
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 13 July 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.890057
This article is part of the Research Topic Advances in the Diagnosis and Prevention of Diabetic Neuropathy View all 15 articles
Aims: To develop and validate a nomogram prediction model for the risk of diabetic foot in patients with type 2 diabetes mellitus (T2DM) and evaluate its clinical application value.
Methods: We retrospectively collected clinical data from 1,950 patients with T2DM from the Second Affiliated Hospital of Xi’an Jiaotong University between January 2012 and June 2021. The patients were divided into training cohort and validation cohort according to the random number table method at a ratio of 7:3. The independent risk factors for diabetic foot among patients with T2DM were identified by multivariate logistic regression analysis. Then, a nomogram prediction model was developed using the independent risk factors. The model performances were evaluated by the area under the receiver operating characteristic curve (AUC), calibration plot, Hosmer–Lemeshow test, and the decision curve analysis (DCA).
Results: Multivariate logistic regression analysis indicated that age, hemoglobin A1c (HbA1c), low-density lipoprotein (LDL), total cholesterol (TC), smoke, and drink were independent risk factors for diabetic foot among patients with T2DM (P < 0.05). The AUCs of training cohort and validation cohort were 0.806 (95% CI: 0.775∼0.837) and 0.857 (95% CI: 0.814∼0.899), respectively, suggesting good discrimination of the model. Calibration curves of training cohort and validation cohort showed a favorable consistency between the predicted probability and the actual probability. In addition, the P values of Hosmer–Lemeshow test for training cohort and validation cohort were 0.826 and 0.480, respectively, suggesting a high calibration of the model. When the threshold probability was set as 11.6% in the DCA curve, the clinical net benefits of training cohort and validation cohort were 58% and 65%, respectively, indicating good clinical usefulness of the model.
Conclusion: We developed and validated a user-friendly nomogram prediction model for the risk of diabetic foot in patients with T2DM. Nomograms may help clinicians early screen and identify patients at high risk of diabetic foot.
Type 2 diabetes mellitus (T2DM), previously referred to as noninsulin-dependent diabetes or adult-onset diabetes and accounting for 90%–95% of all diabetes, is a disease caused by a gradual decrease in insulin secretion from β cells in the context of insulin resistance (1, 2). T2DM is a disease involving the interaction between genetic and environmental risk factors leading to the underlying pathophysiology of beta cell dysfunction as well as insulin resistance in liver and muscle (3, 4). Poorly controlled T2DM can lead to chronic diabetic complications such as microangiopathy (retinopathy and kidney disease), atherosclerotic cardiovascular disease, peripheral neuropathy (sensory dysfunction), and diabetic foot (5–10). In the above complications, the diabetic foot negatively affects the quality of both work and life of patients. The decline in daily living activities of patients with diabetic foot results in substantial physical and psychological burdens on the patients.
Diabetic foot is one of the most serious and costly chronic complications of diabetes. It refers to foot ulcer, infection, or deep tissue destruction related to peripheral neuropathy in the lower extremity and peripheral vascular disease (11, 12). Mild diabetic foot patients usually present with foot deformities, hypoesthesia, skin dryness, and loss of skin elasticity. Patients with severe diabetic foot may develop foot ulcers and gangrene. Diabetic foot is the main reason for non-traumatic amputations in orthopedics. At present, there are many studies on the individual risk factors of diabetic foot in patients with T2DM, but no consensus has been reached. Although there are relevant clinical guidelines as the reference basis for the formulation of clinical treatment plans, how to predict the probability of diabetic foot according to risk factors and determine the timing of interventional treatment is an urgent problem to be solved at present.
The nomogram is drawn by the individual risk factors determined by multivariate logistic regression analysis. The nomogram can graphically represent the numerical relationship between specific disease and risk factors and intuitively predict the incidence of adverse events through a scoring system without any complicated calculation formula (13). The nomogram can provide accurate and individualized risk predictions for each individual. It is convenient for clinicians to effectively screen out high-risk patients and timely take interventions. Therefore, this study aimed to develop a nomogram prediction model for the risk of diabetic foot in patients with T2DM. Early screening and identification of high-risk patients can provide the reliable reference basis for early clinical intervention.
We retrospectively collected and analyzed clinical data from patients with diabetes mellitus from the Second Affiliated Hospital of Xi’an Jiaotong University between January 2012 and June 2021. Baseline-including criteria included (1) T2DM, diagnosis is made according to relevant criteria (fasting plasma glucose ≥ 7.0 mmol/L or 2-h plasma glucose ≥11.1 mmol/L or hemoglobin A1c ≥ 6.5%) (14); (2) both lower extremity arteries (femoral artery, superficial femoral artery, popliteal artery, anterior tibial artery, posterior tibial artery, and dorsalis pedis artery) of patients were examined by color Doppler ultrasonography for intima-media thickness, blood vessel diameter, and filling defects in blood flow; (3) orthopedic examination of the foot and ankle, including visual examination/palpation (skin condition and gait), mobility of foot and ankle (range of motion of the ankle joint, varus/valgus, and pronation/supination), and special examination (ankle anterior drawer test, varus/valgus stress test, and external rotation examination); and (4) patients gave oral informed consent. Baseline-excluding criteria included (1) type 1 diabetes mellitus, (2) gestational diabetes, (3) thromboangiitis obliterans, (4) combined with cancer, and (5) incomplete clinical data.
After the above screening, a total of 1,950 patients with T2DM were enrolled in the study. The patients were divided into training cohort (n = 1,365) and validation cohort (n = 585) according to the random number table method at a ratio of 7:3. The detailed flowchart is shown in Figure 1.
The study was approved by the medical ethics committee of the Second Affiliated Hospital of Xi’an Jiaotong University (Approval number: 2021234), which was consistent with medical ethics. The study was a retrospective cohort study and the data of included patients were anonymous. Oral informed consent was obtained from each enrolled patient before discharge.
Clinical data including gender, age, course of disease, body mass index (BMI), oral glucose tolerance test (OGTT) 2-h plasma glucose, hemoglobin A1c (HbA1c), low-density lipoprotein (LDL), triglyceride (TG), total cholesterol(TC),smoke, drink, hypertension history, family history of T2DM, and exercise of patients were collected. The above data were collected and checked by three researchers to ensure the completeness and validity of the data.
Statistical analysis was performed using SPSS software (Version 25.0, USA) and R software (Version 3.6.2, USA). Continuous variables were presented as means ± standard deviation or median (interquartile range). Categorical variables were presented using counts and percentages. Continuous variables were analyzed by t test or Mann–Whitney U test. Categorical variables were analyzed using the χ2 test or Fisher exact test. The independent risk factors for diabetic foot among patients with T2DM were identified by univariate and multivariate logistic regression analysis of the training cohort. Then, a nomogram prediction model was developed using the independent risk factors. The discrimination, calibration, and clinical usefulness of the nomogram prediction model were validated in training cohort and validation cohort. The area under the receiver operating characteristic curve (AUC) and C index were used to evaluate the discrimination. The calibration was evaluated by calibration plot and Hosmer–Lemeshow test. The clinical usefulness was evaluated by cutoff value combined with decision curve analysis (DCA) curve. P < 0.05 indicated statistically significant differences.
A total of 1,950 patients, including 1,365 patients in training cohort and 585 patients in validation cohort, were enrolled in this study (Table 1). In the training cohort, 203 patients developed diabetic foot, giving a frequency of 14.9%. In the validation cohort, 86 patients developed diabetic foot, giving a frequency of 14.7%. There were no statistically significant differences in gender, age, course of disease, BMI, etc. between training cohort and validation cohort (P > 0.05), indicating comparability between the two groups.
Univariate logistic regression analysis showed that the risk factors with statistically significant differences were age, course of disease, BMI, HbA1c, LDL, TC, smoke, and drink in training cohort (P < 0.05, Table 2). Then, the above risk factors were included in the multivariate logistic regression analysis. The results of multivariate logistic regression analysis showed that the independent risk factors for diabetic foot among patients with T2DM were age, HbA1c, LDL, TC, smoke, and drink (P < 0.05, Table 3).
A nomogram prediction model for the risk of diabetic foot in patients with T2DM was developed using above independent risk factors (Figure 2). The application of the nomogram prediction model was as follows. According to the nomogram, we could obtain the score corresponding to each predictor index, and then the sum of these score was recorded as the total score. The predicted probability corresponding to the total score was the risk of diabetic foot in patients with T2DM.
Receiver operating characteristic (ROC) curves of the training cohort and validation cohort were drawn (Figure 3). The AUC of the training cohort was 0.806 (95% CI: 0.775∼0.837). The cutoff value was 11.6% (P < 0.05). The C index was 0.806. The AUC of the validation cohort was 0.857 (95% CI 0.814∼0.899), (P < 0.05). The C index was 0.857. The C indexes of the nomogram prediction model in the training cohort and validation cohort were greater than 0.75, indicating good discrimination of the model.
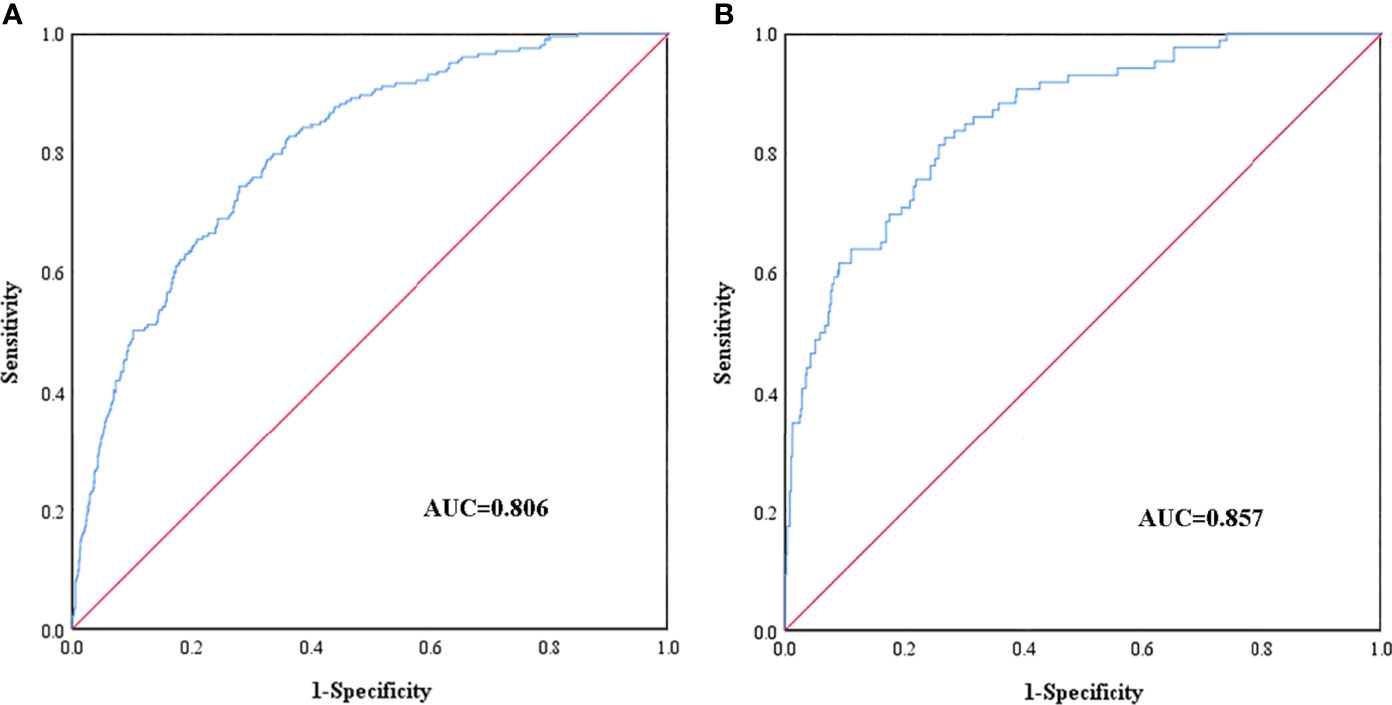
Figure 3 ROC curves of the nomogram prediction for the risk of diabetic foot in patients with T2DM in the training cohort (A) and validation cohort (B).
Calibration curves of the nomogram prediction model in the training cohort and validation cohort showed a favorable consistency between the predicted probability and the actual probability (Figure 4). In addition, the results of Hosmer–Lemeshow test of the nomogram prediction model in training cohort and validation cohort were χ2 = 4.336 (P = 0.826) and χ2 = 7.532 (P = 0.480), respectively. The P values of Hosmer–Lemeshow test of the nomogram prediction model in training cohort and validation cohort were greater than 0.05, suggesting no statistical significance. It indicated that the calibration of the model was high.
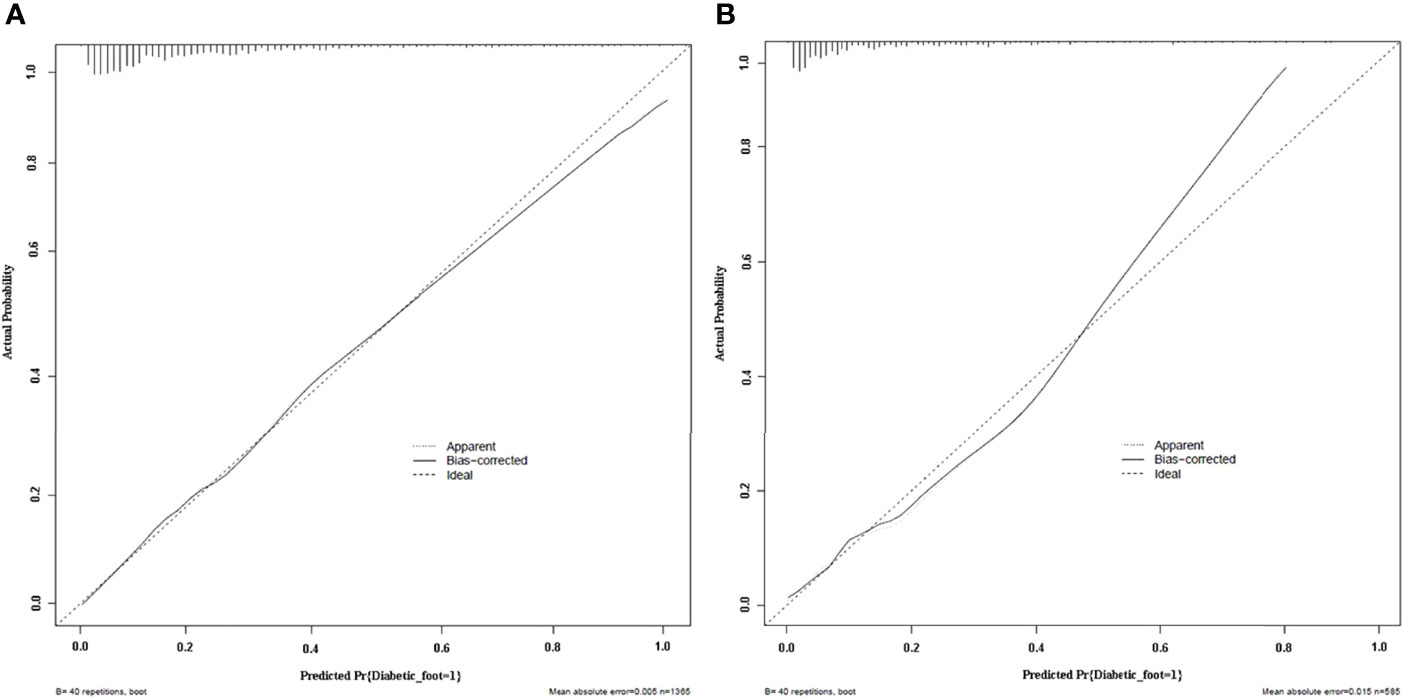
Figure 4 Calibration plots of the nomogram prediction for the risk of diabetic foot in patients with T2DM in the training cohort (A) and validation cohort (B).
DCA curves of the training cohort and validation cohort were drawn (Figure 5). When the threshold probability was in the range of 3%∼62% and 3%∼99%, respectively, the net benefit of patients was higher than that of the other two extreme curves (The horizontal line indicated that no diabetic foot occurred in all patients and no treatment, and the net benefit was 0. The oblique line indicated that all patients developed diabetic foot and received treatment, and the net benefit was a negative slope backslash line.). Within the above range, the nomogram prediction model has good clinical usefulness. The cutoff value (11.6%) obtained from the ROC curve of the training cohort was within the threshold probability range of the above two DCA curves, indicating that the nomogram prediction model has good clinical usefulness. A further analysis of the DCA curves of the nomogram prediction model showed that the net clinical benefit of the training cohort and validation cohort was 58% and 65%, respectively, when 11.6% was set as the threshold probability value for diagnosing diabetic foot and taking intervention. In other words, 58 and 65 of every 100 patients with T2DM who were diagnosed with diabetic foot using the nomogram prediction model in the training cohort and validation cohort would respectively have clinical benefits.
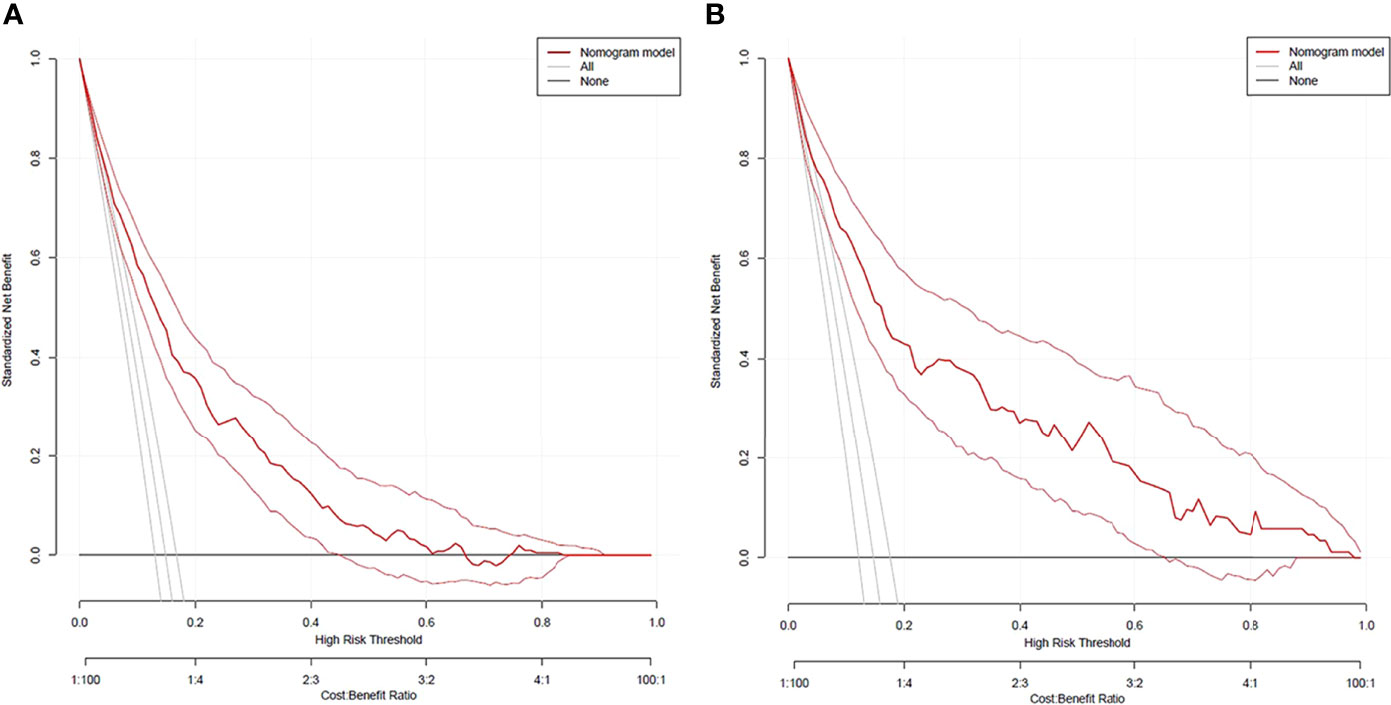
Figure 5 DCA curves of the nomogram prediction for the risk of diabetic foot in patients with T2DM in the training cohort (A) and validation cohort (B).
Take a patient with T2DM as an example, the relevant clinical data of this patient were as follows: age 50, HbA1c 10.2%, LDL 4.7 mmol/L, TC 5.8 mmol/L, smoke, and drink. According to the nomogram prediction model (Figure 6), the predicted risk of diabetic foot for this patient was 22.5%, higher than the threshold probability (11.6%). At this time, according to the DCA curve, we should take intervention to reduce the risk of patients developing diabetic foot.
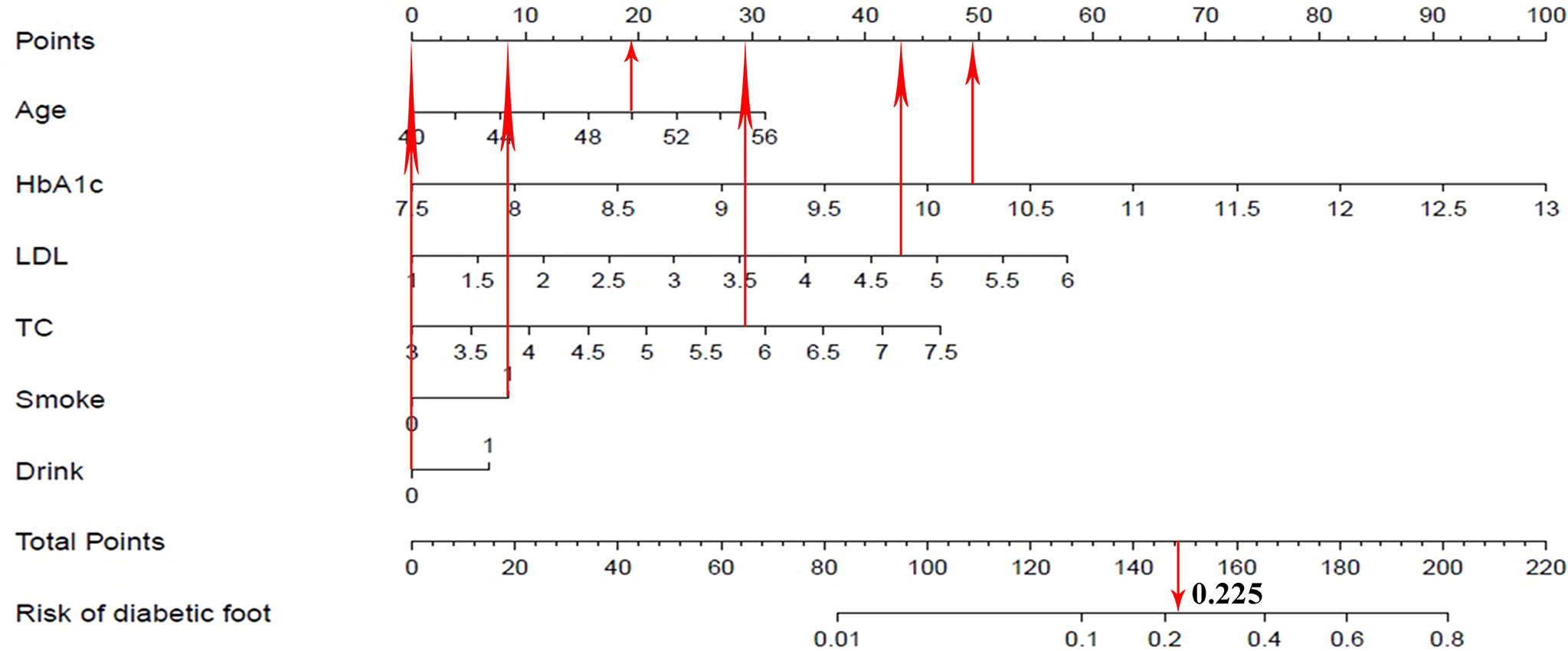
Figure 6 Visualization application of the nomogram prediction for the risk of diabetic foot in patients with T2DM.
Diabetic foot was one of the serious complications of diabetes mellitus. Diabetic foot imposed significant economic burdens on patients and society. T2DM accounted for a large proportion of diabetes mellitus. In patients with T2DM, the incidence of diabetic foot was as high as 12% to 15% (15–17). The overall incidence of diabetic foot in patients with T2DM in this study was approximately 14.9%. The incidence was similar to that reported in the above literature. Patients with T2DM who developed diabetic foot often had no obvious clinical symptoms and signs in the early stage, and sometimes only showed their decline of protective sensation. This made it easy to neglect the condition of the patient (18–20). Therefore, how to predict the risk of diabetic foot in patients with T2DM at an early stage and timely take intervention for high-risk patients is crucial.
Most of the current research on the relevant risk factors of diabetic foot has focused on intervening in the progression of diabetic foot to prevent severe ulcers and amputations (21–24). Although these studies are important, we believe that how to prevent diabetic patients from developing diabetic foot is more important and critical. On the one hand, there are few studies in this area. On the other hand, there is no consensus in this regard. In our study, univariate and multivariate logistic regression analysis found that the independent risk factors for diabetic foot in patients with T2DM were age, HbA1c, LDL, TC, smoke, and drink.
The pathogeneses of diabetic foot in patients with T2DM are as follows:
(1) Peripheral neuropathy
Patients with T2DM have various degree of neuropathy of distal lower extremities. Neuropathy of distal lower extremities mainly includes sensory, motor, and autonomic peripheral neuropathy (25). Sensory neuropathy is mainly characterized by the reduction or loss of vibration sensation (pallhypesthesia) and superficial sensation (pressure and touch) as well as subjective paresthesia. The sensation of pain in the foot is substantially declined as a consequence of chronic sensory neuropathy. As a result, the risk of foot trauma is significantly higher (26–29). Foot injuries and ulcers are neglected by patients and doctors due to the lack of pain symptoms (30, 31). Hence, foot injuries and ulcers often go undetected by doctors for weeks. Motor neuropathy of the foot is characterized by muscle atrophy, motor paralysis, and loss of muscle self-reflexes. The combination of sensory and motor peripheral neuropathy results in severe foot load accompanied by gait abnormality. As the disease progresses, the lesions of the foot will worsen due to neuropathy and increased plantar pressure load. Secretion of sweat is dysfunctional by motor paralysis due to autonomic neuropathy. Perspiration dysfunction could lead to dry skin on the foot and a reduced protective skin function, which increases the risk of injury and ulcers. Through multivariate logistic regression analysis, our study found that age, HbA1c, and drink were related to the occurrence of diabetic foot, and their OR values were 1.103, 2.509, and 1.407, respectively. According to our analysis of the patients, the sensory function of distal lower extremities of elderly patients with T2DM was worse than that of young patients, so the risk of foot injuries and ulcers was higher than that of young patients. HbA1c reflects the level of recent plasma glucose control of patients. The increase of HbA1c beyond the normal range usually indicates that the patient’s level of plasma glucose control is not ideal, leading to the occurrence of hyperglycemia. Previous studies showed that metabolic abnormalities due to hyperglycemia cause neuropathy (32). Hyperglycemia leads to nerve damage through four mechanisms, including increased levels of intracellular advanced glycation end products, activation of protein kinase C, hexosamine pathway, and polyol pathway (33). Alcohol has chronic neurotoxic effects (34), especially in patients with T2DM. This has a certain adverse effect on the sensory nerves of distal lower extremities of patients with T2DM, which leads to a decrease in the damage-sensing capacity of foot and increase the risk of diabetic foot in patients with T2DM.
(2) Peripheral vascular disease
Peripheral vascular disease is often present in the course of T2DM. Peripheral vascular disease is an atherosclerotic occlusive disease of the lower extremity. Patients with T2DM have a higher risk of peripheral vascular disease (35). In patients with T2DM, peripheral vascular disease is an important cause of the occurrence and development of diabetic foot (36). Patients with T2DM have a higher incidence of thickened basement membranes of the capillaries, atherosclerosis, endothelial cell hyperplasia, and arteriolosclerosis (37). As a result, patients with T2DM suffer from a lack of blood supply to their arteries. Poor peripheral blood supply can lead to poor wound healing of foot and worsen the condition. Patients with T2DM have reduced blood perfusion in the foot. As a result, the patients are at risk of ulcers and infections and eventually developing diabetic foot. Through multivariate logistic regression analysis, our study found that LDL, TC, and smoke were related to the occurrence of diabetic foot, and their OR values were 1.796, 1.690, and 1.539, respectively. After analyzing the patients, we believed that patients with T2DM would have a great risk of diabetic foot if their LDL and TC were higher than the normal range.LDL, a cholesterol-rich lipoprotein, is one of the risk factors for atherosclerosis (38). After chemical modification, LDL is ingested by phagocytes, forming foamy cells, and remaining in the vascular wall, resulting in a large amount of cholesterol deposition, which contributes to the formation of atheromatous plaque in the arterial wall (39–41). Thus, elevated LDL increases the risk of peripheral vascular disease in patients with T2DM, resulting in poor blood supply to the foot. In this context, diabetic foot ensues. TC is one of the risk factors for atherosclerosis in clinic (42). Therefore, increased TC will increase the risk of peripheral vascular atherosclerosis in patients. Increased TC has adverse effects on the blood supply to the foot of patients with T2DM, leading to the development of diabetic foot. Smoking can reduce the release of prostacyclin in patients, and then platelets tend to adhere to the arterial wall (43, 44). Smoking can also reduce high density lipoprotein cholesterol and increase TC in blood, resulting in atherosclerosis (45–47). Hence, smoking predisposes the patients with T2DM to atherosclerosis, which reduces peripheral blood supply to the foot. This increases the risk of diabetic foot in patients with T2DM.
At present, the preventive measures for diabetic foot mainly include the following. On the premise of glycemic control, the foot of the patients with T2DM should be checked regularly and protected preventatively (such as wearing loose-fitting shoes and socks). In clinical work, how to identify which patients need early clinical intervention is worth pondering. Meanwhile, there is a lack of related research on the nomogram prediction model for the risk of diabetic foot in patients with T2DM. Hence, we developed and validated a nomogram prediction model for the risk of diabetic foot in patients with T2DM and evaluated its clinical application value. In our study, when the cutoff value (11.6%) was taken as the threshold of DCA curve, we observed that the net clinical benefit of patients was higher than that of the other two extreme curves. This suggests that when the risk of diabetic foot is higher than 11.6% predicted by the nomogram prediction model, immediate intervention will benefit the patients clinically. When the risk of diabetic foot is lower than 11.6% predicted by the nomogram prediction model, doctors and patients can temporarily not take intervention and continue to pay attention to the dynamic changes of the disease. This facilitates clinical decision making in patients with T2DM.
There are some limitations to this study. First of all, it is a retrospective study, which requires further prospective studies in the later stage. Secondly, this study is a single-center study at the present stage. If the data of the patients from multiple centers can be included in the later stage to increase the sample size and the range of observed variables, we will further strengthen the nomogram prediction model.
In conclusion, our study found that the independent risk factors for diabetic foot among patients with T2DM were age, HbA1c, LDL, TC, smoke, and drink. In addition, our study developed an individualized nomogram prediction model, which made the prediction model visualized and easy for clinical application. The nomogram prediction model has good discrimination, calibration, and clinical usefulness in both training cohort and validation cohort. This facilitates early prediction and identification of patients at high risk of developing diabetic foot.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
Study conception and design: JW and HL; data collection and data analysis: JW and TX; manuscript drafting: JW, TX, and SG. All authors were involved in the revision of the manuscript and approved the final version of the paper.
This study was supported by the Fundamental Research Funds for the Central Universities (No. ZRZD2017008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all patients who allowed their data to be used for this study. We gratefully thank the doctors and nurses of orthopedics and internal medicine at the Second Affiliated Hospital of Xi’an Jiaotong University for their help in this study.
1. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care (2010) 33(Suppl 1):S62–9. doi: 10.2337/dc10-S062
2. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care (2020) 43(Suppl 1):S14–31. doi: 10.2337/dc20-S002
3. Defronzo RA. Banting Lecture. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes (2009) 58(4):773–95. doi: 10.2337/db09-9028
4. Cerf ME. Beta Cell Dysfunction and Insulin Resistance. Front Endocrinol (2013) 4:37. doi: 10.3389/fendo.2013.00037
5. Rahman MH, Kamrul-Hasan AB, Islam MR, Hasan AY, Chowdhury FQ, Miah OF, et al. Frequency and Risk Factors of Diabetic Retinopathy Among Patients With Type 2 Diabetes Mellitus: A Single-Center Study From Bangladesh. Mymensingh Med J (2020) 29(4):807–14.
6. Christofides EA, Desai N. Optimal Early Diagnosis and Monitoring of Diabetic Kidney Disease in Type 2 Diabetes Mellitus: Addressing the Barriers to Albuminuria Testing. J Prim Care Community Health (2021) 12:21501327211003683. doi: 10.1177/21501327211003683
7. Raggi P. Screening for Atherosclerotic Cardiovascular Disease in Patients With Type 2 Diabetes Mellitus: Controversies and Guidelines. Can J Diabetes (2020) 44(1):86–92. doi: 10.1016/j.jcjd.2019.08.009
8. Javed S, Hayat T, Menon L, Alam U, Malik RA. Diabetic Peripheral Neuropathy in People With Type 2 Diabetes: Too Little Too Late. Diabetes Med (2020) 37(4):573–9. doi: 10.1111/dme.14194
9. Karki DB, Yadava SK, Pant S, Thusa N, Dangol E, Ghimire S. Prevalence of Sensory Neuropathy in Type 2 Diabetes Mellitus and Its Correlation With Duration of Disease. Kathmandu Univ Med J (2016) 14(54):120–4.
10. Noor S, Zubair M, Ahmad J. Diabetic Foot Ulcer–A Review on Pathophysiology, Classification and Microbial Etiology. Diabetes Metab Syndr (2015) 9(3):192–9. doi: 10.1016/j.dsx.2015.04.007
11. Khanolkar MP, Bain SC, Stephens JW. The Diabetic Foot. QJM (2008) 101(9):685–95. doi: 10.1093/qjmed/hcn027
12. Zubair M, Malik A, Ahmad J. Clinico-Microbiological Study and Antimicrobial Drug Resistance Profile of Diabetic Foot Infections in North India. Foot (Edinb) (2011) 21(1):6–14. doi: 10.1016/j.foot.2010.10.003
13. Peng B, Min R, Liao Y, Yu A. Development of Predictive Nomograms for Clinical Use to Quantify the Risk of Amputation in Patients With Diabetic Foot Ulcer. J Diabetes Res (2021) 2021:6621035. doi: 10.1155/2021/6621035
14. International Expert Committee. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care (2009) 32(7):1327–34. doi: 10.2337/dc09-9033
15. Ahmad J. The Diabetic Foot. Diabetes Metab Syndr (2016) 10(1):48–60. doi: 10.1016/j.dsx.2015.04.002
16. Kalish J, Hamdan A. Management of Diabetic Foot Problems. J Vasc Surg (2010) 51(2):476–86. doi: 10.1016/j.jvs.2009.08.043
17. Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-Based Protocol for Diabetic Foot Ulcers. Plast Reconstr Surg (2006) 117(7 Suppl):193S–209S; discussion 210S-211S. doi: 10.1097/01.prs.0000225459.93750.29
18. Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, et al. Diabetic Foot Ulcer Classifications: A Critical Review. Diabetes Metab Res Rev (2020) 36 Suppl 1:e3272. doi: 10.1002/dmrr.3272
19. Tavee J, Zhou L. Small Fiber Neuropathy: A Burning Problem. Cleve Clin J Med (2009) 76(5):297–305. doi: 10.3949/ccjm.76a.08070
20. Volmer-Thole M, Lobmann R. Neuropathy and Diabetic Foot Syndrome. Int J Mol Sci (2016) 17(6):917. doi: 10.3390/ijms17060917
21. Pastore D, Deja-Simoni A, De Stefano A, Pacifici F, Cela E, Infante M, et al. Risk Factors for Diabetic Foot Ulcers: An Albanian Retrospective Study of Inpatients With Type 2 Diabetes. Eur Rev Med Pharmacol Sci (2022) 26(2):558–72. doi: 10.26355/eurrev_202201_27883
22. Rodrigues BT, Vangaveti VN, Urkude R, Biros E, Malabu UH. Prevalence and Risk Factors of Lower Limb Amputations in Patients With Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Diabetes Metab Syndr (2022) 16(2):102397. doi: 10.1016/j.dsx.2022.102397
23. Hüsers J, Hafer G, Heggemann J, Wiemeyer S, John SM, Hübner U. Development and Evaluation of a Bayesian Risk Stratification Method for Major Amputations in Patients With Diabetic Foot Ulcers. Stud Health Technol Inform (2022) 289:212–5. doi: 10.3233/SHTI210897
24. Yunir E, Hidayah CD, Harimurti K, Kshanti IAM. Three Years Survival and Factor Predicting Amputation or Mortality in Patients With High Risk for Diabetic Foot Ulcer in Fatmawati General Hospital, Jakarta. J Prim Care Community Health (2022) 13:21501319211063707. doi: 10.1177/21501319211063707
25. Gilbey SG. Neuropathy and Foot Problems in Diabetes. Clin Med (Lond) (2004) 4(4):318–23. doi: 10.7861/clinmedicine.4-4-318
26. Zilliox LA, Russell JW. Physical Activity and Dietary Interventions in Diabetic Neuropathy: A Systematic Review. Clin Auton Res (2019) 29(4):443–55. doi: 10.1007/s10286-019-00607-x
27. Peltier A, Goutman SA, Callaghan BC. Painful Diabetic Neuropathy. BMJ (2014) 348:g1799. doi: 10.1136/bmj.g1799
28. Kamenov ZA, Traykov LD. Diabetic Somatic Neuropathy. Adv Exp Med Biol (2012) 771:155–75. doi: 10.1007/978-1-4614-5441-0_14
29. Perrin BM, Southon J, McCaig J, Skinner I, Skinner TC, Kingsley MIC. The Effect of Structured Exercise Compared With Education on Neuropathic Signs and Symptoms in People at Risk of Neuropathic Diabetic Foot Ulcers: A Randomized Clinical Trial. Medicina (Kaunas) (2021) 58(1):59. doi: 10.3390/medicina58010059
30. Marshall A, Alam U, Themistocleous A, Calcutt N, Marshall A. Novel and Emerging Electrophysiological Biomarkers of Diabetic Neuropathy and Painful Diabetic Neuropathy. Clin Ther (2021) 43(9):1441–56. doi: 10.1016/j.clinthera.2021.03.020
31. Azmi S, Petropoulos IN, Ferdousi M, Ponirakis G, Alam U, Malik RA. An Update on the Diagnosis and Treatment of Diabetic Somatic and Autonomic Neuropathy. F1000Res (2019) 8:F1000 Faculty Rev–186. doi: 10.12688/f1000research.17118.1
32. Zochodne DW. The Challenges of Diabetic Polyneuropathy: A Brief Update. Curr Opin Neurol (2019) 32(5):666–75. doi: 10.1097/WCO.0000000000000723
33. Brownlee M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes (2005) 54(6):1615–25. doi: 10.2337/diabetes.54.6.1615
34. de la Monte SM, Kril JJ. Human Alcohol-Related Neuropathology. Acta Neuropathol (2014) 127(1):71–90. doi: 10.1007/s00401-013-1233-3
35. Bandyk DF. The Diabetic Foot: Pathophysiology, Evaluation, and Treatment. Semin Vasc Surg (2018) 31(2-4):43–8. doi: 10.1053/j.semvascsurg.2019.02.001
36. Eleftheriadou I, Tentolouris A, Grigoropoulou P, Tsilingiris D, Anastasiou I, Kokkinos A, et al. The Association of Diabetic Microvascular and Macrovascular Disease With Cutaneous Circulation in Patients With Type 2 Diabetes Mellitus. J Diabetes Complications (2019) 33(2):165–70. doi: 10.1016/j.jdiacomp.2018.10.008
37. Chawla A, Chawla R, Jaggi S. Microvasular and Macrovascular Complications in Diabetes Mellitus: Distinct or Continuum? Indian J Endocrinol Metab (2016) 20(4):546–51. doi: 10.4103/2230-8210.183480
38. Vaverkova H, Karasek D, Novotny D, Jackuliakova D, Lukes J, Halenka M, et al. Apolipoprotein B Versus LDL-Cholesterol: Association With Other Risk Factors for Atherosclerosis. Clin Biochem (2009) 42(12):1246–51. doi: 10.1016/j.clinbiochem.2009.05.004
39. Sokolov AV, Kostevich VA, Runova OL, Gorudko IV, Vasilyev VB, Cherenkevich SN, et al. Proatherogenic Modification of LDL by Surface-Bound Myeloperoxidase. Chem Phys Lipids (2014) 180:72–80. doi: 10.1016/j.chemphyslip.2014.02.006
40. Satchell L, Leake DS. Oxidation of Low-Density Lipoprotein by Iron at Lysosomal Ph: Implications for Atherosclerosis. Biochemistry (2012) 51(18):3767–75. doi: 10.1021/bi2017975
41. Poznyak AV, Nikiforov NG, Markin AM, Kashirskikh DA, Myasoedova VA, Gerasimova EV, et al. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front Pharmacol (2021) 11:613780. doi: 10.3389/fphar.2020.613780
42. Quispe R, Elshazly MB, Zhao D, Toth PP, Puri R, Virani SS, et al. Total Cholesterol/HDL-Cholesterol Ratio Discordance With LDL-Cholesterol and non-HDL-Cholesterol and Incidence of Atherosclerotic Cardiovascular Disease in Primary Prevention: The ARIC Study. Eur J Prev Cardiol (2020) 27(15):1597–605. doi: 10.1177/2047487319862401
43. Nadler JL, Velasco JS, Horton R. Cigarette Smoking Inhibits Prostacyclin Formation. Lancet (1983) 1(8336):1248–50. doi: 10.1016/s0140-6736(83)92698-3
44. Carrier E, Brochu I, de Brum-Fernandes AJ, D'Orléans-Juste P. The Inducible Nitric-Oxide Synthase Modulates Endothelin-1-Dependent Release of Prostacyclin and Inhibition of Platelet Aggregation Ex Vivo in the Mouse. J Pharmacol Exp Ther (2007) 323(3):972–8. doi: 10.1124/jpet.107.125690
45. Mureddu GF, Brandimarte F, De Luca L. High-Density Lipoprotein Levels and Risk of Cardiovascular Events: A Review. J Cardiovasc Med (Hagerstown) (2012) 13(9):575–86. doi: 10.2459/JCM.0b013e32834bb3c8
46. Chelland Campbell S, Moffatt RJ, Stamford BA. Smoking and Smoking Cessation – the Relationship Between Cardiovascular Disease and Lipoprotein Metabolism: A Review. Atherosclerosis (2008) 201(2):225–35. doi: 10.1016/j.atherosclerosis.2008.04.046
Keywords: type 2 diabetes mellitus (T2DM), diabetic foot, orthopedics, nomogram, individual risk prediction model
Citation: Wang J, Xue T, Li H and Guo S (2022) Nomogram Prediction for the Risk of Diabetic Foot in Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 13:890057. doi: 10.3389/fendo.2022.890057
Received: 05 March 2022; Accepted: 13 June 2022;
Published: 13 July 2022.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Angelica Amato, University of Brasilia, BrazilCopyright © 2022 Wang, Xue, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haopeng Li, bGhwXzM5OTNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.