- 1Department of Nephrology, Affiliated Hospital of Weifang Medical University, Weifang, China
- 2Department of Ultrasound, Affiliated Hospital of Weifang Medical University, Weifang, China
- 3Department of Electrophysiology, Affiliated Hospital of Weifang Medical University, Weifang, China
Objective: To observe the feasibility of shear wave elastography (SWE) in the diagnosis of peripheral neuropathy in patients undergoing hemodialysis [chronic kidney disease stage 5 dialysis (CKD5D)].
Methods: Forty patients with CKD5D were divided into a uremic peripheral neuropathy (UPN) group (n = 25) and a non-UPN group (n = 15) according to the results of a neuro-electrophysiological examination. Sixteen healthy control subjects were also enrolled in this study. Two-dimensional ultrasound examination was conducted, and SWE was then performed to measure Young’s modulus of the tibial nerve. The left and right diameters (D1), anterior and posterior diameters (D2), perimeter (C), cross-sectional area (CSA), and Young’s modulus (E) were measured three times at the same non-entrapment site. The average values were recorded and calculated. The following evaluation indices were also analyzed: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristic curve (AUC).
Results: D1, D2, C, and CSA were not significantly different among the three groups (P > 0.05). However, the difference in the E value among the three groups was statistically significant (P < 0.05). The AUC was 0.889 based on the E value. Using a tibial nerve E value of 48.35 kPa as the cutoff value, the sensitivity, specificity, PPV, and NPV were 86.0%, 84.0%, 81.1%, and 88.1%, respectively.
Conclusions: SWE is useful for the diagnosis of peripheral neuropathy in patients with CKD5D. Young’s modulus of 48.35 kPa for the tibial nerve is the optimal cutoff value and has the best diagnostic efficiency for peripheral neuropathy in CKD5D patients.
Introduction
Chronic kidney disease (CKD) is a global health problem that reduces quality of life and disrupts economic development. One of the most common neurological complications of CKD, especially CKD stage 5 dialysis (CKD5D), is peripheral neuropathy, which affects approximately 60% to 90% of patients with CKD (1). Uremic peripheral neuropathy (UPN) has characteristic symptoms or can be detected by clinical examinations. The most common and earliest symptoms are mainly sensory dysfunctions, such as pain and paresthesia; these are followed by limb weakness and atrophy (2). However, some patients with impaired nerve function exhibit no clinical symptoms (3, 4). Thus, a nerve conduction study (NCS) is usually performed to evaluate the function of peripheral nerves. However, the nerve conduction is not sensitive enough to detect peripheral neuropathy, especially in asymptomatic patients (5).
Ultrasound (US) elastography has become an important tool for the evaluation of nerve stiffness. This procedure takes less time to perform and causes less discomfort to patients. US examinations are used to assess the elasticity of the nerve in patients undergoing hemodialysis by measuring the nerve cross-sectional area (CSA) (6, 7). Two-dimensional shear wave elastography (2D-SWE) is a new complementary method to NCS that has been widely applied for the detection of diabetic peripheral neuropathy in recent studies (8, 9). It can reveal minor peripheral nerve lesions that cannot be detected by electrophysiology (8) and has higher sensitivity and specificity than US, which is mainly based on CSA measurement (6). Although research has revealed changes in nerve elasticity in patients undergoing hemodialysis, few studies have used 2D-SWE for the detection of peripheral nerve damage in these patients. Thus, the diagnostic performance of 2D-SWE in patients undergoing hemodialysis was evaluated in the present study.
Materials and Methods
Ethics and Consent
This study was approved by the ethics committee of the Affiliated Hospital of Weifang Medical University. All participants provided written informed consent.
Participants
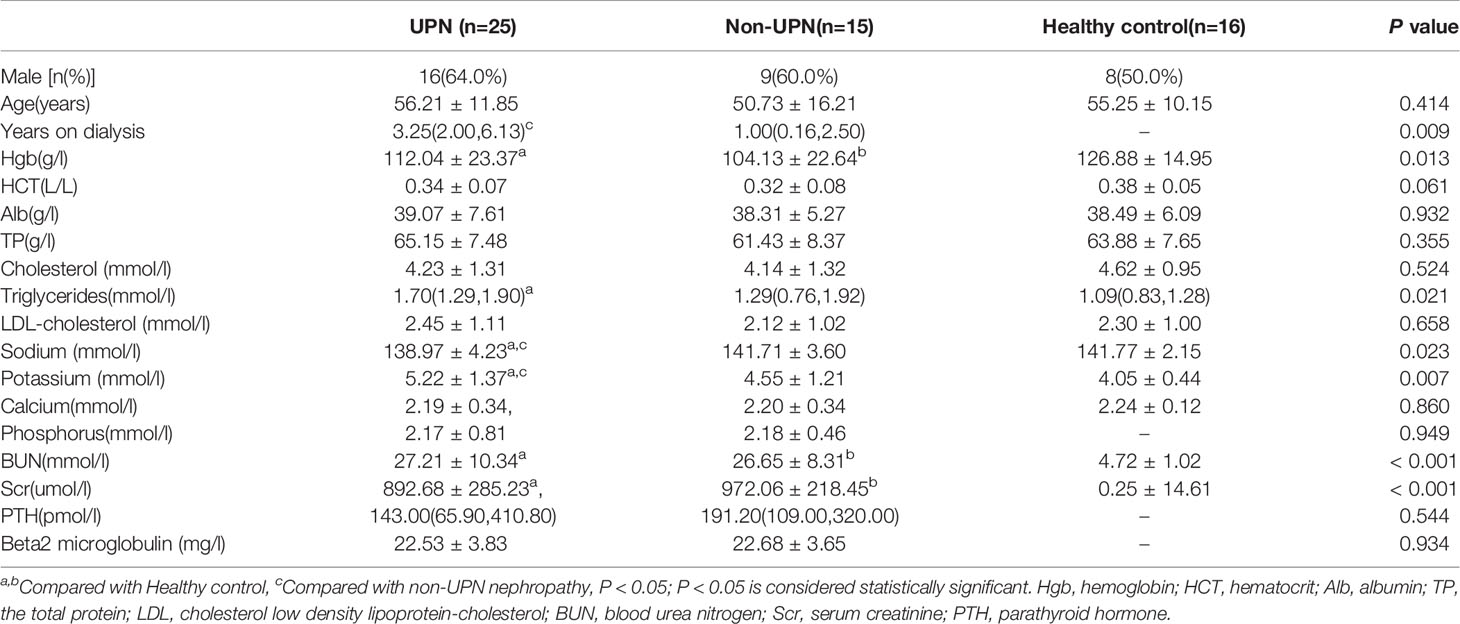
Forty patients (15 women, 25 men) undergoing hemodialysis were recruited from the Affiliated Hospital of Weifang Medical College dialysis center. All patients underwent electrophysiological tests. Sixteen healthy volunteers with no clinical signs or symptoms were enrolled as the control group. All participants underwent US and 2D-SWE examinations. The following basic data were collected for all participants: sex, age, hemoglobin (Hgb), hematocrit (HCT), albumin (Alb), total protein (TP), blood lipid indices, blood urea nitrogen (BUN), serum creatinine (Scr), parathyroid hormone (PTH), beta-2 microglobulin, blood sodium, blood potassium, blood calcium, blood phosphorus, and duration of dialysis. The inclusion criteria were treatment with hemodialysis in our dialysis center and good cognitive function and communication skills; and the age ranged from 18 to 78 years. The exclusion criteria were polyneuropathy caused by diabetes, hereditary factors, alcohol intake, metabolic factors, inflammatory factors, a malignant tumor, or toxic factors; skin lesions or swelling of the ankle; leg or ankle fractures; and damage to the liver, brain, heart, lung, or other important organs.
Diagnosis of UPN
The diagnosis of UPN is based on symptoms and clinical examination findings (10). Patients may have one or more of the following clinical symptoms: paresthesia, restless leg syndrome, increased pain sensation, impaired deep tendon reflexes, imbalance, numbness, and atrophy of the lower limbs (11–15). An NCS is currently regarded as the most effective method to diagnose peripheral neuropathy. Thus, it is used as the gold standard for the diagnosis of UPN (11, 12). In the present study, one neurologist with 10 years of experience in NCSs performed an NCS for all patients. The patients were grouped according to the results.
Electrodiagnostic Studies
Electromyography was performed using a conventional procedure on a standard system (Nicolet EDX; Natus Medical, Middleton, WI, USA). All examinations were performed in the same room at an ambient temperature of 25°C. They were begun 1 hour after a hemodialysis session because normalization of nerve excitability parameters can occur after hemodialysis (16, 17). An NCS of the bilateral tibial nerves was performed in every patient. We obtained all relevant data including latencies, amplitudes, and conduction velocity. The case definition for UPN was based on the results of electromyography, which is the gold standard method (18).
Patient Positioning
All patients were placed in the supine position during the examination. Their ankles were relaxed. To prevent the effect of ankle soft tissue pressure, ankle movement was avoided during the examination.
US and SWE Measurements
The whole study population underwent SWE and US examinations by Sonographer 1 (Zhaoguang Zhang, with 10 years of experience in US and 3 years of experience in elastography). Six of the participants were randomly selected for a second SWE examination by Sonographer 1 after 24 hours and they were performed by Sonographer 2 (Qian Luo, with 8 years of experience in US) by SWE. Both Sonographers were blinded to the participants’ information, including their clinical history, previous examination findings, and NCS results. All SWE examinations were completed within 1 week after the NCS.
The examinations were performed using a device with an L2-9-D transducer (LOGIQ E20; GE Healthcare, Chicago, IL, USA). The transducer was gently placed onto the skin surface. Care was taken to perform the examination with light contact using ample coupling gel. The tibial nerve was scanned upward from the medial malleolus to examine the cross section of the tibial nerve, determine the tibial nerve boundary, observe the internal structure of the tibial nerve, and assess the echo of the nerve bundle. The tibial nerve CSA was measured 4 cm above the medial malleolus. The transducer was then rotated 90° to view the longitudinal imaging plane. The depth of the image was adjusted to find the tibial nerve, and SWE was then performed. Three iterative measurements were obtained at 2-min intervals. The validated measurements of each tibial nerve were taken by each sonographer. Young’s modulus (E) was expressed in kilopascals (kPa).
Statistical Analysis
SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Categorical variables are presented as percentage. Continuous data are presented as mean ± standard deviation or median and interquartile range. An independent t-test and One-way analysis of variance were performed for statistical comparisons, and then the LSD test was used for multiple comparisons. Non-parameter test was used for non-normal distribution data. Kruskal-Wallis test and Mann-Whitney U test were performed to compare variables. The best cut-off value of the nerve E value was obtained by plotting receiver operating characteristic curve. The interclass correlation coefficient (ICC) was used to evaluate the intra- and inter-observer reliability. Statistical significance was accepted at P <0.05.
Results
Clinical Baseline Characteristics
Clinical baseline data were collected from 56 participants (40 patients undergoing hemodialysis and 16 healthy control individuals). These baseline characteristics are displayed in Table 1. The statistical analysis showed no significant differences in age, HCT, Alb, TP, cholesterol, low-density lipoprotein cholesterol, or calcium among the three groups (P > 0.05). BUN and Scr were significantly higher in the CKD5D patients than in the controls (P < 0.05). CKD5D patients had lower Hgb than the controls (P < 0.05). The UPN group had more severe hyponatremia and hyperkalemia than the non-UPN and the control groups (P < 0.05). Triglycerides in the UPN group were higher than in the control group (P < 0.05). However, there were no significant differences in Hgb, BUN, Scr, PTH, phosphorus, triglycerides and beta-2 microglobulin between the UPN group and non-UPN group (P > 0.05). The UPN group had a longer duration of dialysis than the non-UPN group (P < 0.05).
US Features of Tibial Nerve
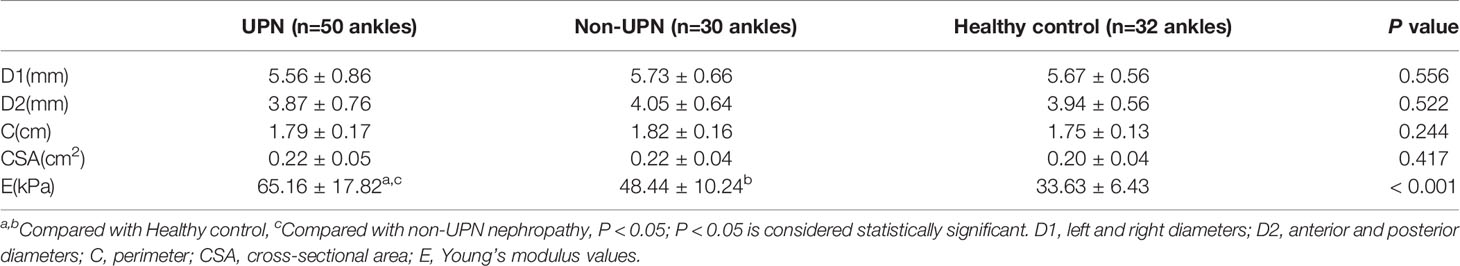
A total of 80 ankles of 40 CKD5D patients and 32 ankles of 16 healthy controls were enrolled in our study. The measurement indices of the tibial nerve in all three groups are displayed in Table 2. There was no significant difference in the left and right diameters, anterior and posterior diameters, perimeter, or CSA of the tibial nerve among the three groups (P > 0.05). The stiffness of the tibial nerve was measured with 2D-SWE and the shear elasticity index was displayed as Young’s modulus(E). The stiffness of the tibial nerve in the UPN group (Figure 1) was significantly different from that in the non-UPN group and control group (P < 0.05) and was significantly different between the non-UPN group (Figure 2) and control group (P < 0.05) (Figure 3).
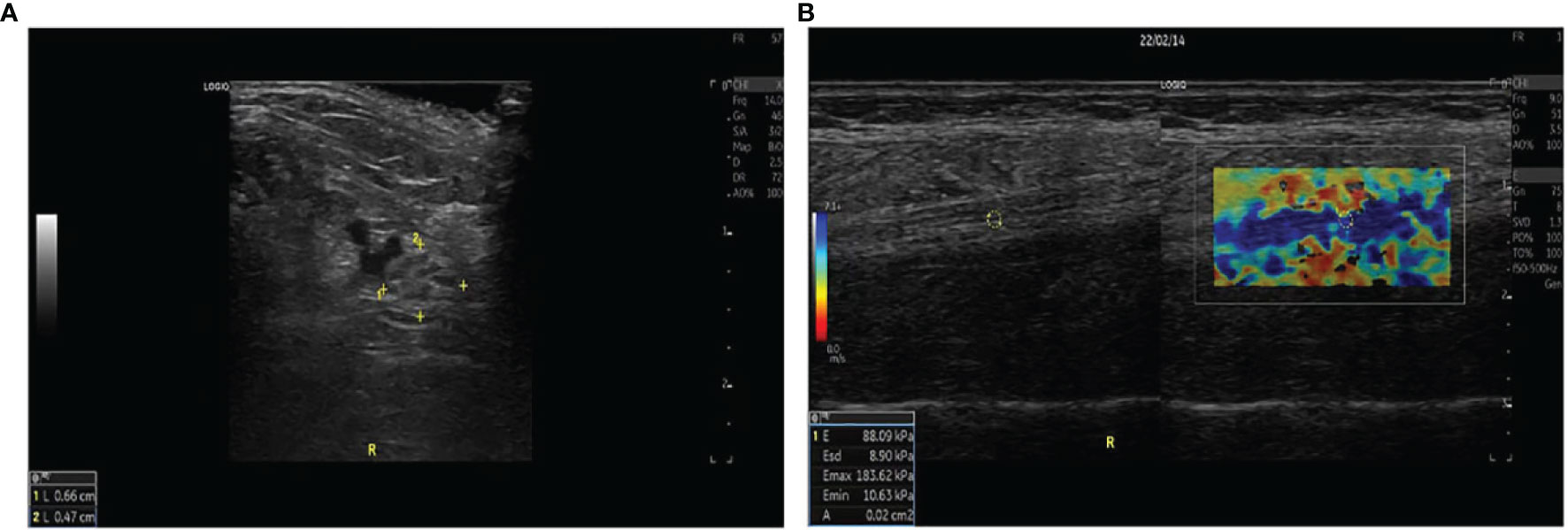
Figure 1 (A) The left and right diameters, anterior and posterior diameters, perimeter, and CSA of the tibial nerve were measured 4 cm proximal to the medial level in a 57-year-old woman with UPN. (B) Two split US and SWE images were obtained at the same longitudinal level in a 39-year-old man with UPN. Quantitative SWE measurements showed that the mean nerve stiffness was 88.09 kPa. R, Right.
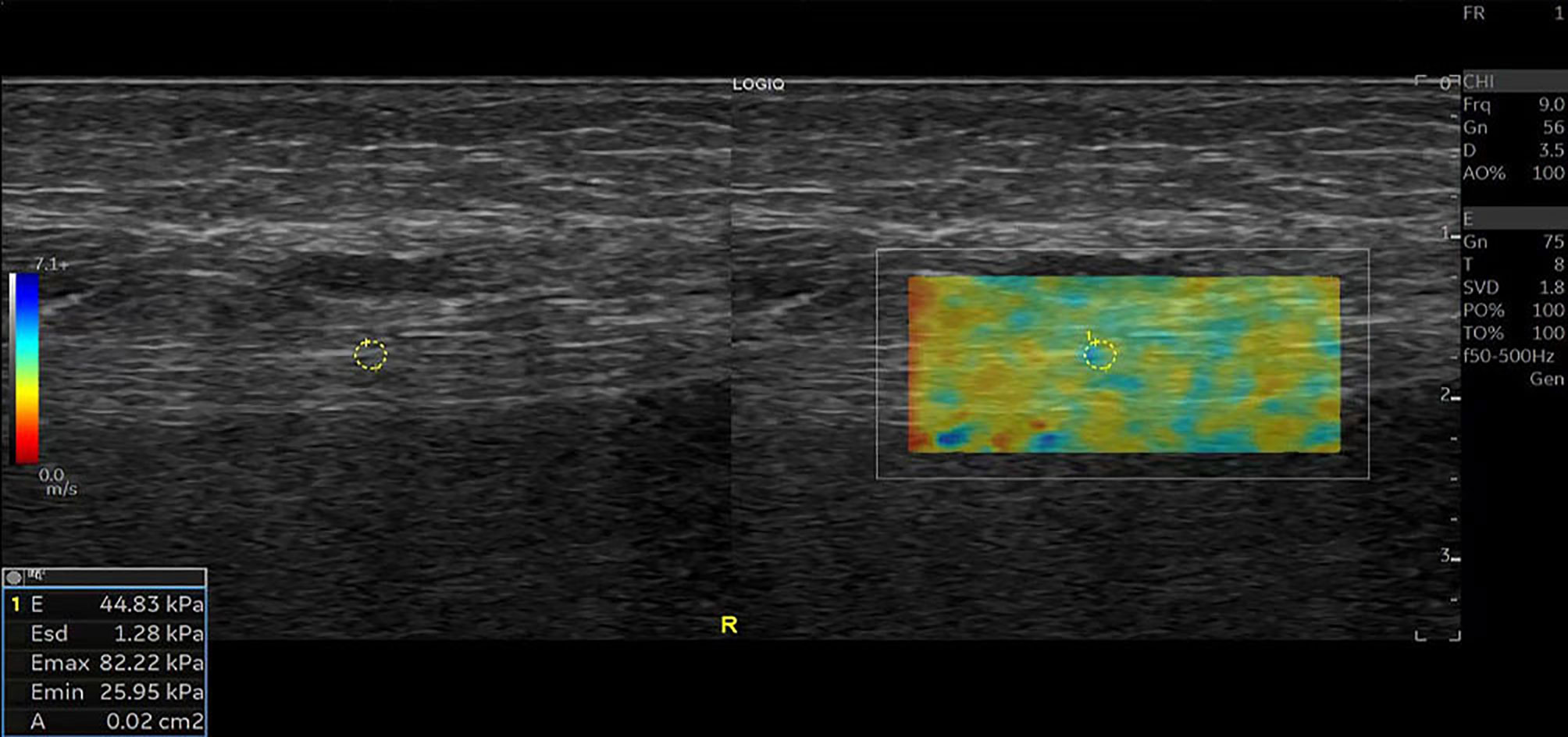
Figure 2 SWE image of the tibial nerve in a 52-year-old man with non-UPN. Quantitative SWE measurements showed that the mean nerve stiffness was 44.83 kPa. R, Right.
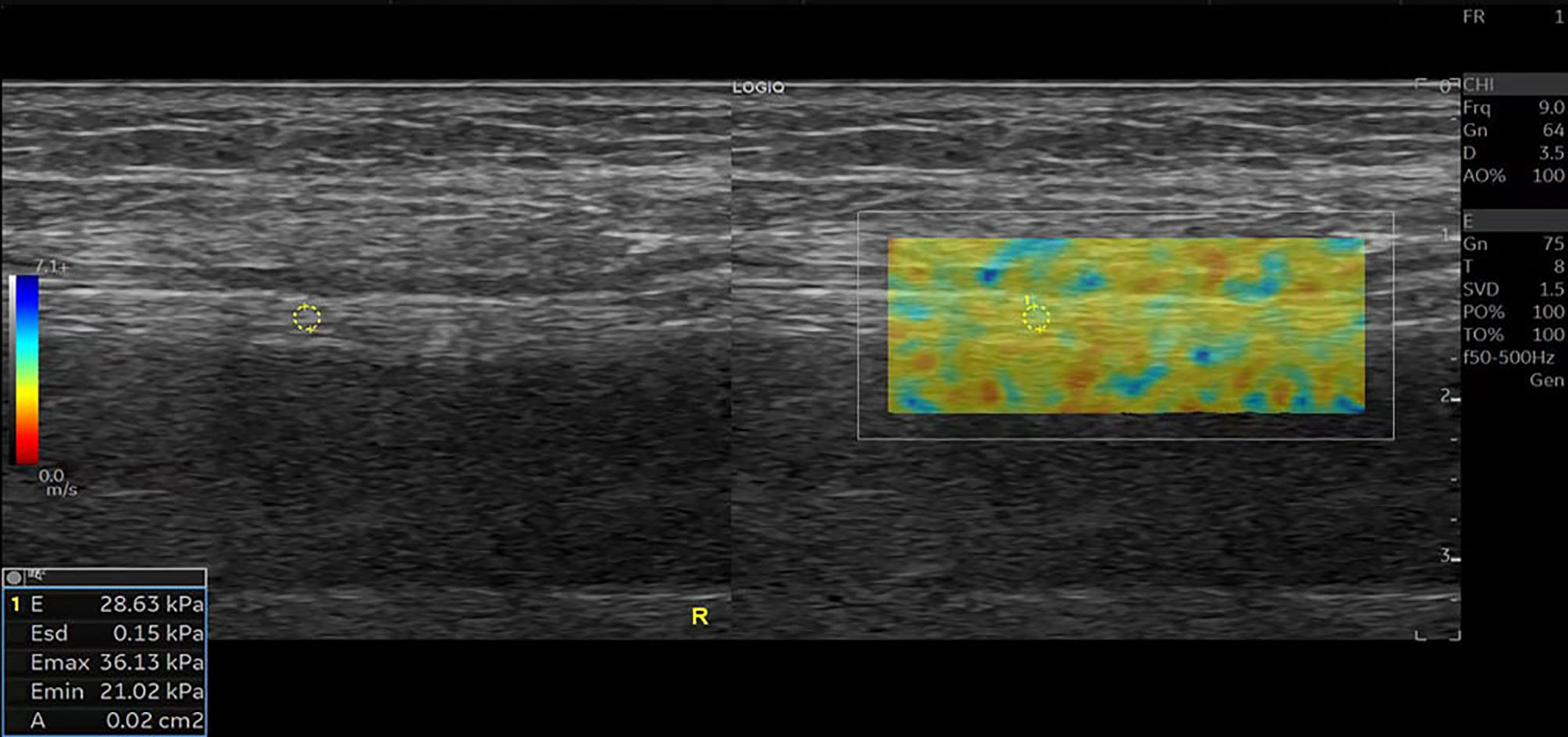
Figure 3 SWE image of the tibial nerve in a 56-year-old healthy man. Quantitative SWE measurements showed that the mean nerve stiffness was 28.63 kPa. R, Right.
Determination of Optimal Cut-Off Value of E for Diagnosis of UPN Based on US and SWE Measurements
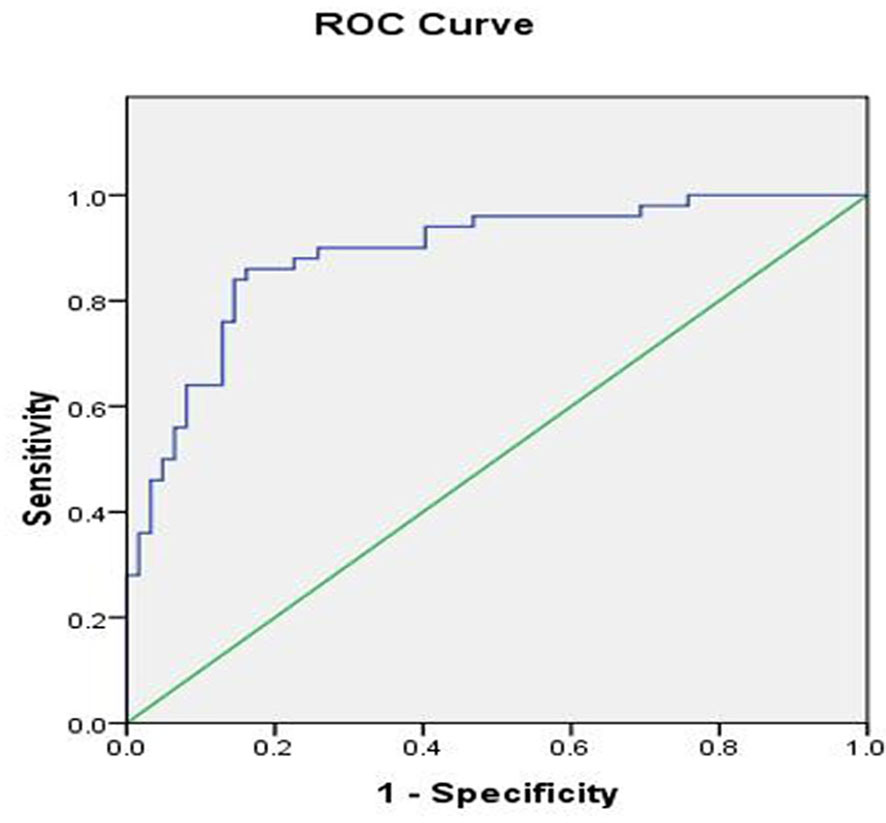
To determine the best cut-off value of E, we performed a receiver operating characteristic curve analysis based on the E value of the tibial nerve as shown in Figure 4. The optimal cutoff value of E for diagnosing UPN was 48.35 kPa. The sensitivities, specificities, positive predictive values, and negative predictive values are summarized in Table 3.
Inter- and Intra-Observer Consistency Analysis
The ICC of the intra-observe was 0.950(95% CI 0.350, 0.990). The inter-observe ICC was 0.931(95% CI 0.786, 0.979). SWE showed very good inter- and intra-observer consistency.
Discussion
Peripheral neurological complications are very common in patients with CKD, with an estimated prevalence of up to 90% in the CKD5D population (19). Such complications present as a slowly progressive sensorimotor neuropathy. The clinical manifestations may include pain, paresthesia, numbness in the distal lower limbs, or loss of sensitivity. Peripheral neuropathy results from a variety of mechanisms. Diabetic neuropathy is one neurological manifestation of end-stage renal disease (ESRD) (12). The mechanisms of diabetic neuropathy are well known and widely reported. With respect to CKD-induced neuropathy, many substrates have been investigated as potential uremic neurotoxins. The retention of neurotoxic solutes with a molecular weight of 300 to 2000 Da, including PTH and beta-2 microglobulin, has been discussed as a cause of UPN because such solutes are slowly dialyzable (20). However, urea, creatine, and uric acid have shown no evidence of causality. Many nerve excitability studies in uremic neuropathy have provided evidence that hyperkalemia is related to nerve dysfunction and contributes to the development of neuropathy (21). Similarly, it showed more sever hyperkalemia in the UPN group than the non-UPN and the control groups in our study. The UPN group had lower sodium than the non-UPN and the control groups. Previous studies showed that hyponatremia may be related to the central nervous system toxicity via multiple pathways (22). It was uncertain whether lower serum sodium levels promoted injury to the peripheral neuropathy, which needed further research. In the present study, the sex distribution among the three groups was not revealed significant difference. However, Said et al. (15) found that uremic neuropathy is more common in male than in female patients. Additionally, Hojs-Fabjan et al. (23) found that polyneuropathy was associated with the patient’s age and duration of dialysis treatment. In our study, the years of dialysis were longer in the UPN group than in the non-UPN group, which is consistent with the study by Hojs-Fabjan et al. (23). No significant difference in age was found among the three groups. The BUN and Scr concentrations were significantly higher in the CKD5D patients than in the healthy controls. Abnormal lipoprotein profile has been reported in CKD5D patients (24). Triglycerides were much lower in the UPN group than in the control group. However, no significant differences were in Hgb, HCT, Alb, TP, cholesterol, low-density lipoprotein cholesterol, calcium, phosphorus, Scr, BUN, PTH, or beta-2 microglobulin between the UPN group and non-UPN group.
The gold standard method for the diagnosis of peripheral neuropathy is an NCS, which is time-consuming and invasive. High-resolution US has recently become more widely used in the detection of neuropathy because of its low cost, noninvasiveness, and ability to depict the location and range of the lesion. Our study showed no significant difference in the anterior and posterior diameters, left and right diameters, perimeter, or CSA of the tibial nerve among the three groups, indicating that nerve damage has little effect on the morphological characteristics of nerves. Consistent with the findings reported by Dikici et al. (8), the morphological changes were not obvious when the nerve was uncharacteristically damaged.
Through the measurement of Young’s modulus, SWE can quantitatively reflect the elasticity of tissue. Harder tissue has a greater Young’s modulus. This measurement index has been widely applied in liver disease (25), thyroid disease (26), diabetic peripheral neuropathy (8), and other conditions. However, few studies have been performed to assess the peripheral nerve elasticity in CKD5D patients. In this study, we evaluated the tibial nerve stiffness in such patients. The inter-observer and intra-observer reproducibility of SWE were excellent. The E value was statistically significant among the three groups. These findings suggest that more severe lesions are associated with greater nerve stiffness. Our group is currently performing animal studies to elucidate the mechanism of this finding. We demonstrated that the E value had high accuracy for identifying UPN. When 48.35 kPa was taken as the optimal value, the sensitivity was high (86.0%), specificity was high (84.0%), Youden’s index was 0.699, and area under the curve was 0.889. Consistent with previous studies, SWE had better sensitivity and specificity than the CSA for diagnosis of neuropathy (27).
This study had some limitations. First, multiple neuropathies may occur in patients with ESRD undergoing hemodialysis; however, we only evaluated the tibial nerve at one point. More nerves and more measurement points of one nerve are needed. Second, we only evaluated patients at a single hemodialysis center, and these patients represent only a fraction of all patients with ESRD. These findings must be validated by large-sample multicenter prospective studies. Finally, we did not investigate the mechanism underlying the increased nerve stiffness in patients with ESRD. The cause of the change in nerve stiffness with time after dialysis is not clear. Therefore, studies in which different time points after dialysis are examined are needed to observe the changes in nerve stiffness.
In conclusion, SWE is useful for the diagnosis of peripheral neuropathy in CKD5D patients. Young’s modulus of 48.35 kPa of the tibial nerve was taken as the optimal cutoff value for the diagnosis of peripheral neuropathy in CKD5D patients, with a sensitivity of 86.0%, specificity of 84.0%, and area under the curve of 0.889.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Affiliated Hospital of Weifang Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XL and HS: writing-original draft preparation, formal analysis and visualization. ZZ, JL, and QL: methodology and investigation. XC and ZG: conceptualization, methodology, funding acquisition and writing-review and editing. HX, LM, HZ, and JLL: resources and data curation. XW and MG: supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Weifang Key Laboratory of Integrated Traditional Chinese and Western Medicine for Chronic renal Failure; the National Science Foundation of Shandong Province (ZR2021MH394 to XW), Medical and health Science and Technology Development Project of Shandong Province (2017WS172 to XC; 2017WS890 to ZZ), Weifang Soft Science Research Plan (2021RKX047 to XL; 2019RKX088 to XC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.899822/full#supplementary-material
References
1. Chung T, Prasad K, Lloyd TE. Peripheral Neuropathy: Clinical and Electrophysiological Considerations. Neuroimaging Clinics North Am (2014) 24(1):49–65. doi: 10.1016/j.nic.2013.03.023
2. Strempska B, Bilinska M, Weyde W, Koszewicz M, Madziarska K, Golebiowski T, et al. The Effect of High-Tone External Muscle Stimulation on Symptoms and Electrophysiological Parameters of Uremic Peripheral Neuropathy. Clin Nephrol (2013) 79 Suppl 1:S24–7. doi: 10.5414/CNX77S103
3. Laaksonen S, Metsarinne K, Voipio-Pulkki LM, Falck B. Neurophysiologic Parameters and Symptoms in Chronic Renal Failure. Muscle Nerve (2002) 25(6):884–90. doi: 10.1002/mus.10159
4. Mallipeddi S, Jasti DB, Apparao A, Vengamma B, Sivakumar V, Kolli S. A Clinical and Electrophysiological Study of Peripheral Neuropathies in Peritoneal Dialysis Patients: Our Experience from Rural South India. Saudi journal of Kidney Diseases and Transplantation: An Official Publication of the Saudi Center for Organ Transplantation, Saudi Arabia (2018) 29(5):1139–49. doi: 10.4103/1319-2442.243942
5. Ezzeldin N, Abdel Galil SM, Said D, Kamal NM, Amer M. Polyneuropathy Associated With Chronic Hemodialysis: Clinical and Electrophysiological Study. Int J Rheum Dis (2019) 22(5):826–33. doi: 10.1111/1756-185X.13462
6. Xin H, Hu HY, Liu B, Liu X, Li X, Li J. Ultrasound Elastographic Evaluation of the Median Nerve in Hemodialysis With Carpal Tunnel Syndrome. J Med Ultrason (2017) 44(1):123–31. doi: 10.1007/s10396-016-0733-x
7. Borire AA, Arnold R, Pussell BA, Kwai NC, Visser LH, Padua L, et al. Haemodialysis Alters Peripheral Nerve Morphology in End-Stage Kidney Disease. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol (2017) 128(1):281–6. doi: 10.1016/j.clinph.2016.09.010
8. Dikici AS, Ustabasioglu FE, Delil S, Nalbantoglu M, Korkmaz B, Bakan S, et al. Evaluation of the Tibial Nerve With Shear-Wave Elastography: A Potential Sonographic Method for the Diagnosis of Diabetic Peripheral Neuropathy. Radiology (2017) 282(2):494–501. doi: 10.1148/radiol.2016160135
9. Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic Performance of Two-Dimensional Shear Wave Elastography for Evaluating Tibial Nerve Stiffness in Patients With Diabetic Peripheral Neuropathy. Eur Radiol (2019) 29(5):2167–74. doi: 10.1007/s00330-018-5858-4
10. Mambelli E, Barrella M, Facchini MG, Mancini E, Sicuso C, Bainotti S, et al. The Prevalence of Peripheral Neuropathy in Hemodialysis Patients. Clin Nephrol (2012) 77(6):468–75. doi: 10.5414/cn107188
11. Kandil MR, Darwish ES, Khedr EM, Sabry MM, Abdulah MA. A Community-Based Epidemiological Study of Peripheral Neuropathies in Assiut, Egypt. Neurol Res (2012) 34(10):960–6. doi: 10.1179/1743132812Y.0000000099
12. Deger SM, Reis KA, Guz G, Bali M, Erten Y. A Case of an Accelerated Uremic Neuropathy. Renal Fail (2011) 33(3):371–2. doi: 10.3109/0886022X.2011.559677
13. Ghazan-Shahi S, Koh TJ, Chan CT. Impact of Nocturnal Hemodialysis on Peripheral Uremic Neuropathy. BMC Nephrol (2015) 16:134. doi: 10.1186/s12882-015-0133-2
14. Witzel II, Jelinek HF, Khalaf K, Lee S, Khandoker AH, Alsafar H. Identifying Common Genetic Risk Factors of Diabetic Neuropathies. Front Endocrinol (2015) 6:88. doi: 10.3389/fendo.2015.00088
15. Said G. Uremic Neuropathy. Handb Clin Neurol (2013) 115:607–12. doi: 10.1016/B978-0-444-52902-2.00035-7
16. Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Bostock H, Kiernan MC. Altered Motor Nerve Excitability in End-Stage Kidney Disease. Brain: J Neurol (2005) 128(Pt 9):2164–74. doi: 10.1093/brain/awh558
17. Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Kiernan MC. Sensory Nerve Excitability and Neuropathy in End Stage Kidney Disease. J Neurol Neurosurg Psychiatry (2006) 77(4):548–51. doi: 10.1136/jnnp.2005.079988
18. Camargo CRS, Schoueri JHM, Alves B, Veiga G, Fonseca FLA, Bacci MR. Uremic Neuropathy: An Overview of the Current Literature. Rev Assoc Med Bras (2019) 65(3):469–74. doi: 10.1590/1806-9282.65.3.469
19. Arnold R, Issar T, Krishnan AV, Pussell BA. Neurological Complications in Chronic Kidney Disease. JRSM Cardiovasc Dis (2016) 5:2048004016677687. doi: 10.1177/2048004016677687
20. Baumgaertel MW, Kraemer M, Berlit P. Neurologic Complications of Acute and Chronic Renal Disease. Handb Clin Neurol (2014) 119:383–93. doi: 10.1016/B978-0-7020-4086-3.00024-2
21. Arnold R, Pussell BA, Howells J, Grinius V, Kiernan MC, Lin CS, et al. Evidence for a Causal Relationship Between Hyperkalaemia and Axonal Dysfunction in End-Stage Kidney Disease. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol (2014) 125(1):179–85. doi: 10.1016/j.clinph.2013.06.022
22. Ayus JC, Achinger SG, Arieff A. Brain Cell Volume Regulation in Hyponatremia: Role of Sex, Age, Vasopressin, and Hypoxia. Am J Physiol Renal Physiol (2008) 295(3):F619–24. doi: 10.1152/ajprenal.00502.2007
23. Hojs-Fabjan T, Hojs R. Polyneuropathy in Hemodialysis Patients: The Most Sensitive Electrophysiological Parameters and Dialysis Adequacy. Wien Klin Wochenschr (2006) 118 Suppl 2:29–34. doi: 10.1007/s00508-006-0547-8
24. Osorio A, Ortega E, de Haro T, Torres JM, Sanchez P, Ruiz-Requena E. Lipid Profiles and Oxidative Stress Parameters in Male and Female Hemodialysis Patients. Mol Cell Biochem (2011) 353(1-2):59–63. doi: 10.1007/s11010-011-0774-9
25. Deffieux T, Gennisson JL, Bousquet L, Corouge M, Cosconea S, Amroun D, et al. Investigating Liver Stiffness and Viscosity for Fibrosis, Steatosis and Activity Staging Using Shear Wave Elastography. J Hepatol (2015) 62(2):317–24. doi: 10.1016/j.jhep.2014.09.020
26. Kim H, Kim JA, Son EJ, Youk JH. Quantitative Assessment of Shear-Wave Ultrasound Elastography in Thyroid Nodules: Diagnostic Performance for Predicting Malignancy. Eur Radiol (2013) 23(9):2532–7. doi: 10.1007/s00330-013-2847-5
Keywords: chronic kidney disease, peripheral neuropathy, cutoff value, tibial nerve, elasticity imaging techniques
Citation: Li X, Sun H, Zhang Z, Liu J, Xu H, Ma L, Zhang H, Li J, Luo Q, Wang X, Guo M, Guo Z and Chen X (2022) Shear Wave Elastography in the Diagnosis of Peripheral Neuropathy in Patients With Chronic Kidney Disease Stage 5. Front. Endocrinol. 13:899822. doi: 10.3389/fendo.2022.899822
Received: 19 March 2022; Accepted: 19 May 2022;
Published: 23 June 2022.
Edited by:
Guiting Lin, University of California, San Francisco, United StatesReviewed by:
Guihua Wang, Southeast University, ChinaZhentao Zhang, Renmin Hospital of Wuhan University, China
Copyright © 2022 Li, Sun, Zhang, Liu, Xu, Ma, Zhang, Li, Luo, Wang, Guo, Guo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuexun Chen, Znl4dWV4dW5fY2hlbkB3Zm1jLmVkdS5jbg==; Zhentao Guo, Z3VvenRAd2ZtYy5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Xuan Li
Xuan Li Haoqi Sun
Haoqi Sun Zhaoguang Zhang
Zhaoguang Zhang Jing Liu
Jing Liu Huiying Xu
Huiying Xu Lin Ma
Lin Ma Haibo Zhang
Haibo Zhang Jialin Li
Jialin Li Qian Luo
Qian Luo Xiangming Wang
Xiangming Wang Min Guo
Min Guo Zhentao Guo
Zhentao Guo Xuexun Chen
Xuexun Chen