrasound Guided Radiofrequency Ablation for Treating Cervical Metastatic Lymph Nodes from Papillary Thyroid Carcinoma. J Cancer Res Clin (2017) 143:1555–62. doi: 10.1007/s00432-017-2386-6
32. Park KW, Shin JH, Han B, Ko EY, Chung JH. Inoperable Symptomatic Recurrent Thyroid Cancers: Preliminary Result of Radiofrequency Ablation. Ann Surg Oncol (2011) 18:2564–8. doi: 10.1245/s10434-011-1619-1
33. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency Ablation of Regional Recurrence from Well-Differentiated Thyroid Malignancy. Surgery (2001) 130:971–7. doi: 10.1067/msy.2001.118708
34. Chen L, Chen L, Liang Z, Shao Y, Sun X, Liu J. Value of Contrast-Enhanced Ultrasound in the Preoperative Evaluation of Papillary Thyroid Carcinoma Invasiveness. Front Oncol (2021) 11:795302. doi: 10.3389/fonc.2021.795302
35. Fang F, Gong Y, Liao L, Ye F, Zuo Z, Li X, et al. Value of Contrast-Enhanced Ultrasound for Evaluation of Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) (2022) 13:812475. doi: 10.3389/fendo.2022.812475
36. Mauri G, Cova L, Tondolo T, Ierace T, Baroli A, Di Mauro E, et al. Percutaneous Laser Ablation of Metastatic Lymph Nodes in the Neck from Papillary Thyroid Carcinoma: Preliminary Results. J Clin Endocrinol Metab (2013) 98:E1203–7. doi: 10.1210/jc.2013-1140
37. Guang Y, He W, Zhang W, Zhang H, Zhang Y, Wan F. Clinical Study of Ultrasonographic Risk Factors for Central Lymph Node Metastasis of Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) (2021) 12:791970. doi: 10.3389/fendo.2021.791970
38. Li QL, Ma T, Wang ZJ, Huang L, Liu W, Chen M, et al. The Value of Contrast-Enhanced Ultrasound for the Diagnosis of Metastatic Cervical Lymph Nodes of Papillary Thyroid Carcinoma: A Systematic Review and Meta-analysis. J Clin Ultrasound (2022) 50:60–9. doi: 10.1002/jcu.23073
39. Wang Y, Duan Y, Zhou M, Liu J, Lai Q, Ye B, et al. The Diagnostic Value of Thyroglobulin In Fine-Needle Aspiration of Metastatic Lymph Nodes In Patients With Papillary Thyroid Cancer and Its Influential Factors. Surg Oncol (2021) 39:101666. doi: 10.1016/j.suronc.2021.101666
40. Liu Z, Wang R, Zhou J, Zheng Y, Dong Y, Luo T, et al. Ultrasound Lymphatic Imaging for the Diagnosis of Metastatic Central Lymph Nodes In Papillary Thyroid Cancer. Eur Radiol (2021) 31:8458–67. doi: 10.1007/s00330-021-07958-y
41. Wang Y, Nie F, Wang G, Liu T, Dong T, Sun Y. Value of Combining Clinical Factors, Conventional Ultrasound, and Contrast-Enhanced Ultrasound Features in Preoperative Prediction of Central Lymph Node Metastases of Different Sized Papillary Thyroid Carcinomas. Cancer Manag Res (2021) 13:3403–15. doi: 10.2147/CMAR.S299157
42. Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 Define Characteristically Different Intralumenal Crawling and Emigration Patterns for Monocytes and Neutrophils In Situ. J Immunol (2010) 185:7057–66. doi: 10.4049/jimmunol.1001638
Keywords: papillary thyroid cancer (PTC), lymph node metastasis (LNM), ablation, ultrasonography, meta-analysis, radiofrequency ablation (RFA), laser ablation (LA), microwave ablation (MWA)
Citation: Zhang X, Ni T and Zhang W (2022) Ultrasonography-Guided Thermal Ablation for Cervical Lymph Node Metastasis of Recurrent Papillary Thyroid Carcinoma: Is it Superior to Surgical Resection? Front. Endocrinol. 13:907195. doi: 10.3389/fendo.2022.907195
Received: 29 March 2022; Accepted: 16 May 2022;
Published: 27 June 2022.
Edited by:
Eleonora Molinaro, University of Pisa, ItalyReviewed by:
Salvatore Sorrenti, Sapienza University of Rome, ItalyDiMing Cai, Sichuan University, China
Dong Xu, University of Chinese Academy of Sciences, China
Copyright © 2022 Zhang, Ni and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Zhang, ZHIueHVfemhhbmdAcXEuY29t
 Xu Zhang
Xu Zhang Tu Ni
Tu Ni Wenzhi Zhang
Wenzhi Zhang