
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 27 July 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.892563
This article is part of the Research Topic Advances in the Diagnosis and Prevention of Diabetic Neuropathy View all 15 articles
 Jianhuai Chen1†
Jianhuai Chen1† Jindan Wu2†
Jindan Wu2† Xinfei Huang1†
Xinfei Huang1† Rui Sun2
Rui Sun2 Ziliang Xiang1
Ziliang Xiang1 Yan Xu1
Yan Xu1 Shi Chen3
Shi Chen3 Weilong Xu3
Weilong Xu3 Jie Yang4,5*
Jie Yang4,5* Yun Chen1*
Yun Chen1*Introduction: Type 2 diabetes mellitus (T2DM) has been found to be associated with abnormalities of the central and peripheral vascular nervous system, which were considered to be involved in the development of cognitive impairments and erectile dysfunction (ED). In addition, altered brain function and structure were identified in patients with ED, especially psychological ED (pED). However, the similarities and the differences of the central neural mechanisms underlying pED and T2DM with ED (DM-ED) remained unclear.
Methods: Diffusion tensor imaging data were acquired from 30 T2DM, 32 ED, and 31 DM-ED patients and 47 healthy controls (HCs). Then, whole-brain structural networks were constructed, which were mapped by connectivity matrices (90 × 90) representing the white matter between 90 brain regions parcellated by the anatomical automatic labeling template. Finally, the method of network-based statistic (NBS) was applied to assess the group differences of the structural connectivity.
Results: Our NBS analysis demonstrated three subnetworks with reduced structural connectivity in DM, pED, and DM-ED patients when compared to HCs, which were predominantly located in the prefrontal and subcortical areas. Compared with DM patients, DM-ED patients had an impaired subnetwork with increased structural connectivity, which were primarily located in the parietal regions. Compared with pED patients, an altered subnetwork with increased structural connectivity was identified in DM-ED patients, which were mainly located in the prefrontal and cingulate areas.
Conclusion: These findings highlighted that the reduced structural connections in the prefrontal and subcortical areas were similar mechanisms to those associated with pED and DM-ED. However, different connectivity patterns were found between pED and DM-ED, and the increased connectivity in the frontal–parietal network might be due to the compensation mechanisms that were devoted to improving erectile function.
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemia, insulin secretion dysfunction, and insulin resistance, which can lead to inflammation, oxidative stress, and endothelial dysfunction (1–3). T2DM has been identified to be associated with a variety of nervous system-related diseases and macro- and microvascular-related complications (4–6). The population-based studies suggested that the incidence of mild cognitive impairment in diabetic patients was around 21.8% in China and varied from 28 to 31.5% worldwide (7–9). An epidemiological study suggested that the prevalence of diabetes was increasing rapidly, and the prevalence of erectile dysfunction (ED) among diabetic patients varied from 35 to 90% (10). Compared with the general population, patients with T2DM have a higher risk for cognitive decline, which is one of the central nervous system complications associated with abnormalities of brain function and structure (11–13). In addition, T2DM patients are at higher risk of developing male sexual dysfunction, including ED and retrograde ejaculation, which are two common peripheral microvascular and neurological complications associated with oxidative stress-induced penile vascular endothelial cell injury and peripheral neuropathy (14–17).
ED is defined, in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) (18), as the inability to achieve and/or maintain an adequate erection until the completion of a sexual activity on 75% of attempts at a partnered sexual activity for satisfactory sexual intercourse or a marked decrease in turgidity ≥6 months with unsatisfactory sexual intercourse. Normal penile erection and detumescence is a complex neurovascular event that is regulated by the balance between the contraction and relaxation of cavernous smooth muscles (19). The etiological factors of ED can be classified as organic (neurogenic, arterial/venous, hormonal, and drug-induced) and psychological (20). Diabetes mellitus is considered as an important cause of organic ED (21, 22), while psychogenic ED (pED) is predominantly and exclusively attributed to psychological or interpersonal factors, such as performance anxiety and relationship stress (23). Hyperglycemia was considered to be associated with the development of impaired vasodilatory signaling, smooth muscle cell hypercontractility, and veno-occlusive disorder, which were all the mechanisms causing ED in T2DM patients and often led to resistance to current therapy (24, 25). Endothelial dysfunction was an important mechanism for the development of T2DM-related ED, and chronic hyperglycemia might lead to inflammation and contribute to the formation of reactive oxygen species, which were related to the development of endothelial dysfunction in T2DM-related ED (24). In addition, pED has been found to be related to impaired activity/functional connectivity and abnormal gray matter/white matter of the brain in recent functional and structural magnetic resonance imaging (MRI) studies (26–29). However, the neural mechanisms underlying T2DM, T2DM with ED (DM-ED), and pED remain unclear.
Diffusion tensor imaging (DTI) is a noninvasive MRI method that can be used to detect microstructural alterations of the white matter, which cannot be revealed by conventional structural MRI scan (30, 31). The integrity of nerve fibers can be measured by the parameter of fractional anisotropy (FA), which indicates the strength and direction of water molecules’ motion within the nerve fibers (32). Decreased FA values (values range from 0 to 1) indicate impaired microstructural tissue integrity of the white matter (33). A variety of white matter regions with microstructural alterations were found in T2DM patients by the technique of DTI (34). In addition, the structural brain networks [two elements: nodes defined by automated anatomical labeling (AAL) template; edges defined by white matter] of pED were constructed by the method of graph theory analysis, and the topological measures were compared with healthy controls (HCs) in our previous DTI study (26). The results showed that white matter fiber tracts connected with the left inferior frontal gyrus(triangular), amygdale, right inferior temporal gyrus, and rolandic operculum exhibited decreased strength of structural connectivity in pED patients, which was measured by FA value-weighted edges in the structural brain network (26).
Network-based statistic (NBS) is a validated nonparametrical statistical approach for elucidating the organization of brain while controlling family‐wise error. It is frequently applied to clinical applications, which can reveal altered connective strength in the brain network. To further identify different structural connections between pED and DM-ED, DTI data were acquired, and the approach of NBS was used in this study. We hypothesized that these patients would show a different structural connectivity located in key regions for sexual behavior regulation of the brain.
In this cross-sectional study, a total of 93 patients, including 30 T2DM, 32 pED, and 31 DM-ED patients, were enrolled in this study. In addition, 47 age- and education-matched HCs were recruited by local advertisements. The protocol and informed consent document were approved by the Medical Ethics Committee of Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine. Written informed consents were obtained from all individuals before their participation in this study.
The inclusion criteria for all subjects were as follows: (1) right-handed, (2) educated for at least 9 years, and (3) aged between 20 and 60 years. The level of HbA1c was measured for the diagnosis of T2DM, and all participants were asked to fill in the five-item version of the international index of erectile function (IIEF-5) questionnaire to determine the presence of ED (35). T2DM patients met the diagnosis of T2DM according to the latest criteria published by the American Diabetes Association (ADA) (2014) (36): (1) fasting plasma glucose (FPG) level ≥7.0 mmol/L or (2) 2‐h oral glucose tolerance test glucose level ≥11.1 mmol/L. Patients with DM met the diagnosis of T2DM based on ADA criteria with IIEF-5 scores >21. Patients with pED met the diagnosis of ED based on DSM-V criteria with IIEF-5 scores ≤21 and normal erection during sleeping (normal morning erection) or masturbation (the penis could maintain an erection until ejaculation during masturbation) was reported by themselves as well as normal penile hemodynamics rated by the color duplex doppler ultrasonography combined with intracavernous injection. DM-ED patients met the diagnosis of T2DM (within 2 years) with the presence of ED (IIEF-5 scores ≤21; abnormal erection during sleeping and masturbation without obvious psychological factors, such as depression, anxiety, etc.). HCs were defined as individuals with normal FPG (<7.0 mmol/L), HbA1c (<6.5) level, and IIEF-5 scores >21.
The exclusion criteria for all individuals were as follows: (1) other types of diabetes, (2) history of severe hyperglycemia coma and hypoglycemia, (3) major medical illnesses or complications, such as severe liver, kidney, or cardiovascular disease or tumors, (4) psychiatric or neurologic disorders, (5) alcohol or other substance abuse, (6) organic brain lesions, such as brain injury, cerebrovascular lesions, or tumors, and (7) any MRI contraindication.
The MRI data were obtained with a 3.0-T MRI scanner (Siemens, Germany). All participants were instructed to relax with their eyes closed, keeping their heads still an avoid deliberate thinking and falling asleep during scanning. High-resolution sagittal three-dimensional T1-weighted images and DTI images were acquired with the parameters that have been described in our previous studies (37–40).
T1-weighted and DTI data were preprocessed using the diffusion toolbox of Functional MRI of the Brain software library (41). Then, whole-brain tractography was performed for the definition of edges in the brain network using the software of Diffusion Toolkit. In addition, the AAL template was used to define the nodes of the brain network (42). The detailed steps of preprocessing and construction of the whole-brain white matter network were performed (Figure 1) as reported in our previous studies (37–40).
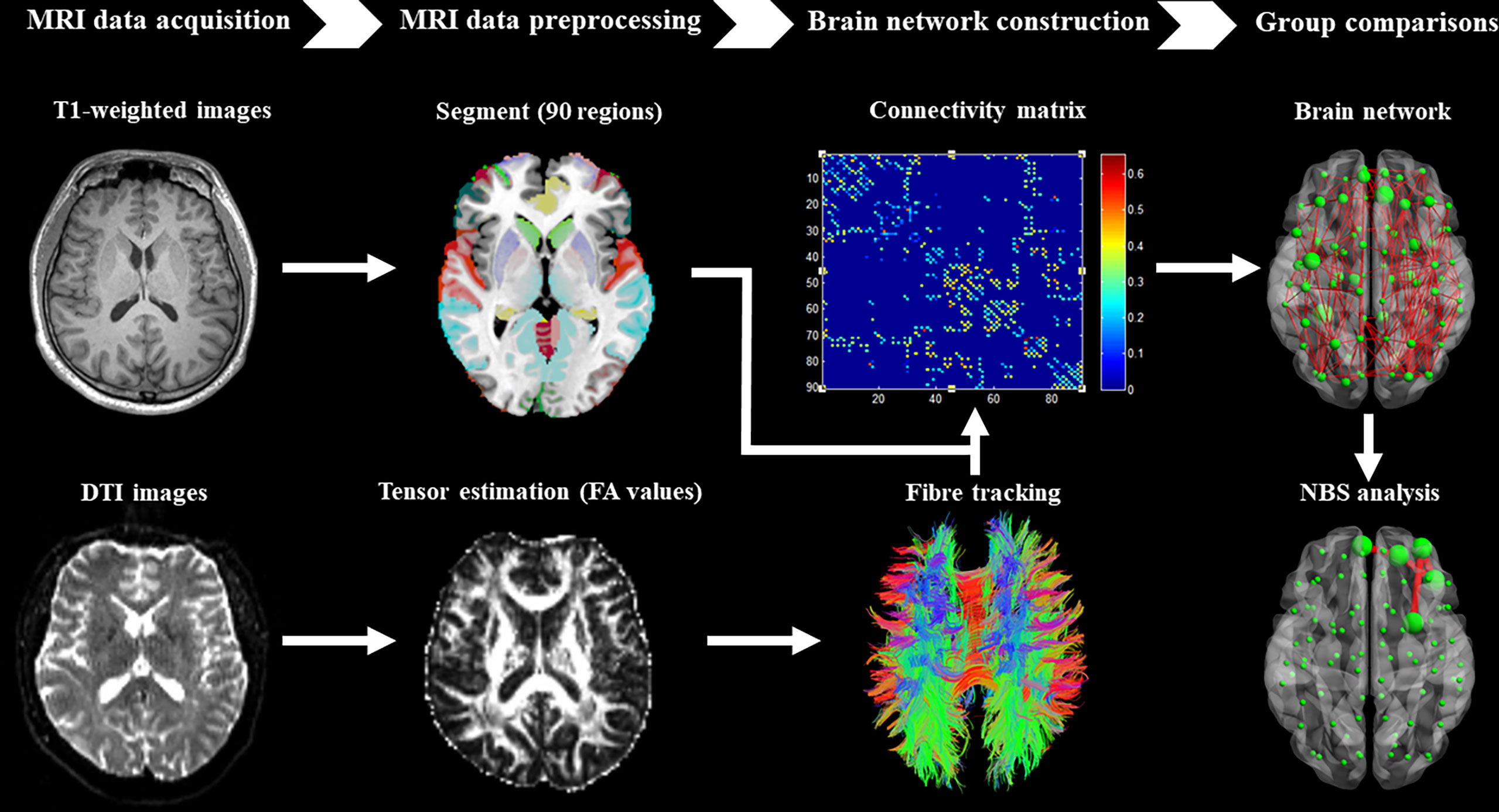
Figure 1 Brief flow chart showing MRI data acquisition, preprocessing, construction of structural brain network, and network-based statistical analysis between groups.
NBS is a statistical method based on the graph theory and is often used to explore differences of the structural connectivity in the brain white matter network, which may be related to the diagnostic statue (43). NBS analysis is usually conducted to identify subnetworks comprising pairs of nodes and connections for which the strength of structural connectivity is significantly different between groups (44, 45). Firstly, two-sample t-tests were performed for all pairs (90 × 89/2 = 4,005) of nodes to test the null hypothesis of equality between groups in mean structural connectivity with respect to the size of interconnected subnetwork/component of edges rather than individually at each connection. Among the structural connections exceeding 2.5 (test–statistic threshold), the search was performed to identify any connected subnetwork, including a collection of regions and a set of suprathreshold connections. The size of the identified subnetwork was determined by the number of suprathreshold connections it comprised. Secondly, permutation tests were conducted to calculate the corrected P-value for each network. The size of the largest subnetwork was recorded, and the null distribution was generated for calculating the family-wise error-corrected statistical threshold across the set of all connections. Finally, the corrected P-value for the identified subnetwork (size = K) in the un-permuted/actual data was computed as the proportion of permutations for which the size of the subnetwork was equal or greater than K. Therefore, NBS is a statistical approach that controls the family-wise error rate across all connections of the brain network, which offers more power than the method of false discovery rate.
The group differences of demographic and clinical variables were compared by using the SPSS software package (IBM, USA). The data normality was evaluated by Kolmogorov–Smirnov test, and the variance homogeneity was measured by Levene’s test. The one-way ANOVA was used to detect demographic and clinical differences among the three groups, while two sample t-test was performed to reveal differences of variables between two groups. The statistical significance threshold was set at P <0.05.
One-way ANOVA and post-hoc analysis with two-sample t-test were applied to identify the group differences of structural connectivity in the white matter brain network by the method of NBS. The connected subnetworks were considered to be significantly different if the corrected P <0.05 at the whole-network level with the preliminary statistic threshold 2.5 (50,000 permutations).
The demographic and clinical characteristics of the three groups are presented in Table 1. No significant differences were found in the age and educational level. Patients with pED and DM-ED had decreased IIEF-5 scores when compared to those with DM and HCs. In addition, there were no significant differences in the level of HbA1c between patients with DM and DM-ED.
As shown in Table 2 and Figure 2, the subnetworks that showed significant differences between groups were identified. Compared to HCs, DM patients showed significantly decreased structural connectivity in a subnetwork comprising four brain regions (four right and zero left) and three connections (zero interhemispheric and three intrahemispheric). This subnetwork involved right middle frontal gyrus (orbital part), thalamus, putamen, and caudate nucleus. A subnetwork comprising five brain regions (four right and one left) and four reduced structural connections (one interhemispheric and three intrahemispheric) was identified in patients with pED when compared with HCs. In this subnetwork, the five well-connected brain regions were the left superior frontal gyrus (medial orbital) and right superior frontal gyrus (orbital part), inferior frontal gyrus (orbital part), middle frontal gyrus (orbital part), and putamen. The NBS analysis also revealed that a subnetwork was significantly different between DM-ED patients and HCs. The subnetwork consisted of four brain regions, including the right middle frontal gyrus (orbital part), thalamus, putamen, pallidum (four right and zero left) and four reduced structural connections (zero interhemispheric and four intrahemispheric).
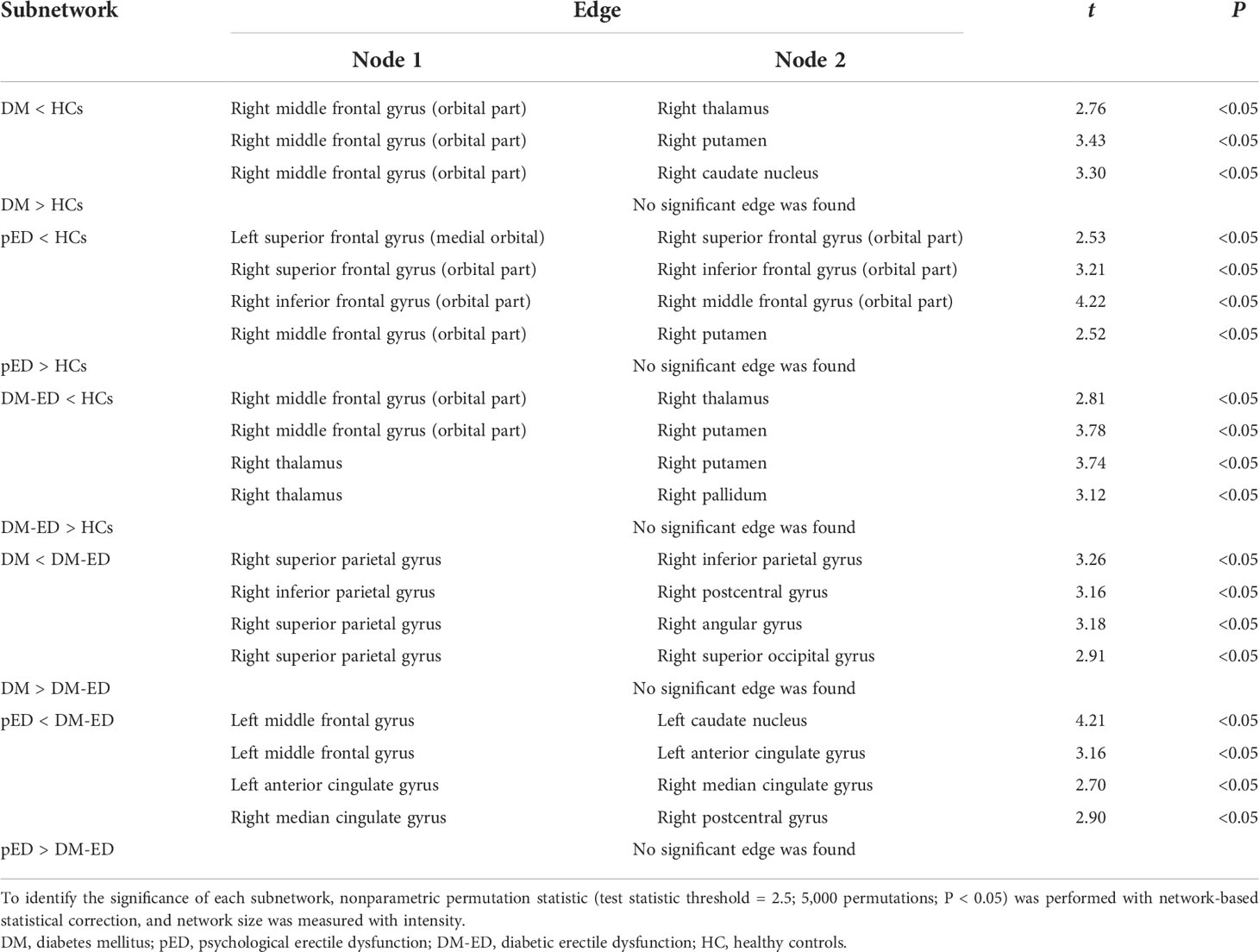
Table 2 Subnetworks identified to be significantly different among the DM, pED, DM-ED, and HC groups using network-based statistical analysis.
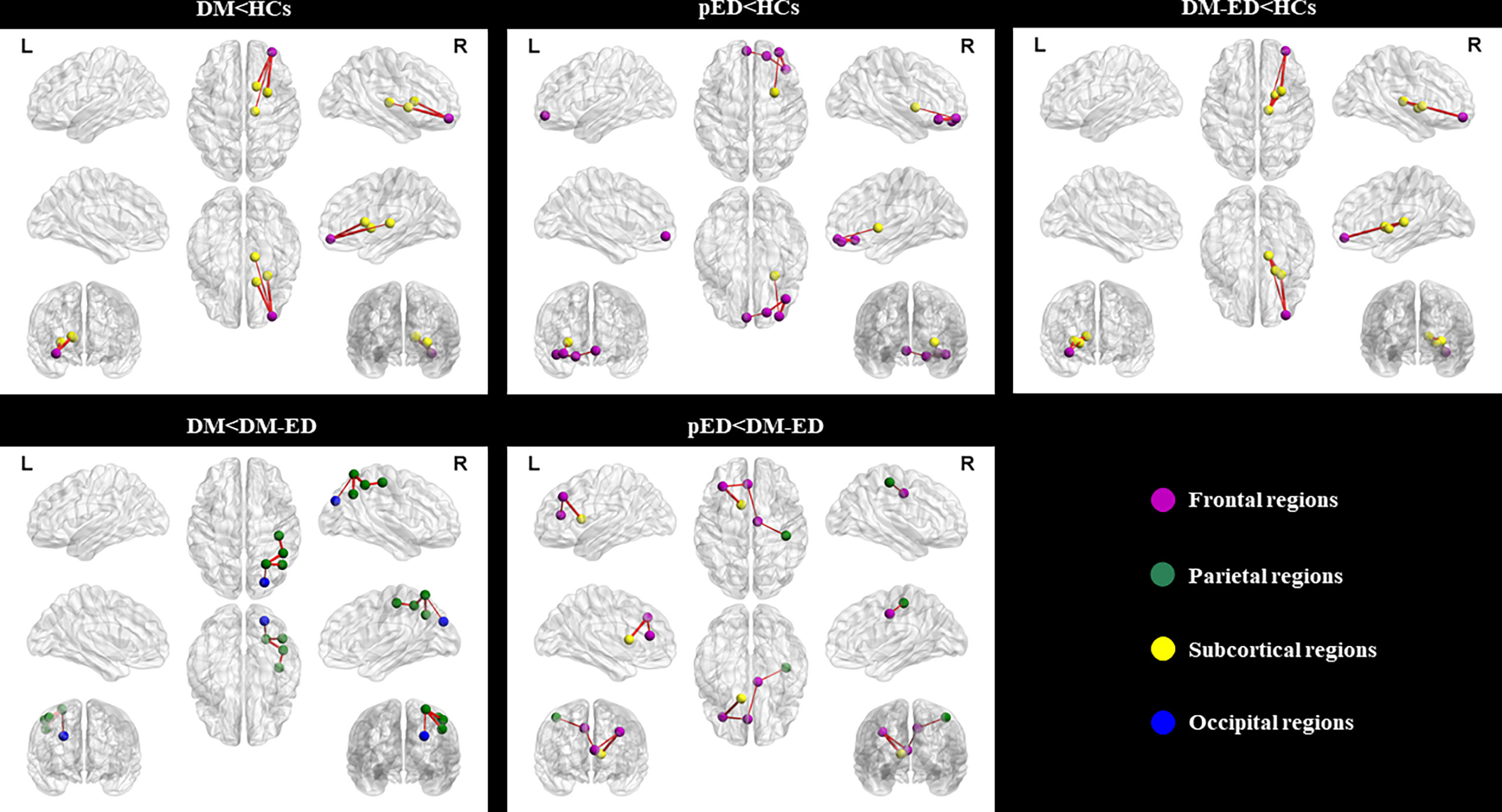
Figure 2 Subnetworks showing differences among the DM, pED, DM-ED, and HC groups using network-based statistical analysis. L, left; R, right; DM, diabetes mellitus; pED, psychogenic erectile dysfunction; DM-ED, diabetic erectile dysfunction; HCs, healthy controls.
In addition, the DM-ED patients demonstrated a subnetwork with five brain regions (five right and zero left) and four increased connections (zero interhemispheric and four intrahemispheric) when compared with DM patients. The regions of this subnetwork were located in the right superior parietal gyrus, inferior parietal gyrus, postcentral gyrus, angular gyrus, and superior occipital gyrus. Moreover, DM-ED patients had a different subnetwork comprising five brain regions (two right and three left) and four increased connections (one interhemispheric and three intrahemispheric) when compared with pED patients. The subnetwork consisted of the left middle frontal gyrus, anterior cingulate gyrus, caudate nucleus, right median cingulate gyrus, and postcentral gyrus.
To the best of our knowledge, this is the first study to explore the differences of structural connectivity between patients with pED and DM-ED by the method of NBS analysis. The findings demonstrated that decreased structural connectivity was found in patients with DM, pED, and DM-ED when compared with HCs. The abnormal brain regions were mainly distributed in the prefrontal and subcortical areas. In addition, DM-ED patients presented increased subnetworks consisting of parietal regions and prefrontal–cingulate areas when compared with DM patients and pED patients, respectively. These findings highlighted the importance of structural network analysis in understanding the different central neural mechanisms underlying diabetic and psychological ED.
In this study, we used DTI data to investigate the different topological properties of brain network between pED and DM-ED. Abnormal structural connectivity of white matter in the brain network were found in DM, pED, and DM-ED patients. The microstructural changes of white matter were speculated to be caused by the compromise of myelin sheath and the impairment or decrement of axons, which might lead to decreased neuronal signal transmission (34). The measure of FA, representing white matter integrity, is more sensitive than structural MRI metrics (33). DTI can detect and quantify subtle abnormalities of white matter before those are detectable by conventional structural MRI scans (30). Therefore, these findings might serve as imaging biomarkers for early diagnosis, monitoring disease progression, and response to therapy of brain disorders (46–48).
In recent years, DTI has been actively used in the investigation of brain structural connectivity alterations in sexual dysfunction patients including ED and premature ejaculation to understand the neuropathophysiology of these two disorders related to some psychological factors (26, 40). Our previous study showed that pED patients had damaged white matter in the left prefrontal and limbic cortex by the method of graph theoretical analysis (26). In addition, white matter microstructural changes were also found in pED patients by the method of tract-based spatial statistics based on DTI data (49). In this study, both pED and DM-ED patients showed lower structural connectivity in the prefrontal and subcortical areas when compared with HCs. Reduced structural connectivity was identified in the left superior frontal gyrus, right frontal regions, and putamen in pED patients, while DM-ED patients exhibited decreased structural connectivity in the right middle frontal gyrus, thalamus, putamen, and pallidum. This finding suggested that pED patients had more impairments in the frontal regions; however, DM-ED patients had more abnormalities in the subcortical areas. Previous studies demonstrated that the subcortical areas and, in particular, the thalamus seemed to be susceptible to T2DM. In addition, pED, owing predominantly to psychological factors including anxiety, depression, and introversion, was found to be more vulnerable to structural and functional changes in the prefrontal regions (26, 27, 38). Therefore, our findings were in agreement with the central neural mechanisms of pED and DM in previous neuroimaging studies (11, 38, 50).
The putamen was a key subcortical region receiving inputs from the prefrontal regions and projecting to other portions of the subcortical areas (51). The putamen, a critical component of the reward network, was considered to facilitate the integration of information from different brain areas and played an important role in reward-related behaviors (52). Sexual behavior was a subjectively pleasurable experience and activity which, in the putamen, could be triggered by visual sexual stimuli, which acted like rewarding stimuli (53–55). In previous neuroimaging studies, activation in the putamen was found to be associated with male sexual arousal and penile turgidity (56, 57). The interactions between the prefrontal and putamen were known to be important for reward and sexual behavior (58, 59). Impaired gray matter of the putamen was found in pED patients (60). The structural connectivity between the prefrontal and putamen might be abnormal and associated with the underlying neural mechanisms of pED. With the exception of the putamen, more subcortical regions, including thalamus and pallidum, were found to have reduced structural connectivity in DM-ED patients. The thalamus was considered as an integration center for different brain regions, and it was found to be a critical structure for cognitive dysfunction in T2DM patients (61, 62). Decreased FA value was found in the thalamus in diabetes mellitus patients when compared with HCs, and the decreased FA was associated with worse neurocognitive performance of patients (63). Both the putamen and pallidum were two important components of the striatum, which played a key role in various brain functions, including cognitive function and reward, through the cortico-striato-thalamo-cortical pathway (64–66). T2DM was often accompanied with ED, which might be also associated with the structural abnormalities in the brain as manifested by decreased structural connectivity in the striato-thalamo-frontal circuit.
In this study, increased structural connectivity was found in the frontal–parietal network of DM-ED when compared with DM and pED. The frontal–parietal network played a vital role in cognitive function, including attention, executive function, and working memory, and it was often activated by executive function-related tasks (67). In previous studies, the inferior parietal lobule was activated in response to visual sexual stimuli, and the regional cerebral blood flow of this region was found to be positively correlated with the level of penile tumescence (56, 68). The initiation and level of penile tumescence in response to visual sexual stimuli was controlled by the frontal–network (69). In addition, increased activation was found in the frontal–parietal network in youth with type 1 diabetes when compared with HCs (70). Therefore, the increased structural connectivity in the frontal–parietal network might indicate compensatory changes for DM-ED patients. However, the complex mechanisms underlying the compensatory changes needed to be explored in further studies with a larger sample size.
In addition, several limitations should be taken into consideration in this study. Firstly, the relatively small sample size and cross-sectional study might limit the generalizability of these findings. Secondly, more demographic and clinical characteristics should be obtained, and their relationships with altered structural connectivity in the brain network should also be explored in our future studies. Finally, future studies entailing longitudinal studies with treatment were needed to evaluate the alterations in brain structural connectivity under treatment and might provide new insight into the treatment strategy of ED.
In summary, this might be the first study to investigate the differences of structural connectivity between diabetic and psychological ED by the method of NBS analysis based on DTI data. Our results showed that both DM-ED and pED had decreased structural connectivity in the frontal-subcortical regions. In addition, DM-ED patients presented increased structural connectivity in the frontal–parietal network, which might be a compensatory mechanism. These findings provided the first evidence of the common and different central neural mechanisms between diabetic and psychological factors related ED.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the medical ethics committee of Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
JC, YC, JY, and JW designed the experiments. JC, JY, JW, XH, RS, ZX, YX, SC, and WX contributed to clinical data collection and assessment. JC, XH, ZX, YX, JW, and JY analyzed the results. JC, JY, JW, and XH wrote the manuscript. JC, YC, JY, and JW approved the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (nos. 81701433 and 81871154), Key Project of Jiangsu Provincial Health Commission (no. ZDA2020025), Natural Science Foundation of Nanjing University of Chinese Medicine (no. XZR2020003), Special Project of Innovation and Development Fund of Jiangsu Province Hospital of Chinese Medicine (no. Y2021CX24), and General Project of Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01A23).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Banik S, Ghosh A. The association of oxidative stress biomarkers with type 2 diabetes mellitus: A systematic review and meta-analysis. Health Sci Rep (2021) 4(4):e389. doi: 10.1002/hsr2.389
2. Zhou Z, Collado A, Sun C, Tratsiakovich Y, Mahdi A, Winter H, et al. Downregulation of erythrocyte miR-210 induces endothelial dysfunction in type 2 diabetes. Diabetes (2022) 71(2):285–97. doi: 10.2337/db21-0093
3. Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity (2022) 55(1):31–55. doi: 10.1016/j.immuni.2021.12.013
4. Pourabbasi A, Tehrani-Doost M, Qavam SE, Arzaghi SM, Larijani B. Association of diabetes mellitus and structural changes in the central nervous system in children and adolescents: a systematic review. J Diabetes Metab Disord (2017) 16:10. doi: 10.1186/s40200-017-0292-8
5. Issar T, Tummanapalli SS, Borire AA, Kwai NCG, Poynten AM, Arnold R, et al. Impact of the metabolic syndrome on peripheral nerve structure and function in type 2 diabetes. Eur J Neurol (2021) 28(6):2074–82. doi: 10.1111/ene.14805
6. Weiss JS, Sumpio BE. Review of prevalence and outcome of vascular disease in patients with diabetes mellitus. Eur J Vasc Endovasc Surg (2006) 31(2):143–50. doi: 10.1016/j.ejvs.2005.08.015
7. Li W, Sun L, Li G, Xiao S. Prevalence, influence factors and cognitive characteristics of mild cognitive impairment in type 2 diabetes mellitus. Front Aging Neurosci (2019) 11:180. doi: 10.3389/fnagi.2019.00180
8. Gorska-Ciebiada M, Saryusz-Wolska M, Ciebiada M, Loba J. Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: prevalence, risk factors, and comorbidity. J Diabetes Res (2014) 2014:179648. doi: 10.1155/2014/179648
9. Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol (2007) 64(4):570–5. doi: 10.1001/archneur.64.4.570
10. Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sexual Med (2009) 6(5):1232–47. doi: 10.1111/j.1743-6109.2008.01168.x
11. Lei H, Hu R, Luo G, Yang T, Shen H, Deng H, et al. Altered structural and functional MRI connectivity in type 2 diabetes mellitus related cognitive impairment: A review. Front Hum Neurosci (2021) 15:755017. doi: 10.3389/fnhum.2021.755017
12. You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetologica (2021) 58(6):671–85. doi: 10.1007/s00592-020-01648-9
13. Rosenberg J, Lechea N, Pentang GN, Shah NJ. What magnetic resonance imaging reveals - a systematic review of the relationship between type II diabetes and associated brain distortions of structure and cognitive functioning. Front Neuroendocrinol (2019) 52:79–112. doi: 10.1016/j.yfrne.2018.10.001
14. Fan J, Peng T, Hui J, Ding W, He B, Zhang H, et al. Erectile dysfunction in type-2 diabetes mellitus patients: Predictors of early detection and treatment. Urologia Internationalis (2021) 105(11-12):986–92. doi: 10.1159/000514700
15. Fedder J, Kaspersen MD, Brandslund I, Højgaard A. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology (2013) 1(4):602–6. doi: 10.1111/j.2047-2927.2013.00083.x
16. Zhou B, Chen Y, Yuan H, Wang T, Feng J, Li M, et al. NOX1/4 inhibitor GKT-137831 improves erectile function in diabetic rats by ROS reduction and endothelial nitric oxide synthase reconstitution. J Sexual Med (2021) 18(12):1970–83. doi: 10.1016/j.jsxm.2021.09.007
17. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care (2003) 26(5):1553–79. doi: 10.2337/diacare.26.5.1553
18. Segraves RT. Considerations for diagnostic criteria for erectile dysfunction in DSM V. J Sexual Med (2010) 7(2 Pt 1):654–60. doi: 10.1111/j.1743-6109.2009.01684.x
19. Giuliano F. Neurophysiology of erection and ejaculation. J Sexual Med (2011) 8 Suppl 4:310–5. doi: 10.1111/j.1743-6109.2011.02450.x
20. Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile dysfunction: AUA guideline. J Urol (2018) 200(3):633–41. doi: 10.1016/j.juro.2018.05.004
21. Yuan C, Jian Z, Gao X, Jin X, Wang M, Xiang L, et al. Type 2 diabetes mellitus increases risk of erectile dysfunction independent of obesity and dyslipidemia: A mendelian randomization study. Andrology (2022) 10(3):518–24. doi: 10.1111/andr.13132
22. Maiorino MI, Bellastella G, Esposito K. Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndrome Obes (2014) 7:95–105. doi: 10.2147/DMSO.S36455
23. Yang Y, Song Y, Lu Y, Xu Y, Liu L, Liu X. Associations between erectile dysfunction and psychological disorders (depression and anxiety): A cross-sectional study in a Chinese population. Andrologia (2019) 51(10):e13395. doi: 10.1111/and.13395
24. Hidalgo-Tamola J, Chitaley K. Review type 2 diabetes mellitus and erectile dysfunction. J Sexual Med (2009) 6(4):916–26. doi: 10.1111/j.1743-6109.2008.01116.x
25. Phé V, Rouprêt M. Erectile dysfunction and diabetes: a review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab (2012) 38(1):1–13. doi: 10.1016/j.diabet.2011.09.003
26. Chen J, Chen Y, Gao Q, Chen G, Dai Y, Yao Z, et al. Brain structural network topological alterations of the left prefrontal and limbic cortex in psychogenic erectile dysfunction. Int J Neurosci (2018) 128(5):393–403. doi: 10.1080/00207454.2017.1387116
27. Chen J, Chen Y, Chen G, Dai Y, Yao Z, Lu Q. Altered brain networks in psychogenic erectile dysfunction: a resting-state fMRI study. Andrology (2017) 5(6):1073–81. doi: 10.1111/andr.12411
28. Ma Z, Ren F, Huang X, Yang X, Li H, Li G, et al. Decreased gray matter volume of the anterior insular cortex in patients with psychogenic erectile dysfunction: A voxel-based morphometry study. J Psychiatr Res (2021) 145:125–31. doi: 10.1016/j.jpsychires.2021.12.006
29. Wang Y, Dong M, Guan M, Wu J, He Z, Zou Z, et al. Aberrant insula-centered functional connectivity in psychogenic erectile dysfunction patients: A resting-state fMRI study. Front Hum Neurosci (2017) 11:221. doi: 10.3389/fnhum.2017.00221
30. Zhao Y, Yang L, Gong G, Cao Q, Liu J. Identify aberrant white matter microstructure in ASD, ADHD and other neurodevelopmental disorders: A meta-analysis of diffusion tensor imaging studies. Prog Neuropsychopharmacol Biol Psychiatry (2022) 113:110477. doi: 10.1016/j.pnpbp.2021.110477
31. Jiang J, Zhao YJ, Hu XY, Du MY, Chen ZQ, Wu M, et al. Microstructural brain abnormalities in medication-free patients with major depressive disorder: a systematic review and meta-analysis of diffusion tensor imaging. J Psychiatry Neurosci (2017) 42(3):150–63. doi: 10.1503/jpn.150341
32. Yang X, Cao D, Liang X, Zhao J. Schizophrenia symptomatic associations with diffusion tensor imaging measured fractional anisotropy of brain: a meta-analysis. Neuroradiology (2017) 59(7):699–708. doi: 10.1007/s00234-017-1844-9
33. Papanikolaou N, Karampekios S, Papadaki E, Malamas M, Maris T, Gourtsoyiannis N. Fractional anisotropy and mean diffusivity measurements on normal human brain: comparison between low-and high-resolution diffusion tensor imaging sequences. Eur Radiol (2006) 16(1):187–92. doi: 10.1007/s00330-005-2833-7
34. Xiong Y, Sui Y, Zhang S, Zhou XJ, Yang S, Fan Y, et al. Brain microstructural alterations in type 2 diabetes: diffusion kurtosis imaging provides added value to diffusion tensor imaging. Eur Radiol (2019) 29(4):1997–2008. doi: 10.1007/s00330-018-5746-y
35. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impotence Res (1999) 11(6):319–26. doi: 10.1038/sj.ijir.3900472
36. American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care (2014) 37 Suppl 1:S14–80. doi: 10.2337/dc14-S014
37. Chen J, Huang X, Liu S, Lu C, Dai Y, Yao Z, et al. Disrupted topological properties of brain networks in erectile dysfunction patients owing predominantly to psychological factors: a structural and functional neuroimaging study. Andrology (2020) 8(2):381–91. doi: 10.1111/andr.12684
38. Chen J, Chen Y, Gao Q, Chen G, Dai Y, Yao Z, et al. Impaired prefrontal-amygdala pathway, self-reported emotion, and erection in psychogenic erectile dysfunction patients with normal nocturnal erection. Front Hum Neurosci (2018) 12:157. doi: 10.3389/fnhum.2018.00157
39. Chen J, Yang J, Huang X, Lu C, Liu S, Dai Y, et al. Variation in brain subcortical network topology between men with and without PE: A diffusion tensor imaging study. J Sexual Med (2020) 17(1):48–59. doi: 10.1016/j.jsxm.2019.10.009
40. Chen J, Yang J, Xiang Z, Huang X, Lu C, Liu S, et al. Graph theory analysis reveals premature ejaculation is a brain disorder with altered structural connectivity and depressive symptom: A DTI-based connectome study. Eur J Neurosci (2021) 53(6):1905–21. doi: 10.1111/ejn.15048
41. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage (2012) 62(2):782–90. doi: 10.1016/j.neuroimage.2011.09.015
42. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage (2002) 15(1):273–89. doi: 10.1006/nimg.2001.0978
43. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage (2010) 53(4):1197–207. doi: 10.1016/j.neuroimage.2010.06.041
44. Zalesky A, Cocchi L, Fornito A, Murray MM, Bullmore E. Connectivity differences in brain networks. NeuroImage (2012) 60(2):1055–62. doi: 10.1016/j.neuroimage.2012.01.068
45. Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry (2014) 76(7):567–74. doi: 10.1016/j.biopsych.2014.02.018
46. Taylor KI, Sambataro F, Boess F, Bertolino A, Dukart J. Progressive decline in Gray and white matter integrity in de novo parkinson's disease: An analysis of longitudinal Parkinson progression markers initiative diffusion tensor imaging data. Front Aging Neurosci (2018) 10:318. doi: 10.3389/fnagi.2018.00318
47. Grieve SM, Korgaonkar MS, Gordon E, Williams LM, Rush AJ. Prediction of nonremission to antidepressant therapy using diffusion tensor imaging. J Clin Psychiatry (2016) 77(4):e436–443. doi: 10.4088/JCP.14m09577
48. Podwalski P, Szczygieł K, Tyburski E, Sagan L, Misiak B, Samochowiec J. Magnetic resonance diffusion tensor imaging in psychiatry: a narrative review of its potential role in diagnosis. Pharmacol Rep (2021) 73(1):43–56. doi: 10.1007/s43440-020-00177-0
49. Zhang P, Liu J, Li G, Pan J, Li Z, Liu Q, et al. White matter microstructural changes in psychogenic erectile dysfunction patients. Andrology (2014) 2(3):379–85. doi: 10.1111/j.2047-2927.2014.00191.x
50. Lu L, Sedor JR, Gulani V, Schelling JR, O'Brien A, Flask CA, et al. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol (2011) 34(5):476–82. doi: 10.1159/000333044
51. Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci (2016) 18(1):7–21. doi: 10.31887/DCNS.2016.18.1/shaber
52. Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci (2007) 27(31):8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007
53. Gola M, Wordecha M, Marchewka A, Sescousse G. Visual sexual stimuli-cue or reward? a perspective for interpreting brain imaging findings on human sexual behaviors. Front Hum Neurosci (2016) 10:402. doi: 10.3389/fnhum.2016.00402
54. Bruijnzeel AW. Kappa-opioid receptor signaling and brain reward function. Brain Res Rev (2009) 62(1):127–46. doi: 10.1016/j.brainresrev.2009.09.008
55. Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AA. Brain activation during human male ejaculation. J Neurosci (2003) 23(27):9185–93. doi: 10.1523/JNEUROSCI.23-27-09185.2003
56. Redouté J, Stoléru S, Grégoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp (2000) 11(3):162–77. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A
57. Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain (2002) 125(Pt 5):1014–23. doi: 10.1093/brain/awf108
58. Wang J, Zhang P, Li W, Wen Q, Liu F, Xu J, et al. Right posterior insula and putamen volume mediate the effect of oxytocin receptor polygenic risk for autism spectrum disorders on reward dependence in healthy adults. Cereb Cortex (New York NY 1991) (2021) 31(2):746–56. doi: 10.1093/cercor/bhaa198
59. Cheng JC, Secondary J, Burke WH, Fedoroff JP, Dwyer RG. Neuroimaging and sexual behavior: identification of regional and functional differences. Curr Psychiatry Rep (2015) 17(7):55. doi: 10.1007/s11920-015-0593-x
60. Cera N, Delli Pizzi S, Di Pierro ED, Gambi F, Tartaro A, Vicentini C, et al. Macrostructural alterations of subcortical grey matter in psychogenic erectile dysfunction. PloS One (2012) 7(6):e39118. doi: 10.1371/journal.pone.0039118
61. Zhou Y, Si X, Chen Y, Chao Y, Lin CP, Li S, et al. Hippocampus- and thalamus-related fiber-specific white matter reductions in mild cognitive impairment. Cereb Cortex (New York NY 1991) (2021). doi: 10.1093/cercor/bhab407 Epub ahead of print.
62. Sanjari Moghaddam H, Ghazi Sherbaf F, Aarabi MH. Brain microstructural abnormalities in type 2 diabetes mellitus: A systematic review of diffusion tensor imaging studies. Front Neuroendocrinol (2019) 55:100782. doi: 10.1016/j.yfrne.2019.100782
63. Xuan DS, Zhao X, Liu YC, Xing QN, Shang HL, Zhu PY, et al. Brain development in infants of mothers with gestational diabetes mellitus: A diffusion tensor imaging study. J Comput Assisted Tomography (2020) 44(6):947–52. doi: 10.1097/RCT.0000000000001110
64. Loonen AJ, Ivanova SA. Circuits regulating pleasure and happiness-mechanisms of depression. Front Hum Neurosci (2016) 10:571. doi: 10.3389/fnhum.2016.00571
65. Gomes FV. Altered ventral striatum-hippocampus connectivity during reward processing as an endophenotype for psychosis. Biol Psychiatry (2022) 91(2):e7–9. doi: 10.1016/j.biopsych.2021.10.019
66. de Kloet SF, Bruinsma B, Terra H, Heistek TS, Passchier EMJ, van den Berg AR, et al. Bi-directional regulation of cognitive control by distinct prefrontal cortical output neurons to thalamus and striatum. Nat Commun (2021) 12(1):1994. doi: 10.1038/s41467-021-22260-7
67. Ray KL, Ragland JD, MacDonald AW, Gold JM, Silverstein SM, Barch DM, et al. Dynamic reorganization of the frontal parietal network during cognitive control and episodic memory. Cognitive Affect Behav Neurosci (2020) 20(1):76–90. doi: 10.3758/s13415-019-00753-9
68. Stoléru S, Redouté J, Costes N, Lavenne F, Bars DL, Dechaud H, et al. Brain processing of visual sexual stimuli in men with hypoactive sexual desire disorder. Psychiatry Res (2003) 124(2):67–86. doi: 10.1016/S0925-4927(03)00068-4
69. Moulier V, Mouras H, Pélégrini-Issac M, Glutron D, Rouxel R, Grandjean B, et al. Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. NeuroImage (2006) 33(2):689–99. doi: 10.1016/j.neuroimage.2006.06.037
Keywords: type 2 diabetes mellitus, erectile dysfunction, diffusion tensor imaging, network-based statistic, psychological erectile dysfunction
Citation: Chen J, Wu J, Huang X, Sun R, Xiang Z, Xu Y, Chen S, Xu W, Yang J and Chen Y (2022) Differences in structural connectivity between diabetic and psychological erectile dysfunction revealed by network-based statistic: A diffusion tensor imaging study. Front. Endocrinol. 13:892563. doi: 10.3389/fendo.2022.892563
Received: 09 March 2022; Accepted: 01 July 2022;
Published: 27 July 2022.
Edited by:
Charumathi Sabanayagam, Singapore Eye Research Institute (SERI), SingaporeReviewed by:
Yan Zhang, Sun Yat-sen University, ChinaCopyright © 2022 Chen, Wu, Huang, Sun, Xiang, Xu, Chen, Xu, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Chen, Y2hlbnl1bm5qdUAxNjMuY29t; Jie Yang, eWoxOTc5MTJAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.