
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 03 June 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.889029
This article is part of the Research TopicSafety and Child Health of Assisted Reproduction Technology (ART)View all 28 articles
Objective: This study aims to evaluate the association between polycystic ovary syndrome (PCOS) phenotypes and adverse perinatal outcomes, comparing the characteristics, ovarian response, and assisted reproductive outcomes in patients with various PCOS phenotypes after in-vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI).
Methods: This study comprised 6,732 patients who underwent the first cycle of IVF/ICSI treatment in our outpatient department from January 2017 to July 2018. Propensity score matching (PSM) was used in PCOS and non-PCOS groups to balance the influence of intergroup confounding factors. After the PSM procedure, 1,186 patients were included in the two groups, and the PCOS patients were further divided into four PCOS phenotype groups based on the Rotterdam criteria.
Results: Patients with various PCOS phenotypes had similar rates of biochemical pregnancy, clinical pregnancy, and live birth (all P-values > 0.05). The overall incidence of adverse pregnancy outcomes (including ectopic pregnancy, miscarriage, preterm birth) was significantly higher in PCOS phenotype A and D groups than in the control group (44% and 46.4% vs. 28.7%, P = 0.027). The rates of hypertensive disorder of pregnancy (HDP) were significantly higher in PCOS phenotype A and C groups than in the control group (9.3% and 12.5% vs. 3.1%, P = 0.037). After adjustment for potential confounders, the differences in adverse pregnancy outcomes persisted (P = 0.025).
Conclusions: The overall incidence of adverse pregnancy outcomes is higher in women with PCOS phenotypes A and D than in women with non-PCOS.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age and the main cause of anovulatory infertility (1–3), which is characterized by obesity, hyperandrogenism, anovulation, insulin resistance, polycystic ovary, and infertility. The global prevalence of PCOS ranges from 6% to 21% (4); however, the etiology of PCOS is unclear (5). Moreover, because anovulation in women with PCOS often results in infertility (6), assisted reproductive technology (ART) is usually required for these women to become pregnant. According to the Rotterdam criteria, PCOS patients can be divided into the following four phenotypes: phenotype A—coexistence of clinical hyperandrogenism/hyperandrogenemia, oligomenorrhea/anovulation, and polycystic ovaries (HA+OA+PCO); phenotype B—clinical hyperandrogenism or hyperandrogenemia and oligomenorrhea/anovulation (HA+OA); phenotype C—clinical hyperandrogenism or hyperandrogenemia and polycystic ovaries (HA+PCO); and phenotype D: oligomenorrhea/anovulation and polycystic ovaries (OA+PCO) (7). For different PCOS phenotypes, the ovarian response to gonadotropin (Gn) is varied in controlled ovarian hyperstimulation (COH) (8), which in turn affects the outcome of ART.
Because PCOS patients have the characteristics of reproductive endocrine dysfunction and metabolic disorder (9), they were more prone to having pregnancy complications (10, 11), which increases the risk of adverse perinatal outcomes (12). Previous studies found that the risk of pregnancy-related complications and adverse pregnancy outcomes via ART was higher than via spontaneous conception (13–15), and a recent meta-analysis showed that patients with PCOS undergoing IVF were associated with higher risks of adverse pregnancy outcomes (16). However, studies on the association between various PCOS phenotypes after IVF/ICSI and adverse perinatal outcomes were relatively small.
The present study retrospectively analyzed the adverse perinatal outcomes of patients with various PCOS phenotypes who underwent IVF/ICSI.
We screened patients who underwent their first IVF/ICSI cycle at the Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University between January 2017 and July 2018. All patients were divided into the PCOS group and the control group. PCOS was defined according to the Rotterdam consensus criteria (2004) (17); that is, PCOS was diagnosed if at least two of the following criteria were present: oligomenorrhea/anovulation (defined as delaying of >35 days or <8 spontaneous hemorrhagic episodes/year), clinical and/or biochemical hyperandrogenism [biochemical hyperandrogenism was defined as total testosterone levels above 48.1 ng/dl detected in patients with no clinical evidence of hyperandrogenism or menstrual disturbances and not taking hormonal medication, and hirsutism was defined as patients with a total score ≥6 by the modified Ferriman–Gallwey score (18)], and polycystic ovary on ultrasonography (≥12 small follicles measuring 2–9 mm in at least one ovary and/or ovarian volume ≥10 cm3), and it is necessary to exclude other endocrine dysfunctions. Furthermore, the PCOS group was classified into four phenotype subgroups as follows (19): phenotype A—HA+OA+PCO, phenotype B—HA+OA, phenotype C—HA+PCO, and phenotype D—OA+PCO. Women in the control group had regular menstrual cycles (21–35 days), without evidence of HA or PCO. All patients with the following conditions were excluded: age >38 years old, serum FSH level >15 IU/L, diabetes, hypertension, abnormal parental karyotypes, severe intrauterine adhesion or uterine abnormality, chronic medical conditions that contraindicated pregnancy or with other endocrine dysfunction (such as Cushing’s syndrome, primary hyperprolactinemia, thyroid dysfunction, congenital adrenal hyperplasia, androgen producing neoplasm), and history of recurrent spontaneous abortion (RSA) or unilateral oophorectomy.
In total, we identified 6,732 women who met the study criteria, consisting of 1,186 in the PCOS group and 5,546 in the control group. This study was approved by the Institutional Review Board of the Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University (2017-53).
All patients underwent clinical history (including but not limited to the menstrual cycle and infertility type), physical examination [including but not limited to body mass index (BMI), Ferriman–Gallwey score, and gynecologic examination], biochemical analysis [including but not limited to the levels of fasting blood glucose (FBG), follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone, total testosterone (To), anti-Müllerian hormone (AMH) and thyroid-stimulating hormone (TSH), and prolactin], and transvaginal ultrasonography for calculated antral follicle count (AFC) on follicular phase. Blood samples were drawn for biochemical analyses on days 2–3 of a spontaneous or progestogen-induced menstrual cycle. All the hormonal assays were made at the Center for Reproductive Medicine Laboratory, Cheeloo College of Medicine, Shandong University.
According to a routine method (18), all patients received a standardized ovarian stimulation regimen; underwent oocyte retrieval, fertilization, and transfer embryos; and were provided luteal phase support. All patients underwent COH with standard long agonist protocol or antagonist protocol [as previously described (20, 21)]. As monitored on ultrasound and based on the level of serum sex hormones (including FSH, LH, E2, progesterone), Gn doses were adjusted based on the ovarian response. Human chorionic gonadotropin (HCG) at a dose of 4,000 to 8,000 IU was administered when at least two follicles were ≥18 mm. Oocyte retrieval was performed 34–36 h later under transvaginal ultrasound guidance. According to sperm quality, IVF/ICSI was performed. The embryo quality was graded according to the number of blastomeres, percent fragmentation, and regularity. Embryos were transferred on day 3 or day 5 after oocyte retrieval according to the patient’s condition (such as embryo quality, abdominal distention, and endocrine examination results). Cycle cancellation is defined if the patient does not have a fresh embryo transfer after oocyte retrieval (and we excluded cycles canceled before HCG triggering). Luteal phase support was provided after oocyte retrieval for those women who planned to transfer fresh embryos, as previously described (18, 20). Fourteen days after embryo transfer, the serum HCG levels were measured. If conception occurred, the luteal phase support was maintained. Transvaginal ultrasonography was performed 35 days after embryo transfer.
In this study, the primary outcome measures were adverse perinatal outcomes, while the secondary outcome measures included biochemical pregnancy, clinical pregnancy (CP), and live birth (LB). Adverse perinatal outcomes were categorized into adverse pregnancy outcomes and pregnancy complications. Adverse pregnancy outcomes included ectopic pregnancy, miscarriage, and premature birth, and pregnancy complications included hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), and others (postpartum hemorrhage, placenta previa, placental abruption, premature rupture of membrane, cardiac diseases complicating pregnancy). Ectopic pregnancy was considered as developing blastocyst implanted outside the endometrial cavity. Miscarriage was defined as clinical pregnancy lost before 28 weeks of gestation. Premature birth was defined as a baby born between the 28th and 37th week of pregnancy. In this study, HDP included gestational hypertension (333 cases) and preeclampsia (1 case). Gestational hypertension and preeclampsia were defined as previously described (22–24). GDM was defined as the variable severity of glucose intolerance with onset or first recognition during pregnancy (25). Biochemical pregnancy was defined as serum HCG level ≥10 IU/L. CP was defined as the presence of gestational sacs by ultrasonography. LB was defined as the delivery of any viable infant at 28 weeks or more of gestation. Additionally, the cycle cancellation rate was calculated as the number of canceled fresh embryo transfer cycles divided by the number of oocyte retrieval cycles. Embryos of grades I and II, with 7–10 cells on day 3, were defined as high-quality embryos, and high-quality embryo rate, defined as the number of high-quality embryo/number of zygotes, was calculated. Fertilization rate (FR) was calculated as the number of 2PN divided by the number of oocyte retrieval, and implantation rate (IR) was calculated as the number of observed gestational sacs divided by the number of transferred embryos.
Comparisons between groups were performed using one-way analysis of variance (with the LSD post-hoc test) for continuous variables and the chi-squared test (or Fisher’s exact test when the expected frequencies were less than five) for categorical variables. The results were expressed as mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables. The study was retrospective to balance basic patient characteristics (including age, infertility type, and stimulation protocol) between groups. We used 1:1 propensity score matching (PSM) to match control patients to PCOS patients, and 0 is the matching caliper of PSM in this study. In the PCOS subgroups, logistic regression was used to evaluate the relationship between PCOS phenotype and IVF/ICSI outcomes while adjusting for relevant confounders, and the results were expressed as odds ratios (OR) with 95% confidence intervals (CI).
All statistical analyses were performed using the Statistical Package for Social Sciences (version 26.0, SPSS Inc., Chicago, USA) and R software. P-value <0.05 was considered statistically significant.
A total of 6,732 patients were recorded, with 1,186 in the PCOS group and 5,546 in the control group. After the PSM procedure, 1,186 patients were included in the control group, and there were 293 cases of phenotype A, 53 cases of phenotype B, 77 cases of phenotype C, and 763 cases of phenotype D in the PCOS groups (Figure 1).
The basic characteristics of the patients among the five groups are shown in Table 1. The results showed significant differences in BMI, FBG, FSH, LH, LH/FSH ratio, To, AMH, and AFC among the five groups (all P < 0.001). Of these, BMI, FBG, LH, and To were higher in the PCOS phenotype A group than in the other groups (all P < 0.001). The basic characteristics before PSM are shown in Supplementary Table 1. In addition, we only compared the 2-h plasma glucose concentrations after OGTT in various PCOS phenotype groups, and the results showed no statistically significant differences between groups (P = 0.633, data not shown).
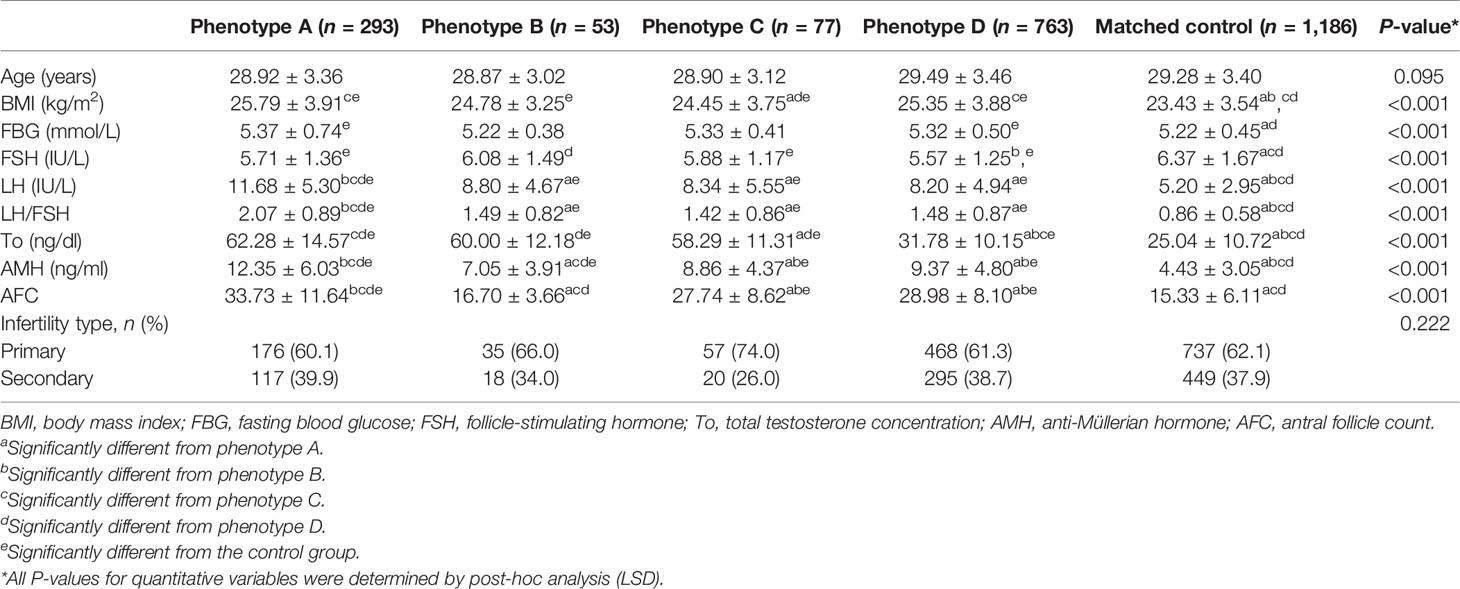
Table 1 Basic characteristics of the patients among the four PCOS phenotype groups and the control group.
The ovarian response and pregnancy outcomes of patients among the four PCOS phenotype groups and the control group are presented in Table 2. Significant differences in HCG dose, endometrial thickness, the number of follicles of diameter ≥14 mm and E2 levels on the trigger day, the number of retrieved oocytes and frozen embryos, and high-quality embryo rate among groups were observed (all P < 0.05). Of these, the number of follicles of diameter ≥14 mm and E2 levels on the trigger day and the number of retrieved oocytes were significantly higher in PCOS phenotype A and C groups compared with the other phenotype groups and the control group (all P < 0.05). It is worth noting that the high-quality embryo rate of PCOS phenotype A and D groups was lower than that of the other groups, especially the control group (P = 0.019). Although there were significant differences in Gn priming dose, stimulation duration, and the number of 2PN among the five groups (all P < 0.001), the total dose of Gn and FR were not statistically different (all P > 0.05). We can see that the cycle cancellation rate of the PCOS phenotype D group is lower than that of PCOS phenotype A and C groups and higher than that of PCOS phenotype B and control groups (62.8% and 68.8% vs. 53.9% vs. 39.6% and 34.8%, P < 0.001). The patients in the five groups had similar biochemical pregnancy rates, CPRs, ectopic pregnancy rates, miscarriage rates, premature birth rates, and LBRs (all P > 0.05). The data on ovarian response and pregnancy outcomes before PSM are shown in Supplementary Table 2. In addition, we compared the incidence of ovarian hyperstimulation syndrome (OHSS) in various PCOS phenotype groups after IVF-ET, and the results showed no statistically significant differences between groups (P = 0.788, data not shown).
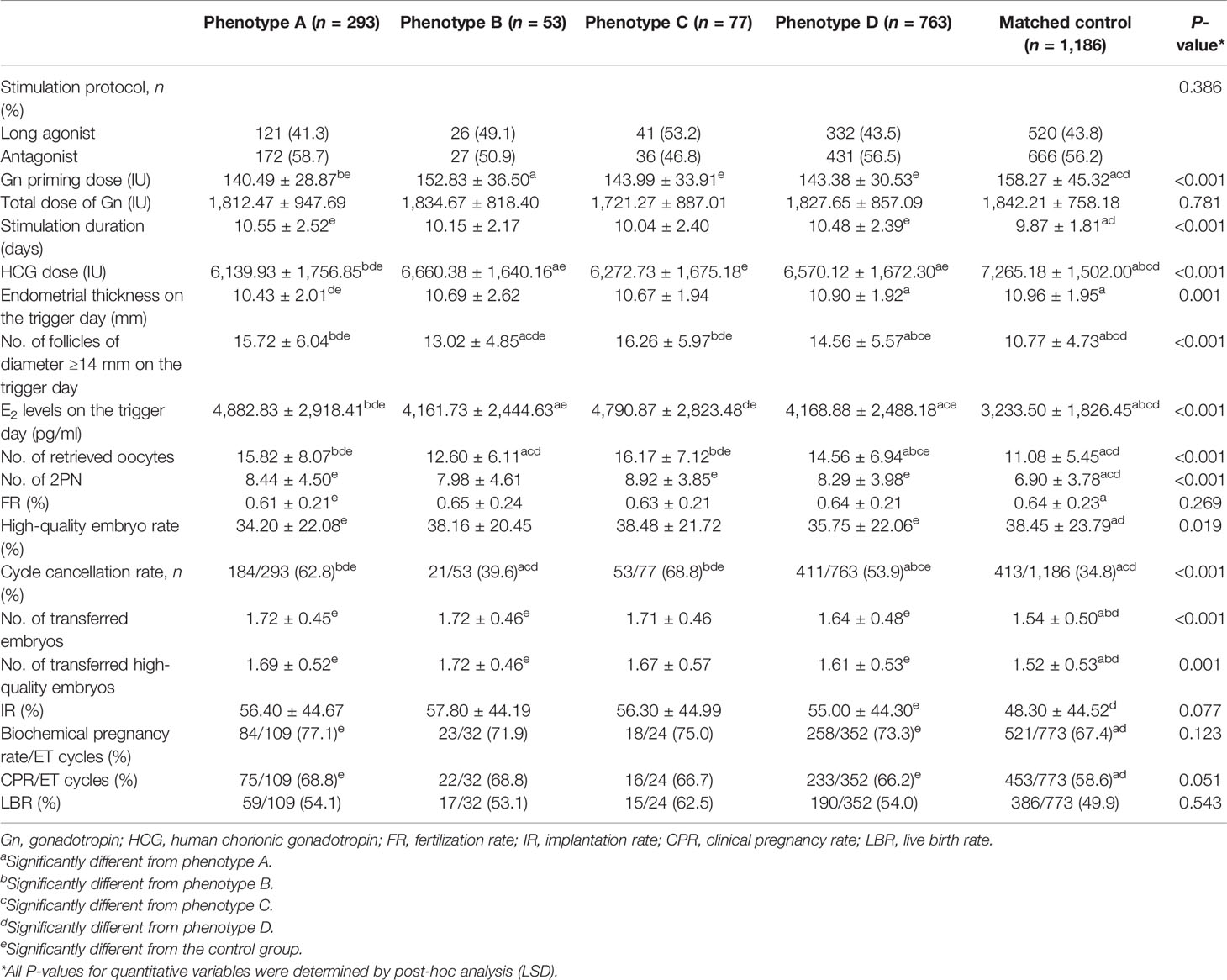
Table 2 Comparison of ovarian response and pregnancy outcomes among the four PCOS phenotype groups and the control group.
The adverse perinatal outcomes of the five groups are displayed in Table 3. The adverse pregnancy outcome rate was higher in PCOS phenotype A and D groups than in the control group (44.0% and 46.4% vs. 28.7%, P = 0.027). Despite the differences in HDP rate of PCOS phenotype A and C groups and the control group (9.3% and 12.5% vs. 3.1%, P = 0.037), the incidence of total pregnancy complications, GDM, or other pregnancy complications was similar among the five groups. There was no difference between the groups for the rates of ectopic pregnancy, miscarriage, premature birth, cesarean section, and multiple births (all P > 0.05). The data on adverse perinatal outcomes before PSM are shown in Supplementary Table 3. In addition, the statistical power of the R×C square test was calculated via the “pwr” package in R software, where the effect size was determined as 0.55 using the ES.w2() function, and the statistical power was calculated as pwr.chisq.test(w = ES.w2(prob), N = 799, df = 4, sig.level = 0.05) >0.99, based on which we admit that the results in Table 3 are accurate.
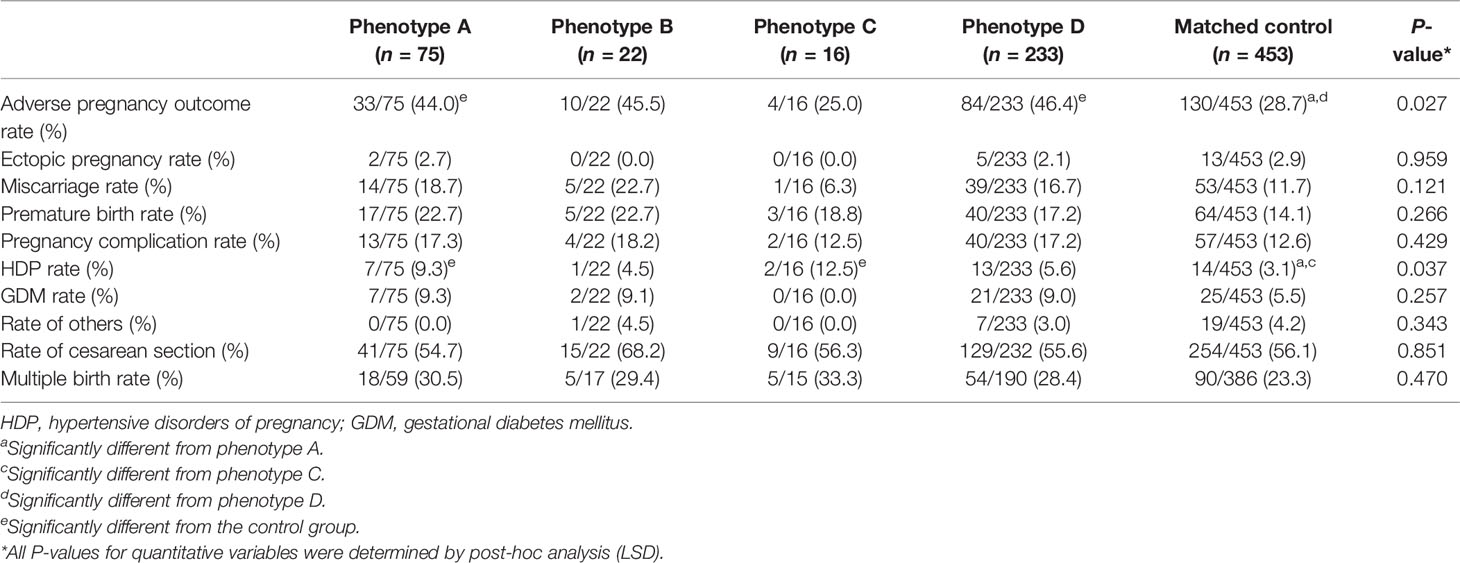
Table 3 Comparison of adverse perinatal outcomes among the four PCOS phenotype groups and the control group.
According to the previous results of adverse perinatal outcomes, a univariate logistic analysis of adverse pregnancy outcomes and HDP was performed. Compared with the control group, PCOS phenotypes A and D were the risk factors for adverse pregnancy outcomes [cOR (crude odds ratio)-A: 1.952, 95% CI-A: 1.185–3.216; cOR-D: 1.401, 95% CI-D: 1.001–1.960] and PCOS phenotype A was the risk factor for HDP (cOR: 3.228, 95% CI: 1.258–8.285). The factors with significant differences in the univariate analysis (these results are shown in Supplementary Tables 4, 5) were included in the multivariate logistic regression analysis. After adjusting for confounding factors, PCOS phenotypes A and D were shown as independent risk factors for adverse pregnancy outcomes (aOR-A: 1.835, 95% CI-A: 1.095–3.075; aOR-D: 1.435, 95% CI-D: 1.025–2.008) (see Table 4).
In this study, the relationship between PCOS phenotypes and pregnancy was retrospectively analyzed in patients who underwent the first cycle of IVF/ICSI treatment. The results revealed that the PCOS phenotype was correlated with adverse pregnancy outcomes (ectopic pregnancy, miscarriage, and premature birth), and PCOS phenotypes A and D were the independent risk factors for adverse pregnancy outcomes. Moreover, CPR and LBR in various PCOS phenotypes were comparable.
Adverse pregnancy outcomes have been the subject of considerable attention, and the relationship between PCOS and adverse pregnancy outcomes has been a topic of great interest in the assisted reproductive field. A meta-analysis of pregnancy-related outcomes and complications in PCOS patients reported that PCOS patients present a high risk of adverse pregnancy outcomes despite the fact that they achieved a better LBR (16). Previous studies concluded that PCOS increased the risk of adverse pregnancy outcomes by affecting the reproductive endocrine and metabolic functions (6, 10, 26, 27). In addition, women with PCOS present with an abnormal endometrial phenotype and function (28), which possibly explains some of the adverse pregnancy outcomes such as miscarriage and premature birth (29).
The results of this study, for the first time, showed that PCOS phenotypes A and D were the independent risk factors for adverse pregnancy outcomes. In other words, higher incidences of adverse pregnancy outcomes occurred in women with PCOS phenotypes A and D. It was found that these two phenotypes of PCOS exist with common characteristics: OA and PCO. We speculated that the higher rates of adverse pregnancy in patients with PCOS result from a combined action of OA and PCO. A menstrual disorder in PCOS patients mainly results from insulin resistance, and it can reflect the degree of metabolic dysfunction (30). Recent findings showed that the menstrual patterns of PCOS patients might be correlated with the higher rates of adverse pregnancy outcomes (27). The result of a retrospective study showed that amenorrhea in PCOS patients was an independent risk factor for adverse pregnancy outcomes. Also, oocyte maturation and fertility rate in women with anovulation were lower than in women with regular cycling, and the development rate of the embryo shared a similar trend (31). Another study involving dairy cattle with anovulation reported that anovulation results in significant alterations in gene expression. Specifically, transcripts linked to the control of energy metabolism and DNA repair were downregulated, whereas genes involved in apoptosis and autophagy were upregulated. It was also found that the risk factors for OA have a direct impact on embryo development and endometrial receptivity (32).
Moreover, several studies suggested that PCO were associated with poor oocyte quality, and they also found elevated levels of homocysteine in the blood of PCOS patients (33–35) and in the follicular fluid of patients with PCO (36). These findings suggested that abnormally high homocysteine levels of follicular fluid were related to the poor quality of oocytes and low fertilization rates, even to the poor quality of embryos and adverse pregnancy outcomes (36). In a previous study, Jia et al. reported that the quality of oocytes in PCO has decreased, which could be due to mtDNA hypermethylation and abnormal activation of one-carbon metabolism (37). In addition, we also found that the high-quality embryo rate of PCOS phenotype A and D groups was lower than that of the other groups, especially the control group. This result supports our speculation. The coexistence of OA and PCO may be associated with higher rates of adverse pregnancy by affecting the quality of oocyte and embryo.
At present, advanced maternal age (38, 39), high levels of BMI (40, 41), and a thin endometrium (42–44) as risk factors for adverse pregnancy outcomes are well recognized in the literature. Therefore, multivariate logistic regression analyses in our study were performed to exclude the potential influences of these confounding factors, but the effect of PCOS phenotypes A and D on adverse pregnancy outcomes persists. In addition, a recent meta-analysis suggested that HA has adverse effects on assisted reproductive outcomes in patients with PCOS (45). However, the contribution of HA to miscarriage is still debated (46, 47). The effect of HA on adverse pregnancy outcomes was not found in our study, but the aOR of PCOS phenotype A (with HA) was higher than that of PCOS phenotype D (without HA) in the logistic analysis of adverse pregnancy outcomes. It was hypothesized that HA may have a role in the incidence of adverse pregnancy outcomes in IVF/ICSI and that this effect would be weak. Simultaneously, OA and PCO were the primary influencers in adverse pregnancy outcomes. As we all know, OHSS is also an important factor affecting adverse pregnancy outcomes (48), and patients with PCOS are at a greater risk to develop OHSS (49). In the present study, we compared the incidence of OHSS in various PCOS phenotype groups after IVF-ET, and the results showed no statistically significant differences between groups. These results were probably due to some PCOS patients with a higher OHSS risk canceling fresh embryo transfer and selecting all-embryo cryopreservation (50).
The results of our study highlight the need for individualized treatment and intensive follow-up after pregnancy in patients with PCOS phenotypes A and D, to decrease the incidence of adverse pregnancy outcomes. However, as with all retrospective data analyses, we were not able to completely rule out all potential confounders. Moreover, our study inevitably suffers from several limitations, even though we used PSM statistical methods to diminish bias. Although we have expanded the sample size compared with those reported in previous studies (8, 51), the sample size of some PCOS phenotypes is still the main limitation of the study. We think that one possible explanation could be the characteristics of the study population. Therefore, further prospective research with a sufficient sample size will be needed to confirm these findings in the future.
Taken together, our data revealed that PCOS phenotypes A and D were the independent risk factors for adverse pregnancy outcomes. Specifically, the higher incidences of adverse pregnancy outcomes occur in women with PCOS phenotypes A and D compared with women with non-PCOS. Therefore, for women with PCOS phenotypes A and D, individualized treatment during assisted reproduction and close follow-up after clinical pregnancy are necessary.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University. The patients/participants provided their written informed consent to participate in this study.
YS, LY, and QW conceived and designed this study. QW contributed to the statistical analysis and interpretation of data and drafting of the manuscript. HW and PL performed the statistical analysis and participated in the discussion. XL and ZW analyzed and interpreted the data. LY and QW participated in the discussion and critically revised the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Key R&D Program of China (2021YFC2700404, 2018YFC1003202).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the clinicians, nurses, and laboratory staff for their contribution. Moreover, the authors thank the infertile couples who participated in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.889029/full#supplementary-material
1. Giampaolino P, Morra I, Della Corte L, Sparice S, Di Carlo C, Nappi C, et al. Serum Anti-Mullerian Hormone Levels After Ovarian Drilling for the Second-Line Treatment of Polycystic Ovary Syndrome: A Pilot-Randomized Study Comparing Laparoscopy and Transvaginal Hydrolaparoscopy. Gynecol Endocrinol (2017) 33(1):26–9. doi: 10.1080/09513590.2016.1188280
2. Della Corte L, Foreste V, Barra F, Gustavino C, Alessandri F, Centurioni MG, et al. Current and Experimental Drug Therapy for the Treatment of Polycystic Ovarian Syndrome. Expert Opin Investig Drugs (2020) 29(8):819–30. doi: 10.1080/13543784.2020.1781815
3. Giampaolino P, De Rosa N, Della Corte L, Morra I, Mercorio A, Nappi C, et al. Operative Transvaginal Hydrolaparoscopy Improve Ovulation Rate After Clomiphene Failure in Polycystic Ovary Syndrome. Gynecol Endocrinol (2018) 34(1):32–5. doi: 10.1080/09513590.2017.1323204
4. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, Prevalence, and Phenotypes of Polycystic Ovary Syndrome. Fertil Steril (2016) 106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003
5. Giampaolino P, Foreste V, Di Filippo C, Gallo A, Mercorio A, Serafino P, et al. Microbiome and PCOS: State-Of-Art and Future Aspects. Int J Mol Sci (2021) 22(4):2048. doi: 10.3390/ijms22042048
6. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic Ovary Syndrome. Nat Rev Dis Primers (2016) 2:16057. doi: 10.1038/nrdp.2016.57
7. Giampaolino P, Corte LD, Rosa ND, Mercorio A, Bruzzese D, Bifulco G. Ovarian Volume and PCOS: A Controversial Issue. Gynecol Endocrinol (2018) 34(3):229–32. doi: 10.1080/09513590.2017.1391205
8. Cela V, Obino MER, Alberga Y, Pinelli S, Sergiampietri C, Casarosa E, et al. Ovarian Response to Controlled Ovarian Stimulation in Women With Different Polycystic Ovary Syndrome Phenotypes. Gynecol Endocrinol (2018) 34(6):518–23. doi: 10.1080/09513590.2017.1412429
9. Gilbert EW, Tay CT, Hiam DS, Teede HJ, Moran LJ. Comorbidities and Complications of Polycystic Ovary Syndrome: An Overview of Systematic Reviews. Clin Endocrinol (Oxf) (2018) 89(6):683–99. doi: 10.1111/cen.13828
10. Bahri Khomami M, Boyle JA, Tay CT, Vanky E, Teede HJ, Joham AE, et al. Polycystic Ovary Syndrome and Adverse Pregnancy Outcomes: Current State of Knowledge, Challenges and Potential Implications for Practice. Clin Endocrinol (Oxf) (2018) 88(6):761–9. doi: 10.1111/cen.13579
11. Li Y, Ruan X, Wang H, Li X, Cai G, Du J, et al. Comparing the Risk of Adverse Pregnancy Outcomes of Chinese Patients With Polycystic Ovary Syndrome With and Without Antiandrogenic Pretreatment. Fertil Steril (2018) 109(4):720–7. doi: 10.1016/j.fertnstert.2017.12.023
12. Kelley AS, Smith YR, Padmanabhan V. A Narrative Review of Placental Contribution to Adverse Pregnancy Outcomes in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2019) 104(11):5299–315. doi: 10.1210/jc.2019-00383
13. Henningsen AK, Pinborg A, Lidegaard Ø, Vestergaard C, Forman JL, Andersen AN. Perinatal Outcome of Singleton Siblings Born After Assisted Reproductive Technology and Spontaneous Conception: Danish National Sibling-Cohort Study. Fertil Steril (2011) 95(3):959–63. doi: 10.1016/j.fertnstert.2010.07.1075
14. Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, et al. Why do Singletons Conceived After Assisted Reproduction Technology Have Adverse Perinatal Outcome? Systematic Review and Meta-Analysis. Hum Reprod Update (2013) 19(2):87–104. doi: 10.1093/humupd/dms044
15. Qin J, Wang H, Sheng X, Liang D, Tan H, Xia J. Pregnancy-Related Complications and Adverse Pregnancy Outcomes in Multiple Pregnancies Resulting From Assisted Reproductive Technology: A Meta-Analysis of Cohort Studies. Fertil Steril (2015) 103(6):1492–1508.e1491-1497. doi: 10.1016/j.fertnstert.2015.03.018
16. Sha T, Wang X, Cheng W, Yang Y. A Meta-Analysis of Pregnancy-Related Outcomes and Complications in Women With Polycystic Ovary Syndrome Undergoing IVF. Reprod BioMed Online (2019) 39(2):281–93. doi: 10.1016/j.rbmo.2019.03.203
17. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil Steril (2004) 81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004
18. Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh Versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. New Engl J Med (2016) 375(6):523–33. doi: 10.1056/NEJMoa1513873
19. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic Ovary Syndrome. Lancet (2007) 370(9588):685–97. doi: 10.1016/S0140-6736(07)61345-2
20. Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of Fresh Versus Frozen Embryos in Ovulatory Women. New Engl J Med (2018) 378(2):126–36. doi: 10.1056/NEJMoa1705334
21. Liu F, Jiang Q, Sun X, Huang Y, Zhang Z, Han T, et al. Lipid Metabolic Disorders and Ovarian Hyperstimulation Syndrome: A Retrospective Analysis. Front Physiol (2020) 11:491892. doi: 10.3389/fphys.2020.491892
22. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol (2000) 183(1):S1–s22.
23. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-Eclampsia. Lancet (London England) (2016) 387(10022):999–1011. doi: 10.1016/S0140-6736(15)00070-7
24. Toyokawa S, Hasegawa J, Ikenoue T, Asano Y, Jojima E, Satoh S, et al. Weekend and Off-Hour Effects on the Incidence of Cerebral Palsy: Contribution of Consolidated Perinatal Care. Environ Health Prev Med (2020) 25(1):52. doi: 10.1186/s12199-020-00889-y
25. Baz B, Riveline JP, Gautier JF. Endocrinology of Pregnancy: Gestational Diabetes Mellitus: Definition, Aetiological and Clinical Aspects. Eur J Endocrinol (2016) 174(2):R43–51. doi: 10.1530/EJE-15-0378
26. Yu HF, Chen HS, Rao DP, Gong J. Association Between Polycystic Ovary Syndrome and the Risk of Pregnancy Complications: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine (2016) 95(51):e4863. doi: 10.1097/MD.0000000000004863
27. Yu T, Wu D, Cao Y, Zhai J. Association Between Menstrual Patterns and Adverse Pregnancy Outcomes in Patients With Polycystic Ovary Syndrome. Front Endocrinol (2021) 12:740377. doi: 10.3389/fendo.2021.740377
28. Giudice LC. Endometrium in PCOS: Implantation and Predisposition to Endocrine CA. Best Pract Res Clin Endocrinol Metab (2006) 20(2):235–44. doi: 10.1016/j.beem.2006.03.005
29. Piltonen TT. Polycystic Ovary Syndrome: Endometrial Markers. Best Pract Res Clin Obstet Gynaecol (2016) 37:66–79. doi: 10.1016/j.bpobgyn.2016.03.008
30. Ezeh U, Ezeh C, Pisarska MD, Azziz R. Menstrual Dysfunction in Polycystic Ovary Syndrome: Association With Dynamic State Insulin Resistance Rather Than Hyperandrogenism. Fertil Steril (2021) 115(6):1557–68. doi: 10.1016/j.fertnstert.2020.12.015
31. Barnes FL, Kausche A, Tiglias J, Wood C, Wilton L, Trounson A. Production of Embryos From In Vitro-Matured Primary Human Oocytes. Fertil Steril (1996) 65(6):1151–6. doi: 10.1016/S0015-0282(16)58330-7
32. Santos JE, Bisinotto RS, Ribeiro ES. Mechanisms Underlying Reduced Fertility in Anovular Dairy Cows. Theriogenology (2016) 86(1):254–62. doi: 10.1016/j.theriogenology.2016.04.038
33. Yarali H, Yildirir A, Aybar F, Kabakçi G, Bükülmez O, Akgül E, et al. Diastolic Dysfunction and Increased Serum Homocysteine Concentrations may Contribute to Increased Cardiovascular Risk in Patients With Polycystic Ovary Syndrome. Fertil Steril (2001) 76(3):511–6. doi: 10.1016/S0015-0282(01)01937-9
34. Badawy A, State O, El Gawad SSA, El Aziz OA. Plasma Homocysteine and Polycystic Ovary Syndrome: The Missed Link. Eur J Obstet Gynecol Reprod Biol (2007) 131(1):68–72. doi: 10.1016/j.ejogrb.2006.10.015
35. Schachter M, Raziel A, Friedler S, Strassburger D, Bern O, Ron-El R. Insulin Resistance in Patients With Polycystic Ovary Syndrome is Associated With Elevated Plasma Homocysteine. Hum Reprod (2003) 18(4):721–7. doi: 10.1093/humrep/deg190
36. Berker B, Kaya C, Aytac R, Satiroglu H. Homocysteine Concentrations in Follicular Fluid are Associated With Poor Oocyte and Embryo Qualities in Polycystic Ovary Syndrome Patients Undergoing Assisted Reproduction. Hum Reprod (2009) 24(9):2293–302. doi: 10.1093/humrep/dep069
37. Jia L, Li J, He B, Jia Y, Niu Y, Wang C, et al. Abnormally Activated One-Carbon Metabolic Pathway is Associated With mtDNA Hypermethylation and Mitochondrial Malfunction in the Oocytes of Polycystic Gilt Ovaries. Sci Rep (2016) 6:19436. doi: 10.1038/srep19436
38. Frick AP. Advanced Maternal Age and Adverse Pregnancy Outcomes. Best Pract Res Clin Obstet Gynaecol (2021) 70:92–100. doi: 10.1016/j.bpobgyn.2020.07.005
39. Kawwass JF, Badell ML. Maternal and Fetal Risk Associated With Assisted Reproductive Technology. Obstet Gynecol (2018) 132(3):763–72. doi: 10.1097/AOG.0000000000002786
40. Lewandowska M, Sajdak S, Więckowska B, Manevska N, Lubiński J. The Influence of Maternal BMI on Adverse Pregnancy Outcomes in Older Women. Nutrients (2020) 12(9):2838. doi: 10.3390/nu12092838
41. Grieger JA, Bianco-Miotto T, Grzeskowiak LE, Leemaqz SY, Poston L, McCowan LM, et al. Metabolic Syndrome in Pregnancy and Risk for Adverse Pregnancy Outcomes: A Prospective Cohort of Nulliparous Women. PloS Med (2018) 15(12):e1002710. doi: 10.1371/journal.pmed.1002710
42. Liu X, Wang J, Fu X, Li J, Zhang M, Yan J, et al. Thin Endometrium is Associated With the Risk of Hypertensive Disorders of Pregnancy in Fresh IVF/ICSI Embryo Transfer Cycles: A Retrospective Cohort Study of 9,266 Singleton Births. Reprod Biol Endocrinol (2021) 19(1):55. doi: 10.1186/s12958-021-00738-9
43. Revel A. Defective Endometrial Receptivity. Fertil Steril (2012) 97(5):1028–32. doi: 10.1016/j.fertnstert.2012.03.039
44. Guo Z, Xu X, Zhang L, Zhang L, Yan L, Ma J. Endometrial Thickness is Associated With Incidence of Small-for-Gestational-Age Infants in Fresh In Vitro Fertilization-Intracytoplasmic Sperm Injection and Embryo Transfer Cycles. Fertil Steril (2020) 113(4):745–52. doi: 10.1016/j.fertnstert.2019.12.014
45. Ma L, Cao Y, Ma Y, Zhai J. Association Between Hyperandrogenism and Adverse Pregnancy Outcomes in Patients With Different Polycystic Ovary Syndrome Phenotypes Undergoing In Vitro Fertilization/Intracytoplasmic Sperm Injection: A Systematic Review and Meta-Analysis. Gynecol Endocrinol (2021) 37(8):694–701. doi: 10.1080/09513590.2021.1897096
46. Yang W, Yang R, Yang S, Li J, Tu B, Gao C, et al. Infertile Polycystic Ovary Syndrome Patients Undergoing In Vitro Fertilization With the Gonadotropin-Releasing Hormone-Antagonist Protocol: Role of Hyperandrogenism. Gynecol Endocrinol (2018) 34(8):715–8. doi: 10.1080/09513590.2018.1431773
47. Sun YF, Zhang J, Xu YM, Cao ZY, Wang YZ, Hao GM, et al. High BMI and Insulin Resistance Are Risk Factors for Spontaneous Abortion in Patients With Polycystic Ovary Syndrome Undergoing Assisted Reproductive Treatment: A Systematic Review and Meta-Analysis. Front Endocrinol (2020) 11:592495. doi: 10.3389/fendo.2020.592495
48. Haas J, Baum M, Meridor K, Hershko-Klement A, Elizur S, Hourvitz A, et al. Is Severe OHSS Associated With Adverse Pregnancy Outcomes? Evidence From a Case-Control Study. Reprod BioMed Online (2014) 29(2):216–21. doi: 10.1016/j.rbmo.2014.04.015
49. Wang F, Dai W, Yang XH, Guo YH, Sun YP. Analyses of Optimal Body Mass Index for Infertile Patients With Either Polycystic or non-Polycystic Ovary Syndrome During Assisted Reproductive Treatment in China. Sci Rep (2016) 6:34538. doi: 10.1038/srep34538
50. Mourad S, Brown J, Farquhar C. Interventions for the Prevention of OHSS in ART Cycles: An Overview of Cochrane Reviews. Cochrane Database Syst Rev (2017) 1(1):Cd012103. doi: 10.1002/14651858.CD012103.pub2
Keywords: polycystic ovarian syndrome, phenotype, assisted reproductive technology, hypertensive disorder of pregnancy, adverse pregnancy outcomes
Citation: Wang Q, Wang H, Li P, Li X, Wang Z, Yan L and Shi Y (2022) Association of Polycystic Ovary Syndrome Phenotypes With Adverse Pregnancy Outcomes After In-Vitro Fertilization/Intracytoplasmic Sperm Injection. Front. Endocrinol. 13:889029. doi: 10.3389/fendo.2022.889029
Received: 03 March 2022; Accepted: 28 April 2022;
Published: 03 June 2022.
Edited by:
Yimin Zhu, Zhejiang University, ChinaReviewed by:
Rong Li, Peking University Third Hospital, ChinaCopyright © 2022 Wang, Wang, Li, Li, Wang, Yan and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Yan, eWFubGVpQHNkdS5lZHUuY24=; Yuhua Shi, c2hpeXVodWEyMDAzQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.