- Endocrinology Department, St George Public Hospital, Sydney, NSW, Australia
We describe a 96-year-old man with insulin-dependent type 2 diabetes mellitus who, despite insulin cessation, presented with recurrent hypoglycemia associated with confirmed inappropriate endogenous hyperinsulinemia. 68Ga-DOTATATE-PET/CT scans demonstrated increased uptake in the pancreatic tail with multiple large intensely active liver metastases. Liver biopsy confirmed the diagnosis of well-differentiated metastatic neuroendocrine tumor. He was unsuitable for surgical resection and long-acting somatostatin analog therapy was ineffective. Subsequent management with four cycles of Lutate [177-Lutetium-DOTA0-Tyr3-octreotate (177Lu-DOTATATE)] resulted in resolution of hypoglycemia and ongoing clinical, biochemical, and radiological response 6 years after. This case is unique due to not only the paradoxical entity of insulinoma in insulin-dependent diabetes but also the positive sustained outcome after 177Lu-DOTATATE, given that unresectable metastatic insulinoma carries a poor prognosis. We review published cases of metastatic insulinoma in patients with diabetes mellitus as well as the literature to-date investigating efficacy and safety of Lutate therapy in metastatic insulinoma.
Introduction
Although insulinomas are the most common functioning pancreatic neuroendocrine tumors (panNETs) and most common cause of endogenous hyperinsulinemic hypoglycemia, they are rare, occurring in approximately one to four per million people annually (1, 2). Insulinoma is exceptionally rare in a patient with pre-existing diabetes mellitus but important not to miss as a cause of recurrent hypoglycemia when iatrogenic causes have been excluded. Insulinomas are predominantly non-metastatic (90%–95%), sporadic, solitary, small (<2cm), and intrapancreatic NETs and most commonly occur in the fifth to sixth decades of life with equal sex distribution (3, 4). Surgical resection is the only cure, with both cure rates and 10-year survival rate >90% in patients with non-metastatic insulinoma following resection (5, 6).
Metastatic insulinoma, however, carries a poor prognosis. A large European registry including 81 patients with metastatic insulinoma reported a 5-year survival rate of 55.6% (7). Data from an American registry of patients (n = 121) revealed a much lower 5-year survival rate in unresectable metastatic insulinoma compared to those who underwent surgery (14% vs. 84%, p < 0.001) (8). Management of recurrent hypoglycemia in these patients is extremely challenging given lack of definitive surgical cure and limitations of available medical options including paucity of data in insulinoma specifically, modest efficacy, and treatment-related side effects and toxicity (9). 177Lu-DOTATATE (Lutate) has an emerging evidence basis in patients with gastroenteropancreatic (GEP) NETs and shows promise in managing hypoglycemia secondary to metastatic unresectable insulinoma; however, further studies are required (10, 11).
We present a 96-year-old man with insulin-dependent type 2 diabetes mellitus (T2DM) and recurrent refractory life-threatening hypoglycemia secondary to metastatic insulinoma. 177Lu-DOTATATE resulted in resolution of hypoglycemia and reduction in metastatic disease burden, with ongoing clinical, biochemical, and radiological response at 6-year follow-up. This case is unique due to not only the paradoxical entity of insulinoma in insulin-dependent diabetes but also the positive sustained well-documented outcome after 177Lu-DOTATATE, given that unresectable metastatic insulinoma carries a poor prognosis. We review the few published cases of metastatic insulinoma in patients with diabetes and detail the limited existing data investigating 177Lu-DOTATATE therapy in patients with metastatic insulinoma.
Case Description
A 96-year-old man was referred to our Endocrinology institution by his general practitioner for difficult management of longstanding T2DM. He had been experiencing recurrent “funny turns” (six episodes in the preceding 12 months) associated with hypoglycemia culminating in an episode of loss of consciousness necessitating hospitalization. During this period, there was significant reduction in diabetic regimen intensity including insulin cessation; however, weight had remained stable. His hypoglycemic episodes were predominantly fasting and relieved with carbohydrate consumption.
Diagnosis, Treatment, and Outcomes
Common differentials such as renal/liver failure (eGFR, 55 ml/min/1.73 m2), hypocortisolemia, growth hormone deficiency, and malabsorption were excluded. He subsequently underwent a 75-g oral glucose tolerance test (OGTT), which indicated abnormal insulin physiology. The OGTT results (Table 1A) revealed inappropriate fasting hyperinsulinemia (23 µIU/ml) in the setting of fasting hypoglycemia (2.6 mmol/L). This was followed by significant hyperglycemia 2 hours after glucose load (15.8 mmol/L) and insufficient insulin response (61 µIU/ml). After 5 hours, he again demonstrated inappropriate hyperinsulinemia (24 µIU/ml) with hypoglycemia (2.5 mmol/L). Thus, the 75-g OGTT indicated a dysregulated relationship between glucose and insulin concentrations, with inappropriate hyperinsulinemia in the setting of hypoglycemia at fasting and 5 hour after glucose load and with significant hyperglycemia and insufficient insulin response at 2 hours after glucose load. During prolonged inpatient fast, several episodes of hypoglycemia occurred during which inappropriate endogenous hyperinsulinemia was confirmed with elevated insulin and C-peptide concentrations during hypoglycemia (Table 1B). Sulfonylurea use and insulin antibodies were excluded, raising suspicion for an insulinoma.
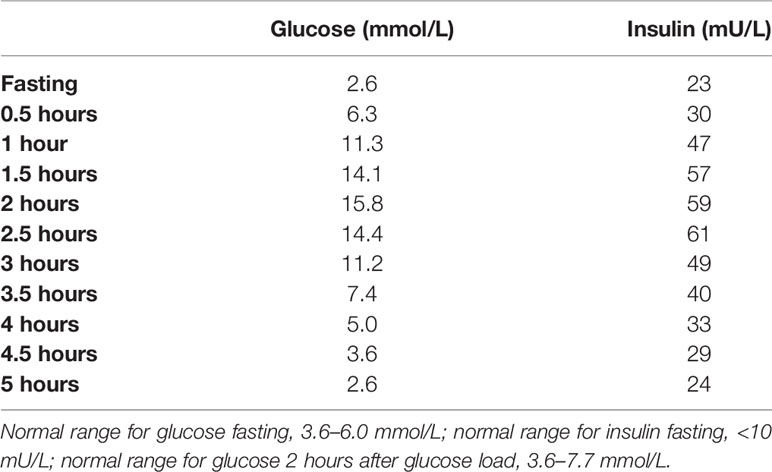
Table 1A Paired serum glucose and insulin concentrations during 5-hour 75-g oral glucose tolerance test (OGTT).
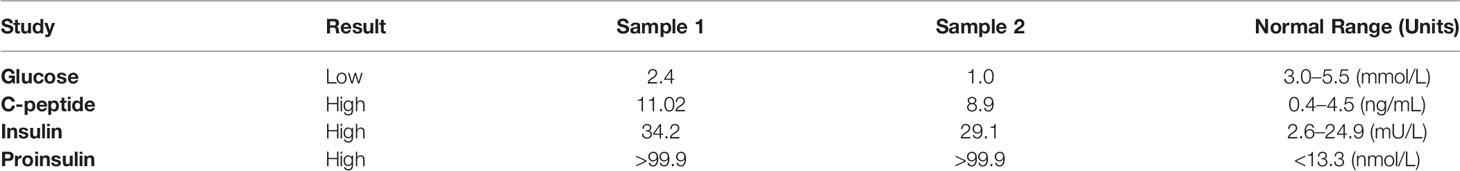
Table 1B Paired glucose, C-peptide, insulin, and proinsulin concentrations during inpatient fasting episodes of hypoglycemia.
Computed tomography (CT) scan of the abdomen could not identify focal or diffuse pancreatic enlargement although detected three arterially enhancing liver lesions, measuring 8.0, 6.0, and 2.0 cm. 68Gallium-DOTATATE–positron emission tomography (PET)/CT scan, however, showed a small focus of moderately intense tracer accumulation in the pancreatic tail (Figure 1) and extensive active metastatic disease in both lobes of the liver (Figures 2, 3). Serum tumor markers α-fetoprotein (AFP), carcinoembryonic antigen (CEA), and cancer antigen (CA)-19.9 were negative. Subsequent liver biopsy revealed a well-differentiated, metastatic NET. Tumor cells were characterized by eccentric round-oval nuclei with mild nuclear pleomorphism and moderate eosinophilic granular cytoplasm. One mitosis per 10 hpf was noted. Tumor cells exhibited strongly positive staining for neuroendocrine markers chromogranin A, synaptophysin, and cluster differentiation (CD)-56, whereas staining was negative for insulin, TTF1, and CDX2. The Ki67 proliferative index was estimated at 2%–3%.
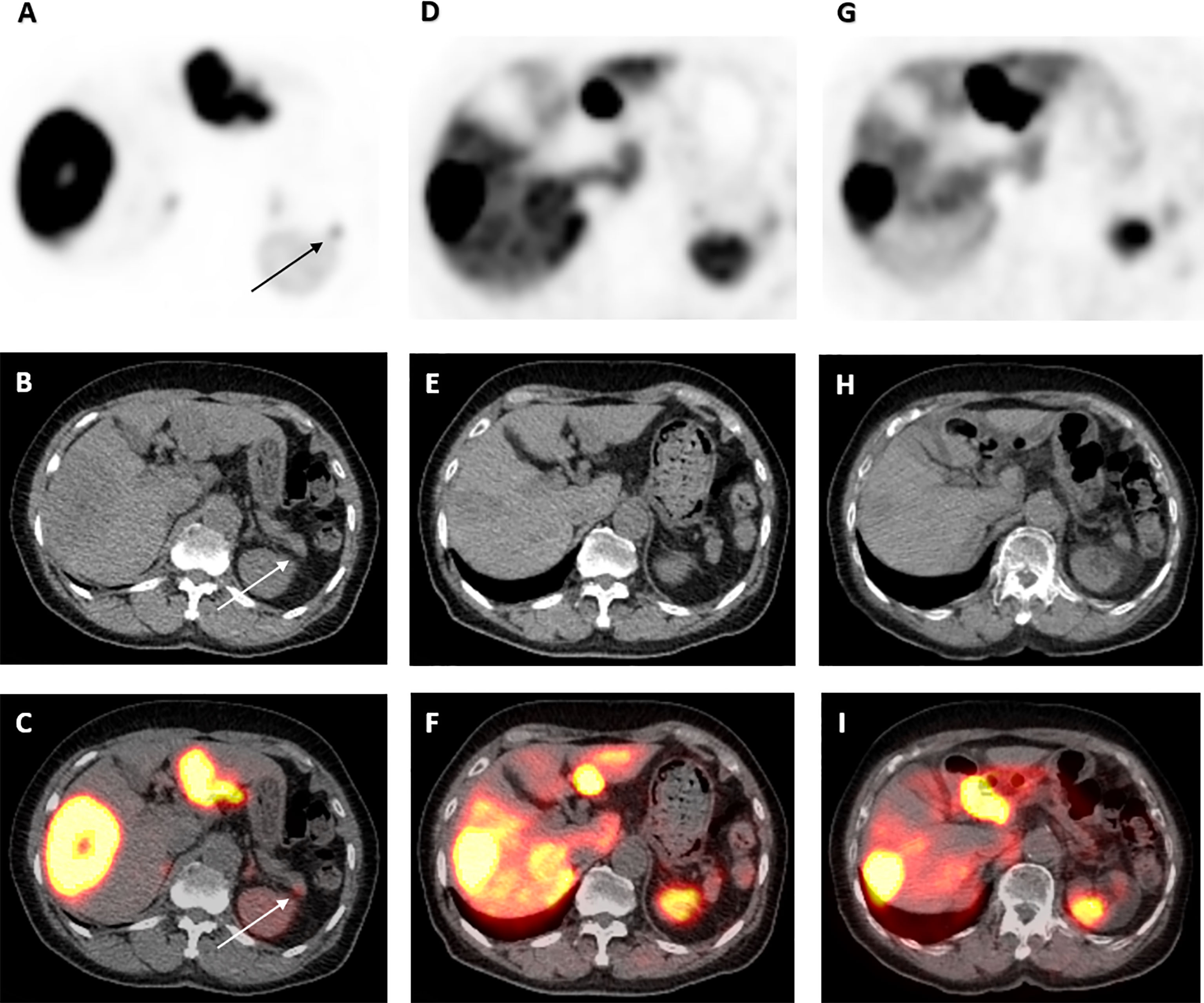
Figure 1 68Ga-DOTATATE PET/CT scan images of pancreatic tail lesion pre- and post-177 Lu-DOTATATE. Axial view images of 68Ga-DOTATATE PET/CT scan demonstrating interval reduction in avidity in moderately intense focus of activity at the tip of the pancreatic tail (arrow) on PET, low-dose CT and PET/CT fusion (top to bottom) from baseline, to 1 and 4 years after 177Lu-DOTATATE therapy (left to right). Physiological uptake in the spleen and remainder of the liver is also visualized.

Figure 2 68Ga-DOTATATE PET/CT scan images of dominant right liver lobe lesion pre- and post-177 Lu-DOTATATE. Axial view images of 68Ga-DOTATATE PET/CT scan demonstrating interval reduction in size in dominant right liver lobe lesion (arrow) on PET, low-dose CT and PET/CT fusion (top to bottom) from baseline, to 1 and 4 years after 177Lu-DOTATATE therapy (left to right). Physiological uptake in the spleen and remainder of the liver is also visualized.
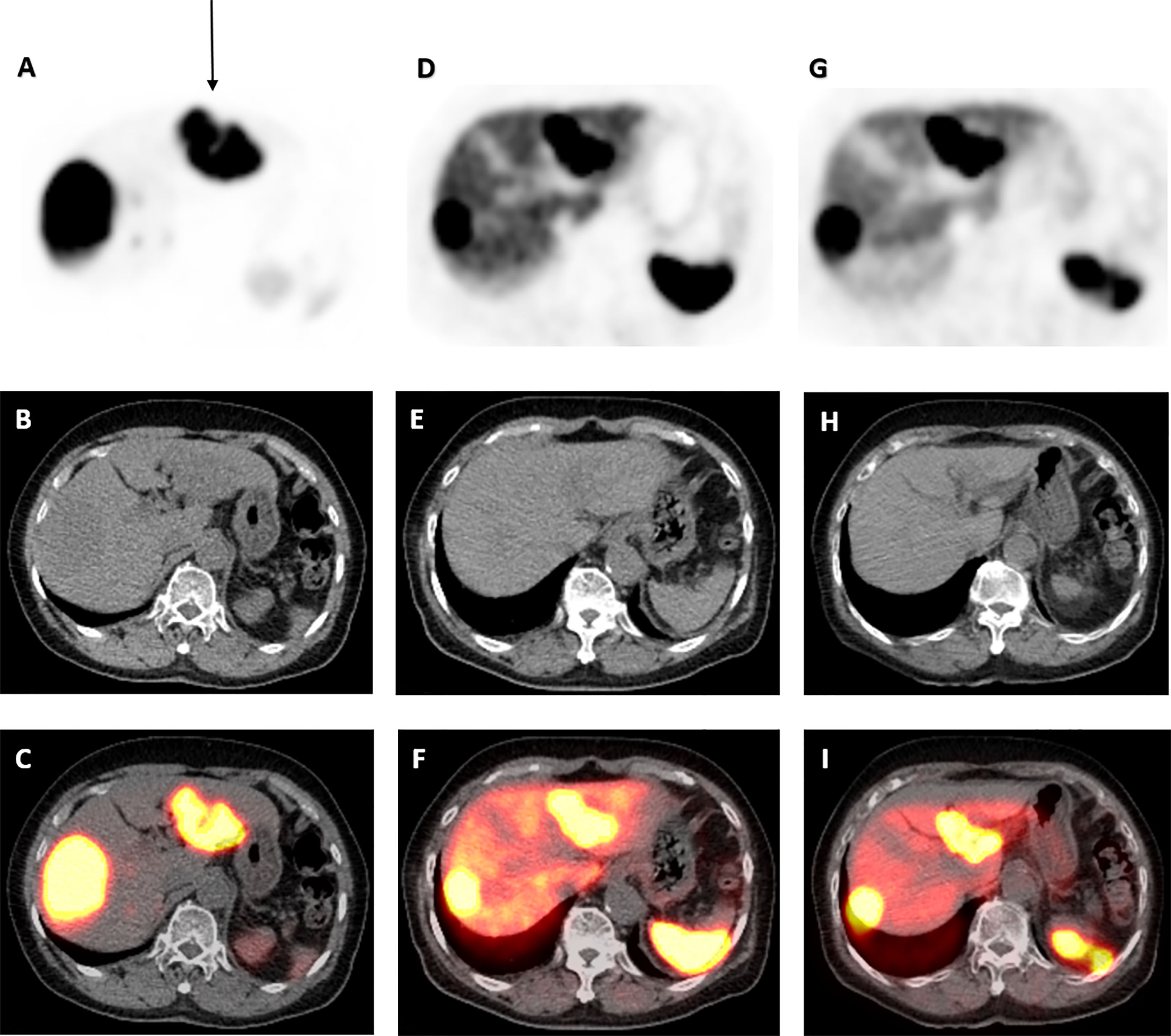
Figure 3 68Ga-DOTATATE PET/CT scan images of dominant left liver lobe lesion pre- and post-177 Lu-DOTATATE. Axial view images of 68Ga-DOTATATE PET/CT scan demonstrating interval reduction in size in dominant left liver lobe lesion (arrow) on PET, low-dose CT, and PET/CT fusion (top to bottom) from baseline, to 1 and 4 years after 177Lu-DOTATATE therapy (left to right). Physiological uptake in the spleen and remainder of the liver is also visualized.
Given advanced age and extensive liver metastases, the patient was deemed unsuitable for surgical resection. He failed initial medical treatment consisting of four weekly intramuscular injections of long-acting Octreotide (30 mg) with another hypoglycemic syncopal event requiring hospital admission. He was then trialed on four cycles of intravenous 177Lu-DOTATATE with cumulative dose of 32 GBq over 5 months. He did not experience any dose-limiting side effects such as acute kidney injury or cytopaenia. Hypoglycemic episodes abated, and after a few months, he developed symptomatic hyperglycemia up to 24 mmol/L, which was insufficiently controlled with re-commencement of oral hypoglycemic therapy.
Paradoxically, this elderly man with endogenous hyperinsulinemia from metastatic insulinoma, having had his metastatic disease controlled on 177Lu-DOTATATE, began to require exogenous insulin administration for glycemic control.
Repeat 68Ga-DOTATATE-PET/CT scans 1 and 4 years after 177Lu-DOTATATE completion demonstrated significant durable response with reduction in uptake in the pancreatic tail (Figure 1) and throughout the liver (Figures 2, 3). Serial chromogranin A levels also declined from 3,992 to 119 ng/ml (normal range ≤104 ng/ml). He is currently well 6 years after completion of 177Lu-DOTATATE for his insulinoma at the age of 96 years with sustained clinical, biochemical, and radiological response.
Timeline
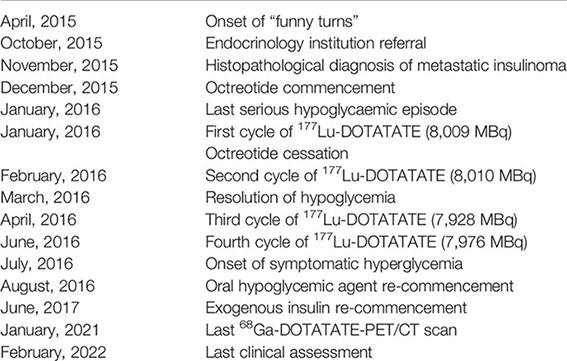
Discussion
Insulinomas are considered functional panNETs as they result in endogenous hyperinsulinemia, producing the clinical syndrome of recurrent hypoglycemia (most commonly fasting) (1). Whipple’s triad, which is well-documented, serves as the trigger for further investigation (12, 13). Biochemical diagnosis is confirmed with evidence of inappropriate endogenous hyperinsulinemia (elevated/non-suppressed insulin and C-peptide concentrations) during either spontaneous or provoked hypoglycemia (during prolonged inpatient 72-hour fast), after excluding sulfonylurea use (12, 13). Given that surgical resection is the only cure, the next step after confirmed biochemical diagnosis is meticulous localization of the culprit tumor. Localization begins with structural imaging including ultrasound, CT, or magnetic resonance imaging (MRI), followed by functional imaging such as 68Ga-DOTATATE-PET/CT scans, which facilitate detection of occult metastases (12, 13). Endoscopic ultrasound (EUS) in combination with fine needle aspiration is the most accurate diagnostic tool for insulinoma with sensitivity and specificity up to 95% and allows histopathological examination of the primary pancreatic tumor and adjacent lymph nodes. EUS, however, is not as sensitive in detecting pancreatic tail tumors (3, 4). Positive tumor cell staining for insulin is supportive but not mandatory for insulinoma diagnosis as up to 20% of patients with pre-operative hyperinsulinemic hypoglycemia and resolution post-tumor resection have negative insulin staining (14). In a retrospective analysis of 80 patients with insulinoma, malignant insulinoma was less likely to stain positive for insulin compared to benign insulinoma (3/7 vs. 66/73, p = 0.015) (15). Hypotheses for lack of insulin staining include defects in insulin storage capacity and sampling errors.
The OGTT is not indicated in the diagnostic algorithm for suspected cases of insulinoma (12), partly due to the occurrence of hypoglycemia during OGTT in some healthy individuals (16). Regardless, the results of a 5-hour OGTT provided the first indication of abnormal insulin physiology in our patient. The OGTT demonstrated inappropriately high fasting insulin during hypoglycemia and insufficient insulin response to hyperglycemia post-glucose ingestion, suggesting that insulin release was occurring largely independent of changes in serum glucose. Under normal physiological conditions, glucose metabolism is intimately coupled with β-cell insulin secretion such that blood glucose concentrations are maintained between 3.5 and 5.5 mmol/L (17), with a sigmoidal pattern of insulin release to higher glucose concentrations in isolated normal human islet cells (18). However, in the presence of an insulinoma, this glucose-insulin coupling becomes dysregulated. Small-scale in vitro studies have shown that cultured human insulinoma cells can have near maximal glucose responsiveness at blood glucose levels of 1.0–3.0 mmol/L (19) with no significant increase in insulin release with rise in glucose concentration from 2.8 to 8.3 mmol/L in one study (20) and plateau at 10.0–15.0 mmol/L in two other studies (19, 21). The autonomous insulin secretion despite low glucose concentrations and the blunted insulin response to higher glucose concentrations can give rise to fasting hypoglycemia and postprandial hyperglycemia, respectively. This abnormal insulin secretion pattern was not only evident in our patient but also the most common pattern in a retrospective analysis published in 2008 of 64 patients who underwent 100-g OGTT prior to insulinoma resection (22).
Insulinoma is an exceptionally rare occurrence in patients with diabetes. Among 313 confirmed insulinoma cases at the Mayo Clinic between 1927 and 1992, there was only one patient with pre-existing diabetes (23), whereas a cohort from Japan of 443 cases of insulinoma included one diabetic patient (24). A single institution in Taiwan reported one patient with diabetes out of 23 insulinoma cases seen between 1984 and 2006 (25). Potential explanations for the low reported incidence of diabetes in insulinoma cases include a) lack of reporting in the literature, b) missed or delayed diagnosis due to difficulty differentiating iatrogenic from insulinoma-induced hypoglycemia, c) insulin resistance or pre-existing hypoglycemia unawareness masking the clinical syndrome, or d) decreased β-cell number and thus decreased potential cellular regeneration for tumor formation. Extensive literature review yielded 13 cases of metastatic insulinoma in patients with pre-existing diabetes in the past 50 years (Supplementary Table 1) (26–37). The majority had pre-existing T2DM (10/13) with mean age 59 years at diagnosis and equal sex distribution. The most common primary site was the pancreatic tail, and majority (7/8) pancreatic lesions were >4 cm in diameter, compared to mean lesion size of 3 cm in the largest published series of malignant insulinoma cases (8), potentially reflecting delayed diagnosis due to the initial requirement of excluding iatrogenic hypoglycemia. The most common metastatic sites were regional lymph nodes (8/13) and liver (11/13). Diazoxide (9/13) and somatostatin analogs (8/13) were the most common medical therapies utilized, whereas 177Lu-DOTATATE has not been reported in a diabetic patient with metastatic insulinoma. Regarding outcomes, six patients died during follow-up, including two patients within 2 weeks from Diazoxide-related toxicity and otherwise due to treatment failure and progressive disease. Six patients had good outcome without recurrence of hypoglycemia; however, this observation is limited by short follow-up length of <1 year in the majority. Thus, our patient shares similarities with previously reported cases of metastatic insulinoma in pre-existing diabetes however is unique given his advanced age, use of 177Lu-DOTATATE, and positive sustained outcome at extended 6-year follow-up.
Given that our patient’s advanced age and extensive unresectable liver metastases, curative surgical resection was unsuitable. This led to the difficult medical management issue of recurrent hypoglycemia from metastatic insulinoma. Various review articles have summarized the available medical therapeutic options for managing recurrent insulinoma-induced hypoglycemia (2, 3, 9, 38). Briefly, these strategies are limited by lack of data particularly in patients with insulinoma, modest efficacy, and treatment-related intolerance and toxicity. Diazoxide is a nondiuretic benzothiazide analog, which opens the ATP-sensitive potassium channel on the pancreatic β-cell membrane, hence facilitating potassium cellular efflux and diminishing membrane depolarization and voltage-gated calcium-dependent exocytosis of insulin-containing vesicles (9). Diazoxide has approximately 50% efficacy in abating hypoglycemia in insulinoma but is not useful in controlling metastatic disease (hence not used in our patient) and is often limited by significant toxicity, e.g., fluid retention, renal/liver failure (3). Somatostatin analogs such as long-acting Octreotide and Lanreotide inhibit insulin release from pancreatic β-cells and have up to 50% efficacy in controlling hypoglycemia with modest tumor regression effect. Somatostatin analog use is often limited by gastrointestinal side effects and tachyphylaxis and in some cases worsens hypoglycemia due to concurrent inhibition of counter-regulatory glucagon release (9). Other less investigated but approved options in unresectable GEP NETs include chemotherapy (5-FU, Doxorubicin, Streptozotocin, Temozolomide, and Capecitabine), mTOR inhibitors such as Everolimus and multiple tyrosine kinase inhibitor Sunitinib, with data particularly scarce in metastatic insulinoma (9, 38).
We successfully trialed 177Lu-DOTATATE in our patient, a form of peptide receptor radionuclide therapy (PRRT). Lutate (177-Lutetium-DOTA0-Tyr3-octreotate) is a radiolabeled somatostatin analog compound consisting of the somatostatin analog Tyr3-octreotate, linked with a radioactive isotope, 177-Lutetium, with DOTA0 acting as the linking agent. Intravenous infusion of 177Lu-DOTATATE allows delivery of targeted cytotoxic ionizing radiation therapy specifically to neuroendocrine tumor cells, taking advantage of their somatostatin receptor (SST) overexpression (particularly SST2) and the low physiological SST expression in normal tissue. Compared to earlier radionuclides, 177-Lutetium emits diagnostic ƴ-radiation, allowing better dosimetry, emits therapeutic β-radiation that has a shorter tissue penetration range (2mm), limiting exposure to neighboring normal tissue, and has nine-fold higher affinity for SST2, and lower hematological/renal toxicity (10, 39).
The Rotterdam group in Netherlands has reported results of 177Lu-DOTATATE in several patients with NETs in the past 20 years. Kwekkeboom et al. investigated 131 patients with metastatic GEP NETs (including two with insulinoma) with median follow-up 16 months (40). Results were favorable with 47% having an objective response, 35% stable disease, and 18% progressive disease, and 177Lu-DOTATATE was considered safe with <2% experiencing serious hematological toxicity and two cases of serious liver/hepatic toxicity. Brabander et al. more recently assessed 177Lu-DOTATATE efficacy in 443 patients with metastatic bronchial and GEP NETs (including 21 patients with functional panNETs) with 78-month median follow-up (41). Progression-free survival was 29 months, time to progression of 36 months, and overall survival of 63 months, with objective response (complete or partial) in 39% and stable disease reached in 43% of patients. Clinically significant hematological toxicity occurred in 10%, including acute leukemia (AL) in 0.7% and myelodysplastic syndrome (MDS) in 1.5% of patients with no treatment-related long-term renal or liver failure observed.
However, given the low prevalence of metastatic insulinoma, data exploring efficacy of 177Lu-DOTATATE in these patients specifically are limited to small case series and single case reports. Our literature review revealed 33 published cases of metastatic insulinoma trialed on 177Lu-DOTATATE therapy, with 32/33 having liver metastases (11, 42–49).
The largest such case series was conducted by Zandee et al. who investigated 177Lu-DOTATATE safety and efficacy in 34 patients with metastatic functioning panNETs including 14 with insulinoma (11). Eight patients had pre-treatment with somatostatin analogs, five had surgery, and two had chemotherapy. Objective response, stable disease, and progressive disease occurred in 50%, 21.4%, and 28.6% of patients, respectively, with approximately 30 months mean progression-free survival and 67% experiencing reduction in hypoglycemia frequency.
In a cohort of 310 patients with GEP NETs managed with 177Lu-DOTATATE between 2000 and 2006, Kwekkeboom et al. included five patients with metastatic insulinoma with partial response in three patients, stable disease in one patient, and progressive disease in the other (42).
Ong et al. described two men with inoperable metastatic insulinoma with severe hypoglycemia, who failed Diazoxide and somatostatin analog therapy (43). Both patients experienced control of hypoglycemia and reduction in size of liver metastases with 177Lu-DOTATATE; however, one was co-treated with Everolimus and both with chemotherapy. One patient was hypoglycaemia-free at 10 months and the other had disease progression at 24 months.
Van Schaik et al. treated four patients with metastatic insulinoma and severe uncontrollable hypoglycemia failing conventional therapy including Octreotide (44). 177Lu-DOTATATE achieved stable disease and euglycemia for mean 22 months (one patient still in remission at 20 months).
Magalhães et al. utilized 177Lu-DOTATATE in four patients with unresectable metastatic insulinoma and refractory hypoglycemia all pre-treated with Diazoxide and Octreotide (45). Two patients had disease progression (mean 14 months) and mortality (mean 20 months), whereas two patients remained asymptomatic at mean follow-up 20 months.
Four single case reports have also outlined positive effects of 177Lu-DOTATATE in metastatic insulinoma with refractory hypoglycemia, such as resolution of hypoglycemia and reduction in metastatic burden with all patients having ongoing disease control and radiological stability at mean follow-up interval of 15 months (46–49).
Hence, 177Lu-DOTATATE is a potential effective and safe option in patients with unresectable metastatic insulinoma with perceived benefits including resolution of hypoglycemia and reduction in radiological metastatic burden. However, given likely positive publication bias and scarce data, further studies (ideally randomized and controlled) exploring177Lu-DOTATATE efficacy in this subset of patients is certainly warranted.
Insulinoma, although a rare cause of hypoglycaemia in a patient with diabetes mellitus, is important not to miss and should especially be considered in patients on minimal glucose-lowering therapy or in whom hypoglycemia continues despite insulin cessation. Surgical management is the only cure and is preferred in suitable patients, whereas medical management in cases of unresectable insulinoma with recurrent hypoglycemia is extremely challenging due to poor prognosis and limitations of available treatment options such as diazoxide, somatostatin analogs, and chemotherapy. 177Lu-DOTATATE, a form of PRRT, has an emerging evidence basis in patients with GEP NETs and shows promise as a potential effective and well-tolerated option in patients with recurrent hypoglycemia secondary to unresectable metastatic insulinoma; however, further studies are needed. Our patient had a successful and sustained response to 177Lu-DOTATATE to the extent that he is now requiring exogenous insulin administration for his previously masked poor diabetic control. Despite not undergoing surgical management, 177Lu-DOTATATE has provided him an exceptional outcome in terms of survival and quality of life considering his advanced age and extent of liver metastases.
Patient Perspective
The patient declined to provide their perspective on the case report, however, provided signed written informed consent for this report to be published.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SK conceived the case report and drafted and critically reviewed the manuscript. MM drafted the manuscript. PR managed the patient and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding and Acknowledgments
The authors have no funding or acknowledgments to disclose.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.906012/full#supplementary-material
References
1. Anderson CW, Bennett JJ. Clinical Presentation and Diagnosis of Pancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am (2016) 25(2):363–74. doi: 10.1016/j.soc.2015.12.003
2. Davi MV, Pia A, Guarnotta V, Pizza G, Colao A, Faggiano A, et al. The Treatment of Hyperinsulinemic Hypoglycaemia in Adults: An Update. J Endocrinol Invest (2017) 40(1):9–20. doi: 10.1007/s40618-016-0536-3
3. Maggio I, Mollica V, Brighi N, Lamberti G, Manuzzi L, Ricci AD, et al. The Functioning Side of the Pancreas: A Review on Insulinomas. J Endocrinol Invest (2020) 43(2):139–48. doi: 10.1007/s40618-019-01091-w
4. Giannis D, Moris D, Karachaliou GS, Tsilimigras D, Karaolanis G, Papalampros A, et al. Insulinomas: From Diagnosis to Treatment. A Review of the Literature. J BUON (2020) 25(3):1302–14.
5. Mehrabi A, Fischer L, Hafezi M, Dirlewanger A, Grenacher L, Diener KM, et al. A Systematic Review of Localization, Surgical Treatment Options, and Outcome of Insulinoma. Pancreas (2014) 43(5):675–86. doi: 10.1097/MPA.0000000000000110
6. Nikfarjam M, Warshaw AL, Axelrod L, Deshpande V, Thayer SP, Ferrone CR, et al. Improved Contemporary Surgical Management of Insulinomas: A 25-Year Experience at the Massachusetts General Hospital. Ann Surg (2008) 247(1):165–72. doi: 10.1097/SLA.0b013e31815792ed
7. Lepage C, Ciccolallo L, De Angelis R, Bouiver AM, Faivre J, Gatta G, et al. European Disparities in Malignant Digestive Endocrine Tumours Survival. Int J Cancer (2010) 126(12):2928–34. doi: 10.1002/ijc.24698
8. Sada A, Glasgow AE, Vella A, Thompson GB, McKenzie TJ, Habermann EB. Malignant Insulinoma: A Rare Form of Neuroendocrine Tumor. World J Surg (2020) 44(7):2288–94. doi: 10.1007/s00268-020-05445-x
9. Matej A, Bujwid H, Wroński J. Glycemic Control in Patients With Insulinoma. Hormones (Athens) (2016) 15(4):489–99. doi: 10.14310/horm.2002.1706
10. Das S, Al-Toubah T, El-Haddad G, Strosberg J. 177lu-DOTATATE for the Treatment of Gastroenteropancreatic Neuroendocrine Tumors. Expert Rev Gastroenterol Hepatol (2019) 13(11):1023–31. doi: 10.1080/17474124.2019.1685381
11. Zandee WT, Brabander T, Blažević A, Kam BLR, Teunissen JJM, Feelders RA, et al. Symptomatic and Radiological Response to 177Lu-DOTATATE for the Treatment of Functioning Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab (2019) 104(4):1336–44. doi: 10.1210/jc.2018-01991
12. Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, et al. Evaluation and Management of Adult Hypoglycemic Disorders: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2009) 94(3):709–28. doi: 10.1210/jc.2008-1410
13. Kittah NE, Vella A. Management of Endocrine Disease: Pathogenesis and Management of Hypoglycemia. Eur J Endocrinol (2017) 177(1):R37–47. doi: 10.1530/EJE-16-1062
14. Zhao YP, Zhan HX, Zhang TP, Cong L, Dai MH, Liao Q, et al. Surgical Management of Patients With Insulinomas: Result of 292 Cases in a Single Institution. J Surg Oncol (2011) 103(2):169–74. doi: 10.1002/jso.21773
15. Andreassen M, Ilett E, Wiese D, Slater EP, Klose M, Hansen CP, et al. Surgical Management, Preoperative Tumor Localization, and Histopathology of 80 Patients Operated on for Insulinoma. J Clin Endocrinol Metab (2019) 104(12):6129–38. doi: 10.1210/jc.2019-01204
16. Lev-Ran A, Anderson RW. The Diagnosis of Postprandial Hypoglycemia. Diabetes (1981) 30(12):996–9. doi: 10.2337/diab.30.12.996
17. Güemes M, Rahman SA, Hussain K. What is a Normal Blood Glucose? Arch Dis Child (2016) 101(6):569–74. doi: 10.1136/archdischild-2015-308336
18. Harrison DE, Christie MR, Gray DW. Properties of Isolated Human Islets of Langerhans: Insulin Secretion, Glucose Oxidation and Protein Phosphorylation. Diabetologia (1985) 28(2):99–103. doi: 10.1007/BF00279924
19. Liao J, Ding F, Luo W, Nie X, He Y, Li G. Using the Secretion Ratios of Insulin and C-peptide During the 2-H Oral Glucose Tolerance Test to Diagnose Insulinoma. Dig Dis Sci (2021) 66(5):1533–9. doi: 10.1007/s10620-020-06379-z
20. Yasunami Y, Funakoshi A, Ono J, Miyazaki K, Jimi A, Konomi K. In Vitro Study of Cultured Human Insulinoma Cells: Evidence of Abnormal Sensitivity to Glucose. J Clin Endocrinol Metab (1987) 65(1):110–5. doi: 10.1210/jcem-65-1-110
21. Henquin JC, Nenquin M, Guiot Y, Rahier J, Sempoux C. Human Insulinomas Show Distinct Patterns of Insulin Secretion In Vitro. Diabetes (2015) 64(10):3543–53. doi: 10.2337/db15-0527
22. Saddig C, Goretzki PE, Starke AA. Differentiation of Insulin Secretion Patterns in Insulinoma. World J Surg (2008) 32(5):918–29. doi: 10.1007/s00268-007-9450-3
23. Kane LA, Grant CS, Nippoldt TB, Service FJ. Insulinoma in a Patient With NIDDM. Diabetes Care (1993) 16(9):1298–300. doi: 10.2337/diacare.16.9.1298
24. Ishii H, Ito T, Moriya S, Horie Y, Tsuchiya M. [Insulinoma—A Statistical Review of 443 Cases in Japan]. Nihon Rinsho (1993) 51 (Suppl):199–206.
25. Lei WY, Wang TE, Chen TL, Chang WH, Yang TL, Wang CY. Insulinoma Causing Hypoglycemia in a Patient With Type 2 Diabetes. J Formos Med Assoc (2007) 106(5):392–6. doi: 10.1016/S0929-6646(09)60324-725
26. Ciacciarelli M, Caruso G, Rengo M, Maceroni P, Misurale C, D’Aremiento E, et al. Malignant Insulinoma With Multiple Liver Metastases and Hypercalcitoninemia in a Patient With Type 2 Diabetes Mellitus Presenting as Recurrent Episodes of Diaphoresis Due to Severe Hypoglycemia. Case Rep Endocrinol (2020) 4239679. doi: 10.1155/2020/4239679
27. Gjelberg HK, Hoem D, Verbeke CS, Eide J, Cooper JG, Molven A. Hypoglycemia and Decreased Insulin Requirement Caused by Malignant Insulinoma in a Type 1 Diabetic Patient: When the Hoof Beats are From a Zebra, Not a Horse. Clin Case Rep (2017) 5(6):761–8. doi: 10.1002/ccr3.927
28. Lablanche S, Chobert-Bakouline M, Risse O, Laverrière MH, Chabre O, Benhamou PY. Malignant Insulinoma may Arise During the Course of Type 1 Diabetes Mellitus: A Case Report. Diabetes Metab (2015) 41(3):258–61. doi: 10.1016/j.diabet.2014.08.004
29. Ademoğlu E, Unlütürk U, Ağbaht K, Karabork A, Corapçioğlu D. Type 2 Diabetes Mellitus in a Patient With Malignant Insulinoma Manifesting Following Surgery. Diabet Med (2012) 29(7):e133–7. doi: 10.1111/j.1464-5491.2012.03603.x29
30. Abbasakoor NO, Healy ML, O’Shea D, Maguire D, Muldoon C, Sheahan K, et al. Metastatic Insulinoma in a Patient With Type 2 Diabetes Mellitus: Case Report and Review of the Literature. Int J Endocrinol (2011) 124078. doi: 10.1155/2011/124078
31. Ferrer-García JC, Tolosa-Torréns M, Hernando-Meliá C, Arribas-Palomar L, Sánchez-Juan C. Everolimus Resolving Hypoglycemia, Producing Hyperglycemia, and Necessitating Insulin Use in a Patient With Diabetes and Nonresectable Malignant Insulinoma. Endocr Pract (2011) 17(2):e17–20. doi: 10.4158/EP10282.CR
32. Grycewicz J, Scibór Z, Cwikła JB, Lewiński A, Cypryk K. Recurrent Hypoglycaemia in a Type 2 Diabetes Patient - Diagnostic Difficulties. Arch Med Sci (2010) 6(1):126–9. doi: 10.5114/aoms.2010.13520
33. Siraj ES, Samuel G, Saber S, Samuel S, Hamrahian AH, Reddy SS. Metastatic Malignant Insulinoma in a Patient With Type 2 Diabetes Mellitus: Case Presentation and Literature Review. Endocr Pract (2006) 12(4):411–6. doi: 10.4158/EP.12.4.411
34. Schmitt J, Boullu-Sanchis S, Moreau F, Drui S, Louis B, Chabrier G, et al. Association of Malignant Insulinoma and Type 2 Diabetes Mellitus: A Case Report. Ann Endocrinol (Paris) (2008) 69(1):69–72. doi: 10.1016/j.ando.2007.11.002
35. Svartberg J, Stridsberg M, Wilander E, Andersson DE, Eriksson B. Tumour-Induced Hypoglycaemia in a Patient With Insulin-Dependent Diabetes Mellitus. J Intern Med (1996) 239(2):181–5. doi: 10.1046/j.1365-2796.1996.405750000.x
36. Atkinson AB, Buchanan KD, Carson DJ, Kennedy T, O’Hare MM, Sloan JM, et al. Insulinoma (Apud Cell Carcinoma) in a Diabetic. Br Med J (1978) 2(6149):1397–8. doi: 10.1136/bmj.2.6149.1397-a
37. Taylor SG 3rd, Schwartz TB, Zannini JJ, Ryan WG. Streptozotocin Therapy for Metastatic Insulinoma. Arch Intern Med (1970) 126(4):654–7. doi: 10.1001/archinte.1970.00310100100012
38. Brown E, Watkin D, Evans J, Yip V, Cuthbertson DJ. Multidisciplinary Management of Refractory Insulinomas. Clin Endocrinol (Oxf) (2018) 88(5):615–24. doi: 10.1111/cen.13528
39. Hennrich U, Kopka K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals (Basel) (2019) 12(3):114. doi: 10.3390/ph12030114
40. Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, Herder WW, Feelders RA, et al. Radiolabeled Somatostatin Analog [177Lu-DOTA0,Tyr3]octreotate in Patients With Endocrine Gastroenteropancreatic Tumors. J Clin Oncol (2005) 23(12):2754–62. doi: 10.1200/JCO.2005.08.066
41. Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, Herder WW, et al. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients With Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res (2017) 23(16):4617–24. doi: 10.1158/1078-0432.CCR-16-2743
42. Kwekkeboom DJ, de Herder WW, Kam BL, van Essen M, Kooij PP, Feelders RA, et al. Treatment With the Radiolabeled Somatostatin Analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, Efficacy, and Survival. J Clin Oncol (2008) 26(13):2124–30. doi: 10.1200/JCO.2007.15.2553
43. Ong GS, Henley DE, Hurley D, Turner JH, Claringbold PG, Fegan PG. Therapies for the Medical Management of Persistent Hypoglycaemia in Two Cases of Inoperable Malignant Insulinoma. Eur J Endocrinol (2010) 162(5):1001–8. doi: 10.1530/EJE-09-1010
44. van Schaik E, van Vliet EI, Feelders RA, Krenning EP, Khan S, Kamp K, et al. Improved Control of Severe Hypoglycemia in Patients With Malignant Insulinomas by Peptide Receptor Radionuclide Therapy. J Clin Endocrinol Metab (2011) 96(11):3381–9. doi: 10.1210/jc.2011-1563
45. Magalhães D, Sampaio IL, Ferreira G, Bogalho P, Martins-Brando D, Santos R, et al. Peptide Receptor Radionuclide Therapy With 177Lu-DOTA-TATE as a Promising Treatment of Malignant Insulinoma: A Series of Case Reports and Literature Review. J Endocrinol Invest (2019) 42(3):249–60. doi: 10.1007/s40618-018-0911-3
46. Iglesias P, Martínez A, Gajate P, Alonso T, Navarro T, Díez JJ. Long-Term Effect Of 177lu-Dotatate on Severe and Refractory Hypoglycemia Associated With Malignant Insulinoma. AACE Clin Case Rep (2019) 5(6):e330–3. doi: 10.4158/ACCR-2019-0086
47. Costa R, Costa R, Bacchi CE, Almeida Filho P. Metastatic Insulinoma Managed With Radiolabeled Somatostatin Analog. Case Rep Endocrinol (2013) 252159. doi: 10.1155/2013/252159
48. Makis W, McCann K, McEwan AJB. Metastatic Insulinoma Pancreatic Neuroendocrine Tumor Treated With 177Lu-DOTATATE Induction and Maintenance Peptide Receptor Radionuclide Therapy: A Suggested Protocol. Clin Nucl Med (2016) 41(1):53–54.49. doi: 10.1097/RLU.0000000000001023
Keywords: hypoglycemia, insulinoma, diabetes, type 2 diabetes, lutate, lutetium, neuroendocrine tumor
Citation: Kumar S, Melek M and Rohl P (2022) Case Report: Hypoglycemia Due to Metastatic Insulinoma in Insulin-Dependent Type 2 Diabetes Successfully Treated With 177 Lu-DOTATATE. Front. Endocrinol. 13:906012. doi: 10.3389/fendo.2022.906012
Received: 28 March 2022; Accepted: 19 April 2022;
Published: 24 May 2022.
Edited by:
Barbara Altieri, University Hospital of Wuerzburg, GermanyReviewed by:
Shaobo Yao, First Affiliated Hospital of Fujian Medical University, ChinaAshwani Sood, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2022 Kumar, Melek and Rohl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shejil Kumar, c2hlamlsX2t1bWFyQGhvdG1haWwuY29t
 Shejil Kumar
Shejil Kumar Mariah Melek
Mariah Melek Peter Rohl
Peter Rohl