
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 16 June 2022
Sec. Bone Research
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.884302
This article is part of the Research TopicOsteoporosis Secondary to Endocrine DisordersView all 8 articles
Purpose: The association between primary aldosteronism (PA) and lower bone mineral density (BMD) has raised a concern, but the contributing factors remain unclear. We aim to explore the risk factors for lower BMD in PA patients.
Methods: We analyzed and compared the data of 60 PA patients with 60 matched essential hypertension (EH) patients. BMD, bone metabolites, and several oxidative stress and inflammation indicators—including C-reactive protein (CRP), superoxide dismutase (SOD), total bilirubin (TBIL), mean platelet volume (MPV), etc.—were assessed and compared in PA and EH patients. Bivariate correlation analysis and multivariate linear regression analysis were performed to explore the factors associated with BMD in PA patients.
Results: The BMD measured by quantitative computed tomography in PA patients was lower than that in EH patients (141.9 ± 34.0 vs. 158.9 ± 55.9 g/cm3, p = 0.047), especially in patients less than 50 years old. BMD was independently negatively associated with age (standardized β = -0.581, p < 0.001), serum phosphorus (standardized β = -0.203, p = 0.008), urinary calcium excretion (standardized β = -0.185, p = 0.031), and MPV (standardized β = -0.172, p = 0.043) and positively associated with SOD (standardized β = 0.205, p = 0.011) and TBIL (standardized β = 0.212, p = 0.015).
Conclusions: The PA patients showed a lower BMD than the EH patients, which was associated with age, serum phosphorus, urinary calcium excretion, MPV, SOD, and TBIL. These variables might be potential markers for the assessment of bone loss and efficacy of treatments in PA patients.
Primary aldosteronism (PA) is the most common endocrine-related hypertension, which frequently manifests as hypertension and hypokalemia (1, 2). Aldosterone overproduction was reported to be a risk factor leading to cardiovascular, renal, and metabolic diseases (3). Recent studies also found that PA was related to a higher risk of impaired bone mass, the most common presentations of which are lower bone mineral density (BMD) and higher risk of fractures (4–6). More and more convincing evidence suggested that PA might be a secondary cause of osteoporosis (7).
Osteoporosis is the most common bone disease characterized by low bone mass and deterioration of bone microstructure, resulting in increased bone brittleness and increased risk of fractures, which as decreases the quality of life of PA patients. Inflammation and oxidative stress were reported to contribute to the pathogenic mechanisms of osteoporosis, mainly through activating osteoclastogenesis and inhibiting osteoblastogenesis (8–10). Besides this, several indicators of inflammation and oxidative stress were increased in PA patients (11, 12), implicating an association between PA and inflammation and oxidative stress. However, the mechanism of association between PA and bone impairment remains unclear, and whether inflammation and oxidative stress were associated with impaired bone mass in PA patients has not been investigated yet.
On the basis of these premises, in this study, we aim to assess the prevalence of impaired bone mass in PA and EH patients and explore whether inflammation and oxidative stress have an impact on BMD in PA patients.
This was a single-center, case-controlled study carried out at the Department of Endocrinology, Sun Yat-sen Memorial Hospital of Sun Yat-sen University in Guangzhou, China. From January 2020 to June 2021, a total of 425 patients referred to our center for screening for cause of hypertension were enrolled in our study. After excluding patients who did not meet the recruitment requirements, the data of 60 PA patients and 60 EH patients matched by sex, age ( ± 2 years), body mass index (BMI, ± 2 kg/m2), duration of hypertension ( ± 2 years), and blood pressure (BP, ± 5 mmHg) were finally analyzed in our study (Figure 1). This study was reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital. Informed consent has been obtained from the patients before enrollment.

Figure 1 Participants’ flow chart of primary aldosteronism and essential hypertension patients in this study.
All enrolled patients obtained a comprehensive evaluation for screening for a secondary cause of hypertension according to clinical features. Briefly, examinations of aldosterone–renin ratio (ARR), serum and urine catecholamine metabolite concentrations, and serum and urine cortisol levels as well as 1-mg overnight dexamethasone suppression test and renal computed tomography angiography were performed routinely to screen for PA, PA with cortisol co-secretion (13, 14), pheochromocytoma, hypercortisolism, and renovascular hypertension. Other examinations were performed to screen for other secondary causes of hypertension as clinically indicated.
The newly confirmed PA patients were included in the PA group. The screening and case definition for PA were according to the PA guidelines (1, 2). Before screening for PA, all drugs that affect the result of ARR were required to be withdrawn to make the ARR convincing. In a nutshell, the diagnostic criteria of PA were defined as a positive screening test for PA, which, in our study, was followed by at least one positive confirmatory test, including captopril challenge test, saline infusion test, furosemide-upright test, and oral salt-loading test. A differential diagnosis of PA forms (bilateral PA and unilateral PA) was derived according to adrenal high-resolution computerized tomography and/or magnetic resonance imaging and by adrenal vein sampling (AVS). Specifically, unilateral PA was confirmed according to the findings of AVS and/or a combination of computerized tomography and/or magnetic resonance imaging finding of pathologically confirmed unilateral adrenocortical adenoma and/or normalization of plasma renin activity (PRA) and plasma aldosterone concentration (PAC) levels after laparoscopic adrenalectomy. The AVS procedure and criteria were according to published guidelines (15).
The EH control group included patients with a history of hypertension and who were receiving treatment such as antihypertensive drugs or with three office BP measurements of systolic blood pressure ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg at different days after excluding a secondary cause of hypertension with comprehensive evaluation.
The exclusion criteria in this study were as follows: (1) patients with a current history of known secondary causes of osteoporosis such as thyroid disorders, inflammatory bowel diseases, rheumatoid arthritis, chronic obstructive pulmonary disease, and primary hyperparathyroidism; (2) patients who were receiving a treatment that affects the results of BMD and bone turnover markers such as glucocorticoid treatment (≥5 mg prednisolone daily or equivalent for 3 months or more), diphosphonate, denosumab, vitamin D, calcium supplementation, and hormone replacement therapy; (3) patients who were pregnant or lactating women; (4) patients with a history of non-osteoporotic fracture within 6 months; (5) patients with incomplete medical records; (6) patients with other confirmed or suspected secondary causes of hypertension except for PA; and (7) PA patients who had been treated with mineralocorticoid receptor antagonists or had been subjected to adrenalectomy.
Information on gender, age, BMI, BP, hypertension duration, smoking history, drinking history, and physical exercise were collected from medical records. BMI was calculated as weight (kg)/height (m)2. BP was defined as the average value of three office BP measurements after the patients had been seated quietly for at least 10 min at different days.
The laboratory parameters of serum potassium, calcium, phosphorus, creatinine, albumin, glycosylated hemoglobin (HbA1c), superoxide dismutase (SOD), total bilirubin (TBIL), uric acid, C-reactive protein (CRP), and urinary calcium and phosphorus concentration were measured by an automatic biochemical analyzer (AU5800, Beckman Coulter, USA). The calculation of estimated glomerular filtration rate was based on the CKD-EPI equation. Peripheral blood cell indices, including the count of white blood cells, lymphocytes, neutrophils, monocytes, platelets, and MCV were measured by an automatic analyzer (DxFLEX, Beckman Coulter, USA). Bone metabolites, including intact parathyroid hormone (iPTH) and calcitonin, were measured by using chemiluminescent immunoassay kits (Siemens, Gwynedd, UK), bone alkaline phosphatase (BLP) and 25-hydroxyvitamin D were measured by commercial ELISA kits (IDS, Boldon, UK), and N-terminal mid osteocalcin (N-MID-OT), type I procollagen N-terminal peptide (PINP), and β-Crosslaps were measured by chemiluminescent immunoassay kits (Roche, Mannheim, Germany). The measurements of PAC and PRA were determined by using chemiluminescent immunoassay kits (Snibe, Shenzhen, China). The reference range values of bone metabolites were as follows: iPTH (11.0–47.0 pg/ml), calcitonin (<2.0 pg/ml), BLP (3.0–15.0 ug/L), 25-hydroxyvitamin D (75.0–250.0 nmol/L), N-MID-OT (11.0–46.0 ng/ml), PINP (14.3–76.3 ng/ml), and β-Crosslaps (≤1.01 ng/ml). The reference range of PAC was 7.0–30.0 ng/dl and that of PRA was 0.10–6.56 ng/ml/h in the standing position.
BMD was measured by quantitative computed tomography (QCT), an effective tool to measure the volumetric BMD of patients and which could measure the volume density of cancellous bone and cortical bone, respectively. Compared with dual-energy X-ray absorptiometry, BMD measured by QCT was not affected by spinal hyperplasia, degeneration, and vascular calcification. In this study, BMD was measured using QCT (Siemens, Germany) with QCT volume model calibration and professional software analysis at average at the lumbar spine (T12-L3), and at least two intact vertebrae were measured (16). According to the guidelines for the diagnosis criteria of osteoporosis with QCT (16, 17), normal bone mass was defined as BMD >120 g/cm3, and 80 g/cm3 ≤ BMD ≤120 g/cm3 was defined as osteopenia, whereas BMD <80g/cm3 was defined as osteoporosis. In this study, we divided the patients into the osteopenia group (BMD ≤120 g/cm3) and the normal bone mass group (BMD >120 g/cm3).
We used SPSS version 25.0 (SPSS, Inc., Chicago, IL, USA) for all statistical analysis. Normality test and analysis of variance were performed on all recorded data. Continuous variables in normal distribution were expressed by mean and standard deviation, and continuous variables in the skew distribution were expressed by median and interquartile range. Frequencies or percentages were expressed in categorical variables. Student’s t-test, Mann–Whitney U-test, or chi-square test were used to test the group differences between PA and EH patients when appropriate. Spearman or Pearson correlation analysis and partial correlation analysis were used to study the correlation between BMD and laboratory parameters. Multivariate linear regression analysis was performed to explore independent association between clinical factors and BMD in the PA group. Assumptions of multivariate linear regression, including linearity, normality, homoscedasticity, and collinearity, were tested and found to be satisfactory. P <0.05 in a two-tailed test was defined as statistical significance.
The clinical and biochemical characteristics of PA and EH groups are shown in Table 1. We found that BMD in PA patients was lower than that in EH patients (141.9 ± 34.0 vs. 158.9 ± 55.9 g/cm3, p = 0.047), while the prevalence of osteopenia showed no significant difference. PA patients presented a higher urinary calcium concentration [6.0 (5.2–6.5) vs. 4.8 (3.4–4.9) mmol/L, p <0.001], higher iPTH [49.5 (39.5–62.5) vs. 36.0 (28.0–48.5) pg/ml, p <0.001], lower serum potassium (3.37 ± 0.34 vs. 3.90 ± 0.30 mmol/L, p <0.001), lower serum phosphorus (1.08 ± 0.14 vs. 1.14 ± 0.16 mmol/L, p = 0.042), and lower TBIL [11.7 (10.5–14.0) vs. 13.0 (11.7–15.9) μmol/L, p = 0.038] than EH patients.
The mean menopausal age in this study was 50 years old (Supplementary Table S1). After the stratification of menopause, the difference of BMD between PA and EH patients only existed in non-menopausal patients (Supplementary Table S1). After applying further stratification according to 50 years old in male patients, the significance for BMD between PA and EH patients was only present in the subgroup of age below 50 years old, while the other variables that presented a significant difference between PA and EH patients were similar to those of the female subgroups (Supplementary Table S2).
Thus, to minimize the influence of menopause (18), we further divided the patients into groups <50 years old and ≥50 years old. As shown in Table 2, the gender proportion and the prevalence of osteopenia showed no significant difference between PA and EH patients in both groups. In the group <50 years old, the PA patients presented a lower BMD (165.0 ± 21.0 vs. 199.7 ± 31.1 g/cm3, p < 0.001), lower serum potassium (3.36 ± 0.39 vs. 3.88 ± 0.32 mmol/L, p < 0.001), lower serum phosphorus (1.08 ± 0.13 vs. 1.17 ± 0.18 mmol/L, p = 0.026), higher urinary calcium excretion [6.0 (4.7–6.1) vs. 4.8 (4.0–5.0) mmol/L, p = 0.033], and higher iPTH [49.8 (40.5–68.5) vs. 36.5 (26.8–50.5) pg/ml, p = 0.003) than the EH control group.
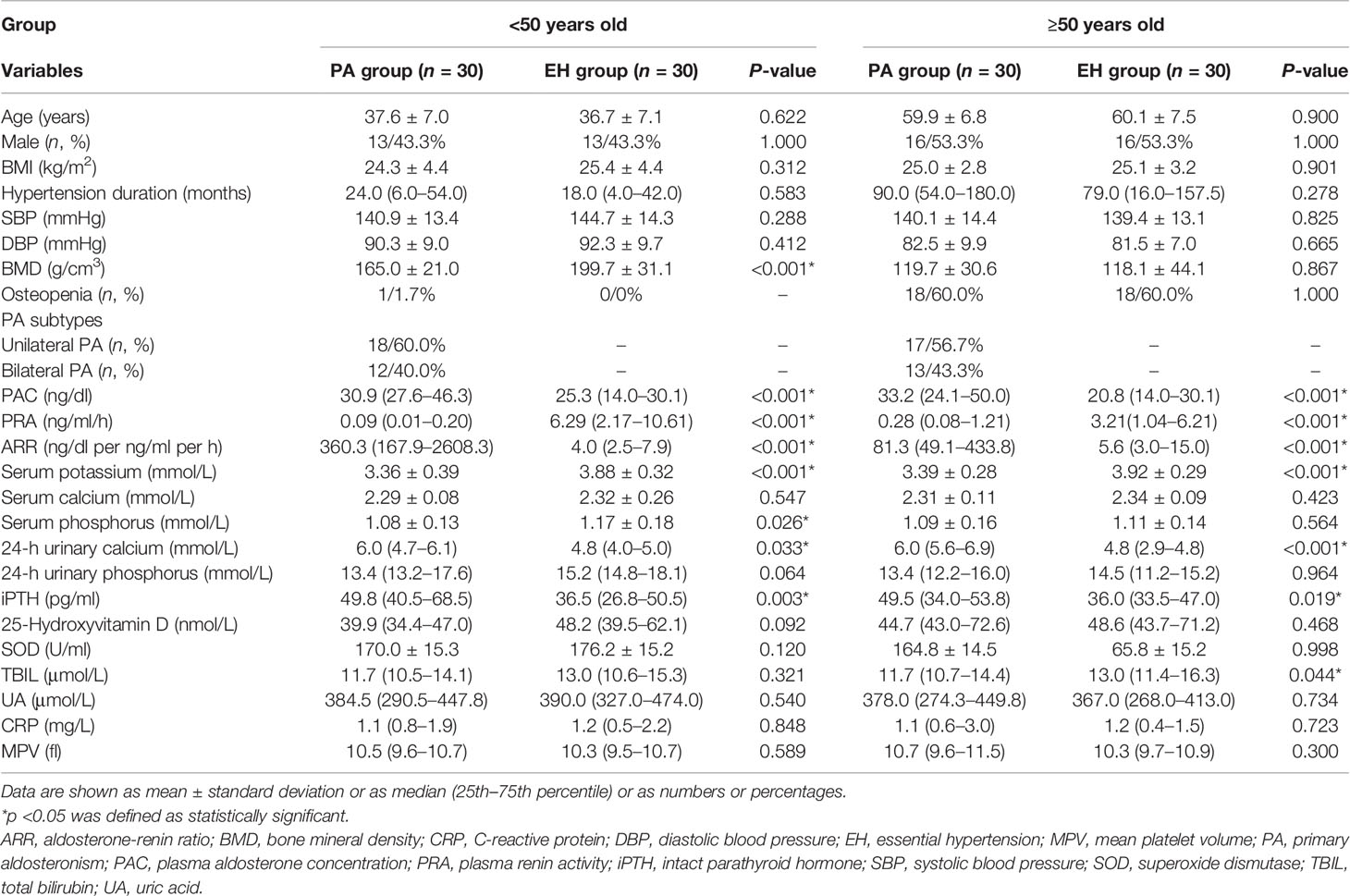
Table 2 Comparison of characteristics between PA and EH patients according to age stratification of 50 years old.
However, in the group of ≥50 years old, no significant difference was found in BMD between PA and EH patients (Table 2). The PA patients merely showed a lower serum potassium (3.39 ± 0.28 vs. 3.92 ± 0.29 mmol/L, p < 0.001), lower TBIL [11.7 (10.7–14.4) vs. 13.0 (11.4–16.3) μmol/L, p = 0.044], higher urinary calcium concentration [6.0 (5.6–6.9) vs. 4.8 (2.9–4.8) mmol/L, p < 0.001], and higher iPTH [49.5 (34.0–53.8) vs. 36.0 (33.5–47.0) pg/ml, p = 0.019] than the EH patients.
Correlation analyses between BMD and clinical and laboratory indices in PA and EH patients were performed. As presented in Table 3, in PA patients, BMD was negatively correlated with age (r = -0.688, p < 0.001), PRA (r = -0.379, p = 0.003), hypertension duration (r = -0.409, p = 0.001), HbA1c (r = -0.311, p = 0.016), serum phosphorus (r = -0.265, p = 0.041), 25-hydroxyvitamin D (r = -0.381, p = 0.003), MPV (r = -0.366, p = 0.004), and urinary calcium concentration (r = -0.418, p = 0.001) and positively correlated with ARR (r = 0.374, p = 0.003), lymphocyte count (r = 0.267, p = 0.039), monocyte count (r = 0.296, p = 0.021), SOD (r = 0.272, p = 0.035), and TBIL (r = 0.349, p = 0.006). Surprisingly, age was positively correlated with PRA and negatively correlated with ARR (Supplementary Table S3). After eliminating the effect of age, BMD showed no partial correlation with PRA or ARR in PA patients (Supplementary Table S4). In EH patients, BMD was negatively correlated with age (r = -0.787, p < 0.001), hypertension duration (r = -0.440, p < 0.001), and HbA1c (r = -0.258, p = 0.047) and positively associated with DBP (r = 0.554, p < 0.001) and SOD (r = 0.313, p = 0.015).
We further performed multivariate linear regression analysis to investigate the clinical factors associated with BMD in PA patients independently. Variables that showed a significant association with BMD in a bivariate correlation analysis were included in the multivariate regression analysis using stepwise regression. As presented in Table 4, we found that BMD was independently negatively associated with age (standardized β = -0.581 p < 0.001), serum phosphorus concentration (standardized β = -0.203, p = 0.008), urinary calcium excretion (standardized β = -0.185, p = 0.031), and MPV (standardized β = -0.172, p = 0.043) and positively associated with SOD (standardized β = 0.205, p = 0.011) and TBIL (standardized β = 0.212, p = 0.015).
In our study, we found that BMD was lower in PA patients than in EH patients, especially in patients less than 50 years old. Moreover, this study implicated that BMD was associated with age, serum phosphorus, urinary calcium excretion, MPV, SOD, and TBIL levels independently in PA patients.
Decreasing BMD was widely known to be closely associated with aging (19, 20). It was reported that about 33% of women over age 50 years would experience osteoporotic fractures as would 20% of men aged over 50 years (21). Osteoporosis was more common in postmenopausal women, which was related to the effect of estrogen. The mean menopausal age in Chinese women was 47.5–49.5 years old (18). In this study, we not only found that BMD was negatively associated with age but also found that PA had more effect on BMD in patients less than 50 years old, which further supported the impact of PA on BMD. However, we could not find a significant difference of BMD between PA and EH patients over 50 years old. A potential explanation could be the impact of age on BMD. It was reported that the prevalence of osteopenia was more than 60% among people over 50 years old in China (22). With an increase of age, the BMD of Czech women and men decreased by 68 and 58%, respectively (23). In our multivariate linear regression analysis, age was the most influential variable that affects BMD. We inferred from the above-mentioned observations that the missing level of significance for BMD between EH and PA patients over 50 years old might be due to the influence of age far exceeding those of other factors, including excess aldosterone.
In the present study, we discovered that BMD was negatively associated with serum phosphorus and urinary calcium excretion, suggesting that BMD was related to calcium and phosphorus metabolism disorders in PA patients. Actually, the association between PA and bone metabolism had been proposed. Several studies had suggested that PA patients presented a lower BMD compared with non-PA controls (6, 24, 25), and these studies unanimously showed higher urinary calcium excretion and higher PTH in PA patients, which was consistent with our results. Besides this, urinary calcium excrement and PTH were reduced and BMD was increased after the initiation of treatments of adrenalectomy or minerolocorticoid antagonists in PA patients (6, 24, 26). The possible mechanism of the increased urinary calcium excretion in PA patients was that volume expansion decreased the reabsorption of calcium by proximal renal tubules, and the amount of calcium secreted by distal renal tubules exceeded the amount of reabsorption, thus resulting in decreased serum calcium (24–27). Higher calcium excretion would decrease serum calcium and then possibly came secondary hyperparathyroidism to stabilize the calcium concentration by promoting the activity of osteoclasts and promoting the absorption of calcium in the intestine, finally resulting in bone loss in PA patients.
Unexpectedly, serum phosphorus was negatively associated with BMD in PA patients, and the 25-hydroxyvitamin D level was positively associated with BMD in a bivariate correlation analysis. The relationship between PA and 25-hydroxyvitamin D levels was controversial in previous studies. A previous study found that the 25-hydroxyvitamin D levels were decreased in PA patients (25), while others found no associations between them (24, 28, 29). The 25-hydroxyvitamin D levels were positively associated with urinary calcium excretion in elderly men (30–32). However, whether higher urinary calcium excretion could induce increased 25-hydroxyvitamin D levels had not been fully discussed. Although a different diet, sunbathing, and sports habits might explain the contradictory association between BMD and 25-hydroxyvitamin D levels, the results of our study raised the possibility that a higher urinary calcium excretion might increase the synthesis of 25-hydroxyvitamin D to maintain calcium and phosphorus homeostasis by promoting the absorption of intestinal calcium and phosphorus in the early stage of PA; further investigations are required to support this opinion. We supposed that the association between serum phosphorus and BMD might be a reflection of the comprehensive effect of PTH and 25-hydroxyvitamin D.
However, although we found a lower BMD in PA patients, the prevalence of osteopenia showed no difference between the PA and EH groups. This study included a relatively small sample size of PA patients, and it was worth noticing that previous studies did not mention the duration of PA, which might be of great significance to BMD. The PA patients in our study were all newly diagnosed, so the bone impairment might be relatively mild.
This was the first study to show that increased MPV was associated with lower BMD in PA patients. The PA patients had a lower MPV than the normotensive subjects (33), which was similar to our results. MPV is a parameter of measurement of platelet size and an early indicator of platelet activation, which is found to be related to osteoporosis (34, 35). Platelet activation was a link between thrombosis and inflammation, and MPV was associated with low-grade inflammation (36) and might be used as a marker to reflect the severity of inflammation (37). PA patients presented increased inflammation markers, such as higher levels of intercellular adhesion molecule-1, interleukin-6, and tumor necrosis factor-alpha messenger RNA and protein (11, 38). It was widely accepted that chronic inflammation took part in the pathophysiological mechanisms of osteoporosis (8, 9). Pro-inflammatory cytokines such as interleukin-1, 6 and tumor necrosis factor-alpha were able to activate osteoclastogenesis and stimulate osteoclastic bone resorption through activating the receptor activator of nuclear factor-kappaB ligand (RANKL) (9). As a result, MPV might be used as a simple marker to reflect the inflammation status in PA patients and connect PA with osteoporosis.
Besides this, we discovered that the SOD and TBIL levels were positively associated with BMD in PA patients, implicating that oxidative stress might have an effect on BMD in PA patients. As we know, SOD was an important regulator in oxidative stress, which catalyzed superoxides to convert into oxygen and hydrogen peroxides to reduce the levels of reactive oxygen species (39). TBIL could be considered as an antioxidant and anti-inflammatory, and the antioxidant capacity was even stronger than that of alpha-tocopherol, SOD, and catalase (40, 41). PA was associated with increased levels of oxidative stress indicators, including plasma NADPH oxidase, oxidized low-density lipoprotein, 8-isoprostane, etc., and lower levels of superoxides and lipid peroxides were observed after spironolactone treatment (12, 42). Oxidative stress played an essential role in bone remodeling. Increased oxidative stress would induce bone loss by increasing RANKL expression and decreasing the differentiation and activities of osteoblasts through regulating kinases and transcription factor activities, including activating c-Jun N terminal kinases, Wnt/β-catenin, nuclear factor-kappaB signaling pathways, etc. (10). Therefore, we considered that the SOD and TBIL levels could act as important indicators of oxidative stress status in PA patients and linked PA to a lower BMD in this study.
It was with regret that we could not find a direct correlation between BMD and the renin–angiotensin–aldosterone system (RAAS) components in our study. We speculated that the small sample size might be one of the reasons. In previous studies, RAAS activation was considered as a potential risk factor for osteoporosis. RAAS and mineralocorticoid receptors had been found to be expressed in human bone tissues, and increased angiotensin II and aldosterone might lead to increased bone turnover and decreased BMD (43, 44). In non-PA patients, it was reported that renin activity was positively and PAC was negatively associated with BMD (45, 46), while similar results were not be found in PA patients. However, the abnormality of RAAS in PA patients was featured by excess aldosterone and a suppressed renin–angiotensin system. An alternative reason for the missing correlation between BMD and RAAS components in PA patients might be the different effects of RAAS components to bone metabolism. As discussed above, potential mechanisms such as hyperparathyroidism, oxidative stress, and inflammation induced by excess aldosterone might play important roles in bone loss in PA patients, while the direct role of excess aldosterone and suppressed renin–angiotensin system in bone metabolism remained to be further investigated.
However, several limitations should be noted in our study. Firstly, this is a retrospective case–control study, so we cannot establish a causal relationship between PA and the lower BMD. Secondly, our study included a relatively small number of patients. Thirdly, we did not collect data on diet or sunbathing habit, which were important to bone metabolism. Therefore, studies that are better designed and with a larger sample size are needed for further investigations in PA and bone metabolism.
In conclusion, PA patients showed a lower BMD than EH patients, especially in patients less than 50 years old. BMD was associated with age, serum phosphorus, urinary calcium excretion, MPV, SOD, and TBIL levels in PA patients, and these might be potential indicators of assessment of bone loss and the effect of treatments. We suggested that early attention should be paid to bone health problems in the PA population, and BMD measurement was recommended in the follow-up to improve the quality of life of the PA population.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital. The patients/participants provided their written informed consent to participate in this study.
XL, HH, and YG conceived and designed the study. XL, CS, HH, and XZ collected and managed the data. XL analyzed the data and wrote the manuscript. YG, HH, LY, and SZ reviewed and edited the manuscript. YG had a primary responsibility for final content. All authors contributed to the article and approved the submitted version.
This work was supported by the Natural Science Foundation of Guangdong Province (grant number 2018A030313596) to YG.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.884302/full#supplementary-material
ARR, aldosterone-renin ratio; AVS, adrenal vein sampling; BLP, bone alkaline phosphatase; BP, blood pressure; BMD, bone mineral density; BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EH, essential hypertension; HbA1c, glycosylation hemoglobin; MPV, mean platelet volume; N-MID-OT, N-terminal mid osteocalcin; PA, primary aldosteronism; PAC, plasma aldosterone concentration; PINP, type I procollagen N-terminal peptide; PRA, plasma renin activity; PTH, parathyroid hormone; QCT, quantitative computed tomography; RAAS, renin-angiotensin-aldosterone system; RANKL, receptor activator of nuclear factor-kappaB ligand; SBP, systolic blood pressure; SOD, superoxide dismutase; TBIL, total bilirubin; UA, uric acid; WBC, white blood cell count.
1. Chinese Society of Endocrinology. Expert Consensus on the Diagnosis and Treatment of Primary Aldosteronism (2020). Chin J Endocrinol Metab (2020) 36(9):727–36.
2. Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, et al. Genetics, Prevalence, Screening and Confirmation of Primary Aldosteronism: A Position Statement and Consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens (2020) 38(10):1919–28. doi: 10.1097/HJH.0000000000002510
3. Buglioni A, Cannone V, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, et al. Aldosterone Predicts Cardiovascular, Renal, and Metabolic Disease in the General Community: A 4-Year Follow-Up. J Am Heart Assoc (2015) 4(12):e002505. doi: 10.1161/JAHA.115.002505
4. Salcuni AS, Carnevale V, Battista C, Palmieri S, Eller-Vainicher C, Guarnieri V, et al. Primary Aldosteronism as a Cause of Secondary Osteoporosis. Eur J Endocrinol (2017) 177(5):431–7. doi: 10.1530/EJE-17-0417
5. Notsu M, Yamauchi M, Yamamoto M, Nawata K, Sugimoto T. Primary Aldosteronism as a Risk Factor for Vertebral Fracture. J Clin Endocrinol Metab (2017) 102(4):1237–43. doi: 10.1210/jc.2016-3206
6. Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, et al. Bone Involvement in Aldosteronism. J Bone Miner Res (2012) 27(10):2217–22. doi: 10.1002/jbmr.1660
7. Shi S, Lu C, Tian H, Ren Y, Chen T. Primary Aldosteronism and Bone Metabolism: A Systematic Review and Meta-Analysis. Front Endocrinol (2020) 11:574151. doi: 10.3389/fendo.2020.574151
8. Mundy GR. Osteoporosis and Inflammation. Nutr Rev (2007) 65(12 Pt 2):S147–51. doi: 10.1111/j.1753-4887.2007.tb00353.x
9. Al-Daghri NM, Aziz I, Yakout S, Aljohani NJ, Al-Saleh Y, Amer OE, et al. Inflammation as a Contributing Factor Among Postmenopausal Saudi Women With Osteoporosis. Med (Baltimore) (2017) 96(4):e5780. doi: 10.1097/MD.0000000000005780
10. Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative Stress in Bone Remodelling and Disease. Trends Mol Med (2009) 15(10):468–77. doi: 10.1016/j.molmed.2009.08.004
11. Kotlyar E, Vita JA, Winter MR, Awtry EH, Siwik DA, Keaney JF Jr, et al. The Relationship Between Aldosterone, Oxidative Stress, and Inflammation in Chronic, Stable Human Heart Failure. J Card Fail (2006) 12(2):122–7. doi: 10.1016/j.cardfail.2005.08.005
12. Queisser N, Schupp N. Aldosterone, Oxidative Stress, and NF-κb Activation in Hypertension-Related Cardiovascular and Renal Diseases. Free Radic Biol Med (2012) 53(2):314–27. doi: 10.1016/j.freeradbiomed.2012.05.011
13. Piaditis GP, Kaltsas GA, Androulakis II, Gouli A, Makras P, Papadogias D, et al. High Prevalence of Autonomous Cortisol and Aldosterone Secretion From Adrenal Adenomas. Clin Endocrinol (Oxf) (2009) 71(6):772–8. doi: 10.1111/j.1365-2265.2009.03551.x
14. Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, et al. High Prevalence of Diabetes in Patients With Primary Aldosteronism (PA) Associated With Subclinical Hypercortisolism and Prediabetes More Prevalent in Bilateral Than Unilateral PA: A Large, Multicenter Cohort Study in Japan. Diabetes Care (2019) 42(5):938–45. doi: 10.2337/dc18-1293
15. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, et al. An Expert Consensus Statement on Use of Adrenal Vein Sampling for the Subtyping of Primary Aldosteronism. Hypertension (2014) 63(1):151–60. doi: 10.1161/HYPERTENSIONAHA.113.02097
16. Cheng XG, Wang L, Wu J. The China Guideline for the Diagnosis Criteria of Osteoporosis With Quantitative Computed Tomography (QCT) (2018). Chin J Osteoporos (2019) 25(06):733–7.
17. American College of Radiology. ACR-SPR-SSR Practice Parameter for the Performance of Musculoskeletal Quantitative Computed Tomography (QCT) (2018). Reston: American College of Radiology. Available at: https://www.acr.org/-/media/ACR/Files/PracticeParameters/QCT.pdf?la=en (Accessed 7 Nov 2018).
18. Lu HB, Yang XZ. Epidemiological Research Status of Perimenopausal Syndrome. Guangxi Med Assoc (2001) 2001(05):1131–4.
19. Johnston CB, Dagar M. Osteoporosis in Older Adults. Med Clin North Am (2020) 104(5):873–84. doi: 10.1016/j.mcna.2020.06.004
20. Aspray TJ, Hill TR. Osteoporosis and the Ageing Skeleton. Subcell Biochem (2019) 91:453–76. doi: 10.1007/978-981-13-3681-2_16
21. Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, et al. Long-Term Risk of Osteoporotic Fracture in Malmö. Osteoporos Int (2000) 11(8):669–74. doi: 10.1007/s001980070064
22. Zeng Q, Li N, Wang Q, Feng J, Sun D, Zhang Q, et al. The Prevalence of Osteoporosis in China, a Nationwide, Multicenter DXA Survey. J Bone Miner Res (2019) 34(10):1789–97. doi: 10.1002/jbmr.3757
23. Henyš P, Vořechovský M, Kuchař M, Heinemann A, Kopal J, Ondruschka B, et al. Bone Mineral Density Modeling via Random Field: Normality, Stationarity, Sex and Age Dependence. Comput Methods Programs Biomed (2021) 210:106353. doi: 10.1016/j.cmpb.2021.106353
24. Ceccoli L, Ronconi V, Giovannini L, Marcheggiani M, Turchi F, Boscaro M, et al. Bone Health and Aldosterone Excess. Osteoporos Int (2013) 24(11):2801–7. doi: 10.1007/s00198-013-2399-1
25. Petramala L, Zinnamosca L, Settevendemmie A, Marinelli C, Nardi M, Concistrè A, et al. Bone and Mineral Metabolism in Patients With Primary Aldosteronism. Int J Endocrinol (2014) 2014:836529. doi: 10.1155/2014/836529
26. Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of Calcium Metabolism and of Parathyroid Function in Primary Aldosteronism, and Their Reversal by Spironolactone or by Surgical Removal of Aldosterone-Producing Adenomas. Am J Hypertens (1995) 8(9):884–93. doi: 10.1016/0895-7061(95)00182-O
27. Kamalov G, Bhattacharya SK, Weber KT. Congestive Heart Failure: Where Homeostasis Begets Dyshomeostasis. J Cardiovasc Pharmacol (2010) 56(3):320–8. doi: 10.1097/FJC.0b013e3181ed064f
28. Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, et al. Hyperparathyroidism in Patients With Primary Aldosteronism: Cross-Sectional and Interventional Data From the GECOH Study. J Clin Endocrinol Metab (2012) 97(1):E75–9. doi: 10.1210/jc.2011-2183
29. Rossi GP, Ragazzo F, Seccia TM, Maniero C, Barisa M, Calò LA, et al. Hyperparathyroidism Can be Useful in the Identification of Primary Aldosteronism Due to Aldosterone-Producing Adenoma. Hypertension (2012) 60(2):431–6. doi: 10.1161/HYPERTENSIONAHA.112.195891
30. Rathod A, Bonny O, Guessous I, Suter PM, Conen D, Erne P, et al. Association of Urinary Calcium Excretion With Serum Calcium and Vitamin D Levels. Clin J Am Soc Nephrol (2015) 10(3):452–62. doi: 10.2215/CJN.12511213
31. Vezzoli G, Soldati L, Arcidiacono T, Terranegra A, Biasion R, Russo CR, et al. Urinary Calcium Is a Determinant of Bone Mineral Density in Elderly Men Participating in the InCHIANTI Study. Kidney Int (2005) 67(5):2006–14. doi: 10.1111/j.1523-1755.2005.00302.x
32. Kim WT, Kim YJ, Yun SJ, Shin KS, Choi YD, Lee SC, et al. Role of 1,25-Dihydroxy Vitamin D3 and Parathyroid Hormone in Urinary Calcium Excretion in Calcium Stone Formers. Yonsei Med J (2014) 55(5):1326–32. doi: 10.3349/ymj.2014.55.5.1326
33. Kurisu S, Shimonaga T, Iwasaki T, Mitsuba N, Ishibashi K, Dohi Y, et al. Mean Platelet Volume in Patients With Primary Aldosteronism and Its Relation to Left Ventricular Hypertrophy. Int J Cardiol (2013) 168(3):3143–4. doi: 10.1016/j.ijcard.2013.04.156
34. Akbal A, Gökmen F, Gencer M, Inceer BS, Kömürcü E. Mean Platelet Volume and Platelet Distribution Width Can be Related to Bone Mineralization. Osteoporos Int (2014) 25(9):2291–5. doi: 10.1007/s00198-014-2764-8
35. Li XS, Zhang JR, Meng SY, Li Y, Wang RT. Mean Platelet Volume is Negatively Associated With Bone Mineral Density in Postmenopausal Women. J Bone Miner Metab (2012) 30(6):660–5. doi: 10.1007/s00774-012-0362-4
36. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean Platelet Volume: A Link Between Thrombosis and Inflammation? Curr Pharm Des (2011) 17(1):47–58. doi: 10.2174/138161211795049804
37. Khaled SAA, NasrEldin E, Makarem YS, Mahmoud HFF. Value of Platelet Distribution Width and Mean Platelet Volume in Disease Activity Score of Rheumatoid Arthritis. J Inflammation Res (2020) 13:595–606. doi: 10.2147/JIR.S265811
38. Wu C, Zhang H, Zhang J, Xie C, Fan C, Zhang H, et al. Inflammation and Fibrosis in Perirenal Adipose Tissue of Patients With Aldosterone-Producing Adenoma. Endocrinology (2018) 159(1):227–37. doi: 10.1210/en.2017-00651
39. Wang Y, Branicky R, Noë A, Hekimi S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J Cell Biol (2018) 217(6):1915–28. doi: 10.1083/jcb.201708007
40. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an Antioxidant of Possible Physiological Importance. Science (1987) 235(4792):1043–6. doi: 10.1126/science.3029864
41. Li WC, Mo LJ, Shi X, Lin ZY, Li YY, Yang Z, et al. Antioxidant Status of Serum Bilirubin, Uric Acid and Albumin in Pemphigus Vulgaris. Clin Exp Dermatol (2018) 43(2):158–63. doi: 10.1111/ced.13289
42. Petramala L, Pignatelli P, Carnevale R, Zinnamosca L, Marinelli C, Settevendemmie A, et al. Oxidative Stress in Patients Affected by Primary Aldosteronism. J Hypertens (2014) 32(10):2022–9. doi: 10.1097/HJH.0000000000000284
43. Beavan S, Horner A, Bord S, Ireland D, Compston J. Colocalization of Glucocorticoid and Mineralocorticoid Receptors in Human Bone. J Bone Miner Res (2001) 16(8):1496–504. doi: 10.1359/jbmr.2001.16.8.1496
44. Mo C, Ke J, Zhao D, Zhang B. Role of the Renin-Angiotensin-Aldosterone System in Bone Metabolism. J Bone Miner Metab (2020) 38(6):772–9. doi: 10.1007/s00774-020-01132-y
45. Kuipers AL, Kammerer CM, Pratt JH, Bunker CH, Wheeler VW, Patrick AL, et al. Association of Circulating Renin and Aldosterone With Osteocalcin and Bone Mineral Density in African Ancestry Families. Hypertension (2016) 67(5):977–82. doi: 10.1161/HYPERTENSIONAHA.115.06837
Keywords: primary aldosteronism, bone mineral density, quantitative CT, inflammation, oxidative stress, MPV, TBIL
Citation: Lv X, Hu H, Shen C, Zhang X, Yan L, Zhang S and Guo Y (2022) Risk Factors Associated With Lower Bone Mineral Density in Primary Aldosteronism Patients. Front. Endocrinol. 13:884302. doi: 10.3389/fendo.2022.884302
Received: 26 February 2022; Accepted: 11 May 2022;
Published: 16 June 2022.
Edited by:
Elżbieta Skowrońska-Jóźwiak, Medical University of Łódź, PolandReviewed by:
Min Sun, Nanjing Medical University, ChinaCopyright © 2022 Lv, Hu, Shen, Zhang, Yan, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Guo, Z3lpbmdAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.