- 1Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Second Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
Context: Thyroid hormones influence glucose homeostasis through central and peripheral regulation. To date, the association between thyroid hormone sensitivity and elevated blood glucose (EBG) in patients with coronary heart disease (CHD) remains unknown. The purpose of this study was to investigate the association between thyroid hormone sensitivity and risk of EBG in patients with CHD, and to further explore their association in different sexes and ages.
Methods: This large multicenter retrospective study included 30,244 patients with CHD (aged 30–80 years) between 1 January 2014 and 30 September 2020. Parameters representing central and peripheral sensitivity to thyroid hormones were calculated. Central sensitivity to thyroid hormones was assessed by calculating the Thyroid Feedback Quantile-based Index (TFQI), Thyroid-stimulating Hormone Index (TSHI), and Thyrotropin Thyroxine Resistance Index (TT4RI), and Parametric Thyroid Feedback Quantile-based Index (PTFQI); peripheral sensitivity to thyroid hormones was evaluated using the ratio of free triiodothyronine (FT3) /free thyroxine (FT4). Taking normal glucose tolerance (NGT) as a reference, logistic regression was used to analyse the relationship between central and peripheral thyroid hormone sensitivity and EBG in patients with CHD.
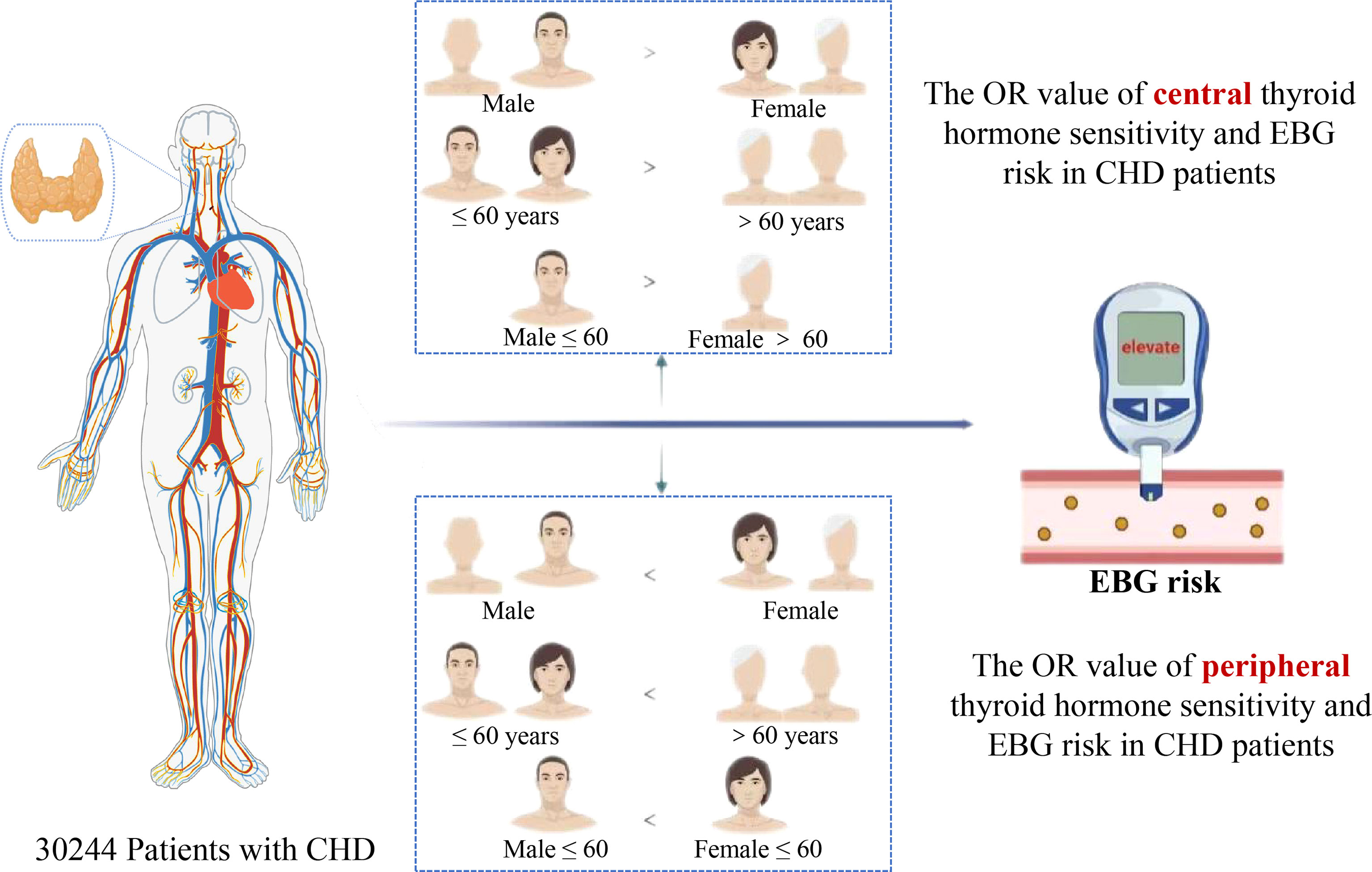
Results: Among the 30,244 participants, 15,493 (51.23%) had EBG. The risk of EBG was negatively correlated with TSHI (OR: 0.91; 95%CI: 0.91 to 0.92; P < 0.001), TT4RI (OR: 0.99; 95% CI: 0.99 to 0.99; P<0.001), TFQI (OR: 0.82; 95%CI: 0.80 to 0.84; P <0.001) and PTFQI (OR: 0.76; 95%CI: 0.74 to 0.78; P<0.001). Compared to males and patients aged 60 and below, the OR value for EBG was lower in females and in patients aged over 60 years old. Conversely, EBG risk was positively associated with FT3/FT4 (OR: 1.08; 95% CI: 1.07 to 1.09; P <0.001) and in the sex-categorized subgroups, males had higher OR values than females.
Conclusions: This study showed that thyroid hormone sensitivity is significantly associated with EBG in patients with CHD. This association is higher in females than in males, and the association in those aged over 60 years old is higher than that in patients aged 60 years and below.
Graphical Abstract Images are from Biorender.com.
Introduction
Cardiovascular diseases are the leading cause of death worldwide (1), seriously affecting the patient’s quality of life and longevity (2, 3). The vast majority of cardiac deaths are caused by coronary heart disease (CHD) secondary to coronary atherosclerosis (4). The relationship between CHD and thyroid hormones is very close, and abnormal thyroid hormones often accompany patients with CHD (5–9). In addition, patients with CHD and EBG are at a higher risk of nephropathy and other cardiovascular diseases. CHD complicated with diabetes is associated with decreased insulin sensitivity, impaired blood pressure regulation, damaged vascular endothelial cells and dysfunction of the fibrinolytic system (10, 11). Lowering blood sugar is extremely important to improve prognosis and prevent the onset of cardiovascular diseases (12–14). Diabetes has been clinically defined as fasting or postprandial hyperglycaemia or abnormally increased glucose excursion in response to an established glucose load. However, this clinical definition defines a relatively late stage in the disease process. Indeed, a defect in glucose homeostasis can be detected long before diabetes occurs.
For early prevention and treatment of secondary elevated blood glucose (EBG) in patients with CHD, identifying the risk factors of prediabetes and diabetes is a critical step. Recent theories show that hypothyroidism and subclinical hypothyroidism are risk factors for diabetes (15). However, some researchers believe that elevated thyroid stimulating hormone (TSH) is a risk factor for diabetes, and an increase in free triiodothyronine (FT3) and free thyroxine (FT4) has a protective effect on the occurrence of diabetes (16). TI de Vries et al. reported that there was no significant relationship between plasma TSH levels in the normal range and the incidence of diabetes in patients at high cardiovascular risk (17). These contradictory results are common. Moreover, almost all previous analyses focused on the influence of TSH and FT4 levels on the risk of prediabetes or diabetes. No research has investigated the association between thyroid hormone sensitivity and EBG in patients with CHD. Furthermore, results of previous studies investigating EBG prevalence in different sexes and ages have been inconsistent (18, 19). Due to inconsistent results, the relationship among EBG, sex, and age remains controversial.
The thyroid hormones regulate glucose homeostasis by interacting with the entire central nervous system and surrounding target organs (20). Therefore, this large-scale, multicenter retrospective study aimed to investigate the association of central and peripheral sensitivity to thyroid hormones in patients with CHD and EBG. Further, we aimed to explore the differences of these associations in different sexes and ages to provide a basis for clinical adjustments of medication according to the situation of individual patients with CHD, and to improve patient conditions.
Methods
Patients
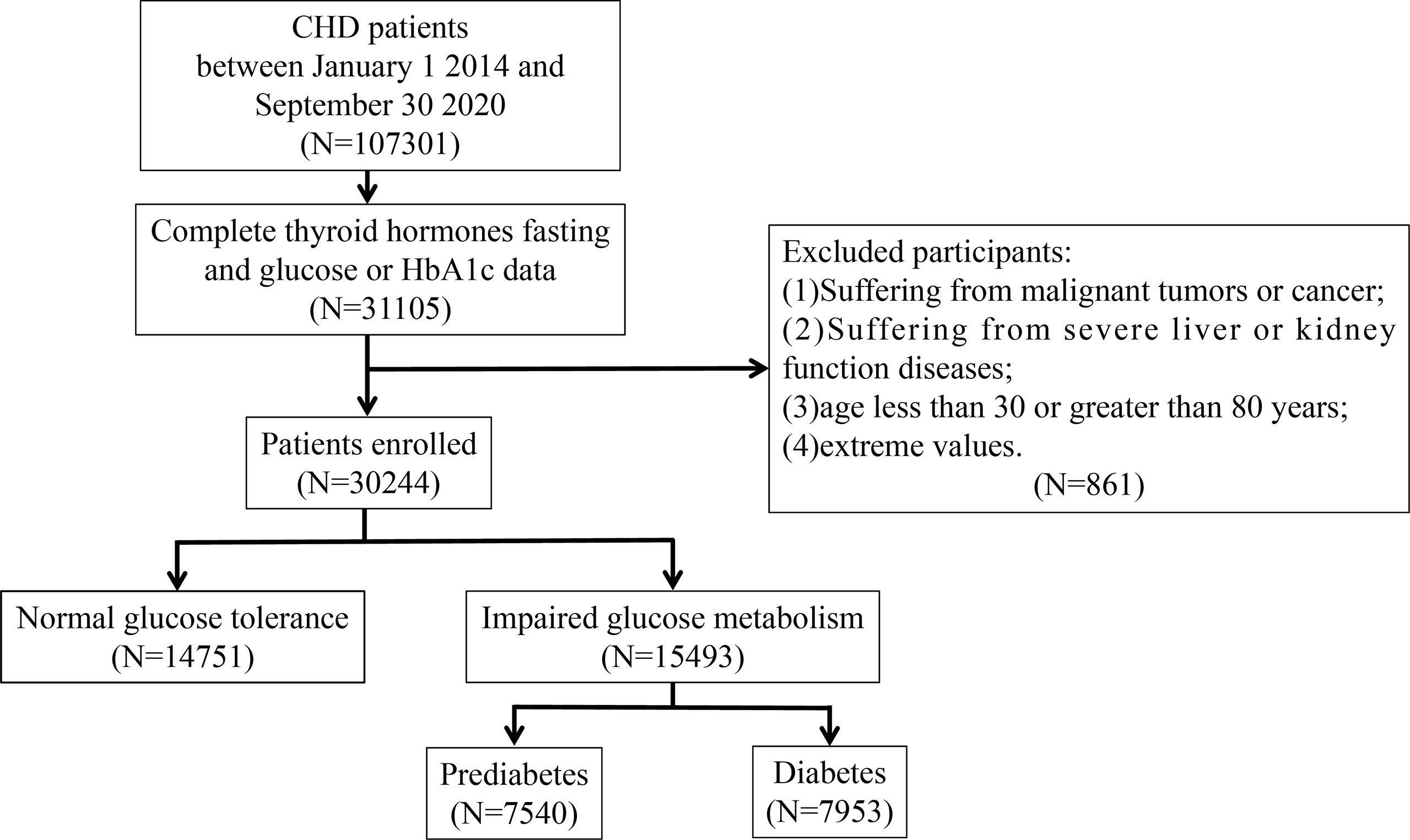
Participants in this study were 107,301 CHD inpatients of cardiology departments from 1 January 2014 to 30 September 2020 from six hospitals in Tianjin. Patients who were younger than 30 years or older than 80 years, had malignancy, infectious or severe liver or kidney disease, incorrect data, and lack of data on TSH, FT3, FT4, fasting blood glucose (FBG), or glycated haemoglobin (HbA1c) were excluded based on the study design. Ultimately, 30,244 participants were included in the study. A flowchart of the patient recruitment process is shown in Figure 1. This study was approved by the ethics committee of Tianjin University of Traditional Chinese Medicine (approval number TJUTCM-EC20190008) and registered with the Chinese Clinical Trial Registry on 14 July 2019 (registration number ChiCTR-1900024535) and ClinicalTrials.gov on 18 July 2019 (registration number NCT04026724).
Data Collection
Trained medical staff collected personal medical history records. These records included information such as age, sex, history of smoking and drinking, which were investigated using standard structured questionnaires (21). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by experienced technicians using automatic blood pressure monitors. Fasting venous blood samples were collected from all participants in the morning. FBG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and HbA1c were measured directly using an automatic haematology analyser. Quality control was conducted in the laboratory according to standard procedures.
Hypertension is defined as elevated blood pressure (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) (22). Dyslipidaemia is defined as TG ≥ 1.7 mmol/L or TC ≥ 5.2 mmol/L or LDL ≥ 3.4 mmol/L or HDL ≤ 1.0 mmol/L (23). Different levels of glucose metabolism, namely normal glucose tolerance (NGT), prediabetes and diabetes, are defined as FBG < 5.6 mmol/L or HbA1c < 5.7%, FBG 5.6 to 6.9 mmol/L or HbA1c 5.7 to 6.4%, and FBG ≥ 7.0 mmol/L or HbA1c ≥ 6.5% (24). Participants with prediabetes or diabetes were assigned to the EBG group and the rest of the participants were assigned to the NGT group (25, 26).
Indices of Thyroid Hormone Sensitivity
Central indices of thyroid hormone sensitivity included the TSH index (TSHI), TSH T4 resistance index (TT4RI), thyroid feedback quantile-based index (TFQI), and parametric thyroid feedback quantile-based index (PTFQI). TSHI was calculated as TSH (mIU/L) + 0.1345 * FT4 (pmol/L) (27). TT4RI was calculated as FT4 (pmol/L) * TSH (mIU/L) (28). TFQI and PTFQI were calculated using the algorithm developed by Laclaustra et al. (29) FT3/FT4 ratio was calculated to evaluate the peripheral thyroid hormone sensitivity.
Statistical Analyses
The characteristics of the participants in the different groups were compared using χ2 (Chi-square) and Mann-Whitney tests. Age, sex, smoking, drinking, hypertension, and hyperlipidaemia were considered as potential confounding factors. The TSHI index was divided into tertiles: T1, TSHI index ≤ 1.5; T2: 1.50 < TSHI index < 2.49; and T3: TSHI index ≥ 2.49. The TT4RI index was divided into tertiles: T1: TT4RI index ≤ 9.01; T2: 9.01 < TT4RI index < 25.58; and T3: TT4RI index ≥ 25.58. The TFQI index was divided into tertiles: T1: TFQI index ≤ -0.23; T2: -0.23 < TFQI index < 0.12; and T3: TFQI index ≥ 0.12. The PTFQI index was divided into tertiles: T1, PTFQI index ≤ -0.13; T2: -0.13 < PTFQI index < 0.24; and T3: PTFQI index ≥ 0.24. The FT3/FT4 index was divided into tertiles: T1: FT3/FT4 index ≤ 0.29; T2: 0.29 < FT3/FT4 index < 0.41; and T3: FT3/FT4 index ≥ 0.41. Logistic regression analysis was used to compare the relationship between thyroid hormone sensitivity and EBG in the different tertiles. Missing values were calculated using the multiple-complement calculation method. All statistical analyses were performed using SPSS 24.0 (IBM Corp., New York, NY, USA).
Results
Baseline Characteristics
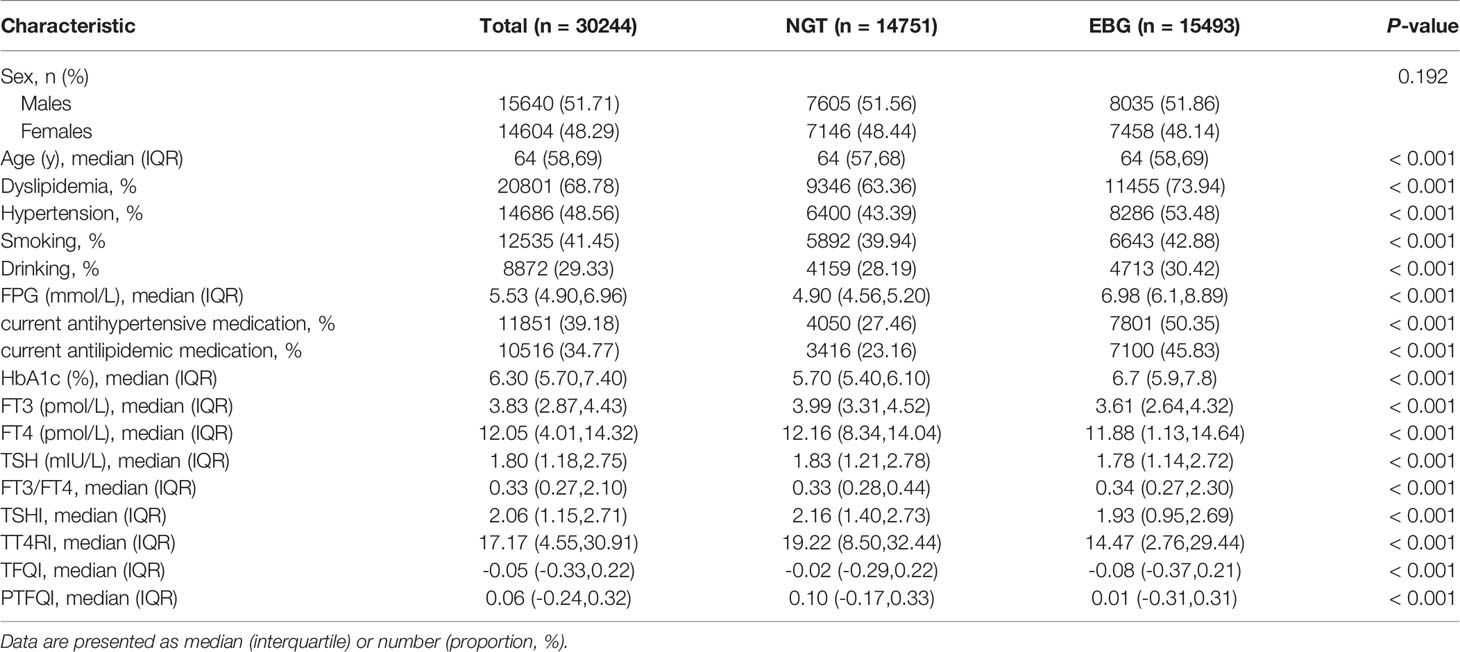
The baseline characteristics of the 30,244 participants are shown in Table 1. The average age of the subjects was 64 years old, and the proportion of females (48.3%) was slightly lower than that of males (51.7%). Among the participants, 15,493 patients demonstrated EBG (51.2%). The subjects were divided into two groups according to glucose metabolism level. Compared with NGT participants, EBG participants were more likely to be older females with hypertension, dyslipidaemia, and a tendency to smoke and drink. Hypertension, dyslipidaemia, age, smoking, and alcohol consumption were positively associated with EBG, and participants with EBG tended to have higher levels of FT3/FT4 and lower levels of TSHI, TT4RI, and PTFQI.
Relationship Between Thyroid Hormone Sensitivity and EBG
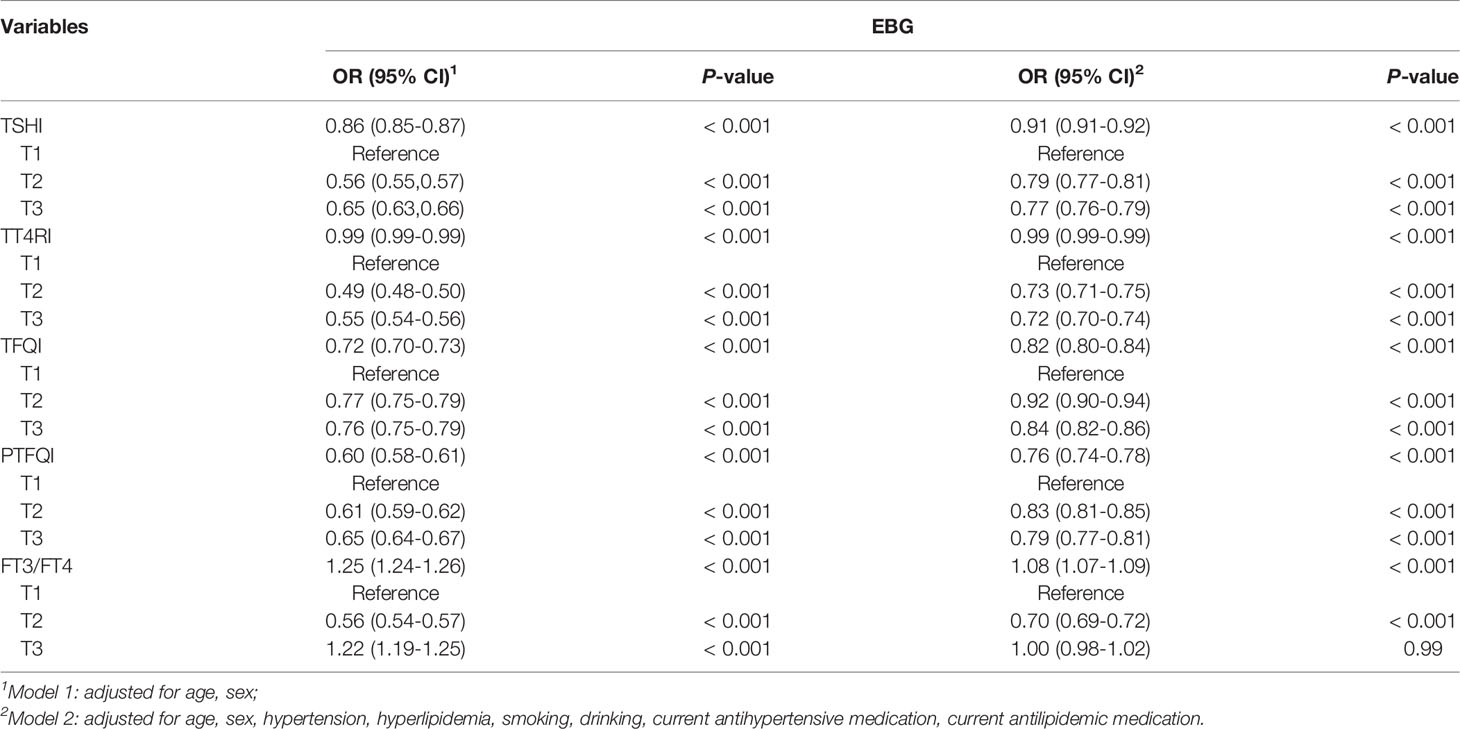
The association between thyroid hormone sensitivity and EBG was estimated by using different logistic regression models (Table 2) After adjusted for sex and age, as a continuous variable, TSHI, TT4RI, PTFQI, and TFQI levels were negatively associated, and FT3/FT4 levels was positively associated with EBG risk. These associations remained significant after multivariate adjustment. In the adjusted model, the OR values of TSHI, TT4RI, and PTFQI were the lowest at T3 when T1 was used as a reference. In addition, when used as a continuous variable, FT3/FT4 level was positively correlated with EBG. Considering the differences between prediabetes and diabetes in the presence of abnormal glucose metabolism, different stratified analyses were performed (Supplementary Tables S1–S3).
Relationship Between Thyroid Hormone Sensitivity and EBG in Different Sexes
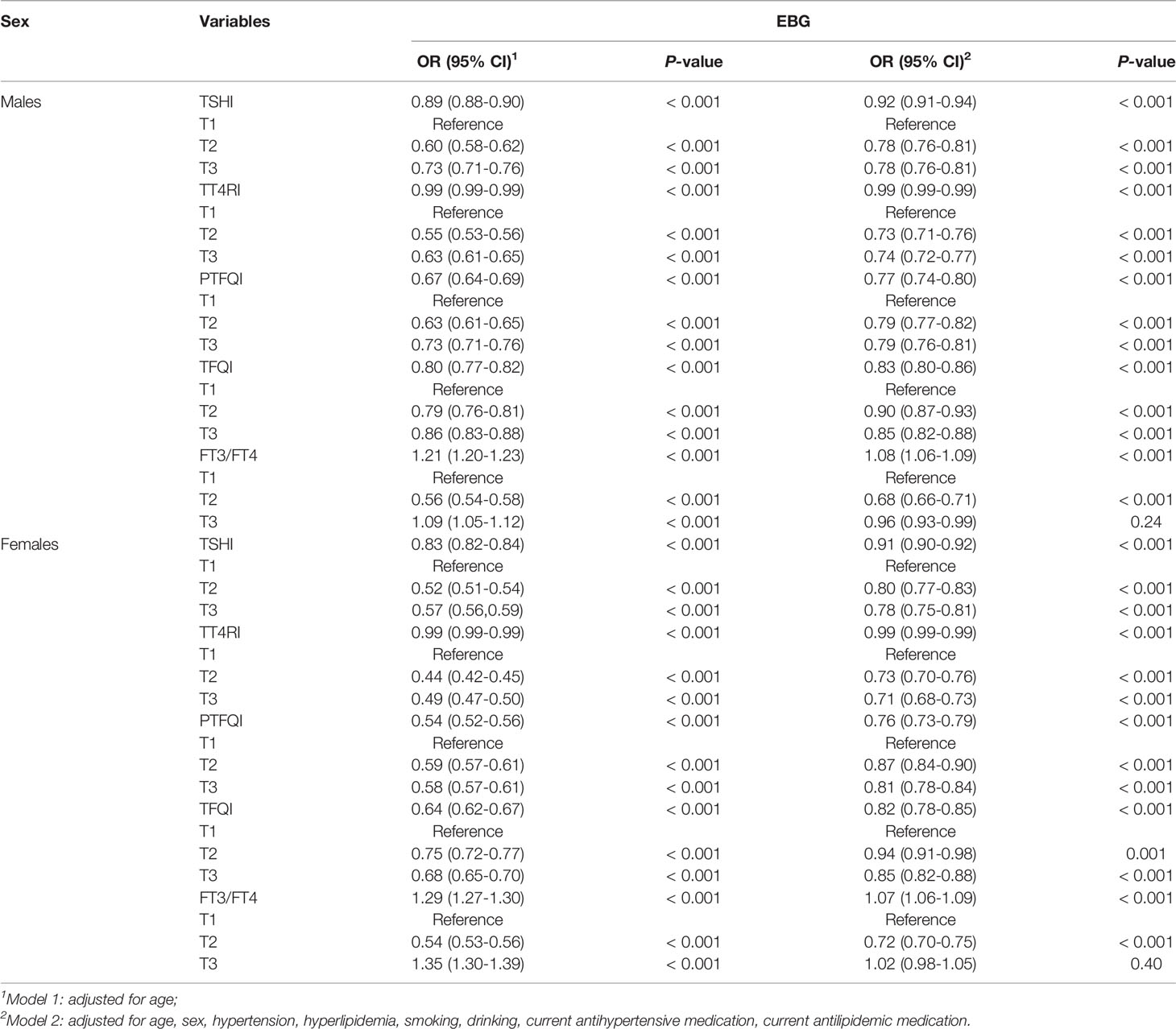
The relationship between thyroid hormone sensitivity and EBG in the different sexes is shown in Table 3. Subgroup analyses stratified by sex showed that thyroid hormone sensitivity was significantly associated with EBG in both sexes. After adjusted for age, as a continuous variable, the OR values of males for TSHI, PTFQI, TFQI, and EBG were slightly higher than those of females. These differences remained significant after adjusting for the model (P <0.001). Of all the indicators representing central thyroid hormone sensitivity, the PTFQI had the lowest OR value, with females (OR: 0.76; 95%CI: 0.73 to 0.79; P <0.001) having a lower PTFQI than males (OR: 0.77; 95%CI: 0.74 to 0.80; P <0.001).
Relationship Between Thyroid Hormone Sensitivity and EBG In Different Age Stratifications
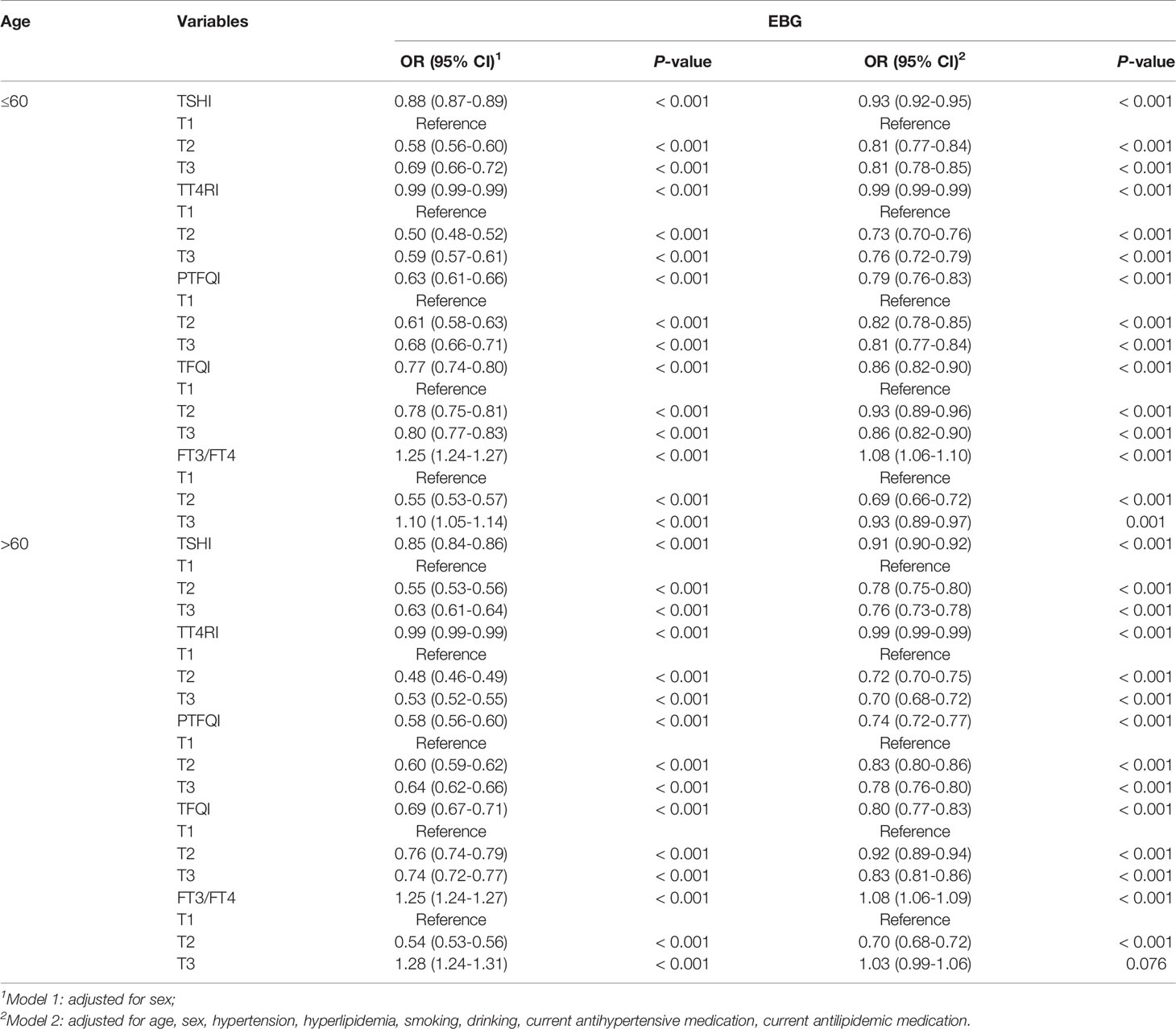
The relationship between thyroid hormone sensitivity and EBG in different age stratifications is shown in Table 4. After adjusted for sex, as a continuous variable, the OR values of TSHI, PTFQI, and TFQI in patients aged 60 years were slightly higher than those aged > 60 years. These differences remained significant after adjusting for the model (P <0.001). Similar to the sex rating and the age rating, PTFQI also had the lowest OR among all measures representing central thyroid hormone sensitivity, and the PTFQI of patients aged over 60 years (OR: 0.74; 95%CI: 0.72 to 0.77; P < 0.001) was lower than that of patients aged 60 years and below (OR: 0.79; 95%CI: 0.76 to 0.83; P < 0.001).
Relationship Between Thyroid Hormone Sensitivity and EBG in Different Sexes and Different Ages
Based on the individual sex and age stratification results, the relationship between thyroid hormone sensitivity and EBG in different sexes and ages was analysed. As shown in Table 5, in the unadjusted and adjusted models, all the central thyroid hormone sensitivity indices had lower OR values with EBG in females aged over 60 years old, and the peripheral thyroid sensitivity index had a higher OR with EBG in females aged 60 and below.
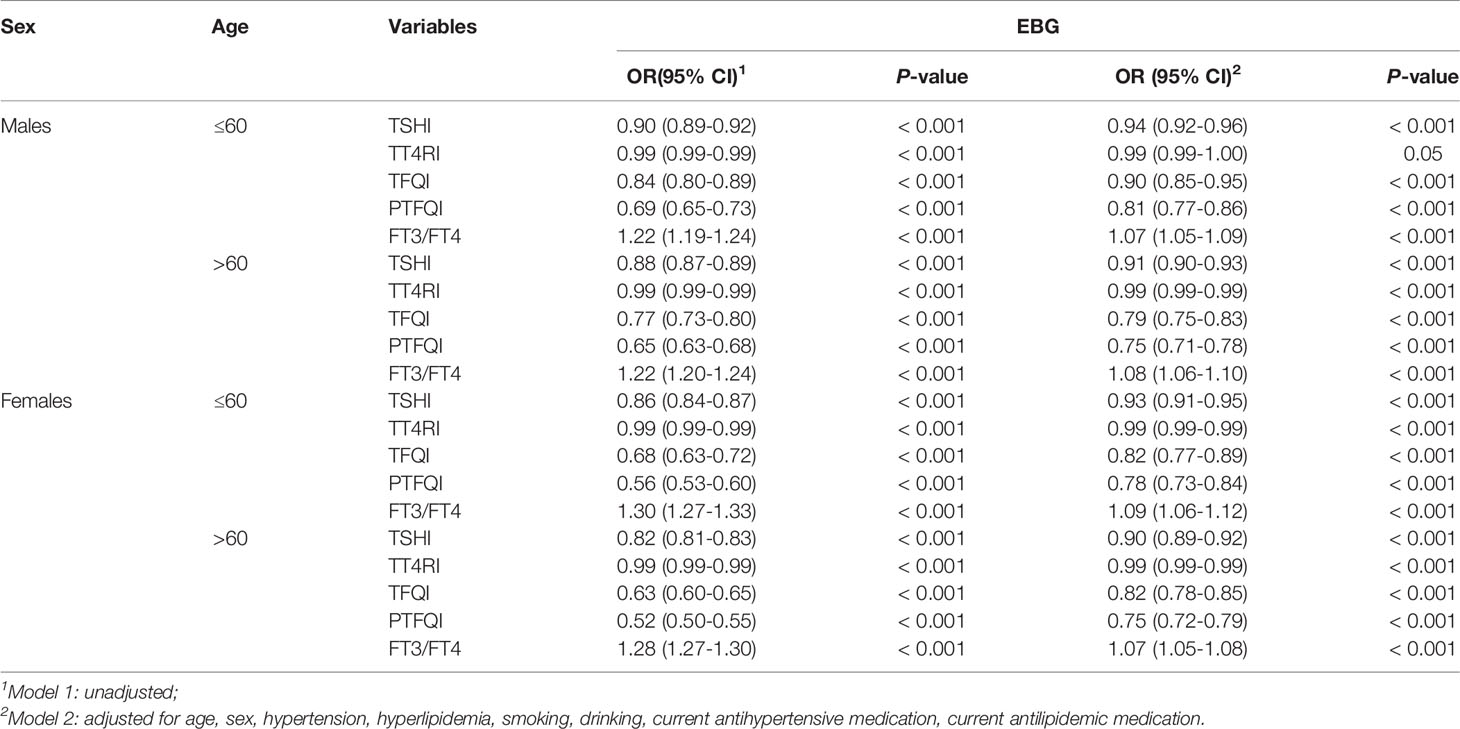
Table 5 Relationship between thyroid hormone sensitivity and EBG in different sexes and different ages.
Discussion
To our knowledge, this is the first study to evaluate and confirm the relationship between central and peripheral sensitivity to thyroid hormone indicators and EBG risk in a large sample of patients in China with CHD. Our study found that the central thyroid hormone sensitivity indices TSHI, TT4RI, TFQI, and PTFQI were negatively associated with EBG risk in patients with CHD. With a gradual increase in TSHI, TT4RI, and PTFQI, the OR value of EBG also gradually decreased. Peripheral thyroid hormone sensitivity index FT3/FT4 was positively associated with EBG. Finally, most associations were observed in different sexes and age groups when considered separately.
Previous studies have shown that almost two-thirds of patients with cardiovascular disease suffer from abnormal glucose metabolism (30). Due to the various changes of thyroid hormones in patients with CHD (31), the various effects of thyroid hormones in patients with CHD and EBG deserve attention. However, previous studies showed that higher TSH and lower FT4 (16, 32, 33), lower TSH (34), higher FT4 (35), and lower FT3 (16, 34) levels were all associated with the risk of EBG. Therefore, TSH or thyroid hormone levels alone may not be sufficient to explain the relationship between the thyroid system and glycaemic disorders. Given these inconsistencies in previously proposed central thyroid hormone sensitivity indices (TSHI, TT4RI) and peripheral thyroid hormone sensitivity indices FT3/FT4 (28, 36–38), in 2019, Laclaustra et al. proposed a new resistance index of central thyroid hormones: TFQI and PTFQI, which approximates TFQI (29). These new indices may have smaller deviations and will not produce extreme values in cases of thyroid dysfunction, which will help to better explain the different associations between the changes in thyroid hormones and diabetes (29).
Based on the new indices, recent studies have found that the increase in TSHI, TT4RI, and PTFQI was associated with reduced prediabetes risk, and the increased FT3/FT4 ratio was associated with an increased risk of prediabetes (23). Unlike our study, the latter had no significant correlation after adjusting for multiple confounding factors. Moreover, our results also suggest that elevated TFQI is associated with a reduced risk of EBG. Laclaustra et al. reported that the cross-section of the TSHI level was not associated with diabetes (29), which is contrary to our results and conclusion. The reasons for these differences are not clear, but may be attributable to confounding factors, differences in study partitions, and sample sizes. Therefore, further studies are needed to validate and confirm these results.
Several pathways may explain the observed association between the thyroid hormone central resistance index and EBG. Previous studies have shown that thyroid dysfunction can increase insulin resistance in muscle and adipose tissue and reduce glucose transport in muscle cells (39, 40). FT3 may also affect the expression of glucose-secreted insulin and an important protein of lipid metabolism (41); lower FT3 and FT4 levels can promote higher insulin resistance in tissues (42). The change in serum TSH may directly affect metabolic parameters and stimulate leptin secretion (43, 44). It is well known that hepatic glucose output is critical for maintaining fasting glucose homeostasis. Leptin has been shown to stimulate hepatic glucose production in vivo and in vitro (45). Moreover, the loss of leptin can lead to problems such as overeating, decreased energy expenditure, and severe obesity, which are important risk factors affecting glucose homeostasis (46). Therefore, the sensitivity of central thyroid hormones may change leptin secretion, affect insulin resistance, and lead to a change in glucose metabolism level. However, the exact regulatory mechanism underlying the relationship between central thyroid hormone sensitivity and leptin remains unclear.
Interestingly, our study also found that when used as a reference in the T1 group, the level of peripheral thyroid hormone sensitivity FT3/FT4 was negatively associated with EBG in the T2 group, but significantly positively associated with EBG when used as a continuous variable. Previously, many studies confirmed the positive effects of elevated FT3/FT4 on diabetes, gestational diabetes, obesity-related inflammatory markers, cardiovascular risk, and arterial stiffness markers (38, 47–49). Regarding the result of the negative correlation of FT3/FT4 with EBG in the T2 group, the promotion of peripheral deiodinase activity may increase the level of FT3/FT4 (38), and the inhibition of peripheral deiodinase activity may reduce the basal metabolic rate, which is closely related to the pathogenesis of diabetes (50, 51).
To address sex- and age-specific differences noted in previous studies (35, 52, 53), we analysed the relationship between thyroid hormone sensitivity and diabetes by sex and age, respectively. The results showed that TSHI, PTFQI, and TFQI of females aged > 60 years had lower OR values for EBG risk, while FT3/FT4 had higher OR values. Sex hormones (such as oestrogen and testosterone) can regulate thyroid function, and oestrogen levels affect the development of diabetes, especially after 60 years of age (54–56). The differences in sex hormones may partly explain the sex differences in the relationship between thyroid hormone sensitivity and EBG found in this study. However, since this study did not measure the levels of sex hormones, further research is needed to explore this concept.
Limitations
There are several limitations to the present study. First, although large scale, this was a cross-sectional study, and causality cannot be inferred. However, the study supports the important hypothesis that adding the examination of thyroid hormone sensitivity levels to the examination of pure thyroid hormone levels may be helpful in assessing the risk of EBG. Second, although we have adjusted for many potential confounding factors, we cannot rule out the possibility that EBG is affected by other lifestyle variables, including iodine supplementation, which is intrinsically related to thyroid hormone levels. Third, this study was conducted among individuals who were Chinese, and racial differences may exist. Fourth, this study did not have a track record of whether participants were undergoing diabetes or thyroid disease-specific treatment. Therefore, well-designed randomized controlled trials are needed to validate these results.
Conclusion
The decrease in central thyroid hormone indices represents an increase in central thyroid hormone sensitivity. This study showed that thyroid hormone sensitivity is significantly associated with EBG in patients with CHD. This association is higher in females than in males, and the association in those aged over 60 years is higher than that in patients aged 60 years and below. This study provides reliable evidence which will improve the prevention strategies and clinical treatment of EBG in patients with CHD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the ethics committee of the Tianjin University of Traditional Chinese Medicine (approval number TJUTCM-EC20190008) and registered with the Chinese Clinical Trial Registry on July 14, 2019 (registration number ChiCTR-1900024535) and in ClinicalTrials.gov on July 18, 2019 (registration number NCT04026724). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CY, LL and QX were responsible for the study protocol and statistical analysis. LY and ZL analysed the data together and drafted the article. RY, GP, QC, YH, YL, FL, MM, TY, YW, JS conducted data collection. YZ and SG revised the article critically. All authors revised the article for important intellectual content and approved the article. The authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Basic Research Program of China (973 project, grant numbers: 2014CB542902).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the participants in the study and the members of the survey teams, as well as the financial support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.895843/full#supplementary-material
Abbreviations
CHD, coronary heart disease; CI, confidence interval; DBP, diastolic blood pressure; EBG, elevated blood glucose; FBG, fasting blood glucose; FT3, free triiodothyronine; FT4, free thyroxine; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NGT, normal glucose tolerance; OR, odds ratio; SBP, Systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglyceride; TH, thyroid hormone; TSH, thyroid stimulating hormone.
References
1. WHO. Cardiovascular Diseases (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
2. Xing DM, Zhu MJ, Liu CX, Wang H. Outcome Measures in Clinical Trials of Traditional Chinese Medicine for Stable Angina Pectoris. Acupunct Herb Med (2021) 1(2):99–106. doi: 10.1097/HM9.0000000000000014
3. Ohman EM. CLINICAL PRACTICE. Chronic Stable Angina. N Engl J Med (2016) 374(12):1167–76. doi: 10.1056/NEJMcp1502240
4. Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The Epidemic of the 20(Th) Century: Coronary Heart Disease. Am J Med (2014) 127(9):807–12. doi: 10.1016/j.amjmed.2014.04.015
5. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Thyroid Studies Collaboration. Subclinical Hypothyroidism and the Risk of Coronary Heart Disease and Mortality. JAMA (2010) 304(12):1365–74. doi: 10.1001/jama.2010.1361
6. Onat A, Aydın M, Can G, Çelik E, Altay S, Karagöz A, et al. Normal Thyroid-Stimulating Hormone Levels, Autoimmune Activation, and Coronary Heart Disease Risk. Endocrine (2015) 48(1):218–26. doi: 10.1007/s12020-014-0269-z
7. Floriani C, Gencer B, Collet TH, Rodondi N. Subclinical Thyroid Dysfunction and Cardiovascular Diseases: 2016 Update. Eur Heart J (2018) 39(7):503–7. doi: 10.1093/eurheartj/ehx050
8. Wang WY, Tang YD, Yang M, Cui C, Mu M, Qian J, et al. Free Triiodothyronine Level Indicates the Degree of Myocardial Injury in Patients With Acute ST-Elevation Myocardial Infarction. Chin Med J (Engl) (2013) 126(20):3926–9. doi: 10.3760/cma.j.issn.0366-6999.20130087
9. Miura S, Iitaka M, Suzuki S, Fukasawa N, Kitahama S, Kawakami Y, et al. Decrease in Serum Levels of Thyroid Hormone in Patients With Coronary Heart Disease. Endocr J (1996) 43(6):657–63. doi: 10.1507/endocrj.43.657
10. Völz S, Svedlund S, Andersson B, Li-Ming G, Rundqvist B. Coronary Flow Reserve in Patients With Resistant Hypertension. Clin Res Cardiol (2017) 106(2):151–7. doi: 10.1007/s00392-016-1043-4
11. Tripolt NJ, Aberer F, Riedl R, Hutz B, Url J, Dimsity G, et al. The Effects of Linagliptin on Endothelial Function and Global Arginine Bioavailability Ratio in Coronary Artery Disease Patients With Early Diabetes: Study Protocol for a Randomized Controlled Trial. Trials (2016) 17(1):495. doi: 10.1186/s13063-016-1627-3
12. Ida S, Kaneko R, Imataka K, Murata K. Relationship Between Frailty and Mortality, Hospitalization, and Cardiovascular Diseases in Diabetes: A Systematic Review and Meta-Analysis. Cardiovasc Diabetol (2019) 18(1):81. doi: 10.1186/s12933-019-0885-2
13. Younis A, Younis A, Tzur B, Peled Y, Shlomo N, Goldenberg I, et al. Metabolic Syndrome Is Independently Associated With Increased 20-Year Mortality in Patients With Stable Coronary Artery Disease. Cardiovasc Diabetol (2016) 15(1):149. doi: 10.1186/s12933-016-0466-6
14. Younis A, Goldkorn R, Goldenberg I, Geva D, Tzur B, Mazu A, et al. Impaired Fasting Glucose Is the Major Determinant of the 20-Year Mortality Risk Associated With Metabolic Syndrome in Nondiabetic Patients With Stable Coronary Artery Disease. J Am Heart Assoc (2017) 6(11):e006609. doi: 10.1161/JAHA.117.006609
15. Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism Is a Risk Factor for New-Onset Diabetes: A Cohort Study. Diabetes Care (2015) 38(9):1657–64. doi: 10.2337/dc14-2515
16. Jun JE, Jee JH, Bae JC, Jin SM, Hur KY, Lee MK, et al. Association Between Changes in Thyroid Hormones and Incident Type 2 Diabetes: A Seven-Year Longitudinal Study. Thyroid (2017) 27(1):29–38. doi: 10.1089/thy.2016.0171
17. de Vries TI, Kappelle LJ, van der Graaf Y, de Valk HW, de Borst GJ, Nathoe HM, et al. Thyroid-Stimulating Hormone Levels in the Normal Range and Incident Type 2 Diabetes Mellitus. Acta Diabetol (2019) 56(4):431–40. doi: 10.1007/s00592-018-1231-y
18. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. China National Diabetes and Metabolic Disorders Study Group. Prevalence of Diabetes Among Males and Females in China. N Engl J Med (2010) 362(12):1090–101. doi: 10.1056/NEJMoa0908292
19. Tian H, Song G, Xie H, Zhang H, Tuomilehto J, Hu G. Prevalence of Diabetes and Impaired Fasting Glucose Among 769,792 Rural Chinese Adults. Diabetes Res Clin Pract (2009) 84(3):273–8. doi: 10.1016/j.diabres.2009.03.015
20. Biondi B, Kahaly GJ, Robertson RP. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr Rev (2019) 40(3):789–824. doi: 10.1210/er.2018-00163
21. Li Z, Cheng Q, Liu Y, Cheng X, Wang S, He Y, et al. Low-/High-Density Lipoprotein Cholesterol Ratio and Carotid Plaques in Patients With Coronary Heart Disease: A Chinese Cohort Study. Lipids Health Dis (2021) 20(1):144. doi: 10.1186/s12944-021-01575-w
22. Lenfant C, Chobanian AV, Jones DW, Roccella EJ, Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): Resetting the Hypertension Sails. Hypertension (2003) 41(6):1178–9. doi: 10.1161/01.HYP.0000075790.33892.AE
23. Liu B, Wang Z, Fu J, Guan H, Lyu Z, Wang W. Sensitivity to Thyroid Hormones and Risk of Prediabetes: A Cross-Sectional Study. Front Endocrinol (Lausanne) (2021) 12:657114. doi: 10.3389/fendo.2021.657114
24. American Diabetes Association. 2. Classification and Diagnosis of Diabetes:Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S15–33. doi: 10.2337/dc21-S002
25. Ongosi AN, Wilunda C, Musumari PM, Techasrivichien T, Wang CW, Ono-Kihara M, et al. Prevalence and Risk Factors of Elevated Blood Pressure and Elevated Blood Glucose Among Residents of Kajiado County, Kenya: A Population-Based Cross-Sectional Survey. Int J Environ Res Public Health (2020) 17(19):6957. doi: 10.3390/ijerph17196957
26. Fu L, Deng H, Lin WD, He SF, Liu FZ, Liu Y, et al. Association Between Elevated Blood Glucose Level and Non-Valvular Atrial Fibrillation: A Report From the Guangzhou Heart Study. BMC Cardiovasc Disord (2019) 19(1):270. doi: 10.1186/s12872-019-1253-6
27. Cappelli C, Rotondi M, Pirola I, Agosti B, Gandossi E, Valentini U, et al. TSH-Lowering Effect of Metformin in Type 2 Diabetic Patients: Differences Between Euthyroid, Untreated Hypothyroid, and Euthyroid on L-T4 Therapy Patients. Diabetes Care (2009) 32(9):1589–90. doi: 10.2337/dc09-0273
28. Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S. Resistance to Thyroid Hormone Caused by Two Mutant Thyroid Hormone Receptors Beta, R243Q and R243W, With Marked Impairment of Function That Cannot Be Explained by Altered In Vitro 3,5,3'-Triiodothyroinine Binding Affinity. J Clin Endocrinol Metab (1997) 82(5):1608–14. doi: 10.1210/jcem.82.5.3945
29. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, Mateo-Gallego R, Casasnovas JA, Guallar-Castillon P, et al. Impaired Sensitivity to Thyroid Hormones Is Associated With Diabetes and Metabolic Syndrome. Diabetes Care (2019) 42(2):303–10. doi: 10.2337/dc18-1410
30. Henareh L, Agewall S. 2-H Postchallenge Plasma Glucose Predicts Cardiovascular Events in Patients With Myocardial Infarction Without Known Diabetes Mellitus. Cardiovasc Diabetol (2012) 11:93. doi: 10.1186/1475-2840-11-93
31. Ertugrul O, Ahmet U, Asim E, Gulcin HE, Burak A, Murat A, et al. Prevalence of Subclinical Hypothyroidism Among Patients With Acute Myocardial Infarction. ISRN Endocrinol (2011) 2011:810251. doi: 10.1186/1475-2840-11-93
32. Chaker L, Ligthart S, Korevaar TI, Hofman A, Franco OH, Peeters RP, et al. Thyroid Function and Risk of Type 2 Diabetes: A Population-Based Prospective Cohort Study. BMC Med (2016) 14(1):150. doi: 10.1186/s12916-016-0693-4
33. Chaker L, Cappola AR, Mooijaart SP, Peeters RP. Clinical Aspects of Thyroid Function During Ageing. Lancet Diabetes Endocrinol (2018) 6(9):733–42. doi: 10.1016/S2213-8587(18)30028-7
34. Taneichi H, Sasai T, Ohara M, Honma H, Nagasawa K, Takahashi T, et al. Higher Serum Free Triiodothyronine Levels Within the Normal Range are Associated With Metabolic Syndrome Components in Type 2 Diabetic Subjects With Euthyroidism. Tohoku J Exp Med (2011) 224(3):173–8. doi: 10.1620/tjem.224.173
35. Gu Y, Li H, Bao X, Zhang Q, Liu L, Meng G, et al. The Relationship Between Thyroid Function and the Prevalence of Type 2 Diabetes Mellitus in Euthyroid Subjects. J Clin Endocrinol Metab (2017) 102(2):434–42. doi: 10.1210/jc.2016-2965
36. Jostel A, Ryder WD, Shalet SM. The Use of Thyroid Function Tests in the Diagnosis of Hypopituitarism: Definition and Evaluation of the TSH Index. Clin Endocrinol (Oxf) (2009) 71(4):529–34. doi: 10.1111/j.1365-2265.2009.03534.x
37. Park SY, Park SE, Jung SW, Jin HS, Park IB, Ahn SV, et al. Free Triiodothyronine/Free Thyroxine Ratio Rather Than Thyrotropin Is More Associated With Metabolic Parameters in Healthy Euthyroid Adult Subjects. Clin Endocrinol (Oxf) (2017) 87(1):87–96. doi: 10.1111/cen.13345
38. Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, et al. Triiodothyronine and Free Thyroxine Levels are Differentially Associated With Metabolic Profile and Adiposity-Related Cardiovascular Risk Markers in Euthyroid Middle-Aged Subjects. Thyroid (2014) 24(2):223–31. doi: 10.1089/thy.2013.0314
39. Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB, et al. Insulin Action in Adipose Tissue and Muscle in Hypothyroidism. J Clin Endocrinol Metab (2006) 91(12):4930–7. doi: 10.1210/jc.2006-0478
40. Maratou E, Hadjidakis DJ, Kollias A, Tsegka K, Peppa M, Alevizaki M, et al. Studies of Insulin Resistance in Patients With Clinical and Subclinical Hypothyroidism. Eur J Endocrinol (2009) 160(5):785–90. doi: 10.1530/EJE-08-0797
41. Jansen MS, Cook GA, Song S, Park EA. Thyroid Hormone Regulates Carnitine Palmitoyltransferase Ialpha Gene Expression Through Elements in the Promoter and First Intron. J Biol Chem (2000) 275(45):34989–97. doi: 10.1074/jbc.M001752200
42. Yang N, Yao Z, Miao L, Liu J, Gao X, Fan H, et al. Novel Clinical Evidence of an Association Between Homocysteine and Insulin Resistance in Patients With Hypothyroidism or Subclinical Hypothyroidism. PloS One (2015) 10(5):e0125922. doi: 10.1371/journal.pone.0125922
43. Jun JE, Jin SM, Jee JH, Bae JC, Hur KY, Lee MK, et al. TSH Increment and the Risk of Incident Type 2 Diabetes Mellitus in Euthyroid Subjects. Endocrine (2017) 55(3):944–53. doi: 10.1007/s12020-016-1221-1
44. Duntas LH, Biondi B. The Interconnections Between Obesity, Thyroid Function, and Autoimmunity: The Multifold Role of Leptin. Thyroid (2013) 23(6):646–53. doi: 10.1089/thy.2011.0499
45. Denroche HC, Huynh FK, Kieffer TJ. The Role of Leptin in Glucose Homeostasis. J Diabetes Investig (2012) 3(2):115–29. doi: 10.1111/j.2040-1124.2012.00203.x
46. Chiu FH, Chuang CH, Li WC, Weng YM, Fann WC, Lo HY, et al. The Association of Leptin and C-Reactive Protein With the Cardiovascular Risk Factors and Metabolic Syndrome Score in Taiwanese Adults. Cardiovasc Diabetol (2012) 11:40. doi: 10.1186/1475-2840-11-40
47. Wang Y, Sun F, Wu P, Huang Y, Ye Y, Yang X, et al. A Prospective Study of Early-Pregnancy Thyroid Markers, Lipid Species, and Risk of Gestational Diabetes Mellitus. J Clin Endocrinol Metab (2022) 107(2):e804–14. doi: 10.1210/clinem/dgab637
48. Qin K, Zhang F, Wu Q, Liu Z, Huang Y, Tan J, et al. Thyroid Hormone Changes in Euthyroid Patients With Diabetes. Diabetes Metab Syndr Obes (2020) 13:2533–40. doi: 10.2147/DMSO.S260039
49. Rawal S, Tsai MY, Hinkle SN, Zhu Y, Bao W, Lin Y, et al. A Longitudinal Study of Thyroid Markers Across Pregnancy and the Risk of Gestational Diabetes. J Clin Endocrinol Metab (2018) 103(7):2447–56. doi: 10.1210/jc.2017-02442
50. Köhrle J. Thyroid Hormone Transporters in Health and Disease: Advances in Thyroid Hormone Deiodination. Best Pract Res Clin Endocrinol Metab (2007) 21(2):173–91. doi: 10.1016/j.beem.2007.04.001
51. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. Cellular and Molecular Basis of Deiodinase-Regulated Thyroid Hormone Signaling. Endocr Rev (2008) 29(7):898–938. doi: 10.1210/er.2008-0019
52. Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function. PloS Genet (2013) 9(2):e1003266. doi: 10.1371/journal.pgen.1003266
53. Krag MØ, Hasselbalch L, Siersma V, Nielsen AB, Reventlow S, Malterud K, et al. The Impact of Gender on the Long-Term Morbidity and Mortality of Patients With Type 2 Diabetes Receiving Structured Personal Care: A 13 Year Follow-Up Study. Diabetologia (2016) 59(2):275–85. doi: 10.1007/s00125-015-3804-4
54. Cho GJ, Lee JH, Park HT, Shin JH, Hong SC, Kim T, et al. Postmenopausal Status According to Years Since Menopause as an Independent Risk Factor for the Metabolic Syndrome. Menopause (2008) 15(3):524–9. doi: 10.1097/gme.0b013e3181559860
55. Boucai L, Hollowell JG, Surks MI. An Approach for Development of Age-, Gender-, and Ethnicity-Specific Thyrotropin Reference Limits. Thyroid (2011) 21(1):5–11. doi: 10.1089/thy.2010.0092
Keywords: elevated blood glucose, coronary heart disease, thyroid hormone sensitivity, diabetes, prediabetes, resistance to thyroid hormones
Citation: Yu L, Li Z, Yang R, Pan G, Cheng Q, He Y, Liu Y, Liu F, Ma M, Yang T, Wang Y, Su J, Zheng Y, Gao S, Xu Q, Li L and Yu C (2022) Impaired Sensitivity to Thyroid Hormones Is Associated With Elevated Blood Glucose in Coronary Heart Disease. Front. Endocrinol. 13:895843. doi: 10.3389/fendo.2022.895843
Received: 14 March 2022; Accepted: 28 April 2022;
Published: 15 June 2022.
Edited by:
Camilla Virili, Sapienza University of Rome, ItalyReviewed by:
Alessandro P. Delitala, Azienda Ospedaliero Universitaria Sassari, ItalyAnthony Martin Gerdes, New York Institute of Technology, United States
Copyright © 2022 Yu, Li, Yang, Pan, Cheng, He, Liu, Liu, Ma, Yang, Wang, Su, Zheng, Gao, Xu, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunquan Yu, eWNxdGp1dGNtQGZveG1haWwuY29t; Lin Li, bGxiaWFuamlAMTYzLmNvbQ==; Qiang Xu, dGNteHVxaWFuZ0Bob3RtYWlsLmNvbQ==
†These authors share first authorship
 Lu Yu1†
Lu Yu1† Shan Gao
Shan Gao Chunquan Yu
Chunquan Yu