- 1Laboratory Immunology of Diabetes, Cochin Institute, Department Endocrinology, Metabolism and Diabetologia (EMD), Institut Nationale de la Santé et de la Recherche Médicale, Unité 1016 (INSERMU1016), Paris, France
- 2Medical Faculty, Paris University, Paris, France
Type 1 Diabetes (T1D) is an autoimmune disease that results from the destruction of pancreatic islet β-cells by auto-reactive T cells. The clinical management of T1D faces the lack of fully predictive biomarkers in its preclinical stage and of antigen-specific therapies to induce or re-induce immune tolerance to β-cell autoantigens and prevent its development. From a therapeutic standpoint, preclinical models of T1D have fallen short of directly translating into humans. To circumvent this limitation, preclinical models are being optimized to allow defining autoantigen epitopes that are presented to T cells and directly apply to the human. In this review, we propose to make a point on the latest available models such as humanized immunodeficient NOD mice models and HLA and autoantigen transgenic mice and their application in the context of T1D.
1 Introduction
Type 1 diabetes (T1D) is a multifactorial autoimmune disease in which T cells destroy the insulin-secreting β-cells of the pancreas. Although initially defined as a juvenile disease, it can occur at any age. It is associated on the long term with the risk of developing micro and macro-vascular complications, which makes it a major public health issue. Current diagnostic strategies in T1D patients rely on detecting anti-insulin, anti-GAD, anti-IA2 and anti-ZnT8 autoantibodies (1). However, 80 to 90% of β-cells are lost by the time of diagnosis and subjects become insulin dependent, requiring lifetime insulin delivery in the absence of therapies to revert or stop the autoimmune process responsible for the destruction of β-cells. Management of T1D remains challenging and effort should be directed towards a better understanding of the disease. Since exploring T1D in humans is difficult, the use of animal models that develop a T1D-like disease is a useful alternative. Among such models, the Non-Obese Diabetic (NOD) mouse model has been a cornerstone in studying T1D. Nevertheless, this model, alike other models in other rodent species such as the BioBreeding BB rat, fails to translate to humans in many aspects. New mouse models are required to develop screening tools and to study targeted immunotherapies in the aim to prevent or cure the disease.
2 The NOD Model
T1D is likely a heterogeneous disease when considering the genetic background on which it develops as well as the severity of the autoimmune process and the T cell subsets involved. The immunological characterization of T1D has been challenging considering the remoteness of the pancreas and the scarcity of autoantigen-specific T cells in the peripheral blood. For many years, the NOD mouse has allowed major advances in delineating the molecular and cellular processes of β-cells autoimmunity (2, 3). This model develops spontaneous autoimmune diabetes that shares several genetic and immunologic traits with the human disease (4). First described in 1974, the NOD mouse was used to study autoantigens, susceptibility genes, and disease initiating events as well as to characterize the nature of involved immune cells (5). It has allowed to define the successive immune steps involved in the disease process and the importance of a progressive imbalance between regulatory and effector T cells in allowing autoimmunity to proceed. NOD mice share with humans, many target autoantigens (insulin, Glutamate Decarboxylase 65, IA2/IA2b and ZnT8) and many genetic susceptibility genes (in particular, the class-II IAg7 gene that is homolog of the high susceptibility HLA-DQ8 class-II molecule in humans). However, NOD mice develop a considerably more extensive insulitis than in human T1D (4, 6). Also, curative strategies that were efficient in the NOD mouse have often failed to translate into a therapy to humans (7). This failure is probably related to the incapacity of this model to fully reproduce the complexity and the heterogeneity of the human disease ( (8). There are important differences between the mouse and the human both in the architecture of the islets of Langerhans and that of the immune system. Moreover, class-I and class-II major histocompatibility complex (MHC) as well as autoantigen genes, although homolog, differ in their sequence between the mouse and the human. Therefore, autoantigen epitopes that are presented by class-I and class-II HLA and H2 molecules to CD8+ and CD4+ T cells, respectively, differ between the two species (7, 9). Evidence that favors the use of antigen-specific immunotherapy to cure T1D highlights the difference in target epitopes in NOD mice as compared to T1D patients. To address these differences, many laboratories have developed preclinical humanized models to fill the gap between mice and humans and to facilitate the translation of novel discoveries to clinical trials (10, 11).
3 Humanized Mouse Models
Humanized mice are defined as mice engrafted with functional human cells or tissues or mice expressing human transgenes. These advanced models are designed to study the pathophysiology of T1D in vivo, to detect new biomarkers and to find new therapeutic targets without putting patients at risk (12).
3.1 HLA Transgenic Mice
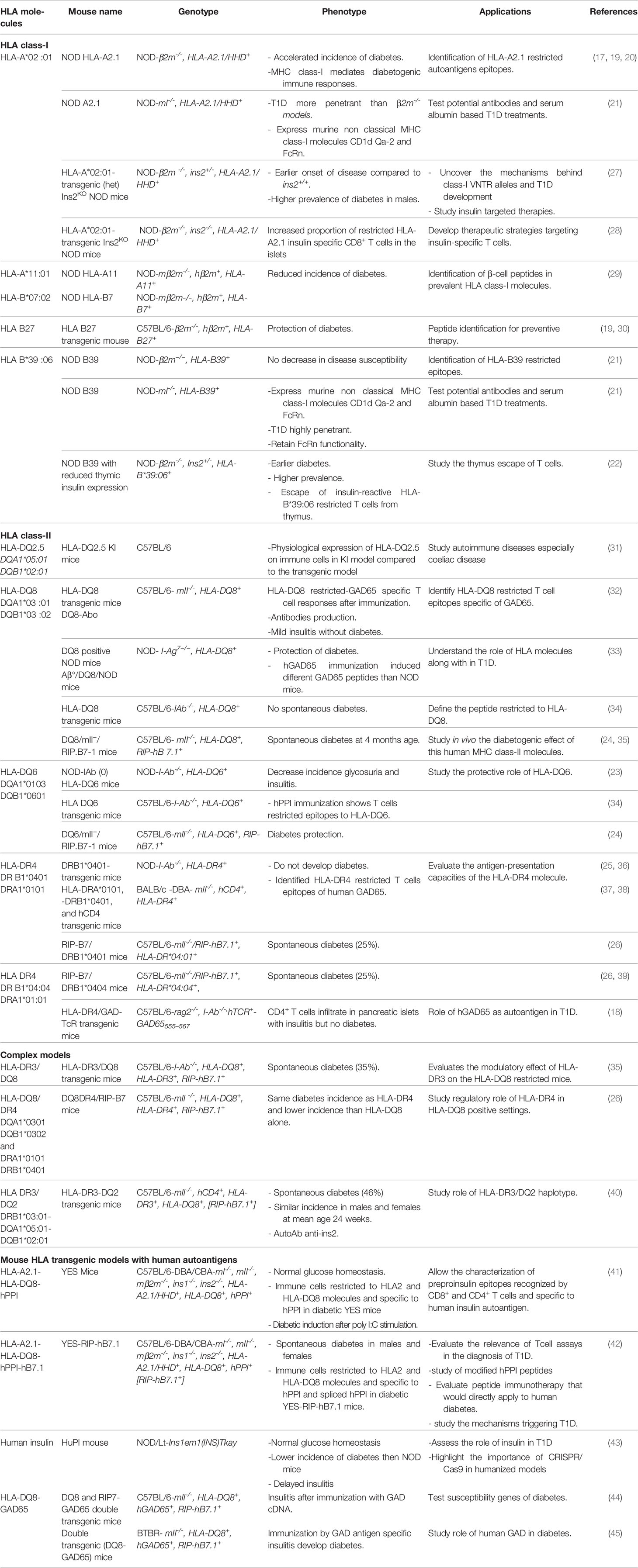
Genetic susceptibility is considered a valuable clue to the molecular mechanisms of T1D. In humans, over 50 gene variants have been identified as carrying a risk for T1D, the most important being HLA genes contributing to 40-50% of the lifetime risk of T1D (13). HLA class-II genes provide the highest susceptibility. They are involved in the initiation of the T cell response to β-cells autoantigens in T1D. Among HLA class-II genes, HLA-DQ8 molecule carries the highest risk and is present in over 40% of at-risk and pediatric T1D patients (14), while HLA-DQ6 molecule is protective with a relative risk of 0.2 (15). HLA-DR molecules carry an independent risk with the particular case of HLA-DR*04:01 (14), and to a lesser extent DR*04:05 and 04:02 (16). HLA class-I molecules provide a lower risk and are associated to the progression of the disease. The strongest class-I susceptibility is conferred by HLA-B*39:06, HLA-B *57:01, HLA- B*18:01, HLA-A*02:01, HLA-A*24:02 and HLA-C*05:01 (15, 16). Class-I and class-II MHC genes directly control the peripheral T cell repertoire and the spectrum of antigen epitopes that are presented to T cells. Thus, introducing HLA transgenes in the mouse allows to characterize HLA-restricted autoantigen peptides at play in human T1D (11). “Humanized mouse” models have been developed by introducing HLA class-I or class-II transgenes into different mouse strains with or without invalidating corresponding murine H2 class-I or class-II genes (11). The expression of HLA class-I transgenes and their interaction with murine CD8+ T cells has been obtained using constructs encoding a human β2-microglobulin (β2m) covalently linked to HLA alpha1 and alpha2 and H2 cytosolic and transmembrane alpha3 chain domains (11, 17, 18). H2 class-I genes have been invalidated by deleting either the murine β2m or the MHC class-I locus. On the NOD genetic background, mice expressing HLA-A2.1 or HLA-B39 transgenes developed accelerated T1D (19–22) while the expression of HLA-DQ6 decreased the incidence of spontaneous diabetes and insulitis (23, 24). HLA-DQ8 or HLA-DR4 transgenic NOD mice depleted for IAg7 were resistant to diabetes probably because T cells shift toward a tolerogenic regulatory profile (25). HLA transgenic mouse models have not been limited to the NOD background. HLA transgenes have been introduced in non-diabetes prone strains, mainly C57/BL6 mice. The expression of T1D susceptibility HLA class-II genes in these mice is not sufficient to induce diabetes. However, immunizing these transgenic mice with β-cells autoantigens allowed homing of T cells to the pancreas and the development of insulitis. The expression of the human costimulatory B7.1 molecule under the control of the rat insulin gene promoter as a transgene in these mice led to the development of diabetes. HLA-DQ8/RIP-B7.1 transgenic mice showed the highest incidence, whereas the genotype HLA-DR*04:01/HLA-DQ8 attenuated this effect and HLA-DQ6/RIP-B7.1 mice were protected from diabetes (24, 26). Table 1 shows the different HLA transgenic models that have been reported.
HLA transgenic mice allowed the identification of key players in T1D development. Adoptive transfer of T cells from HLA-DQ8 transgenic mice immunized with GAD65 and having evidence of insulitis, induced insulitis in recipients (32). Challenging HLA-DR4/RIP-B7.1 mice with murine proinsulin-2 peptides accelerated T1D development (46). The level of thymic insulin 2 gene expression determined the timing and the incidence of T1D in an HLA-B*39:06 transgenic mouse, a similar effect of that of the invalidation of the insulin 2 gene in NOD mice (47) or of the insulin variable number of tandem repeats (VNTR)in humans (22).
3.2 HLA and Human Autoantigen Transgenic Mice
Besides pointing to the role of HLA in T1D development, HLA transgenic models have allowed identifying β-cell peptides recognized by T cells using either T cell hybridomas, or T cell assays or class-I or class-II peptide-MHC tetramers (29, 34, 38, 48). The main autoantigens that are recognized by T cells in T1D patients are insulin and its precursor preproinsulin (PPI) hereafter described as insulin, GAD65, ZnT8, IA2 and islet-glucose-6-phosphatase catalytic subunit-related protein (IGRP) (49, 50). Differences in β-cell peptides have been seen depending on whether a murine or a human autoantigen was expressed (34). Therefore, HLA transgenic mice that express human GAD65 or human PPI have been developed (41, 44).
3.2.1 Humanized Mice That Express Human Insulin
PPI is synthetized in β-cells and translocated to Endoplasmic reticulum (ER) in the form of proinsulin after cleavage of signal peptide sequence by a peptidase. Proinsulin is later converted into mature and bioactive insulin (51). Among the autoantigens, insulin has been ascribed a key role in T1D (52, 53). In infants followed from birth, anti-insulin antibodies were detected early in the diabetes process in at risk subjects (54). The genetic polymorphism of a VNTR 5’ of the INS gene confers a significant risk for T1D development (55). PPI epitopes that are presented by different HLA class-I molecules to CD8+ T cells and by HLA class-II molecules to CD4+ T cells have been characterized in patients and in mouse models (50).
In mice as well as in some fish species, two genes located on different chromosomes encode respectively insulin 1 and insulin 2 (56). However, humans carry a unique Insulin gene that shows homology with the murine Insulin 2 gene. Murine insulin 1 and insulin 2 differ by two amino acids located in the insulin B chain at positions B9 and B29 and by amino acids located in the insulin leader and C-peptide sequences. The Insulin 1 gene lacks an intron that is present in Insulin 2 and in the human Insulin gene. In the mouse, insulin 1 is the main insulin isoform secreted in the pancreas whereas insulin 2 predominates in the thymus. Normal glycemia was maintained in the absence of either insulin1 or insulin2 on conventional mouse genetic backgrounds (43, 57). However, the invalidation of the Insulin 1 or the Insulin 2 gene led respectively to prevent and accelerate T1D development in the NOD mouse (47, 58) while invalidation of GAD or IA2 gene had limited effects. In genetically modified mice, human transgenes are randomly integrated in the genome which may lead to abnormal gene expressions and functions (59). This raises concerns about losing the insulin physiologic function when replacing murine insulin with human insulin. Nevertheless, using a PPI transgene in NOD models invalidated to Insulin 1 and Insulin 2 genes restored the metabolic function of insulin even when switching tyrosine to alanine at position B16 (57). Also, YES mouse that lacks the expression of murine MHC class-I, class-II and insulin genes and expresses human insulin (hPPI), HLA-A*02:01 and HLA-DQ8 transgenes, showed normal β-cell mass and normal glycemia values even after intraperitoneal injection of glucose (41).
3.2.2 Implications of HLA Transgenic Mice Expressing Human Autoantigens
HLA transgenic mice modified to express human autoantigens allow mapping T cell epitopes that match human epitopes, especially in case of autoantigens with low expression in mice (11). These mice highlight the importance of certain antigens in the initiation of diabetes and allow the detection of specific T cells in the pancreas. Immunization with hGAD cDNA induced insulitis and glucose intolerance in HLA-DQ8/mII-/RIPB7.1-hGAD65 transgenic mice (36). When immunized against hPPI, YES mice showed insulin specific T cell responses that are restricted to HLA-A*A2:01 and HLA-DQ8 molecules (41). These mice developed diabetes when injected with polyI:C (Toll-like receptor 3 agonist) and spontaneous diabetes when co-expressing RIP-hB7.1 along with CD8+ and CD4+ T cell responses that largely overlap (41, 42). Thus, in addition to refining the study of human susceptibility genes and human autoantigen epitopes that are targeted by T cells in T1D, humanized models allow evaluating the role of environmental factors in triggering T1D development.
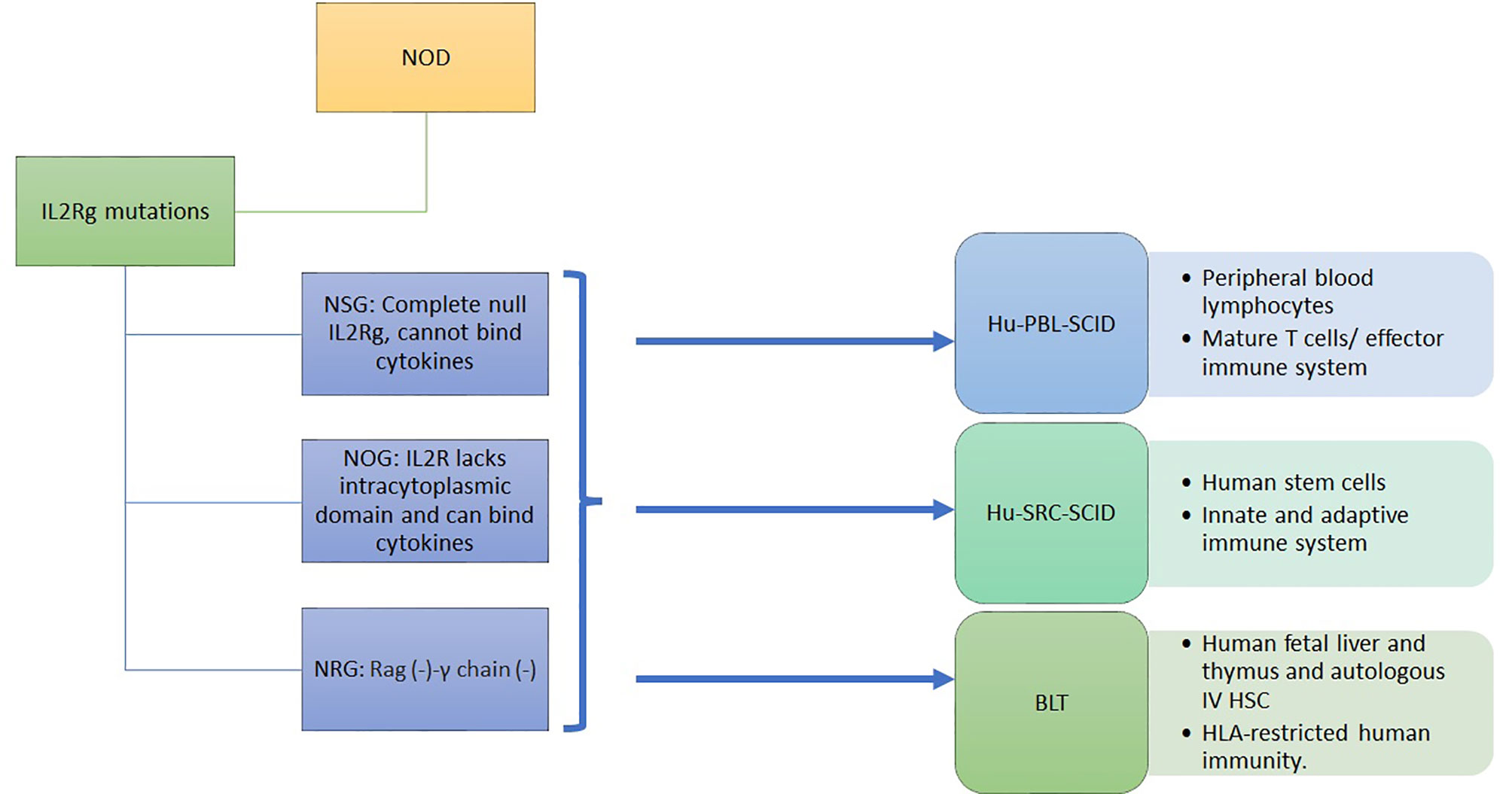
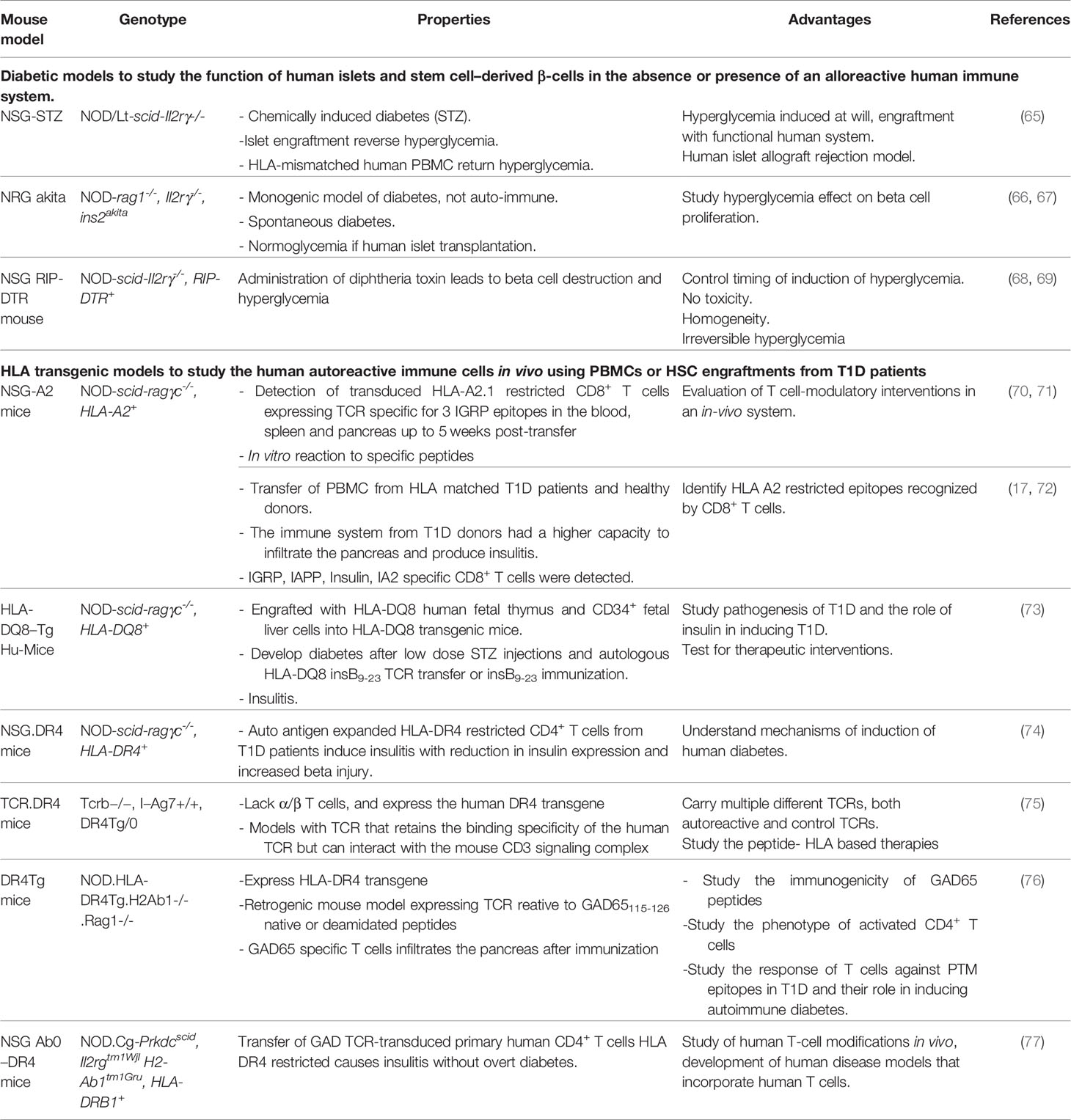
3.3 Humanized Immunodeficient NOD Models
HLA transgenic NOD models has been a unique model to advance our understanding of T1D. However, the genomic inflammatory responses in humans and mice do not overlap, possibly explaining the failure of translating therapies from mice to humans (60). Immunodeficient mice engrafted with human immune cells and tissues provide NOD mice with a humanized functional immune system to overcome this problem. Immunodeficient NOD (NSG) mice were obtained by deleting the IL2-receptor γ C gene, although not the SCID-Prkdc gene, from NOD-SCID mice (61). These mice are engrafted by a human immune system and/or by human islets (8). In NSG mice, the lack of B, T, and NK cells and the poor lymph node organization and development support the engraftment with human cells and tissues. The human immune system engraftment could originate from human peripheral blood monocytes or from human stem cells isolated from the umbilical cord, from fetal liver or mobilized to the periphery through G-CSF. It can also be obtained by transplanting the human fetal liver and autologous thymus fragments under the renal capsule while injecting the autologous human HSC intravenously (8, 12) (Figure 1). This leads to murine models harboring a functional human immune system. The proper technological approach for engraftment and the proper mouse model are chosen depending on study objectives, i.e study of autoreactive or alloreactive T cells, or HLA-restricted epitopes, or induction of autoimmune diabetes. However, the scope of these models is limited by the murine component of many immune determinants: cytokines, murine major histocompatibility complex (H2), homing molecules, poorly developed lymph nodes and in case of diabetes, the cutoff level of a normal glycemia (62, 63).
To optimize these humanized models, mice can be manipulated to induce diabetes or to express human genes such as IL3, MG-CSF, SCF, thrombopoietin, SIRP alpha and HLA class-I or class-II depending on the study outcome (62, 64). Table 2 shows the hyperglycemic and the HLA transgenic NSG mouse models.
These models are valuable to decipher the pathophysiology of T1D. They serve to study T1D triggering factors. Fifty percent of NSG mice transplanted with human islets and infected with cocksackie virus developed hyperglycemia (78). Also, these models serve in studying human β-cells proliferation in vivo. NSG strains have been genetically modified to develop hyperglycemia either spontaneously or chemically (79). These hyperglycemic models could be transplanted with human islets or human stem cells derived from β-cells or progenitor cells to revert the hyperglycemia (66). Engraftment of hyperglycemic NRG Akita mice with human islet cells increased the β-cell proliferation by 6 folds as compared to normoglycemic NRG Akita mice (80).
Additionally, such models allow to identify key players in T1D and mechanisms behind β-cells destruction. Destruction of pancreatic islets and infiltrates with human CD4+ T cells was observed in humanized NSG mice after injection of irradiated monocytes from diabetic NOD mice (81). The adoptive transfer of T cells transduced to express human autoantigen-specific TCR allows to isolate a larger number of human diabetogenic T cells and dissect the role of islet autoantigens in T1D. A mouse model can be engrafted by human or murine stem cells transduced to express autoantigen-specific TCRs to create retrogenic humanized models. The retrogenic mouse model allows to study human autoreactive T cells phenotype and function and to study thymic selection (82). The retrogenic mouse can express multiple autoreactive or control TCRs to better mimic the physiological setting. In a TCR-transgenic humanized mouse model, thymocytes expressing TCRs specific to the HLA-DQ8 restricted peptide hPPI 33-47 (insulin B9-23) were negatively selected in an HLA-DQ8 positive human immune system more efficiently than on an HLA-DQ8 negative immune system (83). In another model, HLA-DR4 retrogenic mouse expressing monoclonal or polyclonal TCRs reactive to native or deamidated GAD-65115-127 peptides showed that post-translational modifications epitopes do not support T reg development (76).
3.4 Applications of Humanized Models in T1D Diagnosis, Treatment and Prevention
The diagnosis of autoimmunity in full-blown T1D is based on the detection of autoantibodies (1). However, in prediabetic individuals, while positivity for three or four different antigenic specificities is highly predictive, positivity for one or two autoantibodies has a low predictive value, highlighting the importance of developing new assays for early and accurate diagnosis (84). Providing the key role of T cells in driving the autoimmune process against β-cells, T cell assays need to be developed. T cell responses to epitopes recognized in the context of HLA class-I and class-II transgenic mice will be useful in helping to develop these assays for diagnosing and immune monitoring in patients under immunotherapy.
Identifying epitopes recognized by T cells in T1D pave the way to developing antigen-specific immunotherapies which are likely to carry a high benefit/risk ratio (85). Among recent examples, injecting NOD-β2mnull HHD mice with a nanoparticle-peptide complex (PSB coupled to HLA-A2 restricted ZnT8 or IGRP epitopes) induced immune tolerance and prevented diabetes by decreasing the numbers of autoreactive CD8+ T cells (86). Altered peptide ligand for insulin B1(5-14) induced antigen-specific anergy in a similar model (87). Vaccinating NSG-HLA-DQ8 transgenic mice with insulin mimotopes stimulated Foxp3+ Tregs in vivo (88).
These models can allow discovering and testing new-targeted therapies. The study of teplizumab in HLA-A2/NSG mice allowed the identification of CCR6+ Treg cells secreting IL-10 which could be considered as a therapeutic target (89). HLA-DQ8/hGAD65 transgenic mice have been used to test a targeted therapy using GC7 molecule which inhibits the eucaryotic translation initiation factor A-1 (eIF5A) activating enzyme. In this model, the onset of T1D was delayed and the function of β-cells improved (90).
In other preclinical models, humanized mice engrafted with human immune precursors have been used to evaluate the translational potential of promising therapies. Currently, pancreatic islet transplantation can restore normoglycemia in patients with long-onset T1D. However, it faces the shortage in human donors and the risk of graft rejection. Manipulation of the hematopoietic stem-cells or PBMC engrafted NSG strain has generated mice in which chemically or spontaneously induced diabetes was reversible by islet engraftment (61, 91). This allowed the identification of new potential therapeutic targets and the study of the mechanisms of islet graft rejection and the means to prevent this rejection (92). Combining human immune system and islet engraftment in these models allow the optimization of protocols for inducing remission in T1D through islet engraftment and suppression of graft rejection. Treatment with IL-2 and rapamycin suppressed effector T cells and stimulated regulatory (CD4+FOXP3+) T cells reducing human islet allograft rejection in NSG mice transfused with human spleen mononuclear cells (93). Combination therapy with ethylcarbodiimide, rituximab and rapamycin limited the rejection of xenogeneic porcine islets in humanized mice (94).
As another approach, costimulation blockade has been shown to prevent the rejection of allogeneic pancreatic endoderm by human PBMCs in a humanized model in vivo (95). Co-transplantation of human bone marrow-derived mesenchymal stem cells (hBMSCs) could prevent immune rejection and improve human islet transplantation in a humanized NSG mouse (96). Another NSG mouse model was created by transferring genetically modified human embryonic stem cells that lacked CIITA and expressed HLA-A2 as the only HLA class-I molecule. The differentiation of these cells into β-cells then the engraftment with human PBMCs allowed to study the immune response and the islet rejection (97). Genetically modified β-cells engraftment is another promising therapy to prevent T1D recurrence post engraftment; human β-cells engineered to express Herpesvirus encoded immune-evasion proteins prevent islet destruction in NSG mice by degrading MHC class-I molecules and inhibiting granzyme B activity (98). Beyond allograft rejection, NSG mice can be used to study xenogeneic GVHD reactions. An option has been developed that replaced human islets by genetically modified porcine islet. Engraftment of neonatal porcine islet-like cell clusters overexpressing CTLA-4 Ig analogue in diabetic Hu-HSC-NSG mice reverted diabetes without a xenogeneic GVHD reaction (99). Despite these advantages, the translation of treatment to humans is not straightforward. The dosing, frequency, and route of administration of immunotherapies are still to be refined.
3.5 Other Humanized Models for T1D
T1D involves an auto-immune destruction of the β-cells. Therefore, a therapeutic approach aiming at modulating the immune response represents an attractive means of treatment approach. So far, therapies have met with varying clinical success despite efficiency in murine preclinical models. At best, the response to short-term treatments such as anti-CD3 antibodies had time limited effect. Pre-clinical models expressing the human targeted molecules might fill this gap and allow the optimization of therapeutic protocols. Immunomodulatory treatments have been attempted. Humanized murine models expressing human CD3ϵ and CD20 were developed to study the therapeutic potential of combined protocols in restoring tolerance in T1D (100). Treating VH125.hCD20/NOD mice with anti-human CD20 delayed diabetes development by reducing the effect of costimulatory molecules on B cells, by decreasing the INFγ production and by limiting T cell activation in the islets. Combining a histone deacetylase inhibitor with low-dose CD3 antibodies abrogated local inflammation, improved pancreatic β-cell survival and metabolic function, and led to long-lasting diabetes remission (101).
β-cell antigen-based therapy is another attractive approach, as it precludes the long-term side effects of immune modulating therapy as being antigen-specific. To study the capacity of dendritic cells to induce an antigen-specific immune tolerance, a humanized mouse model expressing human CD205 on a NOD background was produced. CD205, as the endocytic receptor of antibodies coupled to islet antigens on myeloid dendritic cells, allows antigen processing and presentation by MHC class-I and II and modulate antigen-specific T cell responses (102).
4 Perspective
Humanized HLA and autoantigen transgenic mice allow the identification of the epitopes restricted to HLA class-I and class-II molecules paving the way to antigen-specific immunotherapy and to restoration of immune tolerance in T1D patients. Effort should be made to regenerate β-cell mass after reestablishment of tolerance to autoantigens in T1D patients with a low β-cells mass (103). Using humanized immunodeficient models engrafted with human immune system and human β-cells in the context of human susceptibility genes will allow a better understanding of the pathophysiology. Replacing the current engraftment techniques with the induced pluripotent stem cells (iPS) technology might provide better means to study the disease. These cells can be isolated from T1D patients and can differentiate into β-cells, hematopoietic stem-progenitor and thymic epithelium (61, 104). These models also allow to identify new biomarkers and to design new screening and prognostic biological assays that can apply to humans. This personalized in vivo model provides new insights into the immune function of patients with T1D. This allows to have a better understanding of diabetes in the individual and to overcome the heterogeneity of the disease. It will facilitate the development of peptide-based predictive, diagnostic, and therapeutic strategies and will pave the way to personalized medicine.
Author Contributions
All authors contributed to the article and approved the submitted version. PH designed, did the literature review, and wrote the article. SL designed, reviewed, and edited the review. CB designed, reviewed, and edited the review.
Funding
The publishing of this review is funded by Association Robert Debré pour la recherche médicale (ARDRM). This work was supported by « Fondation Servier » and « Fondation pour la Recherche Médicale » (FRM).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yi L, Swensen AC, Qian W-J. Serum Biomarkers for Diagnosis and Prediction of Type 1 Diabetes. Trans Res (2018) 201:13–25. doi: 10.1016/j.trsl.2018.07.009
2. Driver JP, Serreze DV, Chen Y-G. "Mouse Models for the Study of Autoimmune Type 1 Diabetes: A NOD to Similarities and Differences to Human Disease". In: Seminars in Immunopathology. Springer (2011). p. 67–87.
3. Mcdaniel Mims B, Grisham MB. Humanizing the Mouse Immune System to Study Splanchnic Organ Inflammation. J Physiol (2018) 596:3915–27. doi: 10.1113/JP275325
4. Driver JP, Chen YG, Mathews CE. Comparative Genetics: Synergizing Human and NOD Mouse Studies for Identifying Genetic Causation of Type 1 Diabetes. Rev Diabetes Stud (2012) 9:169–87. doi: 10.1900/RDS.2012.9.169
5. Gvazava IG, Rogovaya OS, Borisov MA, Vorotelyak EA, Vasiliev AV. Pathogenesis of Type 1 Diabetes Mellitus and Rodent Experimental Models. Acta Nat (2018) 10:24–33. doi: 10.32607/20758251-2018-10-1-24-33
6. Von Herrath M, Nepom GT. Animal Models of Human Type 1 Diabetes. Nat Immunol (2009) 10:129–32. doi: 10.1038/ni0209-129
7. In’t Veld P. "Insulitis in Human Type 1 Diabetes: A Comparison Between Patients and Animal Models". In: Seminars in Immunopathology. Springer (2014). p. 569–79.
8. Brehm MA, Powers AC, Shultz LD, Greiner DL. Advancing Animal Models of Human Type 1 Diabetes by Engraftment of Functional Human Tissues in Immunodeficient Mice. Cold Spring Harb Perspect Med (2012) 2:a007757. doi: 10.1101/cshperspect.a007757
9. Creusot RJ, Postigo-Fernandez J, Teteloshvili N. Altered Function of Antigen-Presenting Cells in Type 1 Diabetes: A Challenge for Antigen-Specific Immunotherapy? Diabetes (2018) 67:1481–94. doi: 10.2337/db17-1564
10. Reed JC, Herold KC. Thinking Bedside at the Bench: The NOD Mouse Model of T1DM. Nat Rev Endocrinol (2015) 11:308. doi: 10.1038/nrendo.2014.236
11. Pearson JA, Wong FS, Wen L. The Importance of the Non Obese Diabetic (NOD) Mouse Model in Autoimmune Diabetes. J Autoimmun (2016) 66:76–88. doi: 10.1016/j.jaut.2015.08.019
12. King M, Pearson T, Rossini AA, Shultz LD, Greiner DL. Humanized Mice for the Study of Type 1 Diabetes and Beta Cell Function. Ann New York Acad Sci (2008) 1150:46. doi: 10.1196/annals.1447.009
14. Gan MJ, Albanese-O’neill A, Haller MJ. Type 1 Diabetes: Current Concepts in Epidemiology, Pathophysiology, Clinical Care, and Research. Curr Problems Pediatr Adolesc Health Care (2012) 42:269–91. doi: 10.1016/j.cppeds.2012.07.002
15. Noble JA. Immunogenetics of Type 1 Diabetes: A Comprehensive Review. J Autoimmun (2015) 64:101–12. doi: 10.1016/j.jaut.2015.07.014
16. Noble JA, Valdes AM. Genetics of the HLA Region in the Prediction of Type 1 Diabetes. Curr Diabetes Rep (2011) 11:533. doi: 10.1007/s11892-011-0223-x
17. Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, et al. HLA-A* 0201-Restricted T Cells From Humanized NOD Mice Recognize Autoantigens of Potential Clinical Relevance to Type 1 Diabetes. J Immunol (2006) 176:3257–65. doi: 10.4049/jimmunol.176.5.3257
18. Gebe JA, Unrath KA, Yue BB, Miyake T, Falk BA, Nepom GT. Autoreactive Human T-Cell Receptor Initiates Insulitis and Impaired Glucose Tolerance in HLA DR4 Transgenic Mice. J Autoimmun (2008) 30:197–206. doi: 10.1016/j.jaut.2007.08.001
19. Marron MP, Graser RT, Chapman HD, Serreze DV. Functional Evidence for the Mediation of Diabetogenic T Cell Responses by HLA-A2. 1 MHC Class I Molecules Through Transgenic Expression in NOD Mice. Proc Natl Acad Sci (2002) 99:13753–8. doi: 10.1073/pnas.212221199
20. Serreze DV, Marron MP, Dilorenzo TP. “Humanized” HLA Transgenic NOD Mice to Identify Pancreatic β Cell Autoantigens of Potential Clinical Relevance to Type 1 Diabetes. Ann New York Acad Sci (2007) 1103:103–11. doi: 10.1196/annals.1394.019
21. Racine JJ, Stewart I, Ratiu J, Christianson G, Lowell E, Helm K, et al. Improved Murine MHC-Deficient HLA Transgenic NOD Mouse Models for Type 1 Diabetes Therapy Development. Diabetes (2018) 67:923–35. doi: 10.2337/db17-1467
22. Schloss J, Ali R, Racine JJ, Chapman HD, Serreze DV, Dilorenzo TP. HLA-B* 39: 06 Efficiently Mediates Type 1 Diabetes in a Mouse Model Incorporating Reduced Thymic Insulin Expression. J Immunol (2018) 200:3353–63. doi: 10.4049/jimmunol.1701652
23. Fukui Y, Nishimura Y, Iwanga T, Kimura A, Inamitsu T, Hanaoka Y, et al. GLYCOSURIA AND INSULITIS IN NOD MICE EXPRESSING THE HLA-DQw6 MOLECULE. Int J Immunogenet (1989) 16:445–53. doi: 10.1111/j.1744-313X.1989.tb00493.x
24. Wen L, Wong FS, Tang J, Chen N-Y, Altieri M, David C, et al. In Vivo Evidence for the Contribution of Human Histocompatibility Leukocyte Antigen (HLA)-DQ Molecules to the Development of Diabetes. J Exp Med (2000) 191:97–104. doi: 10.1084/jem.191.1.97
25. Sang LP, Surls J, Mendoza M, Casares S, Brumeanu T. HLA-DR* 0401 Expression in the NOD Mice Prevents the Development of Autoimmune Diabetes by Multiple Alterations in the T-Cell Compartment. Cell Immunol (2015) 298:54–65. doi: 10.1016/j.cellimm.2015.09.003
26. Wen L, Chen N-Y, Tang J, Sherwin R, Wong FS. The Regulatory Role of DR4 in a Spontaneous Diabetes DQ8 Transgenic Model. J Clin Invest (2001) 107:871–80. doi: 10.1172/JCI11708
27. Babad J, Ali R, Schloss J, Dilorenzo TP. An HLA-Transgenic Mouse Model of Type 1 Diabetes That Incorporates the Reduced But Not Abolished Thymic Insulin Expression Seen in Patients. J Diabetes Res (2016) 2016:1–8. doi: 10.1155/2016/7959060
28. Jarchum I, Dilorenzo TP. Ins2 Deficiency Augments Spontaneous HLA-A* 0201–Restricted T Cell Responses to Insulin. J Immunol (2010) 184:658–65. doi: 10.4049/jimmunol.0903414
29. Antal Z, Baker JC, Smith C, Jarchum I, Babad J, Mukherjee G, et al. Beyond HLA-A* 0201: New HLA-Transgenic Nonobese Diabetic Mouse Models of Type 1 Diabetes Identify the Insulin C-Peptide as a Rich Source of CD8+ T Cell Epitopes. J Immunol (2012) 188:5766–75. doi: 10.4049/jimmunol.1102930
30. Taurog JD, Lowen L, Forman J, Hammer RE. HLA-B27 in Inbred and non-Inbred Transgenic Mice. Cell Surface Expression and Recognition as an Alloantigen in the Absence of Human Beta 2-Microglobulin. J Immunol (1988) 141:4020–3.
31. Dewan AE, Koentgen F, Johannesen MK, Du Pre MF, Sollid LM. Generation of an HLA-DQ2.5 Knock-In Mouse. Immunohorizons (2021) 5:25–32. doi: 10.4049/immunohorizons.2000107
32. Wen L, Wong FS, Burkly L, Altieri M, Mamalaki C, Kioussis D, et al. Induction of Insulitis by Glutamic Acid Decarboxylase Peptide-Specific and HLA-DQ8-Restricted CD4 (+) T Cells From Human DQ Transgenic Mice. J Clin Invest (1998) 102:947–57. doi: 10.1172/JCI2723
33. Abraham RS, Wilson SB, De Souza NF Jr., Strominger JL, Munn SR, David CS. NOD Background Genes Influence T Cell Responses to GAD 65 in HLA-DQ8 Transgenic Mice. Hum Immunol (1999) 60:583–90. doi: 10.1016/S0198-8859(99)00057-9
34. Raju R, Munn SR, David CS. T Cell Recognition of Human Pre-Proinsulin Peptides Depends on the Polymorphism at HLA DQ Locus: A Study Using HLA DQ8 and DQ6 Transgenic Mice. Hum Immunol (1997) 58:21–9. doi: 10.1016/S0198-8859(97)00212-7
35. Rajagopalan G, Kudva YC, Chen L, Wen L, David CS. Autoimmune Diabetes in HLA-DR3/DQ8 Transgenic Mice Expressing the Co-Stimulatory Molecule B7-1 in the β Cells of Islets of Langerhans. Int Immunol (2003) 15:1035–44. doi: 10.1093/intimm/dxg103
36. Wicker LS, Chen S-L, Nepom GT, Elliott JF, Freed DC, Bansal A, et al. Naturally Processed T Cell Epitopes From Human Glutamic Acid Decarboxylase Identified Using Mice Transgenic for the Type 1 Diabetes-Associated Human MHC Class II Allele, DRB1* 0401. J Clin Invest (1996) 98:2597–603. doi: 10.1172/JCI119079
37. Fugger L, Michie SA, Rulifson I, Lock CB, Mcdevitt GS. Expression of HLA-DR4 and Human CD4 Transgenes in Mice Determines the Variable Region Beta-Chain T-Cell Repertoire and Mediates an HLA-DR-Restricted Immune Response. Proc Natl Acad Sci (1994) 91:6151–5. doi: 10.1073/pnas.91.13.6151
38. Patel SD, Cope AP, Congia M, Chen TT, Kim E, Fugger L, et al. Identification of Immunodominant T Cell Epitopes of Human Glutamic Acid Decarboxylase 65 by Using HLA-DR (α1* 0101, β1* 0401) Transgenic Mice. Proc Natl Acad Sci (1997) 94:8082–7. doi: 10.1073/pnas.94.15.8082
39. Gebe JA, Unrath KA, Falk BA, Ito K, Wen L, Daniels TL, et al. Age-Dependent Loss of Tolerance to an Immunodominant Epitope of Glutamic Acid Decarboxylase in Diabetic-Prone RIP-B7/DR4 Mice. Clin Immunol (2006) 121:294–304. doi: 10.1016/j.clim.2006.08.002
40. Verhagen J, Yusuf N, Smith EL, Whettlock EM, Naran K, Arif S, et al. Proinsulin Peptide Promotes Autoimmune Diabetes in a Novel HLA-DR3-DQ2-Transgenic Murine Model of Spontaneous Disease. Diabetologia (2019) 62:2252–61. doi: 10.1007/s00125-019-04994-8
41. Luce S, Guinoiseau S, Gadault A, Letourneur F, Blondeau B, Nitschke P, et al. Humanized Mouse Model to Study Type 1 Diabetes. Diabetes (2018) 67:1816–29. doi: 10.2337/db18-0202
42. Luce S, Guinoiseau S, Gadault A, Letourneur F, Nitschke P, Bras M, et al. A Humanized Mouse Strain That Develops Spontaneously Immune-Mediated Diabetes. Front Immunol (2021) 12:748679. doi: 10.3389/fimmu.2021.748679
43. Elso CM, Scott NA, Mariana L, Masterman EI, Sutherland APR, Thomas HE, et al. Replacing Murine Insulin 1 With Human Insulin Protects NOD Mice From Diabetes. PloS One (2019) 14:e0225021. doi: 10.1371/journal.pone.0225021
44. Elagin RB, Balijepalli S, Diacovo MJ, Baekkeskov S, Jaume JC. Homing of GAD65 Specific Autoimmunity and Development of Insulitis Requires Expression of Both DQ8 and Human GAD65 in Transgenic Mice. J Autoimmun (2009) 33:50–7. doi: 10.1016/j.jaut.2009.02.004
45. Elagin RB, Jaume JC. Glucose Intolerance and Diabetes Following Antigen-Specific Insulitis in Diabetes-Susceptible “Humanized” Transgenic Mice. Biochem Biophys Res Commun (2010) 395:99–103. doi: 10.1016/j.bbrc.2010.03.146
46. Verhagen J, Smith EL, Whettlock EM, Macintyre B, Peakman M. Proinsulin-Mediated Induction of Type 1 Diabetes in HLA-DR4-Transgenic Mice. Sci Rep (2018) 8:1–6. doi: 10.1038/s41598-018-32546-4
47. Thebault-Baumont K, Dubois-Laforgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, et al. Acceleration of Type 1 Diabetes Mellitus in Proinsulin 2-Deficient NOD Mice. J Clin Invest (2003) 111:851–7. doi: 10.1172/JCI16584
48. Babad J, Geliebter A, Dilorenzo TP. T-Cell Autoantigens in the non-Obese Diabetic Mouse Model of Autoimmune Diabetes. Immunology (2010) 131:459–65. doi: 10.1111/j.1365-2567.2010.03362.x
49. Mallone R, Brezar V, Boitard C. T Cell Recognition of Autoantigens in Human Type 1 Diabetes: Clinical Perspectives. Clin Dev Immunol (2011) 2011:1–16. doi: 10.1155/2011/513210
50. Han S, Donelan W, Wang H, Reeves W, Yang L-J. Novel Autoantigens in Type 1 Diabetes. Am J Trans Res (2013) 5:379.
51. Liu M, Weiss MA, Arunagiri A, Yong J, Rege N, Sun J, et al. Biosynthesis, Structure, and Folding of the Insulin Precursor Protein. Diabetes Obes Metab (2018) 20 Suppl 2:28–50. doi: 10.1111/dom.13378
52. Zhang L, Nakayama M, Eisenbarth GS. Insulin as an Autoantigen in NOD/human Diabetes. Curr Opin Immunol (2008) 20:111–8. doi: 10.1016/j.coi.2007.11.005
53. Nakayama M. Insulin as a Key Autoantigen in the Development of Type 1 Diabetes. Diabetes Metab Res Rev (2011) 27:773–7. doi: 10.1002/dmrr.1250
54. Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody Appearance and Risk for Development of Childhood Diabetes in Offspring of Parents With Type 1 Diabetes: The 2-Year Analysis of the German BABYDIAB Study. Diabetes (1999) 48:460–8. doi: 10.2337/diabetes.48.3.460
55. Pociot F, Mcdermott MF. Genetics of Type 1 Diabetes Mellitus. Genes Immun (2002) 3:235–49. doi: 10.1038/sj.gene.6363875
56. Deltour L, Leduque P, Blume N, Madsen O, Dubois P, Jami J, et al. Differential Expression of the Two Nonallelic Proinsulin Genes in the Developing Mouse Embryo. Proc Natl Acad Sci USA (1993) 90:527–31. doi: 10.1073/pnas.90.2.527
57. Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime Role for an Insulin Epitope in the Development of Type 1 Diabetes in NOD Mice. Nature (2005) 435:220–3. doi: 10.1038/nature03523
58. Moriyama H, Abiru N, Paronen J, Sikora K, Liu E, Miao D, et al. Evidence for a Primary Islet Autoantigen (Preproinsulin 1) for Insulitis and Diabetes in the Nonobese Diabetic Mouse. Proc Natl Acad Sci USA (2003) 100:10376–81. doi: 10.1073/pnas.1834450100
59. Goodwin G. Type 1 Diabetes Mellitus and Celiac Disease: Distinct Autoimmune Disorders That Share Common Pathogenic Mechanisms. Horm Res Paediatr (2019) 92:285–92. doi: 10.1159/000503142
60. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc Natl Acad Sci USA (2013) 110:3507–12. doi: 10.1073/pnas.1222878110
61. Walsh NC, Kenney LL, Jangalwe S, Aryee K-E, Greiner DL, Brehm MA, et al. Humanized Mouse Models of Clinical Disease. Annu Rev Pathol: Mech Dis (2017) 12:187–215. doi: 10.1146/annurev-pathol-052016-100332
62. Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized Mice for Immune System Investigation: Progress, Promise and Challenges. Nat Rev Immunol (2012) 12:786–98. doi: 10.1038/nri3311
63. Allen TM, Brehm MA, Bridges S, Ferguson S, Kumar P, Mirochnitchenko O, et al. Humanized Immune System Mouse Models: Progress, Challenges and Opportunities. Nat Immunol (2019) 20:770–4. doi: 10.1038/s41590-019-0416-z
64. Shultz LD, Keck J, Burzenski L, Jangalwe S, Vaidya S, Greiner DL, et al. Humanized Mouse Models of Immunological Diseases and Precision Medicine. Mamm Genome (2019) 30:123–42. doi: 10.1007/s00335-019-09796-2
65. King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, et al. A New Hu-PBL Model for the Study of Human Islet Alloreactivity Based on NOD-Scid Mice Bearing a Targeted Mutation in the IL-2 Receptor Gamma Chain Gene. Clin Immunol (2008) 126:303–14. doi: 10.1016/j.clim.2007.11.001
66. Brehm MA, Bortell R, Leif J, Laning J, Cuthbert A, Yang C, et al. Human Immune System Development and Rejection of Human Islet Allografts in Spontaneously Diabetic NOD-Rag1null Il2rγnull Ins2Akita Mice. Diabetes (2010) 59:2265–70. doi: 10.2337/db10-0323
67. Jurczyk A, Yang C, Racki WJ, Brehm MA, Atkinson MA, Powers AC, et al. Hyperglycemia-Induced Proliferation of Adult Human Beta Cells Engrafted Into Spontaneously Diabetic Immunodeficient NOD-Rag1null Il2rγnull Ins2Akita Mice. Pancreas (2011) 40:1147. doi: 10.1097/MPA.0b013e31821ffabe
68. Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of Adult Pancreatic α-Cells to β-Cells After Extreme β-Cell Loss. Nature (2010) 464:1149–54. doi: 10.1038/nature08894
69. Yang C, Loehn M, Jurczyk A, Przewozniak N, Leehy L, Herrera PL, et al. Lixisenatide Accelerates Restoration of Normoglycemia and Improves Human Beta-Cell Function and Survival in Diabetic Immunodeficient nOD–Scid IL-2rgnull riP-DTr Mice Engrafted With Human Islets. Diabetes Metab Syndrome Obes: Targets Ther (2015) 8:387. doi: 10.2147/DMSO.S87253
70. Unger WW, Pearson T, Abreu JR, Laban S, van der Slik AR, Mulder-Van Der Kracht S, et al. Islet-Specific CTL Cloned From a Type 1 Diabetes Patient Cause Beta-Cell Destruction After Engraftment Into HLA-A2 Transgenic NOD/scid/IL2RG Null Mice. PloS One (2012) 7:1–10. doi: 10.1371/journal.pone.0049213
71. Babad J, Mukherjee G, Follenzi A, Ali R, Roep B, Shultz LD, et al. Generation of β Cell-Specific Human Cytotoxic T Cells by Lentiviral Transduction and Their Survival in Immunodeficient Human Leucocyte Antigen-Transgenic Mice. Clin Exp Immunol (2015) 179:398–413. doi: 10.1111/cei.12465
72. Whitfield-Larry F, Young EF, Talmage G, Fudge E, Azam A, Patel S, et al. HLA-A2–matched Peripheral Blood Mononuclear Cells From Type 1 Diabetic Patients, But Not Nondiabetic Donors, Transfer Insulitis to NOD-Scid/γcnull/HLA-A2 Transgenic Mice Concurrent With the Expansion of Islet-Specific CD8+ T Cells. Diabetes (2011) 60:1726–33. doi: 10.2337/db10-1287
73. Tan S, Li Y, Xia J, Jin C-H, Hu Z, Duinkerken G, et al. Type 1 Diabetes Induction in Humanized Mice. Proc Natl Acad Sci (2017) 114:10954–9. doi: 10.1073/pnas.1710415114
74. Milam A, Maher SE, Gibson JA, Lebastchi J, Wen L, Ruddle NH, et al. A Humanized Mouse Model of Autoimmune Insulitis. Diabetes (2014) 63:1712–24. doi: 10.2337/db13-1141
75. Boddul SV, Sharma RK, Dubnovitsky A, Raposo B, Gerstner C, Shen Y, et al. In Vitro and Ex Vivo Functional Characterization of Human HLA-DRB1 *04 Restricted T Cell Receptors. J Transl Autoimmun (2021) 4:100087. doi: 10.1016/j.jtauto.2021.100087
76. Jing Y, Kong Y, Mcginty J, Blahnik-Fagan G, Lee T, Orozco-Figueroa S, et al. TCR/HLA Humanized Mice Reveal Reduced Tolerance and Increased Immunogenicity of Post-Translationally Modified GAD65 Epitope. Diabetes (2022). doi: 10.2337/figshare.19184456
77. Ali R, Babad J, Follenzi A, Gebe JA, Brehm MA, Nepom GT, et al. Genetically Modified Human CD 4+ T Cells Can Be Evaluated In Vivo Without Lethal Graft-Versus-Host Disease. Immunology (2016) 148:339–51. doi: 10.1111/imm.12613
78. Gallagher GR, Brehm MA, Finberg RW, Barton BA, Shultz LD, Greiner DL, et al. Viral Infection of Engrafted Human Islets Leads to Diabetes. Diabetes (2015) 64:1358–69. doi: 10.2337/db14-1020
79. Greiner DL, Brehm MA, Hosur V, Harlan DM, Powers AC, Shultz LD. Humanized Mice for the Study of Type 1 and Type 2 Diabetes. Ann N Y Acad Sci (2011) 1245:55–8. doi: 10.1111/j.1749-6632.2011.06318.x
80. Diiorio P, Jurczyk A, Yang C, Racki WJ, Brehm MA, Atkinson MA, et al. Hyperglycemia-Induced Proliferation of Adult Human Beta Cells Engrafted Into Spontaneously Diabetic Immunodeficient NOD-Rag1null IL2rgammanull Ins2Akita Mice. Pancreas (2011) 40:1147–9. doi: 10.1097/MPA.0b013e31821ffabe
81. Zhao Y, Guo C, Hwang D, Lin B, Dingeldein M, Mihailescu D, et al. Selective Destruction of Mouse Islet Beta Cells by Human T Lymphocytes in a Newly-Established Humanized Type 1 Diabetic Model. Biochem Biophys Res Commun (2010) 399:629–36. doi: 10.1016/j.bbrc.2010.07.128
82. Sprouse ML, Blahnik G, Lee T, Tully N, Banerjee P, James EA, et al. Rapid Identification and Expression of Human TCRs in Retrogenic Mice. J Immunol Methods (2016) 439:29–36. doi: 10.1016/j.jim.2016.08.010
83. Madley R, Nauman G, Danzl N, Borsotti C, Khosravi Maharlooei M, Li HW, et al. Negative Selection of Human T Cells Recognizing a Naturally-Expressed Tissue-Restricted Antigen in the Human Thymus. J Transl Autoimmun (2020) 3:100061. doi: 10.1016/j.jtauto.2020.100061
84. Dimeglio LA, Evans-Molina C, Oram RA. Type 1 Diabetes. Lancet (2018) 391:2449–62. doi: 10.1016/S0140-6736(18)31320-5
85. Peakman M, Von Herrath M. Antigen-Specific Immunotherapy for Type 1 Diabetes: Maximizing the Potential. Diabetes (2010) 59:2087–93. doi: 10.2337/db10-0630
86. Xu X, Bian L, Shen M, Li X, Zhu J, Chen S, et al. Multipeptide-Coupled Nanoparticles Induce Tolerance in ‘Humanised’HLA-Transgenic Mice and Inhibit Diabetogenic CD8+ T Cell Responses in Type 1 Diabetes. Diabetologia (2017) 60:2418–31. doi: 10.1007/s00125-017-4419-8
87. Zhang M, Wang S, Guo B, Meng G, Shu C, Mai W, et al. An Altered CD8+ T Cell Epitope of Insulin Prevents Type 1 Diabetes in Humanized NOD Mice. Cell Mol Immunol (2019) 16:590–601. doi: 10.1038/s41423-018-0058-3
88. Serr I, Fürst RW, Achenbach P, Scherm MG, Gökmen F, Haupt F, et al. Type 1 Diabetes Vaccine Candidates Promote Human Foxp3+ Treg Induction in Humanized Mice. Nat Commun (2016) 7:10991. doi: 10.1038/ncomms10991
89. Waldron-Lynch F, Henegariu O, Deng S, Preston-Hurlburt P, Tooley J, Flavell R, et al. Teplizumab Induces Human Gut-Tropic Regulatory Cells in Humanized Mice and Patients. Sci Trans Med (2012) 4:118ra112–118ra112. doi: 10.1126/scitranslmed.3003401
90. Imam S, Mirmira RG, Jaume JC. Eukaryotic Translation Initiation Factor 5A Inhibition Alters Physiopathology and Immune Responses in a “Humanized” Transgenic Mouse Model of Type 1 Diabetes. Am J Physiology-Endocrinol Metab (2014) 306:E791–8. doi: 10.1152/ajpendo.00537.2013
91. Koboziev I, Jones-Hall Y, Valentine JF, Reinoso Webb C, Furr KL, Grisham MB. Use of Humanized Mice to Study the Pathogenesis of Autoimmune and Inflammatory Diseases. Inflamm Bowel Dis (2015) 21:1652–73. doi: 10.1097/MIB.0000000000000446
92. Ma H, Wert KJ, Shvartsman D, Melton DA, Jaenisch R. Establishment of Human Pluripotent Stem Cell-Derived Pancreatic Beta-Like Cells in the Mouse Pancreas. Proc Natl Acad Sci USA (2018) 115:3924–9. doi: 10.1073/pnas1702059115
93. Hu M, Hawthorne WJ, Nicholson L, Burns H, Qian YW, Liuwantara D, et al. Low-Dose Interleukin-2 Combined With Rapamycin Led to an Expansion of CD4(+)CD25(+)FOXP3(+) Regulatory T Cells and Prolonged Human Islet Allograft Survival in Humanized Mice. Diabetes (2020) 69:1735–48. doi: 10.2337/db19-0525
94. Lee FT, Dangi A, Shah S, Burnette M, Yang YG, Kirk AD, et al. Rejection of Xenogeneic Porcine Islets in Humanized Mice Is Characterized by Graft-Infiltrating Th17 Cells and Activated B Cells. Am J Transplant (2020) 20:1538–50. doi: 10.1111/ajt.15763
95. Szot GL, Yadav M, Lang J, Kroon E, Kerr J, Kadoya K, et al. Tolerance Induction and Reversal of Diabetes in Mice Transplanted With Human Embryonic Stem Cell-Derived Pancreatic Endoderm. Cell Stem Cell (2015) 16:148–57. doi: 10.1016/j.stem.2014.12.001
96. Wu H, Wen D, Mahato RI. Third-Party Mesenchymal Stem Cells Improved Human Islet Transplantation in a Humanized Diabetic Mouse Model. Mol Ther (2013) 21:1778–86. doi: 10.1038/mt.2013.147
97. Parent AV, Faleo G, Chavez J, Saxton M, Berrios DI, Kerper NR, et al. Selective Deletion of Human Leukocyte Antigens Protects Stem Cell-Derived Islets From Immune Rejection. Cell Rep (2021) 36:109538. doi: 10.1016/j.celrep.2021.109538
98. Zaldumbide A, Alkemade G, Carlotti F, Nikolic T, Abreu JR, Engelse MA, et al. Genetically Engineered Human Islets Protected From CD8-Mediated Autoimmune Destruction In Vivo. Mol Ther (2013) 21:1592–601. doi: 10.1038/mt.2013.105
99. Wolf-Van Buerck L, Schuster M, Oduncu F, Baehr A, Mayr T, Guethoff S, et al. LEA29Y Expression in Transgenic Neonatal Porcine Islet-Like Cluster Promotes Long-Lasting Xenograft Survival in Humanized Mice Without Immunosuppressive Therapy. Sci Rep (2017) 7:1–9. doi: 10.1038/s41598-017-03913-4
100. Crespo J, Koh YT, Hu N, Moore PA, Bonvini E, Glasebrook AL, et al. A Humanized CD3epsilon-Knock-in Mouse Model for Pre-Clinical Testing of Anti-Human CD3 Therapy. PloS One (2021) 16:e0245917. doi: 10.1371/journal.pone.0245917
101. Besancon A, Goncalves T, Valette F, Dahllof MS, Mandrup-Poulsen T, Chatenoud L, et al. Oral Histone Deacetylase Inhibitor Synergises With T Cell Targeted Immunotherapy to Preserve Beta Cell Metabolic Function and Induce Stable Remission of New-Onset Autoimmune Diabetes in NOD Mice. Diabetologia (2018) 61:389–98. doi: 10.1007/s00125-017-4459-0
102. Schloss J, Ali R, Babad I, Guerrero-Ros J, Pongsachai L-Z, He T, et al. Development and Characterization of a Preclinical Model for the Evaluation of CD205-Mediated Antigen Delivery Therapeutics in Type 1 Diabetes. ImmunoHorizons 3 (2019) 236–253.
103. Vieira A, Courtney M, Druelle N, Avolio F, Napolitano T, Hadzic B, et al. β-Cell Replacement as a Treatment for Type 1 Diabetes: An Overview of Possible Cell Sources and Current Axes of Research. Diabetes Obes Metab (2016) 18:137–43. doi: 10.1111/dom.12721
Keywords: preclinical model, humanized model mouse, type I diabetes, HLA, autoantigens, antigen-specific immunotherapy, T cell assay, islet engraftment
Citation: Houeiss P, Boitard C and Luce S (2022) Preclinical Models to Evaluate the Human Response to Autoantigen and Antigen-Specific Immunotherapy in Human Type 1 Diabetes. Front. Endocrinol. 13:883000. doi: 10.3389/fendo.2022.883000
Received: 24 February 2022; Accepted: 14 March 2022;
Published: 13 April 2022.
Edited by:
Swetha Gopalakrishnan, University of Helsinki, FinlandReviewed by:
Isabelle Serr, Helmholtz Association of German Research Centres (HZ), GermanyFrancesca Pala, National Institute of Allergy and Infectious Diseases (NIH), United States
Copyright © 2022 Houeiss, Boitard and Luce. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Boitard, Q2hyaXN0aWFuLmJvaXRhcmRAaW5zZXJtLmZy
 Pamela Houeiss
Pamela Houeiss Christian Boitard1,2*
Christian Boitard1,2* Sandrine Luce
Sandrine Luce