- 1Reproduction, Mother and Child Health, Centre de recherche du centre hospitalier universitaire de Québec, Université Laval, Québec City, QC, Canada
- 2Centre de recherche en Reproduction, Développement et Santé Intergénérationnelle, Department of Obstetrics, Gynecology, and Reproduction, Faculty of Medicine, Université Laval, Québec City, QC, Canada
Cell differentiation and acquisition of specialized functions are inherent steps in events that lead to normal tissue development and function. These processes require accurate temporal, tissue, and cell-specific activation or repression of gene transcription. This is achieved by complex interactions between transcription factors that form a unique combinatorial code in each specialized cell type and in response to different physiological signals. Transcription factors typically act by binding to short, nucleotide-specific DNA sequences located in the promoter region of target genes. In males, Leydig cells play a crucial role in sex differentiation, health, and reproductive function from embryonic life to adulthood. To better understand the molecular mechanisms regulating Leydig cell differentiation and function, several transcription factors important to Leydig cells have been identified, including some previously unknown to this specialized cell type. This mini review summarizes the current knowledge on transcription factors in fetal and adult Leydig cells, describing their roles and mechanisms of action.
1 Introduction
Localized in the testicular interstitium, Leydig cells are the principal source of testosterone and insulin-like 3 (INSL3), two hormones that regulate male reproductive development and function. In mammals, there are at least two distinct populations of Leydig cells, fetal Leydig cells (FLC) and adult Leydig cells (ALC), which are responsible for the synthesis of steroid hormones in the prenatal and postnatal testes, respectively [reviewed in (1, 2)]. Steroidogenesis is a multi-step process requiring various transporters and enzymes to convert cholesterol into a steroid hormone [reviewed in (3)]. The expression of the genes coding for these steroidogenic proteins is finely regulated to avoid steroid hormone insufficiency or excess across the lifespan.
Transcription factors (TFs) are fundamental to the regulation of gene expression. They are specialized proteins that recognize and bind to regulatory DNA sequences, modulating the rate of gene transcription [reviewed in (4)]. TFs typically recruit or interact with other TFs forming a unique molecular code that is key for specifying temporal- and tissue-specific gene expression as well as hormone responsiveness in hormone-sensitive target tissues. Moreover, TFs exhibit a dynamic behaviour that is characterized by their ability to interact with various partner proteins and to regulate different target genes according to many determinants such as cell type, development stage, and signal stimulus, among others.
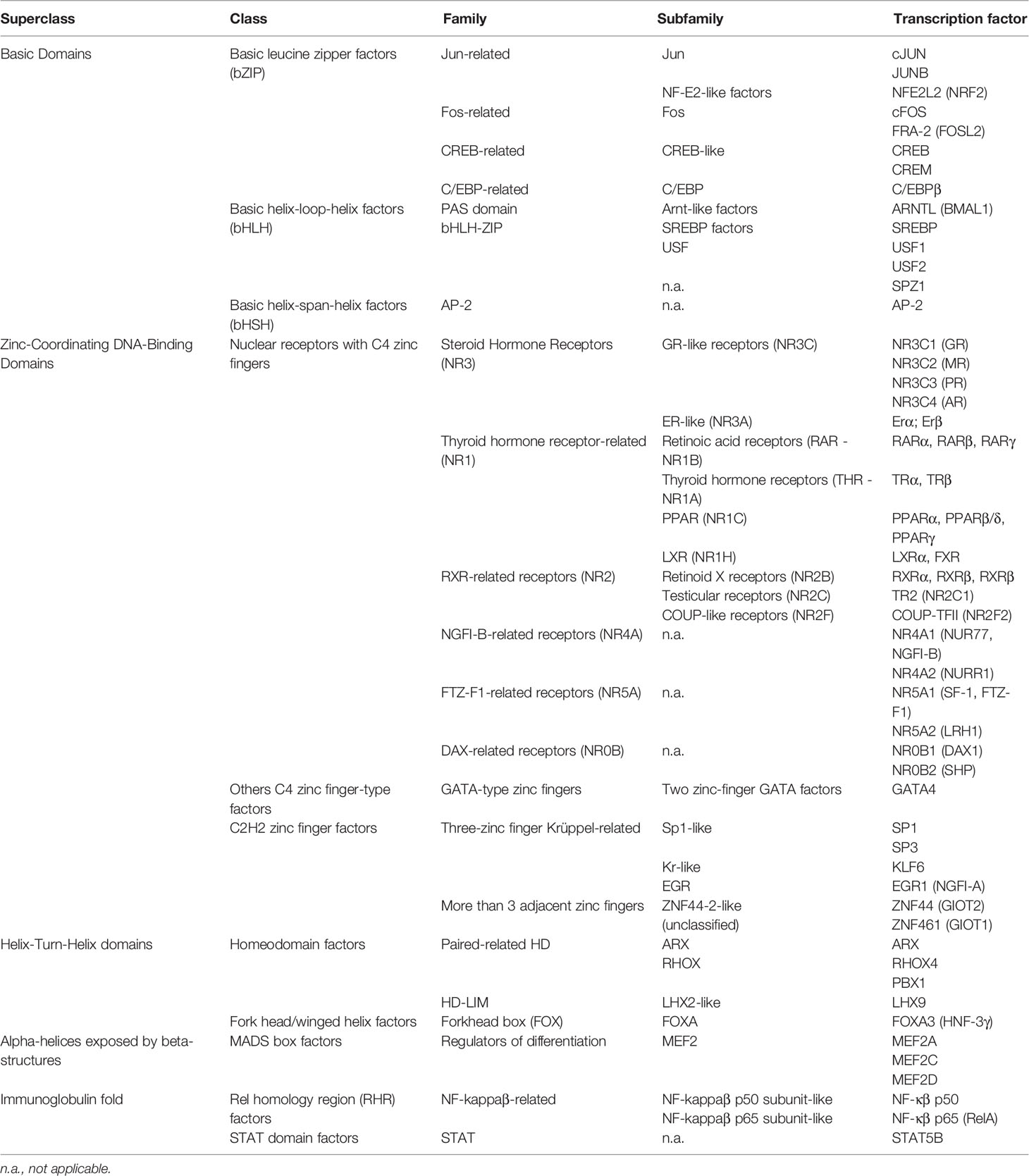
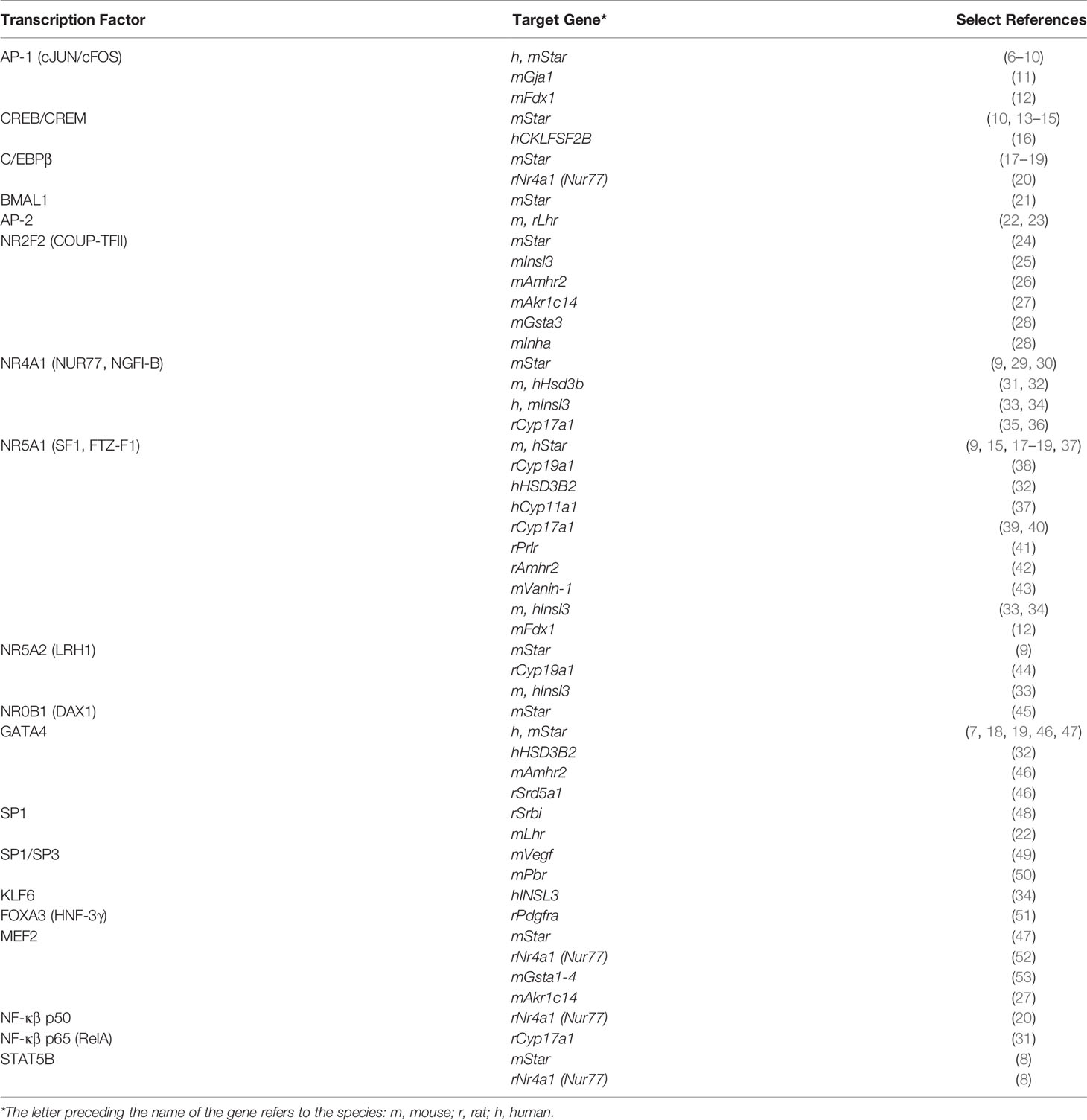
In recent years, the development of novel and powerful methodological approaches in molecular genetics has led to the emergence of new information regarding the role of TFs in the regulation of Leydig cell differentiation and function, and by extension, in male fertility and reproductive health. In this mini review, we provide a brief overview of the roles and mechanisms of action of some of the most characterized TFs in Leydig cells. We have adopted the most recent classification of TFs, which is based both on amino acid sequence homology and the tertiary structure of their DNA-binding domains (5). Using this classification, TFs that have been identified in Leydig cells are presented in Table 1; Table 2 lists the target genes for these TFs in Leydig cells.
2 Superclass of Basic Domains
2.1 Class of Basic Leucine Zipper Factors (BZIP)
2.1.1 AP-1 Factors
The activator protein 1 (AP-1) is a dimeric complex that includes members of the JUN, FOS, activating transcription factor (ATF), and musculoaponeurotic fibrosarcoma (MAF) families of TFs (54). Among the AP-1 members, JUN and FOS are the best characterized. The JUN subfamily comprises three members (cJUN, JUNB, and JUND) while four members compose the FOS subfamily [cFOS, FOSB, Fos-related antigens 1 (FRA-1, FOSL1), and Fos-related antigens 2 (FRA-2, FOSL2)]. Members of the JUN family can homodimerize or heterodimerize, whereas FOS family members only form heterodimers. The DNA sequence recognized by AP-1 members differs according to the dimer involved. JUN : JUN and FOS : JUN dimers recognize the TPA-response element (TRE; TGA(C/G)TCA) and the cAMP-responsive element (CRE; TGACGTCA), whereas ATF dimers preferentially recognize the CRE motif, and MAF dimers bind to MAF recognition elements (MAREs), a long palindromic sequence that contains TRE or CRE motifs (55) [reviewed in (56)].
AP-1 members were first described in Leydig cells in the late 1990s (57). AP-1 factors regulate several genes in Leydig cells such as the steroidogenic acute regulatory protein (Star) gene, which is activated by cJUN (6, 7, 12). In addition, cJUN cooperates with other TFs, including GATA4, STAT5B, and NUR77 leading to a stronger activation of the Star promoter (7–9). Both cJUN and cFOS regulate Star promoter activity by recruiting CREB and CBP (10). Transcription of the gap junction protein alpha1 [Gja1, also known as connexin43 (Cx43)] gene, involved in the initiation and maintenance of sperm production, is also controlled by cJUN, JUNB, and FOSL2, and by a cJUN/cFOS cooperation (11, 58). Furthermore, the ferredoxin 1 (Fdx1) promoter is activated by a cJUN/SF1 cooperation (12). Fdx1 is a partner of Cyp11a1, participating in the conversion of cholesterol into pregnenolone, the first and rate-limiting step in steroidogenesis. It is important to note that the nature of the cJUN dimerization partner influences its role in gene regulation. For example, the combination of either FOSL2 or cFOS with cJUN inhibits the stimulatory effect of cJUN on the Star promoter (6, 10, 59). AP-1 factors in Leydig cells have been reviewed elsewhere (56).
2.1.2 CREB-Related Factors
CREB-related factors include three members: CRE-binding protein (CREB), cAMP response element modulator (CREM), and CRE-activating transcription factor (ATF-1). CREB factors homodimerize and heterodimerize with other CREB members and with other bZIP TFs, such as AP-1 members (60). CREB factors regulate transcription by binding to a CRE motif (TGACGTCA) similar to that recognized by AP-1 members, leading to overlap and redundancy in their activities (61). Although CREM is the most abundant member in MA-10 Leydig cells, all CREB members activate Star transcription through CRE elements located in the proximal promoter region (13, 14). Moreover, CREB factors cooperate with SF1 (NR5A1, Ad4BP) to enhance Star transcription (15). CREB also stimulates CKLFSF2B promoter activity in response to LH/cAMP (16). Cklfsf2b codes for a protein that inhibits steroidogenesis in Leydig cells (16). Therefore, CREB is involved in both activation and repression of steroidogenesis in Leydig cells depending on its target genes.
2.1.3 C/EBP-Related Factors
Members of the CCAAT/enhancer binding protein (C/EBP) subfamily contain a bZIP DNA-binding domain and regulate gene expression by binding to the sequence (A/G)TTGCG(C/T)AA(C/T) as homo- or heterodimers (62). C/EBPβ is the predominant member in Leydig cells (17, 63) where it activates Star transcription alone and in cooperation with SF1 and GATA4 (17–19). C/EBPβ also cooperates with NF-κβ p50 to stimulate Nur77 promoter activity in Leydig cells (20). The Nur77 gene encodes the orphan nuclear receptor NUR77, which regulates several genes involved in steroidogenesis in Leydig cells (see Section 3.1.2, NGFI-B/NR4A Receptors, below).
3 Superclass of Zinc-Coordinating DNA-Binding Domains
3.1 Class of Nuclear Receptors With C4 Zinc Fingers
TFs belonging to the nuclear receptor class respond to extracellular and intracellular signals to regulate gene expression. They also regulate cellular functions within the cytoplasm (64). In this section we present the nuclear receptors for which the roles and mechanisms of action are, or have begun to be, characterized in Leydig cells. Detailed information can be found in a review article dedicated to nuclear receptors in Leydig cells (65).
3.1.1 COUP-Like/NR2F Receptors
The nuclear receptor subclass 2, group F (NR2F) subfamily consists of three members: chicken ovalbumin upstream promoter transcription factor I (COUP-TFI, NR2F1, EAR3), COUP-TFII (NR2F2, ARP1) and COUP-TFIII (NR2F6, EAR2). NR2Fs have been implicated in various physiological and developmental processes by regulating the expression of numerous genes [reviewed in (66, 67)]. Via their double zinc finger DNA-binding domain, NR2F factors bind as monomers to the nuclear receptor element AGGTCA and its variants. They also bind as dimers to direct (DR), inverted (IR), and everted (ER) repeats separated by 1-12 nucleotides (68).
Of the NR2F subfamily members, COUP-TFII is by far the most abundant in Leydig cells. Although COUP-TFII is present in mice interstitial cells from early fetal life throughout adulthood, it is only associated with steroidogenically active ALC in postnatal life (24). COUP-TFII is a marker of stem cells giving rise to the ALC population (24, 69). In vivo studies using mouse models have shown that COUP-TFII is crucial for Leydig cell development and male reproductive function (70, 71). In Leydig cells, COUP-TFII regulates the expression of several genes involved in lipid metabolism, male gonad development, and steroidogenesis (28). COUP-TFII activates Star, Insl3, and Amhr2 expression by binding to their respective promoter sequences (24–26). It cooperates with SF1 on the Star and Insl3 promoters (24, 25) and with SP1 on the Amhr2 promoter (26). The Akr1c14 gene, which codes for the 3α-HSD enzyme that catalyzes the interconversion of dihydrotestosterone (DHT) into 5α-androstane-3α,17β-diol (3α-diol), is activated by COUP-TFII in cooperation with MEF2 (27). COUP-TFII also activates the expression of Gsta3 and Inha, genes involved in the inactivation of reactive oxygen species and in the homeostasis of the hypothalamic-pituitary-gonadal axis, respectively (28). Expression of several other Leydig cell genes including Cyp17a1, Hsd3b1 and Cyp11a1 is reduced in Coup-tfii null mice (71) and in COUP-TFII-depleted MA-10 Leydig cells (28), implying a role for COUP-TFII in their expression.
3.1.2 NGFI-B/NR4A Receptors
The NR4A family consists of three orphan nuclear receptors: neuron-derived clone 77 (NR4A1, NUR77, NGFI-B, TR3), nuclear receptor related 1 (NR4A2, NURR1) and neuron-derived orphan receptor 1 (NR4A3, NOR1). NR4A members can bind to DNA either as monomers, homodimers, or heterodimers. NUR77 and NURR1 also heterodimerize with RXR. As monomers, they bind to a NGFI-B-response element (NBRE; AAAGGTCA), as homodimers and heterodimers to a Nur-response element (NurRE; TGATATTTN6AAATGCCA), and as heterodimers with RXR to a DR5 sequence [reviewed in (72, 73)]. NR4A factors are immediate early response genes involved in the regulation of several physiological and pathological processes, including steroidogenesis (74) [reviewed in (75)].
Leydig cells contain mainly NUR77, followed by NURR1 where both are important regulators of basal and hormone-induced gene transcription (76). Nur77 expression is strongly increased by LH (76) via the CAMKI pathway (29, 77) consistent with its role as a key regulator of several genes in Leydig cells including Cyp17a1 (31, 35), Hsd3b (31), HSD3B2 (32), Insl3 (33, 34), and Star (29, 30). NUR77 regulates the expression of these genes by cooperating with CAMKI (29), cJUN (9), KLF6 (34), and SF1 (34). In Leydig cells, Nur77 expression is controlled by distinct regulatory elements for both basal and hormone-induced expression (77), through mechanisms involving MEF2 (52), STAT5B (8), CREB (77), cJUN (9), C/EBPβ (20), and NF-κβ p50 (20).
3.1.3 FTZ-F1-Related/NR5A Receptors
The nuclear receptor 5A (NR5A) family comprises two members: steroidogenic factor 1 (NR5A1, Ad4BP, SF1) and liver receptor homolog 1 (NR5A2, LRH1, FTF). Both factors share high sequence similarity, bind to the same DNA motif, regulate common target steroidogenic genes, and exhibit overlapping expression in several tissues [reviewed in (78, 79)]. Despite this, they have nonredundant roles and cannot fully compensate for each other [reviewed in (78, 79)]. NR5A members regulate gene expression by binding as monomers to the sequence (T/C)CAAGGTCA located in the promoter region of target genes.
SF1 was initially identified as a tissue-specific activator of several cytochrome P450 steroid hydroxylase genes (38, 80). SF1 is essential for steroidogenesis, reproduction, and male sex differentiation, as revealed by mutations in the SF1 gene in humans and in mouse models where adrenal and gonadal development and function are impaired (37, 81–84) [reviewed in (85, 86)]. Interestingly, Sf1 knockdown in MLTC-1 Leydig cells leads to downregulation of Star and Cyp11a1 and accumulation of neutral lipids and cholesterol (37). Moreover, SF1 is one of only a handful of TFs that can convert fibroblasts into functional Leydig-like cells, revealing the pivotal role of this nuclear receptor in Leydig cells (87, 88).
In vitro analysis of regulatory elements has shown that the expression of several Leydig cell genes is regulated by SF1. These include Star (9, 17, 37), Cyp19a1 (38), HSD3B2 (32), Cyp17a1 (39, 40), Cyp11a1 (37), Prlr (41), Amhr2 (42), Vanin-1 (43), Insl3 (33), and Fdx1 (12). SF1 activity relies on interactions with a long list of protein partners, such as C/EBPβ (17), cJUN (9, 12), DAX1 (45), GATA4 (89), and KLF6 (34).
Like SF1, LRH1 influences steroidogenesis and fertility. To date, only a few genes are known to be regulated by LRH1 in Leydig cells, including Star (in cooperation with cJUN) (9), Cyp19a1 (44), and Insl3 (33).
3.1.4 DAX-Related/NR0B Receptors
The DAX-related receptor (NR0B) family comprises two members: critical region on the X chromosome gene 1 (NR0B1, DAX1) and small heterodimer partner (NR0B2, SHP). They lack the typical zinc finger DNA-binding domain and therefore act mainly as transcriptional repressors by inhibiting the activity of other TFs (90, 91). Both members are present in Leydig cells and act as homodimers or heterodimers (92).
In Dax1-deficient mice, testis cord organization is compromised and FLC development is arrested (93). In vitro studies in Leydig cell lines revealed that DAX1 represses steroidogenesis by inhibiting Star expression, while silencing Dax1 expression increases Star transcription leading to enhanced steroidogenesis (45). DAX1 interacts with and represses the activity of NUR77 and SF1, inhibiting Star expression (36, 45). Interestingly, Dax1 knockdown in MA-10 Leydig cells decreases Cyp11a1 and Star expression suggesting that DAX1 could also act as a coactivator in addition to its repressor role (94).
SHP is a repressor of steroidogenesis. In mouse Leydig cells, Shp expression is reduced by hCG treatment (95). In Shp-deficient mice, testosterone levels as well as Star, Cyp11a1, and Hsd3b1 mRNA levels are increased leading to premature sexual maturation (96). SHP inhibits steroidogenesis by interacting and repressing the activity of LHR1 (96). Shp mRNA levels are significantly reduced in COUP-TFII- and MEF2-depleted Leydig cells, indicating that Shp expression requires these two TFs (28, 97).
3.2 Class of Other C4 Zinc Finger-Type Factors
3.2.1 Two Zinc-Finger GATA Factors
The six GATA members (GATA1 to 6) are crucial for the development and function of several tissues, including the male gonad [reviewed in (98, 99)]. GATA factors regulate gene expression by binding via their two zinc fingers to the DNA sequence (A/T)GATA(A/G) in the promoter region of target genes. Of the six GATA factors, GATA4 is the most abundant in Leydig cells in vivo (100–102). Its expression is also the broadest being present from the onset of testis morphogenesis and into adult life (103). Considered one of the first gonadal markers in both sexes, GATA4 is required for urogenital ridge development in mice and later for mammalian gonadal differentiation (103, 104).
A Sf1-Cre mouse line, which expresses the Cre recombinase in several tissues including Leydig, Sertoli and adrenal cells, was used to conditionally inactivate Gata4. The resulting males were undervirilized and had small testes lacking mature sperm (105), thereby supporting a role for this factor in male reproductive function. Transcriptomic analysis of GATA4-depleted MA-10 Leydig cells revealed several deregulated pathways, including cholesterol metabolism and steroidogenesis (46). Consistent with this, GATA4 stimulates the transcription of several genes expressed in Leydig cells such as HSD3B2 (32), Cyp19a1 (106), Star (46, 106), Inha (106), Sf1 (106), Amhr2 (46), and Srd5a1 (46). GATA4 also cooperates with cJUN, C/EBPβ, and MEF2 to upregulate Star expression (7, 18, 47). These results emphasize the indispensable role of GATA4 in the differentiation and function of FLC and ALC (46, 107). The critical nature of GATA4 in the Leydig cell differentiation is further supported by the demonstration that GATA4, along with SF1 and DMRT1 or NUR77, are sufficient to reprogram fibroblasts toward the Leydig-like cell fate (87, 88).
4 Superclass of Helix-Turn-Helix Domains
4.1 Class of Forkhead/Winged Helix Factors
4.1.1 Forkhead Box (FOX) Factors
The forkhead box A3 (FOXA3) is the only member of the FOXA subfamily present in the testes, mainly in ALC (51, 108, 109). So far, the only direct target identified for FOXA3 in Leydig cells is the gene coding for the platelet-derived growth factor receptor alpha (Pdgfra) (51), that in response to PDGF signaling, acts in Leydig cell differentiation and testis organogenesis (110). In cAMP-induced steroidogenesis, FOXA3 is proposed to repress Nur77 expression, which in turn reduces steroidogenic gene expression and testosterone production (111). These findings indicate that FOXA3 participates actively in the control of Leydig cell function and male fertility.
5 Superclass of α-Helices Exposed by β-Structures
5.1 Class of MADS Box Factors
5.1.1 MEF2 Subfamily
The Myocyte Enhancer Factor 2 (MEF2) factor subfamily comprises four members (MEF2A-2D) that share two highly conserved domains, a MADS box and a MEF2 domain, involved in dimerization and DNA binding [reviewed in (112)]. MEF2 factors form homo- and heterodimers that bind the sequence YTAWWWWTAR (Y=C/T, W=A/T, R=G/A) in the promoter region of their target genes. Because of their conserved DNA-binding domain, MEF2 members share common targets and can compensate for each other. MEF2 members also display unique spatiotemporal patterns in different tissues. Due to their divergent transactivation domain, MEF2 factors respond to different signals and interact with different partners, leading to specific gene expression [reviewed in (112)].
Although widely studied in other organs, the presence of MEF2 in the testes, more specifically in Sertoli and Leydig cells, was only reported in 2014 (52). In Leydig cells, MEF2A and MEF2D and to a lesser extent MEF2C, are expressed from early gonadal development into adulthood (52). MEF2A/2D-depleted MA-10 Leydig cells produce less steroid hormone demonstrating that MEF2 factors have a role in male reproductive function (47). Consistent with this, microarray analysis of MEF2A/2D-depleted MA-10 Leydig cells identified several differently regulated genes known to be involved in fertility, gonad morphology, and steroidogenesis (97). To date, direct gene targets for MEF2 factors in Leydig cells include Nur77 (52), Gsta1-4 (53), Star (involving a MEF2/GATA4 cooperation) (47), and Akr1c14 (through a cooperation with COUP-TFII) (27). The complete network of genes regulated by MEF2 factors in Leydig cells as well as MEF2 interacting partners remain to be fully elucidated.
6 Superclass of Immunoglobulin Fold
6.1 Class of STAT Domain Factors
6.1.1 STAT Factors
The signal transducer and activator of transcription (STAT) family consists of seven proteins [reviewed in (113)]. Cytokines and growth factors activate STAT members through the Janus kinase (JAK) signaling pathway. In the nucleus, STAT factors regulate gene transcription by binding as homo- or heterodimers to the γ-interferon-activated sequence (GAS; TTCN3GAA) in the promoter region of target genes. So far, STAT5B is the only STAT factor identified in Leydig cells (114). In these cells, STAT5B is activated by growth hormone, an important regulator of steroidogenesis (8). STAT5B activates Star expression directly by binding to a GAS element and in cooperation with cJUN (8). STAT5B also activates the Nur77 promoter (8).
7 Other Transcription Factors Present in Leydig Cells
Other TFs have been described in Leydig cells, but their mechanisms of action remain poorly characterized. This includes the nuclear factor E2-related factor-2 (NRF2, NFE2l2), which is an important modulator of reactive oxygen species levels, especially in aging Leydig cells (115–117). Furthermore, the brain and muscle arnt-like protein-1 (BMAL1), a component of the circadian clock system, is also directly involved in the control of Leydig cell function in different species, by regulating the expression of Star, Hsb3b, and Cyp11a1 (21, 118, 119). Finally, members of the nuclear factor kappa-beta (NF-κβ) family, involved in immune and inflammatory responses, also contribute to the regulation of steroidogenesis in Leydig cells (20, 31, 120).
8 Concluding Remarks
As described in this mini review, several TFs belonging to different classes and families are pivotal to ensure proper Leydig cell differentiation and function. This underscores the complex regulatory mechanisms involved. Most of the knowledge acquired so far has relied on in vitro analyses of regulatory elements of genes expressed in Leydig cells. Although we are far from fully understanding all the signals, pathways, and TFs involved, technological advances and novel mouse models will certainly lead to significant discoveries in the coming years.
Author Contributions
KM wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Supported by a grant from the Canadian Institutes of Health Research (funding reference number MOP-81387) to JT. KM is the recipient of a studentship from the Fonds de recherche du Québec-Santé.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shima Y. Development of Fetal and Adult Leydig Cells. Reprod Med Biol (2019) 18:323–30. doi: 10.1002/rmb2.12287
2. Teerds KJ, Huhtaniemi IT. Morphological and Functional Maturation of Leydig Cells: From Rodent Models to Primates. Hum Reprod Update (2015) 21:310–28. doi: 10.1093/humupd/dmv008
3. Selvaraj V, Stocco DM, Clark BJ. Current Knowledge on the Acute Regulation of Steroidogenesis. Biol Reprod (2018) 99:13–26. doi: 10.1093/biolre/ioy102
4. Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, et al. The Human Transcription Factors. Cell (2018) 172:650–65. doi: 10.1016/j.cell.2018.01.029
5. Wingender E, Schoeps T, Haubrock M, Krull M, Dönitz J. TFClass: Expanding the Classification of Human Transcription Factors to Their Mammalian Orthologs. Nucleic Acids Res (2018) 46:D343–7. doi: 10.1093/nar/gkx987
6. Manna PR, Eubank DW, Stocco DM. Assessment of the Role of Activator Protein-1 on Transcription of the Mouse Steroidogenic Acute Regulatory Protein Gene. Mol Endocrinol (2004) 18:558–73. doi: 10.1210/me.2003-0223
7. Martin LJ, Bergeron F, Viger RS, Tremblay JJ. Functional Cooperation Between GATA Factors and cJun on the Star Promoter in MA-10 Leydig Cells. J Androl (2012) 33:81–7. doi: 10.2164/jandrol.110.012039
8. Hébert-Mercier P-O, Bergeron F, Robert NM, Mehanovic S, Pierre KJ, Mendoza-Villarroel RE, et al. Growth Hormone-Induced STAT5B Regulates Star Gene Expression Through a Cooperation With cJun in Mouse MA-10 Leydig Cells. Endocrinology (2022) 163:1–13. doi: 10.1210/endocr/bqab267
9. Martin LJ, Tremblay JJ. The Nuclear Receptors NUR77 and SF1 Play Additive Roles With C-JUN Through Distinct Elements on the Mouse Star Promoter. J Mol Endocrinol (2009) 42:119–29. doi: 10.1677/JME-08-0095
10. Manna PR, Stocco DM. Crosstalk of CREB and Fos/Jun on a Single Cis-Element: Transcriptional Repression of the Steroidogenic Acute Regulatory Protein Gene. J Mol Endocrinol (2007) 39:261–77. doi: 10.1677/JME-07-0065
11. Ghouili F, Martin LJ. Cooperative Regulation of Gja1 Expression by Members of the AP-1 Family cJun and cFos in TM3 Leydig and TM4 Sertoli Cells. Gene (2017) 635:24–32. doi: 10.1016/j.gene.2017.09.017
12. Roumaud P, Rwigemera A, Martin LJ. Transcription Factors SF1 and cJun Cooperate to Activate the Fdx1 Promoter in MA-10 Leydig Cells. J Steroid Biochem Mol Biol (2017) 171:121–32. doi: 10.1016/j.jsbmb.2017.03.003
13. Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, et al. Regulation of Steroidogenesis and the Steroidogenic Acute Regulatory Protein by a Member of the cAMP Response-Element Binding Protein Family. Mol Endocrinol (2002) 16:184–99. doi: 10.1210/mend.16.1.0759
14. Clem BF, Hudson EA, Clark BJ. Cyclic Adenosine 3′,5′-Monophosphate (cAMP) Enhances cAMP-Responsive Element Binding (CREB) Protein Phosphorylation and Phospho-CREB Interaction With the Mouse Steroidogenic Acute Regulatory Protein Gene Promoter. Endocrinology (2005) 146:1348–56. doi: 10.1210/en.2004-0761
15. Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM. Transcriptional Regulation of the Mouse Steroidogenic Acute Regulatory Protein Gene by the cAMP Response-Element Binding Protein and Steroidogenic Factor 1. J Mol Endocrinol (2003) 30:381–97. doi: 10.1677/jme.0.0300381
16. Kumar S, Kang H, Park E, Park HS, Lee K. The Expression of CKLFSF2B Is Regulated by GATA1 and CREB in the Leydig Cells, Which Modulates Testicular Steroidogenesis. Biochim Biophys Acta - Gene Regul Mech (2018) 1861:1063–75. doi: 10.1016/j.bbagrm.2018.10.002
17. Reinhart AJ, Williams SC, Clark BJ, Stocco DM. SF-1 (Steroidogenic Factor-1) and C/EBP Beta (CCAAT/Enhancer Binding Protein-Beta) Cooperate to Regulate the Murine Star (Steroidogenic Acute Regulatory) Promoter. Mol Endocrinol (1999) 13:729–41. doi: 10.1210/mend.13.5.0279
18. Tremblay JJ, Hamel F, Viger RS. Protein Kinase A-Dependent Cooperation Between GATA and CCAAT/Enhancer-Binding Protein Transcription Factors Regulates Steroidogenic Acute Regulatory Protein Promoter Activity. Endocrinology (2002) 143:3935–45. doi: 10.1210/en.2002-220413
19. Hu Y, Dong C, Chen M, Lu J, Han X, Qiu L, et al. Low-Dose Monobutyl Phthalate Stimulates Steroidogenesis Through Steroidogenic Acute Regulatory Protein Regulated by SF-1, GATA-4 and C/EBP-Beta in Mouse Leydig Tumor Cells. Reprod Biol Endocrinol (2013) 11:1–1. doi: 10.1186/1477-7827-11-72
20. El-Asmar B, Giner XC, Tremblay JJ. Transcriptional Cooperation Between NF-Kb P50 and CCAAT/Enhancer Binding Protein Beta Regulates Nur77 Transcription in Leydig Cells. J Mol Endocrinol (2009) 42:131–8. doi: 10.1677/JME-08-0016
21. Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, et al. The Circadian Clock Protein BMAL1 Is Necessary for Fertility and Proper Testosterone Production in Mice. J Biol Rhythm (2008) 23:26–36. doi: 10.1177/0748730407311254
22. Nikula H, Koskimies P, El-Hefnawy T, Huhtaniemi I. Functional Characterization of the Basal Promoter of the Murine LH Receptor Gene in Immortalized Mouse Leydig Tumor Cells. J Mol Endocrinol (2001) 26:21–9. doi: 10.1677/jme.0.0260021
23. Tsai-Morris CH, Geng Y, Xie XZ, Buczko E, Dufau ML. Transcriptional Protein Binding Domains Governing Basal Expression of the Rat Luteinizing Hormone Receptor Gene. J Biol Chem (1994) 269:15868–75. doi: 10.1016/S0021-9258(17)40761-7
24. Mendoza-Villarroel RE, Robert NM, Martin LJ, Brousseau C, Tremblay JJ. The Nuclear Receptor NR2F2 Activates Star Expression and Steroidogenesis in Mouse MA-10 and MLTC-1 Leydig Cells. Biol Reprod (2014) 91:26. doi: 10.1095/biolreprod.113.115790
25. Mendoza-Villarroel RE, Di-Luoffo M, Camiré E, Giner XC, Brousseau C, Tremblay JJ. The INSL3 Gene Is a Direct Target for the Orphan Nuclear Receptor, COUP-TFII, in Leydig Cells. J Mol Endocrinol (2014) 53:43–55. doi: 10.1530/JME-13-0290
26. Mehanovic S, Mendoza-Villarroel RE, Viger RS, Tremblay JJ. The Nuclear Receptor COUP-TFII Regulates Amhr2 Gene Transcription via a GC-rich Promoter Element in Mouse Leydig Cells. J Endocr Soc (2019) 3:2236–57. doi: 10.1210/js.2019-00266
27. Di-Luoffo M, Brousseau C, Tremblay JJ. MEF2 and NR2F2 Cooperate to Regulate Akr1c14 Gene Expression in Mouse MA-10 Leydig Cells. Andrology (2016) 4:335–44. doi: 10.1111/andr.12150
28. Mehanovic S, Mendoza-Villarroel RE, de Mattos K, Talbot P, Viger RS, Tremblay JJ. Identification of Novel Genes and Pathways Regulated by the Orphan Nuclear Receptor COUP-TFII in Mouse MA-10 Leydig Cells. Biol Reprod (2021) 105:1283–306. doi: 10.1093/biolre/ioab131
29. Martin LJ, Boucher N, Brousseau C, Tremblay JJ. The Orphan Nuclear Receptor NUR77 Regulates Hormone-Induced Star Transcription in Leydig Cells Through Cooperation With Ca2+/Calmodulin-Dependent Protein Kinase I. Mol Endocrinol (2008) 22:2021–37. doi: 10.1210/me.2007-0370
30. Manna PR, Huhtaniemi IT, Stocco DM. Mechanisms of Protein Kinase C Signaling in the Modulation of 3’,5’-Cyclic Adenosine Monophosphate-Mediated Steroidogenesis in Mouse Gonadal Cells. Endocrinology (2009) 150:3308–17. doi: 10.1210/en.2008-1668
31. Hong CY, Park JH, Ahn RS, Im SY, Choi H-S, Soh J, et al. Molecular Mechanism of Suppression of Testicular Steroidogenesis by Proinflammatory Cytokine Tumor Necrosis Factor Alpha. Mol Cell Biol (2004) 24:2593–604. doi: 10.1128/mcb.24.7.2593-2604.2004
32. Martin LJ, Taniguchi H, Robert NM, Simard J, Tremblay JJ, Viger RS. GATA Factors and the Nuclear Receptors, Steroidogenic Factor 1/Liver Receptor Homolog 1, Are Key Mutual Partners in the Regulation of the Human 3β-Hydroxysteroid Dehydrogenase Type 2 Promoter. Mol Endocrinol (2005) 19:2358–70. doi: 10.1210/me.2004-0257
33. Robert NM, Martin LJ, Tremblay JJ. The Orphan Nuclear Receptor NR4A1 Regulates Insulin-Like 3 Gene Transcription in Leydig Cells. Biol Reprod (2006) 74:322–30. doi: 10.1095/biolreprod.105.044560
34. Tremblay MA, Mendoza-Villarroel RE, Robert NM, Bergeron F, Tremblay JJ. KLF6 Cooperates With NUR77 and SF1 to Activate the Human INSL3 Promoter in Mouse MA-10 Leydig Cells. J Mol Endocrinol (2016) 56:163–73. doi: 10.1530/JME-15-0139
35. Zhang P, Mellon SH. Multiple Orphan Nuclear Receptors Converge to Regulate Rat P450c17 Gene Transcription: Novel Mechanisms for Orphan Nuclear Receptor Action. Mol Endocrinol (1997) 11:891–904. doi: 10.1210/mend.11.7.9940
36. Song KH, Park YY, Ki CP, Cheol YH, Jin HP, Shong M, et al. The Atypical Orphan Nuclear Receptor DAX-1 Interacts With Orphan Nuclear Receptor Nur77 and Represses Its Transactivation. Mol Endocrinol (2004) 18:1929–40. doi: 10.1210/me.2004-0043
37. Hatano M, Migita T, Ohishi T, Shima Y, Ogawa Y, Morohashi KI, et al. SF-1 Deficiency Causes Lipid Accumulation in Leydig Cells via Suppression of STAR and CYP11A1. Endocrine (2016) 54:484–96. doi: 10.1007/s12020-016-1043-1
38. Lynch JP, Lala DS, Peluso JJ, Luo W, Parker KL, White BA. Steroidogenic Factor 1, an Orphan Nuclear Receptor, Regulates the Expression of the Rat Aromatase Gene in Gonadal Tissues. Mol Endocrinol (1993) 7:776–86. doi: 10.1210/mend.7.6.8395654
39. Givens CR, Zhang P, Bair SR, Mellon SH. Transcriptional Regulation of Rat Cytochrome P450c17 Expression in Mouse Leydig MA-10 and Adrenal Y-1 Cells: Identification of a Single Protein That Mediates Both Basal and cAMP-Induced Activities. DNA Cell Biol (1994) 13:1087–98. doi: 10.1089/dna.1994.13.1087
40. Zhang P, Mellon SH. The Orphan Nuclear Receptor Steroidogenic Factor-1 Regulates the Cyclic Adenosine 3’,5’-Monophosphate-Mediated Transcriptional Activation of Rat Cytochrome P450c17 (17 Alpha-Hydroxylase/C17-20 Lyase). Mol Endocrinol (1996) 10:147–58. doi: 10.1210/mend.10.2.8825555
41. Hu Z, Zhuang L, Guan X, Meng J, Dufau ML. Steroidogenic Factor-1 Is an Essential Transcriptional Activator for Gonad-Specific Expression of Promoter I of the Rat Prolactin Receptor Gene. J Biol Chem (1997) 272:14263–71. doi: 10.1074/jbc.272.22.14263
42. Teixeira J, Kehas DJ, Antun R, Donahoe PK. Transcriptional Regulation of the Rat Müllerian Inhibiting Substance Type II Receptor in Rodent Leydig Cells. Proc Natl Acad Sci USA (1999) 96:13831–8. doi: 10.1073/pnas.96.24.13831
43. Wilson MJ, Jeyasuria P, Parker KL, Koopman P. The Transcription Factors Steroidogenic Factor-1 and SOX9 Regulate Expression of Vanin-1 During Mouse Testis Development. J Biol Chem (2005) 280:5917–23. doi: 10.1074/jbc.M412806200
44. Pezzi V, Sirianni R, Chimento A, Maggiolini M, Bourguiba S, Delalande C, et al. Differential Expression of Steroidogenic Factor-1/Adrenal 4 Binding Protein and Liver Receptor Homolog-1 (LRH-1)/Fetoprotein Transcription Factor in the Rat Testis: LRH-1 as a Potential Regulator of Testicular Aromatase Expression. Endocrinology (2004) 145:2186–96. doi: 10.1210/en.2003-1366
45. Manna PR, Dyson MT, Jo Y, Stocco DM. Role of Dosage-Sensitive Sex Reversal, Adrenal Hypoplasia Congenita, Critical Region on the X Chromosome, Gene 1 in Protein Kinase A and Protein Kinase C-Mediated Regulation of the Steroidogenic Acute Regulatory Protein Expression in Mouse Leydig Tumor. Endocrinology (2009) 150:187–99. doi: 10.1210/en.2008-0368
46. Bergeron F, Nadeau G, Viger RS. GATA4 Knockdown in MA-10 Leydig Cells Identifies Multiple Target Genes in the Steroidogenic Pathway. Reproduction (2015) 149:245–57. doi: 10.1530/REP-14-0369
47. Daems C, Di-Luoffo M, Paradis É, Tremblay JJ. MEF2 Cooperates With Forskolin/cAMP and GATA4 to Regulate Star Gene Expression in Mouse MA-10 Leydig Cells. Endocrinology (2015) 156:2693–703. doi: 10.1210/en.2014-1964
48. Mizutani T, Yamada K, Minegishi T, Miyamoto K. Transcriptional Regulation of Rat Scavenger Receptor Class B Type I Gene. J Biol Chem (2000) 275:22512–9. doi: 10.1074/jbc.M001631200
49. Schwarzenbach H, Chakrabarti G, Paust HJ, Mukhopadhyay AK. Gonadotropin-Mediated Regulation of the Murine VEGF Expression in MA-10 Leydig Cells. J Androl (2004) 25:128–39. doi: 10.1002/j.1939-4640.2004.tb02768.x
50. Giatzakis C, Papadopoulos V. Differential Utilization of the Promoter of Peripheral-Type Benzodiazepine Receptor by Steroidogenic Versus Nonsteroidogenic Cell Lines and the Role of Sp1 and Sp3 in the Regulation of Basal Activity. Endocrinology (2004) 145:1113–23. doi: 10.1210/en.2003-1330
51. Garon G, Bergeron F, Brousseau C, Robert NM, Tremblay JJ. FOXA3 Is Expressed in Multiple Cell Lineages in the Mouse Testis and Regulates Pdgfra Expression in Leydig Cells. Endocrinology (2017) 158:1886–97. doi: 10.1210/en.2016-1736
52. Daems C, Martin LJ, Brousseau C, Tremblay JJ. MEF2 Is Restricted to the Male Gonad and Regulates Expression of the Orphan Nuclear Receptor Nr4a1. Mol Endocrinol (2014) 28:886–98. doi: 10.1210/me.2013-1407
53. Di-Luoffo M, Brousseau C, Bergeron F, Tremblay JJ. The Transcription Factor MEF2 Is a Novel Regulator of Gsta Gene Class in Mouse MA-10 Leydig Cells. Endocrinology (2015) 156:4695–706. doi: 10.1210/en.2015-1500
54. Eferl R, Wagner EF. AP-1: A Double-Edged Sword in Tumorigenesis. Nat Rev Cancer (2003) 3:859–68. doi: 10.1038/nrc1209
55. Kataoka K, Noda M, Nishizawa M. Maf Nuclear Oncoprotein Recognizes Sequences Related to an AP-1 Site and Forms Heterodimers With Both Fos and Jun. Mol Cell Biol (1994) 14:700–12. doi: 10.1128/mcb.14.1.700-712.1994
56. Nguyen HT, Najih M, Martin LJ. The AP-1 Family of Transcription Factors Are Important Regulators of Gene Expression Within Leydig Cells. Endocrine (2021) 74:498–507. doi: 10.1007/s12020-021-02888-7
57. Li X, Hales KH, Watanabe G, Lee RJ, Pestell RG, Hales DB. The Effect of Tumor Necrosis Factor-α and cAMP on Induction of AP-1 Activity in MA-10 Tumor Leydig Cells. Endocrine (1997) 6:317–24. doi: 10.1007/BF02820509
58. Noelke J, Wistuba J, Damm OS, Fietz D, Gerber J, Gaehle M. Brehm R. A Sertoli Cell-Specific Connexin43 Knockout Leads to Altered Interstitial Connexin Expression and Increased Leydig Cell Numbers. Cell Tissue Res (2015) 361:633–44. doi: 10.1007/s00441-015-2126-7
59. Shea-Eaton W, Sandhoff TW, Lopez D, Hales DB, McLean MP. Transcriptional Repression of the Rat Steroidogenic Acute Regulatory (Star) Protein Gene by the AP-1 Family Member C-Fos. Mol Cell Endocrinol (2002) 188:161–70. doi: 10.1016/s0303-7207(01)00715-8
60. Cai DH, Wang D, Keefer J, Yeamans C, Hensley K, Friedman AD. C/EBP Alpha: AP-1 Leucine Zipper Heterodimers Bind Novel DNA Elements, Activate the PU.1 Promoter and Direct Monocyte Lineage Commitment More Potently Than C/EBP Alpha Homodimers or AP-1. Oncogene (2008) 27:2772–9. doi: 10.1038/sj.onc.1210940
61. Rutberg SE, Adams TL, Olive M, Alexander N, Vinson C, Yuspa SH. CRE DNA Binding Proteins Bind to the AP-1 Target Sequence and Suppress AP-1 Transcriptional Activity in Mouse Keratinocytes. Oncogene (1999) 18:1569–79. doi: 10.1038/sj.onc.1202463
62. Osada S, Yamamoto H, Nishihara T, Imagawa M. DNA Binding Specificity of the CCAAT/Enhancer-Binding Protein Transcription Factor Family. J Biol Chem (1996) 271:3891–6. doi: 10.1074/jbc.271.7.3891
63. Nalbant D, Williams SC, Stocco DM, Khan SA. Luteinizing Hormone-Dependent Gene Regulation in Leydig Cells May Be Mediated by CCAAT/Enhancer-Binding Protein-β. Endocrinology (1998) 139:272–9. doi: 10.1210/endo.139.1.5663
64. Zhang Z, Burch PE, Cooney AJ, Lanz RB, Pereira FA, Wu J, et al. Genomic Analysis of the Nuclear Receptor Family: New Insights Into Structure, Regulation, and Evolution From the Rat Genome. Genome Res (2004) 14:580–90. doi: 10.1101/gr.2160004
65. Martin LJ, Tremblay JJ. Nuclear Receptors in Leydig Cell Gene Expression and Function. Biol Reprod (2010) 83:3–14. doi: 10.1095/biolreprod.110.083824
66. Lin FJ, Qin J, Tang K, Tsai SY, Tsai MJ. Coup D’etat: An Orphan Takes Control. Endocr Rev (2011) 32:404–21. doi: 10.1210/er.2010-0021
67. Ashraf UM, Sanchez ER, Kumarasamy S. COUP-TFII Revisited: Its Role in Metabolic Gene Regulation. Steroids (2019) 141:63–9. doi: 10.1016/j.steroids.2018.11.013
68. Cooney AJ, Tsai SY, O’Malley BW, Tsai MJ. Chicken Ovalbumin Upstream Promoter Transcription Factor (COUP-TF) Dimers Bind to Different GGTCA Response Elements, Allowing COUP-TF to Repress Hormonal Induction of the Vitamin D3, Thyroid Hormone, and Retinoic Acid Receptors. Mol Cell Biol (1992) 12:4153–63. doi: 10.1128/mcb.12.9.4153
69. Kilcoyne KR, Smith LB, Atanassova N, Macpherson S, McKinnell C, van den Driesche S, et al. Fetal Programming of Adult Leydig Cell Function by Androgenic Effects on Stem/Progenitor Cells. Proc Natl Acad Sci USA (2014) 111:E1924–32. doi: 10.1073/pnas.1320735111
70. Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The Orphan Nuclear Receptor COUP-TFII Is Required for Angiogenesis and Heart Development. Genes Dev (1999) 13:1037–49. doi: 10.1101/gad.13.8.1037
71. Qin J, Tsai MJ, Tsai SY. Essential Roles of COUP-TFII in Leydig Cell Differentiation and Male Fertility. PloS One (2008) 3:1–11. doi: 10.1371/journal.pone.0003285
72. Maxwell MA, Muscat GEO. The NR4A Subgroup: Immediate Early Response Genes With Pleiotropic Physiological Roles. Nucl Recept Signal (2006) 4:e002. doi: 10.1621/nrs.04002
73. Kurakula K, Koenis DS, van Tiel CM, de Vries CJM. NR4A Nuclear Receptors Are Orphans But Not Lonesome. Biochim Biophys Acta - Mol Cell Res (2014) 1843:2543–55. doi: 10.1016/j.bbamcr.2014.06.010
74. Wilson TE, Mouw AR, Weaver CA, Milbrandt J, Parker KL. The Orphan Nuclear Receptor NGFI-B Regulates Expression of the Gene Encoding Steroid 21-Hydroxylase. Mol Cell Biol (1993) 13:861–8. doi: 10.1128/mcb.13.2.861-868.1993
75. Eells J, Witta J, Otridge J, Zuffova E, Nikodem V. Structure and Function of the Nur77 Receptor Subfamily, a Unique Class of Hormone Nuclear Receptors. Curr Genomics (2005) 1:135–52. doi: 10.2174/1389202003351580
76. Song KH, Park JI, Lee MO, Soh J, Lee K, Choi HS. LH Induces Orphan Nuclear Receptor Nur77 Gene Expression in Testicular Leydig Cells. Endocrinology (2001) 142:5116–23. doi: 10.1210/endo.142.12.8525
77. Martin LJ, Boucher N, El-Asmar B, Tremblay JJ. cAMP-Induced Expression of the Orphan Nuclear Receptor Nur77 in MA-10 Leydig Cells Involves a CaMKI Pathway. J Androl (2009) 30:134–45. doi: 10.2164/jandrol.108.006387
78. Fayard E, Auwerx J, Schoonjans K. LRH-1: An Orphan Nuclear Receptor Involved in Development, Metabolism and Steroidogenesis. Trends Cell Biol (2004) 14:250–60. doi: 10.1016/j.tcb.2004.03.008
79. Meinsohn M-C, Smith OE, Bertolin K, Murphy BD. The Orphan Nuclear Receptors Steroidogenic Factor-1 and Liver Receptor Homolog-1: Structure, Regulation, and Essential Roles in Mammalian Reproduction. Physiol Rev (2019) 99:1249–79. doi: 10.1152/physrev.00019.2018
80. Morohashi K, Zanger UM, Honda S, Hara M, Waterman MR, Omura T. Activation of CYP11A and CYP11B Gene Promoters by the Steroidogenic Cell-Specific Transcription Factor, Ad4BP. Mol Endocrinol (1993) 7:1196–204. doi: 10.1210/mend.7.9.8247022
81. Luo X, Ikeda Y. Parker KL. A Cell-Specific Nuclear Receptor Is Essential for Adrenal and Gonadal Development and Sexual Differentiation. Cell (1994) 77:481–90. doi: 10.1016/0092-8674(94)90211-9
82. Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, et al. Mice Deficient in the Orphan Receptor Steroidogenic Factor 1 Lack Adrenal Glands and Gonads But Express P450 Side-Chain-Cleavage Enzyme in the Placenta and Have Normal Embryonic Serum Levels of Corticosteroids. Proc Natl Acad Sci USA (1995) 92:10939–43. doi: 10.1073/pnas.92.24.10939
83. Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen APN, et al. Cell-Specific Knockout of Steriodogenic Factor 1 Reveals Its Essential Roles in Gonadal Function. Mol Endocrinol (2004) 18:1610–9. doi: 10.1210/me.2003-0404
84. Karpova T, Ravichandiran K, Insisienmay L, Rice D, Agbor V, Heckert LL. Steroidogenic Factor 1 Differentially Regulates Fetal and Adult Leydig Cell Development in Male Mice. Biol Reprod (2015) 93:1–15. doi: 10.1095/biolreprod.115.131193
85. Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, et al. Steroidogenic Factor 1: An Essential Mediator of Endocrine Development. Recent Prog Horm Res (2002) 57:19–36. doi: 10.1210/rp.57.1.19
86. Schimmer BP, White PC. Minireview: Steroidogenic Factor 1: Its Roles in Differentiation, Development, and Disease. Mol Endocrinol (2010) 24:1322–37. doi: 10.1210/me.2009-0519
87. Yang Y, Li Z, Wu X, Chen H, Xu W, Xiang Q, et al. Direct Reprogramming of Mouse Fibroblasts Toward Leydig-Like Cells by Defined Factors. Stem Cell Rep (2017) 8:39–53. doi: 10.1016/j.stemcr.2016.11.010
88. Hou YP, Zhang ZY, Xing XY, Zhou J, Sun J. Direct Conversion of Human Fibroblasts Into Functional Leydig-Like Cells by SF-1, GATA4 and NGFI-B. Am J Transl Res (2018) 10:175–83.
89. Tremblay JJ, Viger RS. Transcription Factor GATA-4 Enhances Mullerian Inhibiting Substance Gene Transcription Through a Direct Interaction With the Nuclear Receptor SF-1. Mol Endocrinol (1999) 13:1388–401. doi: 10.1210/mend.13.8.0330
90. Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, et al. An Unusual Member of the Nuclear Hormone Receptor Superfamily Responsible for X-Linked Adrenal Hypoplasia Congenita. Nature (1994) 372:635–41. doi: 10.1038/372635a0
91. Iyer AK, McCabe ERB. Molecular Mechanisms of DAX1 Action. Mol Genet Metab (2004) 83:60–73. doi: 10.1016/j.ymgme.2004.07.018
92. Iyer AK, Zhang Y-H, McCabe ERB. Dosage-Sensitive Sex Reversal Adrenal Hypoplasia Congenita Critical Region on the X Chromosome, Gene 1 (DAX1) (NR0B1) and Small Heterodimer Partner (SHP) (NR0B2) Form Homodimers Individually, as Well as DAX1-SHP Heterodimers. Mol Endocrinol (2006) 20:2326–42. doi: 10.1210/me.2005-0383
93. Meeks JJ, Russell TA, Jeffs B, Huhtaniemi I, Weiss J, Jameson JL. Leydig Cell-Specific Expression of DAX1 Improves Fertility of the Dax1-Deficient Mouse. Biol Reprod (2003) 69:154–60. doi: 10.1095/biolreprod.102.011429
94. Xu B, Yang W-H, Gerin I, Hu C-D, Hammer GD, Koenig RJ. Dax-1 and Steroid Receptor RNA Activator (SRA) Function as Transcriptional Coactivators for Steroidogenic Factor 1 in Steroidogenesis. Mol Cell Biol (2009) 29:1719–34. doi: 10.1128/mcb.01010-08
95. Vega A, Martinot E, Baptissart M, De Haze A, Saru JP, Baron S, et al. Identification of the Link Between the Hypothalamo-Pituitary Axis and the Testicular Orphan Nuclear Receptor NR0B2 in Adult Male Mice. Endocrinology (2015) 156:660–9. doi: 10.1210/en.2014-1418
96. Volle DH, Duggavathi R, Magnier BC, Houten SM, Cummins CL, Lobaccaro JMA, et al. The Small Heterodimer Partner Is a Gonadal Gatekeeper of Sexual Maturation in Male Mice. Genes Dev (2007) 21:303–15. doi: 10.1101/gad.409307
97. Di-Luoffo M, Daems C, Bergeron F, Tremblay JJ. Novel Targets for the Transcription Factors MEF2 in MA-10 Leydig Cells. Biol Reprod (2015) 93:1–12. doi: 10.1095/biolreprod.114.127761
98. Viger RS, Taniguchi H, Robert NM, Tremblay JJ. Role of the GATA Family of Transcription Factors in Andrology. J Androl (2004) 25:441–52. doi: 10.1002/j.1939-4640.2004.tb02813.x
99. Tremblay M, Sanchez-Ferras O, Bouchard M. GATA Transcription Factors in Development and Disease. Development (2018) 145:1–20. doi: 10.1242/dev.164384
100. Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel JD, et al. Developmental Stage- and Spermatogenic Cycle-Specific Expression of Transcription Factor GATA-1 in Mouse Sertoli Cells. Development (1994) 120:1759–66. doi: 10.1242/dev.120.7.1759
101. Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, et al. Expression and Regulation of Transcription Factors GATA-4 and GATA-6 in Developing Mouse Testis. Endocrinology (1999) 140:1470–80. doi: 10.1210/endo.140.3.6587
102. Robert NM, Tremblay JJ, Viger RS. Friend of GATA (FOG)-1 and FOG-2 Differentially Repress the GATA-Dependent Activity of Multiple Gonadal Promoters. Endocrinology (2002) 143:3963–73. doi: 10.1210/en.2002-220280
103. Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription Factor GATA-4 Is Expressed in a Sexually Dimorphic Pattern During Mouse Gonadal Development and Is a Potent Activator of the Mullerian Inhibiting Substance Promoter. Development (1998) 125:2665–75. doi: 10.1242/dev.125.14.2665
104. Hu YC, Okumura LM, Page DC. Gata4 Is Required for Formation of the Genital Ridge in Mice. PloS Genet (2013) 9:1–12. doi: 10.1371/journal.pgen.1003629
105. Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional Ablation of Gata4 and Fog2 Genes in Mice Reveals Their Distinct Roles in Mammalian Sexual Differentiation. Dev Biol (2011) 353:229–41. doi: 10.1016/j.ydbio.2011.02.032
106. Tremblay JJ, Viger RS. GATA Factors Differentially Activate Multiple Gonadal Promoters Through Conserved GATA Regulatory Elements. Endocrinology (2001) 142:977–86. doi: 10.1210/endo.142.3.7995
107. Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 Is Required for Sex Steroidogenic Cell Development in the Fetal Mouse. Dev Dyn (2007) 236:203–13. doi: 10.1002/dvdy.21004
108. Kaestner KH, Hiemisch H, Luckow B, Schütz G. The HNF-3 Gene Family of Transcription Factors in Mice: Gene Structure, cDNA Sequence, and mRNA Distribution. Genomics (1994) 20:377–85. doi: 10.1006/geno.1994.1191
109. Behr R, Sackett SD, Bochkis IM, Le PP, Kaestner KH. Impaired Male Fertility and Atrophy of Seminiferous Tubules Caused by Haploinsufficiency for Foxa3. Dev Biol (2007) 306:636–45. doi: 10.1016/j.ydbio.2007.03.525
110. Brennan J, Tilmann C, Capel B. Pdgfr-Alpha Mediates Testis Cord Organization and Fetal Leydig Cell Development in the XY Gonad. Genes Dev (2003) 17:800–10. doi: 10.1101/gad.1052503
111. Kim H, Kumar S, Lee K. FOXA3, a Negative Regulator of Nur77 Expression and Activity in Testicular Steroidogenesis. Int J Endocrinol (2021) 2021:1–8. doi: 10.1155/2021/6619447
112. Potthoff MJ, Olson EN. MEF2: A Central Regulator of Diverse Developmental Programs. Development (2007) 134:4131–40. doi: 10.1242/dev.008367
113. Dodington DW, Desai HR, Woo M. JAK/STAT – Emerging Players in Metabolism. Trends Endocrinol Metab (2018) 29:55–65. doi: 10.1016/j.tem.2017.11.001
114. Kanzaki M, Morris PL. Lactogenic Hormone-Inducible Phosphorylation and Gamma-Activated Site-Binding Activities of Stat5b in Primary Rat Leydig Cells and MA-10 Mouse Leydig Tumor Cells. Endocrinology (1998) 139:1872–82. doi: 10.1210/endo.139.4.5956
115. Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortés MM, Hoang YD, et al. Knockout of the Transcription Factor NRF2 Disrupts Spermatogenesis in an Age-Dependent Manner. Free Radic Biol Med (2010) 49:1368–79. doi: 10.1016/j.freeradbiomed.2010.07.019
116. Chen H, Jin S, Guo J, Kombairaju P, Biswal S, Zirkin BR. Knockout of the Transcription Factor Nrf2: Effects on Testosterone Production by Aging Mouse Leydig Cells. Mol Cell Endocrinol (2015) 409:113–20. doi: 10.1016/j.mce.2015.03.013
117. Chung J-Y, Chen H, Zirkin B. Sirt1 and Nrf2: Regulation of Leydig Cell Oxidant/Antioxidant Intracellular Environment and Steroid Formation. Biol Reprod (2021) 105:1307–16. doi: 10.1093/biolre/ioab150
118. Xiao Y, Zhao L, Li W, Wang X, Ma T, Yang L, et al. Circadian Clock Gene BMAL1 Controls Testosterone Production by Regulating Steroidogenesis-Related Gene Transcription in Goat Leydig Cells. J Cell Physiol (2021) 236:6706–25. doi: 10.1002/jcp.30334
119. Ding H, Zhao J, Liu H, Wang J, Lu W. BMAL1 Knockdown Promoted Apoptosis and Reduced Testosterone Secretion in TM3 Leydig Cell Line. Gene (2020) 747:144672. doi: 10.1016/j.gene.2020.144672
Keywords: transcription factors, gene expression, regulatory element, DNA binding motif, steroidogenesis, Leydig cells
Citation: de Mattos K, Viger RS and Tremblay JJ (2022) Transcription Factors in the Regulation of Leydig Cell Gene Expression and Function. Front. Endocrinol. 13:881309. doi: 10.3389/fendo.2022.881309
Received: 22 February 2022; Accepted: 15 March 2022;
Published: 07 April 2022.
Edited by:
Vassilios Papadopoulos, University of Southern California, United StatesReviewed by:
Diane Rebourcet, The University of Newcastle, AustraliaPeter O’Shaughnessy, University of Glasgow, United Kingdom
Copyright © 2022 de Mattos, Viger and Tremblay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacques J. Tremblay, SmFjcXVlcy1KLlRyZW1ibGF5QGNyY2h1ZGVxdWViZWMudWxhdmFsLmNh
 Karine de Mattos
Karine de Mattos Robert S. Viger
Robert S. Viger Jacques J. Tremblay
Jacques J. Tremblay