- 1Department of Endocrinology, Affiliated Hospital 2 of Nantong University and First People’s Hospital of Nantong City, Nantong, China
- 2Department of Ophthalmology, Affiliated Hospital 2 of Nantong University and First People’s Hospital of Nantong City, Nantong, China
Background: Dyslipidemia may contribute to low bone turnover in patients with type 2 diabetes (T2D) through mediating oxidative stress and atherosclerosis. The low-density lipoprotein cholesterol/apoprotein B (LDL-C/Apo B) ratio is a surrogate marker of small and density low-density lipoprotein cholesterol (sd-LDL-C), a most harmful group of LDL-Cs. The present study aimed to investigate the association between the LDL-C/Apo B ratio and bone turnover in patients with T2D.
Methods: This study was a cross-sectional study enrolled patients with T2D from January 2021 to December 2021. Each participant was assessed for lipid profiles, bone turnover markers (BTMs), lumbar spine (L1-L4) and hip dual-energy X-ray absorptiometry (DXA) scans. Osteoporosis was diagnosed as a T-score lower than or equal to -2.5 at the spine or hip.
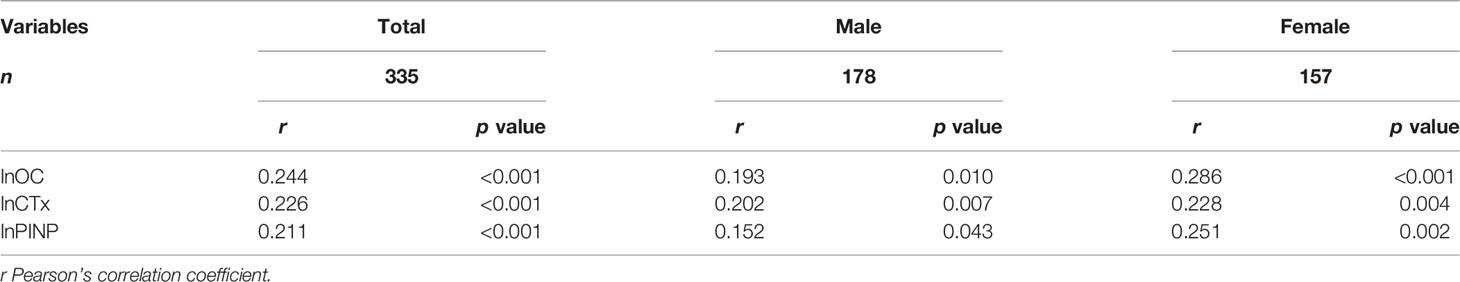
Results: A total of 335 patients with T2D were enrolled in the study, and the LDL-C/Apo B ratio ranged from 0.78 to 4.00. Along with the LDL-C/Apo B ratio tertile ascending, osteocalcin (OC), C-terminal telopeptide (CTx) and N-terminal propeptide of type-I procollagen (PINP) levels gradually increased (all p < 0.05). There were no differences in lumbar spine and hip T-score, proportion of osteoporosis (all p > 0.05) among the three subgroups. The LDL-C/Apo B ratio was positively correlated with lnOC (r = 0.244, p < 0.001), lnCTx (r = 0.226, p < 0.01) and lnPINP (r = 0.211, p < 0.001). These significant positive correlations persisted even when divided into male and female subgroups. Furthermore, three multiple linear regression analyses were constructed to investigate the independent association of the LDL-C/Apo B ratio with the BTMs levels. After adjusting for other clinical parameters, the LDL-C/Apo B ratio was still significantly associated with OC level (β = 0.199, t = 3.348, p < 0.01), CTx level (β = 0.238, t = 4.084, p < 0.001) and PINP level (β = 0.162, t = 2.741, p < 0.01).
Conclusion: The LDL-C/Apo B ratio was significantly and positively associated with BTMs in patients with T2D. In clinical practice, more attention should be paid to the patients with T2D whose LDL-C/Apo B ratio is relatively low for the purpose of maintaining bone health.
Introduction
Type 2 diabetes (T2D) and its chronic complications have posed a great threat to global public health, among which bone fragility has attracted more and more attention due to its high incidence, high disability rate and serious impact on quality of life (1). Bone fragility may contribute to an up to three-fold increased risk of lip fractures and more common wrist and foot fractures in people with T2D than in healthy people (2). However, bone mineral density (BMD) is not sufficient to reflect alternations in bone fragility in patients with T2D, as studies have shown that these patients have a 5-10% increase in BMD compared to their peers without T2D (3). The maintenance of bone health requires the continuous replacement of worn bone tissue with newly synthesized calcified bone matrix throughout life, a process named as bone turnover (4). Bone turnover markers (BTMs) include osteocalcin (OC), C-terminal telopeptide (CTx) and N-terminal propeptide of type-I procollagen (PINP), and a series of studies have consistently concluded that BTMs were significantly reduced and were associated with increased risks of fragility fracture in patients with T2D compared to healthy controls (5). Therefore, it is of great significance to discover and timely intervene the risk factors of low bone turnover in patients with T2D to improve the prognosis of these patients.
T2D is usually accompanied by hyperlipidemia due to insulin resistance, and hyperlipidemia may be involved in multiple chronic complications of T2D (6). In vitro studies showed that oxidized lipids reduced bone turnover by inhibiting osteoblast differentiation and inducing osteoclast differentiation (7). Low-density lipoprotein cholesterol (LDL-C) is a heterogeneous group of lipoproteins, among which with smaller sizes and heavier densities were known as small and density LDL-C (sdLDL-C) (8). Compared with other LDL-Cs, sdLDL-C is characterized by low affinity with LDL receptor, long half-life, susceptible to oxidation, easy to penetrate into the artery wall and so on (9). The LDL-C/apolipoprotein B (LDL-C/Apo B) ratio is a surrogate marker of LDL particle size, and the smaller the LDL-C/Apo B ratio is, the more dominant SD-LDL particles are in LDL particles (10). Due to the methods of detecting sd-LDL-C are laborious, the LDL-C/Apo B ratio is commonly used in clinical work and scientific research (10). Clinical studies revealed that the LDL-C/Apo B ratio was significantly negatively associated with instability of atherosclerotic coronary atherosclerotic plaques (11) and restenosis of coronary stents (12). As bone is highly vascularized connective tissue, affecting the blood supply for bone tissue contributes to the decline of bone turnover (13). The LDL-C/Apo B ratio was closely related to atherosclerosis and oxidative stress, which both were major pathogenesis of low bone turnover in patients with T2D. Therefore, we speculated that the LDL-C/Apo B ratio might be closely associated with BTMs in patients with T2D; those with a lower LDL-C/Apo B ratio may have greater suppression in bone turnover than those with a higher LDL-C/Apo B ratio. However, few studies have explored the relationship between the two.
In the present study, we aimed to estimate the relationship of the LDL-C/Apo B ratio with BTMs in patients with T2D.
Methods
Study Design and Participants
This was an observational cross-sectional study which was performed among patients diagnosed with T2D at the Second Affiliated Hospital of Nantong University between January 2021 to October 2021 (14). The main exclusion criteria were as follows: type 1 diabetes, previous use of steroids and anabolic steroids, previous use of antiosteoporosis drugs (e.g., vitamin D, calcium tablet, bisphosphonates, denosumab and selective estrogen receptor modulators), previous or current received antiandrogen therapy, previous use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, previous or current malignant tumors, chronic hepatitis and heart failure, acute diabetic complications, history of lumbar surgery, history of thyroid or parathyroid disease. Finally, total 335 patients with T2D were included in the present study. Written informed consent was given after each subject fully understood the present study protocol. The study followed the Declaration of Helsinki thoroughly and was approved by the medical research ethics committee of the Second Affiliated Hospital of Nantong University.
Basic Data Collection
Clinical data including the demographic data, lifestyle, medication history and diagnosis history of diseases were collected by interviewing and examining each participant upon enrollment. Body mass index (BMI) was calculated as the weight/height squared. Blood pressure was measured by a standard mercury sphygmomanometer, and the average of three recordings was recorded.
Laboratory Examination
Fasting blood samples and fresh first-void morning urine samples were collected for respective measurement of blood indexes, urinary albumin and urinary creatinine after enrollment. The urinary albumin-to-creatinine ratio (UACR) was calculated as the ratio of urinary albumin to urinary creatinine. Lipid profiles such as triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL-C), LDL-C, apoprotein A (Apo A) and apoprotein B (Apo B) levels, glucose, uric acid (UA), creatinine (Cr) and cystatin C levels were measured using an automated biochemical analyzer (Model 7600, Hitachi) with the inter-assay and intra-assay coefficients of variation (CVs) < 2.8%, and the LDL-C/Apo B ratio was calculated as the ratio of LDL-C to Apo B ratio. According to the CKD-EPI creatinine-cystatin C equation, estimated glomerular filtration rate (eGFR) was calculated (2012) (15). Chronic kidney disease (CKD) was defined based on an eGFR < 60 ml/min/1.73 m2 or a UACR ≥ 30 mg/g lasting more than 3 months (16). HbA1c levels were measured with an ion exchange-based high-performance liquid chromatography (HPLC) method in a hemoglobin analysis system (D-10, Bio-Rad) with the inter-assay and intra-assay CVs < 3.0%. Serum C-peptide (CP) levels were measured by the chemiluminescence method in an immunoassay system (DxI 800, Beckman Coulter) with the inter-assay and intra-assay CVs < 4.0%. In order to eliminate the influence of exogenous insulin, HOMA-IRCP which was defined as (fasting glucose × fasting CP)/22.5 was adopted as an indicator of insulin resistance (17). Serum OC, CTx, PINP, parathyroid hormone (PTH) and vitamin D levels were analyzed on an automated immunoassay system (iSYS, Immunodiagnostic Systems Ltd., Boldon) using a chemiluminescence method.
The corresponding CVs from the manufacturers as follows: OC < 2.0%, CTx < 5.4%, PINP < 4.5%, PTH < 4.5%, and vitamin D < 4.0%.
Diagnosis of Peripheral Artery Disease
The ankle-brachial index (ABI) was detected in each participant using the color Doppler blood flow device (Chioy Medical, Beijing) under the operation of an experienced physician. Then peripheral artery disease (PAD) was diagnosed with reference to the Inter-Society Consensus for the Management of Peripheral Arterial Disease guideline and based the ABI values (18).
Measurement of Bone Mineral Density
All participants underwent spine (L1-L4) and hip dual-energy X-ray absorptiometry (DXA) scans on Prodigy Scanners (GE-Healthcare, Madison) by trained investigators, and the results were analyzed according to the manufacturer’s recommendations. The calculation of T-score was based on the peak BMD in healthy young people of the same race and gender, and the calculation formula of T-score was (measured BMD - peak BMD in healthy young people of the same race and gender)/standard deviation of peak BMD in healthy young people of the same race and sex. The daily CV value of DXA was controlled below 0.24%. Average T-scores of L1-L4 and T-scores of hips were recorded for further analyses. Osteoporosis was diagnosed as a T-score lower than or equal to -2.5 at the spine or hip (19).
Statistical Analyses
The total participants were divided into three subgroups based on the LDL-C/Apo B ratio. Kolmogorov-Smirnov test was first conducted to test whether continuous variables conformed to normal distribution. In order to achieve a normal distribution for further analysis, a natural logarithm transformation (ln) was applied, such as lnOC, lnCTX and lnPNIP. The normally and skewed distributed continuous variables and the categorical variables were respectively described as mean ± SD, median (25 and 75% interquartile) and frequencies (percentages). We adopted the one-way analysis of variance, the Kruskal-Wallis test and the chi-square test to compare differences in normally distributed data, skewed distributed data and categorical data, respectively. Pearson’s bivariate correlation analyses were applied to investigate the correlations of the LDL-C/Apo B ratio with BTMs in the total population and separately in men and women. Furthermore, we constructed three multiple linear regression analyses to investigate the independent association of the LDL-C/Apo B ratio with the BTMs levels. Before conducting the linear regression analyses, the case analyses screening outliers were carried out first. If the standardized residual values were not in the range of -3 to 3, the outliers should be considered. Later, the case records were retrieved to exclude the abnormal data as a result of typing errors, and if not, the corresponding data were removed. Data analyses were performed on SPSS statistical software 18.0 (IBM SPSS Inc., USA). A value of p < 0.05 was defined as statistical significance.
Results
Clinical Characteristics of the Study Participants
Table 1 showed the clinical characteristics of the total population and the three subgroups based on the LDL-C/Apo B ratio tertiles. Along with the LDL-C/Apo B ratio ascending, lnOC, lnCTx and lnPINP levels gradually increased (all p < 0.05). BMI, proportion of peripheral artery disease, statins use, use of acarbose and TG level were the highest in T1, followed by T2 and T3, while TC and Apo B levels were the highest in T3, followed by T2 and T1 (all p < 0.05). Among the three subgroups, there were significant differences in percentage of hypertension, use of β-blocker and Apo A level (all p < 0.05). However, there were no differences in age, proportion of males, diabetic duration, systolic/diastolic blood pressure, use of other antidiabetic treatments and other antihypertensive treatments, HbA1c level, HOMA-IRCP level, HDL-C level, LDL-C level, UACR level, eGFR level, proportion of diabetic kidney disease, PTH level, vitamin D level, lumbar spine and hip T-score, proportion of osteoporosis (all p > 0.05).
Relationships Between the LDL-C/Apo B Ratio and BTMs
As illustrated in Table 2, the LDL-C/Apo B ratio was positively associated with lnOC, lnCTx and lnPINP levels (r = 0.244, 0.226 and 0.211, respectively, all p < 0.001). These significant and positive correlations persisted even when divided into male and female subgroups.
Multiple Linear Regression Models Displayed Independent Associations of the LDL-C/Apo B Ratio With BTMs Levels
In Table 3, the LDL-C/Apo B ratio was significantly and positively associated with lnOC level (β = 0.244, t = 4.582, p < 0.001, R2 = 0.059), lnCTx level (β = 0.226, t = 4.220, p < 0.001, R2 = 0.051) and lnPINP level (β = 0.211, t = 3.933, p < 0.001, R2 = 0.045). After adding the other clinical covariates in each model step by step, the R2 was gradually increased. In the model 1, after adjusting for age, sex, diabetic duration, BMI, systolic/diastolic blood pressure and smoking history, the LDL-C/Apo B ratio was significantly and positively associated with lnOC level (β = 0.193, t = 3.426, p < 0.01, R2 = 0.073), lnCTx level (β = 0.213, t = 3.885, p < 0.001, R2 = 0.127) and lnPINP level (β = 0.158, t = 2.860, p < 0.01, R2 = 0.109). Antidiabetic treatments, antihypertensive treatments and statins medications were then added as clinical covariates in the model 2, and the LDL-C/Apo B ratio was significantly and positively associated with lnOC level (β = 0.192, t = 3.238, p < 0.01, R2 = 0.107), lnCTx level (β = 0.219, t = 3.833, p < 0.001, R2 = 0.175) and lnPINP level (β = 0.155, t = 2.644, p < 0.01, R2 = 0.131). The fully adjusted model 3 further adjusted for HbA1c, HOMA-IRCP and eGFR, the LDL-C/Apo B ratio was still significantly and positively associated with lnOC level (β = 0.199, t = 3.348, p < 0.01, R2 = 0.171), lnCTx level (β = 0.238, t = 4.084, p < 0.001, R2 = 0.202) and lnPINP level (β = 0.162, t = 2.741, p < 0.01, R2 = 0.172).
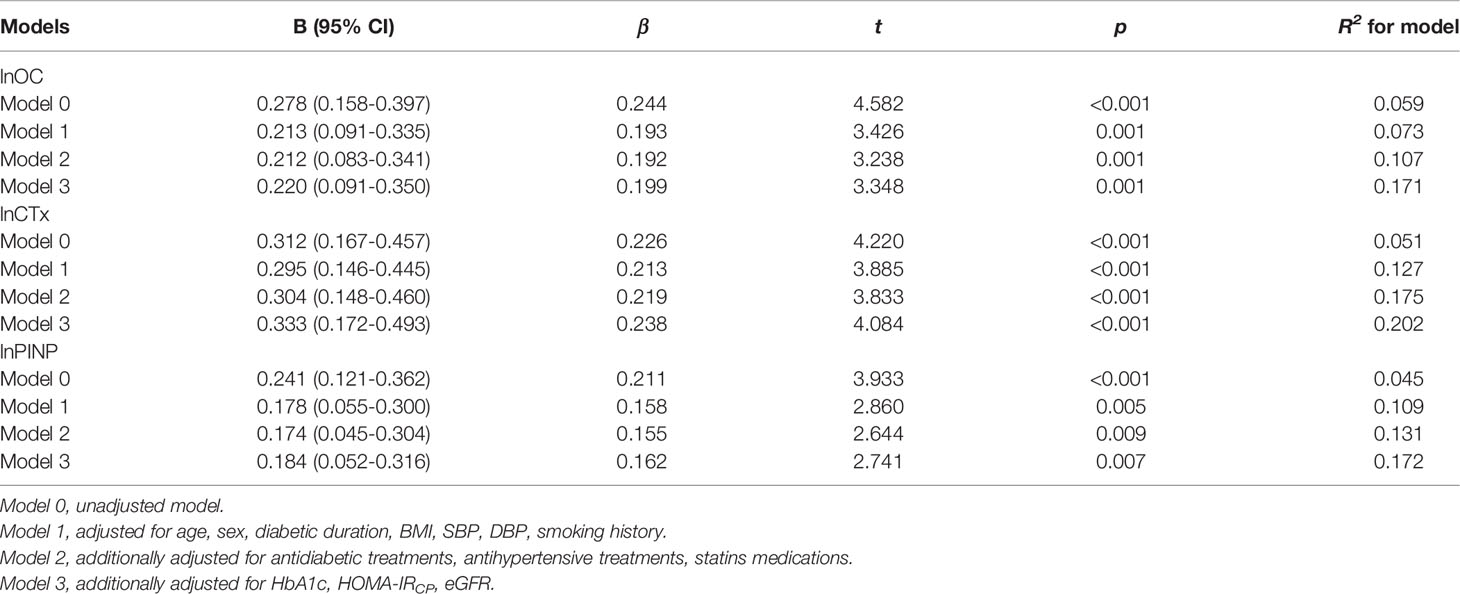
Table 3 Multiple linear regression models displayed independent associations of the LDL-C/Apo B ratio with BTMs levels.
Discussion
In the present study, we investigated the association between the LDL-C/Apo B ratio with BTMs in patients with T2D. The main findings are as follows: first, with the increase of the LDL-C/Apo B ratio tertile, serum OC, CTx and PINP levels gradually increased; second, the LDL-C/Apo B ratio was significantly and positively related to serum OC, CTx and PINP levels; third, after adjusting for sex, age, diabetic duration and other clinical factors via multiple linear regression analysis, the LDL-C/Apo B ratio was still significantly and positively associated with serum OC, CTx and PINP level. Collectively, a decreased LDL-C/Apo B ratio is independently associated with low bone turnover in patients with T2D.
Existing guidelines recommend detecting BMD (T-score) via DXA for the initial diagnosis of osteoporosis, and osteoporosis is diagnosed based on a T-score lower than or equal to -2.5 at the spine or hip (19). However, the Rotterdam study showed an increased risk of fracture despite higher BMD in patients with T2D or impaired glucose tolerance (IGT) compared with those with normal glucose metabolism (20). A meta-analysis got similar results that with BMDs in patients with T2D were 4-5% higher than in healthy population (21). These results suggested that BMD should not be the only concern for bone health in patients with T2D. Manavalan et al. performed bone biopsies in patients with T2D and controls, and found significant reduced bone formation rate, osteoid surface, and osteoblast surface in T2D patients, suggesting the importance of bone turnover (22). Most studies demonstrated that bone turnover in patients with T2D was decreased (5, 23, 24). Hence, the present study aimed to explore the relationship between the LDL-C/Apo B ratio and BTMs in patients with T2D.
Risk factors for low bone turnover in patients with T2D include glucose control, use of hypoglycemic drugs, hyperlipidemia, advanced glycation end products (AGEs), bone microvascular disease and so on (25). Poor glycemic control is the initiating factor of the onset and progression of diabetic chronic complications, and a cross-sectional study of men with T2D revealed that a HbA1c greater than 7% was closely associated with decreased bone turnover (26). Reactive oxygen species (ROS) is an important mediator of diabetic chronic complications induced by hyperglycemia, hyperlipemia and AGEs (27). ROS can directly inhibit Wnt/β-catenin signaling pathway, which is critical for bone formation (28). ROS is also a key signaling molecule mediating inflammation, which induces the expression of Dickkopf-related protein 1 (DKK1) (29). Subsequently, DKK1 can bind to lipoprotein receptor-related protein 6 (LRP6) to form a complex contributing to the internalization of LRP6. LRP6, a member of the LDLR gene family, is a co-receptor of Wnt, and the internalization of LRP6 can lead to the inhibition of Wnt/β-catenin signaling pathway (30). In addition, in hyperlipidemia rat model hyperlipidemia could inhibit dishevelled-2 expression and its phosphorylation, thus inhibiting Wnt/β-catenin signaling pathway (31). Consistently, the present study found that a low LDL-C/Apo B ratio was an independent contributor to low bone turnover in patients with T2D.
LDL-C is an important risk factor for atherosclerosis, and a study revealed that serum LDL-C was an independent risk factor for fragility fractures in postmenopausal women (32). Compared with other LDL-Cs, sd-LDL-C has delayed catabolism and increased oxidation susceptibility (33). In high-fat fed rats, oxidized LDL could inhibit bone formation by blocking osteoblast progenitor cells differentiation and inhibiting OC expression in marrow (34). Similarly, in our study the LDL-C/Apo B ratio was positively associated with serum OC and PINP levels. Additionally, we also observed a positive relationship between the LDL-C/Apo B ratio and CTx level. However, oxidative lipids could promote the differentiation of marrow preosteoclasts in vitro, so oxidized lipids might be positively related to serum CTx level (35). This discrepancy may be explained by other mechanisms of low bone turnover in T2D.
Bone microvascular disease is also a vital pathogenesis of decreased bone turnover in patients with T2D (13). As well as peripheral vessels, a histological study showed that intraosseous arterioles also occurred atherosclerosis plagues (36). Legs without atherosclerotic plaques had higher BMD than those with atherosclerotic plaque (37). In addition, the accumulation of oxidized lipoprotein particles was observed in the subcutaneous space surrounding blood vessels (38). In the present study, proportion of peripheral artery disease (PAD) increased from the first tertile to the third tertile of the LDL-C/Apo B ratio. These results highlighted the role of lipids in affecting bone turnover by inducing bone microvascular injury.
In this study, the LDL-C/Apo B ratio was significantly and positively correlated with BTMs levels even after adjusting for other covariates. In line with these results, a review suggested that dyslipidemia might aggravate atherosclerosis independently of serum LDL-C levels, possibly due to the presence of sd-LDL-C (39). Worse, uses of statin did not significantly reduce serum sd-LDL-C levels in patients with acute ischemic stroke (40). Similarly, the present study also observed that proportion of statin use was highest in the first tertile of the LDL-C/Apo B ratio. Therefore, adequate attention should be paid to the LDL-C/Apo B ratio in patients with T2D.
Although the present study observed an independent positive correlation between the LDL-C/Apo B ratio and BTMs, we failed to observe a correlation between the LDL-C/Apo B ratio and T-score. This was similar to other studies (26, 41), which might ascribe to the fact that BMD or T-score could not fully reflect bone alternations in T2D.
Several limitations of this study should be pointed out. First, the causal relationship between the LDL-C/Apo B ratio and bone turnover could not be concluded based on the present study, which was an observational cross-sectional study. Longitudinal and intervention studies are needed to address the limitation. Second, although we intended to present the trend of BTMs among the LDL-C/Apo B ratio tertiles in Table 1, grouping by the tertiles of LDL-C/Apo B ratio had no biological significance. In future studies, the association between the LDL-C/Apo B ratios and sd-LDL-C levels needs to be evaluated, and then grouping may be more meaningful than grouping directly by the tertiles of this ratio. Third, the LDL-C/Apo B ratio is a substitute index rather than the gold standard for evaluating sd-LDL-C, but the relationship between LDL-C/Apo B ratio and sd-LDL-C has been fully verified by multiple clinical studies. Fourth, high-resolution peripheral quantitative computed tomography (HRpQCT) may reflect bone microarchitectural properties better than DXA, so HRpQCT should be carried out simultaneously in future studies. Fifth, the generalization of this study was limited by the fact that all subjects enrolled in this study were Chinese.
In a word, the LDL-C/Apo B ratio was significantly and positively associated with BTMs in patients with T2D. In clinical practice, more attention should be paid to the patients with T2D whose LDL-C/Apo B ratio is relatively low for the purpose of maintaining bone health. This study is a hypothesis generating study, and we hypothesis that a predominance of sd-LDL-C in LDL-Cs may be an important risk factor for low bone turnover in patients with T2D by inducing oxidative stress and artery trauma. This hypothesis requires further follow-up and intervention studies to evaluate the effects of sd-LDL-C on bone turnover in patients with T2D, and to explore the mechanisms of sd-LDL-C inducing low bone turnover in animal and cell experiments.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the medical research ethics committee of Second Affiliated Hospital of Nantong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Cf-L and W-sL participated in the design of the study, data collection, analysis of the data, and drafting of the manuscript. X-qW and J-bS conceived of the study, participated in its design and revised the manuscript. X-qG, H-yH, and L-yH participated in data collection. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Medical Research Project of Health Commission of Nantong (MB2020012) and the Science and Technology Support Program of Nantong (HS2020005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li G, Prior JC, Leslie WD, Thabane L, Papaioannou A, Josse RG, et al. Frailty and Risk of Fractures in Patients With Type 2 Diabetes. Diabetes Care (2019) 42:507–13. doi: 10.2337/dc18-1965
2. Eller-Vainicher C, Cairoli E, Grassi G, Grassi F, Catalano A, Merlotti D, et al. Pathophysiology and Management of Type 2 Diabetes Mellitus Bone Fragility. J Diabetes Res (2020) 22:7608964. doi: 10.1155/2020/7608964
3. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, et al. Mechanisms of Diabetes Mellitus-Induced Bone Fragility. Nat Rev Endocrinol (2017) 13:208–19. doi: 10.1038/nrendo.2016.153
4. Clarke B. Normal Bone Anatomy and Physiology. Clin J Am Soc Nephrol (2008) 3 Suppl 3:S131–9. doi: 10.2215/CJN.04151206
5. Starup-Linde J, Eriksen SA, Lykkeboe S, Handberg A, Vestergaard P. Biochemical Markers of Bone Turnover in Diabetes Patients–a Meta-Analysis, and a Methodological Study on the Effects of Glucose on Bone Markers. Osteoporos Int (2014) 25:1697–708. doi: 10.1007/s00198-014-2676-7
6. Opazo-Ríos L, Mas S, Marín-Royo G, Mezzano S, Gómez-Guerrero C, Moreno JA, et al. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int J Mol Sci (2020) 21:2632. doi: 10.3390/ijms21072632
7. Laroche M, Pécourneau V, Blain H, Breuil V, Chapurlat R, Cortet B, et al. Osteoporosis and Ischemic Cardiovascular Disease. Joint Bone Spine (2017) 84:427–32. doi: 10.1016/j.jbspin.2016.09.022
8. Hirano T. Pathophysiology of Diabetic Dyslipidemia. J Atheroscler Thromb (2018) 25:771–82. doi: 10.5551/jat.RV17023
9. Mikhailidis DP, Elisaf M, Rizzo M, Berneis K, Griffin B, Zambon A, et al. "European Panel on Low Density Lipoprotein (LDL) Subclasses": A Statement on the Pathophysiology, Atherogenicity and Clinical Significance of LDL Subclasses. Curr Vasc Pharmacol (2011) 9:533–71. doi: 10.2174/157016111796642661
10. Cavalcante Lda S, da Silva EL. Application of a Modified Precipitation Method for the Measurement of Small Dense LDL-Cholesterol (Sd-LDL-C) in a Population in Southern Brazil. Clin Chem Lab Med (2012) 50:1649–56. doi: 10.1515/cclm-2011-0797
11. Rabizadeh S, Rajab A, Mechanick JI, Moosaie F, Rahimi Y, Nakhjavani M, et al. LDL/apo B Ratio Predict Coronary Heart Disease in Type 2 Diabetes Independent of ASCVD Risk Score: A Case-Cohort Study. Nutr Metab Cardiovasc Dis (2021) 31:1477–85. doi: 10.1016/j.numecd.2021.01.013
12. Akutsu N, Hori K, Mizobuchi S, Ogaku A, Koyama Y, Fujito H, et al. Clinical Importance of the LDL-C/apolipoprotein B Ratio for Neointimal Formation After Everolimus-Eluting Stent Implantations. J Atheroscler Thromb (2022) 29:536–50. doi: 10.5551/jat.60954
13. Griffith JF, Yeung DK, Tsang PH, Choi KC, Kwok TC, Ahuja AT, et al. Compromised Bone Marrow Perfusion in Osteoporosis. J Bone Miner Res (2008) 23:1068–75. doi: 10.1359/jbmr.080233
14. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care (2013) 36 Suppl 1:S67–74. doi: 10.2337/dc13-S067
15. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating Glomerular Filtration Rate From Serum Creatinine and Cystatin C. N Engl J Med (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
16. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Diabetes Care (2014) 37:2864–83. doi: 10.2337/dc14-1296
17. Wang XH, Xu F, Cheng M, Wang X, Zhang DM, Zhao LH, et al. Fasting Serum Total Bile Acid Levels Are Associated With Insulin Sensitivity, Islet β-Cell Function and Glucagon Levels in Response to Glucose Challenge in Patients With Type 2 Diabetes. Endocr J (2020) 67:1107–17. doi: 10.1507/endocrj.EJ20-0201
18. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr Pract (2020) 26(Suppl 1):1–46. doi: 10.4158/GL-2020-0524SUPPL
19. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC Ii). J Vasc Surg (2007) 45 Suppl S:S5–67. doi: 10.1016/j.jvs.2006.12.037
20. de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone Mineral Density and Fracture Risk in Type-2 Diabetes Mellitus: The Rotterdam Study. Osteoporos Int (2005) 16:1713–20. doi: 10.1007/s00198-005-1909-1
21. Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML. Type 2 Diabetes and the Skeleton: New Insights Into Sweet Bones. Lancet Diabetes Endocrinol (2016) 4:159–73. doi: 10.1016/S2213-8587(15)00283-1
22. Manavalan JS, Cremers S, Dempster DW, Zhou H, Dworakowski E, Kode A, et al. Circulating Osteogenic Precursor Cells in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab (2012) 97:3240–50. doi: 10.1210/jc.2012-1546
23. Jiajue R, Jiang Y, Wang O, Li M, Xing X, Cui L, et al. Suppressed Bone Turnover was Associated With Increased Osteoporotic Fracture Risks in non-Obese Postmenopausal Chinese Women With Type 2 Diabetes Mellitus. Osteoporos Int (2014) 25(8):1999–2005. doi: 10.1007/s00198-014-2714-5
24. Colleluori G, Aguirre L, Dorin R, Robbins D, Blevins D, Barnouin Y, et al. Hypogonadal Men With Type 2 Diabetes Mellitus Have Smaller Bone Size and Lower Bone Turnover. Bone (2017) 99:14–9. doi: 10.1016/j.bone.2017.03.039
25. Napoli N, Strollo R, Paladini A, Briganti SI, Pozzilli P, Epstein S. The Alliance of Mesenchymal Stem Cells, Bone, and Diabetes. Int J Endocrinol (2014) 2014:690783. doi: 10.1155/2014/690783
26. Joad S, Ballato E, Deepika F, Gregori G, Fleires-Gutierrez AL, Colleluori G, et al. Hemoglobin A1c Threshold for Reduction in Bone Turnover in Men With Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne) (2021) 12:788107. doi: 10.3389/fendo.2021.788107
27. Panigrahy SK, Bhatt R, Kumar A. Reactive Oxygen Species: Sources, Consequences and Targeted Therapy in Type 2 Diabetes. J Drug Target (2017) 25:93–101. doi: 10.1080/1061186X.2016.1207650
28. Duan P, Bonewald LF. The Role of the Wnt/β-Catenin Signaling Pathway in Formation and Maintenance of Bone and Teeth. Int J Biochem Cell Biol (2016) 77:23–9. doi: 10.1016/j.biocel.2016.05.015
29. Dong X, Wang Z, Wang H, Lan J. The Research of Dishevelled-2 in Dental Implant Osseointegration of Hyperlipidemic Rats. Int J Oral Maxillofac Implants (2018) 33:351–6. doi: 10.11607/jomi.6015
30. Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, et al. Interferon-Gamma Regulates Intestinal Epithelial Homeostasis Through Converging Beta-Catenin Signaling Pathways. Immunity (2010) 32:392–402. doi: 10.1016/j.immuni.2010.03.001
31. Jeong W, Jho EH. Regulation of the Low-Density Lipoprotein Receptor-Related Protein LRP6 and Its Association With Disease: Wnt/β-Catenin Signaling and Beyond. Front Cell Dev Biol (2021) 9:714330. doi: 10.3389/fcell.2021.714330
32. Yamauchi M, Yamaguchi T, Nawata K, Tanaka K, Takaoka S, Sugimoto T. Increased Low-Density Lipoprotein Cholesterol Level Is Associated With non-Vertebral Fractures in Postmenopausal Women. Endocrine (2015) 48:279–86. doi: 10.1007/s12020-014-0292-0
33. Norata GD, Raselli S, Grigore L, Garlaschelli K, Vianello D, Bertocco S, et al. Small Dense LDL and VLDL Predict Common Carotid Artery IMT and Elicit an Inflammatory Response in Peripheral Blood Mononuclear and Endothelial Cells. Atherosclerosis (2009) 206:556–62. doi: 10.1016/j.atherosclerosis.2009.03.017
34. Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic High-Fat Diet Reduces Bone Mineralization in Mice. J Bone Miner Res (2001) 16:182–8. doi: 10.1359/jbmr.2001.16.1.182
35. Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 Enhances Receptor-Activated NFkappa B Ligand (RANKL)-Dependent Osteoclastic Potential of Marrow Hematopoietic Precursors via the cAMP Pathway. J Biol Chem (2002) 277:14221–6. doi: 10.1074/jbc.M111551200
36. Laroche M, Ludot I, Thiechart M, Arlet J, Pieraggi M, Chiron P, et al. Study of the Intraosseous Vessels of the Femoral Head in Patients With Fractures of the Femoral Neck or Osteoarthritis of the Hip. Osteoporos Int (1995) 5:213–7. doi: 10.1007/BF01774009
37. Pennisi P, Russo E, Gaudio A, Veca R, D'Amico F, Mangiafico RA, et al. The Association Between Carotid or Femoral Atherosclerosis and Low Bone Mass in Postmenopausal Women Referred for Osteoporosis Screening. Does Osteoprotegerin Play a Role?. Maturitas (2010) 67:358–62. doi: 10.1016/j.maturitas.2010.07.013
38. Pirih F, Lu J, Ye F, Bezouglaia O, Atti E, Ascenzi MG, et al. Adverse Effects of Hyperlipidemia on Bone Regeneration and Strength. J Bone Miner Res (2012) 27:309–18. doi: 10.1002/jbmr.541
39. Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-Based Chronic Disease, Adiposity and Dysglycemia Drivers: JACC State-Of-the-Art Review. Am Coll Cardiol (2020) 75:525–38. doi: 10.1016/j.jacc.2019.11.044
40. Yao T, Long Q, Li J, Li G, Ding Y, Cui Q, et al. Small Dense Low-Density Lipoprotein Cholesterol Is Strongly Associated With NIHSS Score and Intracranial Arterial Calcification in Acute Ischemic Stroke Subjects. Sci Rep (2020) 10:7645. doi: 10.1038/s41598-020-64715-9
Keywords: type 2 diabetes, bone turnover, low-density lipoprotein cholesterol/apolipoprotein B ratio, small and density low-density lipoprotein cholesterol, osteocalcin, C-terminal telopeptide, N-terminal propeptide of type-I procollagen
Citation: Lu C-f, Liu W-s, Huang H-y, Ge X-q, Hua L-y, Wang X-q and Su J-b (2022) The Positive Relationship Between the Low-Density Lipoprotein Cholesterol/Apoprotein B Ratio and Bone Turnover Markers in Patients With Type 2 Diabetes. Front. Endocrinol. 13:903336. doi: 10.3389/fendo.2022.903336
Received: 24 March 2022; Accepted: 22 April 2022;
Published: 09 June 2022.
Edited by:
Jakob Starup-Linde, Aarhus University Hospital, DenmarkReviewed by:
Peter Vestergaard, Aalborg University Hospital, DenmarkKok Yong Chin, National University of Malaysia, Malaysia
Copyright © 2022 Lu, Liu, Huang, Ge, Hua, Wang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-feng Lu, bGNmMDQxM2N3bDEwMzBAMTI2LmNvbQ==; Xue-qin Wang, d2FuZ3h1ZXFpbjEwOEAxNjMuY29t; Jian-bin Su, c3VqYnpqeEAxNjMuY29t
†These authors have contributed equally to this work
 Chun-feng Lu
Chun-feng Lu Wang-shu Liu1†
Wang-shu Liu1† Jian-bin Su
Jian-bin Su