- 1Department of Clinical Medicine and Surgery, Endocrinology Unit, University of Naples “Federico II”, Naples, Italy
- 2Dipartimento di Scienze Umanistiche, Università Telematica Pegaso, Naples, Italy
- 3Centro Italiano per la cura e il Benessere del Paziente con Obesità (C.I.B.O), Department of Clinical Medicine and Surgery, Endocrinology Unit, Federico II University Medical School of Naples, Naples, Italy
- 4UNESCO Chair “Education for Health and Sustainable Development”, University of Naples “Federico II”, Naples, Italy
Menopause is a natural event occurring in a woman’s life that is often accompanied by symptoms that might affect the quality of life. Diet has been shown to influence menopausal-related symptoms. Therefore, the present study aimed to investigate whether the adherence to the Mediterranean Diet (MD) might influence menopausal symptoms in women with obesity. This cross-sectional study involved postmenopausal women with obesity. Anthropometric and clinical parameters, and lifestyle habits were evaluated. All participants underwent interview questionnaires to assess: the adherence to the MD (PREDI PREvencion con DIetaMEDiterranea, PREDIMED), sleep quality (Pittsburgh Sleep Quality Index, PSQI), and severity of menopausal symptoms (Menopausal Rating Scale, MRS). One hundred postmenopausal women were enrolled (age 57.1 ± 7.3 years, BMI 35.0 ± 5.5 kg/m2). The mean PREDIMED score was 7.82 ± 1.66 showing moderate adherence to MD. Women in the marked MRS class had a significantly lower PREDIMED score than the none-to-moderate MRS class (p=0.036). The intake of legumes was associated with a lower MRS class (r= -0.201, p=0.045). In addition, the intake of extra-virgin olive oil inversely correlated with psychological symptoms (r= -0.230 p=0.021). Finally, 79% of participants were poor sleepers (mean PSQI score was 8.68 ± 3.6) and women in the severe MRS class had a worse sleep quality compared to other MRS classes. Post-menopausal women with marked menopausal symptoms had low adherence to MD. Legume consumption was associated with lower menopausal symptoms severity while extra virgin olive oil consumption was associated with lower psychological symptoms.
1 Introduction
Menopause is the physiological cessation of reproductive capacity in a woman’s life. Usually, it occurs between 45-55 years unless pathological conditions induce premature or early menopause (1).
Menopausal transition (also known as climacteric) starts with the onset of menstrual abnormalities and ends with the last menstrual period. Physiological changes during climacteric -mainly related to hormonal variations - may trigger a variety of menopausal symptoms that might last years in the postmenopausal period (2, 3).
Menopausal symptoms include psychological (depressive mood, irritability, anxiety, tiredness), somato-vegetative (hot flushes, palpitations, insomnia, muscle and joint pain), and urogenital (sexual problems, bladder problems, and vaginal dryness) disorders (1–3). Notably, several cohort studies have demonstrated an association between menopausal symptoms and lower quality of life (4–8).
In addition, hormonal changes during menopause contribute significantly to weight gain, increased visceral fat mass, and the development of abdominal obesity (3). Therefore, postmenopausal women may also present obesity-related metabolic disorders (i.e., insulin-resistance, dyslipidemia, and metabolic syndrome) that associate with an increased risk of type 2 diabetes, cardiovascular disease, and other chronic-degenerative diseases (9–11).
The Mediterranean diet (MD) is a healthy dietary pattern that has been associated with a reduced risk of cardiovascular events, morbidity and overall mortality, and the improvement of body weight, metabolic profile, and cognitive function (11–14).
MD is characterized by a wide variety of plant foods, such as vegetables, fruits, legumes, nuts, and whole-grain cereals, and the use of extra-virgin olive oil as the main source of fat (15). Therefore, MD provides low amounts of saturated fats in favor of unsaturated fats, and many bioactive compounds with anti- inflammatory and antioxidant activities (omega 3 fatty acids and vitamins, minerals, and phytochemicals, respectively) (15). More in detail, most of MD energy intake comes from carbohydrate (55%), close to 30% from fat, and the remaining part by protein (15%). Fats are mainly represented by 19% of monounsaturated fatty acids (MUFA), followed by saturated fatty acids (SFA, 9%) and polyunsaturated fatty acids (PUFA, 5%), and cholesterol is 300 mg/day (15).
Several studies suggested that MD might play a role in the reduction of body weight and menopausal symptoms in postmenopausal women by virtue of its dietary composition. In particular, a cross-sectional study in 481 postmenopausal women showed that a high adherence to the MD, evaluated by the Mediterranean Diet Score (MDS), was negatively associated with body weight, waist circumference, and waist-to-height ratio (16). Moreover, a cross-sectional study in 8.954 Spanish perimenopausal-post-menopausal women showed that high adherence to the MD was associated with a lower prevalence and risk of being overweight/obese (17).
As for menopausal symptoms, Herber-Gast and colleagues (18) investigated the relationship between MD and risk of vasomotor symptoms (hot flushes and night sweats) in the Australian Longitudinal Study on Women’s Health, a cohort study involving 6,040 postmenopausal women with a 9 year- follow-up. In this study a higher adherence to MD was inversely associated with vasomotor menopausal symptoms, thus suggesting that MD might be useful to prevent vasomotor menopausal symptoms.
Against this background, the aim of the present study was to investigate the impact of adherence to MD on the severity of menopausal symptoms in postmenopausal women with obesity. In addition, we explored the possible association between postmenopausal symptoms and the main foodstuffs characterizing MD.
2 Methods
2.1 Study Population
All postmenopausal women with obesity attending the Outpatient Clinic of the Unit of Endocrinology, Department of Clinical Medicine and Surgery, University of Naples “Federico II” from May 2021 to October 2021 were screened for eligibility. The inclusion criteria were menopause status established after 1 year since the last menstrual period (amenorrhea for at least 12 months in the absence of treatment with hormonal contraceptives, or hysterectomized women with menopausal symptoms) and obesity (BMI >30 kg/m2). The exclusion criteria were the following: normal weight or overweight (BMI< 30 kg/m2), premenopausal status, hormonal therapy, type 1 diabetes mellitus, women with diabetes on insulin therapy, any other chronic disease (cardiopulmonary, brain, kidney, or liver disease), severe mental illness (depression, anxiety, or schizophrenia), women with allergy/intolerance or following a specific dietary regimen.
The aim of the study was clearly explained to all potential participants and written informed consent was obtained. The study was approved by the Local Ethical Committee and carried out in accordance with the Declaration of Helsinki.
2.2 Study Design
This cross-sectional study was conducted in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist (19).
Trained medical doctors and nutritionists collected demographic information, medical history, lifestyle habits (smoke, alcohol use, physical activity), and anthropometric parameters. All participants underwent interview questionnaires to assess: adherence to MD (PREDI PREvencion con DIetaMEDiterranea, PREDIMED) (20), sleep quality (Pittsburgh Sleep Quality Index, PSQI) (21), and severity of menopausal symptoms (Menopausal Rating Scale, MRS) (22).
2.2.1 Demographic Information, Medical History, Lifestyle Habits
A brief medical history was used to assess the presence of diseases (type 2 diabetes, cardiovascular diseases, hypertension, dyslipidemia) and pharmacological therapy. In addition, information about age at menarche, years since menopause, type of menopause (natural or induced), weight before menopause, weight gain after menopause, and full-term pregnancies were collected.
As for lifestyle habits, smoking at least one cigarette/day identified “current smokers”. Participants habitually engaged in at least 30 minutes/day of aerobic exercise were defined as physically active. Alcohol use was identified with a threshold of more than 20 g/day.
2.2.2 Anthropometric Measurements
Anthropometric measurements were performed by the same operator (a nutritionist experienced in providing nutritional assessment and body composition), according to standard procedures (23, 24).
All measurements (weight, height, waist and hip circumferences) were performed on participants wearing light clothing and no shoes, after an overnight fast, according to standard procedures (the subject was standing upright with the feet together and the arms hanging loosely by the sides, with the subject standing and breathing normally, as previously reported (25–27). Waist (WC) and hip circumferences (HC) were used to calculate the waist-hip ratio (WHR). Weight and height were used to calculate the body mass index (BMI). Obesity was classified according to World Health Organization’s criteria (28): Obesity grade I (BMI: 30.0-34.9 kg/m2); Obesity grade II (BMI: 35.0-39.9 kg/m2); Obesity grade III (BMI >40.0 kg/m2).
2.2.3 Interview Questionnaires
The adherence to MD was assessed using the PREDIMED questionnaire, consisting in 14 items (intake and amount of extra-virgin olive oil, frequency of fruit, vegetables, nuts, legumes, red meat, poultry, fish, animal fat, sweetened beverages, sweets, and sofrito). A qualified nutritionist administered this questionnaire with the same face-to-face interview used in previous studies (29). PREDIMED score was calculated by assigning a score of 1 and 0 for each item. According to the PREDIMED score, participants were classified as follows: 0–5, lowest adherence; score 6–9, average adherence; and score ≥10, highest adherence (20).
Overall sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI). Poor sleepers were defined as participants with PSQI score ≥ 5 whereas good sleepers were defined as participants with PSQI score < 5 (21).
The Menopausal Rating Scale (MRS) consisted in a list of 11 symptoms (22). The respondents had a choice of score between 0 (no symptoms) and 4 points (severe symptoms), depending on subjective perception. Three groups of symptoms can be detected with MRS: psychological (depression, irritability, anxiety, physical and mental exhaustion), somato-vegetative (sweating and hot flushes, cardiac complaints, sleeping disorders, joint and muscle complaints), and urogenital symptoms (sexual problems, urinary complaints, vaginal dryness). Therefore, the total MRS score ranged between 0 (asymptomatic) and 44 (highest degree of complaints). According to the MRS score, five categories can be identified: no symptoms (0-4), mild (5-8), moderate (9-12), marked (13-16), and severe (>17). Nevertheless, previous studies (30, 31) reported that respondents might have difficulties in rating the perceptions of low-to-moderate symptoms. Therefore, since our primary aim was to investigate the impact of MD on the severity of menopausal symptoms, the classes of “no symptoms”, “mild”, and “moderate” were combined in a single class (“none-to-moderate symptoms”).
2.2.4 Sample Size Justification and Power
The calculation of the sample size was performed a priori by considering the effect size 0.3 with a type I error of 0.05 and a power of 80%. The number of subjects to be enrolled was found to be 82. During the study, 100 women were considered eligible for the study. This sample size met the at least necessary number of subjects and would further support the result. Therefore, we decided to include all subjects in the statistical analysis. The calculation of the Sample Size was performed using G Power Software.
2.2.5 Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) whereas categorical variables were reported as numbers and percentages (%). Differences between groups were analyzed by analysis of variance (one-way ANOVA) and post hoc analyses (least significant difference test, LSD). Chi-squared test for independence was used to assess the association between outcomes and categorical variables. Correlations between study variables were performed using Spearman rank correlation. A p value <0.05 was considered significant. Statistical analysis was performed according to standard methods using the Statistical Package for Social Sciences software 26.0 (SPSS/PC; SPSS, Chicago, IL, USA).
3 Results
A total of 100 postmenopausal women with obesity (BMI 35.0 ± 5.5 kg/m2, mean age 57.2 ± 7.3) were included in the analyses. The main characteristics of the study participants (demographic, anthropometric and medical data, lifestyle habits) and the mean score of the interview questionnaires (PREDIMED and PSQI) were reported in Table 1.
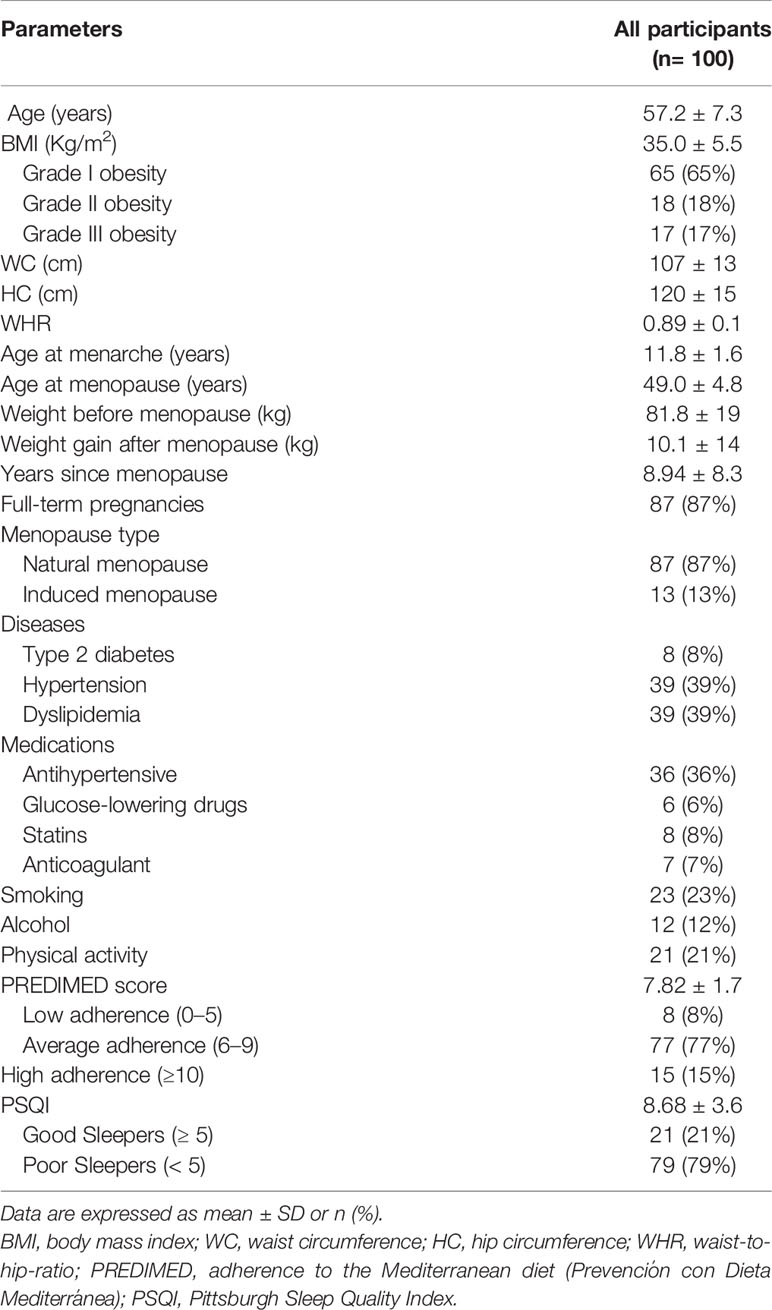
Table 1 Demographic, anthropometric and medical data, lifestyle habits, adherence to the Mediterranean diet, and sleep quality of all study participants.
Seventy-five (65%) participants presented grade I obesity whereas women with grade II and grade III were 18 (18%) and 17 (17%), respectively.
WC was 107.4 ± 13 cm, HC was 120.3 ± 14.8, and WHR was 0.89 ± 0.06. As for lifestyle habits, 23 women (23%) were smokers, and most of the participants were sedentary 79 (79%). The prevalence of diseases was: 8% type 2 diabetes, 39% hypertension, and 39% dyslipidemia. Participants taking medications were 36% antihypertensive, 6% antidiabetic, 8% hypolipidemic medications, and 7% anticoagulant medications.
The mean PREDIMED score was 7.82 ± 1.7. Eight (8%) participants presented low adherence, 77 (77%) had average adherence, while 15 (15%) had high adherence. The mean PSQI score was 8.68 ± 3.6. Twenty-one participants (21%) were good sleepers, while 79 (79%) were poor sleepers.
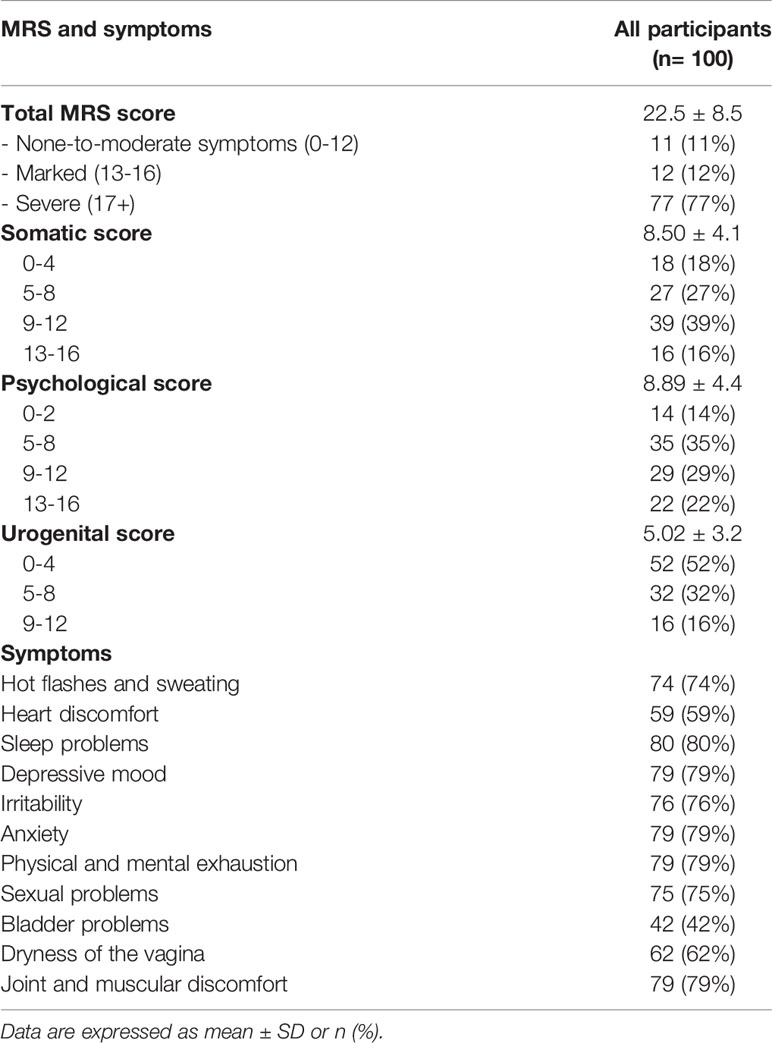
Total MRS scores and the severity of symptoms are reported in Table 2. The mean MRS score was 22.5 ± 8.5, with the following prevalence of the severity of symptoms: 11 women (11%) none-to-moderate symptoms, 12 (12%) marked symptoms, and 77 (77%) severe symptoms. Regarding the somatic and psychological symptoms, most of the participants were in the third class of symptoms severity (39% and 29%, respectively), whereas 52% of participants were in the first class of severity for urogenital symptoms. As for the specific menopausal symptom, the most frequent disorders were sleep problems (80%), depressive mood (79%), anxiety (79%), physical and mental exhaustion (79%), and joint and muscular discomfort (79%).
Table 3 reported the population’s characteristics stratified by severity of menopausal symptoms identified according to the MRS score. No significant differences were observed for age, BMI, obesity grade, other anthropometric measures (WC, HC, and WHR), lifestyle habits (smoking, alcohol, physical activity), and medical history (prevalence of diseases, medications, and menopause-related data). However, a significantly lower PREDIMED score we observed in the “marked symptoms” class than the “none-to-moderate symptoms” class (7.08 ± 1.2 vs. 9.50 ± 0.7, respectively; p=0.036). The “severe symptoms” class presented a lower PREDIMED score than the “none-to-moderate symptoms” class (7.83 ± 1.6 vs. 9.50 ± 0.7, respectively; p=0.181), although the difference did not reach the conventional level of statistical significance.
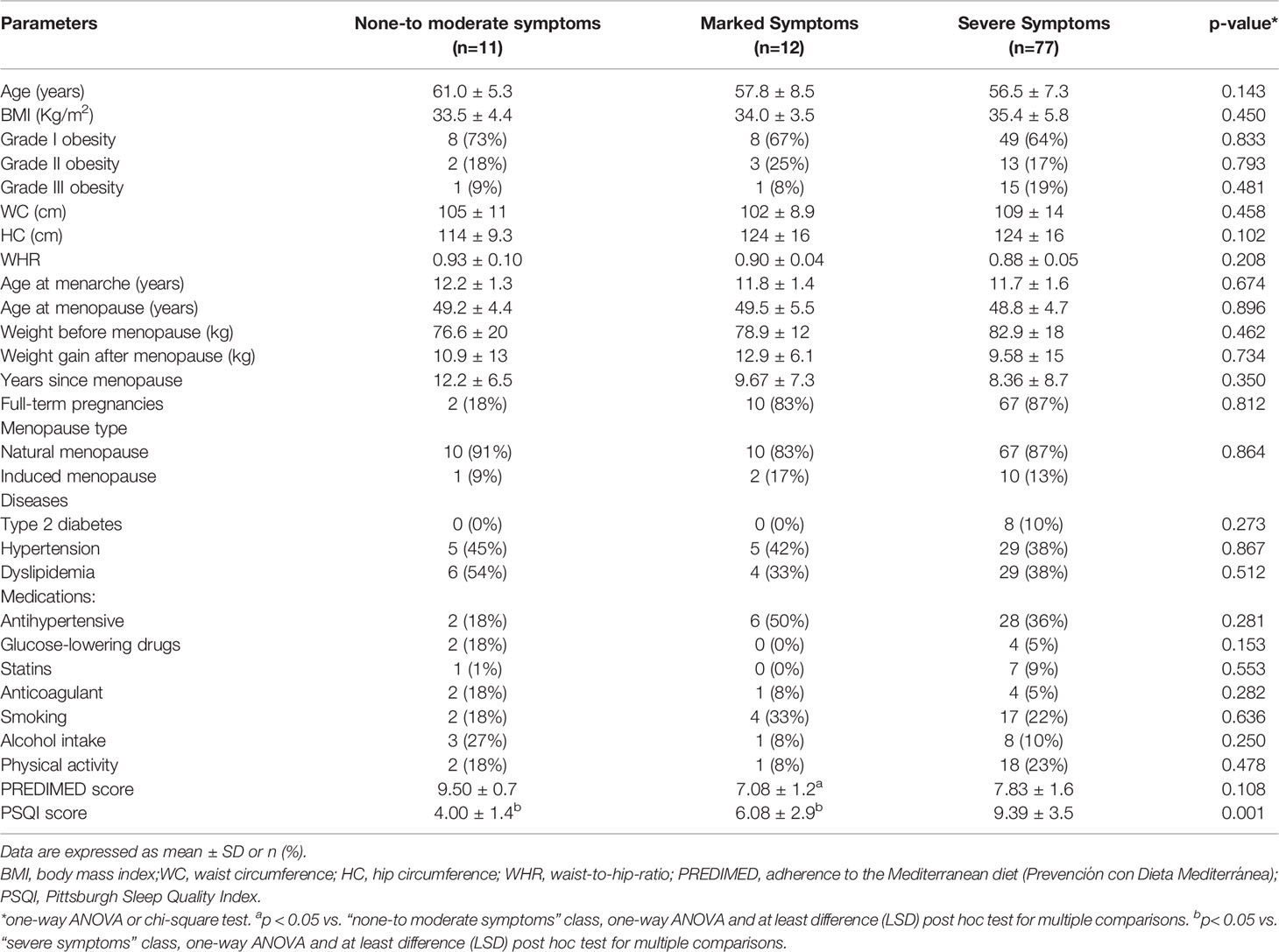
Table 3 Demographic, anthropometric and medical, lifestyle habits, adherence to the Mediterranean diet, and sleep quality of participants stratified by severity of menopausal symptoms (MRS).
PSQI score was significantly lower in the “severe symptoms” class than in “marked symptoms” (p=0.012) and “none-to-moderate symptoms” (p=0.002) classes.
Correlation analysis was performed to assess the association of menopausal symptoms with PREDIMED items. The intake of legumes (≥3 times/week) was inversely correlated to the MRS score (r= -0.201, p=0.045). The intake of extra-virgin olive oil was inversely correlated with psychological symptoms (r: -0.230, p=0.021), mainly related to reduced depressive mood (r= -0.205, p=0.041). No other significant correlations with PREDIMED items and menopausal symptoms were observed.
4 Discussion
The present study showed that the severity of menopausal symptoms (assessed by MRS) was inversely associated with the adherence to MD. In particular, women in the marked MRS class had a significantly lower adherence to MD than women in the none-to-moderate MRS class. Therefore, we investigated what food component characterizing MD was mostly involved in this association. The results showed that the intake of legumes and extra-virgin olive oil was associated with lower severity of total menopausal symptoms and psychological symptoms, respectively. Furthermore, women in the severe MRS class presented a lower sleep quality compared to other classes.
The association between menopausal symptoms and adherence to MD has been previously reported in two large cohort studies in postmenopausal women with overweight/obesity. Interestingly, a 2-years clinical trial in demonstrated that postmenopausal women with overweight/obesity (n=116, aged 56.6 ± 3.8 years, BMI 29.6 ± 3.8 kg/m2) advised a MD-resembling diet (low intake of meat, pastries, cakes, and sweets, while high intake of virgin olive oil, nuts, vegetables, legumes, and fruits) experienced an improvement of menopausal symptoms (32).
The present study added information about the specific components of MD that might explain the association with post-menopausal symptoms. Although no previous studies focused on the consumption of legumes intake and the severity of menopausal symptoms, two meta-analyses of randomized controlled trials (33, 34) reported an inverse association between post-menopausal symptoms and soy intake - known to be a legume. The mechanism behind this effect seems to be related to the activity of isoflavones. Indeed, isoflavones are biologically active compounds, with a chemical structure resembling estrogens. Therefore, they can bind estrogen-receptors thus positively influencing menopausal symptoms (35, 36). Dietary intake of isoflavones ranges from 5 to 80 mg/day in Asia, whereas in Western populations the reported daily intake is usually less than 3 mg/day (37). However, a study carried out in 14.029 individuals living in Southern Italy (38), with a strong Mediterranean gastronomic background, reported an isoflavone intake ranging 18-31 mg/day.
Regarding the use of extra-virgin olive oil, in this study women who consumed extra virgin olive oil experienced less psychological symptoms, in particular depressive mood.
Although this finding has not been observed in postmenopausal women, a 12-week nutritional trial carried out in 149 individuals with severe obesity (BMI > 35 Kg/m2) showed that daily consumption of extra virgin olive oil (52ml/day) significantly reduced depression and anxiety (39). In line with this, Foshati and colleagues demonstrated that the consumption of extra-virgin olive oil (25 ml/day for 7 weeks) significantly improved depressive symptoms in 73 patients with severe depression (40).
Although the psychological implications of diet are not completely clear (41, 42), pro-inflammatory foods such as those rich in saturated fats may induce the release of pro-inflammatory cytokines impairing the activity of the dopaminergic system, while the intake of monounsaturated fatty acids has shown to improve brain function (39).
Finally, as expected, participants in the severe MRS class presented a worsen sleep quality than other MRS classes. This finding is in line with a previous study carried out in 385 postmenopausal women aged <45 years investigating sleep quality by PSQI and its association with symptoms related to the menopausal period. In this study women who presented greater severity of menopausal symptoms reported greater impairment in sleep quality (43). The etiology of sleep disorders in menopausal women is still controversial. Some evidence suggested a role of the reduction of estrogens levels that can induce depression (44) and vasomotor symptoms (45). In addition, weight gain and particularly increased visceral adiposity can influence sleep quality through the secretion of cytokines or indirectly through obstructive sleep apnea that is frequently in individuals with severe obesity (46–48).
Although no correlation was found between sleep disorders and adherence to MD, nutrition might play a role in the modulation of sleep quality. Indeed, the intake of phytoestrogens has been associated with a reduction of the frequency of insomnia (49). Moreover, a high intake of omega-3 has been reported to decrease depression and anxiety which could contribute to improve sleep quality (50).
Nevertheless, we can not exclude the possibility that sleep disorders would worse the menopausal symptoms, at least the psychological aspects.
The present study had both strengths and limitations. It included a relatively large sample of postmenopausal women for exploring associations between MD and menopausal symptoms. However, it was a convenience sample, as participants were consecutively recruited from women attending the Outpatient Clinic of the Unit of Endocrinology, Department of Clinical Medicine and Surgery, University of Naples “Federico II”. In addition, the cross-sectional design of the study did not allow for cause-effect conclusions. Finally, all questionnaires used in this study were translated in Italian from the English version with no forward-backward translation method to guarantee the conceptual equivalence (51). Nevertheless, the participants were interviewed by expert operators thus increasing the reliability of the collected data.
In conclusion, in the present study, the adherence to MD was associated to lower severity of menopausal symptoms in women with obesity. Notably, legume consumption was associated with lower menopausal symptoms severity, and extra virgin olive oil was associated with a reduction in psychological symptoms. These findings highlight the importance of the MD as an ideal nutritional strategy in the management of menopause.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Naples Federico II Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The authors’ responsibilities were as follows CV, LB, and GM: were responsible for the concept and design of the study, interpreted data and drafted the manuscript. CV, LB, and RR: conducted statistical analyses. RR, LV, GA, AD, and RA: collected data. AC, SS, and GM: provided a critical review of the manuscript. All authors contributed to and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the study participants.
References
2. Keller C, Larkey L, Distefano JK, Boehm-Smith E, Records K, Robillard A, et al. Perimenopausal Obesity. J Womens Health (Larchmt) (2010) 19(5):987–96. doi: 10.1089/jwh.2009.1547
3. Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, et al. Understanding Weight Gain at Menopause. Climacteric (2012) 15(5):419–29. doi: 10.3109/13697137.2012.707385
4. Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare Seeking and Treatment for Menopausal Symptoms in the United States. Maturitas (2007) 58(4):348–58. doi: 10.1016/j.maturitas.2007.09.006
5. Karaçam Z, Seker SE. Factors Associated With Menopausal Symptoms and Their Relationship With the Quality of Life Among Turkish Women. Maturitas (2007) 58(1):75–82. doi: 10.1016/j.maturitas.2007.06.004
6. Avis NE, Colvin A, Bromberger JT, Hess R, Matthews KA, Ory M, et al. Change in Health-Related Quality of Life Over the Menopausal Transition in a Multiethnic Cohort of Middle-Aged Women: Study of Women's Health Across the Nation. Menopause (2009) 16(5):860–9. doi: 10.1097/gme.0b013e3181a3cdaf
7. Gallicchio L, Miller S, Zacur H, Flaws JA. Race and Health-Related Quality of Life in Midlife Women in Baltimore, Maryland. Maturitas (2009) 63(1):67–72. doi: 10.1016/j.maturitas.2009.02.001
8. Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-Specific Questionnaire Assessment in US Population-Based Study Shows Negative Impact on Health-Related Quality of Life. Maturitas (2009) 62(2):153–9. doi: 10.1016/j.maturitas.2008.12.006
9. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation (2020) 142(25):e506–32. doi: 10.1161/CIR.0000000000000912
10. Nappi RE, Simoncini T. Menopause Transition: A Golden Age to Prevent Cardiovascular Disease. Lancet Diabetes Endocrinol (2021) 9(3):135–7. doi: 10.1016/S2213-8587(21)00018-8
11. Hirahatake KM, Jiang L, Wong ND, Shikany JM, Eaton CB, Allison MA, et al. Diet Quality and Cardiovascular Disease Risk in Postmenopausal Women With Type 2 Diabetes Mellitus: The Women's Health Initiative. J Am Heart Assoc (2019) 8(19):e013249. doi: 10.1161/JAHA.119.013249
12. Shannon OM, Ashor AW, Scialo F, Saretzki G, Martin-Ruiz C, Lara J, et al. Mediterranean Diet and the Hallmarks of Ageing. Eur J Clin Nutr (2021) 75(8):1176–92. doi: 10.1038/s41430-020-00841-x
13. Grosso G, Marventano S, Yang J, Micek A, Pajak A, Scalfi L, et al. A Comprehensive Meta-Analysis on Evidence of Mediterranean Diet and Cardiovascular Disease: Are Individual Components Equal? Crit Rev Food Sci Nutr (2017) 57(15):3218–32. doi: 10.1080/10408398.2015.1107021
14. Altieri B, Barrea L, Modica R, Muscogiuri G, Savastano S, Colao A, et al. Nutrition and Neuroendocrine Tumors: An Update of the Literature. Rev Endocr Metab Disord (2018) 19(2):159–67. doi: 10.1007/s11154-018-9466-z
15. Barrea L, Pugliese G, Laudisio D, Colao A, Savastano S, Muscogiuri G. Mediterranean Diet as Medical Prescription in Menopausal Women With Obesity: A Practical Guide for Nutritionists. Crit Rev Food Sci Nutr (2021) 61(7):1201–11. doi: 10.1080/10408398.2020.1755220
16. Papavagelis C, Avgeraki E, Augoulea A, Stamatelopoulos K, Lambrinoudaki I, Yannakoulia M. Dietary Patterns, Mediterranean Diet and Obesity in Postmenopausal Women. Maturitas (2018) 110:79–85. doi: 10.1016/j.maturitas.2018.02.001
17. Sayón-Orea C, Santiago S, Cuervo M, Martínez-González MA, Garcia A, Martínez JA. Adherence to Mediterranean Dietary Pattern and Menopausal Symptoms in Relation to Overweight/Obesity in Spanish Perimenopausal and Postmenopausal Women. Menopause (2015) 22(7):750–7. doi: 10.1097/GME.0000000000000378
18. Herber-Gast GC, Mishra GD. Fruit, Mediterranean-Style, and High-Fat and -Sugar Diets Are Associated With the Risk of Night Sweats and Hot Flushes in Midlife: Results From a Prospective Cohort Study. Am J Clin Nutr (2013) 97(5):1092–9. doi: 10.3945/ajcn.112.049965
19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet (2007) 370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X
20. Martínez-González MA, García-Arellano A, Toledo E, Salas-Salvadó J, Buil-Cosiales P, Corella D, et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes Among High-Risk Subjects: The PREDIMED Trial. PloS One (2012) 7(8):e43134. doi: 10.1371/journal.pone.0043134
21. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res (1989) 28(2):193–213. doi: 10.1016/0165-1781(89)90047-4
22. Schneider HP, Heinemann LA, Rosemeier HP, Potthoff P, Behre HM. The Menopause Rating Scale (MRS): Reliability of Scores of Menopausal Complaints. Climacteric (2000) 3(1):59–64. doi: 10.3109/13697130009167600
23. Barrea L, Muscogiuri G, Pugliese G, Laudisio D, de Alteriis G, Graziadio C, et al. Phase Angle as an Easy Diagnostic Tool of Meta-Inflammation for the Nutritionist. Nutrients (2021) 13(5):1446. doi: 10.3390/nu13051446
24. Khazem S, Itani L, Kreidieh D, El Masri D, Tannir H, Citarella R, et al. Reduced Lean Body Mass and Cardiometabolic Diseases in Adult Males With Overweight and Obesity: A Pilot Study. Int J Environ Res Public Health (2018) 15(12):2754. doi: 10.3390/ijerph15122754
25. Barrea L, Muscogiuri G, Di Somma C, Annunziata G, Megna M, Falco A, et al. Coffee Consumption, Metabolic Syndrome and Clinical Severity of Psoriasis: Good or Bad Stuff? Arch Toxicol (2018) 92(5):1831–45. doi: 10.1007/s00204-018-2193-0
26. Barrea L, Di Somma C, Macchia PE, Falco A, Savanelli MC, Orio F, et al. Influence of Nutrition on Somatotropic Axis: Milk Consumption in Adult Individuals With Moderate-Severe Obesity. Clin Nutr (2017) 36(1):293–301. doi: 10.1016/j.clnu.2015.12.007
27. Barrea L, Tarantino G, Somma CD, Muscogiuri G, Macchia PE, Falco A, et al. Adherence to the Mediterranean Diet and Circulating Levels of Sirtuin 4 in Obese Patients: A Novel Association. Oxid Med Cell Longev (2017) 2017:6101254. doi: 10.1155/2017/6101254
28. World Health Organization. Body Mass Index - BMI. Available at: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (Accessed November 15, 2021).
29. Barrea L, Muscogiuri G, Di Somma C, Tramontano G, De Luca V, Illario M, et al. Association Between Mediterranean Diet and Hand Grip Strength in Older Adult Women. Clin Nutr (2019) 38(2):721–9. doi: 10.1016/j.clnu.2018.03.012
30. Tao M, Shao H, Li C, Teng Y. Correlation Between the Modified Kupperman Index and the Menopause Rating Scale in Chinese Women. Patient Prefer Adherence (2013) 7:223–9. doi: 10.2147/PPA.S42852
31. Rahman SA, Zainudin SR, Mun VL. Assessment of Menopausal Symptoms Using Modified Menopause Rating Scale (MRS) Among Middle Age Women in Kuching, Sarawak, Malaysia. Asia Pac Fam Med (2010) 9(1):5. doi: 10.1186/1447-056X-9-5
32. Llaneza P, Gonzalez C, Fernandez-Iñarrea J, Alonso A, Diaz-Fernandez MJ, Arnott I, et al. Soy Isoflavones, Mediterranean Diet, and Physical Exercise in Postmenopausal Women With Insulin Resistance. Menopause (2010) 17(2):372–8. doi: 10.1097/gme.0b013e3181ba56fa
33. Taku K, Melby MK, Kronenberg F, Kurzer MS, Messina M. Extracted or Synthesized Soybean Isoflavones Reduce Menopausal Hot Flash Frequency and Severity: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Menopause (2012) 19(7):776–90. doi: 10.1097/gme.0b013e3182410159
34. Franco OH, Chowdhury R, Troup J, Voortman T, Kunutsor S, Kavousi M, et al. Use of Plant-Based Therapies and Menopausal Symptoms: A Systematic Review and Meta-Analysis. JAMA (2016) 315(23):2554–63. doi: 10.1001/jama.2016.8012
35. Taylor M. Complementary and Alternative Approaches to Menopause. Endocrinol Metab Clin North Am (2015) 44(3):619–48. doi: 10.1016/j.ecl.2015.05.008
36. Russell L, Hicks GS, Low AK, Shepherd JM, Brown CA. Phytoestrogens: A Viable Option? Am J Med Sci (2002) 324(4):185–8. doi: 10.1097/00000441-200210000-00004
37. Lahmann PH, Hughes MC, Ibiebele TI, Mulligan AA, Kuhnle GG, Webb PM. Estimated Intake of Dietary Phyto-Oestrogens in Australian Women and Evaluation of Correlates of Phyto-Oestrogen Intake. J Nutr Sci (2012), 1:e11. doi: 10.1017/jns.2012.11
38. Pounis G, Di Castelnuovo A, Bonaccio M, Costanzo S, Persichillo M, Krogh V, et al. Flavonoid and Lignan Intake in a Mediterranean Population: Proposal for a Holistic Approach in Polyphenol Dietary Analysis, the Moli-Sani Study. Eur J Clin Nutr (2016) 70(3):338–45. doi: 10.1038/ejcn.2015.178
39. Canheta ABS, Santos ASEAC, Souza JD, Silveira EA. Traditional Brazilian Diet and Extra Virgin Olive Oil Reduce Symptoms of Anxiety and Depression in Individuals With Severe Obesity: Randomized Clinical Trial. Clin Nutr (2021) 40(2):404–11. doi: 10.1016/j.clnu.2020.05.046
40. Foshati S, Ghanizadeh A, Akhlaghi M. Extra-Virgin Olive Oil Improves Depression Symptoms Without Affecting Salivary Cortisol and Brain-Derived Neurotrophic Factor in Patients With Major Depression: A Double-Blind Randomized Controlled Trial. J Acad Nutr Diet (2022) 122(2):284–297.e1. doi: 10.1016/j.jand.2021.07.016
41. El Ghoch M, Calugi S, Dalle Grave R. The Effects of Low-Carbohydrate Diets on Psychosocial Outcomes in Obesity/Overweight: A Systematic Review of Randomized, Controlled Studies. Nutrients (2016) 8(7):402. doi: 10.3390/nu8070402
42. Calugi S, Marchesini G, El Ghoch M, Gavasso I, Dalle Grave R. The Influence of Weight-Loss Expectations on Weight Loss and of Weight-Loss Satisfaction on Weight Maintenance in Severe Obesity. J Acad Nutr Diet (2017) 117(1):32–8. doi: 10.1016/j.jand.2016.09.001
43. Blümel JE, Chedraui P, Aedo S, Fica J, Mezones-Holguín E, Barón G, et al. Obesity and Its Relation to Depressive Symptoms and Sedentary Lifestyle in Middle-Aged Women. Maturitas (2015) 80(1):100–5. doi: 10.1016/j.maturitas.2014.10.007
44. Parry BL, Fernando Martínez L, Maurer EL, López AM, Sorenson D, Meliska CJ. Sleep, Rhythms and Women's Mood. Part II. Menopause. Sleep Med Rev (2006) 10(3):197–208. doi: 10.1016/j.smrv.2005.09.004
45. Lampio L, Polo-Kantola P, Polo O, Kauko T, Aittokallio J, Saaresranta T. Sleep in Midlife Women: Effects of Menopause, Vasomotor Symptoms, and Depressive Symptoms. Menopause (2014) 21(11):1217–24. doi: 10.1097/GME.0000000000000239
46. Perrini S, Cignarelli A, Quaranta VN, Falcone VA, Kounaki S, Porro S, et al. Correction of Intermittent Hypoxia Reduces Inflammation in Obese Subjects With Obstructive Sleep Apnea. JCI Insight (2017) 2(17):e94379. doi: 10.1172/jci.insight.94379
47. Muscogiuri G, Barrea L, Annunziata G, Di Somma C, Laudisio D, Colao A, et al. Obesity and Sleep Disturbance: The Chicken or the Egg? Crit Rev Food Sci Nutr (2019) 59(13):2158–65. doi: 10.1080/10408398.2018.1506979
48. Naufel MF, Frange C, Andersen ML, Girão MJBC, Tufik S, Beraldi Ribeiro E, et al. Association Between Obesity and Sleep Disorders in Postmenopausal Women. Menopause (2018) 25(2):139–44. doi: 10.1097/GME.0000000000000962
49. Hachul H, Brandão LC, D'Almeida V, Bittencourt LR, Baracat EC, Tufik S. Isoflavones Decrease Insomnia in Postmenopause. Menopause (2011) 18(2):178–84. doi: 10.1097/gme.0b013e3181ecf9b9
50. Abshirini M, Siassi F, Koohdani F, Qorbani M, Khosravi S, Aslani Z, et al. Higher Intake of Dietary N-3 PUFA and Lower MUFA Are Associated With Fewer Menopausal Symptoms. Climacteric (2019) 22(2):195–201. doi: 10.1080/13697137.2018.1547700
Keywords: menopause, menopausal symptoms, Mediterranean diet, sleep quality, PREDIMED score, MRS
Citation: Vetrani C, Barrea L, Rispoli R, Verde L, De Alteriis G, Docimo A, Auriemma RS, Colao A, Savastano S and Muscogiuri G (2022) Mediterranean Diet: What Are the Consequences for Menopause? Front. Endocrinol. 13:886824. doi: 10.3389/fendo.2022.886824
Received: 01 March 2022; Accepted: 21 March 2022;
Published: 25 April 2022.
Edited by:
Marwan El Ghoch, Beirut Arab University, LebanonReviewed by:
Massimo Pellegrini, Università degli Studi di Modena e Reggio Emilia, ItalyLeila Itani, Beirut Arab University, Lebanon
Copyright © 2022 Vetrani, Barrea, Rispoli, Verde, De Alteriis, Docimo, Auriemma, Colao, Savastano and Muscogiuri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanna Muscogiuri, Z2lvdmFubmEubXVzY29naXVyaUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Claudia Vetrani
Claudia Vetrani Luigi Barrea
Luigi Barrea Rosa Rispoli1
Rosa Rispoli1 Ludovica Verde
Ludovica Verde Annamaria Docimo
Annamaria Docimo Giovanna Muscogiuri
Giovanna Muscogiuri