- 1Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease, Women and Children’s Medical Center, Department of Pediatric Surgery, Guangzhou Institute of Pediatrics, Guangzhou Guangzhou Medical University, Guangzhou, China
- 3Department of Cancer Immunology and Virology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, United States
- 4The Third Affiliated Hospital of Southern Medical University, Guangzhou, China
- 5The 2nd Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Osteoporosis (OP) is a systemic metabolic skeletal disease which can lead to reduction in bone mass and increased risk of bone fracture due to the microstructural degradation. Traditional Chinese medicine (TCM) has been applied in the prevention and treatment of osteoporosis for a long time. Terpenoids, a class of natural products that are rich in TCM, have been widely studied for their therapeutic efficacy on bone resorption, osteogenesis, and concomitant inflammation. Terpenoids can be classified in four categories by structures, monoterpenoids, sesquiterpenoids, diterpenoids, and triterpenoids. In this review, we comprehensively summarize all the currently known TCM-derived terpenoids in the treatment of OP. In addition, we discuss the possible mechanistic-of-actions of all four category terpenoids in anti-OP and assess their therapeutic potential for OP treatment.
Introduction
As a systemic skeletal disease, Osteoporosis (OP) is characterized by increased risk of bone fragility, chronic pain, and even disability, leading to decreased life quality. Especially, OP strongly affects postmenopausal women and elderly population. About 30-50% of women and those who are more than 70 years old suffer from OP-induced fractures throughout their lives (1–3). In health condition, osteoblasts (OBs, bone-forming cells) and osteoclasts (OCs, bone-resorbing cells) form a balance for bone homeostasis. The lack of OB function or over-activated OC status will disturb the balance and induce OP.
In recent years, there has been a growing interest in traditional Chinese medicine (TCM) for the treatment of OP, such as Liu-Wei-Di-Huang Wan (formula), Morindae Officinalis Radix (herb), Longspur epimedium glycoside (natural product) (4). TCM has accumulated extensive experience for thousands of years and owns fewer adverse effects during a long-term usage comparing to some chemically synthesized medicines (5). Chinese herbal medicines usually play their therapeutic roles through a “multi-components, multi-targets, multi-pathway” mode, which is compatible with the multifactorial nature of OP. Plenty of evidence suggest that targeting OCs with TCM is an efficient strategy for the treatment of OP (6–8).
According to the theory of TCM on the pathogenesis and symptoms of OP, the kidney stores essence, turns it into bone marrow, nourishes bones to strengthen the skeleton, and promotes bone growth and repair. Therefore, ‘kidney deficiency’ is regarded as the underlying cause of all skeletal pathologies (9, 10). Many classic and empirical formulas of TCM used to tonify the kidney are clinically applied in OP treatment, TCMs like Liu Wei Di Huang Wan, Qing E Wan, Jiawei Yanghe Decoction, Er Zhi Wan, Qiangji Jianli Yin, Zuo Gui Wan, Rongjin Tablets, and You Gui Wan showed excellent anti-OP efficacy through reinforcing the kidney (8). Modern pharmacological studies have shown that these classic formulas significantly inhibited OC formation and bone resorption, and promoted bone formation to increase bone mineral density (BMD) (8, 9). Moreover, many individual herbs that make up the formulas of TCM are beneficial for bone formation since they are bone-specific drugs for the treatment of bone fractures and bone loss diseases (11). Rehmanniae Radix has been clinically used for more than 3,000 years in Chinese medicine, which has an anti-OP effect through modulating the kidney and liver functions and improving blood circulation (12). Over 140 individual compounds have been isolated from Rehmanniae Radix, and iridoid glycosides (a kind of monoterpenoids) are vital for the anti-OP activity of Rehmanniae Radix (6).
Terpenoids are structurally diverse and may represent the most diverse source of essential chemotherapeutic drugs. They are isoprene units (C5H8)n-based nature products and are classified into monoterpenes, sesquiterpenes, diterpenes, triterpenes, and tetraterpenes. To date, more than 40,000 different terpenoids have been obtained in nature (13, 14). Terpenoids are also reported to have anti-inflammatory, anti-cancer, and neuroprotective effects, with beneficial effects on human health. Although the treatment of OP using TCM has a long history and natural terpenoids have been extensively studied for their therapeutic activities against bone resorption (15), less attention has been given to the whole series of terpenoids in the treatment of OP. Therefore, we here summarize anti-OP advances and molecular mechanisms of terpenoids isolated from TCM.
Natural Terpenoids Against OP
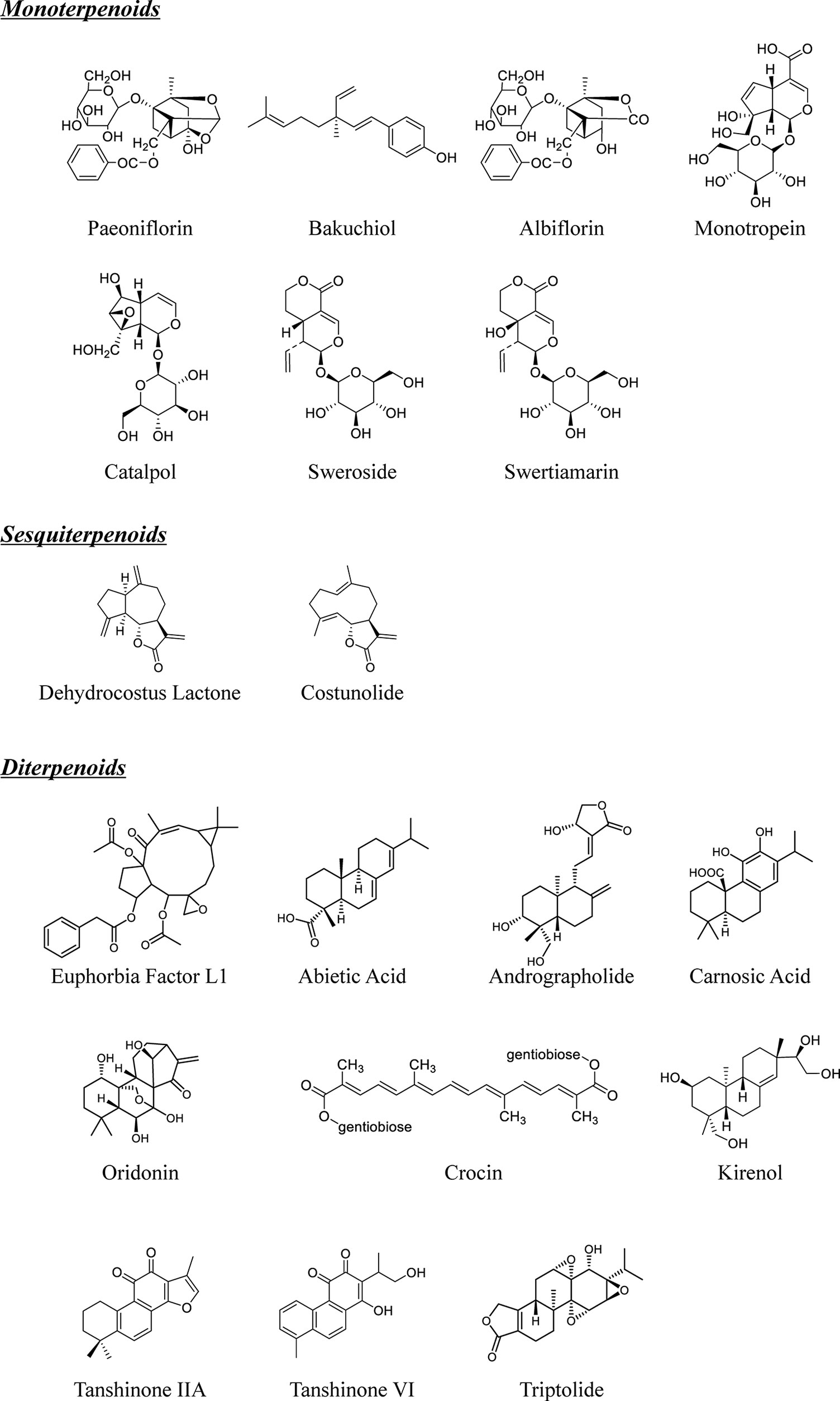
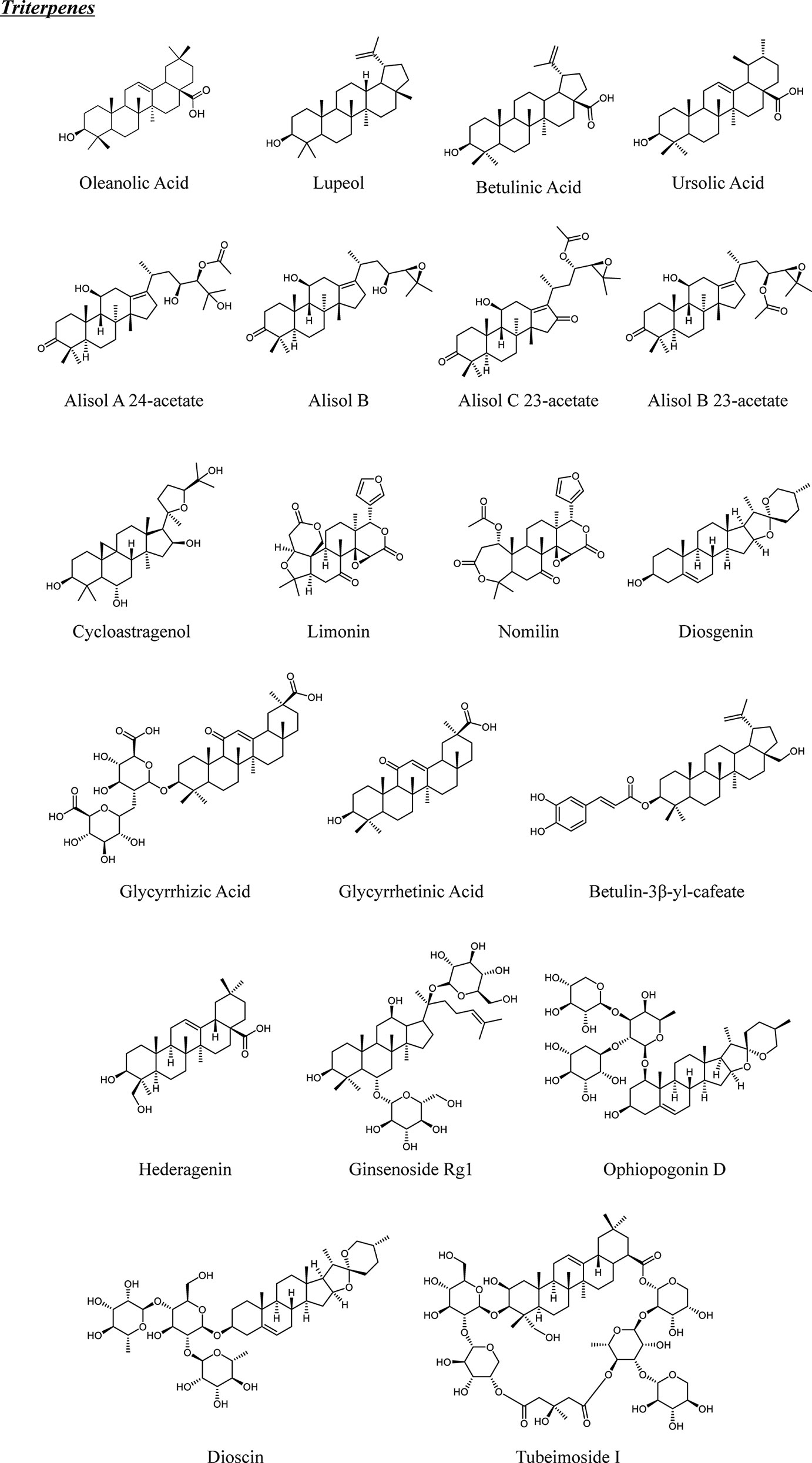
Terpenoids are classified as monoter-, sesquiter-, diter-, triter-, and tetra-penoids according to different structures (Figures 1 and 2). Although few natural terpenoids exhibit genotoxicity or carcinogenicity based on epigenetic mechanism, most are beneficial to humans (15). Natural terpenoids from TCM have been reported to regulate OBs and OCs via different signaling pathways (concluded in Figure 3 and Table 1), such as nuclear factor-κB (NF-κB), Wnt/β-catenin, mitogen-activated protein kinases (MAPK), and receptor activator of nuclear factor-κB ligand (RANKL)/receptor activator of nuclear factor-κB (RANK). We will provide a comprehensive review of natural terpenoids from TCM and their potential in OP therapy.
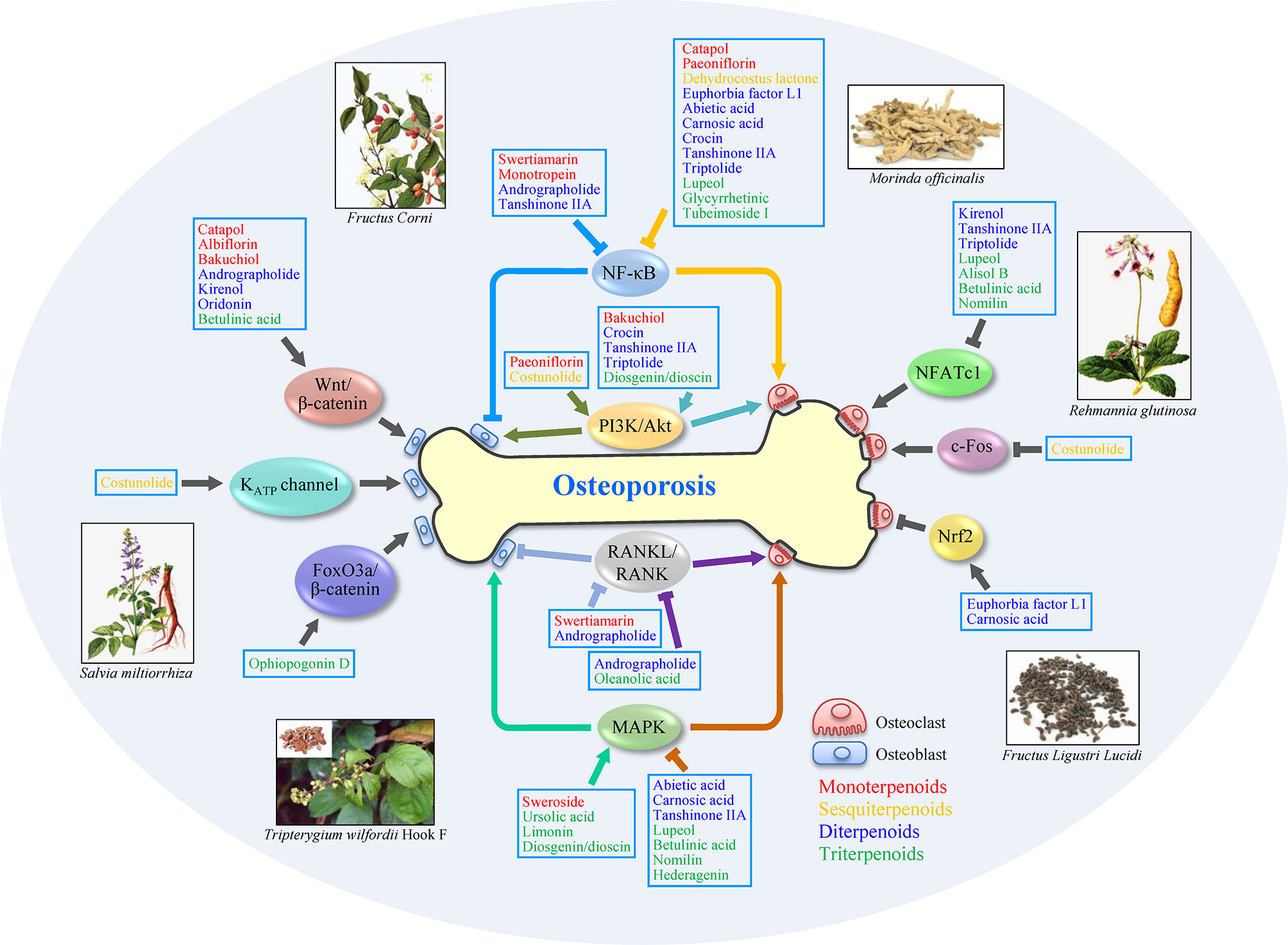
Figure 3 Schematic of anti-osteoporosis mechanisms of terpenoid on osteoblasts and osteoclasts. The activation of MAPK, PI3K/Akt, Wnt/β-catenin signaling pathways and so on, or inhibition of NF-κB and RANKL/RANK signaling pathways, can promote cell proliferation or differentiation in osteoblasts, which benefits osteoporosis treatment. Meanwhile, the inhibition of MAPK, NF-κB, RANKL/RANK, and NFATc1 signaling pathways, or activation of PI3K/Akt and Nrf2 signaling pathways, also exerts potential therapeutic efficacy via regulating osteoclasts. Some terpenoids, such as andrographolide and tanshinone IIA, show anti-osteoporosis effect by modifying multi-targets. Arrows (↓) indicate activation of a factor or positive effect on indicated cell type, while inverted T marks (⊥) indicate inhibition or negative effect. Subclass of terpenoids is distinguished with different colors: monoterpenoids (red), sesquiterpenoids (yellow), diterpenoids (blue), and triterpenoids (green).
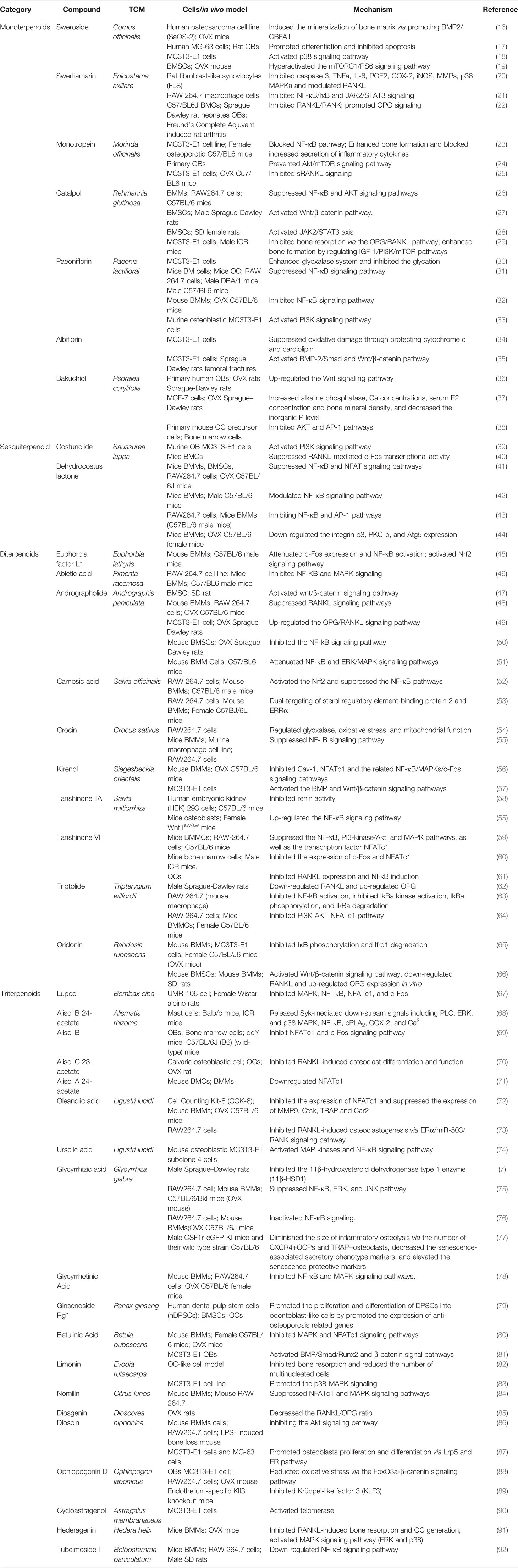
Table 1 Summary of studies for the antiosteoporotic effects of natural terpenoids from natural Chinese medicine.
Monoterpenoids
Sweroside, an iridoid glycoside obtained from Cornus officinalis Sieb. et Zucc. (Shan Zhu Yu in Chinese), is commonly used in TCM for treating OP in postmenopausal women or elderly men (93). Emerging evidences demonstrated that sweroside increased the proliferation and suppressed the apoptosis of human MG-63 cells and rat OBs (17). Yan et al. observed that sweroside effectively promoted OB differentiation in bone marrow mesenchymal stem cells (BMSCs) through hyperactivating the mechanistic target of rapamycin complex 1 (mTORC1)/pS6 signaling pathway (19). Additionally, sweroside treatment induced the mineralization of bone matrix via modulating the expression of bone morphogenetic protein (BMP)-2/core binding factor alpha 1 (CBFA1)-mediated molecules in postmenopausal OP. Meanwhile, sweroside promoted the mineralization of MC3T3-E1 cells by activating p38 signaling pathway (16, 18). Swertiamarin, a structural analog of sweroside, is a secoiridoid glycoside extracted from Enicostemma axillere subsp. axillere (Gentianaceae) (94). It was evidenced that swertiamarin could promote OB differentiation and exhibit anti-inflammatory activity by regulating NF-κB/inhibitor of κB (IκB) and Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathways. In addition, swertiamarin treatment markedly reduced RANKL/RANK expression and elevated osteoprotegerin (OPG) level, showing an excellent anti-osteoclastogenic activity (20–22).
Morinda officinalis HOW (Ba Ji Tian in Chinese) has been continuously used for more than 2,000 years in China as a tonic to nourish the kidney, strengthen bones, and enhance immune function in the treatment of OP (95, 96). It has been reported that the root extracts of Morinda officinalis showed therapeutic effect by suppressing bone resorption and enhancing bone formation on OP rat model induced by sciatic neurectomy and ovariectomy (97). He et al. observed that monotropein, a natural iridoid glycoside in the root extracts of Morinda officinalis, effectively attenuated lipopolysaccharide (LPS)- and ovariectomy-induced bone loss, and reduced inflammatory responses in MC3T3-E1 cells via inhibiting the activation of NF-κB (23). Furthermore, monotropein showed anti-osteoporotic effect by increasing bone mineral content (BMC), BMD, bone volume fraction (BVF), and decreasing the levels of interleukin (IL)-1, IL-6 and soluble RANKL in the serum of ovariectomized (OVX) mice (25). Meanwhile, monotropein treatment attenuated oxidative stress and increased the proliferation of OBs (24, 25).
Catalpol, the major bioactive iridoid glycoside isolated from Rehmannia glutinosa (Gaertn.) Libosch. ex Fisch. et C. A. Mey. Root (Dihuang in Chinese), is clinically used for OP treatment in China (6). Meng et al. showed that catapol suppressed RANKL-induced bone resorption in bone marrow-derived macrophages (BMMs) and RAW264.7 cells by reducing the ubiquitination of phosphatase and tensin homolog (PTEN), which subsequently inhibited the activations of NF-κB and protein kinase B (Akt) (26). Other reports also proved that catalpol treatment promoted the osteogenic ability of BMSCs and BMSC-dependent angiogenesis, partly via activation of JAK2/STAT3 axis and Wnt/β-catenin pathway (27, 28). Furthermore, Zhao et al. observed that catalpol could protect diabetic OP induced by high glucose treatment in MC3T3-E1 cells through regulating the migration and differentiation of OBs (29).
As a water-soluble monoterpene glucoside, paeoniflorin is the major bioactive components extracted from the root of Paeonia lactifloral Pall (98).. In antimycin A treated osteoblastic MC3T3-E1 cells, paeoniflorin attenuated cytotoxicity via improving the mitochondrial function. In addition, paeoniflorin also increased the differentiation of MC3T3-E1 cells and inhibited oxidative stress induced by methylglyoxal in the same cell model (30, 33, 98). In rats fed on high-carbohydrate/high-fat (HCHF) diet, paeoniflorin exhibited multiple pharmacological activities to prevent hyperlipidemia-induced OP. Intriguingly, paeoniflorin increased the trabecular and cortical parameters, as well as width and length of femur. Simultaneously, paeoniflorin rescued OB differentiation and the proliferation activities of bone turnover markers (99). Xu et al. reported that paeoniflorin suppressed bone destruction in collagen-induced arthritis (CIA) and decreased OC differentiation in vitro by down-regulating the activation of NF-κB (31). Wang et al. demonstrated that paeoniflorin suppressed OC generation and promoted OB formation via regulating NF-κB signaling pathway in BMMs and OVX mice (32).
Albiflorin, a monoterpene glycoside isolated from the roots of Paeonia lactifloral Pall., owns the ability to increase the differentiation of osteoblastic MC3T3-E1 cells (98). Kwang et al. found that albiflorin maintained mitochondrial function by reducing cytochrome c loss and cardiolipin peroxidation in MC3T3-E1 cells, which contributed to the inhibition of antimycin A-induced oxidative stress and toxicity (34). Another study showed that albiflorin treatment promoted the generation of OBs and expression of runt-related transcription factor 2 (RUNX2) through activating BMP-2/Smad and Wnt/β-catenin signaling pathways (35). Meanwhile, albiflorin up-regulated the levels of various osteogenic genes, such as osteocalcin (OCN), osteopontin (OPN), osteonectin (OSN), bone sialoprotein (BSP), and AP. In femur fracture rat model, albiflorin stimulated the expression of osteogenic genes in femoral tissue and promoted callus formation at the early stage during fracture recovery. Additionally, albiflorin could increase the expression of bone-related genes (35). This finding suggested that albiflorin motivated bone calcification, osteogenesis and bone formation, resulting in improving the fracture healing.
Bakuchiol is a prenylated phenolic monoterpene in the fruit of Psoralea corylifolia (L.) Medik (37, 100). And Psoralea corylifolia was used in TCM formulas to treat osteoporosis for a long history time (101). Recent researches indicated that Psoralea corylifolia and its major active ingredient bakuchiol possessed anti-OP activity (100, 102). Bakuchiol treatment significantly inhibited bone resorption and OC differentiation via the inhibition of Akt phosphorylation and c-jun nuclear translocation induced by macrophage colony stimulating factor (M-CSF) plus RANKL (38). In OVX Sprague-Dawley (SD) rats, bakuchiol treatment reduced bone loss through increasing Ca2+ and serum E2 concentrations, AP activity, and BMD, along with reduced inorganic P level (37). Li et al. found that bakuchiol significantly stimulated OB proliferation and differentiation (103). In addition, bakuchiol treatment prevented bone loss in OVX rats induced by estrogen deficiency and induced OB differentiation by up-regulating the Wnt signaling pathway (36).
Collectively, monoterpenoids can protect bone from erosion via targeting different signaling pathways. In OBs, catapol, albiflorin, and bakuchiol can activate Wnt/β-catenin signaling pathway; paeoniflorin and sweroside stimulate PI3K/Akt and MAPK signaling pathways respectively; swertiamarin inhibits RANKL/RANK signaling pathway; monotropein and swertiamarin suppress NF-κB signaling pathway. In OCs, catapol and paeoniflorin depress NF-κB signaling pathway; bakuchiol enhances PI3K/Akt signaling pathway.
Sesquiterpenoids
Costunolide is sesquiterpene lactones derived from Saussurea lappa C.B. Clarke roots. A recent research showed that costunolide markedly induced bone mineralization and differentiation and increased cell growth, AP activity, and collagen synthesis in osteoblastic MC3T3-E1 cells via targeting diverse key proteins, such as estrogen receptor (ER), phosphoinositide 3-kinase (PI3K), extracellular signal-regulated kinase (ERK), protein kinase C (PKC), mitochondrial ATP-sensitive K+ channel, p38, and c-Jun N-terminal kinase (JNK) (39). Moreover, Cheon et al. observed that costunolide suppressed RANKL-induced OC differentiation via suppressing c-Fos transcriptional activity without affecting c-Fos expression (40).
Saussurea lappa C.B. Clarke has been used in clinic for decades as a TCM (104). Sesquiterpenes and sesquiterpene lactones are main bioactive constituent of this herb. As a member of sesquiterpene lactones, dehydrocostus lactone is extracted from the roots of Saussurea lappa and has been reported to exert various pharmacological activities including anti‐ulcer, anti‐tumor, anti‐inflammatory, and immunomodulation (42, 105). In mouse BMMs, dehydrocostus lactone attenuated the RANKL-dependent OC differentiation through modulating IκB kinase (IKK), JNK, nuclear factor of activated T cell cytoplasmic 1 (NFATc1), and nuclear factor-erythroid 2-related factor 2 (Nrf2). Moreover, it suppressed the activation of OCs through down-regulating the expression of integrin β3, PKC-β, and autophagy related 5 (43, 44). Besides, dehydrocostus lactone reduced RANKL‐induced OC formation and differentiation via modulating NF‐κB signaling pathway both in vitro and in vivo (41, 42).
Therefore, costunolide owns the ability to increase bone formation by modulating KATP channel and activating PI3K/Akt signaling pathway in OBs, and dehydrocostus lactone can decrease OC differentiation via inhibiting NF-κB signaling pathway.
Diterpenoids
Euphorbia factor L1 (EFL1) is an active diterpenoid composition extracted from the seed oil of Chinese herb Euphorbia lathyrism L. (Qian Jin Zi in Chinese) (106). EFL1 inhibited RANKL-induced osteoclastogenesis by inhibiting c-Fos expression and NF-κB activation. Meanwhile, apoptosis induced by EFL1 in differentiated OCs resulted from caspase activation and enhanced Fas ligand expression. In mice, EFL1 ameliorated bone destruction induced by inflammation and ovariectomy. These findings demonstrated that EFL1 can block OC differentiation through modulating inflammatory responses and trigger Fas-regulated apoptosis, which offers the potential to treat OP caused by excessive Ocs (45).
Abietic acid is a bioactive diterpene isolated from Pimenta racemosa var. grissea which exhibits anti-obesity and anti-inflammatory activities (107). In RAW264.7 cells and mouse BMMs, abietic acid inhibited RANKL-induced OC formation via suppressing NF-κB and MAPK signaling pathways. It also decreased the expression of osteoclastic genes, such as NFATc1, tartrate-resistant acid phosphatase (TRAP), dendritic cell specific transmembrane protein (DC-STAMP), and c-Fos. In C57/BL6 male mice of osteolysis model induced by LPS, abietic acid significantly reduced the number of Ocs and the levels of inflammatory cytokines, including tumor necrosis factor (TNF)-α and IL-6 (46).
As a bicyclic diterpenoid lactone, andrographolide can be isolated from the leaves of traditional herb Andrographis aniculate (Burm. F.) Wall. Ex Nees in Wallich (Chuan Xin Lian). According to previous study, andrographolide has extensive pharmacological activities, such as anti-inflammation, anti-oxidation, anti-platelet aggregation, immunomodulation, and potential antineoplastic properties partly by targeting NF-κB (108–111). Andrographolide showed the capacity to protect breast cancer-induced bone loss (112) and inflammatory osteolysis (51, 113). Furthermore, andrographolide depressed osteoclastogenesis in BMMs by decreasing the expression of OC-related genes induced by RANKL and inhibiting bone loss and inflammation in OVX mice (48, 51). In addition, andrographolide promoted osteogenesis of mouse and rat BMSCs and blocked the inhibitory effect of TNF-α on OB formation and mineralization (47, 50). Other study indicated that andrographolide increased OPG expression and suppressed OC differentiation in MC3T3-E1 cells. It also stimulated the differentiation and survival of OBs, which increased bone deposition. Meanwhile, the study confirmed that andrographolide prevented bone loss and improved bone turnover rate in OVX rat model (49).
Carnosic acid, an abietane diterpenoid extracted from Rosmarinus officinalis (rosemary) and Salvia officinalis (common sage), displayed anti-angiogenic, anti-neoplastic, anti-oxidant and anti-HIV activities (114). Recent study had suggested the protective effect of rosemary against OP through effectively mitigated bone loss induced by calcium deficiency (115). Both in RAW 264.7 cells and mouse BMMs, carnosic acid decreased the osteoclastogenesis and reactive oxygen species (ROS) generation via activating Nrf2 and suppressing NF-κB and MAPK signaling pathways. The same results were also detected in C57BL/6 male mice of LPS-induced OP (52). Furthermore, Zheng et al. found that carnosic acid played a dual role via targeting sterol regulatory element-binding protein 2 (SREBP2) and estrogen-related receptor alpha (ERRα) to suppress RANKL-mediated osteoclastogenesis and restrained bone loss induced by ovariectomy (53).
Crocin, a diterpenoid glycoside carotenoid component of Crocus sativus L., shows various pharmacological activities (116, 117). It was observed that crocin treatment mitigated bone loss in metabolic syndrome-induced OP rat model (118). Meanwhile, this research showed anti-inflammatory and anti-oxidative activities of crocin which significantly decreased the production of IL-6, TNF-α, reduced glutathione (GSH), and superoxide dismutase (SOD). In RAW264.7 cells, crocin attenuated the dysfunction of OCs induced by methylglyoxal via modulating glyoxalase I, oxidative stress, and mitochondrial function (54). Moreover, Fatemeh et al. observed that crocin could effectively improve the differentiation of BMSCs, by inhibiting NF-κB signaling pathway activation, crocin treatment suppressed RANKL-induced bone resorption and OC formation (55, 119).
Kirenol is a bioactive diterpenoid compound derived from Siegesbeckia orientalis L. that was used as an anti-rheumatic TCM (120, 121). Kim et al. demonstrated that kirenol stimulated OB differentiation via activation of BMP and Wnt/β-catenin signaling pathways in MC3T3-E1 cells, which increased the levels of AP, OPN, type I collagen, and OB differentiation markers, as well as the OPG/RANKL ratio (57). Furthermore, kirenol treatment suppressed RANKL-induced OC formation and the NFATc1/Cav-1 signaling pathway in BMMs and OVX rats, consequently preventing ovariectomy-induced OP (56).
Tanshinone IIA is an abietane diterpenoid isolated from Salvia miltiorrhiza Bunge (Danshen) that is used for the treatment of trauma and fractures in clinical according to the dispelling stasis theory of TCM (122). 36 clinical trials used Salvia miltiorrhiza to treat different kinds of osteoporosis displayed high efficacy and low toxicity (123). Modern pharmacological studies showed that the ethanol extract of Salvia miltiorrhiza could inhibit trabecular bone loss by restraining bone resorption both in OVX and naturally menopaused mice (124). Tanshinone IIA blocked dexamethasone induced OB apoptosis through the suppression on NADPH oxidase (Nox) 4-derived ROS production. In addition, it blocked RANKL-mediated OC differentiation by decreasing the expression of c-Fos and NFATc1 (60). Tanshinone IIA could attenuate the formation of OCs by depressing the NF-κB, PI3K/Akt, and MAPK signaling pathways in OVX mice model (59). Zhu et al. found that tanshinone IIA administration prevented the harmfulness of oxidative stress and promoted the activity and functions of OBs in genetic OP model, Wnt1sw/sw mice, through regulating the NF−κB signaling pathway (125). Recently, in streptozotocin (STZ)-induced C57BL/6 diabetic mice, tanshinone IIA treatment restrained the activity of renin that resulted in protecting OP (58). As another abietane diterpenoid constituent obtained from Salvia miltiorrhiza, tanshinone VI significantly suppressed the differentiation of OCs and bone resorption via down-regulating the expression of RANKL and activation of NF−κB (61).
Triptolide, the major active diterpenoid component isolated from Tripterygium wilfordii Hook F, has been used in TCM for hundreds of years to treat cancer and bone loss (126, 127). A recent study suggested that triptolide effectively suppressed the activation of NF-κB induced by RANKL, as well as tumor cell- and RANKL-induced OC formation (63). Triptolide showed the protective effects on bone loss both in old male rats and OVX C57BL/6 mice (62, 64). Triptolide could suppress RANKL-induced OC formation and prevented the bone resorption of OCs in BMSCs and RAW264.7 cells, resulting from inhibiting PI3K/Akt/NFATc1 signaling pathway.
Oridonin is an ent-kaurane diterpenoid extracted from the TCM herb Rabdosia rubescens (Hemsl.) Hara (128). As a plant metabolite, oridonin acts as an anti-tumor agent, angiogenesis inhibitor, apoptosis inducer, anti-asthmatic agent, and anti-bacterial agent (129, 130). Recent studies demonstrated that oridonin could maintain bone homeostasis (65, 66). In ovariectomy-induced OP mouse model, oridonin could protect bone loss via inhibiting osteoclastogenesis and enhancing osteogenesis by inhibiting interferon-related development regulator 1 (Ifrd1) and IκBα-mediated p65 nuclear translocation. Simultaneously, in vitro study revealed that oridonin motivated osteogenesis by Wnt/β-catenin signaling pathway and suppressed RANKL-induced OC formation in BMSCs.
In conclusion, diterpenoids are mostly investigated terpenoids that exert superior anti-OP efficacy by affecting various signaling pathways. In OBs, andrographolide, kirenol, and oridonim activate Wnt/β-catenin signaling pathway; andrographolide inhibits RANKL/RANK and NF-κB signaling pathways; tanshinone IIA blocks NF-κB signaling pathway. In OCs, euphorbia factor L1, abietic acid, carnosic acid, crocin, tanshinone IIA, and triptolide depress NF-κB signaling pathway; crocin, tanshinone IIA, and triptolide activate PI3K/Akt signaling pathway; andrographolide inhibits RANKL/RANK signaling pathway; abietic acid, carnosic acid, and tanshinone IIA inhibit MAPK signaling pathway; kirenol, tanshinone IIA, and triptolide depress NFATc1 signaling pathway; euphorbia factor L1 and carnosic acid promote Nrf2 signaling pathway.
Triterpenoids
Lupeol is a major active lupine-type pentacyclic triterpenoid of Sorbus commixta Hedlund and Celastrus orbiculatus Thunb (131). Recently, lupeol has attracted the attention of researchers for its osteogenic activity. On one hand, lupeol significantly suppressed OC differentiation and bone resorption mediated by 1α, 25-(OH)2D3 and prostaglandin E2 (PGE2) via inhibiting the activities of MAPK and transcription factors (NF-κB, NFATc1, and c-Fos). On another hand, lupeol decreased hypercalcemic mediated bone loss in C57BL/6 mice (67). In addition, lupeol in bombax ceiba contributed to relieve bone fragility and fracture (132).
Alismatis Rhizoma is a famous traditional Chinese herb, which has been used for hepatoprotective, diuretic, hypolipidemic, anti-tumor, anti-inflammatory and anti-diabetic treatments for more than ten centuries (133, 134). More and more researches reported that the terpenoids constituents of this herb, such as the protostane triterpenes compounds Alisol B (69), Alisol A 24-acetate (71, 135), Alisol B 23-acetate (68), and Alisol C 23-acetate (70), own the protective activity against bone loss. Alisol A 24-acetate suppressed OC differentiation mediated by RANKL through downregulating NFATc1 and restraining the DC-STAMP and cathepsin K expression in mouse BMMs (71). Moreover, in OVX mice, alisol A 24-acetate and alisol C 23-acetate could effectively protect bone loss (70, 135). Alisol B suppressed the RANKL-induced osteoclastogenesis in mouse BMMs and stopped bone loss in 2-methylene-19-nor-(20S)-1a,25(OH)2D3 (2MD)-induced hypercalcemia mouse model (69).
As a member of the pentacyclic triterpenoids, oleanolic acid is a free acid or triterpenoid saponins in many Chinese herbs, such as Nvzhenzi (Ligustri lucidi W. T. Aiton), Baihuasheshecao (Hedyotis diffusa), Renshen (Panax ginseng C. A. Meyer), and Sanqi (Panax Notoginseng (Burk.) F.H.Chen). Nvzhenzi has been clinically applied in the treatment of OP for over 1,000 years (136). Chen et al. summarized more than 150 articles and reviews on the anti-osteoporosis activity of Ligustri lucidi. In TCM, Ligustri lucidi is believed to have anti-osteoporosis effects, improve liver and kidney deficiency and reduce lower back pain. Pharmacological experiments showed Ligustri lucidi improved bone metabolism and bone quality in OVX, growing, aged and diabetic rats via regulating PTH/FGF-23/1,25-(OH)2D3/CaSR, Nox4/ROS/NF-κB, and OPG/RANKL/cathepsin K signaling pathways (137) Oleanolic acid could suppress RANKL-mediated osteoclastogenesis in BMMs, and attenuate bone loss through decreasing the quantity of OC in C57BL/6 OVX mouse model (72). Furthermore, it has been proved that oleanolic acid modulated the ER alpha/miR-503/RANK signaling pathway to inhibit RANKL-induced osteoclastogenesis in RAW264.7 cells (138). In aged female rats and mature OVX mice, oleanolic acid regulated vitamin D metabolism to exhibit osteoprotective effect (73). The investigation with high-throughput metabolomics showed that oleanolic acid ameliorated the disordered metabolism state in glucocorticoid-induced OP rats (139). In addition, five oleanolic acid glycosides of Achyranthes bidentata also exerted inhibitory effect on the formation of OC-like multinucleated cells (OCLs) induced by 1α, 25-(OH)2D3 (140).
Ursolic acid, as the isomer of oleanolic acid, is a ubiquitous active triterpenoids constituent in traditional Chinese medicinal herbs, such as Salvia miltiorrhiza (141, 142), Fructus ligustri lucidi (143), and Eriobotrya japonica (144, 145). Ursolic acid exhibited multiple pharmacological activities, including anti-cancer, anti-inflammation, anti-anaphylaxis, and anti-aging (146–148). In recent years, ursolic acid has attracted the attention of researchers for its osteogenic activity. Lee et al. proved that ursolic acid induced the expression of OB-specific genes by activating NF-κB, MAPK, and activator protein-1. Moreover, they demonstrated the osteogenic activity of ursolic acid in a mouse calvarial bone model (74). As the two most abundant ingredients in Fructus ligustri lucidi, both ursolic acid and oleanolic acid regulated the expression of bone turnover markers and calcium balance in mature OVX rats. In addition, the combination of these two compounds significantly improved bone properties and vitamin D metabolism in aged female rats (143, 149). Tan et al. observed that ursolic acid prevents OC differentiation induced by RANKL in RAW 264.7 cells through targeting XPO5 (150).
Glycyrrhizic acid, as well as glycyrrhetinic acid, are extracted from the root of Glycyrrhiza glabra L., and glycyrrhizic acid is formed by the combination of pentacyclic triterpenoid glycoside and glycyrrhetinic acid (151). Both of them showed protective effects on glucocorticoid-induced OP (152). Glycyrrhizic acid and glycyrrhetinic acid could act as the ligands for glucocorticoid receptor (GR), which further modulated glucocorticoid resistance and ameliorated inflammatory responses by disrupting the GR-heat shock protein 90 (HSP90) (76, 153). Glycyrrhizic acid prevented glucocorticoid-induced OP in male SD rats through inhibiting the 11β-hydroxysteroid dehydrogenase type 1 enzyme (11β-HSD1) (75). Furthermore, Yamada et al. found that in an aging mouse model of periprosthetic osteolysis, glycyrrhizic acid alleviated inflammatory bone loss and increased senescence-protective sirtuins expression (77). In OVX mice model, glycyrrhizic acid treatment improved bone metabolism and suppressed OC differentiation via modulating NF-κB, ERK, and JNK signaling pathways (7, 154). Glycyrrhetinic acid inhibited osteoclastogenesis via decreasing RANKL-mediated association of RANK and TNF receptor associated factor 6 (TRAF6), and consequently inactivating the NF-κB and MAPK signaling pathways in vitro (BMMs and RAW264.7 cells) and in vivo (OVX C57BL/6 mice) (78).
Betulinic acid is a pentacyclic lupane-type triterpene derivative of Betula pubescens Ehrh., exhibiting multiple biological effects including osteogenic activity. Betulinic acid could enhance the proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 through regulating the BMP/Smad/Runx2 and β-catenin signal pathways (81). Furthermore, betulinic acid reduced RANKL-associated osteoclastogenesis via suppressing the MAPK and NFATc1 signaling pathways in BMMs isolated from C57BL/6 mice. In the osteoporotic C57/BL6 mice, betulinic acid prevented ovariectomy-induced bone loss (80).
Ginsenoside Rg1, a tetracyclic triterpenoid, is an active compound in Panax ginseng C. A. Meyer and Panax japonicus (T. Nees) C. A. Meyer, which acts as a neuroprotective agent and pro-angiogenic agent. Ginsenoside Rg1 promoted the proliferation and odontogenic/osteogenic differentiation of human dental pulp stem cells (hDPSCs), stimulated the proliferation of BMSCs, and suppressed the maturation and differentiation of OCs (79). Zishen Jiangtang Pill (ZJP) is a formula of Chinese medicine, which regulated bone metabolism in diabetic OP (DOP) and consequently exhibited a protective effect. As the primary active ingredient of ZJP, Ginsenoside Rg1 improved the ultrastructure and histomorphology of bone and islets in DOP rats (155).
Limonin is a tetracyclic triterpenoid of various TCM and fruits, such as Evodia rutaecarpa, Coptidis rhizoma, Cortex chinensis phellodendri, bergamot, Aurantii fructus immaturus, Citri reticulatae pericarpium, and citrus fruits (156). Early study showed that limonin significantly inhibited bone resorption and reduced the number of multinucleated cells with TRAP-positive nature in OC-like cell model (82). Otherwise, limonin treatment modulated the ERK and p38-MAPK signaling in osteoblastic MC3T3-E1 cell line to induce osteogenic differentiation (83).
Nomilin, a furan-containing triterpenoid isolated from medicinal citrus, showed inhibitory effects on RANKL-stimulated OC differentiation and bone resorption in RAW 246.7 cells and mouse BMMs cells, resulting from the inhibition of-NFATc1 and MAPK signaling pathways (84).
Diosgenin and dioscin are steroid sapogenin triterpenoids, which are extracted from Dioscorea nipponica Makino (157).. It was reported that diosgenin could suppress osteoclastogenesis and bone resorption. Meanwhile, it enhanced the osteogenic activity of OBs that contributed to increased bone formation in vitro, and anti-osteoporotic effect in vivo (85, 158–162). Diosgenin ameliorated bone loss by decreasing the RANKL/OPG ratio in OVX rats (85, 163) and retinoic acid-induced OP rats (164). Similarly, dioscin enhanced osteoblastogenesis and inhibited osteoclastogenesis to prevent ovariectomy-induced bone loss (165). In addition, dioscin blocked OC differentiation and bone resorption via inhibiting the activation of Akt signaling pathway (86). In human and mouse OB-like cell lines, dioscin promoted the proliferation and differentiation of OBs via Lrp5 and ER pathway (87).
Ophiopogonin D is a saposins triterpenoid extracted from the TCM Ophiopogon japonicus (L. f.) Ker-Gawl. and has been applied in clinical use for a long time. Ophiopogonin D suppressed ROS generation to exert anti-OP effects via the FoxO3a/β-catenin signaling pathway in both RAW264.7 and MC3T3-E1 cells. In RAW264.7 cells, ophiopogonin D decreased the expression of Osteoclastic genes and the activity of CTX1 and TRAP, which are bone degradation markers in serum. In MC3T3-E1 cells, ophiopogonin D significantly promoted cell proliferation and increased the gene levels of some osteogenic markers (88). Furthermore, Yang et al. highlighted that ophiopogonin D owned the ability to inhibit Krüppel-like factor 3 (KLF3), resulting in increased abundance of vessels in the bone tissue for bone formation (89).
As a pentacyclic triterpenoid compound, cycloastragenol is the aglycone derivative of astragaloside IV isolated from the root of Astragalus membranaceus (Fisch.) Bunge, which is a TCM used for thousands of years (166). Recent study reported that cycloastragenol might be a candidate drug to treat glucocorticoid-induced OP (GIOP) through alleviating the inhibition of osteogenic differentiation induced by dexamethasone (90). Yu et al. also observed that cycloastragenol treatment could improve bone formation, protect bone microstructure from degradation, reduce OC number, and augment bone biomechanical properties in both bone loss models induced by aging and D-galactose. Furthermore, cycloastragenol promoted the differentiation, viability, and mineralization of osteoblastic MC3T3-E1 cells. Cycloastragenol could also alleviate bone loss through increasing osteoactivin expression (167).
Hederagenin is a pentacyclic triterpenoid sapogenin extracted from Hedera helix (common ivy). In BMM cell model, hederagenin depressed the formation and bone (hydroxyapatite) resorption of OC induced by RANKL. Mechanism study revealed that hederagenin reduced the production of intracellular reactive oxygen species (ROS) and the activation of MAPK signaling pathway (ERK and p38), causing decreased induction of c-Fos and NFATc1. Similar to the in vitro effects, hederagenin treatment significantly prevented bone loss in OVX mice via inhibiting RANKL-induced bone resorption and OC generation (91). Meanwhile, hederagenin 3-O-(2-O-acetyl)-α-L-arabinopyranoside remarkably elevated the protein levels of BSP and osteocalcin and augmented AP activity (168).
Tubeimoside I, isolated from the Chinese medicinal herb Bolbostemma paniculatum (Maxim) Franquet (Cucurbitaceae), is a natural pentacyclic triterpenoid, and traditionally used for the treatment of snake venoms and inflammation. Recently, it was reported that tubeimoside I could inhibit the formation and function of OCs, as well as type 2 diabetes-induced decrease of bone mass in SD rats, resulting from down-regulating IκBα degradation which subsequently suppressed NF-κB transcriptional activity (92).
In summary, triterpenoids are potential anti-OP candidates with multi-target characteristics. In OBs, betulinic acid can activate Wnt/β-catenin signaling pathway; ophiopogonin D stimulates FoxO3a/β-catenin signaling pathway; ursolic acid, limonin, diosgenin, and dioscin promote MAPK signaling pathway. In OCs, diosgenin and dioscin enhance PI3K/Akt signaling pathway; lupeol, glycyrrhetinic, and tubeimoside I inhibit NF-κB signaling pathway; oleanolic acid inhibits RANKL/RANK signaling pathway; lupeol, betulinic acid, nomilin, and hederagenin depress MAPK signaling pathway; lupeol, alisol B, betulinic acid, and nomilin block NFATc1 signaling pathway.
Conclusion and Prospects
TCM has been widely used around the world for thousands of years to treat various diseases. These in vivo and in vitro findings discussed above demonstrate that terpenoids in natural Chinese medicine own the potential ability to provide therapeutic benefits for OP treatment.
Although terpenoids are beneficial for OP treatment, some terpenoids have been reported to be toxic. Cantharidin, a monoterpene obtained from Mylabris phalerata showed nephrotoxicity by suppressing the lactate dehydrogenase expression and intracellular release (169). Diterpene compound Pekinenin C and pekinenal also exhibited serious cytotoxicity intestinal toxicity (170). Thus, modification of their structures for lower toxicity and stronger efficacy are needed. For example, the quinoxaline derivative of oleanolic acid, QOA-8a, could not only inhibit bone resorption but also stimulate bone formation, playing dual roles in anti-OP (171). Meanwhile, the addition of quinoxaline contributed to lower cytotoxicity (172). Comparing with andrographolide itself, its derivative 14-deoxy-11,12-didehydroandrographolide showed stronger anti-osteoclastogenesis effect with significantly reduced cytotoxicity (173, 174). Therefore, structure modification will be an optional strategy for anti-OP drug development based on natural terpenoids. In addition, other problems, such as poor water solubility, short half-life, poor stability, and low bioavailability, severely limit the development and clinical use of TCM. The application of modern technologies (nanotechnology and co-crystallization) can overcome these short comings (175–177). Hence, for those terpenoids with perfect anti-OP efficacy but poor water solubility, we can apply nanoparticles in the drug delivery.
Nowadays, though a massive of studies reveal the anti-OP effects and molecular mechanisms of terpenoids, most of their direct targets as well as regulation mechanisms have not been illustrated. Several advanced technologies, such as proteomics (178) and systems pharmacology-based approaches (179, 180), have offered effective tools to identify potential targets of natural terpenoids. Proteomics and systems pharmacology-based approaches could perform the large-scale study of proteins and the major targets of most compounds. On the one hand, it is helpful to explain the exact pharmacological mechanism for pre-clinical drug development. On the other hand, the screening of terpenoids targeted proteins in OP treatment benefits researchers for understanding the pathogenesis of osteoporosis.
Moreover, TCM not only exerted anti-OP functions alone through diverse signaling pathways, but also showed enhancing effects via combining with clinically used hormones (estrogen or growth hormone) to prevent bone loss (181). This combination can avoid possible toxic side-effects and improve clinical efficacy (182). In the future, more in-depth and high-quality clinical researches are essential to ensure the safety, efficacy, and specificity of the terpenoids, which will provide more evidence for the candidates in efficiently anti-osteoporotic applications.
Author Contributions
JF and YZ: conceptualization. YZ and ML: writing — original draft preparation. QJ, HK, QL, and L-FZ: editing, and revising. JF: supervision. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the National Natural Science Foundation of China (No.82074278), the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110584), Special Foundation of Guandong Educational Committe (No. 2021ZDZX2001), and Guangdong Province Science and Technology Plan International Cooperation Project (No. 2020A0505100052).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Qadir A, Liang S, Wu Z, Chen Z, Hu L, Qian A. Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells. Int J Mol Sci (2020) 21:349. doi: 10.3390/ijms21010349
2. An J, Yang H, Zhang Q, Liu C, Zhao J, Zhang L, et al. Natural Products for Treatment of Osteoporosis: The Effects and Mechanisms on Promoting Osteoblast-Mediated Bone Formation. Life Sci (2016) 147:46–58. doi: 10.1016/j.lfs.2016.01.024
3. Melton LJ 3rd. How Many Women Have Osteoporosis Now? J Bone Miner Res (1995) 10:175–7. doi: 10.1002/jbmr.5650100202
4. Liu Y, Liu JP, Xia Y. Chinese Herbal Medicines for Treating Osteoporosis. Cochrane Database Syst Rev (2014) 3:CD005467. doi: 10.1002/14651858.CD005467.pub2
5. Bu L, Dai O, Zhou F, Liu F, Chen JF, Peng C, et al. Traditional Chinese Medicine Formulas, Extracts, and Compounds Promote Angiogenesis. BioMed Pharmacother (2020) 132:110855. doi: 10.1016/j.biopha.2020.110855
6. Liu C, Ma R, Wang L, Zhu R, Liu H, Guo Y, et al. Rehmanniae Radix in Osteoporosis: A Review of Traditional Chinese Medicinal Uses, Phytochemistry, Pharmacokinetics and Pharmacology. J Ethnopharmacol (2017) 198:351–62. doi: 10.1016/j.jep.2017.01.021
7. Yin Z, Zhu W, Wu Q, Zhang Q, Guo S, Liu T, et al. Glycyrrhizic Acid Suppresses Osteoclast Differentiation and Postmenopausal Osteoporosis by Modulating the Nf-Kappab, Erk, and Jnk Signaling Pathways. Eur J Pharmacol (2019) 859:172550. doi: 10.1016/j.ejphar.2019.172550
8. Zhang ND, Han T, Huang BK, Rahman K, Jiang YP, Xu HT, et al. Traditional Chinese Medicine Formulas for the Treatment of Osteoporosis: Implication for Antiosteoporotic Drug Discovery. J Ethnopharmacol (2016) 189:61–80. doi: 10.1016/j.jep.2016.05.025
9. Wang SJ, Yue W, Rahman K, Xin HL, Zhang QY, Qin LP, et al. Mechanism of Treatment of Kidney Deficiency and Osteoporosis Is Similar by Traditional Chinese Medicine. Curr Pharm Des (2016) 22:312–20. doi: 10.2174/1381612822666151112150346
10. Gao Z, Lu Y, Halmurat U, Jing J, Xu D. Study of Osteoporosis Treatment Principles Used Historically by Ancient Physicians in Chinese Medicine. Chin J Integr Med (2013) 19:862–8. doi: 10.1007/s11655-013-1328-z
11. He J, Li X, Wang Z, Bennett S, Chen K, Xiao Z, et al. Therapeutic Anabolic and Anticatabolic Benefits of Natural Chinese Medicines for the Treatment of Osteoporosis. Front Pharmacol (2019) 10:1344. doi: 10.3389/fphar.2019.01344
12. Tan W, Yu KQ, Liu YY, Ouyang MZ, Yan MH, Luo R, et al. Anti-Fatigue Activity of Polysaccharides Extract From Radix Rehmanniae Preparata. Int J Biol Macromol (2012) 50:59–62. doi: 10.1016/j.ijbiomac.2011.09.019
13. Withers ST, Keasling JD. Biosynthesis and Engineering of Isoprenoid Small Molecules. Appl Microbiol Biotechnol (2007) 73:980–90. doi: 10.1007/s00253-006-0593-1
14. Ateba SB, Mvondo MA, Ngeu ST, Tchoumtchoua J, Awounfack CF, Njamen D, et al. Natural Terpenoids Against Female Breast Cancer: A 5-Year Recent Research. Curr Med Chem (2018) 25:3162–213. doi: 10.2174/0929867325666180214110932
15. Bellavia D, Caradonna F, Dimarco E, Costa V, Carina V, De Luca A, et al. Terpenoid Treatment in Osteoporosis: This Is Where We Have Come in Research. Trends Endocrinol Metab (2021) 32:846–61. doi: 10.1016/j.tem.2021.07.011
16. Choi Y, Kim MH, Yang WM. Promotion of Osteogenesis by Sweroside Via Bmp2-Involved Signaling in Postmenopausal Osteoporosis. Phytother Res (2021) 35:7050–63. doi: 10.1002/ptr.7336
17. Sun H, Li L, Zhang A, Zhang N, Lv H, Sun W, et al. Protective Effects of Sweroside on Human Mg-63 Cells and Rat Osteoblasts. Fitoterapia (2013) 84:174–9. doi: 10.1016/j.fitote.2012.11.010
18. Wu QC, Tang XY, Dai ZQ, Dai Y, Xiao HH, Yao XS. Sweroside Promotes Osteoblastic Differentiation and Mineralization Via Interaction of Membrane Estrogen Receptor-Alpha and Gpr30 Mediated P38 Signalling Pathway on Mc3t3-E1 Cells. Phytomedicine (2020) 68:153146. doi: 10.1016/j.phymed.2019.153146
19. Ding Y, Jiang H, Meng B, Zhu B, Yu X, Xiang G. Sweroside-Mediated Mtorc1 Hyperactivation in Bone Marrow Mesenchymal Stem Cells Promotes Osteogenic Differentiation. J Cell Biochem (2019) 120:16025–36. doi: 10.1002/jcb.28882
20. Saravanan S, Islam VI, Thirugnanasambantham K, Pazhanivel N, Raghuraman N, Paulraj MG, et al. Swertiamarin Ameliorates Inflammation and Osteoclastogenesis Intermediates in Il-1beta Induced Rat Fibroblast-Like Synoviocytes. Inflammation Res (2014) 63:451–62. doi: 10.1007/s00011-014-0717-5
21. Saravanan S, Islam VI, Babu NP, Pandikumar P, Thirugnanasambantham K, Chellappandian M, et al. Swertiamarin Attenuates Inflammation Mediators Via Modulating Nf-Kappab/I Kappab and Jak2/Stat3 Transcription Factors in Adjuvant Induced Arthritis. Eur J Pharm Sci (2014) 56:70–86. doi: 10.1016/j.ejps.2014.02.005
22. Hairul-Islam MI, Saravanan S, Thirugnanasambantham K, Chellappandian M, Simon Durai Raj C, Karikalan K, et al. Swertiamarin, a Natural Steroid, Prevent Bone Erosion by Modulating Rankl/Rank/Opg Signaling. Int Immunopharmacol (2017) 53:114–24. doi: 10.1016/j.intimp.2017.10.022
23. He YQ, Yang H, Shen Y, Zhang JH, Zhang ZG, Liu LL, et al. Monotropein Attenuates Ovariectomy and Lps-Induced Bone Loss in Mice and Decreases Inflammatory Impairment on Osteoblast Through Blocking Activation of Nf-Kappab Pathway. Chem Biol Interact (2018) 291:128–36. doi: 10.1016/j.cbi.2018.06.015
24. Shi Y, Liu XY, Jiang YP, Zhang JB, Zhang QY, Wang NN, et al. Monotropein Attenuates Oxidative Stress Via Akt/Mtor-Mediated Autophagy in Osteoblast Cells. BioMed Pharmacother (2020) 121:109566. doi: 10.1016/j.biopha.2019.109566
25. Zhang Z, Zhang Q, Yang H, Liu W, Zhang N, Qin L, et al. Monotropein Isolated From the Roots of Morinda Officinalis Increases Osteoblastic Bone Formation and Prevents Bone Loss in Ovariectomized Mice. Fitoterapia (2016) 110:166–72. doi: 10.1016/j.fitote.2016.03.013
26. Meng J, Zhang W, Wang C, Zhang W, Zhou C, Jiang G, et al. Catalpol Suppresses Osteoclastogenesis and Attenuates Osteoclast-Derived Bone Resorption by Modulating Pten Activity. Biochem Pharmacol (2020) 171:113715. doi: 10.1016/j.bcp.2019.113715
27. Zhu Y, Wang Y, Jia Y, Xu J, Chai Y. Catalpol Promotes the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells Via the Wnt/Beta-Catenin Pathway. Stem Cell Res Ther (2019) 10:37. doi: 10.1186/s13287-019-1143-y
28. Chen L, Zhang RY, Xie J, Yang JY, Fang KH, Hong CX, et al. Stat3 Activation by Catalpol Promotes Osteogenesis-Angiogenesis Coupling, Thus Accelerating Osteoporotic Bone Repair. Stem Cell Res Ther (2021) 12:108. doi: 10.1186/s13287-021-02178-z
29. Zhao L, Du W, Zhao D, Ji X, Huang Y, Pang Y, et al. Catalpol Protects Against High Glucose-Induced Bone Loss by Regulating Osteoblast Function. Front Pharmacol (2021) 12:626621. doi: 10.3389/fphar.2021.626621
30. Choi EM, Suh KS, Rhee SY, Kim YS. Inhibitory Effect of Paeoniflorin on Methylglyoxal-Mediated Oxidative Stress in Osteoblastic Mc3t3-E1 Cells. Phytomedicine (2014) 21:1170–7. doi: 10.1016/j.phymed.2014.05.008
31. Xu H, Cai L, Zhang L, Wang G, Xie R, Jiang Y, et al. Paeoniflorin Ameliorates Collagen-Induced Arthritis Via Suppressing Nuclear Factor-Kb Signalling Pathway in Osteoclast Differentiation. Immunology (2018) 154:593–603. doi: 10.1111/imm.12907
32. Wang Y, Dai J, Zhu Y, Zhong W, Lu S, Chen H, et al. Paeoniflorin Regulates Osteoclastogenesis and Osteoblastogenesis Via Manipulating Nf-Kb Signaling Pathway Both In Vitro and In Vivo. Oncotarget (2018) 9:7372. doi: 10.18632/oncotarget.23677
33. Choi EM, Lee YS. Paeoniflorin Isolated From Paeonia Lactiflora Attenuates Osteoblast Cytotoxicity Induced by Antimycin A. Food Funct (2013) 4:1332–8. doi: 10.1039/c3fo60147a
34. Suh KS, Choi EM, Lee YS, Kim YS. Protective Effect of Albiflorin Against Oxidative-Stress-Mediated Toxicity in Osteoblast-Like Mc3t3-E1 Cells. Fitoterapia (2013) 89:33–41. doi: 10.1016/j.fitote.2013.05.016
35. Kim JH, Kim M, Hong S, Kim EY, Lee H, Jung HS, et al. Albiflorin Promotes Osteoblast Differentiation and Healing of Rat Femoral Fractures Through Enhancing Bmp-2/Smad and Wnt/Beta-Catenin Signaling. Front Pharmacol (2021) 12:690113. doi: 10.3389/fphar.2021.690113
36. Weng ZB, Gao QQ, Wang F, Zhao GH, Yin FZ, Cai BC, et al. Positive Skeletal Effect of Two Ingredients of Psoralea Corylifolia L. On Estrogen Deficiency-Induced Osteoporosis and the Possible Mechanisms of Action. Mol Cell Endocrinol (2015) 417:103–13. doi: 10.1016/j.mce.2015.09.025
37. Lim SH, Ha TY, Kim SR, Ahn J, Park HJ, Kim S. Ethanol Extract of Psoralea Corylifolia L. And Its Main Constituent, Bakuchiol, Reduce Bone Loss in Ovariectomised Sprague-Dawley Rats. Br J Nutr (2009) 101:1031–9. doi: 10.1017/S0007114508066750
38. Chai L, Zhou K, Wang S, Zhang H, Fan N, Li J, et al. Psoralen and Bakuchiol Ameliorate M-Csf Plus Rankl-Induced Osteoclast Differentiation and Bone Resorption Via Inhibition of Akt and Ap-1 Pathways in Vitro. Cell Physiol Biochem (2018) 48:2123–33. doi: 10.1159/000492554
39. Lee YS, Choi EM. Costunolide Stimulates the Function of Osteoblastic Mc3t3-E1 Cells. Int Immunopharmacol (2011) 11:712–8. doi: 10.1016/j.intimp.2011.01.018
40. Cheon YH, Song MJ, Kim JY, Kwak SC, Park JH, Lee CH, et al. Costunolide Inhibits Osteoclast Differentiation by Suppressing C-Fos Transcriptional Activity. Phytother Res (2014) 28:586–92. doi: 10.1002/ptr.5034
41. Li Z, Yuan G, Lin X, Liu Q, Xu J, Lian Z, et al. Dehydrocostus Lactone (Dhc) Suppresses Estrogen Deficiency-Induced Osteoporosis. Biochem Pharmacol (2019) 163:279–89. doi: 10.1016/j.bcp.2019.02.002
42. Hu B, Wu F, Shi Z, He B, Zhao X, Wu H, et al. Dehydrocostus Lactone Attenuates Osteoclastogenesis and Osteoclast-Induced Bone Loss by Modulating Nf-Kappab Signalling Pathway. J Cell Mol Med (2019) 23:5762–70. doi: 10.1111/jcmm.14492
43. Lee HI, Lee GR, Lee J, Kim N, Kwon M, Kim HJ, et al. Dehydrocostus Lactone Inhibits Nfatc1 Via Regulation of Ikk, Jnk, and Nrf2, Thereby Attenuating Osteoclastogenesis. BMB Rep (2020) 53:218–22. doi: 10.5483/BMBRep.2020.53.4.220
44. Lee HI, Lee J, Hwang D, Lee GR, Kim N, Kwon M, et al. Dehydrocostus Lactone Suppresses Osteoclast Differentiation by Regulating Nfatc1 and Inhibits Osteoclast Activation Through Modulating Migration and Lysosome Function. FASEB J (2019) 33:9685–94. doi: 10.1096/fj.201900862R
45. Hong SE, Lee J, Seo DH, In Lee H, Ri Park D, Lee GR, et al. Euphorbia Factor L1 Inhibits Osteoclastogenesis by Regulating Cellular Redox Status and Induces Fas-Mediated Apoptosis in Osteoclast. Free Radic Biol Med (2017) 112:191–9. doi: 10.1016/j.freeradbiomed.2017.07.030
46. Thummuri D, Guntuku L, Challa VS, Ramavat RN, Naidu VGM. Abietic Acid Attenuates Rankl Induced Osteoclastogenesis and Inflammation Associated Osteolysis by Inhibiting the Nf-Kb and Mapk Signaling. J Cell Physiol (2018) 234:443–53. doi: 10.1002/jcp.26575
47. Jiang T, Zhou B, Huang L, Wu H, Huang J, Liang T, et al. Andrographolide Exerts Pro-Osteogenic Effect by Activation of Wnt/Beta-Catenin Signaling Pathway in Vitro. Cell Physiol Biochem (2015) 36:2327–39. doi: 10.1159/000430196
48. Wang T, Liu Q, Zhou L, Yuan JB, Lin X, Zeng R, et al. Andrographolide Inhibits Ovariectomy-Induced Bone Loss Via the Suppression of Rankl Signaling Pathways. Int J Mol Sci (2015) 16:27470–81. doi: 10.3390/ijms161126039
49. Tantikanlayaporn D, Wichit P, Suksen K, Suksamrarn A, Piyachaturawat P. Andrographolide Modulates Opg/Rankl Axis to Promote Osteoblastic Differentiation in Mc3t3-E1 Cells and Protects Bone Loss During Estrogen Deficiency in Rats. BioMed Pharmacother (2020) 131:110763. doi: 10.1016/j.biopha.2020.110763
50. Yongyun C, Jingwei Z, Zhiqing L, Wenxiang C, Huiwu L. Andrographolide Stimulates Osteoblastogenesis and Bone Formation by Inhibiting Nuclear Factor Kappa-Beta Signaling Both In Vivo and In Vitro. J Orthop Translat (2019) 19:47–57. doi: 10.1016/j.jot.2019.02.001
51. Zhai ZJ, Li HW, Liu GW, Qu XH, Tian B, Yan W, et al. Andrographolide Suppresses Rankl-Induced Osteoclastogenesis In Vitro and Prevents Inflammatory Bone Loss In Vivo. Br J Pharmacol (2014) 171:663–75. doi: 10.1111/bph.12463
52. Thummuri D, Naidu VGM, Chaudhari P. Carnosic Acid Attenuates Rankl-Induced Oxidative Stress and Osteoclastogenesis Via Induction of Nrf2 and Suppression of Nf-Kappab and Mapk Signalling. J Mol Med (Berl) (2017) 95:1065–76. doi: 10.1007/s00109-017-1553-1
53. Zheng ZG, Cheng HM, Zhou YP, Zhu ST, Thu PM, Li HJ, et al. Dual Targeting of Srebp2 and Erralpha by Carnosic Acid Suppresses Rankl-Mediated Osteoclastogenesis and Prevents Ovariectomy-Induced Bone Loss. Cell Death Differ (2020) 27:2048–65. doi: 10.1038/s41418-019-0484-5
54. Suh KS, Chon S, Jung WW, Choi EM. Crocin Attenuates Methylglyoxal-Induced Osteoclast Dysfunction by Regulating Glyoxalase, Oxidative Stress, and Mitochondrial Function. Food Chem Toxicol (2019) 124:367–73. doi: 10.1016/j.fct.2018.12.031
55. Fu L, Pan F, Jiao Y. Crocin Inhibits Rankl-Induced Osteoclast Formation and Bone Resorption by Suppressing Nf-Kappab Signaling Pathway Activation. Immunobiology (2017) 222:597–603. doi: 10.1016/j.imbio.2016.11.009
56. Zou B, Zheng J, Deng W, Tan Y, Jie L, Qu Y, et al. Kirenol Inhibits Rankl-Induced Osteoclastogenesis and Prevents Ovariectomized-Induced Osteoporosis Via Suppressing the Ca(2+)-Nfatc1 and Cav-1 Signaling Pathways. Phytomedicine (2021) 80:153377. doi: 10.1016/j.phymed.2020.153377
57. Kim MB, Song Y, Hwang JK. Kirenol Stimulates Osteoblast Differentiation Through Activation of the Bmp and Wnt/Beta-Catenin Signaling Pathways in Mc3t3-E1 Cells. Fitoterapia (2014) 98:59–65. doi: 10.1016/j.fitote.2014.07.013
58. Zhang J, Cai Z, Yang M, Tong L, Zhang Y. Inhibition of Tanshinone Iia on Renin Activity Protected Against Osteoporosis in Diabetic Mice. Pharm Biol (2020) 58:219–24. doi: 10.1080/13880209.2020.1738502
59. Cheng L, Zhou S, Zhao Y, Sun Y, Xu Z, Yuan B, et al. Tanshinone Iia Attenuates Osteoclastogenesis in Ovariectomized Mice by Inactivating Nf-Kb and Akt Signaling Pathways. Am J Transl Res (2018) 10:1457–68.
60. Kwak HB, Yang D, Ha H, Lee JH, Kim HN, Woo ER, et al. Tanshinone Iia Inhibits Osteoclast Differentiation Through Down-Regulation of C-Fos and Nfatc1. Exp Mol Med (2006) 38:256–64. doi: 10.1038/emm.2006.31
61. Nicolin V, Dal Piaz F, Nori SL, Narducci P, De Tommasi N. Inhibition of Bone Resorption by Tanshinone Vi Isolated From Salvia Miltiorrhiza Bunge. Eur J Histochem (2010) 54:e21. doi: 10.4081/ejh.2010.e21
62. Luo D, Ren H, Zhang H, Zhang P, Huang Z, Xian H, et al. The Protective Effects of Triptolide on Age-Related Bone Loss in Old Male Rats. BioMed Pharmacother (2018) 98:280–5. doi: 10.1016/j.biopha.2017.12.072
63. Park B. Triptolide, a Diterpene, Inhibits Osteoclastogenesis, Induced by Rankl Signaling and Human Cancer Cells. Biochimie (2014) 105:129–36. doi: 10.1016/j.biochi.2014.07.003
64. Cui J, Li X, Wang S, Su Y, Chen X, Cao L, et al. Triptolide Prevents Bone Loss Via Suppressing Osteoclastogenesis Through Inhibiting Pi3k-Akt-Nfatc1 Pathway. J Cell Mol Med (2020) 24:6149–61. doi: 10.1111/jcmm.15229
65. Xie Z, Yu H, Sun X, Tang P, Jie Z, Chen S, et al. A Novel Diterpenoid Suppresses Osteoclastogenesis and Promotes Osteogenesis by Inhibiting Ifrd1-Mediated and Ikappabalpha-Mediated P65 Nuclear Translocation. J Bone Miner Res (2018) 33:667–78. doi: 10.1002/jbmr.3334
66. Zhou L, Huang Y, Zhao J, Yang H, Kuai F. Oridonin Promotes Osteogenesis Through Wnt/Beta-Catenin Pathway and Inhibits Rankl-Induced Osteoclastogenesis in Vitro. Life Sci (2020) 262:118563. doi: 10.1016/j.lfs.2020.118563
67. Im NK, Lee DS, Lee SR, Jeong GS. Lupeol Isolated From Sorbus Commixta Suppresses 1alpha,25-(Oh)2d3-Mediated Osteoclast Differentiation and Bone Loss In Vitro and In Vivo. J Nat Prod (2016) 79:412–20. doi: 10.1021/acs.jnatprod.5b01088
68. Shao C, Fu B, Ji N, Pan S, Zhao X, Zhang Z, et al. Alisol B 23-Acetate Inhibits Ige/Ag-Mediated Mast Cell Activation and Allergic Reaction. Int J Mol Sci (2018) 19:4092. doi: 10.3390/ijms19124092
69. Lee JW, Kobayashi Y, Nakamichi Y, Udagawa N, Takahashi N, Im NK, et al. Alisol-B, A Novel Phyto-Steroid, Suppresses the Rankl-Induced Osteoclast Formation and Prevents Bone Loss in Mice. Biochem Pharmacol (2010) 80:352–61. doi: 10.1016/j.bcp.2010.04.014
70. Jia X, Zhu H, Li G, Lan M, Li X, Huang M, et al. Anti-Osteoporotic Effects of Alisol C 23-Acetate Via Osteoclastogenesis Inhibition. BioMed Pharmacother (2021) 137:111321. doi: 10.1016/j.biopha.2021.111321
71. Kim KJ, Leutou AS, Yeon JT, Choi SW, Kim SH, Yee ST, et al. The Inhibitory Effect of Alisol a 24-Acetate From Alisma Canaliculatum on Osteoclastogenesis. Int J Endocrinol (2015) 2015:132436. doi: 10.1155/2015/132436
72. Zhao D, Li X, Zhao Y, Qiao P, Tang D, Chen Y, et al. Oleanolic Acid Exerts Bone Protective Effects in Ovariectomized Mice by Inhibiting Osteoclastogenesis. J Pharmacol Sci (2018) 137:76–85. doi: 10.1016/j.jphs.2018.03.007
73. Cao S, Dong XL, Ho MX, Yu WX, Wong KC, Yao XS, et al. Oleanolic Acid Exerts Osteoprotective Effects and Modulates Vitamin D Metabolism. Nutrients (2018) 10:247. doi: 10.3390/nu10020247
74. Lee SU, Park SJ, Kwak HB, Oh J, Min YK, Kim SH. Anabolic Activity of Ursolic Acid in Bone: Stimulating Osteoblast Differentiation in Vitro and Inducing New Bone Formation in Vivo. Pharmacol Res (2008) 58:290–6. doi: 10.1016/j.phrs.2008.08.008
75. Ramli ES, Suhaimi F, Asri SF, Ahmad F, Soelaiman IN. Glycyrrhizic Acid (Gca) as 11beta-Hydroxysteroid Dehydrogenase Inhibitor Exerts Protective Effect Against Glucocorticoid-Induced Osteoporosis. J Bone Miner Metab (2013) 31:262–73. doi: 10.1007/s00774-012-0413-x
76. Kao TC, Shyu MH, Yen GC. Glycyrrhizic Acid and 18beta-Glycyrrhetinic Acid Inhibit Inflammation Via Pi3k/Akt/Gsk3beta Signaling and Glucocorticoid Receptor Activation. J Agric Food Chem (2010) 58:8623–9. doi: 10.1021/jf101841r
77. Yamada C, Ho A, Akkaoui J, Garcia C, Duarte C, Movila A. Glycyrrhizin Mitigates Inflammatory Bone Loss and Promotes Expression of Senescence-Protective Sirtuins in an Aging Mouse Model of Periprosthetic Osteolysis. BioMed Pharmacother (2021) 138:111503. doi: 10.1016/j.biopha.2021.111503
78. Chen X, Zhi X, Yin Z, Li X, Qin L, Qiu Z, et al. 18beta-Glycyrrhetinic Acid Inhibits Osteoclastogenesis in Vivo and in Vitro by Blocking Rankl-Mediated Rank-Traf6 Interactions and Nf-Kappab and Mapk Signaling Pathways. Front Pharmacol (2018) 9:647. doi: 10.3389/fphar.2018.00647
79. Wang P, Wei X, Zhang F, Yang K, Qu C, Luo H, et al. Ginsenoside Rg1 of Panax Ginseng Stimulates the Proliferation, Odontogenic/Osteogenic Differentiation and Gene Expression Profiles of Human Dental Pulp Stem Cells. Phytomedicine (2014) 21:177–83. doi: 10.1016/j.phymed.2013.08.021
80. Wei J, Li Y, Liu Q, Lan Y, Wei C, Tian K, et al. Betulinic Acid Protects From Bone Loss in Ovariectomized Mice and Suppresses Rankl-Associated Osteoclastogenesis by Inhibiting the Mapk and Nfatc1 Pathways. Front Pharmacol (2020) 11:1025. doi: 10.3389/fphar.2020.01025
81. Lo YC, Chang YH, Wei BL, Huang YL, Chiou WF. Betulinic Acid Stimulates the Differentiation and Mineralization of Osteoblastic Mc3t3-E1 Cells: Involvement of Bmp/Runx2 and Beta-Catenin Signals. J Agric Food Chem (2010) 58:6643–9. doi: 10.1021/jf904158k
82. Li H, Miyahara T, Tezuka Y, Namba T, Nemoto N, Tonami S, et al. The Effect of Kampo Formulae on Bone Resorption in Vitro and in Vivo. I. Active Constituents of Tsu-Kan-Gan. Biol Pharm Bull (1998) 21:1322–6. doi: 10.1248/bpb.21.1322
83. Dahye L, Eunjoo J, Jiyun A, Jintaek H, Jinyoung H, Taeyoul H, et al. Limonin Enhances Osteoblastogenesis and Prevents Ovariectomy-Induced Bone Loss. J Funct Foods (2016) 23:105–14. doi: 10.1016/j.jff.2016.02.008
84. Kimira Y, Taniuchi Y, Nakatani S, Sekiguchi Y, Kim HJ, Shimizu J, et al. Citrus Limonoid Nomilin Inhibits Osteoclastogenesis in Vitro by Suppression of Nfatc1 and Mapk Signaling Pathways. Phytomedicine (2015) 22:1120–4. doi: 10.1016/j.phymed.2015.08.013
85. Zhang Z, Song C, Fu X, Liu M, Li Y, Pan J, et al. High-Dose Diosgenin Reduces Bone Loss in Ovariectomized Rats Via Attenuation of the Rankl/Opg Ratio. Int J Mol Sci (2014) 15:17130–47. doi: 10.3390/ijms150917130
86. Qu X, Zhai Z, Liu X, Li H, Ouyang Z, Wu C, et al. Dioscin Inhibits Osteoclast Differentiation and Bone Resorption Though Down-Regulating the Akt Signaling Cascades. Biochem Biophys Res Commun (2014) 443:658–65. doi: 10.1016/j.bbrc.2013.12.029
87. Zhang C, Peng J, Wu S, Jin Y, Xia F, Wang C, et al. Dioscin Promotes Osteoblastic Proliferation and Differentiation Via Lrp5 and Er Pathway in Mouse and Human Osteoblast-Like Cell Lines. J BioMed Sci (2014) 21:30. doi: 10.1186/1423-0127-21-30
88. Huang Q, Gao B, Wang L, Zhang HY, Li XJ, Shi J, et al. Ophiopogonin D: A New Herbal Agent Against Osteoporosis. Bone (2015) 74:18–28. doi: 10.1016/j.bone.2015.01.002
89. Yang M, Guo Q, Peng H, Xiao YZ, Xiao Y, Huang Y, et al. Kruppel-Like Factor 3 Inhibition by Mutated Lncrna Reg1cp Results in Human High Bone Mass Syndrome. J Exp Med (2019) 216:1944–64. doi: 10.1084/jem.20181554
90. Wu J, Zeng Z, Li Y, Qin H, Zuo C, Zhou C, et al. Cycloastragenol Protects Against Glucocorticoid-Induced Osteogenic Differentiation Inhibition by Activating Telomerase. Phytother Res (2021) 35:2034–44. doi: 10.1002/ptr.6946
91. Tian K, Su Y, Ding J, Wang D, Zhan Y, Li Y, et al. Hederagenin Protects Mice Against Ovariectomy-Induced Bone Loss by Inhibiting Rankl-Induced Osteoclastogenesis and Bone Resorption. Life Sci (2020) 244:117336. doi: 10.1016/j.lfs.2020.117336
92. Yang M, Xie J, Lei X, Song Z, Gong Y, Liu H, et al. Tubeimoside I Suppresses Diabetes-Induced Bone Loss in Rats, Osteoclast Formation, and Rankl-Induced Nuclear Factor-Kappab Pathway. Int Immunopharmacol (2020) 80:106202. doi: 10.1016/j.intimp.2020.106202
93. Wang SF, Chen XG, Hu ZD, Ju Y. Analysis of Three Effective Components in Fructus Corni and Its Preparations by Micellar Electrokinetic Capillary Chromatography. BioMed Chromatogr (2003) 17:306–11. doi: 10.1002/bmc.247
94. Magora HB, Rahman M, Gray AI, Cole MD. Swertiamarin From Enicostemma Axillare Subsp. Axillare (Gentianaceae). Biochem Systematics Ecol (2003) 31:553–5. doi: 10.1016/S0305-1978(02)00200-4
95. Heffels P, Muller L, Schieber A, Weber F. Profiling of Iridoid Glycosides in Vaccinium Species by Uhplc-Ms. Food Res Int (2017) 100:462–8. doi: 10.1016/j.foodres.2016.11.018
96. Seo BI, Ku SK, Cha EM, Park JH, Kim JD, Choi HY, et al. Effect of Mornidae Radix Extracts on Experimental Osteoporosis in Sciatic Neurectomized Mice. Phytother Res (2005) 19:231–8. doi: 10.1002/ptr.1683
97. Li N, Qin LP, Han T, Wu YB, Zhang QY, Zhang H. Inhibitory Effects of Morinda Officinalis Extract on Bone Loss in Ovariectomized Rats. Molecules (2009) 14:2049–61. doi: 10.3390/molecules14062049
98. Yen PH, Van Kiem P, Nhiem NX, Tung NH, Quang TH, Van Minh C, et al. A New Monoterpene Glycoside From the Roots of Paeonia Lacti-Flora Increases the Differentiation of Osteoblastic Mc3t3-E1 Cells. Arch Pharm Res (2007) 30:1179–85. doi: 10.1007/BF02980258
99. Wang Y, Zhu Y, Lu S, Hu C, Zhong W, Chai Y. Beneficial Effects of Paeoniflorin on Osteoporosis Induced by High-Carbohydrate, High-Fat Diet-Associated Hyperlipidemia in Vivo. Biochem Biophys Res Commun (2018) 498:981–7. doi: 10.1016/j.bbrc.2018.03.093
100. Xin Z, Wu X, Ji T, Xu B, Han Y, Sun M, et al. Bakuchiol: A Newly Discovered Warrior Against Organ Damage. Pharmacol Res (2019) 141:208–13. doi: 10.1016/j.phrs.2019.01.001
101. Zhang X, Zhao W, Wang Y, Lu J, Chen X. The Chemical Constituents and Bioactivities of Psoralea Corylifolia Linn.: A Review. Am J Chin Med (2016) 44:35–60. doi: 10.1142/S0192415X16500038
102. Zhang CZ, Wang SX, Zhang Y, Chen JP, Liang XM. In Vitro Estrogenic Activities of Chinese Medicinal Plants Traditionally Used for the Management of Menopausal Symptoms. J Ethnopharmacol (2005) 98:295–300. doi: 10.1016/j.jep.2005.01.033
103. Li WD, Yan CP, Wu Y, Weng ZB, Yin FZ, Yang GM, et al. Osteoblasts Proliferation and Differentiation Stimulating Activities of the Main Components of Fructus Psoraleae Corylifoliae. Phytomedicine (2014) 21:400–5. doi: 10.1016/j.phymed.2013.09.015
104. Jeong SJ, Itokawa T, Shibuya M, Kuwano M, Ono M, Higuchi R, et al. Costunolide, a Sesquiterpene Lactone From Saussurea Lappa, Inhibits the Vegfr Kdr/Flk-1 Signaling Pathway. Cancer Lett (2002) 187:129–33. doi: 10.1016/s0304-3835(02)00361-0
105. Lee HK, Song HE, Lee HB, Kim CS, Koketsu M, Ngan LT, et al. Growth Inhibitory, Bactericidal, and Morphostructural Effects of Dehydrocostus Lactone From Magnolia Sieboldii Leaves on Antibiotic-Susceptible and -Resistant Strains of Helicobacter Pylori. PloS One (2014) 9:e95530. doi: 10.1371/journal.pone.0095530
106. Bicchi C, Appendino G, Cordero C, Rubiolo P, Ortelli D, Veuthey JL. Hplc-Uv and Hplc-Positive-Esi-Ms Analysis of the Diterpenoid Fraction From Caper Spurge (Euphorbia Lathyris) Seed Oil. Phytochem Anal (2001) 12:255–62. doi: 10.1002/pca.592
107. Gao Y, Zhaoyu L, Xiangming F, Chunyi L, Jiayu P, Lu S, et al. Abietic Acid Attenuates Allergic Airway Inflammation in a Mouse Allergic Asthma Model. Int Immunopharmacol (2016) 38:261–6. doi: 10.1016/j.intimp.2016.05.029
108. Zhao J, Yang G, Liu H, Wang D, Song X, Chen Y. Determination of Andrographolide, Deoxyandrographolide and Neoandrographolide in the Chinese Herb Andrographis Paniculata by Micellar Electrokinetic Capillary Chromatography. Phytochem Anal (2002) 13:222–7. doi: 10.1002/pca.644
109. Abu-Ghefreh AA, Canatan H, Ezeamuzie CI. In Vitro and in Vivo Anti-Inflammatory Effects of Andrographolide. Int Immunopharmacol (2009) 9:313–8. doi: 10.1016/j.intimp.2008.12.002
110. Liu J, Jiang T, He M, Fang D, Shen C, Le Y, et al. Andrographolide Prevents Human Nucleus Pulposus Cells Against Degeneration by Inhibiting the Nf-Kappab Pathway. J Cell Physiol (2019) 234:9631–9. doi: 10.1002/jcp.27650
111. Reabroi S, Chairoungdua A, Saeeng R, Kasemsuk T, Saengsawang W, Zhu W, et al. A Silyl Andrographolide Analogue Suppresses Wnt/Beta-Catenin Signaling Pathway in Colon Cancer. BioMed Pharmacother (2018) 101:414–21. doi: 10.1016/j.biopha.2018.02.119
112. Zhai Z, Qu X, Yan W, Li H, Liu G, Liu X, et al. Andrographolide Prevents Human Breast Cancer-Induced Osteoclastic Bone Loss Via Attenuated Rankl Signaling. Breast Cancer Res Treat (2014) 144:33–45. doi: 10.1007/s10549-014-2844-7
113. Al Batran R, Al-Bayaty FH, Al-Obaidi MM. In-Vivo Effect of Andrographolide on Alveolar Bone Resorption Induced by Porphyromonas Gingivalis and Its Relation With Antioxidant Enzymes. BioMed Res Int (2013) 2013:276329. doi: 10.1155/2013/276329
114. Birtic S, Dussort P, Pierre FX, Bily AC, Roller M. Carnosic Acid. Phytochemistry (2015) 115:9–19. doi: 10.1016/j.phytochem.2014.12.026
115. Elbahnasawy AS, Valeeva ER, El-Sayed EM, Rakhimov II. The Impact of Thyme and Rosemary on Prevention of Osteoporosis in Rats. J Nutr Metab (2019) 2019:1431384. doi: 10.1155/2019/1431384
116. Bukhari SI, Manzoor M, Dhar MK. A Comprehensive Review of the Pharmacological Potential of Crocus Sativus and Its Bioactive Apocarotenoids. BioMed Pharmacother (2018) 98:733–45. doi: 10.1016/j.biopha.2017.12.090
117. Hosseinzadeh H, Nassiri-Asl M. Avicenna’s (Ibn Sina) the Canon of Medicine and Saffron (Crocus Sativus): A Review. Phytother Res (2013) 27:475–83. doi: 10.1002/ptr.4784
118. Algandaby MM. Crocin Attenuates Metabolic Syndrome-Induced Osteoporosis in Rats. J Food Biochem (2019) 43:e12895. doi: 10.1111/jfbc.12895
119. Kalalinia F, Ghasim H, Amel Farzad S, Pishavar E, Ramezani M, Hashemi M. Comparison of the Effect of Crocin and Crocetin, Two Major Compounds Extracted From Saffron, on Osteogenic Differentiation of Mesenchymal Stem Cells. Life Sci (2018) 208:262–7. doi: 10.1016/j.lfs.2018.07.043
120. Wu B, Huang XY, Li L, Fan XH, Li PC, Huang CQ, et al. Attenuation of Diabetic Cardiomyopathy by Relying on Kirenol to Suppress Inflammation in a Diabetic Rat Model. J Cell Mol Med (2019) 23:7651–63. doi: 10.1111/jcmm.14638
121. Lu Y, Xiao J, Wu ZW, Wang ZM, Hu J, Fu HZ, et al. Kirenol Exerts a Potent Anti-Arthritic Effect in Collagen-Induced Arthritis by Modifying the T Cells Balance. Phytomedicine (2012) 19:882–9. doi: 10.1016/j.phymed.2012.04.010
122. Chin A, Yang Y, Chai L, Wong RW, Rabie AB. Effects of Medicinal Herb Salvia Miltiorrhiza on Osteoblastic Cells in Vitro. J Orthop Res (2011) 29:1059–63. doi: 10.1002/jor.21376
123. Guo Y, Li Y, Xue L, Severino RP, Gao S, Niu J, et al. Salvia Miltiorrhiza: An Ancient Chinese Herbal Medicine as a Source for Anti-Osteoporotic Drugs. J Ethnopharmacol (2014) 155:1401–16. doi: 10.1016/j.jep.2014.07.058
124. Lee SR, Jeon H, Kwon JE, Suh H, Kim BH, Yun MK, et al. Anti-Osteoporotic Effects of Salvia Miltiorrhiza Bunge Etoh Extract Both in Ovariectomized and Naturally Menopausal Mouse Models. J Ethnopharmacol (2020) 258:112874. doi: 10.1016/j.jep.2020.112874
125. Zhu S, Wei W, Liu Z, Yang Y, Jia H. Tanshinoneiia Attenuates the Deleterious Effects of Oxidative Stress in Osteoporosis Through the Nfkappab Signaling Pathway. Mol Med Rep (2018) 17:6969–76. doi: 10.3892/mmr.2018.8741
126. Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an Extract of Tripterygium Wilfordii Hook F in Patients With Rheumatoid Arthritis: A Double-Blind, Placebo-Controlled Study. Arthritis Rheum (2002) 46:1735–43. doi: 10.1002/art.10411
127. Wang Y, Wang B, Yang X. The Study of Cellular Mechanism of Triptolide in the Treatment of Cancer, Bone Loss and Cardiovascular Disease and Triptolide’s Toxicity. Curr Stem Cell Res Ther (2020) 15:18–23. doi: 10.2174/1574888X14666190301155810
128. Li D, Han T, Liao J, Hu X, Xu S, Tian K, et al. Oridonin, a Promising Ent-Kaurane Diterpenoid Lead Compound. Int J Mol Sci (2016) 17:1395. doi: 10.3390/ijms17091395
129. Hsieh TC, Wijeratne EK, Liang JY, Gunatilaka AL, Wu JM. Differential Control of Growth, Cell Cycle Progression, and Expression of Nf-Kappab in Human Breast Cancer Cells Mcf-7, Mcf-10a, and Mda-Mb-231 by Ponicidin and Oridonin, Diterpenoids From the Chinese Herb Rabdosia Rubescens. Biochem Biophys Res Commun (2005) 337:224–31. doi: 10.1016/j.bbrc.2005.09.040
130. Ku CM, Lin JY. Anti-Inflammatory Effects of 27 Selected Terpenoid Compounds Tested Through Modulating Th1/Th2 Cytokine Secretion Profiles Using Murine Primary Splenocytes. Food Chem (2013) 141:1104–13. doi: 10.1016/j.foodchem.2013.04.044
131. Vu TO, Tran PT, Seo W, Lee JH, Min BS, Kim JA. Triterpenoids From Celastrus Orbiculatus Thunb. Inhibit Rankl-Induced Osteoclast Formation and Bone Resorption Via C-Fos Signaling. J Nat Med (2021) 75:56–65. doi: 10.1007/s11418-020-01444-3
132. Chauhan S, Sharma A, Upadhyay NK, Singh G, Lal UR, Goyal R. In-Vitro Osteoblast Proliferation and in-Vivo Anti-Osteoporotic Activity of Bombax Ceiba With Quantification of Lupeol, Gallic Acid and Beta-Sitosterol by Hptlc and Hplc. BMC Complement Altern Med (2018) 18:233. doi: 10.1186/s12906-018-2299-1
133. Xiu RJ. Microcirculation and Traditional Chinese Medicine. JAMA (1988) 260:1755–7. doi: 10.1001/jama.260.12.1755
134. Zhang LL, Xu W, Xu YL, Chen X, Huang M, Lu JJ. Therapeutic Potential of Rhizoma Alismatis: A Review on Ethnomedicinal Application, Phytochemistry, Pharmacology, and Toxicology. Ann N Y Acad Sci (2017) 1401:90–101. doi: 10.1111/nyas.13381
135. Hwang YH, Kang KY, Lee SJ, Nam SJ, Son YJ, Yee ST. The Protective Effects of Alisol a 24-Acetate From Alisma Canaliculatum on Ovariectomy Induced Bone Loss in Vivo. Molecules (2016) 21:74. doi: 10.3390/molecules21010074
136. Pang Z, Zhi-yan Z, Wang W, Ma Y, Feng-ju N, Zhang X, et al. The Advances in Research on the Pharmacological Effects of Fructus Ligustri Lucidi. BioMed Res Int (2015) 2015:281873. doi: 10.1155/2015/281873
137. Chen B, Wang L, Li L, Zhu R, Liu H, Liu C, et al. Fructus Ligustri Lucidi in Osteoporosis: A Review of Its Pharmacology, Phytochemistry, Pharmacokinetics and Safety. Molecules (2017) 22:1469. doi: 10.3390/molecules22091469
138. Xie BP, Shi LY, Li JP, Zeng Y, Liu W, Tang SY, et al. Oleanolic Acid Inhibits Rankl-Induced Osteoclastogenesis Via Er Alpha/Mir-503/Rank Signaling Pathway in Raw264.7 Cells. BioMed Pharmacother (2019) 117:109045. doi: 10.1016/j.biopha.2019.109045
139. Xu Y, Chen S, Yu T, Qiao J, Sun G. High-Throughput Metabolomics Investigates Anti-Osteoporosis Activity of Oleanolic Acid Via Regulating Metabolic Networks Using Ultra-Performance Liquid Chromatography Coupled With Mass Spectrometry. Phytomedicine (2018) 51:68–76. doi: 10.1016/j.phymed.2018.09.235
140. Li JX, Hareyama T, Tezuka Y, Zhang Y, Miyahara T, Kadota S. Five New Oleanolic Acid Glycosides From Achyranthes Bidentata With Inhibitory Activity on Osteoclast Formation. Planta Med (2005) 71:673–9. doi: 10.1055/s-2005-871275
141. Orgah JO, He S, Wang Y, Jiang M, Wang Y, Orgah EA, et al. Pharmacological Potential of the Combination of Salvia Miltiorrhiza (Danshen) and Carthamus Tinctorius (Honghua) for Diabetes Mellitus and Its Cardiovascular Complications. Pharmacol Res (2020) 153:104654. doi: 10.1016/j.phrs.2020.104654
142. Steinkamp-Fenske K, Bollinger L, Voller N, Xu H, Yao Y, Bauer R, et al. Ursolic Acid From the Chinese Herb Danshen (Salvia Miltiorrhiza L.) Upregulates Enos and Downregulates Nox4 Expression in Human Endothelial Cells. Atherosclerosis (2007) 195:e104–11. doi: 10.1016/j.atherosclerosis.2007.03.028
143. Cao S, Wastney ME, Lachcik PJ, Xiao HH, Weaver CM, Wong MS. Both Oleanolic Acid and a Mixture of Oleanolic and Ursolic Acids Mimic the Effects of Fructus Ligustri Lucidi on Bone Properties and Circulating 1,25-Dihydroxycholecalciferol in Ovariectomized Rats. J Nutr (2018) 148:1895–902. doi: 10.1093/jn/nxy242
144. Tan H, Furuta S, Nagata T, Ohnuki K, Akasaka T, Shirouchi B, et al. Inhibitory Effects of the Leaves of Loquat (Eriobotrya Japonica) on Bone Mineral Density Loss in Ovariectomized Mice and Osteoclast Differentiation. J Agric Food Chem (2014) 62:836–41. doi: 10.1021/jf402735u
145. Tan H, Ashour A, Katakura Y, Shimizu K. A Structure-Activity Relationship Study on Antiosteoclastogenesis Effect of Triterpenoids from the Leaves of Loquat (Eriobotrya Japonica). Phytomedicine (2015) 22:498–503. doi: 10.1016/j.phymed.2015.03.002
146. Pitaloka DAE, Cooper AM, Artarini AA, Damayanti S, Sukandar EY. Regulation of Mitogen-Activated Protein Kinase Signaling Pathway and Proinflammatory Cytokines by Ursolic Acid in Murine Macrophages Infected With Mycobacterium Avium. Infect Dis Rep (2020) 12:8717. doi: 10.4081/idr.2020.8717
147. Tohme MJ, Gimenez MC, Peralta A, Colombo MI, Delgui LR. Ursolic Acid: A Novel Antiviral Compound Inhibiting Rotavirus Infection In Vitro. Int J Antimicrob Agents (2019) 54:601–9. doi: 10.1016/j.ijantimicag.2019.07.015
148. Tan H, Sonam T, Shimizu K. The Potential of Triterpenoids From Loquat Leaves (Eriobotrya Japonica) for Prevention and Treatment of Skin Disorder. Int J Mol Sci (2017) 18:1030. doi: 10.3390/ijms18051030
149. Cao S, Tian XL, Yu WX, Zhou LP, Dong XL, Favus MJ, et al. Oleanolic Acid and Ursolic Acid Improve Bone Properties and Calcium Balance and Modulate Vitamin D Metabolism in Aged Female Rats. Front Pharmacol (2018) 9:1435. doi: 10.3389/fphar.2018.01435
150. Tan H, Zhao C, Zhu Q, Katakura Y, Tanaka H, Ohnuki K, et al. Ursolic Acid Isolated From the Leaves of Loquat (Eriobotrya Japonica) Inhibited Osteoclast Differentiation Through Targeting Exportin 5. J Agric Food Chem (2019) 67:3333–40. doi: 10.1021/acs.jafc.8b06954
151. Kiratipaiboon C, Tengamnuay P, Chanvorachote P. Glycyrrhizic Acid Attenuates Stem Cell-Like Phenotypes of Human Dermal Papilla Cells. Phytomedicine (2015) 22:1269–78. doi: 10.1016/j.phymed.2015.11.002
152. Zhang L, Li X, Ying T, Wang T, Fu F. The Use of Herbal Medicines for the Prevention of Glucocorticoid-Induced Osteoporosis. Front Endocrinol (Lausanne) (2021) 12:744647. doi: 10.3389/fendo.2021.744647
153. Kao TC, Wu CH, Yen GC. Glycyrrhizic Acid and 18beta-Glycyrrhetinic Acid Recover Glucocorticoid Resistance Via Pi3k-Induced Ap1, Cre and Nfat Activation. Phytomedicine (2013) 20:295–302. doi: 10.1016/j.phymed.2012.10.013
154. Tang Y, Lv XL, Bao YZ, Wang JR. Glycyrrhizin Improves Bone Metabolism in Ovariectomized Mice Via Inactivating Nf-Kappab Signaling. Climacteric (2021) 24:253–60. doi: 10.1080/13697137.2020.1828853
155. Chu S, Liu D, Zhao H, Shao M, Liu X, Qu X, et al. Effects and Mechanism of Zishen Jiangtang Pill on Diabetic Osteoporosis Rats Based on Proteomic Analysis. Evid Based Complement Alternat Med (2021) 2021:7383062. doi: 10.1155/2021/7383062
156. Fan S, Zhang C, Luo T, Wang J, Tang Y, Chen Z, et al. Limonin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Molecules (2019) 24:3679. doi: 10.3390/molecules24203679
157. Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. Antioxidative and Hypolipidemic Effects of Diosgenin, a Steroidal Saponin of Yam (Dioscorea Spp.), on High-Cholesterol Fed Rats. Biosci Biotechnol Biochem (2007) 71:3063–71. doi: 10.1271/bbb.70472
158. Folwarczna J, Zych M, Nowinska B, Pytlik M, Bialik M, Jagusiak A, et al. Effect of Diosgenin, a Steroidal Sapogenin, on the Rat Skeletal System. Acta Biochim Pol (2016) 63:287–95. doi: 10.18388/abp.2015_1095
159. Shishodia S, Aggarwal BB. Diosgenin Inhibits Osteoclastogenesis, Invasion, and Proliferation Through the Downregulation of Akt, I Kappa B Kinase Activation and Nf-Kappa B-Regulated Gene Expression. Oncogene (2006) 25:1463–73. doi: 10.1038/sj.onc.1209194
160. Alcantara EH, Shin MY, Sohn HY, Park YM, Kim T, Lim JH, et al. Diosgenin Stimulates Osteogenic Activity by Increasing Bone Matrix Protein Synthesis and Bone-Specific Transcription Factor Runx2 in Osteoblastic Mc3t3-E1 Cells. J Nutr Biochem (2011) 22:1055–63. doi: 10.1016/j.jnutbio.2010.09.003
161. Zhang Z, Chen Y, Xiang L, Wang Z, Xiao GG, Ju D. Diosgenin Protects Against Alveolar Bone Loss in Ovariectomized Rats Via Regulating Long Non-Coding Rnas. Exp Ther Med (2018) 16:3939–50. doi: 10.3892/etm.2018.6681
162. Yen ML, Su JL, Chien CL, Tseng KW, Yang CY, Chen WF, et al. Diosgenin Induces Hypoxia-Inducible Factor-1 Activation and Angiogenesis Through Estrogen Receptor-Related Phosphatidylinositol 3-Kinase/Akt and P38 Mitogen-Activated Protein Kinase Pathways in Osteoblasts. Mol Pharmacol (2005) 68:1061–73. doi: 10.1124/mol.104.010082
163. Chiang SS, Chang SP, Pan TM. Osteoprotective Effect of Monascus-Fermented Dioscorea in Ovariectomized Rat Model of Postmenopausal Osteoporosis. J Agric Food Chem (2011) 59:9150–7. doi: 10.1021/jf201640j
164. Zhao S, Niu F, Xu CY, Liu Y, Ye L, Bi GB, et al. Diosgenin Prevents Bone Loss on Retinoic Acid-Induced Osteoporosis in Rats. Ir J Med Sci (2016) 185:581–7. doi: 10.1007/s11845-015-1309-2
165. Tao X, Qi Y, Xu L, Yin L, Han X, Xu Y, et al. Dioscin Reduces Ovariectomy-Induced Bone Loss by Enhancing Osteoblastogenesis and Inhibiting Osteoclastogenesis. Pharmacol Res (2016) 108:90–101. doi: 10.1016/j.phrs.2016.05.003
166. Li L, Hou X, Xu R, Liu C, Tu M. Research Review on the Pharmacological Effects of Astragaloside Iv. Fundam Clin Pharmacol (2017) 31:17–36. doi: 10.1111/fcp.12232
167. Yu Y, Wu J, Li J, Liu Y, Zheng X, Du M, et al. Cycloastragenol Prevents Age-Related Bone Loss: Evidence in D-Galactose-Treated and Aged Rats. BioMed Pharmacother (2020) 128:110304. doi: 10.1016/j.biopha.2020.110304
168. Kim BS, Kim YC, Zadeh H, Park YJ, Pi SH, Shin HS, et al. Effects of the Dichloromethane Fraction of Dipsaci Radix on the Osteoblastic Differentiation of Human Alveolar Bone Marrow-Derived Mesenchymal Stem Cells. Biosci Biotechnol Biochem (2011) 75:13–9. doi: 10.1271/bbb.100379
169. Xiang JY, Chi YY, Han JX, Xiang H, Xie Q. The Toxicity and Attenuation Methods of Toxic Chinese Materia Medica for Its Reasonable Application: A Review. Am J Chin Med (2021) 49:41–67. doi: 10.1142/S0192415X21500038
170. Cao Y, Cheng F, Yao W, Bao B, Zhang K, Zhang L, et al. Toxicity of Pekinenin C From Euphorbia Pekinensis Radix on Rat Small Intestinal Crypt Epithelial Cell and Its Apoptotic Mechanism. Int J Mol Sci (2016) 17:850. doi: 10.3390/ijms17060850
171. Wu J, Shen Q, Cui W, Zhao Y, Huai Y, Zhang YC, et al. Dual Roles of Qoa-8a in Antiosteoporosis: A Combination of Bone Anabolic and Anti-Resorptive Effects. Acta Pharmacol Sin (2018) 39:230–42. doi: 10.1038/aps.2017.63
172. Zhao Y, Huai Y, Jin J, Geng M, Li JX. Quinoxaline Derivative of Oleanolic Acid Inhibits Osteoclastic Bone Resorption and Prevents Ovariectomy-Induced Bone Loss. Menopause (2011) 18:690–7. doi: 10.1097/gme.0b013e3181fd7f4b
173. Zhang S, Zhang Y, Fang Y, Chen H, Hao M, Tan Q, et al. Synthesis and Evaluation of Andrographolide Derivatives as Potent Anti-Osteoporosis Agents in Vitro and in Vivo. Eur J Med Chem (2021) 213:113185. doi: 10.1016/j.ejmech.2021.113185
174. Zhao C, Huang D, Li R, Xu Y, Su S, Gu Q, et al. Identifying Novel Anti-Osteoporosis Leads With a Chemotype-Assembly Approach. J Med Chem (2019) 62:5885–900. doi: 10.1021/acs.jmedchem.9b00517
175. Smith AJ, Kavuru P, Wojtas L, Zaworotko MJ, Shytle RD. Cocrystals of Quercetin With Improved Solubility and Oral Bioavailability. Mol Pharm (2011) 8:1867–76. doi: 10.1021/mp200209j
176. Wang R, Han J, Jiang A, Huang R, Fu T, Wang L, et al. Involvement of Metabolism-Permeability in Enhancing the Oral Bioavailability of Curcumin in Excipient-Free Solid Dispersions Co-Formed With Piperine. Int J Pharm (2019) 561:9–18. doi: 10.1016/j.ijpharm.2019.02.027
177. Zuo W, Qu W, Li N, Yu R, Hou Y, Liu Y, et al. Fabrication of Multicomponent Amorphous Bufadienolides Nanosuspension With Wet Milling Improves Dissolution and Stability. Artif Cells Nanomed Biotechnol (2018) 46:1513–22. doi: 10.1080/21691401.2017.1375938
178. Zhuo Y, Zhang Y, Li M, Wu H, Gong S, Hu X, et al. Hepatotoxic Evaluation of Toosendanin Via Biomarker Quantification and Pathway Mapping of Large-Scale Chemical Proteomics. Food Chem Toxicol (2021) 153:112257. doi: 10.1016/j.fct.2021.112257
179. Gao L, Cao M, Li JQ, Qin XM, Fang J. Traditional Chinese Medicine Network Pharmacology in Cardiovascular Precision Medicine. Curr Pharm Des (2021) 27:2925–33. doi: 10.2174/1381612826666201112142408
180. Fang J, Liu C, Wang Q, Lin P, Cheng F. In Silico Polypharmacology of Natural Products. Brief Bioinform (2018) 19:1153–71. doi: 10.1093/bib/bbx045
181. Audran M. Drug Combination Strategies for Osteoporosis. Joint Bone Spine (2006) 73:374–8. doi: 10.1016/j.jbspin.2006.02.004
Keywords: osteoporosis, traditional Chinese medicine, terpenoids, osteoblast, osteoclast
Citation: Zhuo Y, Li M, Jiang Q, Ke H, Liang Q, Zeng L-F and Fang J (2022) Evolving Roles of Natural Terpenoids From Traditional Chinese Medicine in the Treatment of Osteoporosis. Front. Endocrinol. 13:901545. doi: 10.3389/fendo.2022.901545
Received: 22 March 2022; Accepted: 13 April 2022;
Published: 16 May 2022.
Edited by:
Bo Liu, Guangdong Provincial Hospital of Chinese Medicine, ChinaReviewed by:
Xiao-Jia Chen, University of Macau, ChinaJinhua Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Huajun Wang, Jinan University, China
Copyright © 2022 Zhuo, Li, Jiang, Ke, Liang, Zeng and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Zhuo, emh1b3l1ZUBnenVjbS5lZHUuY24=; Ling-Feng Zeng, emVuZ2xmNjc3OEAxNjMuY29t; Jiansong Fang, ZmFuZ2pzQGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Yue Zhuo
Yue Zhuo Meng Li2†
Meng Li2† Ling-Feng Zeng
Ling-Feng Zeng Jiansong Fang
Jiansong Fang