- 1Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai, China
Background & Aims: Primary hyperparathyroidism(PHPT) has been evolving into a milder asymptomatic disease. No study has assessed the association between famine exposure and such a shift. We aim to explore the effects of China’s Great Famine exposure on the changing pattern of PHPT phenotypes.
Methods: 750 PHPT patients diagnosed from 2000 to 2019 were studied. The clinical presentations were compared between them in recent 10 years (2010-2019) and previous 10 years (2000-2009). Participants were then categorized into fetal, childhood, adolescent, adult exposure, and unexposed groups. Logistic regression was used to estimate the odds ratios (ORs) and confidence intervals (CIs) of famine exposure as factors contributing to the changes in the clinical presentations of PHPT.
Results: Serum levels of PTH, albumin-corrected Ca, tumor size, eGFR, BMDs (all P<0.001), and clinical symptoms became milder in recent 10 years. Famine exposure (72.6% vs 58.4%, P<0.001), especially the adult exposure (18.8% vs 4.1%, P<0.001)was significant less in recent 10 years. The ORs (95%CIs) of having upper 3rd tertile PTH were 2.79(1.34,5.8), 2.07(1.04,4.11), 3.10(1.15,8.38) and 8.85(2.56,30.56) for patients with fetal, childhood, adolescent and adult famine exposure, respectively. The ORs (95%CIs) of upper 3rd tertile albumin-corrected Ca and upper 3rd tertile of tumor size was 4.78(1.39, 16.38) and 4.07(1.12,14.84) for participants with adult famine exposure, respectively. All these associations were independent of age, sex, disease duration and other confounders.
Conclusions: The clinical manifestations of PHPT in China continue to be milder. Exposure to famine is associated with PHPT. Less famine exposure might be responsible for the mile form of PHPT in recent years.
Introduction
Primary hyperparathyroidism (PHPT) is the third largest endocrine disease in the world with the main features of hyperparathyroidism and hypercalcemia (1, 2). The classical symptoms of PHPT involve bone, kidney, gastrointestinal manifestations, such as bone pain, fracture, osteoporosis, urolithiasis, nausea, vomiting, and etc. There are also some non-classical features, including neuropsychiatric, cardiovascular and metabolic abnormities (3).
Since 1970s, the clinical presentations of PHPT have changed around the world, especially in most Western countries and some developing countries in Asia, but not in Africa (4), from a symptomatic disorder to a mild or asymptomatic one (5–9). It was generally accepted that such a change is due to the widely routine measurement of electrolytes including serum calcium levels with automatic biochemical analyzers and neck ultrasonography which incidentally identifies parathyroid lesion(s) (8, 10). However, it is still of interest to ask whether there is any other factor responsible for such a change. Why the mild cases are mostly observed in developed Western countries, and in those developing countries with better social-economic condition, could it be related with nutritional status?
In fact, previous studies have already shown that nutritional status has a profound impact on many diseases. Numerous studies provided evidence that long-term malnutrition, such as famine, is closely related to chronic and metabolic diseases, such as cardiovascular diseases (11), diabetes (12, 13), non-alcoholic fatty liver disease (14),thyroid disorders (15) and osteoporosis (16). It is noteworthy that most such studies investigated the impacts of famine exposure in early life (fetal, childhood, adolescence exposure) on the risk of adulthood diseases, a few is reported on the impact of famine exposure during adulthood (17, 18).
The China’s Great Famine occurred in 1959-1962 (19), which was the world’s largest famine, it provides a unique opportunity to explore the effects of nutritional status on human health and diseases. Therefore, using the data collected from Jan, 2000-Dec, 2019 of PHPT patients in our center, we aimed to explore the changing clinical pattern of PHPT, and most importantly, the impact of famine exposure at different stages of life (fetal, childhood, adolescence and adult) on the changes of clinical phenotypes of PHPT in 20 years. We hypothesized that famine exposure in early and adult life is associated with the biochemical and clinical severity of PHPT.
Materials and Methods
From Jan, 2000 to Dec, 2019, there were 810 cases of PHPT patients in Department of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. However, patients with parathyroid carcinoma (N=60) were excluded. For those with secondary hyperparathyroidism due to vitamin D deficiency, they were managed in the out-patient department; for those with secondary and tertiary hyperparathyroidism caused by renal failure, they were handled by doctors from the Department of Nephrology. Multiple endocrine neoplasia is caused by genetic abnormalities which are pathologically different from sporadic PHPT, and thus is beyond the purpose of the current study. Therefore, these patients were also not included in the current study.
A total of 750 patients with PHPT in our center were enrolled in this study. The diagnosis of parathyroid adenoma was established based on histopathology and immunohistochemistry. A mass was considered an adenoma if it fulfilled all of the following three criteria: 1) absence of fat cells within the mass; 2) absence of a lobular pattern; 3)presence of a well-defined demarcation between it and the surrounding parathyroid tissue with no demonstrable blending between the two (20).
In the current study, we included PHPT patients who were born between 1921 and 1995, and collected their baseline data. Participants were categorized into four exposure-age cohorts that defined as famine exposure in different periods of life: fetal exposure (born between 1959 and 1961, N=78), childhood exposure (exposed at ages 0-9 years, born between 1949 and 1959, N=239), adolescent exposure (exposed at ages 10-17 years, born between 1941 and 1949, N=90), adulthood exposure (exposed at ages over18 years, bore before 1941, N=58) and unexposed (born after 1962, N=285).
This study was approved by the Medical Ethics Committee at the Institutional Review Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Data Collection
All the information pertaining to demographic characteristics, symptoms of PHPT (osteoporosis, bone pain, fracture history, urolithiasis, gastritis and gastric ulcer, nausea and vomiting) and disease duration were recorded. Height was measured to the nearest 0.1 cm, and weight was recorded to the nearest 0.1 kg with the participant wearing light clothing. BMI was calculated as weight in kilograms divided by height in meters squared.
All participants were required to fast for at least 10 hours before blood samples were collected to precede biochemical evaluation. The biochemical blood parameters that were tested included serum concentrations of calcium (Ca), phosphorus (P), albumin (Alb), parathyroid hormone (PTH), 25-hydroxyvitamin D [25(OH)D], creatinine-based glomerular filtration rate (GFR) as estimated GFR (eGFR), 24-hour urinary calcium (24hUCa) and phosphorus (24hUP) excretion. Serum Ca and P were measured using an automatic biochemical analyzer (Modular E170, Roche, Basel, Switzerland). Serum level of PTH was measured by intact immunoradiometric assay (ARCHITECT i2000sr, Abbott, Chicago, IL). The serum 25(OH)D concentration was measured with an electrochemiluminescence immunoassay (Roche Cobas 601)[Vitamin D3(25-OH) kit before 2011, and after that, Vitamin D total kit]. Fasting plasma glucose (FPG) was tested by the glucose oxidase method on an autoanalyzer (Modular P800; Roche, Basel, Switzerland). The albumin-corrected serum calcium level (mmol/L) was calculated using the following formula: albumin-corrected Ca(mmol/L) = measured total serum calcium(mmol/L) + [0.02 × (40 - serum albumin concentration(g/L)]. The formula for the calculation of eGFR is eGFR(ml/(min*1.73m2)=186×(Scr)-1.154×(age)-0.203×(0.742 if Female) (Scr, Serum creatinine).
Bone mineral densities (BMDs) at lumbar spine 1-4 (L1-L4), femoral neck (FN) and total hip (TH) were measured using a dual-energy X-ray absorptiometer (DXA)(Lunar Prodigy; GE Medical Systems, Madison, Wisconsin). Complete DXA data were available for 511 patients. The disease duration was recorded based on the first recognition of symptom(s) related to hyperparathyroidism in symptomatic patients or the first abnormal serum calcium or PTH level discovered by laboratory tests or parathyroid lesion found by neck ultrasonography in asymptomatic patients.
Statistical Analysis
All statistical analyses were carried out with R 4.0.0 software (R Foundation, Vienna, Austria). The Shapiro-Wilk test was used to test whether continuous variables are normally distributed. Continuous variables were represented using the mean ± SD or mean ± SEM as otherwise specified for normally distributed variables and medians with 25th and 75th percentiles for non-normally distributed variables. Categorical variables were represented by frequency (percentage). For normal distribution data, the comparison between the two groups used the independent sample t test, the comparison between more than two groups used ANOVA, and the multiple comparison used Tukey’s fixed gap test (Tukey HSD); if the data was skewed, the Wilcoxon rank sum test was used for comparison, Kruskal-Wallis rank sum test was used for comparison between more than two groups, Holm’s method was used for multiple comparisons; chi-square test was used for comparison between categorical variables groups. Covariance analysis was used to correct confounders between groups. Spearman correlation was used to test the correlation between target variables and related variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by using multivariable adjusted logistic regression model to analyze the associations of famine exposure with PHPT clinical phenotype. The accepted level of statistical significance was p<0.05.
Results
Demographic, Clinical Phenotypes and Famine Exposure in PHPT Patients From 2000-2019
750 patients with PHPT were divided into two groups: group A (187 patients diagnosed from 2000 to 2009) and group B (563 patients diagnosed from 2010 to 2019). The study included 540 females (72.0%) and 210 males (28.0%) with a sex-ratio of female to male 2.57:1. The clinical characteristics of two groups are summarized in Table 1.
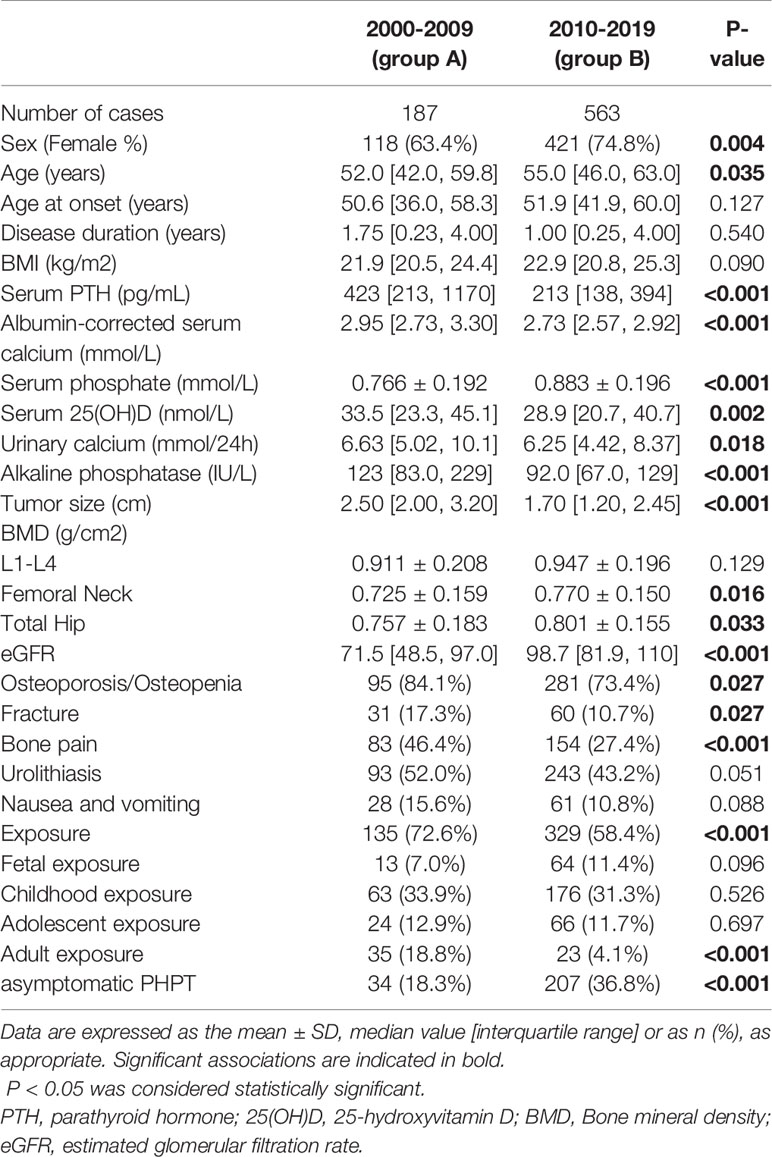
Table 1 Changes over time in demographic, clinical findings and famine exposure in patients with PHPT from 2000-2019.
The mean age of the whole group was 54.0[46.0,62.0] years. Patients in group B had an older age than those in group A (P=0.035). There was no difference in the age at onset and disease duration between two groups. Comparing previous 10 years, there were significant decreases in serum levels of PTH, albumin corrected-Ca and alkaline phosphatase (ALP), as well as tumor size over time (all P<0.001), whilst serum P concentration (P<0.001), eGFR (P<0.001) and BMDs at FN(P=0.016) and TH (P=0.033) increased in recent 10 years.
Changes of clinical manifestations over time included a decrease in the rate of bone and digestive system involvement (Table 1). Compare with group A, osteoporosis/osteopenia was significantly less frequent in group B (73.4% vs. 84.1%; P=0.027). As to fracture, 91 (12.1%) subjects in total developed fragility fractures previously. The declining percentages of fracture were noticed between the two groups (17.3% in group A and 10.7% in group B, P=0.027). The proportion of bone pain witnessed a sharp drop (46.4% in group A vs 27.4% in group B, P<0.001). Nausea and vomiting showed a non-significant decreased incidence in group B (10.8%) as compared with group A(15.6%)(P=0.088). 336 PHPT patients (44.8%) developed urolithiasis at presentation with a marginal significant decrease in recent 10 years (43.2% vs 52.0%, P=0.051). There was an improvement in eGFR over time (P<0.001). The percentages of asymptomatic PHPT were 18.3% in group A (2000-2009) and 36.8% in group B (2010-2019)(P<0.001),respectively.
Also, as shown in Table 1, compared with previous 10 years, the famine exposure in recent 10 years showed a significant decrease from 72.6% to 58.4% (P<0.001), especially the adult exposure from 18.8% to 4.1% (P<0.001).
The Relationship Between Famine Exposure and Clinical Phenotypes of PHPT
As shown in Table 2, compared with the unexposed group, after adjustment for age and sex, serum levels of PTH, albumin-corrected Ca, and ALP in the childhood exposure, adolescent exposure and adult exposure group were significantly increased, while BMDs at L1-L4, FN and TH in these exposure groups were significantly reduced. PHPT patients with fetal exposure also had higher circulating PTH level than unexposed ones. PHPT patients with famine exposure also had more clinical symptoms, such as osteoporosis/osteopenia, fracture history, bone pain, nausea and vomiting, except for urolithiasis. But there was no difference of the percentages of asymptomatic PHPT in famine exposure at different stages of life.
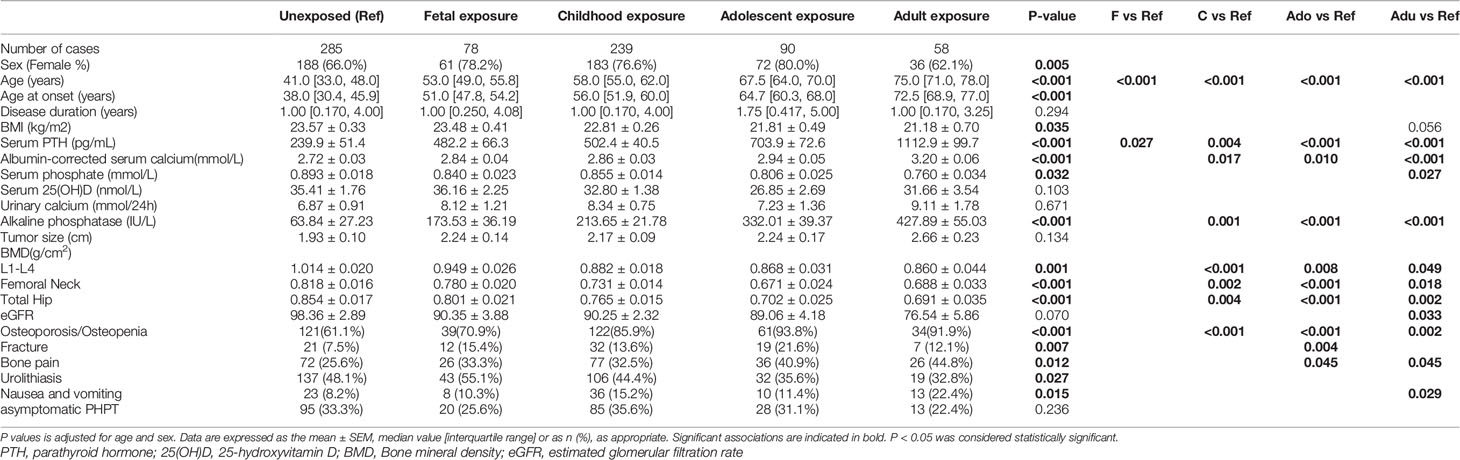
Table 2 The clinical data of primary hyperparathyroidism (PHPT) patients in famine exposure at different stages of life.
For the PHPT related symptoms (Table 3), after adjusting age, gender, BMI, disease duration and eGFR, as compared with non-exposure group, the famine exposure was associated with a higher ORs of having osteoporosis/osteopenia, fracture, bone pain and urolithiasis. The ORs (95%CIs) of osteoporosis/osteopenia were 4.51 (1.97,10.31), 8.04 (2.05,31.49) and 5.71 (1.07,30.42) for participants with childhood (P<0.001), adolescent(P=0.003) and adult famine exposure (P=0.041), respectively. The ORs (95%CIs) of fracture were 3.25 (1.11,9.47), 3.7 (1.28,10.72) and 6.51 (1.53,27.75) for participants with fetal(P=0.031), childhood (P=0.016) and adolescent famine exposure (P=0.011), respectively. The ORs (95%CIs) of bone pain was 4.33 (1.27,14.81) for participants with adult famine exposure(P=0.020). The ORs (95%CIs) of urolithiasis was 1.83 (1.03,3.25) for participants with famine exposure(P=0.038). It was also revealed that famine exposure in childhood and adult was related with more digestive symptoms, such as nausea and vomiting after adjusting age and gender in model 2, however, this association was lost when disease duration and eGFR were further adjusted in model 3.
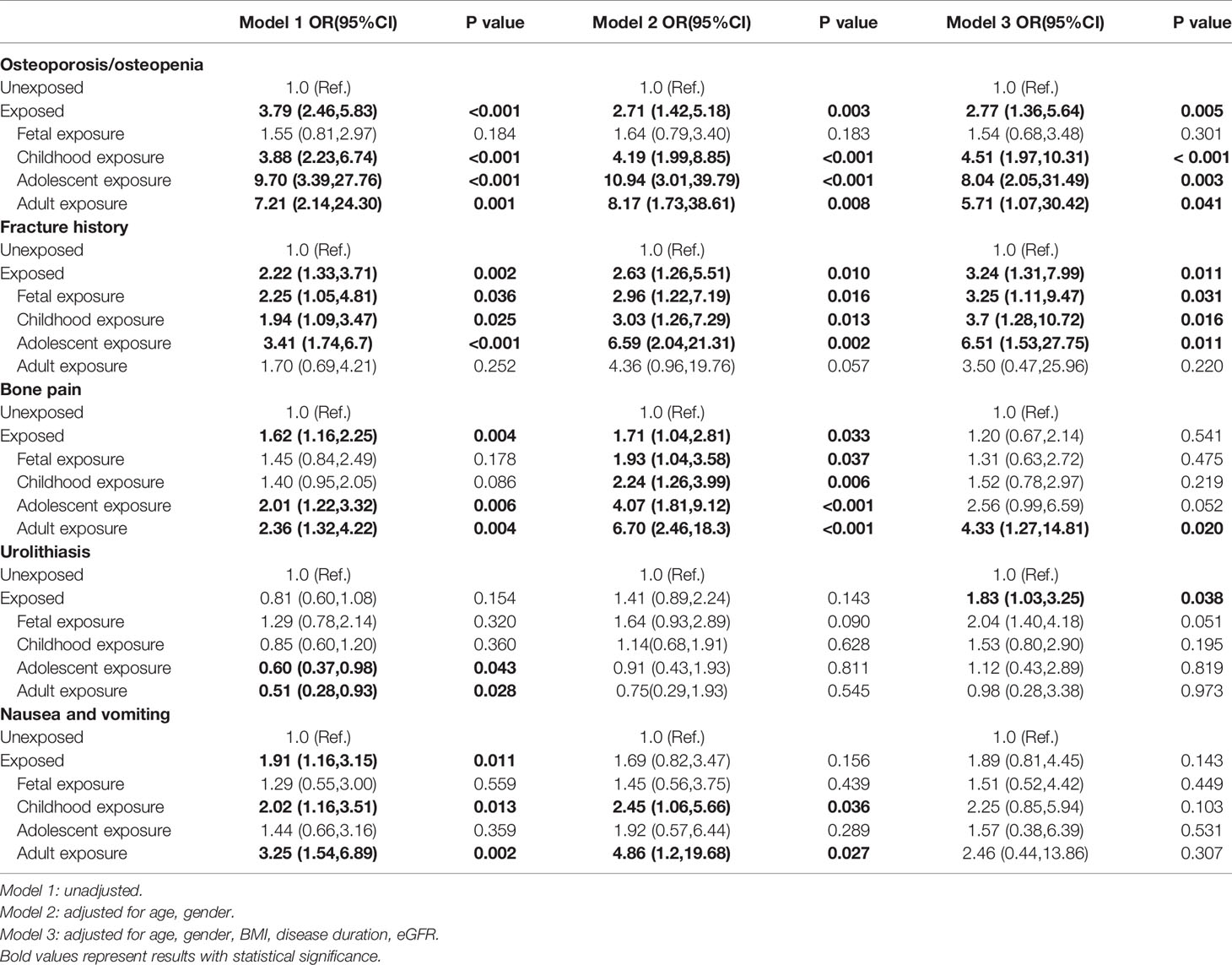
Table 3 ORs (95%CIs) for related symptoms in PHPT patients with famine exposure at different stages of life.
The Relationships Between Famine Exposure and the Severity of PHPT
To evaluate the association between famine exposure and severity of PHPT, patients were subgrouped according to the tertiles of serum PTH, albumin-corrected Ca levels and tumor size. As shown in Table 4, after adjustment for age, sex, BMI, disease duration and eGFR, the ORs (95%CIs) of having upper 3rd tertile PTH were 2.79(1.34,5.8), 2.07(1.04,4.11), 3.10(1.15,8.38) and 8.85(2.56,30.56) for participants with fetal, childhood, adolescent and adult famine exposure, respectively. The ORs (95%CIs) of upper 3rd tertile albumin-corrected Ca was 4.78(1.39, 16.38) for participants with adult famine exposure. The ORs (95%CIs) of upper 3rd tertile of tumor size was 4.07(1.12,14.84) for participants with adult famine exposure.
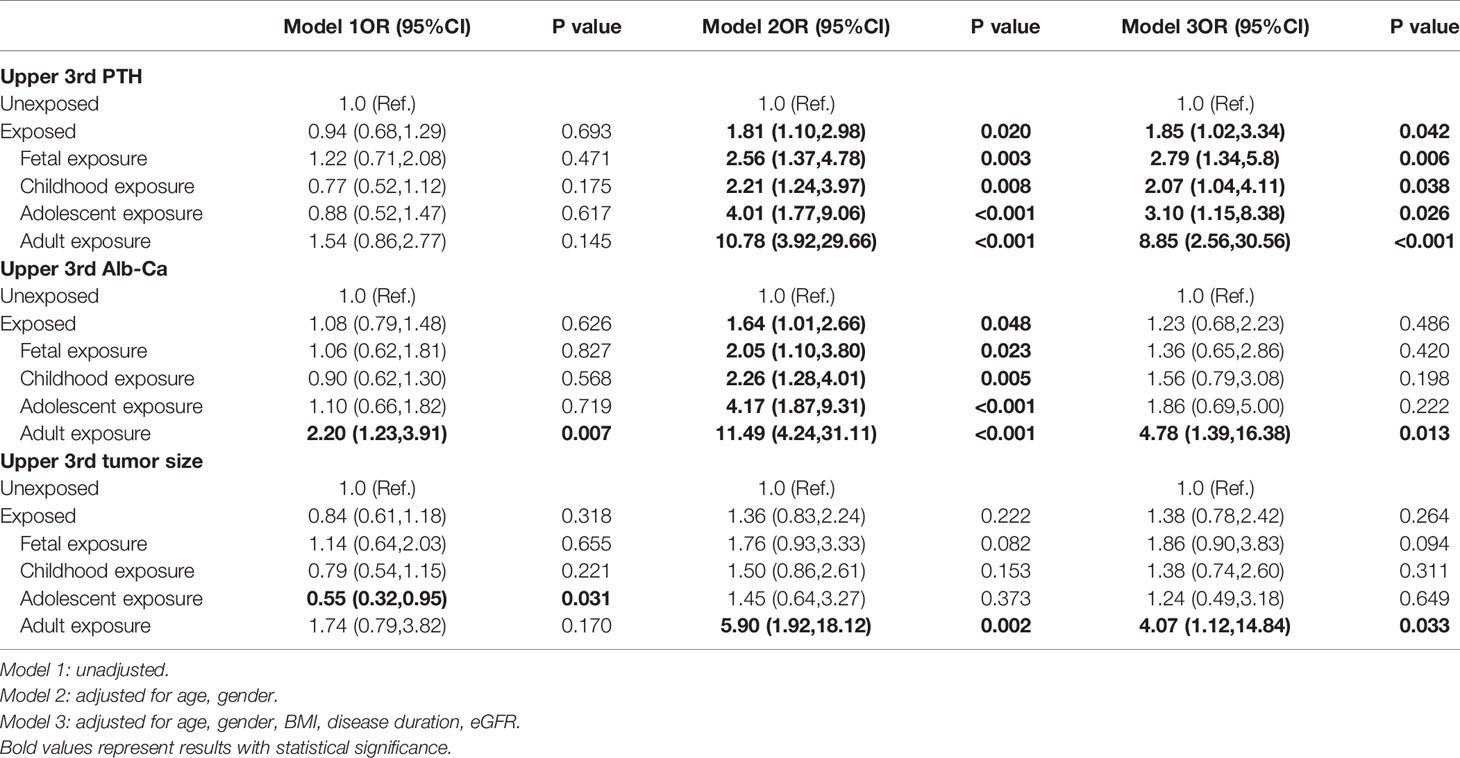
Table 4 ORs (95%CIs) for upper 3rd serum PTH, albumin-corrected calcium levels and tumor size in PHPT patients with famine exposure at different stages of life.
Discussion
The main findings of the study are: 1) The clinical and biochemical manifestations of PHPT in China are trending toward even milder in recent 10 years, and 2) both the early life and adult exposure to famine are associated the changing pattern of PHPT in this 20 years. To the best of our knowledge, this is the first study to evaluate the association between famine exposure and PHPT in adulthood.
In our previous study, we found that PHPT in China was evolving into a mild form from 2000 to 2010 (8). In the current study, such a changing patter continued. Comparing with the previous 10 years from 2000-2009, the biochemical features in PHPT patients, such as serum PTH, albumin-corrected Ca, ALP concentrations all became lower; and tumor size to be smaller; kidney function and BMDs turned to be better in recent 10 years from 2010-2019. Such a picture echoes the most recent findings from another hospital in our city (9) and other areas in Western countries.
Since the major purpose of this study is to evaluate whether famine exposure during early and adult life has anything related with PHPT, we then investigated the rate and type of exposures in different years. It was demonstrated that famine exposure, especially adult exposure was more pronounced in previous 10 years than recent 10 years. Detail analysis further revealed that even with the adjustment of age, gender, eGFR, and other confounders, our study revealed that exposure to famine conferred increased risk of severer PHPT, as evidenced by higher PTH, albumin-corrected Ca levels, lower BMDs, higher risk of osteoporosis/osteopenia, fracture and renal involvement. It was noteworthy that our results seemed to be more prominent in adult exposure group, while the findings from a recent meta-analysis involving 1.6 million subjects showing that early life exposure (fetal, childhood and adolescent) were more prone to develop diabetes as compared with adult exposure (21). The possible explanation for such a difference might be that adequate nutrition during the stage of growth and development is important for metabolic diseases (21), while for a tumor disease like PHPT, in addition to nutrition status, other factors may also play some role (see below for more discussion).
Bone is a continuous remodeling organ with critical bone development during adolescence, and peak bone mass obtained during early adulthood. Sufficient calcium, VitD and protein intake in early life, and even intrauterine or early postnatal life, is crucial for bone accretion (22). Experiencing malnutrition in these periods will compromise the genetic determined bone growth and maturation, leading to low bone mass, compromised bone micro-architecture as well as bone strength, and subsequently higher fracture risk (23). Such an explanation is supported by the evidence that famine exposure in early life is associated with lower BMDs, poorer bone quality as revealed by heal quantitative ultrasound (QUS), and higher risk of vertebral fracture as evidenced by a 2-cm height loss in adulthood (16). Even with the adjustment of calcium intake, age, and other factors, famine exposure in Hong Kong Chinese man and women is associated with decreased DXA measured BMD at FN and a 25% increased risk of osteoporosis (24).
In this study, we found that famine exposure is related with higher odds of being at upper 3rd tertile of serum level of PTH and albumin-corrected Ca, which are considered to be responsible for the symptoms and severity of PHPT. However, it is difficult to explain this phenomenon at mechanism level. In this study, PHPT patients with pathological confirmed adenoma were enrolled. The molecular pathogenesis of sporadic parathyroid adenomatosis is complex (25) not only involving mutations in CCND1/PRAD1, CDC73/HRPT2, MEN1, CaSR (25–27) and several other genes identified by the next-generation sequencing techniques (28) but also involving epigenetic alterations such as DNA methylation and histone modifications in sporadic parathyroid adenoma (29–31). Studies in etiology or mechanism of famine-related tumorigenesis and disease severity are not fully understood. The appropriate mechanism(s) linking genetic alteration, environmental changes and human health and disease might be epigenetics (18, 32) and two-hit hypothesis or multiple-hit model (33–35).
In a human study, it was demonstrated that prenatal famine exposure is associated with the hypomethylation of IGF2 differentially methylated region (DMR) 60 years later (36), and disruption of IGF-2 gene expression may contribute to tumor progression and aggressive phenotype in MEN-1 (37). As a disease of tumor, it would be reasonable to hypothesize that in genetic predisposed individual, experiencing calories deprivation in early life and/or adult life as one hit, together with epigenetic modifications resulting from famine (18, 38), environmental insults related with famine, such as life style changes, alcohol drinking, tobacco smoking (38–41), and economic status (42), will induce cumulative and interactive responses (43) and culminate in the development of a disease or even a tumor. In addition, the accumulation of a series of gene alterations and epigenetic modifications as multiple hits, is not only responsible for the tumorigenesis but also the functionality of the tumor, the aggressiveness of the clinical course, and the recurrence of the tumor, even independent of histopathology score (39, 44–48). Although these findings are derived from other tumors, such as gastric cancer (39, 44, 45) and adrenocortical carcinoma (46–48), such hypothesis is still suggestive for this study and should be investigated in detail in clinical and basic researches of PHPT.
PHPT is also a disease of aberrant set-point for PTH secretion with the curve of inverse relationship between PTH release and calcium level shifts to the right (49). Previously, it was reported that prenatal nutrition deprivation may result in in utero resetting the hypothalamus-pituitary-adrenal axis by a so called fetal reprograming mechanism, and leading to overproduction of cortisol, however, this was not confirmed in a later experiment at least at adrenal level (50). Nevertheless, the role of famine in dysregulation of set-point of PTH secretion could also be investigated.
It could be argued that in this study, age rather than famine itself is a contributing factor. It is expected that age was definitely different among various exposure groups from fetal to adult exposure. It was reported that in PHPT, serum calcium level is more closely related with cell number, tumor volume and serum PTH concentration, while age and sex are not contributing factors (51). In our analysis, age and disease duration were adjusted, and the results were still supportive the relationship between famine exposure and PHPT biochemical as well as clinical manifestations.
If the famine exposure is finally proved to be true to explain the occurrence and severity of PHPT, then it could be used to explain the changing pattern of PHPT in this study. In our cohort, significantly less proportion of PHPT patients in recent 10 years experienced famine than that of previously 10 years. The fact that there was a more than 19.6% decrease of exposed group in recent 10 years from 72.6% to 58.4%, and 78.2% relative reduction in adult exposure from 18.8% to 4.1%, which accounted for most of the severer clinical and laboratory findings of PHPT independent of age, disease duration, eGFR and other confounders should not be neglected and might be a factor responsible for the continuous milder form of PHPT in recent 10 years.
Our study has some limitations. First, we did not perform an analysis on the effects of famine severity, duration and geographical distribution on the phenotypes and severity of PHPT, we did not record the regions where our patients were living during the famine period. Second, we could not distinguish fetal exposed from infant exposed group, since some individuals experienced famine during both fetal period and infancy. Third, famine length might not be consistent across all included patients, which may influence the stability of our results. Fourth, as mentioned in the Method, the methodologies for the measurement of serum 25(OH)D concentration in the two periods were different and thus not comparable. Using more accurate method, such as Liquid Chromatography (LC)-Mass Spectroscopy (MS)/MS, may reflect the real Vitamin D status in human bodies.
In summary, the results from the current study demonstrate that PHPT in Chinese patients are still changing and become milder. Exposure to China’ Great Famine during early life and adult life is associated with clinical presentations of PHPT, and might be another explanation for the milder form of PHPT observed in recent years in Chinese patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ruijin Hospital Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Study design – J-mL, BT, and T-jY. Data extraction and analysis – T-jY. Writing of the manuscript – All authors. Critical review – J-mL, BT and L-hS. Final approval – All authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary Hyperparathyroidism. Nat Rev Dis Primers (2016) 19:16033. doi: 10.1038/nrdp.2016.33
2. Bilezikian JP. Primary Hyperparathyroidism. J Clin Endocrinol Metab (2018) 103:3993–4004. doi: 10.1210/jc.2018-01225
3. Wang SM, He Y, Zhu MT, Tao B, Zhao HY, Sun LH, et al. The Associations of Serum Osteocalcin and Cortisol Levels With the Psychological Performance in Primary Hyperparathyroidism Patients. Front Endocrinol (Lausanne) (2021) 12:692722. doi: 10.3389/fendo.2021.692722
4. Paruk IM, Esterhuizen TM, Maharaj S, Pirie FJ, Motala AA. Characteristics, Management and Outcome of Primary Hyperparathyroidism in South Africa: A Single-Centre Experience. Postgrad Med J (2013) 89:626–31. doi: 10.1136/postgradmedj-2012-131707
5. Pallan S, Rahman MO, Khan AA. Diagnosis and Management of Primary Hyperparathyroidism. BMJ (2012) 19:e1013. doi: 10.1136/bmj.e1013
6. Lowe H, McMahon DJ, Rubin MR, Bilezikian JP, Silverberg SJ. Normocalcemic Primary Hyperparathyroidism: Further Characterization of a New Clinical Phenotype. J Clin Endocrinol Metab (2007) 92:3001–5. doi: 10.1210/jc.2006-2802
7. Yu N, Donnan PT, Murphy MJ, Leese GP. Epidemiology of Primary Hyperparathyroidism in Tayside, Scotland, Uk. Clin Endocrinol (Oxf) (2009) 71:485–93. doi: 10.1111/j.1365-2265.2008.03520.x
8. Zhao L, Liu JM, He XY, Zhao HY, Sun LH, Tao B, et al. The Changing Clinical Patterns of Primary Hyperparathyroidism in Chinese Patients: Data From 2000 to 2010 in a Single Clinical Center. J Clin Endocrinol Metab (2013) 98:721–8. doi: 10.1210/jc.2012-2914
9. Lin X, Fan Y, Zhang Z, Yue H. Clinical Characteristics of Primary Hyperparathyroidism: 15-Year Experience of 457 Patients in a Single Center in China. Front Endocrinol (Lausanne) (2021) 12:602221. doi: 10.3389/fendo.2021.602221
10. Zhu CY, Sturgeon C, Yeh MW. Diagnosis and Management of Primary Hyperparathyroidism. JAMA (2020) 323:1186–7. doi: 10.1001/jama.2020.0538
11. van Abeelen AF, Elias SG, Bossuyt PM, Grobbee DE, van der Schouw YT, Roseboom TJ, et al. Cardiovascular Consequences of Famine in the Young. Eur Heart J (2012) 33:538–45. doi: 10.1093/eurheartj/ehr228
12. Botden IP, Zillikens MC, de Rooij SR, Langendonk JG, Danser AH, Sijbrands EJ, et al. Variants in the SIRT1 Gene may Affect Diabetes Risk in Interaction With Prenatal Exposure to Famine. Diabetes Care (2012) 35:424–6. doi: 10.2337/dc11-1203
13. Shi Z, Ji L, Ma RCW, Zimmet P. Early Life Exposure to 1959-1961 Chinese Famine Exacerbates Association Between Diabetes and Cardiovascular Disease. J Diabetes (2020) 12(2):134–41. doi: 10.1111/1753-0407.12975
14. Qi H, Hu C, Wang S, Zhang Y, Du R, Zhang J, et al. Early Life Famine Exposure, Adulthood Obesity Patterns and the Risk of Nonalcoholic Fatty Liver Disease. Liver Int (2020) 40:2694–705. doi: 10.1111/liv.14572
15. Guo J, Teng D, Shi X, Li Y, Ba J, Chen B, et al. Exposure to the Chinese Great Famine in Early Life and Thyroid Function and Disorders in Adulthood: A Cross-Sectional Study. Thyroid (2021) 31:563–71. doi: 10.1089/thy.2020.0325
16. Zong L, Cai L, Liang J, Lin W, Yao J, Huang H, et al. Exposure To Famine In Early Life And The Risk Of Osteoporosis In Adulthood: A Prospective Study. Endocr Pract (2019) 25:299–305. doi: 10.4158/EP-2018-0419
17. Schouten LJ, van Dijk BA, Lumey LH, Goldbohm RA, van den Brandt PA. Energy Restriction During Childhood and Early Adulthood and Ovarian Cancer Risk. PLoS One (2011) 6:e27960. doi: 10.1371/journal.pone.0027960
18. Hughes LA, van den Brandt PA, de Bruïne AP, Wouters KA, Hulsmans S, Spiertz A, et al. Early Life Exposure to Famine and Colorectal Cancer Risk: A Role for Epigenetic Mechanisms. PLoS One (2009) 4:e7951. doi: 10.1371/journal.pone.0007951
19. Lin JY, Yang DT. Food Availability, Entitlements and the Chinese Famine of 1959–61. Economic J (2000) 110:136–58.doi: 10.1111/1468-0297.00494
20. Ghandur-Mnaymneh L, Kimura N. The Parathyroid Adenoma. A Histopathologic Definition With a Study of 172 Cases of Primary Hyperparathyroidism. Am J Pathol (1984) 115(1):70–83.
21. Liu H, Chen X, Shi T, Qu G, Zhao T, Xuan K, et al. Association of Famine Exposure With the Risk of Type 2 Diabetes: A Meta-Analysis. Clin Nutr (2020) 39:1717–23. doi: 10.1016/j.clnu.2019.08.002
22. Cooper C, Fall C, Egger P, Hobbs R, Eastell R, Barker D. Growth in Infancy and Bone Mass in Later Life. Ann Rheum Dis (1997) 56:17–21. doi: 10.1136/ard.56.1.17
23. Kueper J, Beyth S, Liebergall M, Kaplan L, Schroeder JE. Evidence for the Adverse Effect of Starvation on Bone Quality: A Review of the Literature. Int J Endocrinol (2015) 2015:628740. doi: 10.1155/2015/628740
24. Kin CF, Shan WS, Shun LJ, Chung LP, Jean W. Experience of Famine and Bone Health in Post-Menopausal Women. Int J Epidemiol (2007) 36:1143–50. doi: 10.1093/ije/dym149
25. Arnold A, Shattuck TM, Mallya SM, Krebs LJ, Costa J, Gallagher J, et al. Molecular Pathogenesis of Primary Hyperparathyroidism. J Bone Miner Res (2002) 17:N30–6.
26. Hong YA, Park KC, Kim BK, Lee J, Sun WY, Sul HJ, et al. Analyzing Genetic Differences Between Sporadic Primary and Secondary/Tertiary Hyperparathyroidism by Targeted Next-Generation Panel Sequencing. Endocr Pathol (2021) 32:501–12. doi: 10.1007/s12022-021-09686-x
27. Zhao L, Sun LH, Liu DM, He XY, Tao B, Ning G, et al. Copy Number Variation in CCND1 Gene is Implicated in the Pathogenesis of Sporadic Parathyroid Carcinoma. World J Surg (2014) 38:1730–7. doi: 10.1007/s00268-014-2455-9
28. Juhlin CC, Erickson LA. Genomics and Epigenomics in Parathyroid Neoplasia: From Bench to Surgical Pathology Practice. Endocr Pathol (2021) 32:17–34. doi: 10.1007/s12022-020-09656-9
29. Starker LF, Svedlund J, Udelsman R, Dralle H, Akerström G, Westin G, et al. The DNA Methylome of Benign and Malignant Parathyroid Tumors. Genes Chromosomes Cancer (2011) 50(9):735–45. doi: 10.1002/gcc.20895
30. Arya AK, Bhadada SK, Singh P, Sachdeva N, Saikia UN, Dahiya D, et al. Promoter Hypermethylation Inactivates CDKN2A, CDKN2B and RASSF1A Genes in Sporadic Parathyroid Adenomas. Sci Rep (2017) 7(1):3123. doi: 10.1038/s41598-017-03143-8
31. Singh P, Bhadada SK, Dahiya D, Arya AK, Saikia UN, Sachdeva N, et al. Reduced Calcium Sensing Receptor (CaSR) Expression Is Epigenetically Deregulated in Parathyroid Adenomas. J Clin Endocrinol Metab (2020) 105(9):3015–24. doi: 10.1210/clinem/dgaa419
32. Cavalli G, Heard E. Advances in Epigenetics Link Genetics to the Environment and Disease. Nature (2019) 571:489–99. doi: 10.1038/s41586-019-1411-0
33. Aoshiba K, Tsuji T, Yamaguchi K, Itoh M, Nakamura H. The Danger Signal Plus DNA Damage Two-Hit Hypothesis for Chronic Inflammation in COPD. Eur Respir J (2013) 42:1689–95. doi: 10.1183/09031936.00102912
34. Wang LH, Wu CF, Rajasekaran N, Shin YK. Loss of Tumor Suppressor Gene Function in Human Cancer: An Overview. Cell Physiol Biochem (2018) 51:2647–93. doi: 10.1159/000495956
35. Buzzetti E, Pinzani M, Tsochatzis EA. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism (2016) 65:1038–48. doi: 10.1016/j.metabol.2015.12.012
36. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent Epigenetic Differences Associated With Prenatal Exposure to Famine in Humans. Proc Natl Acad Sci USA (2008) 105:17046–9. doi: 10.1073/pnas.0806560105
37. Raef H, Zou M, Baitei EY, Al-Rijjal RA, Kaya N, Al-Hamed M, et al. A Novel Deletion of the MEN1 Gene in a Large Family of Multiple Endocrine Neoplasia Type 1 (MEN1) With Aggressive Phenotype. Clin Endocrinol (Oxf) (2011) 75:791–800. doi: 10.1111/j.1365-2265.2011.04134.x
38. Zimmet P, Shi Z, El-Osta A, Ji L. Epidemic T2DM, Early Development and Epigenetics: Implications of the Chinese Famine. Nat Rev Endocrinol (2018) 14:738–46. doi: 10.1038/s41574-018-0106-1
39. Xie SH, Lagergren J. A Possible Link Between Famine Exposure in Early Life and Future Risk of Gastrointestinal Cancers: Implications From Age-Period-Cohort Analysis. Int J Cancer (2017) 140:636–45. doi: 10.1002/ijc.30485
40. van Noord PA. Breast Cancer and the Brain: A Neurodevelopmental Hypothesis to Explain the Opposing Effects of Caloric Deprivation During the Dutch Famine of 1944-1945 on Breast Cancer and its Risk Factors. J Nutr (2004) 134:3399S–406S. doi: 10.1093/jn/134.12.3399S
41. Meng R, Yu C, Guo Y, Bian Z, Si J, Nie J, et al. Early Famine Exposure and Adult Disease Risk Based on a 10-Year Prospective Study of Chinese Adults. Heart (2020) 106:213–20. doi: 10.1136/heartjnl-2019-315750
42. Wang Y, Zhang W, Xia F, Wan H, Chen C, Chen Y, et al. Moderation Effect of Economic Status in the Association Between Early Life Famine Exposure and MAFLD in Adulthood. Liver Int (2022) 42:299–308. doi: 10.1111/liv.15088
43. Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, et al. A Review of Vulnerability and Risks for Schizophrenia: Beyond the Two Hit Hypothesis. Neurosci Biobehav Rev (2016) 65:185–94. doi: 10.1016/j.neubiorev.2016.03.017
44. Li QD, Li H, Li FJ, Wang MS, Li ZJ, Han J, et al. Nutrition Deficiency Increases the Risk of Stomach Cancer Mortality. BMC Cancer (2012) 12:315. doi: 10.1186/1471-2407-12-315
45. Abnet CC, Corley DA, Freedman ND, Kamangar F. Diet and Upper Gastrointestinal Malignancies. Gastroenterology (2015) 148:1234–43.e4. doi: 10.1053/j.gastro.2015.02.007
46. Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell (2016) 30:363. doi: 10.1016/j.ccell.2016.07.013
47. Alshabi AM, Vastrad B, Shaikh IA, Vastrad C. Identification of Important Invasion and Proliferation Related Genes in Adrenocortical Carcinoma. Med Oncol (2019) 36:73. doi: 10.1007/s12032-019-1296-7
48. Assié G, Jouinot A, Fassnacht M, Libé R, Garinet S, Jacob L, et al. Value of Molecular Classification for Prognostic Assessment of Adrenocortical Carcinoma. JAMA Oncol (2019) 5:1440–7. doi: 10.1001/jamaoncol.2019.1558
49. Gómez Sáez JM. Primary Hyperparathyroidism Focused on Molecular Pathogenesis. Eur Endocrinol (2014) 10:153–6. doi: 10.17925/EE.2014.10.02.153
50. Welberg LA, Seckl JR. Prenatal Stress, Glucocorticoids and the Programming of the Brain. J Neuroendocrinol (2001) 13:113–28. doi: 10.1046/j.1365-2826.2001.00601.x
Keywords: primary hyperparathyroidism, clinical phenotype, famine, adult exposure, early life exposure
Citation: Yuan T-j, Yang Y-y, Zhu M-t, He Y, Zhao L, Zhou W-z, Su T-w, Zhao H-y, Sun L-h, Tao B and Liu J-m (2022) Association of Famine Exposure on the Changing Clinical Phenotypes of Primary Hyperparathyroidism in 20 years. Front. Endocrinol. 13:907019. doi: 10.3389/fendo.2022.907019
Received: 29 March 2022; Accepted: 12 May 2022;
Published: 17 June 2022.
Edited by:
Melissa Orlandin Premaor, Federal University of Minas Gerais, BrazilReviewed by:
Brendan Stack, Southern Illinois University Carbondale, United StatesAshutosh Kumar Arya, All India Institute of Medical Sciences, India
Copyright © 2022 Yuan, Yang, Zhu, He, Zhao, Zhou, Su, Zhao, Sun, Tao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-hao Sun, bGVvc3VuOEAxNjMuY29t; Bei Tao, dGFvYmVpMTk4MUBob3RtYWlsLmNvbQ==; Jian-min Liu, bGptMTA1ODZAcmpoLmNvbS5jbg==
†Present address: Tian-jiao Yuan, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China, Department of General Practice, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Yang He, Department of Endocrinology, Zhong Shang Hospital, Fudan University, Shanghai, China
Lin Zhao, Department of Endocrinology, Zhong Shang Hospital, Fudan University, Shanghai, China
 Tian-jiao Yuan
Tian-jiao Yuan Yu-ying Yang
Yu-ying Yang Min-ting Zhu1,2†
Min-ting Zhu1,2† Yang He
Yang He Jian-min Liu
Jian-min Liu