
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 27 July 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.893506
This article is part of the Research Topic Fertility Preservation: From Next-generation Technologies to Clinical Practice View all 11 articles
Objective: We aimed to evaluate the future outcomes of patients undergoing their first IVF (in vitro fertilization) attempt with no oocyte retrieved, no normal zygotes formed, or no embryos available for transfer and to identify factors affecting the live birth rate.
Methods: Patients who underwent no transplantable embryo in their first IVF cycles but carried out several consecutive cycles between January 2012 to December 2020 were retrospectively enrolled and divided into three groups:group A (no egg retrieval), group B (no normal zygotes formed), and group C (no embryos available to transfer). The patients were also divided into the live birth group and non-live birth group according to whether they got a live baby or not. The clinical data and the cumulative clinical outcomes of groups were compared.
Results: 496 patients met the inclusion criteria and enrolled, with 121 patients with no oocytes retrieved in group A, 138 patients with no normal zygotes formed in group B, and 237 patients with no embryos available to transfer in group C. The age [(34.75(5.82) vs 31.91(5.31), P<0.001; 34.75(5.82) vs 32.25(5.72), P<0.001)] and baseline FSH level [(13.04(8.82) vs 10.52(7.39), P=0.005; 13.04(8.82) vs 9.91(5.95), P<0.001)] of women in group A were significantly higher than those in groups B and C. The stable cumulative live birth rate/patient of three groups achieved 18.18% (after 5 cycles, group A), 28.98% (after 3 cycles, group B) and 20.25% (after 7 cycles, group C). Moreover, the multivariate regression analysis showed that female age and basic FSH were main factors affecting live birth outcome of patients with no embryo transfer in their first IVF cycle attempts.
Conclusions: The future clinical outcome may be better in women with no normal zygotes than those with no oocyte retrieved or no available embryo at their first IVF cycle attempts. The main factors influencing the live birth are age and ovarian reserve.
Embryo transfer is a key step for successful pregnancy of women through assisted reproduction, but this process may face cycle cancellation because of no oocyte retrieved, no normal zygote formed, or no available embryos. Considering the inherent poor outcomes, most studies have excluded patients with cycle cancellation at the beginning of research (1–3). The ESPART study reported the prevalence of cycle cancellation was 4.7% in poor responders (4), and another survey showed the risk of cycle cancellation caused by poor ovarian response was about 20% (5). However, the prevalence in the general population receiving in vitro fertilization (IVF) is obscure. A French study examined medical factors associated with early cessation of IVF in 5135 couples and found that couples who did undergo no embryo transfer during the first IVF cycle attempt were more likely to stop treatment early (6). Other studies (7–10) have found that the psychological burden of failure in non-pregnancy treatment is the reason for withdrawing from further treatment. There are currently few reports on the clinical outcome of follow-up treatment in these patients, but it is necessary to provide these patients with information about the final clinical outcome in all institutions carrying out the artificial reproduction technology (ART).
Based on the above observational studies and to answer the consultation of patients with no transplantable embryo in their first IVF cycle attempt, this study was performed to investigate the outcome of future fertility of patients undergoing their first IVF cycle attempt with no embryos transplanted as well as to identify factors that might affect the possibility to get a baby in subsequent IVF cycle attempt.
The retrospective study was performed in consecutive women attending the IVF procedure in our hospital from January 2012 to December 2020. Inclusion criteria were women who wanted more cycle attempts after their first IVF cycles had been cancelled for some reasons even if they had reached the oocyte pick-up (OPU) stage and had undergone egg retrieval in their first IVF cycle. The first delivery was used as the end point of the study. Exclusion criteria include: (i) chromosomal abnormalities in either their spouse or pre-implantation genetic testing cycles; (ii) patients who did not continue the IVF cycle after the first cancelled cycle; (iii) patients who had no clinical outcome at the end of follow-up. This study was approved by the ethics committee of our hospital, and all patients had signed the informed consent to participate (Figure 1).
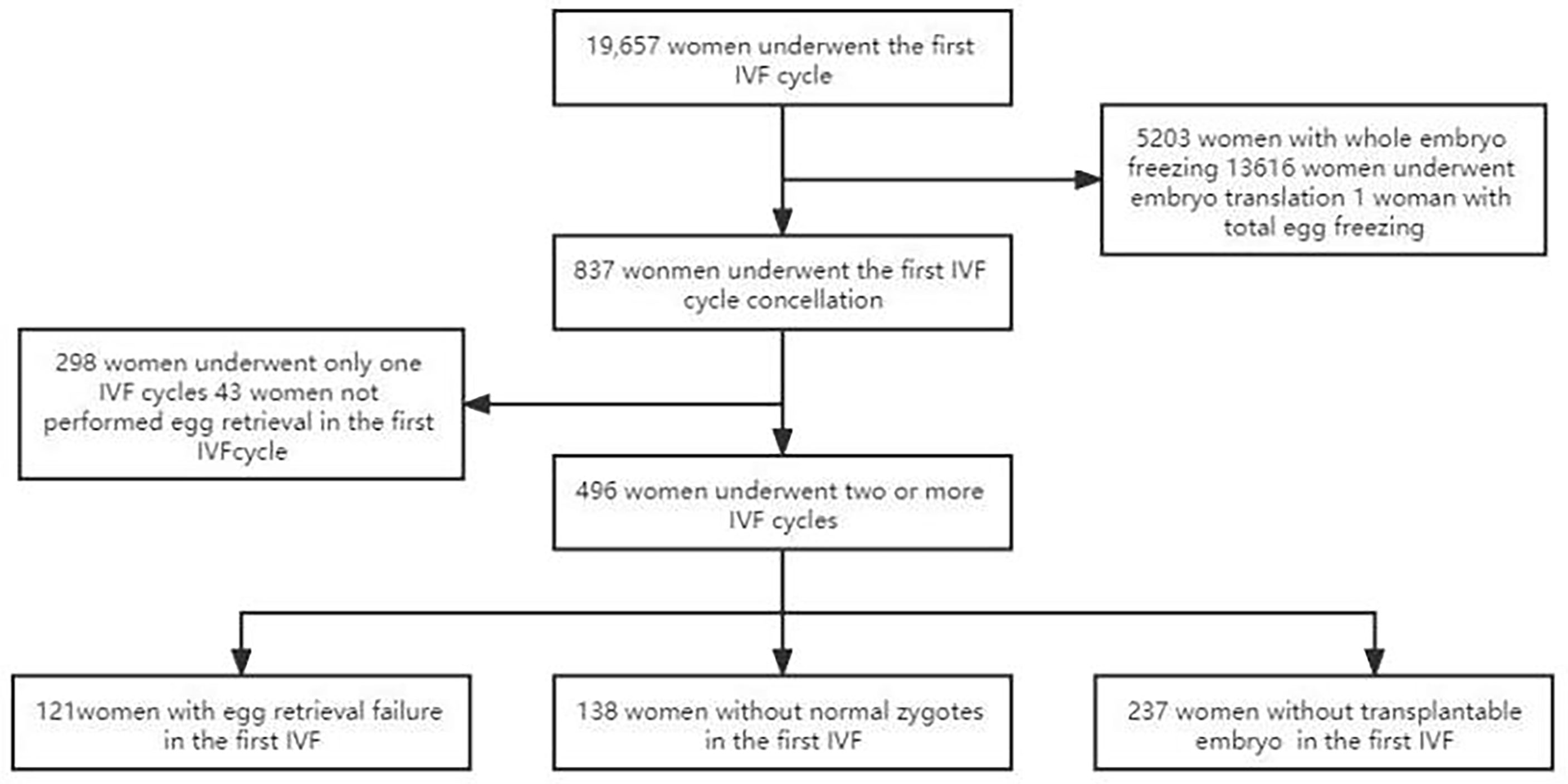
Figure 1 Flowchart of patient selection. A total of 19,657 couples underwent IVF cycle with oocytes to pick up, and 837 patients had cycle cancellation in their first IVF cycle attempts. 298 women underwent only one IVF cycle, of which 50 women were with no egg retrieved, 48 women with no normal zygotes formed and 200 women with the cancellation cycle due to poor embryo quality. 496 women met the study inclusion criteria and were enrolled.
The stimulation protocol was performed according to our center’s guidelines and was determined by the treating clinician based on individualized conditions. The stimulation protocol in fresh cycles included GnRH-a long protocol (luteal phase short-acting GnRH-a long protocol), short-acting GnRH-a protocol, GnRH-ant protocol (GnRH antagonist protocol), EFLL protocol (early-follicular phase long-acting GnRH agonist long protocol), PPOS protocol (progestin-primed ovarian stimulation protocol), and milder ovarian stimulation protocol. Different protocles may be used in the same person in different cycles.
Follicle growth and hormone levels were continuously monitored by ultrasound and blood tests. Human chorionic gonadotropin (hCG) was injected when the largest follicle diameter was bigger than 18 mm or the diameter of at least three follicles was bigger than 17 mm during the fresh cycle, and the oocytes were collected 36-38h after hCG injection. The oocytes were inseminated through conventional IVF/ICSI (intracytoplasmic sperm injection), and fertilization was observed 16-18h after insemination. Seventy-two hours after oocyte retrieval, whether to transplant or to freeze the embryo was based on embryo grading and the individual clinical situation of each patient. Blood β-hCG (+) was measured 14d after transfer as biochemical pregnancy, and clinical pregnancy was determined by ultrasonography at 28-30d when a gestational sac and primordial ventricular pulsation were observed.
The ASEBIR scoring criteria was used to assess the embryo at the cleavage stage (11). Embryos were classified into grades I-IV according to the number of blastomeres on day 3, proportion of fragmentation, uniformity of blastomeres, multinucleation, number of vacuoles, and normalness of the zona pellucida. The criteria for good quality embryos were 2PN (2 pronucleus) origin, 7-9 cells, fragmentation <10%, and basic homogeneity of the cleavage. Non-transferable embryos were defined as embryos with grade IV with no fused embryos formed in further culture at Day3; non-insemination was defined as no MII (mature) eggs 2 hours after degranulation; non-fertilization was defined as no normal progenitor nuclei observed after IVF fertilization with no oocyte cleavage. Group B (no normal zygote formation group) included non-fertilization and no normal progenitor nuclei.
Patients were divided into three groups depending on the cause for the cancellation in their first IVF cycle: group A (no egg retrieval group), group B (no normal zygote formation group) including patients with non-insemination and non-fertilization, and group C (no embryos available to transfer). According to whether they had got a live baby or not, the patients were divided into live birth and non-live birth groups. The patients’ age, BMI (Body mass index), infertility years, infertility type, infertility factors (male or female), basic FSH or LH level, Gn (gonadotropin) dose and days, cumulative clinical pregnancy rate, and cumulative live birth rate of the three groups were compared. The Gn days, Gn dose, mean Oocytes/opu, total oocytes retrieved, average number of 2PN fertilization/opu, average number of transferred embryos/opu, cumulative clinical pregnancy rate/opu, cumulative clinical pregnancy rate/patient, https://fanyi.baidu.com/translate?aldtype=16047&query=%E7%AC%AC%E4%B8%80%E5%91%A8%E6%9C%9F%E6%82%A3%E8%80%85%E7%89%B9%E5%BE%81&keyfrom=baidu&smartresult=dict&lang=auto2zh - ##cumulative live birth rate/opu, https://fanyi.baidu.com/translate?aldtype=16047&query=%E7%AC%AC%E4%B8%80%E5%91%A8%E6%9C%9F%E6%82%A3%E8%80%85%E7%89%B9%E5%BE%81&keyfrom=baidu&smartresult=dict&lang=auto2zh - ##cumulative live birth rate/patient were recorded, respectively, among three groups according to the cycle rank, and the basic characteristics including age, BMI, basic FSH or LH level, Gn dose and days between the live birth and non-live birth groups in the first cycle were investigated.
The SPSS23.0 software (IBM, Chicago, IL, USA) was used for statistical analysis. Continuous variables were expressed as mean (standard deviation or SD) and categorical variable data were expressed as numbers and percentages. One-Way ANOVA was used to analyze the mean difference among groups, the Chi squre test was used to compare the classified data between groups, and the binary Logistic regression (Enter) was used to calculate the OR (odds ratio) value. The statistically significant difference was set at P<0.05.
During the study period, a total of 19,657 couples underwent the first IVF cycle and with oocytes being picked up, and 837 patients had cycle cancellation in their first IVF cycle attempts, resulting in a cancellation rate of 4.26% (837/19657). Among the 837 patients with cycle cancellation, 496 women who met the study inclusion criteria, with no embryo transfer, were enrolled to attend more cycles attempts. Among them, 121 cases of egg retrieval failure were assigned to group A, 138 cases who failed to form normal zygotes (including 83 cases of non-fertilization, 21 cases of abnormal fertilization, 33 cases of non-insemination, 1 case of non-cleavage) were assigned group B, and 237 without available embryo were assigned to group C. Baseline clinical characteristics and clinical outcomes of the three groups in the first IVF cycle are summarized in Table 1. Characteristics of the subsequent IVF cycles and the final reproductive outcomes stratified according to the IVF cycle rank between three groups are presented in Tables 2.1–2.3 and Figure 2. The characteristics of live birth and non-live birth groups are showed in Table 3, and the outcomes of multivariate regression analysis between the two groups are demonstrated in Table 4.
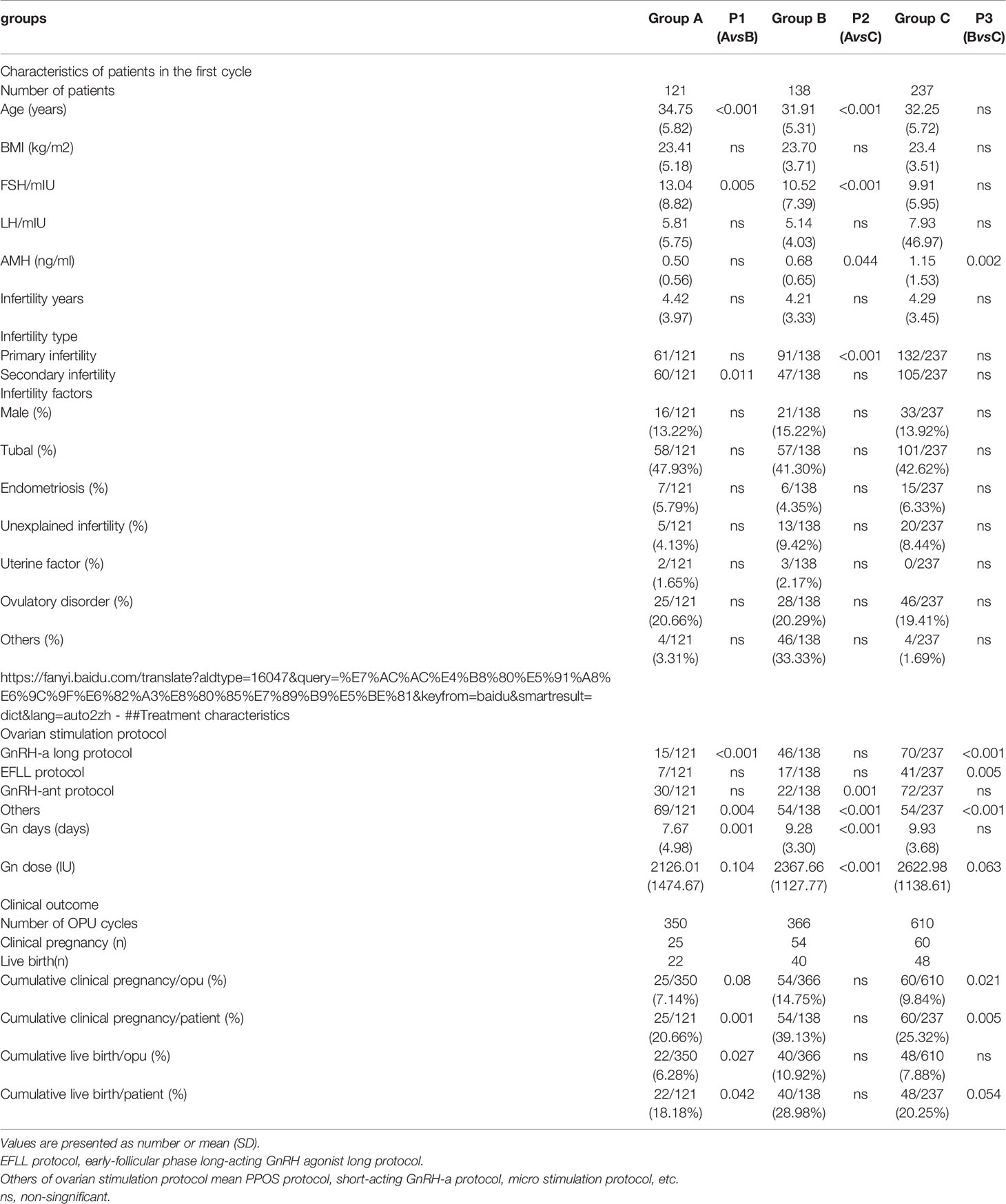
Table 1 Comparison of baseline values and final reproductive outcomes in the first IVF cycle among the three groups.
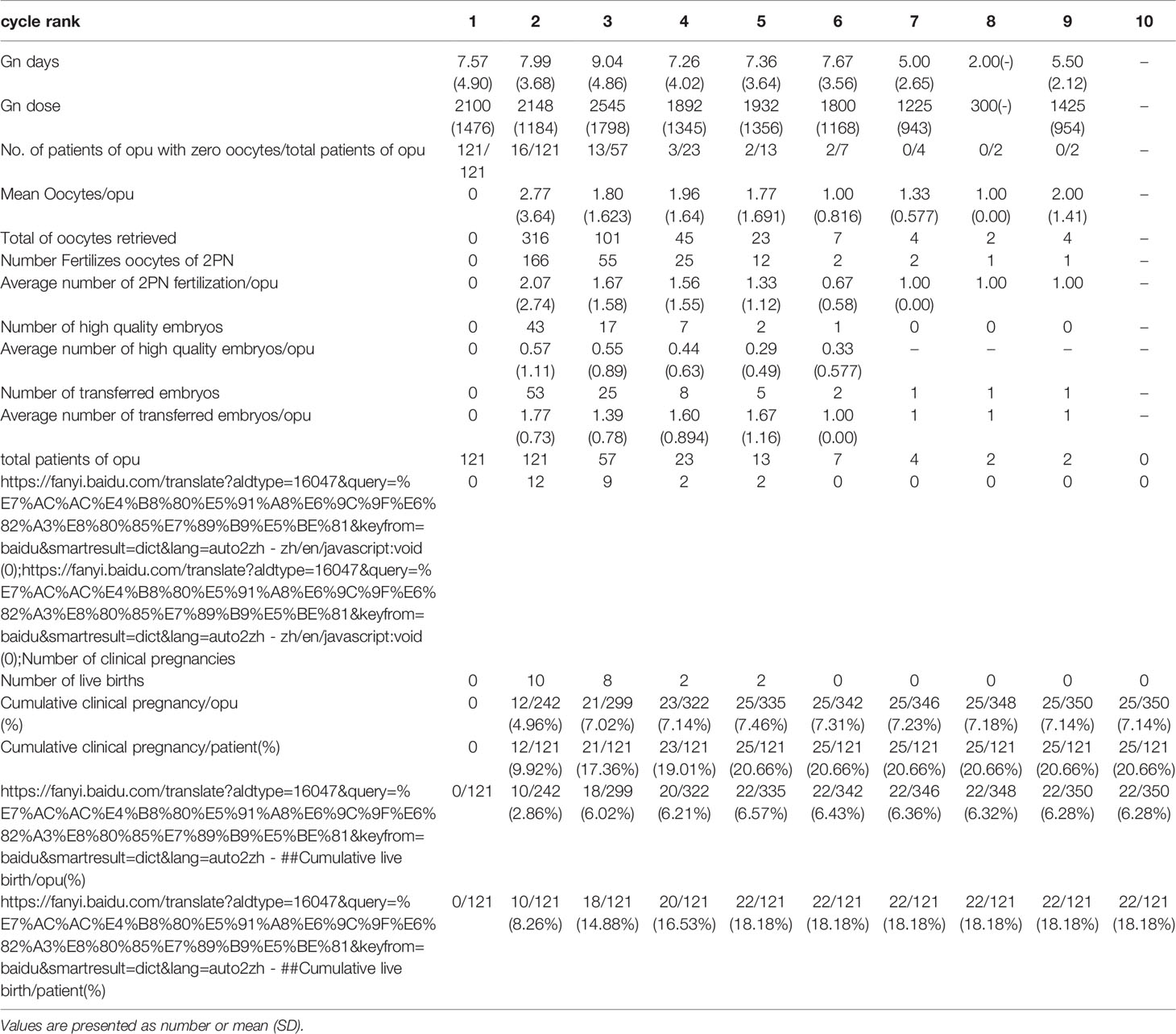
Table 2.1 IVF cycles’ characteristics and their final reproductive outcomes of group A stratified according to IVF cycle rank.
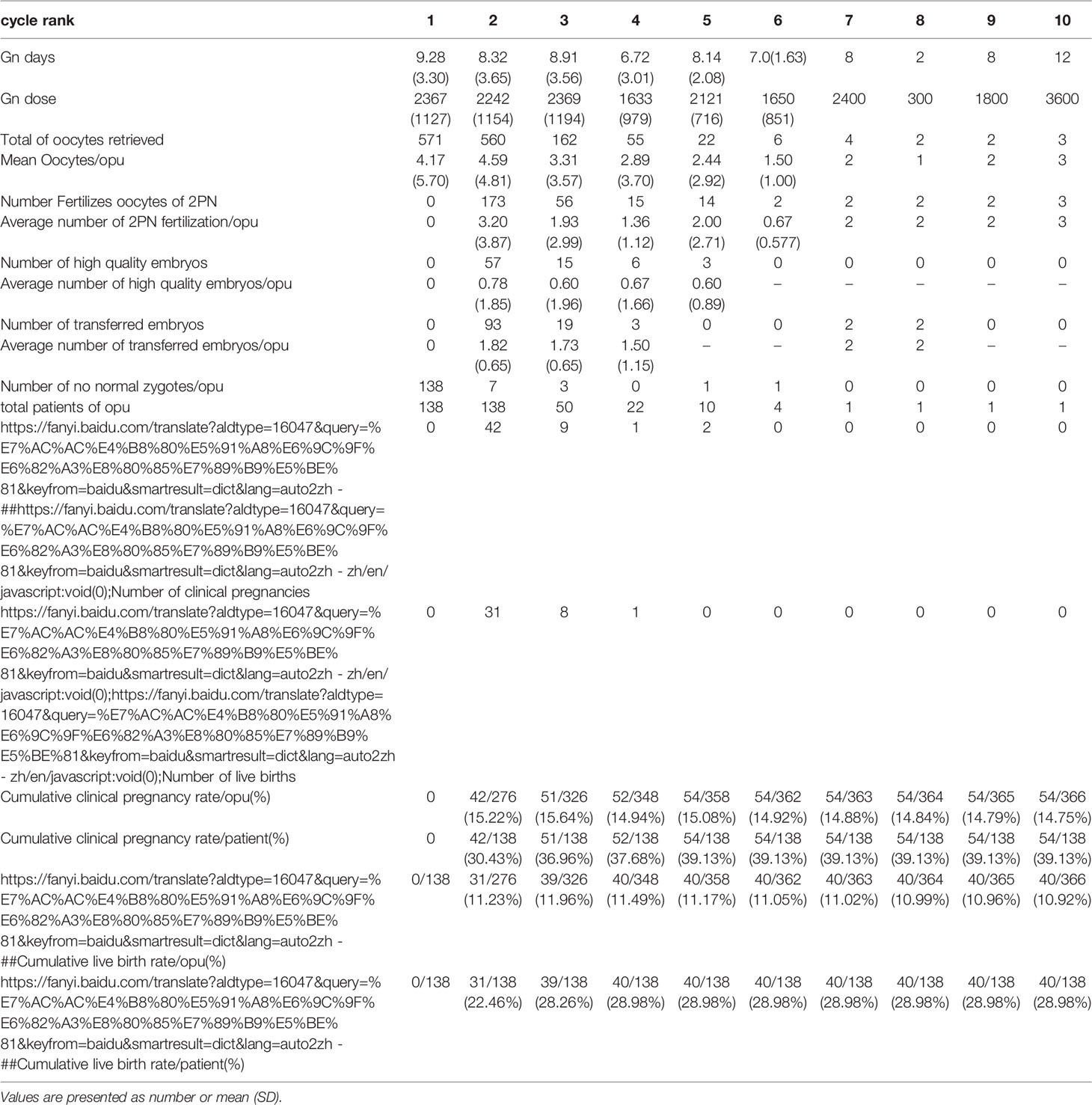
Table 2.2 IVF cycles’ characteristics and their final reproductive outcomes of group B stratified according to IVF cycle rank.
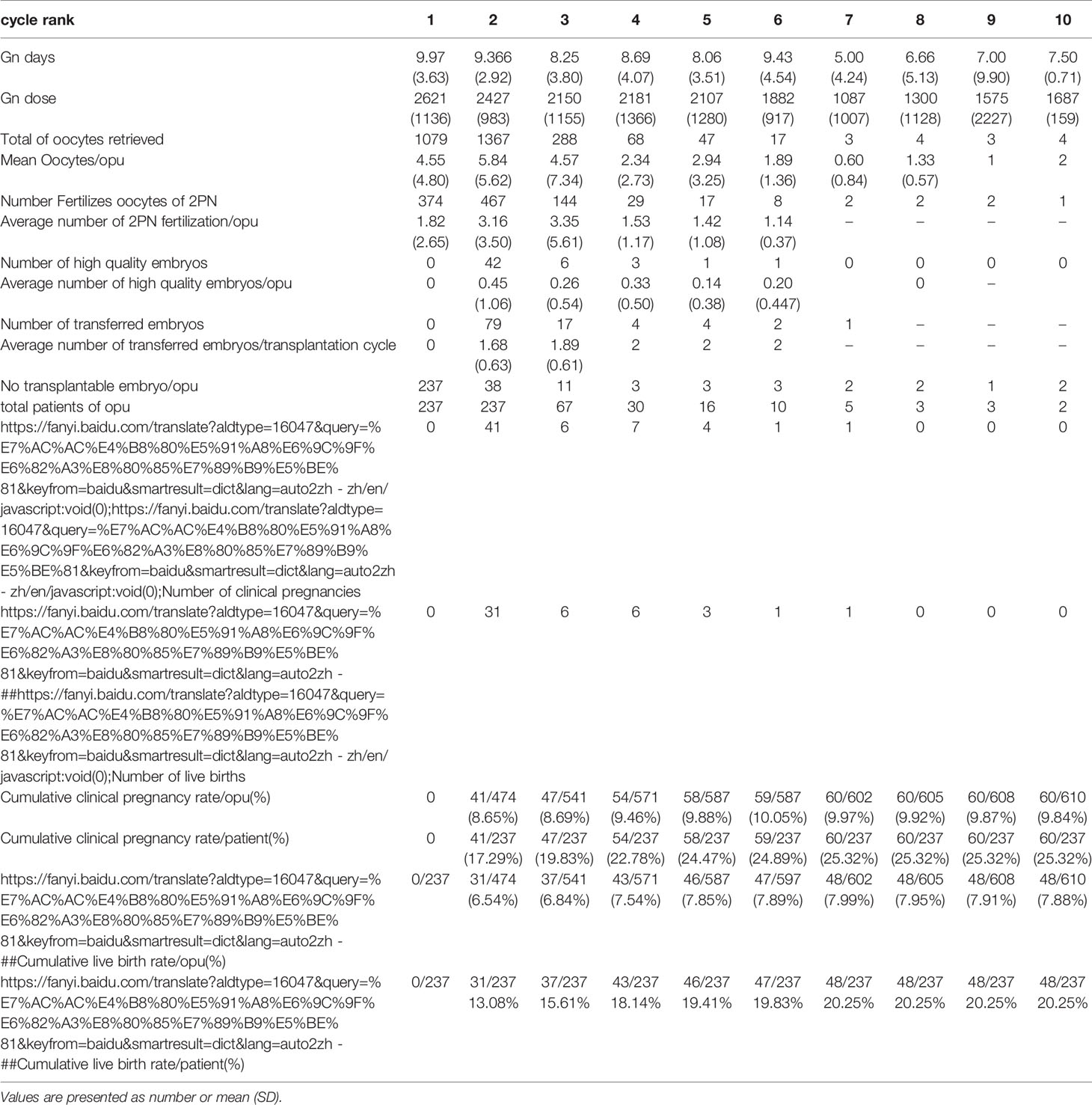
Table 2.3 IVF cycles’ characteristics and their final reproductive outcomes of group c stratified according to IVF cycle rank.
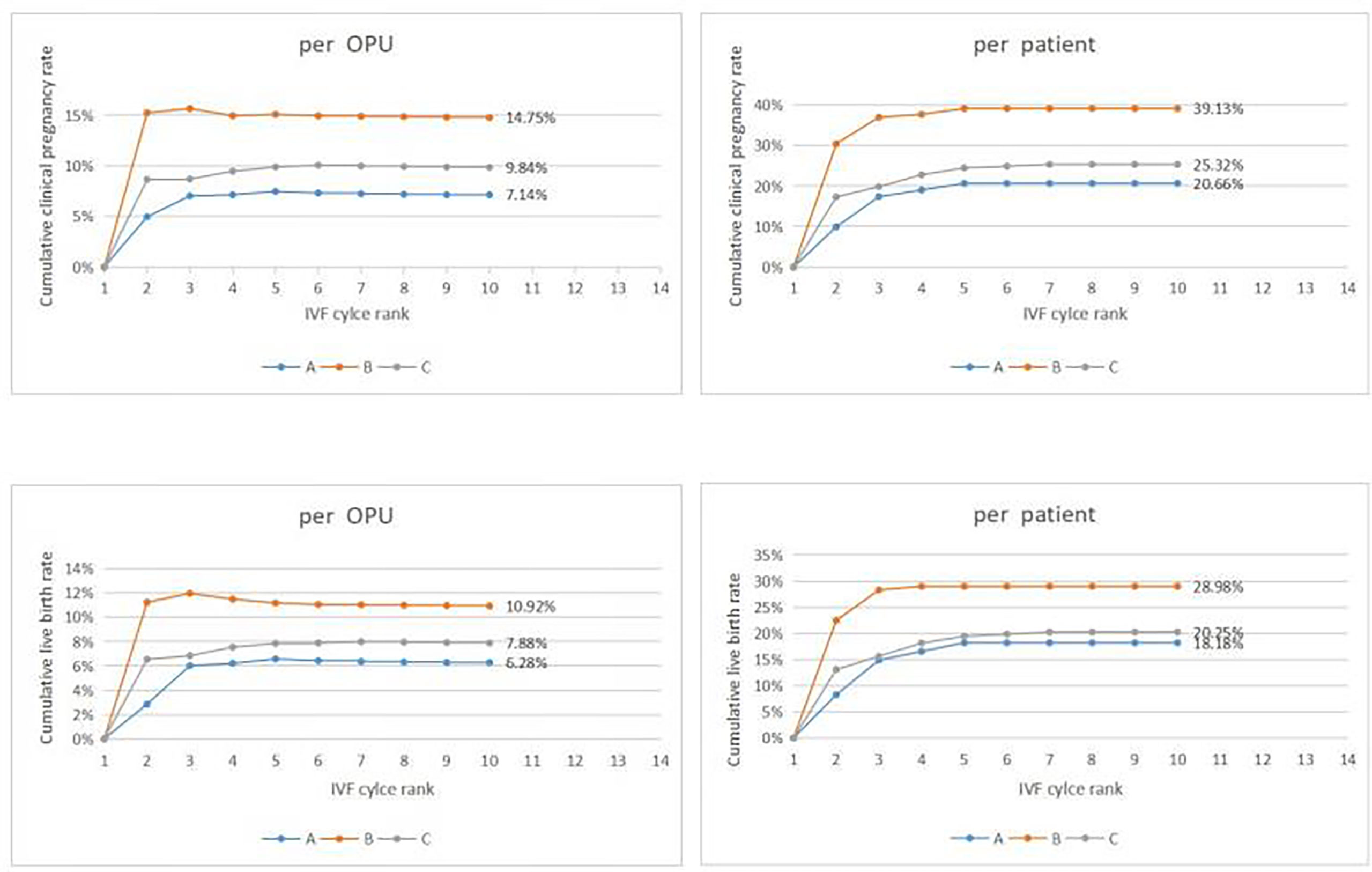
Figure 2 Cumulative clinical outcomes at cycle rank. In group A, The cumulative clinical pregnancy rates were 7.14% per OPU and 20.66% per patient, and the cumulative live birth rates were 6.28% per OPU and 18.18% per patient, with both rates reaching a plateau at the 5th OPU cycle. The cumulative live birth rate/patient in group B was 28.98%, which did not increase any more after cycle 3, whereas the cumulative live birth rates/patient in group C was 20.25% and reached a plateau after cycle 7.
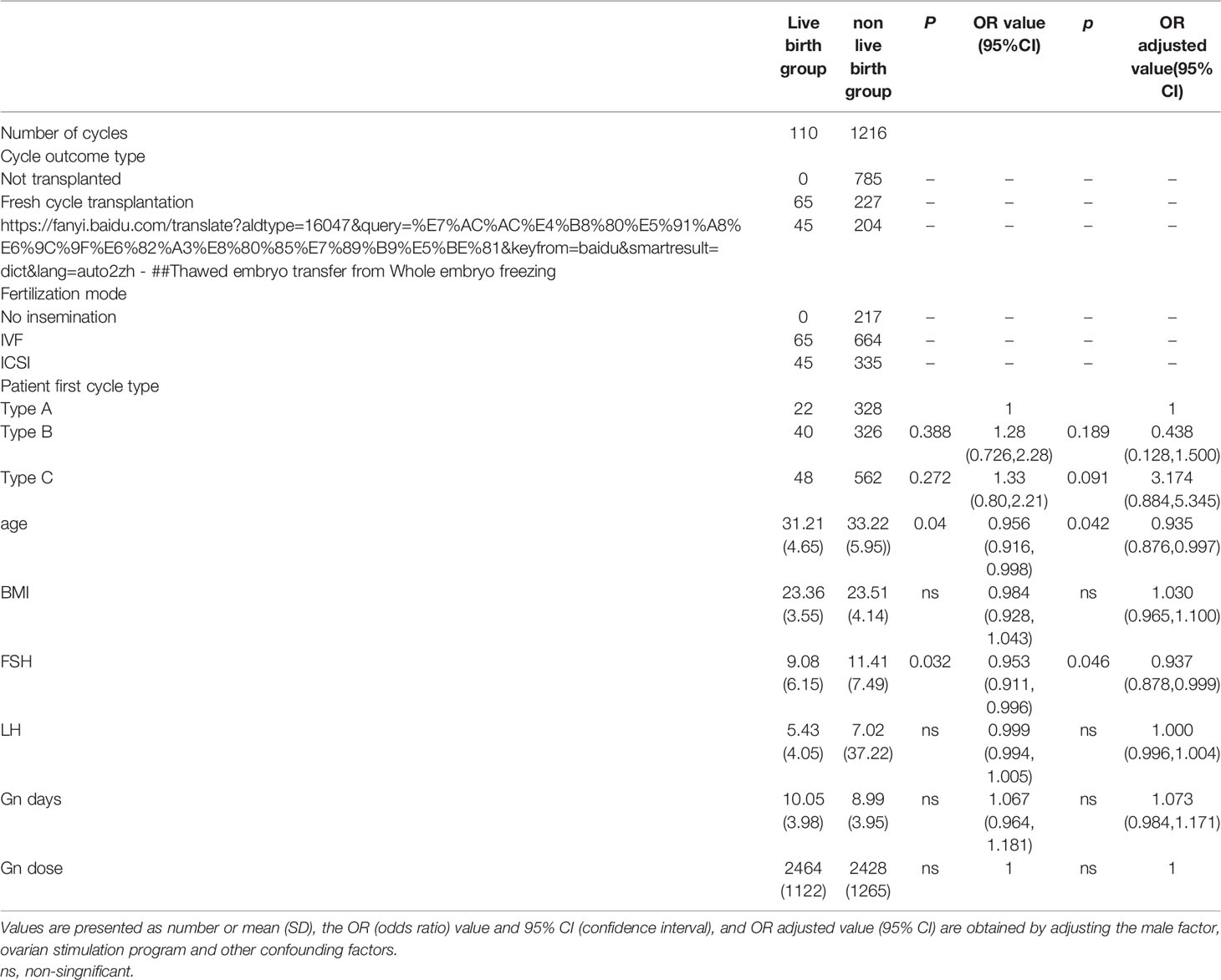
Table 4 Multivariate regression analysis of IVF cycle between the live birth group and non-live birth group.
A significant difference existed in female age, basic FSH level and Gn days between group A and group B (P< 0.001, P=0.005, P=0.001)as well as between groups A and C (P< 0.001, P< 0.001, P< 0.001). Cumulative clinical pregnancy rates per OPUand per patient in group B were 14.75% and 39.13%, respectively, which were better than those in other two groups. Cumulative live birth rates per OPU and per patient were 10.92% and 28.98%, respectively, in group B, which were significantly higher than those in group A (P=0.027, P=0.042), but not significantly (P>0.05)different from those in group C. Both cumulative pregnancy rate and cumulative live birth rates were not significantly (P>0.05) different between group A and C (Table 1).
In group A, the cumulative clinical pregnancy rates were 7.14% per OPU and 20.66% per patient, and the cumulative live birth rates were 6.28% per OPU and 18.18% per patient, all of which reached a plateau at the 5th OPU cycle (Table 2.1 and Figure 2). The cumulative live birth rates/patient in group B was 28.98% and did not increase any more after cycle 3 (Table 2.2 and Figure 2), whereas the cumulative live birth rates/patient in group C was 20.25% and reached a plateau after cycle 7 (Table 2.3 and Figure 2).
Both age (31.21 ± 4.65 vs 33.22 ± 5.95, P=0.001) and basic FSH level (9.08 ± 6.15 vs 11.41 ± 7.49, P=0.004) in the live birth group were statistically signficantly lower than those in other groups (Table 3). The multivariate regression analysis showed that age and basic FSH level were predict factors for live birth (ORadjusted 0.935, 95% CI 0.876-0.997, P=0.042; ORadjusted 0.937, 95% CI 0.878-0.999, P=0.046) (Table 4).
This study evaluated the chance of a live birth in women with no embryo transfer at their first IVF cycle attempt over their subsequent IVF treatment course and factors affecting the live birth.
In our center, the total cycle cancellation rate was nearly 10%, and the cancellation rate of the first cycle was 4.9%. This may be related to embryo transfer strategies as well as the ovarian stimulation protocols used in our hospital. For example, the cycle cancellation rate of low ovarian response with minimal stimulation was 10% while that with long protocol was 2.9% (12). A meta-analysis in 2021 showed that the cycle cancellation rate of mild stimulation was higher than that of conventional ovarian stimulation in the low ovarian response group (13). Siristatidis et al (14) also found that the cycle cancellation rate of mild stimulation was significantly higher than that of long-term and antagonist regimens (36.4% vs 12%). A recent study showed that participants experienced an overall feeling of “loss and loneliness” when they had no experience of embryo transfer. The situation was described as a shock unprepared for them. Their need for relevant information has not been met (15). The results of the present study showed that some data could provide these patients with some information in terms of no embryo transfer in their first IVF cycle attempt.
In the group with no egg retrieved, the proportion of the patients in the first IVF cycle cancellation was 21.54% [(121 + 50)/(496 + 298)], and 22 of these women achieved a live birth in their subsequent cycles. Furthermore, there was no significant difference in the cycle source and the mode of fertilization through analysis of the original data. In patients with no eggs being retrieved in our study, the ovarian response was low, and their average age was higher than those in the other two groups. In the end, a 18% cumulative live birth rate was achieved in these patients after the 5th IVF cycle, which was similar to that of the study by Raoul Orvieto et al. (16). For these patients, the reproductive outcome will not change after the 5th IVF OPU cycle being tried, and the next ovulation stimulation cycle is suggested to be abandoned. Nonetheless, the patient’s own decision should always be respected.
The proportion of women with no normal zygotes formed in the first IVF cycle cancellation was 23.43% [(138 + 48)/(496 + 298)], and 40 of the 138 patients achieved a live birth eventually. In our hospital, when the number of eggs retrieved was less than or equal to 3, ICSI was not rescued. The majority of these patients had fewer eggs retrieved, which might be one reason for fertilization failure. Even though there was no significant difference in the fertilization methods (IVF vs ICSI) in the live birth cycle (9.46%vs13.27%), 54 cases were changed to ICSI after the first cycle of IVF, and 13 cases obtained a live birth, with the live birth rate of 24.07%. According to these outcomes, changing the mode of insemination in the second cycle may bring a better outcome. Some scholars (17) found that compared with the hCG single trigger group, the oocyte fertilization rate (73.1% vs 58.6%), clinical pregnancy rate (33% vs 20.7%), live birth rate (26.9% vs 14.5%), abortion rate (17.4% vs 37.0%), and embryo transfer elimination rate (6.1% vs 15.4%) in the double trigger group were significantly different. This may suggest that changing the trigger scheme, grasping the correct trigger time so as to get the target follicles as far as possible, and getting the MII (mature) eggs may improve the pregnancy outcomes. Furthermore, some patients may improve the clinical outcomes by using some new techniques like AOA (assisted oocyte activation), IVM (in vitro maturation), and testing for associated genes. A retrospective study (18) showed that AOA intervention after ICSI fertilization failure significantly increased the normal fertilization rate (52.1%), the cumulative clinical pregnancy rate (47.1%) and the live birth rate (29.4%).
The proportion of patients with the cancellation cycle caused by poor embryo quality in the first IVF cycle was 55.04% [(237 + 200)/(496 + 298)]. These patients are more challenging for the ART. In our study, these patients’ age was not high, they had more eggs, but the CLBR was low. In the ovulation induction of these patients, 25 cases with the antagonist regimen obtained a live birth, whereas 23 cases with other protocols did not. It seems that the antagonist regimen had a trend of increasing the live birth rate, but no significant difference was found in our study (10.25% vs 6.28%, P =0.07). Similarly, the fertilization mode of the live birth cycle was not signfiicantly different. Moreover, although no differences were found in various infertility factors among these patients in our study, a recent study (19) reported that AOA could improve the clinical pregnancy and live birth rate in patients with male factors (oligoasthenospermia [OAT]), advanced age, polycystic ovary syndrome (PCOS), and unexplained infertility. In current literature, blastocyst transfer after ICSI-AOA according to different infertility factors may help to improve the clinical outcome of these patients. Besides, shortening the embryo culture time to the first or second day and carrying out gamete transfer or zygote transfer may be a goodway to improve the reproductive outcome of patients with embryonic development block from the embryologists’ point of view.
Female age and ovarian reserve are still the main factors influencing the live birth of patients with first IVF cycle cancellation. However, clinical quality control, personalized treatment, or changes in ovulation induction and fertilization mode may help them achieve a live birth. Therefore, for patients with good financial resources, the findings of the present study suggest extending the number of OPU cycles up to the 3rd, 5th, or 7th cycle even if the reasons for cancellation of the first IVF cycle are different.
Some limitations existed in this study, including the retrospective and one-center design, a small cohort of patients, and Chinese patients enrolled only. Moreover, the reasons for patient cycle cancellation are multifarious and complex, and patients with non-fertilization, fertilization failure and abnormal fertilization might have been assigned to one group for analysis, which limits the ability to control for potential unknown confounding factors. All these factors may affect the generalization of the outcome of this study. Future studies will have to resolve all these issues for better outcomes.
In conclusion, future clinical outcomes may be better in women with no normal zygotes than those with no oocyte retrieved or no available embryo at their first IVF cycle attempts. The main factors influencing the live birth are age and ovarian reserve.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of The Second Hospital of Hebei Medical University. The patients/participants provided their written informed consent to participate in this study.
Study design: ZZ and GH. Data collection: XZ, MC, ZY, and PL. Data analysis: XZ and MC. Supervision: YX. Writing the original article: MC. Revision: ZZ. All authors contributed to the article and approved the submitted version.
This study was supported by S&T Program of Hebei (22377742D), Natural Science Foundation of Hebei Province(Beijing-Tianjin-Hebei Cooperation Special Project) (H2019206712).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional Ovarian Stimulation and Single Embryo Transfer for IVF/ICSI. How Many Oocytes do We Need to Maximize Cumulative Live Birth Rates After Utilization of All Fresh and Frozen Embryos. Hum Reprod (2016) 31(2):370–6. doi: 10.1093/humrep/dev316
2. Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative Live Birth Rates According to the Number of Oocytes Retrieved After the First Ovarian Stimulation for In Vitro Fertilization/Intracytoplasmic Sperm Injection: A Multicenter Multinational Analysis Including 15,000 Women. Fertil Steril (2018) 110(4):661–70. doi: 10.1016/j.fertnstert.2018.04.039
3. Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A, et al. Association Between the Number of Eggs and Live Birth in IVF Treatment: An Analysis of 400135 Treatment Cycles. Hum Reprod (2011) 26(7):1768–74. doi: 10.1093/humrep/der106
4. Humaidan P, Chin W, Rogoff D, D'Hooghe T, Longobardi S, Hubbard J, et al. ESPART Study Investigators. Efficacy and Safety of Follitropin Alfa/Lutropin Alfa in ART: A Randomized Controlled Trial in Poor Ovarian Responders. Hum Reprod (2017) 32(3):544–55. doi: 10.1093/humrep/dew360
5. Giovanale V, Pulcinelli FM, Ralli E, Primiero FM, Caserta D. Poor Responders in IVF: An Update in Therapy. Gynecol Endocrinol (2015) 31(4):253–7. doi: 10.3109/09513590.2014.987228
6. Troude P, Guibert J, Bouyer J, de la Rochebrochard E, DAIFI Group. Medical Factors Associated With Early IVF Discontinuation. Reprod Biomed Online (2014) 28(3):321–9. doi: 10.1016/j.rbmo.2013.10.018
7. Olivius C, Friden B, Borg G, Bergh C. Psychological Aspects of Discontinuation of In Vitro Fertilization Treatment. Fertil Steril (2004) 81(2):276. doi: 10.1016/j.fertnstert.2003.09.026
8. Smeenk JM, Verhaak CM, Braat DD. Psychological Interference in In Vitro Fertilization Treatment. Fertil Steril (2004) 81(2):277. doi: 10.1016/j.fertnstert.2003.09.025
9. Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do Patients Discontinue Fertility Treatment? A Systematic Review of Reasons and Predictors of Discontinuation in Fertility Treatment. Hum Reprod Update (2012) 18(6):652–69. doi: 10.1093/humupd/dms031
10. Domar AD, Rooney K, Hacker MR, Sakkas D, Dodge LE. Burden of Care is the Primary Reason Why Insured Women Terminate In Vitro Fertilization Treatment. Fertil Steril (2018) 109(6):1121–6. doi: 10.1016/j.fertnstert.2018.02.130
11. Alpha scientists in reproductive medicine and ESHRE special interest group of embryology. The Istanbul Consensus Workshop on Embryo Assessment: Proceedings of an Expert Meeting. Hum Reprod (2011) 26(6):1270–83. doi: 10.1093/humrep/der037
12. Baka S, Makrakis E, Tzanakaki D, Konidaris S, Hassiakos D, Moustakarias T, et al. Poor Responders in IVF: Cancellation of a First Cycle is Not Predictive of a Subsequent Failure. Ann N Y Acad Sci (2006) 1092:418–25. doi: 10.1196/annals.1365.040
13. Datta AK, Maheshwari A, Felix N, Campbell S, Nargund G. Mild Versus Conventional Ovarian Stimulation for IVF in Poor, Normal and Hyper-Responders: A Systematic Review and Meta-Analysis. Hum Reprod Update (2021) 27(2):229–53. doi: 10.1093/humupd/dmaa035
14. Siristatidis C, Salamalekis G, Dafopoulos K, Basios G, Vogiatzi P, Papantoniou N. Mild Versus Conventional Ovarian Stimulation for Poor Responders Undergoing IVF/ICSI. In Vivo (Athens Greece) (2017) 31(2):231–7. doi: 10.21873/invivo.11050
15. Holter H, Bergh C, Gejervall AL. Lost and Lonely: A Qualitative Study of Women's Experiences of No Embryo Transfer Owing to non-Fertilization or Poor Embryo Quality. Hum Reprod Open (2021) 19(1):hoaa062. doi: 10.1093/hropen/hoaa062
16. Orvieto R, Farhi J, Nahum R, Basch S, Haas J, Aizer A. Future Fertility of Patients With Zero Oocytes Yield in Their First IVF Cycle Attempt. PloS One (2021) 16(2):e0246889. doi: 10.1371/journal.pone.0246889
17. Lin MH, Wu FS, Hwu YM, Lee RK, Li RS, Li SH. Dual Trigger With Gonadotropin Releasing Hormone Agonist and Human Chorionic Gonadotropin Significantly Improves Live Birth Rate for Women With Diminished Ovarian Reserve. Reprod Biol Endocrinol (2019) 17(1):7. doi: 10.1186/s12958-018-0451-x
18. Lam KKW, Wong JYY, Cheung TM, Li RHW, Ng EHY, Yeung WSB. A Retrospective Analysis of Artificial Oocyte Activation in Patients With Low or No Fertilisation in Intracytoplasmic Sperm Injection Cycles. J Obstet Gynaecol (2021) 12:1–6. doi: 10.1080/01443615.2021.1922878
Keywords: in vitro fertilization, no embryo transfer, cycle cancellation rate, poor ovarian response, cumulative live birth rate
Citation: Zhu X, Cao M, Yao Z, Lu P, Xu Y, Hao G and Zhao Z (2022) Future Fertility of Patients With No Embryo Transfer in Their First IVF Cycle Attempts. Front. Endocrinol. 13:893506. doi: 10.3389/fendo.2022.893506
Received: 10 March 2022; Accepted: 27 May 2022;
Published: 27 July 2022.
Edited by:
Yuting Fan, Boston IVF, United StatesReviewed by:
Alice Albu, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2022 Zhu, Cao, Yao, Lu, Xu, Hao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiming Zhao, ZG9jdG9yX3poYW96aGFvQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.