- 1Centre de recherche en Reproduction, Développement et Santé Intergénérationnelle and Department of Obstetrics, Gynecology, and Reproduction, Faculty of Medicine, Université Laval, Quebec City, QC, Canada
- 2Reproduction, Mother and Child Health, Centre de recherche du centre hospitalier universitaire de Québec—Université Laval, Quebec City, QC, Canada
Defining how genes get turned on and off in a correct spatiotemporal manner is integral to our understanding of the development, differentiation, and function of different cell types in both health and disease. Testis development and subsequent male sex differentiation of the XY fetus are well-orchestrated processes that require an intricate network of cell-cell communication and hormonal signals that must be properly interpreted at the genomic level. Transcription factors are at the forefront for translating these signals into a coordinated genomic response. The GATA family of transcriptional regulators were first described as essential regulators of hematopoietic cell differentiation and heart morphogenesis but are now known to impact the development and function of a multitude of tissues and cell types. The mammalian testis is no exception where GATA factors play essential roles in directing the expression of genes crucial not only for testis differentiation but also testis function in the developing male fetus and later in adulthood. This minireview provides an overview of the current state of knowledge of GATA factors in the male gonad with a particular emphasis on their mechanisms of action in the control of testis development, gene expression in the fetal testis, testicular disease, and XY sex differentiation in humans.
Introduction
The Vertebrate Family of GATA Transcription Factors
The GATA family of transcriptional regulators comprises six factors (GATA1 to 6) in vertebrates. GATA factors regulate gene transcription by binding to a consensus nucleotide sequence—A/TGATAA/G—called the GATA motif that is scattered throughout the genome. The six GATA proteins are typically classified based on their amino acid sequence and tissue distribution patterns. GATA1/2/3 are hallmarks of hematopoietic cell lineages, and consequently are essential for the differentiation of multiple blood cell types (erythrocytes, leukocytes, megakaryocytes) [reviewed in (1, 2)]. By contrast, the GATA4/5/6 proteins (known as the cardiac family of GATA factors) populate tissues of mesodermal and endodermal origin such as the heart, organs of the digestive tract, and gonads [reviewed in (1–5)]. Initial insights into the cardiac subfamily of GATA factors came from global gene inactivation studies in mice. Loss of GATA4 precipitates early embryo lethality because of defects in ventral morphogenesis of the embryo and its subsequent inability to form a functional linear heart tube (6, 7). Lack of Gata6 expression arrests embryo development at an even earlier stage due to defects in extraembryonic development (8). Unlike GATA4 and GATA6, global loss of GATA5 function is not embryonic lethal; rather Gata5-/- mice exhibit prominent genitourinary tract abnormalities in females but not males (9). Identification of GATA gene mutations associated with clinical disease has helped to highlight the essential nature of GATA function in humans. For the GATA4 gene alone, more than 100 mutations have been linked to a large spectrum of cardiac abnormalities [reviewed in (2, 10)].
All GATA proteins share a highly conserved zinc finger DNA-binding domain. The specificity of GATA action is conferred in part through interactions with specific partners [reviewed in (2–4)]. The most notable are the Friend of GATA (FOG) proteins [reviewed in (11)]. GATA factors also interact and functionally cooperate with a multitude of other transcription factors that not only amplify GATA activity but also limit their scope of action to select target genes. This is true for the testis where many potential GATA-interacting partners have been identified (12–18). GATA activity can also be positively or negatively modulated by direct post-translational modifications such as sumoylation, acetylation, and phosphorylation. Post-translational modifications alter GATA4 transcriptional activity by changing either its nuclear localization, DNA-binding, stability, and/or cofactor recruitment [reviewed in (10, 19)].
GATA Factors in the Testis
Developmental and Cell Type-Specific Expression Patterns
Three GATA factors (GATA1/4/6) are found in the testis and their expression profiles differ based on testicular cell type and timing of expression. Gata1 was the first reported to be abundantly transcribed in the testis (20). It is also the only GATA factor limited to a single testicular cell type: the postnatal Sertoli cell (21). GATA1 is absent from the fetal testis and as such is not involved in early testis development (22). The onset of Gata1 expression in mouse Sertoli cells coincides with the first wave of spermatogenesis; thereafter GATA1 levels remain constant and independent of hormones but become progressively spermatogenic stage-dependent (21, 22). GATA1 is therefore an excellent marker of mature Sertoli cells; however, its functions appear to be dispensable for Sertoli cells, at least in mice (23). GATA4 is one of the earliest markers of gonadal development (22, 24). Expression begins in the coelomic epithelial layer of the presumptive genital ridges of both the mouse and chick (24–26). After testis differentiation, GATA4 levels are upregulated in Sertoli, myoid, and Leydig cells [(22, 26, 27) and reviewed in (28)]. High GATA4 levels persist throughout fetal development and into adulthood (22, 27). Like Gata4, the Gata6 gene is transcribed in Sertoli cells of both fetal and adult testes (29, 30). However, unlike Gata4, Sertoli cell Gata6 transcripts only become upregulated during the later fetal stages (29, 30). While Gata6 expression has been documented in at least one Leydig cell line (29), data showing that the gene is also expressed in Leydig cells in vivo is less robust. A review article published in 2014 citing unpublished observations nonetheless reported that GATA6 is present in both late fetal and postnatal Leydig cells in the mouse (5).
Roles in Early Mammalian Gonad Development and Male Sex Determination
Of the three GATA factors expressed in testis, only GATA4 is involved in early gonad development—in the absence of GATA4, coelomic thickening that permits the subsequent growth of the genital ridge is arrested (24). In the same study, the authors also demonstrated that GATA4 acts early being upstream of LHX9 and SF1, two other critical regulators of genital ridge development (31, 32). GATA4 function in gonadal development does not end at the genital ridge stage as revealed by elegant gene inactivation studies in mice, which showed that GATA4 continues to play multiple essential roles with the timing of Gata4 deletion being critical to the phenotypic outcome [reviewed in detail in (5)]. Gata4 deletion prior to sex determination completely blocks testis differentiation and results in XY sex reversal (33). By contrast, Gata4 deletion after male sex determination allows testis differentiation to proceed unfettered (33). In this same model, testis cord development is nonetheless disrupted due to diminished Dmrt1 expression (described more below), and males are undervirilized and eventually become infertile (33). GATA4 action is also required in the postnatal testis where it controls male fertility and steroidogenesis (34–39). Its role in male fertility appears to be linked to regulation of blood-testis barrier integrity and Sertoli cell genes critical for this function (35, 40).
GATA4 initiates testis differentiation by regulating at least two genes: Sex determining region Y chromosome (Sry) and Sry-related homeobox gene 9 (Sox9); both genes are markedly downregulated in newly differentiated testes that lack a fully functional GATA4 protein (41–43). However, as for many of the proposed GATA target genes in the testis, it remains to be seen whether they are indeed direct targets. For the Sry gene, evidence suggests that this might be the case. A detailed analysis of Sry 5’ flanking sequences from 17 mammalian species identified a single broadly conserved 106 bp region termed the Sry proximal conserved interval (SPCI). The SPCI contains a conserved binding motif for WT1, a known activator of both mouse and human SRY transcription (44–46). WT1 has also been reported to directly cooperate with GATA4 on the mouse, pig, and human SRY promoters (15). The requirement for GATA4 in Sry and Sox9 transcription has raised the important question as to whether it also participates in the specification of the Sertoli cell lineage at the time of sex determination. Mice lacking a functional GATA4 protein that can no longer interact with FOG2 do not express any Sertoli cell markers (41), indicating that the GATA4-FOG2 complex (and by extension GATA4 itself) is required for the differentiation of pre-Sertoli cells. Additional insights have come from studies that have reprogrammed fibroblasts into embryonic Sertoli cells. Initial studies showed that GATA4 along with other transcription factors (SF1, WT1, DMRT1, SOX9, DMRT1) are sufficient to induce the formation of embryonic Sertoli-like cells (47–49). A later study refined the mechanism of GATA4 induction of the Sertoli cell fate by showing that it acts upstream of SF1, LHX9, and the anti-Müllerian hormone (AMH) (50).
GATA Factors in Fetal Testis Gene Expression and Function
GATA Control of Fetal Sertoli Cell Gene Expression
Following sex determination, the newly formed testis (by producing key fetal hormones) assumes several important functions in masculinizing the developing male embryo. GATA factors continue to play an important role in regulating genes that participate in this fundamental developmental process. Sertoli cells begin to produce AMH, a glycoprotein hormone belonging to the transforming growth factor beta (TGFβ) superfamily. AMH is most recognized for its role in blocking the Müllerian ducts from developing into female reproductive structures in typical XY males (51). The AMH promoter from multiple species contain at least one consensus GATA motif that is activated by GATA4 (22, 52–55). In cell lines, the mechanism of GATA4 action on AMH transcription has been proposed to involve synergistic interactions with other transcription factors including SF1 and WT1 (12, 15, 56). A more recent in vivo study that inactivated the GATA regulatory motif in the Amh promoter in the mouse has validated not only the Amh gene as a direct target for GATA4 but also the requirement of a GATA4/SF1 interaction for Amh expression in fetal Sertoli cells (57). Endogenous Amh expression is also controlled by direct binding of SF1 and SOX9 to the Amh promoter (58). Unlike SOX9, however, which is required to initiate the production of Amh transcripts in newly differentiated Sertoli cells, GATA4 appears to function like SF1 as an amplifier of Amh transcription (57, 58).
While mutagenesis of the Amh promoter has highlighted the requirement of GATA binding for Amh transcription, simultaneous deletion of Gata4 and Gata6 in Sertoli and Leydig cells in the fetal testis has favored the opposite view (38). GATA1 was first proposed to disrupt GATA4 action at the Amh promoter thereby decreasing Amh expression (59). A separate study reported that AMH levels remained high beyond its normal period of decline in postnatal mouse testis lacking both GATA4 and GATA6 (38). GATA1 was also absent in this model (38). These observations led to the suggestion that GATA factors negatively regulate Amh gene transcription, at least in postnatal Sertoli cells (38). However, male gonads lacking GATA4 and GATA6 also have compromised steroidogenic gene expression during the fetal period, and a reported reduction of serum testosterone beyond puberty (38). Testosterone, acting through the androgen receptor, is known to attenuate AMH production in Sertoli cells (60, 61). This might account for the differences in Amh expression seen in a GATA4/6-deficient mouse testis model where androgen production is notably reduced (38), in comparison to a mouse model where the GATA binding motif in the Amh promoter is mutated and androgen levels are not affected (57).
The Dmrt1 gene has been proposed to be another GATA4 target in the fetal testis (33). DMRT1 is an evolutionarily conserved transcription factor that regulates testis development in all vertebrates (62). Testes from Dmrt1-/- mice, and animals where Gata4 was conditionally inactivated in testes after sex determination, share a similar testicular cord defect beginning at the mid-fetal stage (33). This suggested that Dmrt1 gene expression in Sertoli cells is directly regulated by GATA4. In support of this, the cis-regulatory elements for the Dmrt1 gene have been mapped in primary Sertoli cells cultures and shown to contain multiple active GATA binding motifs (63). However, GATA4 is not required to maintain somatic Dmrt1 expression after fetal development as adult Sertoli cells lacking GATA4 continue to express Dmrt1 (33). Moreover, while both GATA4 and FOG2 are required for the differentiation of pre-Sertoli cells, Dmrt1 expression in fetal Sertoli cells continues to require GATA4 but not FOG2 (33).
Several other Sertoli cell-expressed genes have been proposed to be regulated by GATA factors. These include inhibin α (Inha), inhibin β B (Inhbb), reproductive homeobox 5 (Rhox5), and follicle stimulating hormone receptor (Fshr) (14, 29, 64–69). The GATA-dependence of these additional putative GATA targets have been limited to promoter activation assays and studies where GATA levels have been manipulated in immortalized cell lines. Whether these genes are in fact genuine GATA targets in vivo and what contribution GATA factors have to these genes in terms of fetal Sertoli cell expression remain unanswered.
GATA Control of Fetal Leydig Cell Differentiation and Gene Expression
Leydig cells regulate male sex differentiation during fetal development, and fertility in adults, through the production of two hormones: insulin-like 3 (INSL3) and testosterone. INSL3 initiates the first of two phases of testicular descent during embryogenesis (70, 71). Testosterone is a steroid hormone made from the multistep enzymatic conversion of cholesterol. Testosterone regulates the second phase of testis descent and promotes the masculinization of the Wolffian ducts into organs of the male reproductive tract. In mammals, there are two distinct populations of Leydig cells: fetal Leydig cells (FLCs) present during fetal life and shortly after birth, and adult Leydig cells (ALCs) present in the postnatal testis. FLCs are proposed to derive from progenitor cells originating within the adreno-gonadal primordium and other sources within the mesonephros, neural crest cells, coelomic epithelium [reviewed in (72)]. Many transcription factors have been implicated in FLC differentiation and function [reviewed in (73, 74)]. GATA4 appears to play an integral role in both of these processes. First, XY Gata4−/− embryonic stem (ES) cells fail contribute to FLCs in chimeric mouse embryos (75). Second, teratomas derived from XY Gata4++, but not XY Gata4−/− ES cells, express steroidogenic markers when grown in gonadectomized nude mice (75). Together, these two experimental models provided the first evidence that GATA4 is likely required for the cell autonomous differentiation of FLCs. XY fetuses lacking GATA4 in testes at the time of male sex determination are undervirilized with partially descended testes but whose steroidogenic function is apparently intact (33). A major reduction in the number of cells expressing P450 side chain cleavage enzyme (CYP11A1) and 3β-hydroxysteroid dehydrogenase (3βHSD) only occurs in the testicular interstitium of mice lacking both GATA4 and GATA6 (38), suggesting that FLC differentiation and/or their steroidogenic function requires both factors. These mice also have reduced Insl3 expression; no other in vitro or in vivo data exists, however, that would suggest the Insl3 gene to be a direct transcriptional target for GATA factors. In this same model, steroidogenic expression observed in postnatal testes was notably increased but was accompanied by a significant decrease in circulating testosterone (38). The authors of this study further showed that the effects on postnatal testicular steroidogenesis and androgen production were not driven from FLCs or ALCs but rather from an expansion of adrenocortical-like cells residing in the testicular interstitium—thus making the direct contribution of GATA4/6 to Leydig cell gene expression and function in this model difficult to assess.
Independent groups have postulated that GATA4 functions as a master regulator of Leydig cell steroidogenesis based on data generated in steroidogenic cell lines (34, 36, 76). Blockade of Gata4 expression in Leydig cell lines leads to a suppression of the steroidogenic program and a decrease in hormone production (34, 76). Loss of both GATA4 and GATA6 in primary Leydig cells (77), or disruption of their transcriptional activities in mice (36), has also been shown to suppress steroidogenesis by reducing the expression of several genes involved in androgen biosynthesis, including steroidogenic acute regulatory protein (Star), Cyp11a1, 3β-hydroxysteroid dehydrogenase 1 (Hsd3b1), 17α-hydroxylase (Cyp17a1), and 17β-hydroxysteroid dehydrogenase 3 (Hsd17b3)—many of these genes have conserved GATA motifs in their cis-regulatory regions [reviewed in (4, 18)]. Since some of these genes are also reduced in fetal testes lacking GATA factors but only when the Gata4 and Gata6 genes are simultaneously deleted, both factors likely compensate for one another in regulating FLC gene expression. Transcription of several other Leydig cell-expressed genes have been proposed to be targeted by either GATA4 and/or GATA6 [reviewed in (4, 18)]. The GATA-dependence of these additional targets has been mostly limited to characterization of their cis-regulatory regions. Therefore, their validation as genuine in vivo and/or direct targets of GATA factors remains to be determined.
Regulation of GATA Factors in the Fetal Testis
The regulation of GATA gene expression and GATA transcriptional activity in the fetal testis remains poorly understood. The transcriptional regulation of the Gata4 gene was first reported in 2006 (78). In that initial study, activity of the first 124 bp upstream of the mouse Gata4 transcriptional start site in Leydig and Sertoli cell lines was shown to be dependent on conserved GC-rich and E-box elements (78). Similar findings highlighting the importance of GC-rich and E-box elements were later reported in context of 5 kb of the rat Gata4 promoter (25). The same 5 kb of rat Gata4 promoter was also shown to be sufficient to drive GFP expression in the male gonad of transgenic mice from the onset of testis differentiation to adulthood (25). Subsequent analysis showed high transgene expression in both Sertoli and Leydig cells, and at all times (both fetal and mature testis) (79). Targeted mutagenesis of the Gata4 E-box element in the mouse confirmed the importance of this motif for endogenous Gata4 expression in both fetal and adult testis (80). Like other GATA genes, the rodent and human GATA4 genes are expressed as multiple transcripts that differ in their 5’ untranslated exons, which are driven by alternative promoters (81). Thus, in addition to the typical Gata4 transcript driven by the E-box dependent proximal Gata4 promoter, the fetal testis also expresses a second Gata4 transcript driven by an alternative promoter sequence located nearly 30 kb upstream of the proximal promoter (81, 82). The distal Gata4 promoter appears to be autoregulated by GATA4 itself in a regulatory mechanism proposed to ensure sufficient Gata4 expression in the testis at critical times such as during testis differentiation (82). Characterization of the cis-regulatory regions required for Gata6 expression in the testis have not been reported.
Despite their profound roles in testis physiology, the hormonal regulation of GATA factors in the testis have received comparably little attention. Nonetheless, treatment of Sertoli and Leydig cell lines with gonadotropins causes a modest increase in Gata4 expression (29, 83). Gonadotropins and androgens, however, do not appear to be required for basal Gata4 or Gata6 expression in the testis as their expression is retained in different models that perturb these hormones (29). In contrast to the paucity of information on hormones that regulate GATA factors in the testis, much more is known about the signaling pathways activated following hormone stimulation and their impacts on the transcriptional activity of GATA factors, especially GATA4. Two GATA4 phosphorylation sites have been well described: serine 105 (S105) targeted by mitogen-activated protein kinases (MAPK) and serine 261 (S261) targeted by protein kinase A (PKA) (84–87). In the testis, GATA4 phosphorylation by PKA is particularly informative since it helps to better understand the acute gonadotropic regulation of multiple testis-expressed genes that lack classic cAMP response elements (CREs), but contain GATA regulatory motifs, in their respective promoters. For both S105 and S261, in vitro studies have shown that GATA4 phosphorylation stimulates GATA4 transcriptional activity on multiple testis target genes (87, 88). GATA4 S105 phosphorylation by MAPK has also been proposed to be essential for GATA4 action in the upregulation of Sry expression during testis differentiation (89, 90). A study published in 2019, addressed the in vivo role of GATA4 phosphorylation through the detailed characterization of two mouse models (GATA4 S105A and GATA4 S261A) that carry mutations that specifically block GATA4 phosphorylation at either site. One of these mutations (S105A) was associated with a male hormonal defect characterized by a deficiency in testosterone production in adult but not fetal males (36). Interestingly, male sex determination was unaffected in GATA4 S105A mutants (36), which questions the requirement for GATA4 phosphorylation (at least on S105) in the testis differentiation process.
GATA Factors in Human Testis and Testicular Pathologies
Several studies have investigated the expression and potential role of GATA factors in normal human testis development and function, as well as the pathogenesis of testicular tumors [(91–94) and reviewed in (95)]. Much like rodents and other mammalian species, GATA4 and GATA6 appear to be the predominant GATA factors of the human fetal testis. In Sertoli cells, GATA4 is present during both fetal and postnatal development (91). GATA4 is also expressed in fetal and postpubertal Leydig cells which correlates with its proposed role in steroidogenesis (91). Interestingly, GATA4 expression is significantly higher in both Sertoli and Leydig cell tumors (91), suggesting that GATA4 might influence cell proliferation during tumorigenesis in human testicular somatic cells. Unlike rodents, GATA4 is a marker of a subset of early fetal gonocytes (91), as well as multiple testicular germ cell neoplasia that include both precursor carcinoma in situ (CIS) testis cells and definite testicular germ cell tumors (seminomas, yolk sac tumors, teratomas) (93). Testicular seminomas and yolk sac tumors also express GATA6 (93). The presence of GATA4 and/or GATA6 in these cells has suggested a role for these factors in early human germ cell differentiation. GATA6 is also expressed in normal human testis, at least during fetal development. GATA6 in fetal human testis is most abundant in Sertoli and Leydig cells (92), where it may have overlapping roles with GATA4 if one transposes the comparable rodent data to humans (38).
Several mutations in either the GATA4 or FOG2/ZFPM2 genes have been identified in human cases of differences of sexual development (DSD) (96–100). While most of the reported GATA4 and FOG2/ZFPM2 variants appear to have a benign impact on XY DSDs (98), at least three FOG2/ZFPM2 (p.S402R; p.R260Q; p.R260Q/M544I) (97), and two GATA4 missense variants (p.G221R; p.W228C) (96, 98), are considered to be pathogenic with reduced transcriptional activities on the human AMH promoter, a known GATA4 target. The fact that most of the reported variants are heterozygous in nature suggests that correct functional dosage of these transcription factors is critical for typical XY sex differentiation in humans. In support of this, haploinsufficiency of either Gata4 or ZFPm2/Fog2 is also known to induce gonadal sex-reversal on specific genetic backgrounds in mice (43).
Conclusion
GATA factors are incontrovertibly essential regulators of developmental and functional processes in multiple organs systems. The mammalian testis is no exception where at least three GATA factors (1/4/6) play essential, and sometimes overlapping roles, in controlling the initiation of male gonad development, testis differentiation at the time of sex differentiation, and gene expression and function in both the fetal and mature testis (an illustrative overview in presented in Figure 1). Over the years, much of our knowledge of the mechanisms of GATA action in the testis have come from genetic manipulation of the different Gata genes in mice and the analysis of the cis-regulatory regions of many of its proposed target genes in cell lines. From this point forward, the challenge will be to fine-tune this knowledge base by ascertaining not only what testis genes are regulated by GATA factors but more so, by identifying the genes that are directly targeted by these factors. The recent development and applicability of new technologies has made this a reality as we are now capable of probing the role of individual cis-regulatory elements in suspected genes by genome editing. Therefore, understanding GATA function in the testis is far from complete, but rather has just begun.
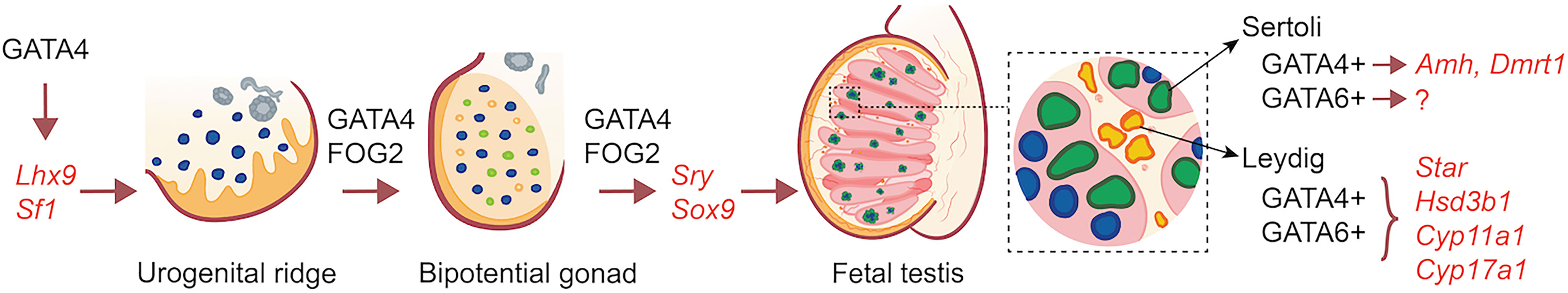
Figure 1 Overview of the action of GATA factors in XY gonad development and fetal testis gene expression.
Author Contributions
RV and JT drafted and edited the final version of the manuscript. KM prepared the illustration and participated in the final editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Open-access fees for this publication were provided by project grant PJT-166131 from the Canadian Institutes of Health Research to RV.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tremblay M, Sanchez-Ferras O, Bouchard M. GATA Transcription Factors in Development and Disease. Development (2018) 145:dev164384. doi: 10.1242/dev.164384
2. Whitcomb J, Gharibeh L, Nemer M. From Embryogenesis to Adulthood: Critical Role for GATA Factors in Heart Development and Function. IUBMB Life (2020) 72:53–67. doi: 10.1002/iub.2163
3. Molkentin JD. The Zinc Finger-Containing Transcription Factors GATA-4, -5, and -6. Ubiquitously Expressed Regulators of Tissue-Specific Gene Expression. J Biol Chem (2000) 275:38949–52. doi: 10.1074/jbc.R000029200
4. Viger RS, Mazaud Guittot S, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA Family of Transcription Factors in Endocrine Development, Function, and Disease. Mol Endocrinol (2008) 22:781–98. doi: 10.1210/me.2007-0513
5. Tevosian SG. Transgenic Mouse Models in the Study of Reproduction: Insights Into GATA Protein Function. Reproduction (2014) 148:R1–R14. doi: 10.1530/REP-14-0086
6. Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the Transcription Factor GATA-4 for Heart Tube Formation and Ventral Morphogenesis. Genes Dev (1997) 11:1061–72. doi: 10.1101/gad.11.8.1061
7. Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, et al. GATA-4 Transcription Factor is Required for Ventral Morphogenesis and Heart Tube Formation. Genes Dev (1997) 11:1048–60. doi: 10.1101/gad.11.8.1048
8. Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The Transcription Factor GATA-6 is Essential for Early Extraembryonic Development. Development (1999) 126:723–32. doi: 10.1242/dev.126.9.723
9. Molkentin JD, Tymitz KM, Richardson JA, Olson EN. Abnormalities of the Genitourinary Tract in Female Mice Lacking GATA-5. Mol Cell Biol (2000) 20:5256–60. doi: 10.1128/MCB.20.14.5256-5260.2000
10. Zhou P, He A, Pu WT. Regulation of GATA4 Transcriptional Activity in Cardiovascular Development and Disease. Curr Top Dev Biol (2012) 100:143–69. doi: 10.1016/B978-0-12-387786-4.00005-1
11. Chlon TM, Crispino JD. Combinatorial Regulation of Tissue Specification by GATA and FOG Factors. Development (2012) 139:3905–16. doi: 10.1242/dev.080440
12. Tremblay JJ, Viger RS. Transcription Factor GATA-4 Enhances Müllerian Inhibiting Substance Gene Transcription Through a Direct Interaction With the Nuclear Receptor SF-1. Mol Endocrinol (1999) 13:1388–401. doi: 10.1210/mend.13.8.0330
13. Bouchard MF, Taniguchi H, Viger RS. Protein Kinase A-Dependent Synergism Between GATA Factors and the Nuclear Receptor, Liver Receptor Homolog-1, Regulates Human Aromatase (CYP19) PII Promoter Activity in Breast Cancer Cells. Endocrinology (2005) 146:4905–16. doi: 10.1210/en.2005-0187
14. Robert NM, Miyamoto Y, Taniguchi H, Viger RS. LRH-1/NR5A2 Cooperates With GATA Factors to Regulate Inhibin Alpha-Subunit Promoter Activity. Mol Cell Endocrinol (2006) 257-258:65–74. doi: 10.1016/j.mce.2006.06.011
15. Miyamoto Y, Taniguchi H, Hamel F, Silversides DW, Viger RS. A GATA4/WT1 Cooperation Regulates Transcription of Genes Required for Mammalian Sex Determination and Differentiation. BMC Mol Biol (2008) 9:44. doi: 10.1186/1471-2199-9-44
16. Martin LJ, Bergeron F, Viger RS, Tremblay JJ. Functional Cooperation Between GATA Factors and cJUN on the Star Promoter in MA-10 Leydig Cells. J Androl (2012) 33:81–7. doi: 10.2164/jandrol.110.012039
17. Daems C, Di-Luoffo M, Paradis E, Tremblay JJ. MEF2 Cooperates With Forskolin/cAMP and GATA4 to Regulate Star Gene Expression in Mouse MA-10 Leydig Cells. Endocrinology (2015) 156:2693–703. doi: 10.1210/en.2014-1964
18. Tremblay JJ, Viger RS. Nuclear Receptor Dax1 Represses the Transcriptional Cooperation Between GATA-4 and SF-1 in Sertoli Cells. Biol Reprod (2001) 64:1191–9. doi: 10.1095/biolreprod64.4.1191
19. Suzuki YJ. Cell Signaling Pathways for the Regulation of GATA4 Transcription Factor: Implications for Cell Growth and Apoptosis. Cell Signal (2011) 23:1094–9. doi: 10.1016/j.cellsig.2011.02.007
20. Ito E, Toki T, Ishihara H, Ohtani H, Gu L, Yokoyama M, et al. Erythroid Transcription Factor GATA-1 is Abundantly Transcribed in Mouse Testis. Nature (1993) 362:466–8. doi: 10.1038/362466a0
21. Imai T, Kawai Y, Tadokoro Y, Yamamoto M, Nishimune Y, Yomogida K. In Vivo and In Vitro Constant Expression of GATA-4 in Mouse Postnatal Sertoli Cells. Mol Cell Endocrinol (2004) 214:107–15. doi: 10.1016/j.mce.2003.10.065
22. Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription Factor GATA-4 is Expressed in a Sexually Dimorphic Pattern During Mouse Gonadal Development and is a Potent Activator of the Müllerian Inhibiting Substance Promoter. Development (1998) 125:2665–75. doi: 10.1242/dev.125.14.2665
23. Lindeboom F, Gillemans N, Karis A, Jaegle M, Meijer D, Grosveld F, et al. A Tissue-Specific Knockout Reveals That Gata1 is Not Essential for Sertoli Cell Function in the Mouse. Nucleic Acids Res (2003) 31:5405–12. doi: 10.1093/nar/gkg723
24. Hu YC, Okumura LM, Page DC. Gata4 is Required for Formation of the Genital Ridge in Mice. PloS Genet (2013) 9:e1003629. doi: 10.1371/journal.pgen.1003629
25. Mazaud Guittot S, Tetu A, Legault E, Pilon N, Silversides DW, Viger RS. The Proximal Gata4 Promoter Directs Reporter Gene Expression to Sertoli Cells During Mouse Gonadal Development. Biol Reprod (2007) 76:85–95. doi: 10.1095/biolreprod.106.055137
26. Oreal E, Mazaud S, Picard JY, Magre S, Carre-Eusebe D. Different Patterns of Anti-Müllerian Hormone Expression, as Related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 Expression, in the Chick Differentiating Gonads. Dev Dyn (2002) 225:221–32. doi: 10.1002/dvdy.10153
27. McCoard SA, Wise TH, Fahrenkrug SC, Ford JJ. Temporal and Spatial Localization Patterns of GATA-4 During Porcine Gonadogenesis. Biol Reprod (2001) 65:366–74. doi: 10.1095/biolreprod65.2.366
28. Viger RS, Taniguchi H, Robert NM, Tremblay JJ. Role of the GATA Family of Transcription Factors in Andrology. J Androl (2004) 25:441–52. doi: 10.1002/j.1939-4640.2004.tb02813.x
29. Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, et al. Expression and Regulation of Transcription Factors GATA-4 and GATA-6 in Developing Mouse Testis. Endocrinology (1999) 140:1470–80. doi: 10.1210/endo.140.3.6587
30. Robert NM, Tremblay JJ, Viger RS. FOG-1 and FOG-2 Differentially Repress the GATA-Dependent Activity of Multiple Gonadal Promoters. Endocrinology (2002) 143:3963–73. doi: 10.1210/en.2002-220280
31. Luo X, Ikeda Y, Parker KL. A Cell-Specific Nuclear Receptor is Essential for Adrenal and Gonadal Development and Sexual Differentiation. Cell (1994) 77:481–90. doi: 10.1016/0092-8674(94)90211-9
32. Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, et al. The LIM Homeobox Gene Lhx9 is Essential for Mouse Gonad Formation. Nature (2000) 403:909–13. doi: 10.1038/35002622
33. Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional Ablation of Gata4 and Fog2 Genes in Mice Reveals Their Distinct Roles in Mammalian Sexual Differentiation. Dev Biol (2011) 353:229–41. doi: 10.1016/j.ydbio.2011.02.032
34. Schrade A, Kyronlahti A, Akinrinade O, Pihlajoki M, Hakkinen M, Fischer S, et al. GATA4 is a Key Regulator of Steroidogenesis and Glycolysis in Mouse Leydig Cells. Endocrinology (2015) 156:1860–72. doi: 10.1210/en.2014-1931
35. Kyrönlahti A, Euler R, Bielinska M, Schoeller EL, Moley KH, Toppari J, et al. GATA4 Regulates Sertoli Cell Function and Fertility in Adult Male Mice. Mol Cell Endocrinol (2011) 333:85–95. doi: 10.1016/j.mce.2010.12.019
36. Bergeron F, Boulende Sab A, Bouchard MF, Taniguchi H, Souchkova O, Brousseau C, et al. Phosphorylation of GATA4 Serine 105 But Not Serine 261 is Required for Testosterone Production in the Male Mouse. Andrology (2019) 7:357–72. doi: 10.1111/andr.12601
37. Tremblay JJ, Viger RS. Novel Roles for GATA Transcription Factors in the Regulation of Steroidogenesis. J Steroid Biochem Mol Biol (2003) 85:291–8. doi: 10.1016/S0960-0760(03)00211-5
38. Padua MB, Jiang T, Morse DA, Fox SC, Hatch HM, Tevosian SG. Combined Loss of the GATA4 and GATA6 Transcription Factors in Male Mice Disrupts Testicular Development and Confers Adrenal-Like Function in the Testes. Endocrinology (2015) 156:1873–86. doi: 10.1210/en.2014-1907
39. Chen SR, Tang JX, Cheng JM, Li J, Jin C, Li XY, et al. Loss of Gata4 in Sertoli Cells Impairs the Spermatogonial Stem Cell Niche and Causes Germ Cell Exhaustion by Attenuating Chemokine Signaling. Oncotarget (2015) 6:37012–27. doi: 10.18632/oncotarget.6115
40. Schrade A, Kyrönlahti A, Akinrinade O, Pihlajoki M, Fischer S, Rodriguez VM, et al. GATA4 Regulates Blood-Testis Barrier Function and Lactate Metabolism in Mouse Sertoli Cells. Endocrinology (2016) 157:2416–31. doi: 10.1210/en.2015-1927
41. Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal Differentiation, Sex Determination and Normal Sry Expression in Mice Require Direct Interaction Between Transcription Partners GATA4 and FOG2. Development (2002) 129:4627–34. doi: 10.1242/dev.129.19.4627
42. Manuylov NL, Fujiwara Y, Adameyko II, Poulat F, Tevosian SG. The Regulation of Sox9 Gene Expression by the GATA4/FOG2 Transcriptional Complex in Dominant XX Sex Reversal Mouse Models. Dev Biol (2007) 307:356–67. doi: 10.1016/j.ydbio.2007.04.040
43. Bouma GJ, Washburn LL, Albrecht KH, Eicher EM. Correct Dosage of Fog2 and Gata4 Transcription Factors is Critical for Fetal Testis Development in Mice. Proc Natl Acad Sci USA (2007) 104:14994–9. doi: 10.1073/pnas.0701677104
44. Shimamura R, Fraizer GC, Trapman J, Lau Y, Saunders GF. The Wilms' Tumor Gene WT1 can Regulate Genes Involved in Sex Determination and Differentiation: SRY, Müllerian-Inhibiting Substance, and the Androgen Receptor. Clin Cancer Res (1997) 3:2571–80.
45. Hossain A, Saunders GF. The Human Sex-Determining Gene SRY is a Direct Target of WT1. J Biol Chem (2001) 276:16817–23. doi: 10.1074/jbc.M009056200
46. Matsuzawa-Watanabe Y, Inoue J, Semba K. Transcriptional Activity of Testis-Determining Factor SRY is Modulated by the Wilms' Tumor 1 Gene Product, WT1. Oncogene (2003) 22:7900–4. doi: 10.1038/sj.onc.1206717
47. Buganim Y, Itskovich E, Hu YC, Cheng AW, Ganz K, Sarkar S, et al. Direct Reprogramming of Fibroblasts Into Embryonic Sertoli-Like Cells by Defined Factors. Cell Stem Cell (2012) 11:373–86. doi: 10.1016/j.stem.2012.07.019
48. Xu C, Mohsin A, Luo Y, Xie L, Peng Y, Wang Q, et al. Differentiation Roadmap of Embryonic Sertoli Cells Derived From Mouse Embryonic Stem Cells. Stem Cell Res Ther (2019) 10:81. doi: 10.1186/s13287-019-1180-6
49. Liang J, Wang N, He J, Du J, Guo Y, Li L, et al. Induction of Sertoli-Like Cells From Human Fibroblasts by NR5A1 and GATA4. Elife (2019) 8:e48767. doi: 10.7554/eLife.48767
50. Xu C, Dai Y, Mohsin A, Hang H, Zhuang Y, Guo M. Mapping Molecular Pathways for Embryonic Sertoli Cells Derivation Based on Differentiation Model of Mouse Embryonic Stem Cells. Stem Cell Res Ther (2020) 11:85. doi: 10.1186/s13287-020-01600-2
51. Mullen RD, Behringer RR. Molecular Genetics of Müllerian Duct Formation, Regression and Differentiation. Sex Dev (2014) 8:281–96. doi: 10.1159/000364935
52. Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK. Endogenous Expression of Müllerian Inhibiting Substance in Early Postnatal Rat Sertoli Cells Requires Multiple Steroidogenic Factor-1 and GATA-4-Binding Sites. Proc Natl Acad Sci USA (2000) 97:1624–9. doi: 10.1073/pnas.97.4.1624
53. Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. FOG-2 and GATA-4 Are Coexpressed in the Mouse Ovary and can Modulate Mullerian-Inhibiting Substance Expression. Biol Reprod (2003) 68:1333–40. doi: 10.1095/biolreprod.102.008599
54. Thurisch B, Liang SY, Sarioglu N, Schomburg L, Bungert J, Dame C. Transgenic Mice Expressing Small Interfering RNA Against Gata4 Point to a Crucial Role of Gata4 in the Heart and Gonads. J Mol Endocrinol (2009) 43:157–69. doi: 10.1677/JME-09-0030
55. Yang S, Deng Y, Chen D, Hu S, Zhang Y, Huang H, et al. Promoter Identification and Transcriptional Regulation of the Goose AMH Gene. Animals (2019) 9:816. doi: 10.3390/ani9100816
56. Tremblay JJ, Viger RS. A Mutated Form of Steroidogenic Factor 1 (SF-1 G35E) That Causes Sex Reversal in Humans Fails to Synergize With Transcription Factor GATA-4. J Biol Chem (2003) 278:42637–42. doi: 10.1074/jbc.M305485200
57. Bouchard MF, Bergeron F, Grenier Delaney J, Harvey LM, Viger RS. In Vivo Ablation of the Conserved GATA Binding Motif in the Amh Promoter Impairs Amh Expression in the Male Mouse. Endocrinology (2019) 160:817–26. doi: 10.1210/en.2019-00047
58. Arango NA, Lovell-Badge R, Behringer RR. Targeted Mutagenesis of the Endogenous Mouse Mis Gene Promoter: In Vivo Definition of Genetic Pathways of Vertebrate Sexual Development. Cell (1999) 99:409–19. doi: 10.1016/S0092-8674(00)81527-5
59. Beau C, Rauch M, Joulin V, Jegou B, Guerrier D. GATA-1 is a Potential Repressor of Anti-Mullerian Hormone Expression During the Establishment of Puberty in the Mouse. Mol Reprod Dev (2000) 56:124–38. doi: 10.1002/(SICI)1098-2795(200006)56:2<124::AID-MRD2>3.0.CO;2-J
60. Al-Attar L, Noel K, Dutertre M, Belville C, Forest MG, Burgoyne PS, et al. Hormonal and Cellular Regulation of Sertoli Cell Anti-Mullerian Hormone Production in the Postnatal Mouse. J Clin Invest (1997) 100:1335–43. doi: 10.1172/JCI119653
61. Edelsztein NY, Racine C, di Clemente N, Schteingart HF, Rey RA. Androgens Downregulate Anti-Mullerian Hormone Promoter Activity in the Sertoli Cell Through the Androgen Receptor and Intact Steroidogenic Factor 1 Sites. Biol Reprod (2018) 99:1303–12. doi: 10.1093/biolre/ioy152
62. Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, et al. Evidence for Evolutionary Conservation of Sex-Determining Genes. Nature (1998) 391:691–5. doi: 10.1038/35618
63. Lei N, Heckert LL. Gata4 Regulates Testis Expression of Dmrt1. Mol Cell Biol (2004) 24:377–88. doi: 10.1128/MCB.24.1.377-388.2004
64. Feng ZM, Wu AZ, Zhang Z, Chen CL. GATA-1 and GATA-4 Transactivate Inhibin/Activin Beta-B-Subunit Gene Transcription in Testicular Cells. Mol Endocrinol (2000) 14:1820–35. doi: 10.1210/me.14.11.1820
65. Hermann BP, Heckert LL. Silencing of Fshr Occurs Through a Conserved, Hypersensitive Site in the First Intron. Mol Endocrinol (2005) 19:2112–3. doi: 10.1210/me.2004-0244
66. Kim JS, Griswold MD. E2F and GATA-1 are Required for the Sertoli Cell-Specific Promoter Activity of the Follicle-Stimulating Hormone Receptor Gene. J Androl (2001) 22:629–39. doi: 10.1002/j.1939-4640.2001.tb02223.x
67. Feng ZM, Wu AZ, Chen CL. Testicular GATA-1 Factor Up-Regulates the Promoter Activity of Rat Inhibin Alpha-Subunit Gene in MA-10 Leydig Tumor Cells. Mol Endocrinol (1998) 12:378–90. doi: 10.1210/me.12.3.378
68. Tremblay JJ, Viger RS. GATA Factors Differentially Activate Multiple Gonadal Promoters Through Conserved GATA Regulatory Elements. Endocrinology (2001) 142:977–86. doi: 10.1210/endo.142.3.7995
69. Bhardwaj A, Rao MK, Kaur R, Buttigieg MR, Wilkinson MF. GATA Factors and Androgen Receptor Collaborate to Transcriptionally Activate the Rhox5 Homeobox Gene in Sertoli Cells. Mol Cell Biol (2008) 28:2138–53. doi: 10.1128/MCB.01170-07
70. Nef S, Parada LF. Cryptorchidism in Mice Mutant for Insl3. Nat Genet (1999) 22:295–9. doi: 10.1038/10364
71. Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, et al. Targeted Disruption of the Insl3 Gene Causes Bilateral Cryptorchidism. Mol Endocrinol (1999) 13:681–91. doi: 10.1210/mend.13.5.0272
72. Rotgers E, Jorgensen A, Yao HH. At the Crossroads of Fate-Somatic Cell Lineage Specification in the Fetal Gonad. Endocr Rev (2018) 39:739–59. doi: 10.1210/er.2018-00010
73. Tremblay JJ. Molecular Regulation of Steroidogenesis in Endocrine Leydig Cells. Steroids (2015) 103:3–10. doi: 10.1016/j.steroids.2015.08.001
74. de Mattos KV, Viger RS, Tremblay JJ. Transcription Factors in the Regulation of Leydig Cell Gene Expression and Function. Front Endocrinol (2022) 13:881309. doi: 10.3389/fendo.2022.881309
75. Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is Required for Sex Steroidogenic Cell Development in the Fetal Mouse. Dev Dyn (2007) 236:203–13. doi: 10.1002/dvdy.21004
76. Bergeron F, Nadeau G, Viger RS. GATA4 Knockdown in MA-10 Leydig Cells Identifies Multiple Target Genes in the Steroidogenic Pathway. Reproduction (2015) 149:245–57. doi: 10.1530/REP-14-0369
77. Penny GM, Cochran RB, Pihlajoki M, Kyronlahti A, Schrade A, Hakkinen M, et al. Probing GATA Factor Function in Mouse Leydig Cells via Testicular Injection of Adenoviral Vectors. Reproduction (2017) 154:455–67. doi: 10.1530/REP-17-0311
78. Ohara Y, Atarashi T, Ishibashi T, Ohashi-Kobayashi A, Maeda M. GATA-4 Gene Organization and Analysis of its Promoter. Biol Pharm Bull (2006) 29:410–9. doi: 10.1248/bpb.29.410
79. Nel-Themaat L, Jang CW, Stewart MD, Akiyama H, Viger RS, Behringer RR. Sertoli Cell Behaviors in Developing Testis Cords and Postnatal Seminiferous Tubules of the Mouse. Biol Reprod (2011) 84:342–50. doi: 10.1095/biolreprod.110.086900
80. Boulende Sab A, Bouchard MF, Beland M, Prud'homme B, Souchkova O, Viger RS, et al. An Ebox Element in the Proximal Gata4 Promoter is Required for Gata4 Expression In Vivo. PloS One (2011) 6:e29038. doi: 10.1371/journal.pone.0029038
81. Mazaud Guittot S, Bouchard MF, Robert-Grenon JP, Robert C, Goodyer CG, Silversides DW, et al. Conserved Usage of Alternative 5' Untranslated Exons of the GATA4 Gene. PloS One (2009) 4:e8454. doi: 10.1371/journal.pone.0008454
82. Mazaud-Guittot S, Prud'homme B, Bouchard MF, Bergeron F, Daems C, Tevosian SG, et al. GATA4 Autoregulates its Own Expression in Mouse Gonadal Cells via its Distal 1b Promoter. Biol Reprod (2014) 90:25. doi: 10.1095/biolreprod.113.113290
83. Heikinheimo M, Ermolaeva M, Bielinska M, Rahnman NA, Narita N, Huhtaniemi IT, et al. Expression and Hormonal Regulation of Transcription Factors GATA-4 and GATA-6 in the Mouse Ovary. Endocrinology (1997) 138:3505–14. doi: 10.1210/endo.138.8.5350
84. Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD. The Transcription Factor GATA-4 is Activated by Extracellular Signal- Regulated Kinase 1- and 2-Mediated Phosphorylation of Serine 105 in Cardiomyocytes. Mol Cell Biol (2001) 21:7460–9. doi: 10.1128/MCB.21.21.7460-7469.2001
85. Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, et al. Tissue-Specific GATA Factors are Transcriptional Effectors of the Small GTPase RhoA. Genes Dev (2001) 15:2702–19. doi: 10.1101/gad.915701
86. Tenhunen O, Sarman B, Kerkela R, Szokodi I, Papp L, Toth M, et al. Mitogen-Activated Protein Kinases P38 and ERK 1/2 Mediate the Wall Stress-Induced Activation of GATA-4 Binding in Adult Heart. J Biol Chem (2004) 279:24852–60. doi: 10.1074/jbc.M314317200
87. Tremblay JJ, Viger RS. Transcription Factor GATA-4 is Activated by Phosphorylation of Serine 261 via the cAMP/PKA Pathway in Gonadal Cells. J Biol Chem (2003) 278:22128–35. doi: 10.1074/jbc.M213149200
88. Tremblay JJ, Hamel F, Viger RS. Protein Kinase A-Dependent Cooperation Between GATA and C/EBP Transcription Factors Regulates StAR Promoter Activity. Endocrinology (2002) 143:3935–45. doi: 10.1210/en.2002-220413
89. Warr N, Carre GA, Siggers P, Faleato JV, Brixey R, Pope M, et al. Gadd45gamma and Map3k4 Interactions Regulate Mouse Testis Determination via P38 MAPK-Mediated Control of Sry Expression. Dev Cell (2012) 23:1020–31. doi: 10.1016/j.devcel.2012.09.016
90. Gierl MS, Gruhn WH, von Seggern A, Maltry N, Niehrs C. GADD45G Functions in Male Sex Determination by Promoting P38 Signaling and Sry Expression. Dev Cell (2012) 23:1032–42. doi: 10.1016/j.devcel.2012.09.014
91. Ketola I, Pentikainen V, Vaskivuo T, Ilvesmaki V, Herva R, Dunkel L, et al. Expression of Transcription Factor GATA-4 During Human Testicular Development and Disease. J Clin Endocrinol Metab (2000) 85:3925–31. doi: 10.1210/jcem.85.10.6900
92. Ketola I, Toppari J, Vaskivuo T, Herva R, Tapanainen JS, Heikinheimo M. Transcription Factor GATA-6, Cell Proliferation, Aaoptosis, and Apoptosis-Related Proteins Bcl-2 and Bax in Human Fetal Testis. J Clin Endocrinol Metab (2003) 88:1858–65. doi: 10.1210/jc.2002-021647
93. Salonen J, Rajpert-De Meyts E, Mannisto S, Nielsen JE, Graem N, Toppari J, et al. Differential Developmental Expression of Transcription Factors GATA-4 and GATA-6, Their Cofactor FOG-2 and Downstream Target Genes in Testicular Carcinoma In Situ and Germ Cell Tumors. Eur J Endocrinol (2010) 162:625–31. doi: 10.1530/EJE-09-0734
94. Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh AL, et al. Characterization and Functionality of Proliferative Human Sertoli Cells. Cell Transplant (2011) 20:619–35. doi: 10.3727/096368910X536563
95. Pihlajoki M, Farkkila A, Soini T, Heikinheimo M, Wilson DB. GATA Factors in Endocrine Neoplasia. Mol Cell Endocrinol (2016) 421:2–17. doi: 10.1016/j.mce.2015.05.027
96. Lourenco D, Brauner R, Rybczynska M, Nihoul-Fekete C, McElreavey K, Bashamboo A. Loss-Of-Function Mutation in GATA4 Causes Anomalies of Human Testicular Development. Proc Natl Acad Sci USA (2011) 108:1597–602. doi: 10.1073/pnas.1010257108
97. Bashamboo A, Brauner R, Bignon-Topalovic J, Lortat-Jacob S, Karageorgou V, Lourenco D, et al. Mutations in the FOG2/ZFPM2 Gene are Associated With Anomalies of Human Testis Determination. Hum Mol Genet (2014) 23:3657–65. doi: 10.1093/hmg/ddu074
98. van den Bergen JA, Robevska G, Eggers S, Riedl S, Grover SR, Bergman PB, et al. Analysis of Variants in GATA4 and FOG2/ZFPM2 Demonstrates Benign Contribution to 46,XY Disorders of Sex Development. Mol Genet Genomic Med (2020) 8:e1095. doi: 10.1002/mgg3.1095
99. Finelli P, Pincelli AI, Russo S, Bonati MT, Recalcati MP, Masciadri M, et al. Disruption of Friend of GATA 2 Gene (FOG-2) by a De Novo T(8;10) Chromosomal Translocation is Associated With Heart Defects and Gonadal Dysgenesis. Clin Genet (2007) 71:195–204. doi: 10.1111/j.1399-0004.2007.00752.x
Keywords: transcription, GATA, hormone, testis, fetal, gene expression
Citation: Viger RS, de Mattos K and Tremblay JJ (2022) Insights Into the Roles of GATA Factors in Mammalian Testis Development and the Control of Fetal Testis Gene Expression. Front. Endocrinol. 13:902198. doi: 10.3389/fendo.2022.902198
Received: 22 March 2022; Accepted: 22 April 2022;
Published: 26 May 2022.
Edited by:
Rodolfo A. Rey, Hospital de Niños Ricardo Gutiérrez (CONICET), ArgentinaReviewed by:
Anu Bashamboo, Institut Pasteur, FranceMarkku Heikinheimo, University of Helsinki, Finland
Copyright © 2022 Viger, de Mattos and Tremblay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert S. Viger, cm9iZXJ0LnZpZ2VyQGNyY2h1ZGVxdWViZWMudWxhdmFsLmNh
 Robert S. Viger
Robert S. Viger Karine de Mattos
Karine de Mattos Jacques J. Tremblay
Jacques J. Tremblay