- 1Biomedical Research and Innovation Platform, South African Medical Research Council, Cape Town, South Africa
- 2Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
- 3Center for Cardio-Metabolic Research in Africa (CARMA), Division of Medical Physiology, Faculty of Health Sciences, Stellenbosch University, Cape Town, South Africa
Maternal diabetes is associated with pregnancy complications and poses a serious health risk to both mother and child. Growing evidence suggests that pregnancy complications are more frequent and severe in pregnant women with pregestational type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) compared to women with gestational diabetes mellitus (GDM). Elucidating the pathophysiological mechanisms that underlie the different types of maternal diabetes may lead to targeted strategies to prevent or reduce pregnancy complications. In recent years, microRNAs (miRNAs), one of the most common epigenetic mechanisms, have emerged as key players in the pathophysiology of pregnancy-related disorders including diabetes. This review aims to provide an update on the status of miRNA profiling in pregnancies complicated by maternal diabetes. Four databases, Pubmed, Web of Science, EBSCOhost, and Scopus were searched to identify studies that profiled miRNAs during maternal diabetes. A total of 1800 articles were identified, of which 53 are included in this review. All studies profiled miRNAs during GDM, with no studies on miRNA profiling during pregestational T1DM and T2DM identified. Studies on GDM were mainly focused on the potential of miRNAs to serve as predictive or diagnostic biomarkers. This review highlights the lack of miRNA profiling in pregnancies complicated by T1DM and T2DM and identifies the need for miRNA profiling in all types of maternal diabetes. Such studies could contribute to our understanding of the mechanisms that link maternal diabetes type with pregnancy complications.
Introduction
Maternal diabetes is associated with an increased risk of pregnancy complications and is a significant cause of morbidity for both mother and child (1–3). The prevalence of diabetes during pregnancy is increasing globally, paralleling the obesity and type 2 diabetes mellitus (T2DM) epidemics (4). According to recent estimates, ~16.7% of live births (21.1 million) are associated with maternal diabetes, of which 80.3% are due to gestational diabetes mellitus (GDM), 10.6% due to pre-existing type 1 diabetes mellitus (T1DM) or T2DM, and 9.1% due to T1DM and T2DM first detected in pregnancy (5). All types of maternal diabetes are associated pregnancy complications, with several studies reporting that the frequency and severity of adverse pregnancy outcomes are related with the degree of hyperglycaemia (6, 7). Women with pregestational T1DM and T2DM have a higher risk of pregnancy complications including fetal and neonatal loss, congenital malformations, preterm delivery, macrosomia, preeclampsia and caesarean deliveries, compared to women with GDM (8, 9). The more severe effects of pregestational diabetes compared to GDM are most likely attributed to the pre-conceptual hyperglycaemic environment, longer intrauterine exposure to hyperglycaemia, and the different pathophysiological mechanisms that underlie the different types of maternal diabetes (10, 11).
MiRNAs are short, highly conserved, non-coding RNA molecules that are approximately 22 nucleotides in length. They were first identified in Caenorhabditis elegans in 1993 (12) and have emerged as powerful epigenetic mediators of diverse biological processes including development, proliferation, differentiation, apoptosis and metabolism (13). To date over 2 500 miRNAs have been identified in humans (14, 15), which together regulate ~ 60% of genes in the genome (Zhang and Wang, 2017). MiRNAs regulate gene expression through post-transcriptional mechanisms, by binding to the 3’ untranslated region (UTR) of messenger RNA (mRNA) and inducing degradation or by translational repression of the mRNA transcript (16). Furthermore, recent studies have proposed an important role for circulating miRNAs in cell-to-cell communication, suggesting that these extracellular miRNAs may similarly regulate biological processes (17, 18). The dysregulated expression of miRNAs is associated with the development of metabolic disease and conditions including cancer, obesity, T2DM and cardiovascular disease (19; 20).
In recent years, miRNAs have been identified as key regulators of metabolic adaptation during pregnancy (21–23). They regulate several biological processes that are critical during pregnancy and may reflect the physiological state of the pregnancy and fetal development. A growing body of evidence have reported on the association between maternal miRNAs and pregnancy complications, including placental weight (24), placental abruption (25), placental previa (26), preeclampsia and gestational hypertension (27), and intrauterine growth restriction (28), macrosomia (29) and GDM (30). Therefore, miRNA profiling may aid in elucidating the pathophysiological mechanisms that underlie the different types of maternal diabetes. This review aims to provide an update on the status of miRNA profiling in pregnancies complicated by maternal diabetes. Four databases, Pubmed, Web of Science, EBSCOhost, and Scopus, were searched to identify published studies reporting miRNA profiling during maternal diabetes between the date of inception to January 2022. The search terms “type 1 diabetes”, “type 2 diabetes”, “gestational diabetes mellitus”, “pregestational diabetes”, “maternal diabetes”, “microRNA”, and “pregnancy”, including corresponding synonyms and associated terms for each word were used. Studies were considered eligible if they were original articles, investigated miRNA patterns during maternal diabetes, and if the study was published in English. Reference lists of included studies were also searched to identify other potentially eligible studies.
Characteristics of included studies
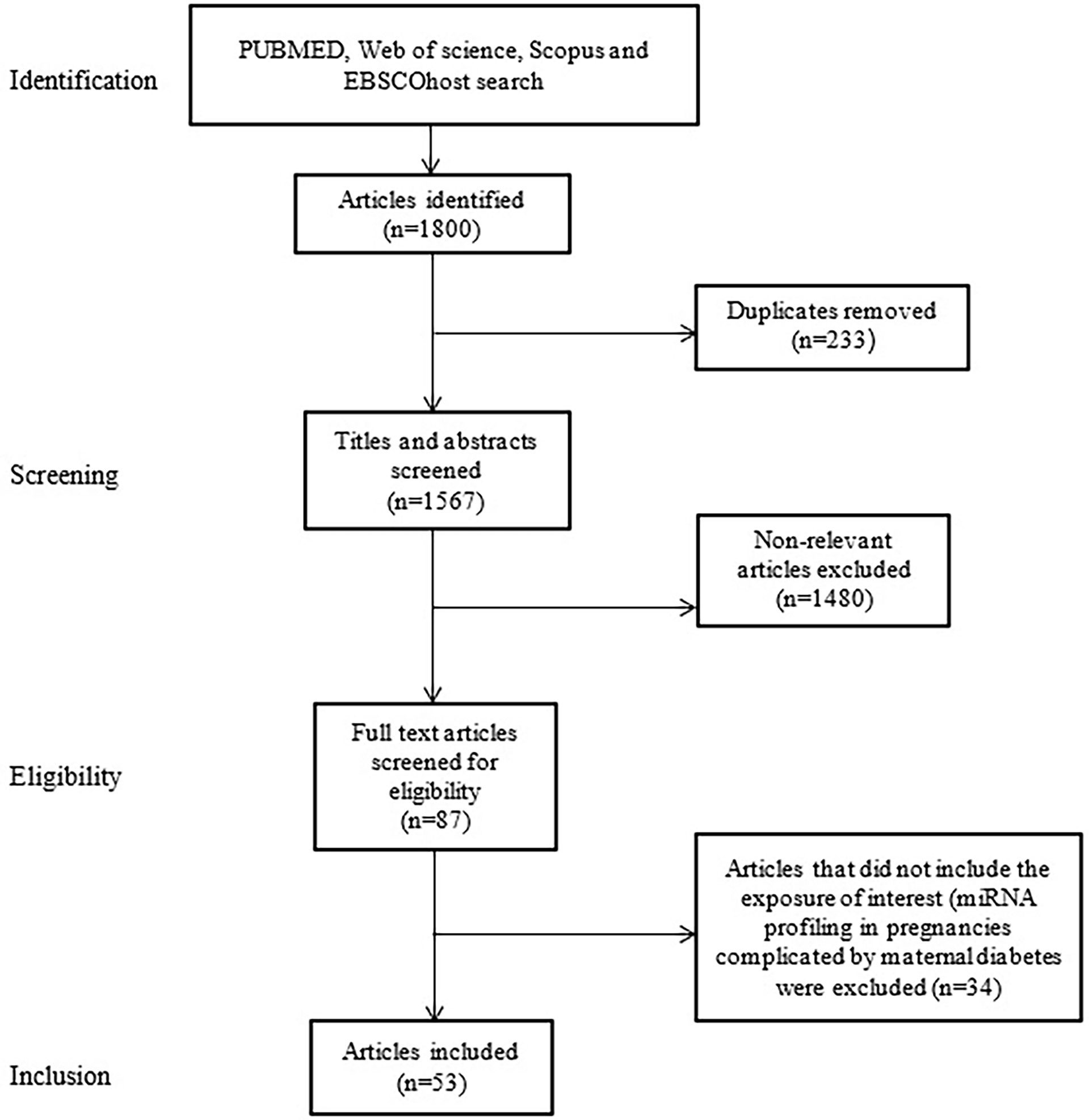
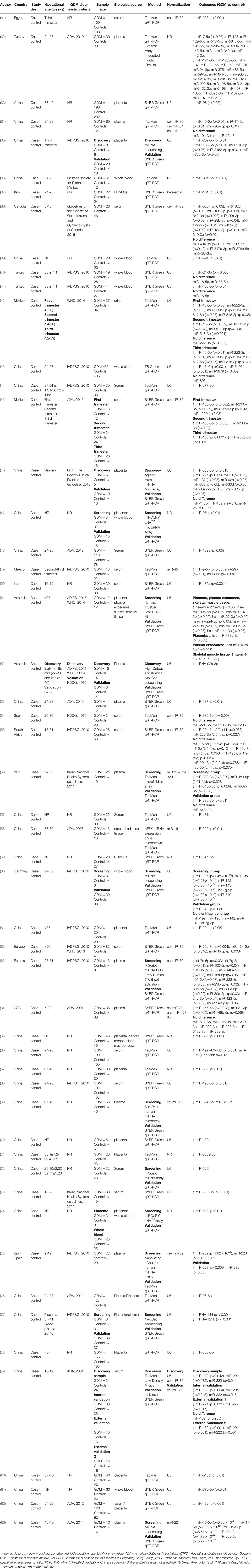
A total of 1800 articles were identified from the search strategy, of which 53 met the inclusion criteria and are included in the review (Figure 1). The 53 included studies were case-control studies on GDM conducted between 2011 and 2022 (Table 1). Studies were conducted across five continents (Africa, Asia, Australia, Europe and North America), with studies conducted in different countries, such as Australian (n = 2), Canada (n = 1), China (n = 33), Estonia (n = 1), Egypt (n = 1), Germany (n = 1), Mexico (n = 3), Iran (n = 1), Italy (n = 2), Italy/Spain (n = 1), South Africa (n = 1), Spain (n = 1), Turkey (n = 3), United States of America (USA) (n = 1) and different places in Europe (n = 1). The sample size of studies ranged from three to 204 women. Studies profiled miRNAs in different biological sources including human umbilical vein endothelial cells (HUVECs) (n = 2), omental adipose tissue (n = 1), plasma (n = 10), placenta/plasma (n = 2), placenta (n = 9), placenta/plasma exosomes/skeletal muscle tissue (n = 1), placenta/whole blood (n = 2), placental-derived mononuclear macrophages (n = 1), serum (n = 16), serum/placenta (n = 1), skeletal muscle tissue (n = 1), urine (n = 1) and whole blood (n = 7). Different measurement platforms and techniques were employed across studies. Studies profiled miRNAs using quantitative real-time PCR (qRT-PCR) with SYBR Green (n = 29), Taqman probes (n = 20), TB Green (n = 1) and qRT-PCR not referenced (NR) (n = 3), for the validation groups. Other studies also used techniques such as miRNA sequencing (n = 5), Taqman Low Density Arrays (n = 1), miRCURY LNA™ Array (n = 2), miScript Array T & B cell activation (n = 1), miScript miRNA Array (n = 2), Dynamic Array Integrated Fluidic Circuits (n = 1), Agilent miRNA Microarrays (n = 1), TaqMan Microarray (n = 2), SurePrint human miRNA Microarray (n = 1), AFFX chip Microarrays (n = 1), NanoString nCounter human miRNA assay (n = 1) and NR (n = 35) for the screening groups. Gestational age at the time of miRNA profiling ranged between 6-40 weeks. Studies used different normalization controls, with the majority using U6 (n = 30) and C. elegans miR-39 (n = 12) for circulating miRNAs.
Qualitative synthesis of studies
All the included studies profiled miRNAs during GDM with no studies on pregestational T1DM and T2DM identified. A total of 32 miRNAs were assessed in two or more studies and are discussed below. These included miR-9 (n = 3), miR-16 (n = 7), miR-17 (n = 4), miR-19a (n = 5), miR-19b (n = 5), miR-20a (n = 3), miR-21 (n = 3), miR-29a (n = 7), miR-29b (n = 3), miR-30d (n = 3), miR-92a (n = 3), miR-96 (n = 2), miR-125b (n = 2), miR-132 (n = 5), miR-137 (n = 3), miR-142 (n = 2), miR-143 (n = 2), miR-155 (n = 3), miR-195 (n = 2), miR-197 (n = 2), miR-210 (n = 4), miR-222 (n = 7), miR-223 (n = 3), miR-330 (n = 4), miR-342 (n = 3), miR-483 (n = 2), miR-494 (n = 2), miR-517 (n = 3), miR-520h (n = 2), miR-657 (n = 2), miR-1323 (n = 2) and let-7g (n = 2).
Three studies that reported on the expression of miR-9. Of the three studies, one study reported higher levels of miR-9 in the serum of Mexican women with GDM compared to controls (49). In contrast, two studies profiling miRNAs in placental tissue of Chinese women (46) and in plasma samples of Turkish women (32) reported lower expression of miR-9 in women with GDM compared to controls. Of the seven studies reporting on miR-16, three studies demonstrated higher expression in serum and plasma samples of Chinese and European women with GDM compared to controls (34, 62, 83). In contrast, Herrera et al. (42) reported lower expression of miR-16 in urine samples of Mexican women with GDM compared to controls in the third trimester, and higher expression in the first and second trimesters (42). Three studies conducted in Turkey and South Africa reported no difference in miR-16 expression in women with GDM compared to controls (40, 41, 55). Four studies investigated miR-17 during GDM. Of these, three studies reported that miR-17 expression was higher in plasma samples of Chinese and Turkish women with GDM compared to controls (32, 34, 83). In contrast, Pheiffer et al. (55) showed no significant difference in miR-17 expression in the serum of South African women with GDM compared to controls (55). Five studies profiled miR-19a and miR-19b during GDM. Two studies reported that miR-19a and miR-19b expression was higher in serum and plasma samples of Chinese women with GDM compared to pregnant women without GDM (66, 83). However, two studies reported that miR-19a and miR-19b expression did not differ in plasma and serum samples of women with GDM compared to controls (34, 55). Stirm et al. (60) demonstrated higher expression of miR-19a and miR-19b in the whole blood of German women with GDM compared to controls in the screening group, however, this difference was not validated in a larger sample (60).
Three studies profiled miR-20a, of which two studies reported higher expression of miR-20a in Chinese women with GDM when compared to controls (34, 83), while Pheiffer et al. (55) reported lower expression of miR-20a in South African women during GDM compared to controls (55). For miR-21, Wander et al. (64) reported higher expression in plasma samples of American women with GDM compared to controls (64), while two studies reported lower expression of miR-21 in whole blood and plasma samples of Turkish women with GDM compared to controls (32, 40). MiR-29a was investigated in seven studies. Of these, three studies showed higher serum expression of miR-29a during GDM in women from Canada, Mexico, and different regions in Europe (38, 49, 62). Two studies reported lower levels of miR-29a in serum and plasma of Chinese and Turkish women with GDM compared to controls (32, 79), and two studies reported no difference in miR-29a expression in serum and plasma samples of American and South African women with GDM compared to controls (55, 64). Of the three studies that reported on miR-29b expression during GDM, two studies reported higher expression in serum and plasma samples of Canadian and Turkish women with GDM compared to controls (32, 38), while Sun etal. (61) reported lower expression of miR-29b in Chinese women with GDM compared to controls (61).
Three studies investigated miR-30d during GDM. Of these, two studies reported higher expression of miR-30d in plasma and placenta of Estonian and Chinese women with GDM compared to controls (63, 78), while one study reported lower expression in placenta samples of Chinese women with GDM compared to controls (46). Three studies reported on miR-92a during GDM. Of these, two studies reported higher expression in plasma of miR-92a during GDM (52, 63), while Lie at al. (46) reported lower expression in the placenta of Chinese women with GDM compared to controls (46). The two studies that investigated miR-96, both reported lower expression in plasma/placenta/whole blood of Chinese women with GDM compared to controls (47, 76). Two studies reported contradicting results for miR-125b. Lamadrid-Romero et al. (45) reported higher expression of miR-125b in serum samples of Mexican women with GDM compared to controls (45), while Balci et al. (32) reported lower expression in plasma samples of Turkish women with GDM compared to controls. Of the five studies, only one study reported a higher expression of miR-132 in the serum samples of Canadian women (38). Contradictingly, three studies reported a lower expression of miR-132 in serum and plasma samples of Chinese and Turkish women with GDM (32, 79, 82). However, Pheiffer et al. (55) observed no significant change in expression of miR-132 in serum samples of South African women with GDM when compared to controls (55).. All three studies that investigated miR-137 reported lower expression in plasma and placenta samples of Chinese and Turkish women with GDM compared to controls. (32, 46, 53). Two studies reported on the expression of miR-142 and miR-143 during GDM. One study reported higher expression of miR-142 and miR-143 in plasma of Turkish women with GDM compared to controls (32). Stirm et al. (60) reported higher expression of miR-142 and miR-143 in the whole blood of German women with GDM in the screening group, however, these findings were not validated in a larger sample. Of the three studies that investigated miR-155, one study reported higher expression in plasma samples of American women with GDM compared to controls (64). Hocaoglu et al. (40) reported no change in the expression of miR-155 in whole blood of Turkish women with GDM compared to controls (40). However, a more recent study by the same authors reported lower expression of miR-155 in whole blood of Turkish women with GDM compared to controls (41). Both studies that investigated miR-195 reported higher expression in plasma samples of Estonian and Chinese women with GDM compared to controls (63, 68). Contradicting results were reported for the expression miR-197. Nair et al. (51) reported higher expression of miR-197 in placenta, exosomes and skeletal muscle tissue samples of Australian women with GDM compared to controls (51), while Balci et al. (32) reported lower expression of miR-197 in plasma samples of Turkish women with GDM compared to controls (32).
Four studies reported on the expression of miR-210. Of these, one study reported higher levels of miR-210 in serum samples of Canadian women with GDM compared to controls (38), while lower levels of miR-210 was observed in placental and plasma samples of Chinese and Turkish women with GDM (32, 35). Wander at al. observed no difference in miR-210 expression in plasma samples of American women with GDM compared to controls (64). Of the seven studies that reported on miR-222 expression during GDM, two reported higher expression of miR-222 in omental adipose tissue and plasma of Chinese women with GDM compared to controls (58, 63), while three studies observed lower expression of miR-222 in serum of Chinese, South African and Turkish women with GDM compared to controls (32, 55, 79). Wander et al. (64) observed no difference in the expression of miR-222 in plasma of American women with GDM compared to controls (64). Herrera-Van Oostdam et al. (42) demonstrated higher expression of miR-222 in urine samples of Mexican women with GDM compared to controls in the first trimester and observed no significant difference in the second trimester and lower expression in the third trimester (42). Two studies reported increased levels of miR-223 in serum and plasma of women during GDM from Italy/Spain and Egypt (31, 75), however, Wander et al. (64) reported no difference in the expression of miR-223 in plasma of American women with GDM compared to controls (64).
All four of the studies that profiled miR-330 reported higher levels in serum and plasma of Italian, Mexican, Spanish and Turkish women with GDM compared to controls (49, 54, 56, 74). All three studies that profiled miR-342 demonstrated higher expression in serum and plasma of Estonian, Canadian and Turkish women with GDM compared to controls (32, 38, 63). Sebastiani et al. (56) reported higher expression of miR-483 in plasma of Italian women with GDM compared to controls (56), while Gillet et al. (38) showed no difference in expression of miR-483 in serum samples of Canadian women with GDM compared to controls (38). He et al. (39) reported lower expression of miR-494 in whole blood samples of Chinese women with GDM compared to controls (38), while Gillet et al. (39) demonstrated no difference in the expression of miR-494 in serum samples of Canadian women with GDM compared to controls (38). Of the three studies that investigated miR-517, Herrera-Van Oostdam et al. (42) demonstrated higher expression in women with GDM compared to controls in the first and second trimesters but lower expression in the third trimester (42). The other study that profiled miR-517 showed no difference in expression in serum of women with GDM compared to controls (35, 64). Both studies that profiled miR-520h reported higher expression in serum of Canadian and Chinese women with GDM compared to controls (38, 72). Two studies investigated miR-657 during GDM, and both studies reported higher expression in placental and placental-derived mononuclear macrophages of Chinese women with GDM compared to controls (65, 67). Both studies reporting on miR-1323 observed higher levels in the serum of Canadian and Chinese women with GDM compared to controls (38, 48). Two studies reported on the expression of let-7g. Tagoma et al. (63) reported higher expression of let-7g in plasma samples of Estonian women with GDM compared to controls (57). Stirm et al. (60) reported conflicting results on the expression of let-7g. These authors reported higher expression of let-7g in the screening group, however, no difference was observed in the validation group in whole blood samples of German women with GDM compared to controls in the screening group (60)
Other articles included in this review reported differential miRNA expression, yet these miRNAs were identified in single studies only (36, 37, 43, 44, 48, 50, 57, 59, 69, 71, 74, 76, 77, 80, 81).
Discussion
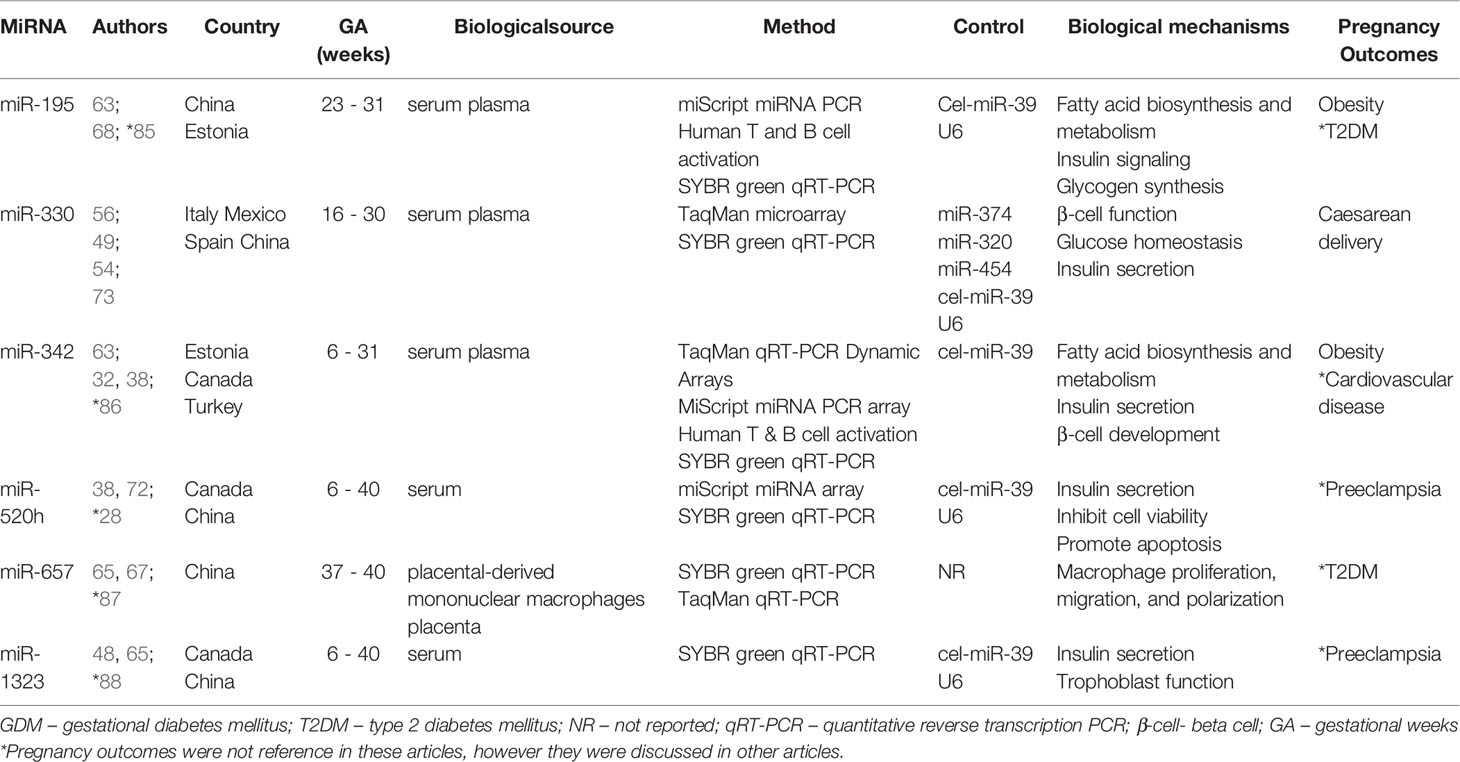
MiRNA profiling in pregnancies complicated by diabetes may aid in elucidating the pathophysiological mechanisms that underlie T1DM, T2DM, and GDM (21–23, 84). This review provides an update on the status of miRNA profiling in pregnancies complicated by maternal diabetes. The main finding of this review is the lack of studies that have profiled miRNAs in pregnant women with pregestational T1DM and T2DM. All the included studies investigated GDM only. Of these, six miRNAs [miR-195 (n = 2), miR-330 (n = 4), miR-342 (n = 3), miR-520h (n = 2), miR-657 (n = 2) and miR-1323 (n = 2)] were similarly differentially expressed in pregnant women with GDM compared to controls in two or more studies (Table 2). The consistency of expression of these miRNAs across diverse populations and gestational ages and using different methodologies and measuring platforms support their candidacy as biomarkers of GDM.
Despite our search identifying 53 articles on miRNA profiling during maternal diabetes, none investigated pregestational T1DM and T2DM. Previously, Collares et al. (89) profiled miRNAs in non-pregnant individuals with T1DM and T2DM, and in women with GDM (89). These authors identified several miRNAs that were unique to each diabetes type. Eleven miRNAs, let-7f, let-7g, miR-103, miR-1260, miR-1274a, miR-1274b, miR-130a, miR-150, miR-20b, miR-21 and miR-720 were unique to T1DM. Five miRNAs, miR-140-3p, miR-199a-3p, miR-222, miR-30e and miR-451 were unique to T2DM. Ten miRNAs, miR-101, miR-1180, miR-1268, miR-181a, miR-181d, miR-26a, miR-29a, miR-29c, miR-30b and miR-595 were unique to GDM (89). Specific miRNAs may represent biological markers for each type of diabetes, warranting further investigation as potential mechanisms that underlie the different diabetes types. Collares et al. (89) assessed miRNA expression in both females and males, and included non-pregnant individuals, thus their results do not reflect placental-derived miRNAs and pregnancy pathophysiology. Ibarra et al. (90) profiled miRNAs during pregestational T1DM and T2DM (90). This research was reported as a conference abstract only and was not included in this review. Data from the abstract report that miR-19a, miR-125b, miR-20a and a miRNA on Chr11-134 were unique to placenta samples of women with T1DM and were not expressed in pregnant women with T2DM (90). Our review highlights the lack of studies profiling miRNAs in pregnancies complicated by pregestational T1DM and T2DM. We propose that future studies on miRNA profiling include all types of maternal diabetes, which may contribute to elucidating the different pathophysiological mechanisms that underlie pregestational T1DM and T2DM, and GDM.
Studies on miRNA profiling during GDM were mainly related to biomarker discovery. These studies identified six miRNAs that were consistently expressed at higher levels in serum, plasma, placenta and placental-derived mononuclear macrophages in women with GDM compared to controls in different populations, using different methodologies and measurement platforms, and during different gestational ages. These include miR-195 (n = 2), miR-330 (n = 4), miR-342 (n = 3), miR-520h (n = 2), miR-657 (n = 2) and miR-1323 (n = 2) (Table 2). MiR-195 levels were reported to be consistently higher in the serum and plasma samples of women with GDM compared to controls across two studies conducted in China and Estonia using miScript miRNA PCR Human T and B cell activation, and SYBR green qRT-PCR (63, 68). Previous studies observed high levels of miR‐195 associated with fatty acid biosynthesis and metabolism, insulin signaling cascade and glycogen synthesis (63, 85), suggestive of miR-195 candidacy as a biomarker for GDM. Furthermore, upregulation of miR-195 in women with GDM was shown to be associated with the development of T2DM (85) and obesity (63, 68). Interestingly, circulating levels of miR-330 was consistently higher in the serum and plasma samples of women with GDM compared to controls across four studies conducted in Italy, Mexico, Spain and China using TaqMan microarray, TaqMan and SYBR green qRT-PCR (49, 54, 56, 73). MiR-330 regulates genes involved in beta-cell (β-cell) function and glucose homeostasis, suggesting that increased miR-330 expression may lead to impaired β-cell proliferation and insulin secretion (56). Furthermore, upregulation of miR-330 was shown to be associated with caesarean delivery in women with GDM (54, 56). MiR-342 levels were reported to be higher in serum and plasma of Estonian, Canadian and Turkish women with GDM compared to controls using TaqMan qRT-PCR, MiScript miRNA PCR array Human T & B cell activation and SYBR green qRT-PCR (32, 38, 63). MiR-342 has been associated with the regulation of fatty acid biosynthesis and metabolism (63), impaired insulin secretion (38) and β-cell development (32). Furthermore, upregulation of miR-342 in women with GDM was shown to be associated with obesity (63) and cardiovascular disease in children born to mothers with GDM (86). MiR-520h levels were reported to be higher in serum of Canadian and Chinese women with GDM compared to controls using miScript miRNA array and SYBR green qRT-PCR (38, 72). MiR-520h is implicated in impaired insulin secretion in pancreatic β-cells (38), and has been demonstrated to inhibit cell viability and promote apoptosis (72). Furthermore, the upregulation of plasma miR-520h during the first trimester was associated with the onset of preeclampsia (28). MiR-657 levels were reported to be higher in placental-derived mononuclear macrophages and placenta samples of women with GDM in a Chinese population using SYBR green qRT-PCR and TaqMan qRT-PCR (65, 67). MiR-657 regulates inflammation via targeting Interleukin-37/Nuclear factor-κB signalling axis, that is responsible for the regulation of inflammatory responses (65). Furthermore, the upregulation of miR-657 was associated with the pathogenesis of T2DM (87). MiR-1323 was expressed at higher levels in serum samples of women with GDM compared to controls in studies conducted in Canada and China using SYBR green qRT-PCR (38, 48). MiR-1323 regulates insulin secretion (38) and trophoblast cell activity crucial for placental cell development (48). MiR-1323 was implicated in patients with preeclampsia (88). MiRNAs that are commonly expressed across diverse populations and gestational ages, biological samples and using different measurement platforms present opportunities as biomarkers for GDM. Although it could be argued that miRNAs offer little advantage over measurement of glucose concentrations, the oral glucose tolerance test, the gold standard for GDM diagnosis, is associated with several disadvantages which include the requirement for fasting, multiple blood draws, and association with nausea, vomiting and bloating, lead to decreased patient compliance (30). Furthermore, as discussed above, these miRNAs have been reported to be associated with adverse pregnancy outcomes, supporting their use as biomarkers to predict pregnancy outcomes.
Findings from this review show heterogenous miRNA expression across studies with a general lack of reproducibility. MiRNA heterogeneity may be attributed to factors such as diet, physical activity, medication use, population differences such as ethnicity, socioeconomic status, environmental factors and viral infections (91–95), and differing gestational ages between women (42). Furthermore, different GDM diagnostic criteria and glucose cut-off values across studies may have also contributed to miRNA variability. Pre-analytical and analytical factors such as sample collection and storage, miRNA isolation procedures, measurement platform, and normalisation methods (96–98) affect miRNA expression analysis. The development of optimized protocols for standardizing sample collection, transport, and storage, as well as miRNA isolation procedures and data analysis for the diversity of technological methods used are important to improve reproducibility across studies. Importantly, the non-specificity of miRNAs is another factor that may limit its clinical applicability. MiRNAs are able to regulate multiple genes across different biological pathways in different diseases (99, 100), therefore, miRNA signatures based on a pool of miRNAs may have more clinical applicability than individual miRNAs. Although rapid technological advances could facilitate the use of miRNAs as inexpensive, point-of-care biomarkers in the future, at present, miRNA profiling during GDM remains inconclusive, largely due to poor reproducibility between studies. Many pre-analytical, analytical and biological challenges must be addressed before miRNAs can become clinically applicable. Although it could be argued that miRNAs offer little advantage over measurement of glucose concentrations, the oral glucose tolerance test, the gold standard for GDM diagnosis, is associated with several disadvantages which include the requirement for fasting, multiple blood draws, and association with nausea, vomiting and bloating, which leads to decreased patient compliance (30). Furthermore, as discussed above, these miRNAs have been reported to be associated with adverse pregnancy outcomes, supporting their use as biomarkers to predict pregnancy outcomes.
Conclusion and future perspectives
This review highlights the lack of studies profiling miRNA expression in pregnancies complicated by pregestational T1DM and T2DM. Future studies should prioritise miRNA profiling in all types of maternal diabetes, which may aid in identifying the mechanisms that underlie the different types of diabetes during pregnancy. Such studies could contribute to unravelling the link between diabetes type and pregnancy outcomes. Furthermore, this review confirms the growing evidence supporting the potential of miRNAs to serve as biomarkers of GDM. Six miRNAs with similar expression in women with GDM compared to controls in two or more studies, across different populations and gestational ages, using different methodologies and measuring platforms are highlighted. These six miRNAs represent candidates as future GDM biomarkers and should be prioritized in future studies.
Author Contributions
MM and CP, conceptualization and original draft. MM and NM, literature search and study selection. MM and CP, data extraction. MM, SD, NM, SA, and CP, manuscript writing and approval of the final draft. All authors contributed to the article and approved the submitted version.
Funding
Research Foundation (NRF) Competitive Programme for Rated Researchers Grant No: 120832 to C Pheiffer. Baseline funding from the South African Medical Research Council (SAMRC) is also acknowledged. Mr Masete is a recipient of the SAMRC Division of Research and Capacity Development internship scholarship funding programme. The content hereof is the sole responsibility of the authors and do not represent the official views of the NRF or SAMRC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Capobianco G, Gulotta A, Tupponi G, Dessole F, Pola M, Virdis G, et al. Materno-Fetal and Neonatal Complications of Diabetes in Pregnancy: A Retrospective Study. J Clin Med (2020) 9(9):2707. doi: 10.3390/jcm9092707
2. Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes During Pregnancy: A Maternal Disease Complicating the Course of Pregnancy With Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int J Mol Sci (2021) 22(6):2965. doi: 10.3390/ijms22062965
3. Kakotrichi P, Mantsiou C, Bekiari E, Tsapas A. Long-Term Maternal Complications. In: Goulis DG, editor. Comprehensive Clinical Approach to Diabetes During Pregnancy Cham: Springer International Publishing (2022). p. 361–74. doi: 10.1007/978-3-030-89243-2_19
4. Choudhury AA, Devi Rajeswari V. Gestational Diabetes Mellitus - A Metabolic and Reproductive Disorder. Biomed Pharmacother (2021) 143:112183. doi: 10.1016/j.biopha.2021.112183
5. International Diabetes Federation. IDF Diabetes Atlas (2021). Available at: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF (Accessed 3 March 2022).
6. Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia (2019) 62:2118–28. doi: 10.1007/s00125-019-4961-7
7. Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, et al. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus With Congenital Anomalies of the Newborn. Diabetes Care (2020) 43:2983–90. doi: 10.2337/dc20-0261
8. Sugrue R, Zera C. Pregestational Diabetes in Pregnancy. Obstet Gynecology Clin North Am (2018) 45(2):315–31. doi: 10.1016/j.ogc.2018.01.002
9. Shub A, Lappas M. Pregestational Diabetes in Pregnancy: Complications, Management, Surveillance, and Mechanisms of Disease—A Review. PrenatDiagn (2020) 40(9):1092–8. doi: 10.1002/pd.5718
10. Huynh J, Yamada J, Beauharnais C, Wenger JB, Thadhani RI, Wexler D, et al. Type 1, Type 2 and Gestational Diabetes Mellitus Differentially Impact Placental Pathologic Characteristics of Uteroplacental Malperfusion. Placenta (2015) 36(10):1161–6. doi: 10.1016/j.placenta.2015.08.004
11. Xiang AH, Wang X, Martinez MP, Getahun D, Page KA, Buchanan TA, et al. Maternal Gestational Diabetes Mellitus, Type 1 Diabetes, and Type 2 Diabetes During Pregnancy and Risk of ADHD in Offspring. Diabetes Care (2018) 41(12):2502. doi: 10.2337/dc18-0733
12. Lee RC, Feinbaum RL, Ambros V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs With Antisense Complementarity to Lin-14. Cell (1993) 75(5):843–54. doi: 10.1016/0092-8674(93)90529-y
13. O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (2018) 9:402. doi: 10.3389/fendo.2018.00402
14. Satake E, Pezzolesi MG, Md Dom ZI, Smiles AM, Niewczas MA, Krolewski AS. Circulating miRNA Profiles Associated With Hyperglycemia in Patients With Type 1 Diabetes. Diabetes (2018) 67:1013–23. doi: 10.2337/db17-1207
15. Alles J, Fehlmann T, Fischer U, Backes C, Galata V, Marie M. An estimate of the total number of true human miRNAs. Nucleic Acids Res (2019) 47(7):3353–64. doi: 10.1093/nar/gkz097
16. Zhang F, Wang D. The Pattern of microRNA Binding Site Distribution. Genes (2017) 8:E296. doi: 10.1155/2017/6972732
17. Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA Target Predictions. Nat Genet (2005) 37:495–500. doi: 10.1038/ng1536
18. Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab (2017) 19:Suppl 1:137–46. doi: 10.1111/dom.13027
19. Jiménez-Lucena R, Rangel-Zúñiga OA, Alcalá-Díaz JF, López-Moreno J, Roncero-Ramos I, Molina-Abril H, et al. Circulating miRNAs as Predictive Biomarkers of Type 2 Diabetes Mellitus Development in Coronary Heart Disease Patients from the CORDIOPREV Study. Mol Ther - Nucleic Acids (2018) 12:146–57. doi: 10.1016/j.omtn.2018.05.002
20. Méndez-Mancilla A, Lima-Rogel V, Toro-Ortíz JC, Escalante-Padrón F, Monsiváis-Urenda AE, Noyola DE, et al. Differential Expression Profiles of Circulating Micrornas in Newborns Associated to Maternal Pregestational Overweight and Obesity: Newborns Mirnas Alterations. Pediatr Obes (2018) 13:168–74. doi: 10.1111/ijpo.12247
21. Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, et al. Placental Exosomes in Normal and Complicated Pregnancy. Am J Obstet Gynecol (2015) 213:S173–81. doi: 10.1016/j.ajog.2015.07.001
22. Cai M, Kolluru GK, Ahmed A. Small Molecule, Big Prospects: MicroRNA in Pregnancy and Its Complications. J Pregnancy (2017) 2017:6972732. doi: 10.1155/2017/6972732
23. Poirier C, Desgagné V, Guérin R, Bouchard L. MicroRNAs in Pregnancy and Gestational Diabetes Mellitus: Emerging Role in Maternal Metabolic Regulation. Curr Diabetes Rep (2017) 17(5):35. doi: 10.1007/s11892-017-0856-5
24. Miura K, Morisaki S, Abe S, Higashijima A, Hasegawa Y, Miura S, et al. Circulating Levels of Maternal Plasma Cell-Free Pregnancy-Associated Placenta-Specific microRNAs Are Associated With Placental Weight. Placenta (2014) 35(10):848–51. doi: 10.1016/j.placenta.2014.06.002
25. Miura K, Higashijima A, Murakami Y, Fuchi N, Tsukamoto O, Abe S, et al. Circulating Levels of Pregnancy-Associated, Placenta-Specific microRNAs in Pregnant Women With Placental Abruption. Reprod Sci Thousand Oaks Calif (2016) 24(1):148–55. doi: 10.1177/1933719116653837
26. Hasegawa Y, Miura K, Higashijima A, Abe S, Miura S, Yoshiura K, et al. Increased Levels of Cell-Free miR-517a and Decreased Levels of Cell-Free miR-518b in Maternal Plasma Samples From Placenta Previa Pregnancies at 32 Weeks of Gestation. Reprod Sci Thousand Oaks Calif (2015) 22(12):1569–76. doi: 10.1177/1933719115589407
27. Hromadnikova I, Kotlabova K, Ondrackova M, Kestlerova A, Novotna V, Hympanova L, et al. Circulating C19MC microRNAs in Preeclampsia, Gestational Hypertension, and Fetal Growth Restriction. Mediators Inflammation (2013) 2013:186041. doi: 10.1155/2013/186041
28. Hromadnikova I, Kotlabova K, Ivankova K, Krofta L. First Trimester Screening of Circulating C19MC microRNAs and the Evaluation of Their Potential to Predict the Onset of Preeclampsia and IUGR. PloS One (2017) 12(2):e0171756. doi: 10.1371/journal.pone.0171756
29. Jiang H, Wen Y, Hu L, Miao T, Zhang M, Dong J. Serum MicroRNAs as Diagnostic Biomarkers for Macrosomia. Reprod Sci (2015) 22(6):664–71. doi: 10.1177/1933719114561557
30. Pheiffer C, Dias S, Rheeder P, Adam S. Screening for Gestational Diabetes Mellitus: The Potential of microRNAs. IntechOpen (2018). doi: 10.5772/intechopen.82102
31. Abdeltawab A, Zaki ME, Abdeldayem Y, Mohamed AA, Zaied SM. Circulating Micro RNA-223 and Angiopoietin-Like Protein 8 as Biomarkers of Gestational Diabetes Mellitus. Br J Biomed Science (2020) 78(1):12–7. doi: 10.1080/09674845.2020.1764211
32. Balci S, Gorur A, Yildirim DD, Cayan F, Tamer L. Expression Level of miRNAS in Patients With Gestational Diabetes. Turk J Biochem-Turk Biyokim Derg (2020) 45(6):825–31. doi: 10.1515/tjb-2019-0157
33. Cao JL, Zhang L, Li J, Tian S, Lv X-D, Wang X-Q, et al. Up-Regulation of miR-98 and Unraveling Regulatory Mechanisms in Gestational Diabetes Mellitus. Sci Rep (2016) 6(1):32268. doi: 10.1038/srep32268
34. Cao YL, Jia YJ, Xing BH, Shi D-D, Dong X-J. Plasma microRNA-16-5p, -17-5p and -20a-5p: Novel Diagnostic Biomarkers for Gestational Diabetes Mellitus. J Obstet Gynaecol Res (2017) 43(6):974–81. doi: 10.1111/jog.13317
35. Ding R, Guo F, Zhang Y, Liu X-M, Xiang Y-Q, Zhang C, et al. Integrated Transcriptome Sequencing Analysis Reveals Role of miR-138-5p/ TBL1X in Placenta From Gestational Diabetes Mellitus. Cell Physiol Biochem (2018) 51(2):630–46. doi: 10.1159/000495319
36. Feng Y, Qu X, Chen Y, Feng Q, Zhang Y, Hu J, et al. MicroRNA-33a-5p Sponges to Inhibit Pancreatic β-Cell Function in Gestational Diabetes Mellitus LncRNA DANCR. Reprod Biol Endocrinol (2020) 18(1):61. doi: 10.1186/s12958-020-00618-8
37. Floris I, Descamps B, Vardeu A, Mitić T, Posadino AM, Shantikumar S, et al. Gestational Diabetes Mellitus Impairs Fetal Endothelial Cell Functions Through a Mechanism Involving microRNA-101 and Histone Methyltransferase Enhancer of Zester Homolog-2. Arterioscler Thromb Vasc Biol (2015) 35(3):664–74. doi: 10.1161/ATVBAHA.114.304730
38. Gillet V, Ouellet A, Stepanov Y, Rodosthenous RS, Croft EK, Brennan K, et al. miRNA Profiles in Extracellular Vesicles From Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J Clin Endocrinol Metab (2019) 104(11):5157–69. doi: 10.1210/jc.2018-02693
39. He Y, Bai J, Liu P, Dong J, Tang Y, Zhou J, et al. miR-494 Protects Pancreatic β-Cell Function by Targeting PTEN in Gestational Diabetes Mellitus. Exp Clin Sci J (2017) 16:1297–307. doi: 10.17179/excli2017-491
40. Hocaoglu M, Demirer S, Senturk H, Turgut A, Komurcu-Bayrak E. Differential Expression of Candidate Circulating microRNAs in Maternal Blood Leukocytes of the Patients With Preeclampsia and Gestational Diabetes Mellitus. Pregnancy Hypertens (2019) 17:5–11. doi: 10.1016/j.preghy.2019.04.004
41. Hocaoglu M, Demirer S, Loclar Karaalp I, Kaynak E, Attar E, Turgut A, et al. Identification of Mir-16-5p and Mir-155-5p Micrornas Differentially Expressed in Circulating Leukocytes of Pregnant Women With Polycystic Ovary Syndrome and Gestational Diabetes. Gynecol Endocrinol (2020) 37:216–20. doi: 10.1080/09513590.2020.1843620
42. Herrera-Van Oostdam AS, Toro-Ortíz JC, López JA, Noyola DE, Garcí-López DA, Durán‑Figueroa NV, et al. Placental Exosomes Isolated From Urine of Patients With Gestational Diabetes Exhibit a Differential Profile Expression of microRNAs Across Gestation. Int J Mol Med (2020) 46:546–60. doi: 10.3892/ijmm.2020.4626
43. Hu J, Mu H, Gao L, Pan Y, Wu C, Zhang D, et al. Diagnostic Value of Candidate Noncoding RNAs in Leukocytes of Patients With Gestational Diabetes Mellitus. Exp Ther Med (2021) 21:145. doi: 10.3892/etm.2020.9576
44. Hua Z, Li D, Wu A, Cao T, Luo S. miR-377 Inhibition Enhances the Survival of Trophoblast Cells via Upregulation of FNDC5 in Gestational Diabetes Mellitus. Open Med Wars Pol (2021) 16:464–71. doi: 10.1515/med-2021-0247
45. Lamadrid-Romero M, Solís KH, Cruz-Reséndiz MS, Pérez JE, Díaz NF, Flores-Herrera H, et al. Central Nervous System Development-Related microRNAs Levels Increase in the Serum of Gestational Diabetic Women During the First Trimester of Pregnancy. Neurosci Res (2018) 130:8–22. doi: 10.1016/j.neures.2017.08.003
46. Li J, Song L, Zhou L, Wu J, Sheng C, Chen H, et al. MicroRNA Signature in Gestational Diabetes Mellitus Associated With Risk of Macrosomia. Cell Physiol Biochem (2015) 37:243–52. doi: 10.1159/000430349
47. Li L, Wang S, Li H, Wan J, Zhou Q, Zhou Y, et al. microRNA-96 Protects Pancreatic β-Cell Function by Targeting PAK1 in Gestational Diabetes Mellitus. BioFactors (2018) 44:539–547. doi: 10.1002/biof.1461
48. Liu L, Zhang J, Liu Y. MicroRNA-1323 Serves as a Biomarker in Gestational Diabetes Mellitus and Aggravates High Glucose-Induced Inhibition of Trophoblast Cell Viability by Suppressing TP53INP1. Exp Ther Med (2021) 21:230. doi: 10.3892/etm.2021.9661
49. Martínez-Ibarra A, Martínez-Razo LD, Vázquez-Martínez ER, Martínez-Cruz N, Flores-Ramírez R, García-Gómez E, et al. Unhealthy Levels of Phthalates and Bisphenol a in Mexican Pregnant Women With Gestational Diabetes and Its Association to Altered Expression of miRNAs Involved With Metabolic Disease. Int J Mol Sci (2019) 20(13):3343. doi: 10.3390/ijms20133343
50. Lewis BP, Burge CB, Bartel DP. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are microRNA Targets. Cell (2005) 120:15–20. doi: 10.1016/j.cell.2004.12.035
51. Nair S, Jayabalan N, Guanzon D, Palma C, Scholz-Romero K, Elfeky O, et al. Human Placental Exosomes in Gestational Diabetes Mellitus Carry a Specific Set of miRNAs Associated With Skeletal Muscle Insulin Sensitivity. Clin Sci (2018) 132:2451–67. doi: 10.1042/CS20180487
52. Nair S, Guanzon D, Jayabalan N, Lai A, Scholz-Romero K, Kalita de Croft P, et al. Extracellular Vesicle-Associated miRNAs Are an Adaptive Response to Gestational Diabetes Mellitus. J Transl Med (2021) 19:360. doi: 10.1186/s12967-021-02999-9
53. Peng H-Y, Li H-P, Li M-Q. High Glucose Induces Dysfunction of Human Umbilical Vein Endothelial Cells by Upregulating miR-137 in Gestational Diabetes Mellitus. Microvasc Res (2018) 118:90–100. doi: 10.1016/j.mvr.2018.03.002
54. Pfeiffer S, Sanchez-Lechuga B, Donovan P, Halang L, Prehn JHM, Campos-Caro A, et al. Circulating miR-330-3p in Late Pregnancy Is Associated With Pregnancy Outcomes Among Lean Women With GDM. Sci Rep (2020) 10:908. doi: 10.1038/s41598-020-57838-6
55. Pheiffer C, Dias S, Rheeder P, Adam S. Decreased Expression of Circulating miR-20a-5p in South African Women With Gestational Diabetes Mellitus. Mol Diagn Ther (2018) 22(3):345–52. doi: 10.1007/s40291-018-0325-0
56. Sebastiani G, Guarino E, Grieco GE, Formichi C, Delli Poggi C, Ceccarelli E, et al. Circulating microRNA (miRNA) Expression Profiling in Plasma of Patients With Gestational Diabetes Mellitus Reveals Upregulation of miRNA miR-330-3p. Front Endocrinol (2017) 8:345. doi: 10.3389/fendo.2017.00345
57. Shen H, Sun J, Liu J, Wang L, Dong L. miR-181d Promotes Pancreatic Beta Cell Dysfunction by Targeting IRS2 in Gestational Diabetes Mellitus. Ginekol Pol (2021) 92:563–70. doi: 10.5603/GP.a2021.0077
58. Shi Z, Zhao C, Guo X, Ding H, Cui Y, Shen R, Liu J. Differential Expression of microRNAs in Omental Adipose Tissue From Gestational Diabetes Mellitus Subjects Reveals miR-222 as a Regulator of ERα Expression in Estrogen-Induced Insulin Resistance. Endocrinology (2014) 155:1982–90. doi: 10.1210/en.2013-2046
59. Song F, Cai A, Ye Q, Chen X, Lin L, Hao X. MiR-34b-3p Impaired HUVECs Viability and Migration via Targeting PDK1 in an in Vitro Model of Gestational Diabetes Mellitus. Biochem Genet (2021) 59:1381–95. doi: 10.1007/s10528-021-10064-9
60. Stirm L, Huypens P, Sass S, Batra R, Fritsche L, Brucker S, et al. Maternal Whole Blood Cell miRNA-340 Is Elevated in Gestational Diabetes and Inversely Regulated by Glucose and Insulin. Sci Rep (2018) 8(1):1366. doi: 10.1038/s41598-018-19200-9
61. Sun D-G, Tian S, Zhang L, Hu Y, Guan C-Y, Ma X, et al. The miRNA-29b Is Downregulated in Placenta During Gestational Diabetes Mellitus and May Alter Placenta Development by Regulating Trophoblast Migration and Invasion Through a HIF3A-Dependent Mechanism. Front Endocrinol (2020) 11. doi: 10.3389/fendo.2020.00169
62. Sørensen A, van Poppel M, Desoye G, Damm P, Simmons D, Jensen D, et al. The DALI Core Investigator Group. the Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort. Cells (2021) 10:170. doi: 10.3390/cells10010170
63. Tagoma A, Alnek K, Kirss A, Uibo R, Haller-Kikkatalo K. MicroRNA Profiling of Second Trimester Maternal Plasma Shows Upregulation of miR-195-5p in Patients With Gestational Diabetes. Gene (2018) 672:13. doi: 10.1016/j.gene.2018.06.004
64. Wander PL, Boyko EJ, Hevner K, Parikh VJ, Tadesse MG, Sorensen TK, et al. Circulating Early- and Mid-Pregnancy microRNAs and Risk of Gestational Diabetes. Diabetes Res Clin Pract (2017) 132:1–9. doi: 10.1016/j.diabres.2017.07.024
65. Wang P, Wang H, Li C, Zhang X, Xiu X, Teng P, et al. Dysregulation of microRNA-657 influences inflammatory response via targeting interleukin-37 in gestational diabetes mellitus: WANG et al. J Cell Physiol (2018) 234:7141–7148. doi: 10.1002/jcp.27468
66. Wang F, Zhang X, Zhou H. Role of Cell Free microRNA-19a and microRNA-19b in Gestational Diabetes Mellitus Patients. 3 Biotech (2019) 9:406. doi: 10.1007/s13205-019-1952-9
67. Wang P, Wang Z, Liu G, Jin C, Zhang Q, Man S, et al. miR-657 Promotes Macrophage Polarization Toward M1 by Targeting FAM46C in Gestational Diabetes Mellitus. Mediators Inflamm (2019) 1–9. doi: 10.1155/2019/4851214
68. Wang J, Pan Y, Dai F, Wang F, Qiu H, Huang X. Serum miR-195-5p Is Upregulated in Gestational Diabetes Mellitus. J Clin Lab Anal (2020) 34:e23325. doi: 10.1002/jcla.23325
69. Wang F, Li Z, Zhao M, Ye W, Wu H, Liao Q, et al. Circulating miRNAs miR-574-5p and miR-3135b Are Potential Metabolic Regulators for Serum Lipids and Blood Glucose in Gestational Diabetes Mellitus. Gynecol Endocrinol (2021) 37:665–71. doi: 10.1080/09513590.2021.1908990
70. Wang S, Wei D, Sun X, Li Y, Li D, Chen B, et al. MiR-190b Impedes Pancreatic β Cell Proliferation and Insulin Secretion by Targeting NKX6-1 and May Associate to Gestational Diabetes Mellitus. J Recept Signal Transduct Res (2021) 41:349–56. doi: 10.1080/10799893.2020.1810705
71. Wang P, Ma Z, Wang Z, Wang X, Zhao G, Wang Z. MiR-6869-5p Induces M2 Polarization by Regulating PTPRO in Gestational Diabetes Mellitus. Mediators Inflamm (2021) 2021:e6696636. doi: 10.1155/2021/6696636
72. Wen J, Bai X. miR-520h Inhibits Cell Survival by Targeting mTOR in Gestational Diabetes Mellitus. Acta Biochim Pol (2021) 68:65–70. doi: 10.18388/abp.2020_5389
73. Xiao Y, Ding J, Shi Y, Lin L, Huang W, Shen D, et al. MiR-330-3p Contributes to INS-1 Cell Dysfunction by Targeting Glucokinase in Gestational Diabetes Mellitus. J Obstet Gynaecol Res (2020) 46:864–75. doi: 10.1111/jog.14249
74. Xu K, Bian D, Hao L, Huang F, Xu M, Qin J, et al. microRNA-503 Contribute to Pancreatic Beta Cell Dysfunction by Targeting the mTOR Pathway in Gestational Diabetes Mellitus. EXCLI J (2017) 16:1177–87. doi: 10.17179/excli2017-738
75. Yoffe L, Polsky A, Gilam A, Raff C, Mecacci F, Ognibene A, et al. Early Diagnosis of Gestational Diabetes Mellitus Using Circulating microRNAs. Eur J Endocrinol (2019) 181:565–77. doi: 10.1530/EJE-19-0206
76. Yu X, Liu Z, Fang J, Qi H. miR-96-5p: A Potential Diagnostic Marker for Gestational Diabetes Mellitus. Medicine (Baltimore) (2021) 100:e25808. doi: 10.1097/MD.0000000000025808
77. Zhang L, Zhang T, Sun D, Cheng G, Ren H, Hong H, et al. Diagnostic Value of Dysregulated Microribonucleic Acids in the Placenta and Circulating Exosomes in Gestational Diabetes Mellitus. J Diabetes Investig (2021) 12:1490–1500. doi: 10.1111/jdi.13493
78. Zhang L, Li K, Tian S, Wang X, Li J, Dong Y, et al. Down-Regulation of microRNA-30d-5p Is Associated With Gestational Diabetes Mellitus by Targeting RAB8A. J Diabetes Complications (2021) 35:107959. doi: 10.1016/j.jdiacomp.2021.107959
79. Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu Y, et al. Early Second-Trimester Serum miRNA Profiling Predicts Gestational Diabetes Mellitus. PloS One (2011) 6:e23925. doi: 10.1371/journal.pone.0023925
80. Zhao C, Zhang T, Shi Z, Ding H, Ling X. MicroRNA-518d Regulates PPARα Protein Expression in the Placentas of Females With Gestational Diabetes Mellitus. Mol Med Rep (2014) 9:2085–90. doi: 10.3892/mmr.2014.2058
81. Zhang Y-L, Chen X-Q. Dysregulation of microRNA-770-5p Influences Pancreatic-β-Cell Function by Targeting TP53 Regulated Inhibitor of Apoptosis 1 in Gestational Diabetes Mellitus. Eur Rev Med Pharmacol Sci (2020) 24:793–801. doi: 10.26355/eurrev_202001_20062
82. Zhou X, Xiang C, Zheng X. miR-132 Serves as a Diagnostic Biomarker in Gestational Diabetes Mellitus and Its Regulatory Effect on Trophoblast Cell Viability. Diagn Pathol (2019) 14(1):119. doi: 10.1186/s13000-019-0899-9
83. Zhu Y, Tian F, Li H, Zhou Y, Lu J, Ge Q. Profiling Maternal Plasma microRNA Expression in Early Pregnancy to Predict Gestational Diabetes Mellitus. Int J Gynecol Obstet (2015) 130:49–53. doi: 10.1016/j.ijgo.2015.01.010
84. Dornhorst A, Banerjee AA. ”Diabetes in Pregnancy.”, Textbook of Diabetes Blackwell(2010). p. 888–921.
85. Yang W-M, Jeong H-J, Park S-Y, Lee W. Saturated Fatty Acid-Induced miR-195 Impairs Insulin Signaling and Glycogen Metabolism in HepG2 Cells. FEBS Lett (2014) 588:3939–46. doi: 10.1016/j.febslet.2014.09.006
86. Hromadnikova I, Kotlabova K, Krofta L, Sirc J. Association Analysis in Children Born from Normal and Complicated Pregnancies-Cardiovascular Disease Associated microRNAs and the Incidence of Prehypertension/Hypertension, Overweight/Obesity, Valve Problems and Heart Defects. Int J Mol Sci (2020) 21:8413. doi: 10.3390/ijms21218413
87. Lv Z, Guo Y. Metformin and Its Benefits for Various Diseases. Front Endocrinol (2020) 11:191. doi: 10.3389/fendo.2020.00191
88. He X, Zhao L, Yue L, Zhang W, Wang W, Fu Y, et al. The Relationship Between IGF1 and the Expression Spectrum of miRNA in the Placenta of Preeclampsia Patients. Ginekol Pol (2019) 90:596–603. doi: 10.5603/GP.2019.0096
89. Collares CV, Evangelista AF, Xavier DJ, Rassi DM, Arns T, Foss-Freitas MC, et al. Identifying Common and Specific microRNAs Expressed in Peripheral Blood Mononuclear Cell of Type 1, Type 2, and Gestational Diabetes Mellitus Patients. BMC Res Notes (2013) 6:491. doi: 10.1186/1756-0500-6-491
90. Ibarra A, Vega Guedes B, Armas Roca M, Gonzalez Garcia-Cano D, Perera S, Horres R, et al. Placental microRNA Expression Patterns in Pregestational Diabetes and Identification of Specific Potential Biomarkers. Diabetol (2018) 161(1):S87 SSN0012–186X V. [Accessed November 8, 2021].
91. Pheiffer C, Dias S, Rheeder P, Adam S. MicroRNA Profiling in HIV-Infected South African Women with Gestational Diabetes Mellitus. Mol Diagn Ther (2019) 23:499–505. doi: 10.1007/s40291-019-00404-2
92. Florio MC, Magenta A, Beji S, Lakatta EG, Capogrossi MC. Aging, MicroRNAs, and Heart Failure. Curr Probl Cardiol (2020) 45:100406. doi: 10.1016/j.cpcardiol.2018.12.003
93. Huang RS, Gamazon ER, Ziliak D, Wen Y, Im HK, Zhang W, et al. Combinatorial microRNA Target Predictions. Nat Genet (2005) 37:495–500. doi: 10.1038/ng1536
94. Bovell LC, Shanmugam C, Putcha B-DK, Katkoori VR, Zhang B, Bae S, et al. The Prognostic Value of microRNAs Varies With Patient Race/ethnicity and Stage of Colorectal Cancer. Clin Cancer Res Off J Am Assoc Cancer Res (2013) 19:3955–65. doi: 10.1158/1078-0432.CCR-12-3302
95. Karere GM, Cox LA, Bishop AC, South AM, Shaltout HA, Mercado-Deane M-G, et al. Sex Differences in miRNA Expression and Cardiometabolic Risk Factors in Hispanic Adolescents With Obesity. J Pediatr (2021) 235:138–43.e5. doi: 10.1016/j.jpeds.2021.03.070
96. Pritchard CC, Cheng HH, Tewari M. MicroRNA Profiling: Approaches and Considerations. Nat Rev Genet (2012) 13:358–69. doi: 10.1038/nrg3198
97. Wang K, Yuan Y, Cho J-H, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA Spectrum Between Serum and Plasma. PLoS ONE (2012) 7. doi: 10.1371/journal.pone.0041561
98. Dias S, Hemmings S, Muller C, Louw J, Pheiffer C. MicroRNA Expression Varies according to Glucose Tolerance, Measurement Platform, and Biological Source. BioMed Res Int (2017) 2017:1080157. doi: 10.1155/2017/1080157
99. Krek A, Grün D, Poy MN, Wolf L, Rosenberg C, Epstein EJ, et al. Combinatorial microRNA Target Predictions. Nat Genet (2005) 37:495–500. doi: 10.1038/ng1536
Keywords: microRNAs, pregnancy, type 1 diabetes mellitus, type 2 diabetes mellitus, gestational diabetes mellitus
Citation: Masete M, Dias S, Malaza N, Adam S and Pheiffer C (2022) A Big Role for microRNAs in Gestational Diabetes Mellitus. Front. Endocrinol. 13:892587. doi: 10.3389/fendo.2022.892587
Received: 09 March 2022; Accepted: 24 June 2022;
Published: 25 July 2022.
Edited by:
Anca Dana Dobrian, Eastern Virginia Medical School, United StatesReviewed by:
Laura Dearden, University of Cambridge, United KingdomEwa Wender-Ozegowska, Poznan University of Medical Sciences, Poland
Copyright © 2022 Masete, Dias, Malaza, Adam and Pheiffer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmen Pheiffer, Y2FybWVuLnBoZWlmZmVyQG1yYy5hYy56YQ==
 Matladi Masete
Matladi Masete Stephanie Dias
Stephanie Dias Nompumelelo Malaza1,2
Nompumelelo Malaza1,2 Sumaiya Adam
Sumaiya Adam Carmen Pheiffer
Carmen Pheiffer