- 1Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Emergency, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 3Department of Urology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 4Department of Cardiology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Background: Catecholamine excess arising from pheochromocytomas and paragangliomas (PPGLs) can cause a wide spectrum of cardiac manifestations. Although there are reviews of reported cases, these reviews lack detailed data, which makes it impossible to perform an accurate analysis. In this study, we conducted a comprehensive analysis of cardiovascular complications (CCs), including PPGL-related myocardial injury, cardiogenic shock, and arrhythmias requiring antiarrhythmic therapy, in a large cohort of patients with PPGL.
Methods: We retrospectively analyzed the clinical data of consecutive patients with PPGL admitted between January 2018 and June 2020. The prevalence and the characteristics of patients with CCs were investigated. Moreover, comparisons were made between patients with and without CCs.
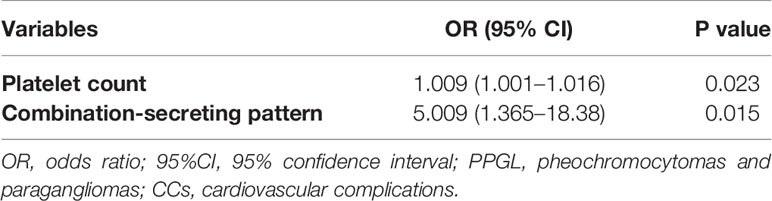
Results: Compared with the non-CC group, the percentage of men was significantly lower (14/41 vs.92/175, 34.1% vs. 52.6%, p = 0.034) and the proportion of patients with paroxysmal hypertension was significantly higher (13/41 vs.29/173, 31.7% vs.16.8%, p = 0.03) in the CC group. More patients showed excessive sweating (19/41 vs 64/175, 46.3% vs. 24.0%, p = 0.004) and PPGL crisis (7/41 vs. 10/175, 17.1% vs.5.7%, p=0.035) in the CC group. In terms of laboratory findings, higher white blood cell [7.36 (6.49, 20.23) vs. 5.95 (5.1, 6.97)×109/L, p<0.001] and platelet [339.28 ± 108.54 vs. 250.66 ± 70.83(×109/L), p = 0.021] counts were more common in the CC group. There was also a higher prevalence of combination-producing PPGL in the CC group (13/24 vs.20/149, 54.2% vs.13.4%, p<0.001). However, the tumor size, invasive behavior on histology, and hemorrhage or necrosis on histology did not differ between the two groups. Platelet count [odds ratio (OR): 1.009; 95% confidence interval (CI) 1.001–1.016; p=0.023] and combination-secreting PPGL (OR: 5.009; 95% CI 1.365–18.38; p=0.015) are independent risk factors for CCs in patients with PPGL.
Conclusions: In patients with PPGL, even in the absence of signs and symptoms of CCs, a work up of cardiology should be strongly considered. Importantly, if patients with PPGLs have higher platelet counts and the combination-secreting pattern, they are more likely to have CCs. Thus, a careful cardiac evaluation should be performed.
Introduction
Pheochromocytomas and paragangliomas (PHEOs and PGLs, PPGLs) are a group of rare catecholamine (CA)-secreting neuroendocrine tumors that are separately derived from the adrenal medulla and the extra-adrenal sympathetic or parasympathetic nervous system (1). The joint annual incidence of PPGL is estimated to be 2–8 cases per million inhabitants (1). The clinical manifestations of PPGLs include hypertension (HTN), headache, excessive sweating, chest pain and so on, which are due to excessive CA secretion (2). However, the clinical presentation of PPGLs can also be asymptomatic and vague, which may explain the delay in diagnosis in many cases.
PPGLs are associated with a wide variety of cardiovascular complications (CCs), which have been reviewed previously (3). Briefly, CCs related to PPGLs include arrhythmias (3, 4),Takotsubo-like cardiomyopathy (5–7), dilated cardiomyopathy, and acute coronary syndrome and so on (3). Although thought to arise from the incident CA excess, the exact mechanism of CCs induced by PPGLs remains elusive (7). CCs in patients with PPGLs may be life-threatening; hence, timely diagnosis and surgery can be life-saving (3). Different types of CCs may occur in one fifth to more than one third of patients with PPGLs (4, 8–10). Given the potential reversibility of these CCs, early diagnosis and resection of PPGLs are crucial because delayed diagnosis may lead to irreversible cardiac remodeling and death. However, the diagnosis of PPGL-related CCs is often delayed due to the atypical presentation in many cases.
Although there are reviews of reported cases (11, 12), these reviews lack detailed data, which make it impossible to perform an accurate analysis, and publication bias due to the tendency to report more severe cases is inevitable (13, 14). In this study, we retrospectively reviewed patients diagnosed with PPGLs at a single center and screened these patients for CCs. The prevalence and features of PPGLs with CCs were assessed. Furthermore, comparisons between patients with and without CCs were made to identify the clinical features associated with CCs to aide in further understanding this clinical entity.
Materials and Methods
Study Population
Consecutive patients who were diagnosed with PPGLs and admitted to the hospital between January 2018 and June 2020 were included. PPGLs were diagnosed in 407 patients. Forty-six patients referred for recurrence or metastasis, 21 patients with pathologically confirmed non-PPGLs, 41 patients who did not undergo surgery because they had metastatic PPGL or refused surgery, 10 patients aged <18 years, 6 patients with congenital heart disease (1 with Kawasaki disease, 1 with left cor triatriatum, 1 with congenitally corrected transposition of the great arteries, 2 with atrial defects, and 1 with congenital single ventricle), 12 patients finally diagnosed with coronary heart disease, and 55 patients with incomplete clinical data were excluded. Thus, 216 patients with histopathological evidence were included in the analysis. The flowchart of patient recruitment is shown in Supplementary Figure 1.
Clinical Assessment
Electronic health records were reviewed, and clinical history data, biochemical test results, imaging results, surgical reports, and pathology diagnoses were extracted and analyzed. Smoking history was defined as current smoking. Drinking history was defined as current drinking. Diabetes mellitus (DM) was defined as: 1) fasting plasma glucose value ≥ 7.0 mmol/L or a 2-h plasma glucose value ≥ 11.1 mmol/L during a 75 g OGTT or HbA1c ≥ 6.5% confirmed by repeated testing in an asymptomatic patient; or 2) random plasma glucose ≥ 11.1mmol/L in a patient with classic symptoms of hyperglycemia; or 3) preexisting diagnosis of DM with established antidiabetic diet/treatment (15). The patterns of HTN included normal, sustained (just as essential HTN), paroxysmal (paroxysms of HTN on a background of normal blood pressure [BP]), mixed (paroxysms of HTN on a background of sustained HTN), and unknown (16). HTN was based on the diagnosis at admission and preoperative BP readings, as BP can decline after surgery. A secreting tumor was defined by a CA or metanephrine concentration at least twice the upper limit of normal (ULN). The pattern of secretion was classified as non-secreting, epinephrine predominant (≥2 × ULN), norepinephrine predominant (≥2 × ULN), dopamine predominant (≥2 × ULN), or combination (epinephrine and norepinephrine ≥2 × ULN) (9). The maximum tumor diameters were comprehensively determined from gross pathological specimens. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI method (17).
CCs were diagnosed if at least one of the following clinical situations was present before surgery: (1) biochemical, electrocardiographic, and/or echocardiographic evidence of myocardial ischemia with left ventricular systolic dysfunction, with absence of significant coronary artery stenosis confirmed by coronary artery angiography or coronary computed tomography angiography (18); (2) heart failure of unknown etiology, but most likely attributed to CA release from PPGL, requiring hospitalization or intravenous diuretic therapy; (3) arrhythmia requiring hospitalization or antiarrhythmic therapy. The comparison group was composed of patients with PPGL who did not present with CCs.
All patient records were anonymized before analysis. The study protocol was approved by the ethics committee of the institution and followed the principles of the Helsinki Declaration.
Statistical Analysis
Continuous variables were assessed for normality using the Shapiro–Wilk test and the Kolmogorov–Smirnov test. Continuous data are expressed as mean ± standard deviation or median (25th, 75th percentile), as appropriate. The mean values were compared using the two-tailed Student’s t-test or, in the case of non-normally distributed data, the rank-sum test. Categorical variables are presented as number (percentage), and differences were detected using Pearson’s chi-square test or Fisher’s exact test. The parameters identified as statistically significant in the univariate logistic regression analysis (p < 0.1) were included in the multivariate logistic regression analysis to identify independent predictors. Parameters with odds ratios (ORs) of >1 were considered as risk factors, whereas parameters with ORs of <1 were considered as protective factors. Two-sided p values of <0.05 were considered statistically significant. All analyses were performed using SPSS statistical software, version 25.0 (IBM Corp.).
Results
Characteristics of Patients With and Without CCs
The clinical profiles of patients with PPGL are summarized in Table 1. A total of 216 patients were included in the analysis. Among the study subjects, 41 patients with PPGLs had CCs, while 175 patients did not. Men and women with PPGL were roughly equally represented, while the percentage of men in the non-CC group (92/175, 52.6%) was significantly higher than in the CC group (14/41, 34.1%) (p = 0.034). There were no differences between the two groups in terms of age at diagnosis of PPGL, history of smoking and alcohol abuse. DM occurred in 48 patients (22.2%). As expected, more patients with CCs had DM (14/41 vs. 34/175, 34.1% vs. 19.5%, p = 0.043). Higher levels of plasma glucose [6.1 (4.8, 7.6) vs. 5.3(4.7, 6.1) (mmol/L), p = 0.025] were more common in the CC group.
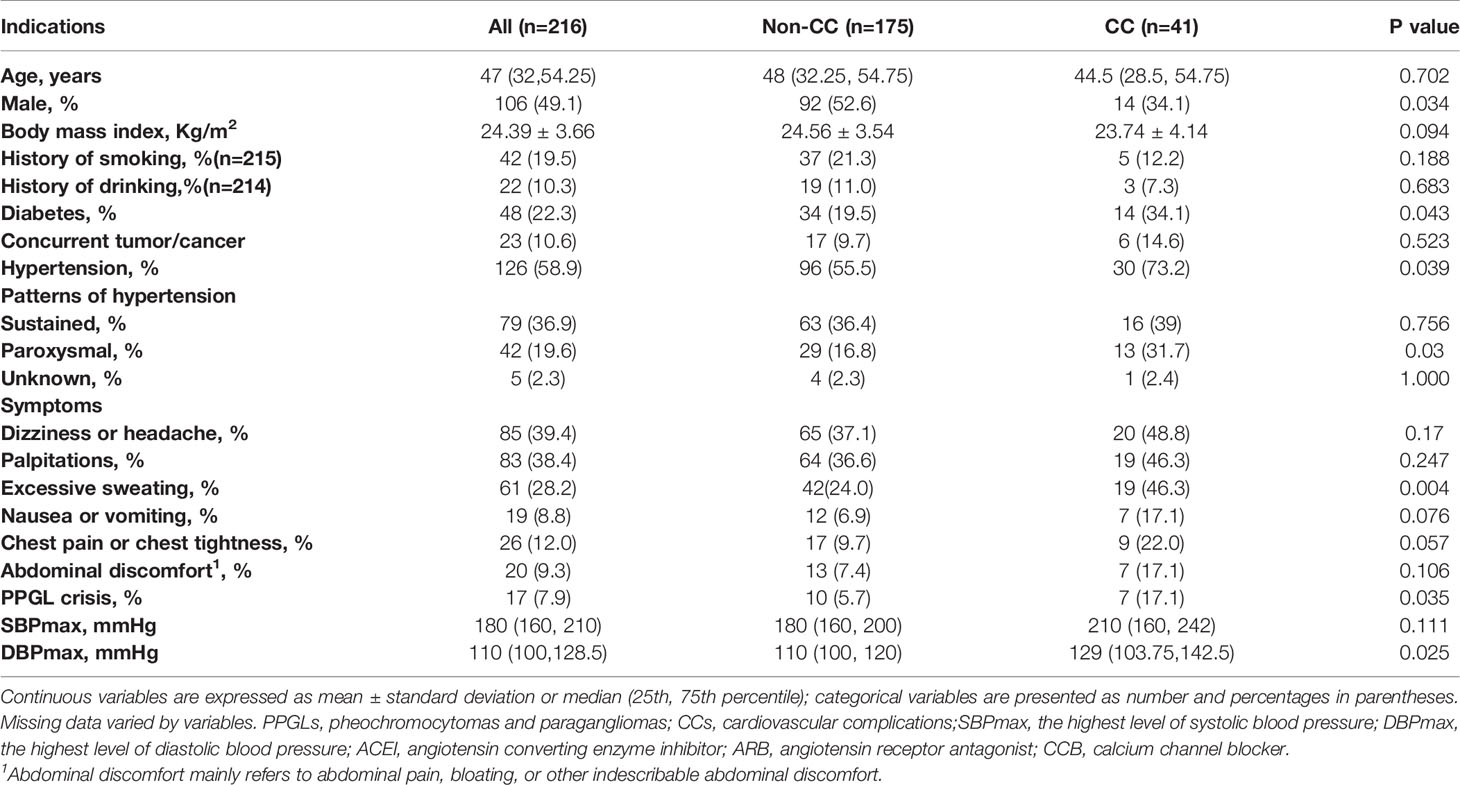
Table 1 Clinical profiles of patients diagnosed with PPGL and comparisons between the non-CC and CC groups.
The results of BP measurements were obtained in 214 patients, including 41 in the CC group and 173 in the non-CC group. HTN occurred in 126 patients (58.3%), more patients with CCs had HTN (30/41 vs. 96/173, 73.2% vs. 55.5%, p = 0.039); the proportion of patients with paroxysmal HTN in the CC group was significantly higher than non-CC group (13/41 vs. 29/173, 31.7% vs 16.8%, p = 0.03). However, the sustained (p = 0.756) and unknown (p = 1.000) patterns of HTN did not differ between the two groups. The maximum systolic BP (SBP) and diastolic BP (DBP) values were higher in the CC group than in the non-CC group, but only the DBP was significantly different between the two groups (p = 0.025). We collected data on preoperative antihypertensive drug use in 211 patients, of whom 163 patients (77.3%) received alpha-adrenergic receptor blockers, including 28 in the CC group (28/37, 75.7%) and 135 in the non-CC group (135/174, 77.6%). Beta-receptor blockers were used in 67 patients (67/211, 31.8%), including 23 in the CC group (23/37, 62.2%) and 44 in the non-CC group (44/174, 25.3%). Calcium channel blockers were used in 9 patients (4.3%), including 1 in the CC group (1/37, 2.7%) and 8 in the non-CC group (8/174, 4.6%). Angiotensin converting enzyme inhibitors/angiotensin receptor antagonists were used in 3 patients (1.4%), all in the non-CC group. Patients took alpha-adrenergic receptor blockers for 7-14 days before surgery to normalize BP and heart rate. Preoperative coadministration of beta-receptor blockers was indicated to control tachycardia only after administration of alpha-adrenergic receptor blockers (19).
Regarding to the symptoms documented in medical records, 25 patients (25/216, 11.6%) were asymptomatic. Dizziness or headache, palpitations, profuse sweating, nausea or vomiting, chest pain or tightness, and abdominal discomfort appeared in 85 (39.4%), 83 (38.4%), 61 (28.2%), 19 (8.8%), 26 (12.0%), and 20 (9.3%) patients, respectively. PPGL crisis occurred in 17 patients (7.9%). Surprisingly, the proportions of patients who displayed dizziness or headache (65/175 vs.20/41, 37.1% vs. 48.8%, p = 0.17), palpitations (64/175 vs.19/41, 36.6% vs. 46.3%, p = 0.247), nausea or vomiting (12/175 vs.7/41, 6.9% vs. 17.1%, p = 0.076), chest pain or tightness (17/175 vs. 9/41, 9.7% vs. 22.0%, p = 0.057), or abdominal discomfort (13/175 vs. 7/41, 7.4% vs. 17.1%, p = 0.106) were similar between the two groups. Nevertheless, significantly more patients demonstrated profuse sweating (19/41 vs.42/175, 46.3% vs. 24.0%, p = 0.004) and PPGL crisis (7/41 vs. 10/175, 17.1% vs. 5.7%, p = 0.035) in the CC group. There were 3 patients presented with cardiogenic shock in the PPGL-CC group (3/41, 7.3%). PPGL was incidentally found in 72 patients (33.3%), and other symptoms (anxiety, sweaty palms, fever, etc.) were present in about 20% of our cohort.
In terms of laboratory findings, higher white blood cell (WBC) [7.36 (6.49, 20.23) vs. 5.95 (5.1, 6.97)×109/L, p<0.001] and platelet (339.28 ± 108.54 vs. 250.66 ± 70.83(×109/L), p = 0.021) counts were more frequently observed in the CC group (Table 2). Hemoglobin and eGFR were not significantly different between the two groups. The concentrations of 24-hour urine epinephrine, 24-hour urine norepinephrine and 24-hour urine dopamine were available for 173 patients, including 24 patients in the CC group and 149 patients in the non-CC group. Among them, the non-secreting pattern appeared in 64 patients (37.0%). The types of secretion were epinephrine predominant in 19 patients (11.0%), norepinephrine predominant in 56 patients (32.4%), and combination in 33 patients (19.1%). One case demonstrated a dopamine-predominant pattern. There was no difference in the frequency of non-secreting, epinephrine-predominant, or norepinephrine-predominant patterns between the two groups; however, a higher proportion of patients had combination-secreting pattern (13/24 vs 20/149, 54.2% vs. 13.4%, p <0.001) in the CC group (Table 2).
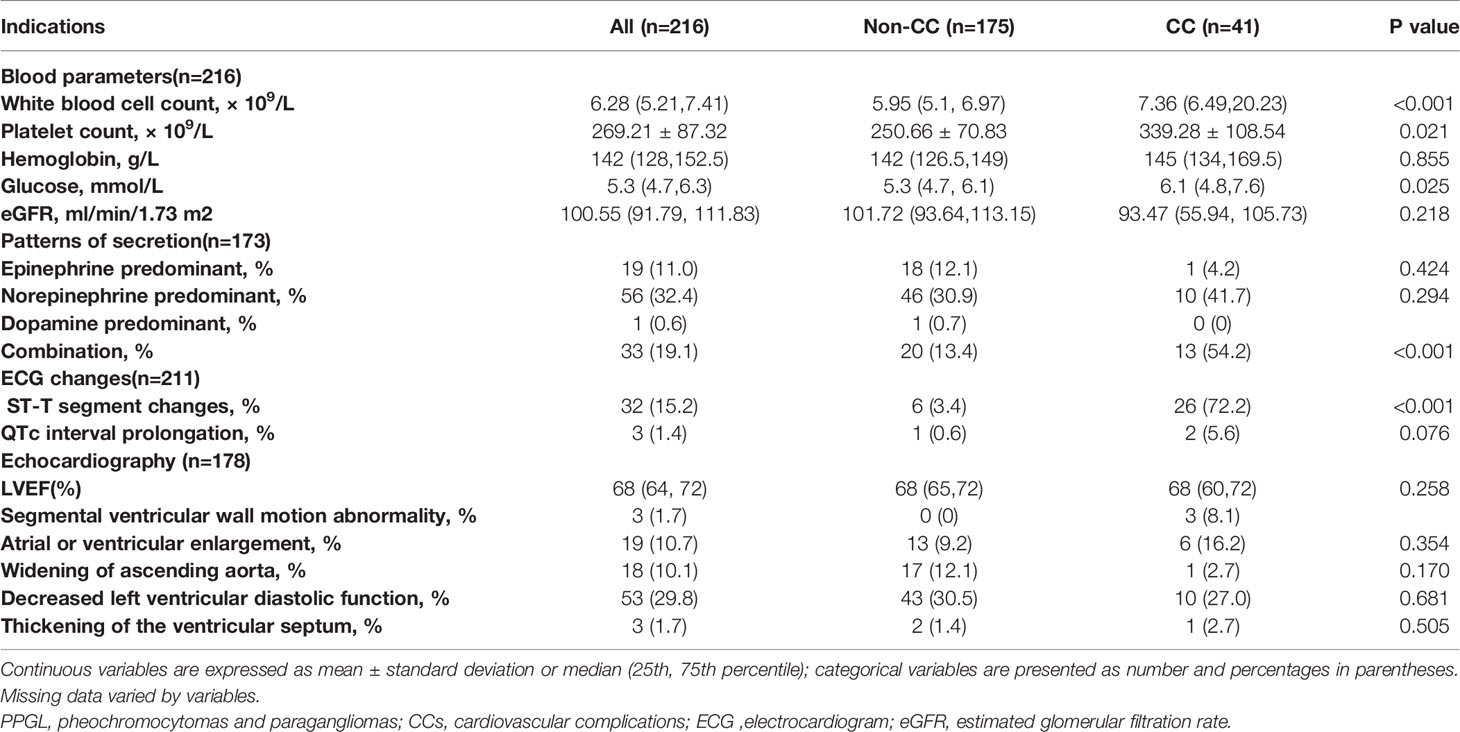
Table 2 Laboratory, electrocardiographic, and echocardiographic findings of patients diagnosed with PPGLs and comparisons between the non-CC and CC groups.
Electrocardiography (ECG) was found in 211 of 216 patients (97.7%), including 175 patients in the non-CC group and 36 patients in the CC group. The most common ECG changes was ST-T change (15.2%) and it was more common in the CC group (6/175 vs. 26/36, 3.4% vs. 72.2%, p<0.001), while QTc prolongation (1/175 vs. 2/36, 0.6% vs. 5.6%, p = 0.076) presented similarly in patients with and without CCs. Arrhythmias occurred in 21 patients (21/211, 10.0%), including sinus tachycardia in 5 subjects, atrial fibrillation (AF) in 2 subjects, premature atrial beats in 8 cases, occasional premature ventricular beats in 3 patients, and atrioventricular block in 1 subject. Two patients had both premature atrial beats and premature ventricular beats. AF and atrioventricular block all occurred in the CC group. Atrial arrhythmias and ventricular arrhythmias were more likely in the CC group (CC group vs. non-CC group: 8/36 vs. 4/175, 22.2% vs. 2.3%, p<0.001; 4/36 vs.1/175, 11.1% vs. 0.6%, p=0.003, respectively). Among these, all occasional premature atrial beats occurred in the non-CC group, and three patients in CC group had frequent premature atrial beats. The incidence of sinus tachycardia was not statistically different between the two groups (4/175 vs. 1/36, 2.3% vs. 2.8%, p = 1.000). 178 patients in the study had echocardiograms. The two groups did not differ significantly in terms of the proportions of patients with atrial or ventricular enlargement (13/141 vs. 6/37, 9.2% vs. 16.2%, p = 0.354), widening of the ascending aorta (17/141 vs. 1/37, 12.1% vs. 2.7%, p = 0.170), decreased left ventricular diastolic function (43/141 vs. 10/37, 30.5% vs. 27.0%, p = 0.681), and thickening of the ventricular septum (2/141 vs. 1/37, 1.4% vs. 2.7%, p = 0.505) (Table 2).
Of the 216 patients, 104 patients (48.1%) had PGLs only, 111 patients (51.4%) had PHEOs only, and 1 patient had both (PPGL). Four patients (3.6%) had bilateral PHEOs. There was no statistical difference between PHEOs and PGLs for inducing CCs (p = 0.473). The proportion of bilateral PHEOs and the tumor size were not statistically different between the two groups. Regarding to the locations of PGLs, forty patients (40/216, 18.5%) were head and neck PGLs, including 8 (8/41, 19.5%) in the CC group and 32 (32/175, 18.3%) in the non-CC group; fifty-one patients(51/216, 23.6%) had intra-abdominal PGL, including 12 (12/41, 29.3%)in the CC group and 39 (39/175, 22.3%) in the non-CC group; nine patients (9/216, 4.2%) were bladder PGLs, including 2 patients (2/41, 4.9%)in the CC group and 7 patients(7/175, 4.0%) in the non-CC group; there were also 5 patients (5/216, 2.3%) with PGLs elsewhere, including 2 with cardiac PGLs, 1 with mediastinal PGL, 1 with bone PGL, and 1 with pelvic PGL. The locations of the PGL were not statistically different between the two groups. Invasive behavior and the presence of hemorrhage/necrosis on pathology were described in 6.0% and 9.7% of patients, respectively. More patients in the CC group demonstrated invasive behavior (4/41 vs. 9/175, 9.8% vs. 5.1%, p = 0.451) and hemorrhage/necrosis (7/41 vs.14/175, 17.1% vs. 8.0%, p = 0.141) on pathology than in the non-CC group, but they were not statistically significant (Table 3).
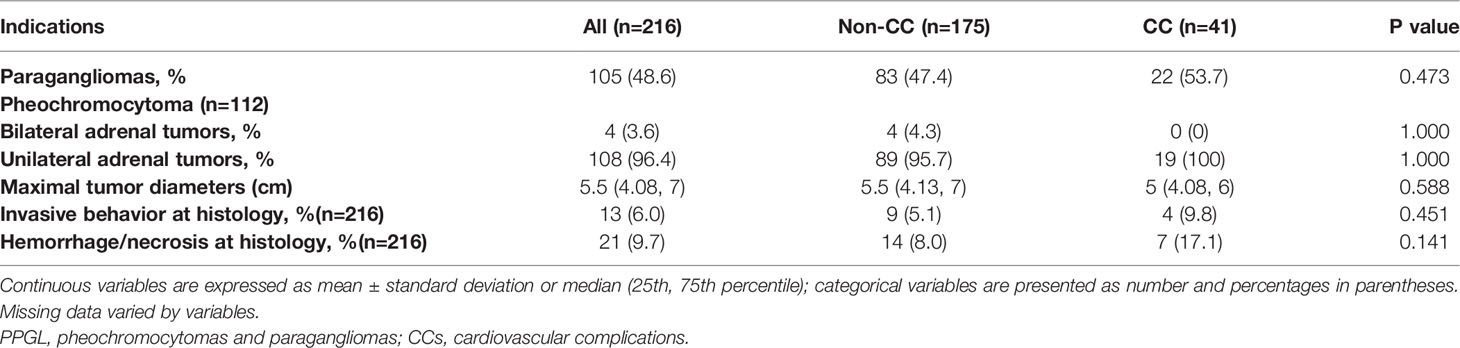
Table 3 Tumor characteristics of patients diagnosed with PPGLs and comparisons between the non-CC and CC groups.
We also followed up 196 patients for 19.4 ± 11.3 months, and three patients were confirmed with metastatic PPGL (one each with pancreatic metastases, bone metastases, pulmonary and pelvic metastases). Twenty-three patients had concurrent tumors, including 17 in the non-CC group and 6 in the CC group, with no statistical difference between the two groups (17/175 vs. 6/41, 9.7% vs.14.6%, p=0.523). The types of concurrent tumors were medullary thyroid cancer (n=6, 26.1%), lung cancer (n=4, 17.4%), gastrointestinal stromal tumor (n=3, 13.0%), hematologic tumors (n=2, 8.7%), cervical cancers (n=2, 8.7%), ovarian borderline serous cystadenoma (n=1, 4.3%), ovarian teratoma (n=1, 4.3%), gastric cancer (n=1, 4.3%), kidney cancer (n=1, 4.3%), breast cancer (n=1, 4.3%) and meningioma (n=1, 4.3%).
Potential Risk Factors for CCs in Patients With PPGL
To explore the potential risk factors for CCs among patients with PPGL, parameters with p values of <0.1 were included in the multivariate logistic regression analysis. The clinical symptoms of patients with PPGL occurred due to CA secretion. Thus, interactions among these parameters were possible. As such, only the patterns of secretion were included in the multivariate logistic regression analysis. Finally, sex, age, DM, BMI, maximum SBP, maximum DBP, WBC count, platelet count, hemoglobin, plasma glucose and secretion pattern (epinephrine, norepinephrine, and combination) were included in the multivariate logistic regression analysis. The multivariate logistic regression analysis showed that platelet count [OR: 1.009, 95% confidence interval (CI): 1.001–1.016, p = 0.023] and the combination pattern of secretion (OR: 5.009, 95% CI: 1.365–18.380, p = 0.015) were independent risk factors for CCs in patients with PPGL (Table 4).
Discussion
In this retrospective study, we summarized our experience of PPGL associated with CCs. Our study provides insights into the frequency, clinical characteristics, and predictors of PPGL associated with CCs. We observed a relatively high prevalence (almost 19.0%) of CCs in patients with PPGL, suggesting the universality of CA-related cardiac damage. Some abnormalities that did not require medical treatment on ECG, including sinus tachycardia, premature complexes, and non-specific ST-T changes, were not classified as CCs because they are non-specific for PPGL-related cardiac damage; hence, the occurrence rate of CCs might have been underestimated. To our knowledge, we are the first to identify that platelet count and the combination-secreting pattern are independent risk factors for CCs in patients with PPGL.
In a recent study, the rate of classical triad symptoms in patients with PPGL was as low as 17% and less than 10% of PPGLs in general has no symptoms at all (8). In our study, 11.6% of the patients had no clinical symptoms, this result was consistent with what has been reported (8, 20). About 20% of the patients had mild nonspecific symptoms, such as anxiety, sweaty palms, etc., this should remind clinicians not to rely on the classical triad as a diagnostic threshold. Three patients (1.4%) presented with cardiogenic shock in our study, which was similar to the reported rates (5, 21). Unfortunately, we could not conduct further statistical analyses due to the small number of cases. However, there was an interesting phenomenon that Sattler et al (22)found a trend towards a lower risk for cardiogenic shock in Takotsubo cardiomyopathy (TTC) patients taking beta-blockers at admission. This may suggest that beta-blocker intake prior to the TTS event might protect against the deleterious effects of CAs (23). However, further studies are needed to determine the role of beta-blockers in this situation.
Due to the rarity of PPGL, many patients were only screened in the presence of characteristic symptoms. However, because of the widespread use of cross-sectional imaging, the main method used to identify PPGL has changed; in this study, 33.3% of PPGL cases were identified incidentally. However, in recent studies, there are about 60% of PPGLs were discovered incidentally (8, 24). The reason for the low proportion of incidental PPGLs in our data may be due to the fact that computed tomography (CT) is not carried out as a routine physical examination in China and patients may refuse to undergo CT due to concerns about the damage of radiation to the body; the recent emphasis on the treatment of HTN, has increased attention to the reasons of secondary HTN, wider knowledge of PPGLs means that doctors in our hospital are more likely to consider PPGLs as a differential diagnosis in patients with non-specific symptoms, which helps to improve the diagnosis of PPGLs.
In our study, 22.2% of the participants had DM, which corresponds to previously published data (15, 25, 26). Some researchers have suggested the pathogenesis of DM in PPGL is multifactorial and includes impaired insulin secretion and insulin deficiency, defects in insulin signalling and response, as well as increase endogenous glucose production and impaired exogenous glucose uptake (27). Novel contributing mechanisms are also emerging, such as the indirect effects of CAs on insulin sensitivity through adiponectin (27).DM is known to be a risk factor for cardiovascular diseases (28), which may explain the more patients in PPGL-CCs group suffered from DM. Sattler. et al (23) presented a strong association between TTC and malignant diseases, which was due to the high catecholaminergic state. Screening for concurrent tumors is therefore recommended for all patients with PPGL. Notably, PPGL can be one clinical phenotype of specific clinical syndromes, such as multiple endocrine neoplasia type 2(MEN2) (29). This may lead to unique features in the distribution of comorbid tumor types in PPGL. For example, in our study, medullary thyroid carcinoma was the most common concurrent tumor in PPGL patients who were subsequently diagnosed with MEN2. Therefore, we recommend timely concurrent tumors screening for PPGL patients, especially for tumors related to PPGL-related syndromes.
The total proportion of arrhythmias in PPGL patients in this study was similar to Zelinka ‘s study (4). There were only 2 patients suffering from AF. However, previous studies (30, 31) have shown that the in-hospital morbidity and mortality rates were significantly higher in TTC with than without AF (31). TTC and PPGL-TTC are believed to have similar physiopathology (5, 6, 32), and excessive CAs have also been reported to play an important role in the occurrence of adverse arrhythmias (33–36).Therefore, it is important to pay attention to the arrhythmias in PPGL. Although QTc interval prolongation in PPGL is rare, it has been reported in several cases (37, 38). The QTc prolongation could be also explained by elevated catecholamines, which can increase late sodium current and reduce the transient outward current (33, 34).
Notably, norepinephrine-predominant secretion was present in most patients with PPGL, and combination secretion was present in most patients with CCs. PPGLs with epinephrine-predominant secretion may present with hypotension and shock due to interplay between multiple factors, including intravascular volume depletion, abrupt cessation of CA secretion due to tumor necrosis, desensitization of adrenergic receptors, and hypocalcemia (21, 39). In the current study, almost half of the patients had PHEOs, with a similar proportion of PHEOs to PGLs as the reported studies (9, 40). However, another study showed that PGLs were less common than PHEOs (41). This discrepancy may be due to the limitations of retrospective single-center studies. For example, the locations of the PGLs, such as retroperitoneal or mediastinal, may make surgery difficult, and patients were thus often referred to our hospital for surgery, which may have increased the relative ratio of PGLs. Besides, since head and neck PGLs were included in our study, this did increase the proportion of PGLs. In agreement with the recognition that all PPGLs have a metastatic risk, we reported a higher rate of invasive behavior on pathology in PPGLs with CCs (9.8%), but the results of our study did not reach statistical significance. This may be due to the lack of standardized interpretation of the pathology results. Also, the presence of hemorrhage/necrosis on histology was more often seen in subjects with CCs than in those without CCs, this result is consistent with previous studies (10, 41). This conclusion should thus be cautiously promoted, and more research is necessary for definitive clarification.
It has been frequently maintained that PPGL-associated CCs are rare (42, 43). In the present study, almost 19.0% of patients with PPGL presented with CCs. Recent studies have also shown that the probability of CCs in PPGL is 10.8%-20.9% (10, 41, 44), and our results are consistent with this. To explore the potential risk factors for CCs among patients with PPGL, we conducted the multivariate logistic regression analysis. We found that a higher platelet count and a combination-secreting pattern were independent risk factors for CCs in patients with PPGL. Platelets play an interesting role in the development of CCs in PPGL. Human platelets express both adrenergic and dopaminergic receptors (45, 46). CAs modulate thrombopoiesis (47) and platelet function (48) through platelet α2-adrenergic or dopaminergic receptors. High concentrations of CAs are sufficient alone to induce human platelet aggregation, granule secretion, and release of platelet markers (49). In addition, high levels of epinephrine also increase the number of circulating platelet, which has been attributed to an adrenaline action on spleen blood flow (46). Platelet activation and aggregation are critically involved in the pathophysiology of various diseases such as HTN, DM and atherosclerosis (50, 51). Clinical data also support the role of platelets in regulation of the immune response. The inappropriate and activation of a proinflammatory reaction has been linked to atherosclerosis (52). These theories may explain that increased platelet count in PPGLs is a risk factor for CCs in patients with PPGLs.
The pathogenesis of CCs associated with PPGL is generally thought to be similar to that of CA cardiomyopathy (7, 53). The pathophysiology of CA excess in the myocardium includes functional hypoxia due to increased contractility and coronary spasm leading to decreased blood flow, increased oxygen consumption due to excessive free fatty acid-induced mitochondrial uncoupling, intracellular calcium excess, stimulation of cell growth and cardiomyocyte hypertrophy, induction of interstitial fibrosis and scarring, chronic inflammation, direct toxicity, and generation of oxidative stress (54–56). However, CAs are unlikely to be the only mediators of CCs because some patients with very high levels of biochemical markers did not have CCs; in addition, individual patients respond variably to CAs, which may be due to the differential expression of adrenergic receptors (57, 58). The real etiologic link of PPGL-CCs remains unclear and seems to be multifactorial.
Our study has several limitations. First, the sample size was small. However, this is related to the rare incidence of PPGL. Second, bias was inevitable based on the retrospective and single-center study design. For example, BP classification was based on 24-hour ambulatory BP measurements if available, or otherwise on medical records. If paroxysmal BP elevation was detected during the course of the disease by the patients or by the medical staff during hospitalization, the patient was then classified into the “paroxysmal HTN” group. The limitations of retrospective studies mean that this classification may under-record patients with paroxysmal HTN. Third, cardiac magnetic resonance imaging (MRI) was not systematically evaluated. Relying solely on echocardiography may have resulted in some cases of myocardial involvement being missed. However, the limited availability of cardiac MRI makes it unsuitable for use as primary imaging modality for all patients. Fourth, the possibility of reporting bias with overestimation of uncharacteristic presentations and non-representative cases cannot be ruled out. This is again unavoidable due to the infrequent occurrence of this disease. Finally, these results should be generalized with caution owing to center-specific referral bias.
Conclusion
PPGL can lead to life-threatening events and sometimes death; however, timely diagnosis and surgery can be life-saving. In all patients with PPGLs, even in the absence of signs and symptoms of CCs, a work up of cardiology should be strongly considered. Importantly, if patients with PPGLs have higher platelet counts and the combination-secreting pattern, they are more likely to have CCs. Thus, a careful cardiac evaluation should be performed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital. The requirement for informed consent was waived because of the retrospective nature of the study.
Author Contributions
ZL, MX, and LYC designed the study. ZL collected patients'data. ZL, MX, and MQM performed the analyses and wrote the paper. FH assisted with data collection and analysis. ZXL, ZHD, and ZSY assisted with the study design. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the Science and Technology Innovation 2030 “New Generation Artificial Intelligence” Major Project (Ministry of Science and Technology) (2020AAA0109600), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320057), the CAMS Innovation Fund for Medical Sciences (2016-I2M-1-002), and the National Key Research and Development Program of China (2016YFC1300100). The funding sources had no involvement in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all of the study patients for their participation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.877341/full#supplementary-material
References
1. Garcia-Carbonero R, Matute Teresa F, Mercader-Cidoncha E, Mitjavila-Casanovas M, Robledo M, Tena I, et al. Multidisciplinary Practice Guidelines for the Diagnosis, Genetic Counseling and Treatment of Pheochromocytomas and Paragangliomas. Clin Trans Oncol (2021) 23(10):1995–2019. doi: 10.1007/s12094-021-02622-9
2. Y-Hassan S, Falhammar H. Clinical Features, Complications, and Outcomes of Exogenous and Endogenous Catecholamine-Triggered Takotsubo Syndrome: A Systematic Review and Meta-Analysis of 156 Published Cases. Clin Cardiol (2020) 43(5):459–67. doi: 10.1002/clc.23352
3. Y-Hassan S, Falhammar H. Cardiovascular Manifestations and Complications of Pheochromocytomas and Paragangliomas. J Clin Med (2020) 9(8):2435. doi: 10.3390/jcm9082435
4. Zelinka T, Petrák O, Turková H, Holaj R, Strauch B, Kršek M, et al. High Incidence of Cardiovascular Complications in Pheochromocytoma. Hormone Metab Res = Hormon- Und Stoffwechselforschung = Hormones Metabol (2012) 44(5):379–84. doi: 10.1055/s-0032-1306294
5. Santos JRU, Brofferio A, Viana B, Pacak K. Catecholamine-Induced Cardiomyopathy in Pheochromocytoma: How to Manage a Rare Complication in a Rare Disease? Hormone Metab Res = Hormon- Und Stoffwechselforschung = Hormones Metabol (2019) 51(7):458–69. doi: 10.1055/a-0669-9556
6. Agarwal V, Kant G, Hans N, Messerli FH. Takotsubo-Like Cardiomyopathy in Pheochromocytoma. Int J Cardiol (2011) 153(3):241–8. doi: 10.1016/j.ijcard.2011.03.027
7. Y-Hassan S, Falhammar H. Pheochromocytoma- and Paraganglioma-Triggered Takotsubo Syndrome. Endocrine (2019) 65(3):483–93. doi: 10.1007/s12020-019-02035-3
8. Falhammar H, Kjellman M, Calissendorff J. Initial Clinical Presentation and Spectrum of Pheochromocytoma: A Study of 94 Cases From a Single Center. Endocrine Connect (2018) 7(1):186–92. doi: 10.1530/EC-17-0321
9. Gagnon N, Mansour S, Bitton Y, Bourdeau I. Takotsubo-Like Cardiomyopathy In A Large Cohort Of Patients With Pheochromocytoma And Paraganglioma. Endocrine Pract (2017) 23(10):1178–92. doi: 10.4158/EP171930.OR
10. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute Catecholamine Cardiomyopathy in Patients With Phaeochromocytoma or Functional Paraganglioma. Heart (2013) 99(19):1438–44. doi: 10.1136/heartjnl-2013-304073
11. Zhang R, Gupta D, Albert SG. Pheochromocytoma as a Reversible Cause of Cardiomyopathy: Analysis and Review of the Literature. Int J Cardiol (2017) 249:319–23. doi: 10.1016/j.ijcard.2017.07.014
12. Shi F, Sun LX, Long S, Zhang Y. Pheochromocytoma as a Cause of Repeated Acute Myocardial Infarctions, Heart Failure, and Transient Erythrocytosis: A Case Report and Review of the Literature. World J Clin Cases (2021) 9(4):951–9. doi: 10.12998/wjcc.v9.i4.951
13. Y-Hassan S. Recurrent Takotsubo Syndrome Triggered by Undiagnosed Pheochromocytoma. Int J Cardiol (2015) 187:369–71. doi: 10.1016/j.ijcard.2015.03.220
14. Y-Hassan S, Falhammar H. Stumbling Broke the Spleen and Unveiled Pheochromocytoma, Which in Turn Broke the Heart. Endocrine (2020) 67(3):727–8. doi: 10.1007/s12020-019-02169-4
15. Elenkova A, Matrozova J, Vasilev V, Robeva R, Zacharieva S. Prevalence and Progression of Carbohydrate Disorders in Patients With Pheochromocytoma/Paraganglioma: Retrospective Single-Center Study. Annales d'endocrinol (2020) 81(1):3–10. doi: 10.1016/j.ando.2020.01.001
16. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet (2005) 366(9486):665–75. doi: 10.1016/S0140-6736(05)67139-5
17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
18. Bybee KA, Prasad A. Stress-Related Cardiomyopathy Syndromes. Circulation (2008) 118(4):397–409. doi: 10.1161/CIRCULATIONAHA.106.677625
19. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2014) 99(6):1915–42. doi: 10.1210/jc.2014-1498
20. Kopetschke R, Slisko M, Kilisli A, Tuschy U, Wallaschofski H, Fassnacht M, et al. Frequent Incidental Discovery of Phaeochromocytoma: Data From a German Cohort of 201 Phaeochromocytoma. Eur J Endocrinol (2009) 161(2):355–61. doi: 10.1530/EJE-09-0384
21. Bergland BE. Pheochromocytoma Presenting as Shock. Am J Emergency Med (1989) 7(1):44–8. doi: 10.1016/0735-6757(89)90084-3
22. Sattler K, El-Battrawy I, Gietzen T, Kummer M, Lang S, Zhou XB, et al. Improved Outcome of Cardiogenic Shock Triggered by Takotsubo Syndrome Compared With Myocardial Infarction. Can J Cardiol (2020) 36(6):860–7. doi: 10.1016/j.cjca.2019.10.012
23. Sattler K, El-Battrawy I, Lang S, Zhou X, Schramm K, Tülümen E, et al. Prevalence of Cancer in Takotsubo Cardiomyopathy: Short and Long-Term Outcome. Int J Cardiol (2017) 238:159–65. doi: 10.1016/j.ijcard.2017.02.093
24. Gruber LM, Hartman RP, Thompson GB, McKenzie TJ, Lyden ML, Dy BM, et al. Pheochromocytoma Characteristics and Behavior Differ Depending on Method of Discovery. J Clin Endocrinol Metab (2019) 104(5):1386–93. doi: 10.1210/jc.2018-01707
25. Khatiwada S, Agarwal S, Kandasamy D, Jyotsna VP, Kumar R, Kumar Bansal V, et al. Diabetes Mellitus in Pheochromocytoma and Paraganglioma: Prevalence, Dynamics of Insulin Secretion / Sensitivity and Predictors of Remission. Diabetes Metab Syndr (2020) 14(6):2169–75. doi: 10.1016/j.dsx.2020.10.030
26. Komada H, Hirota Y, So A, Nakamura T, Okuno Y, Fukuoka H, et al. Insulin Secretion and Insulin Sensitivity Before and After Surgical Treatment of Pheochromocytoma or Paraganglioma. J Clin Endocrinol Metab (2017) 102(9):3400–5. doi: 10.1210/jc.2017-00357
27. Mesmar B, Poola-Kella S, Malek R. The Physiology Behind Diabetes Mellitus in Patients With Pheochromocytoma: A Review of the Literature. Endocrine Pract (2017) 23(8):999–1005. doi: 10.4158/EP171914.RA
28. UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control With Sulphonylureas or Insulin Compared With Conventional Treatment and Risk of Complications in Patients With Type 2 Diabetes (UKPDS 33). Lancet (1998) 352(9131):837–53. doi: 10.1016/S0140-6736(98)07019-6
29. Neumann HP, Young WF Jr., Krauss T, Bayley JP, Schiavi F, Opocher G, et al. 65 Years of the Double Helix: Genetics Informs Precision Practice in the Diagnosis and Management of Pheochromocytoma. Endocrine-related Cancer (2018) 25(8):T201–t19. doi: 10.1530/ERC-18-0085
30. El-Battrawy I, Lang S, Ansari U, Behnes M, Hillenbrand D, Schramm K, et al. Impact of Concomitant Atrial Fibrillation on the Prognosis of Takotsubo Cardiomyopathy. Europace (2017) 19(8):1288–92. doi: 10.1093/europace/euw293
31. El-Battrawy I, Cammann VL, Kato K, Szawan KA, Di Vece D, Rossi A, et al. Impact of Atrial Fibrillation on Outcome in Takotsubo Syndrome: Data From the International Takotsubo Registry. J Am Heart Assoc (2021) 10(15):e014059. doi: 10.1161/JAHA.119.014059
32. Batisse-Lignier M, Pereira B, Motreff P, Pierrard R, Burnot C, Vorilhon C, et al. Acute and Chronic Pheochromocytoma-Induced Cardiomyopathies: Different Prognoses?: A Systematic Analytical Review. Med (Baltimore) (2015) 94(50):e2198. doi: 10.1097/MD.0000000000002198
33. El-Battrawy I, Santoro F, Stiermaier T, Möller C, Guastafierro F, Novo G, et al. Prevalence, Management, and Outcome of Adverse Rhythm Disorders in Takotsubo Syndrome: Insights From the International Multicenter GEIST Registry. Heart Fail Rev (2020) 25(3):505–11. doi: 10.1007/s10741-019-09856-4
34. El-Battrawy I, Zhao Z, Lan H, Schünemann JD, Sattler K, Buljubasic F, et al. Estradiol Protection Against Toxic Effects of Catecholamine on Electrical Properties in Human-Induced Pluripotent Stem Cell Derived Cardiomyocytes. Int J Cardiol (2018) 254:195–202. doi: 10.1016/j.ijcard.2017.11.007
35. Huang M, Yang Z, Li Y, Lan H, Cyganek L, Yuecel G, et al. Dopamine D1/D5 Receptor Signaling Is Involved in Arrhythmogenesis in the Setting of Takotsubo Cardiomyopathy. Front Cardiovasc Med (2021) 8:777463. doi: 10.3389/fcvm.2021.777463
36. El-Battrawy I, Lang S, Ansari U, Tülümen E, Schramm K, Fastner C, et al. Prevalence of Malignant Arrhythmia and Sudden Cardiac Death in Takotsubo Syndrome and its Management. Europace (2018) 20(5):843–50. doi: 10.1093/europace/eux073
37. van der Heide K, de Haes A, Wietasch GJ, Wiesfeld AC, Hendriks HG. Torsades De Pointes During Laparoscopic Adrenalectomy of a Pheochromocytoma: A Case Report. J Med Case Rep (2011) 5:368. doi: 10.1186/1752-1947-5-368
38. Chakraborty P, Bhattacharjeee HK, Anandaraja S. Palpitition, Presyncope and Abdominal Mass. Indian Heart J (2010) 62(5):447–8.
39. Olson SW, Deal LE, Piesman M. Epinephrine-Secreting Pheochromocytoma Presenting With Cardiogenic Shock and Profound Hypocalcemia. Ann Intern Med (2004) 140(10):849–51. doi: 10.7326/0003-4819-140-10-200405180-00033
40. Leung AA, Pasieka JL, Hyrcza MD, Pacaud D, Dong Y, Boyd JM, et al. Epidemiology of Pheochromocytoma and Paraganglioma: Population-Based Cohort Study. Eur J Endocrinol (2021) 184(1):19–28. doi: 10.1530/EJE-20-0628
41. Zhou J, Xuan H, Miao Y, Hu J, Dai Y. Acute Cardiac Complications and Subclinical Myocardial Injuries Associated With Pheochromocytoma and Paraganglioma. BMC Cardiovasc Disord (2021) 21(1):203. doi: 10.1186/s12872-021-02013-6
42. Yu R, Nissen NN, Chopra P, Dhall D, Phillips E, Wei M. Diagnosis and Treatment of Pheochromocytoma in an Academic Hospital From 1997 to 2007. Am J Med (2009) 122(1):85–95. doi: 10.1016/j.amjmed.2008.08.021
43. Manger WM. An Overview of Pheochromocytoma: History, Current Concepts, Vagaries, and Diagnostic Challenges. Ann New York Acad Sci (2006) 1073:1–20. doi: 10.1196/annals.1353.001
44. Yu R, Nissen NN, Bannykh SI. Cardiac Complications as Initial Manifestation of Pheochromocytoma: Frequency, Outcome, and Predictors. Endocrine Pract (2012) 18(4):483–92. doi: 10.4158/EP11327.OR
45. Ricci A, Bronzetti E, Mannino F, Mignini F, Morosetti C, Tayebati SK, et al. Dopamine Receptors in Human Platelets. Naunyn-Schmiedeberg's Arch Pharmacol (2001) 363(4):376–82. doi: 10.1007/s002100000339
46. Anfossi G, Trovati M. Role of Catecholamines in Platelet Function: Pathophysiological and Clinical Significance. Eur J Clin Invest (1996) 26(5):353–70. doi: 10.1046/j.1365-2362.1996.150293.x
47. Chen S, Du C, Shen M, Zhao G, Xu Y, Yang K, et al. Sympathetic Stimulation Facilitates Thrombopoiesis by Promoting Megakaryocyte Adhesion, Migration, and Proplatelet Formation. Blood (2016) 127(8):1024–35. doi: 10.1182/blood-2015-07-660746
48. Tschuor C, Asmis LM, Lenzlinger PM, Tanner M, Härter L, Keel M, et al. In Vitro Norepinephrine Significantly Activates Isolated Platelets From Healthy Volunteers and Critically Ill Patients Following Severe Traumatic Brain Injury. Crit Care (London England) (2008) 12(3):R80. doi: 10.1186/cc6931
49. Amadio P, Zarà M, Sandrini L, Ieraci A, Barbieri SS. Depression and Cardiovascular Disease: The Viewpoint of Platelets. Int J Mol Sci (2020) 21(20):7560. doi: 10.3390/ijms21207560
50. Beckman JA, Creager MA, Libby P. Diabetes and Atherosclerosis: Epidemiology, Pathophysiology, and Management. Jama (2002) 287(19):2570–81. doi: 10.1001/jama.287.19.2570
51. Ouviña SM, La Greca RD, Zanaro NL, Palmer L, Sassetti B. Endothelial Dysfunction, Nitric Oxide and Platelet Activation in Hypertensive and Diabetic Type II Patients. Thromb Res (2001) 102(2):107–14. doi: 10.1016/S0049-3848(01)00237-7
52. Camilli M, Iannaccone G, La Vecchia G, Cappannoli L, Scacciavillani R, Minotti G, et al. Platelets: The Point of Interconnection Among Cancer, Inflammation and Cardiovascular Diseases. Expert Rev Hematol (2021) 14(6):537–46. doi: 10.1080/17474086.2021.1943353
53. Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular Manifestations of Phaeochromocytoma. J Hypertens (2011) 29(11):2049–60. doi: 10.1097/HJH.0b013e32834a4ce9
54. Adameova A, Abdellatif Y, Dhalla NS. Role of the Excessive Amounts of Circulating Catecholamines and Glucocorticoids in Stress-Induced Heart Disease. Can J Physiol Pharmacol (2009) 87(7):493–514. doi: 10.1139/Y09-042
55. Galetta F, Franzoni F, Bernini G, Poupak F, Carpi A, Cini G, et al. Cardiovascular Complications in Patients With Pheochromocytoma: A Mini-Review. BioMed Pharmacother (2010) 64(7):505–9. doi: 10.1016/j.biopha.2009.09.014
56. Fan X, Yang G, Kowitz J, Akin I, Zhou X, El-Battrawy I. Takotsubo Syndrome: Translational Implications and Pathomechanisms. Int J Mol Sci (2022) 23(4):1951. doi: 10.3390/ijms23041951
57. Wilcox CS, Aminoff MJ. Blood Pressure Responses to Noradrenaline and Dopamine Infusions in Parkinson's Disease and the Shy-Drager Syndrome. Br J Clin Pharmacol (1976) 3(2):207–14. doi: 10.1111/j.1365-2125.1976.tb00594.x
Keywords: pheochromocytoma, paraganglioma, cardiovascular complications, hypertension, catecholamines (CAs)
Citation: Zhao L, Meng X, Mei Q, Fan H, Liu Y, Zhou X, Zhu H and Zhang S (2022) Risk Factors for Cardiac Complications in Patients With Pheochromocytoma and Paraganglioma: A Retrospective Single-Center Study. Front. Endocrinol. 13:877341. doi: 10.3389/fendo.2022.877341
Received: 16 February 2022; Accepted: 12 April 2022;
Published: 01 June 2022.
Edited by:
Peiyuan Yin, Dalian Medical University, ChinaReviewed by:
Henrik Falhammar, Karolinska Institutet (KI), SwedenIbrahim El-Battrawy, Ruhr University Bochum, Germany
Copyright © 2022 Zhao, Meng, Mei, Fan, Liu, Zhou, Zhu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: YeCheng Liu, cHRjYWxpdUBzaW5hLmNvbQ==; XianLiang Zhou, emhvdXhpYW5saWFuZzAzMjZAaG90bWFpbC5jb20=; HuaDong Zhu, ZHJ6aHVoZEBzaW5hLmNvbQ==; ShuYang Zhang, c2h1eWFuZ3poYW5nMTAzQG5yZHJzLm9yZw==
†These authors have contributed equally to this work and share first authorship
 Lin Zhao
Lin Zhao Xu Meng
Xu Meng QiMin Mei2†
QiMin Mei2† YeCheng Liu
YeCheng Liu HuaDong Zhu
HuaDong Zhu