
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Endocrinol., 29 April 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.874968
This article is part of the Research TopicMeal Replacement Impacts on Individuals with DiabetesView all 5 articles
A commentary has been posted on this article:
Commentary: Is there a role for diabetes-specific nutrition formulas as meal replacements in type 2 diabetes?
 Jarvis C. Noronha1,2*
Jarvis C. Noronha1,2* Jeffrey I. Mechanick3
Jeffrey I. Mechanick3Nutrition therapy plays an integral role in the prevention and management of patients with type 2 diabetes (T2D). A potential strategy is the utilization of diabetes-specific nutrition formulas (DSNFs) as meal replacements. In this article, we distinguish DSNFs from standard nutrition formulas, review the clinical data examining the effectiveness of DSNFs, and propose an evidence-based algorithm for incorporating DSNFs as part of nutrition therapy in T2D. DSNFs contain slowly-digestible carbohydrates, healthy fats (e.g., monounsaturated fatty acids), and specific micronutrients, which provide added benefits over standard nutrition formulas. In short- and long-term clinical trials, DSNFs demonstrate improvements in postprandial glycemic responses translating into sustainable benefits in long-term glycemic control (e.g., hemoglobin A1c and glycemic variability) and various cardiometabolic outcomes. To facilitate the delivery of DSNFs in a clinical setting, the transcultural diabetes nutrition algorithm can be utilized based on body weight (underweight, normal weight, or overweight) and level of glycemic control (controlled or uncontrolled).
Type 2 diabetes (T2D) is a well-established risk factor for cardiovascular disease, the leading cause of death in the world (1–3). Individuals with T2D are also at a significant risk for developing microvascular complications including, retinopathy, nephropathy and neuropathy. The International Diabetes Federation estimates that 643 million people or 11.3% of the global population will have diabetes by 2030. If these trends continue, this estimate is predicted to rise to 783 million or 12.2% of the global population by 2045 (4). Effective strategies are urgently needed to slow the burden of disease in this burgeoning population.
Nutrition therapy plays an integral role in the prevention and treatment of T2D. Core principles of nutrition and T2D include (1): individualized medical nutrition therapy (provided by a registered dietitian nutritionist whenever possible) that achieves healthy body composition, eating patterns, and macronutrient distributions based on preventative/therapeutic goals (with an emphasis on fiber, pulses, unprocessed whole foods, healthy fats, and limits on added sugars, refined grains, and alcohol); (2) recommendation of dietary supplements and nutraceuticals only when they are shown to have proven benefits that outweigh risks; and (3) synchronization of carbohydrate with insulin or secretagogue (e.g., sulfonylureas and glinides) therapy (5). In addition, clinical practice guidelines by the American Association of Clinical Endocrinology (formerly known as American Association of Clinical Endocrinologists), American Diabetes Association (ADA), Diabetes Canada (formerly known as Canadian Diabetes Association), and the European Association for the Study of Diabetes (EASD) recommend consumption of a low glycemic index (GI) diet that is high in fibre and monounsaturated fatty acids as part of healthy eating in T2D (6, 7).
Micronutrient deficiency states are common in T2D and should also be addressed: prevalence rates can range from 58-63% for vitamin B6 (pyridoxine), 13-55% for vitamin C, 85-91% for vitamin D, and 19% for zinc (8). These deficiency states are associated with increased oxidative stress, inflammation, and immune abnormalities (9). In T2D, nutritional deficiencies are associated with the progression of β-cell dysfunction, to loss of β-cell mass, and then to insulin signaling impairment and hyperinsulinemia (9). Nevertheless, as with any nutritional epidemiological finding and clinical correlate, the presence of a micronutrient deficiency (e.g., vitamin D deficiency) does not directly infer that clinically significant outcomes (e.g., improved glycemic control) will result with an intervention (e.g., vitamin D supplementation).
Notwithstanding the above caveat, diabetes-specific nutrition formulas (DSNFs) provide an opportunity to deliver essential macronutrients and micronutrients that align with clinical practice guideline recommendations for nutrition therapy in T2D. In this article, the role of DSNFs in T2D will be clarified based on the extant scientific literature. Specifically, DSNFs will be distinguished from standard nutrition formulas, clinical data on the effectiveness of DSNFs critically assessed, and an evidence-based algorithm for incorporating DSNFs as part of nutrition therapy in T2D proposed.
DSNFs have a defined macronutrient and micronutrient composition to manage dysglycemia, malnutrition, and other cardiometabolic risk factors. These formulas contain modified carbohydrates that have a low GI and complementary dietary recommendations for patients with T2D. In addition, DSNFs generally contain fiber, unsaturated fatty acids, proteins, vitamins and minerals in palatable and calorie-controlled portions. These formulas are typically used as iso- or hypocaloric meal/snack replacements, hypercaloric supplementation for malnourished patients, enteral nutrition support, and in the context of very-low-calorie diets, as determined by clinical circumstances and the discretion of prescribing healthcare professionals. The macronutrient distribution (carbohydrates:fats:proteins) of DSNFs [37–55%:30–45%:15–19%, respectively and averaged from four common formulas (10–13)] compare well with distributions from various eating patterns, such as the Mediterranean Diet (40–50%:35–40%:15–20%, respectively) and clinical practice guidelines by professional societies (e.g., ADA: 45%:20–35%:15–20%, respectively) (9). In contrast, standard formulas may be high in rapidly digested carbohydrates (high GI), with varying fat content, and therefore compromising glycemic control.
The role of DSNFs in nutrition management of patients with T2D with respect to glycemic control and cardiometabolic risks can be clarified through a critical evaluation of the scientific data (Table 1).
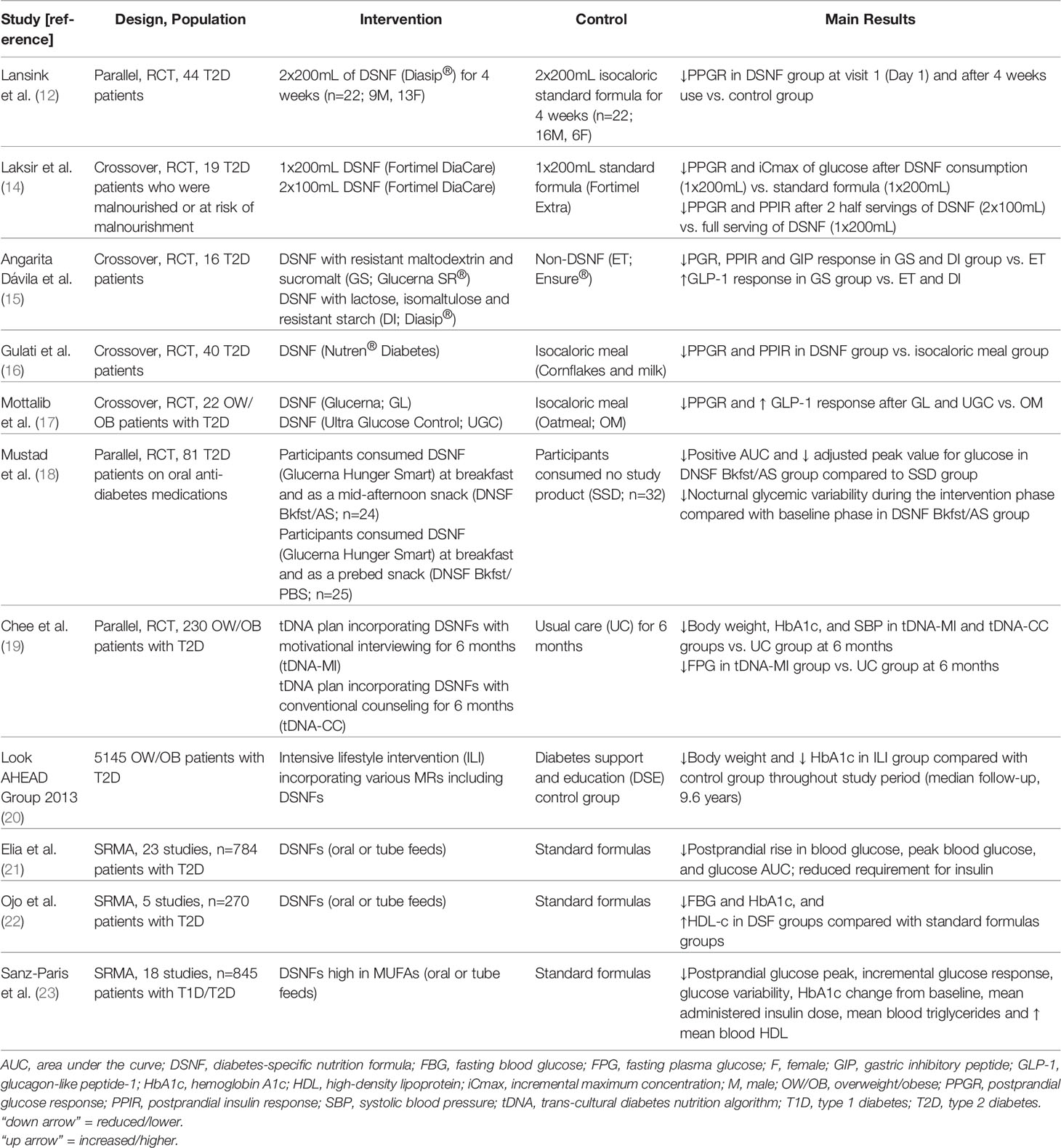
Table 1 Effect of Diabetes-Specific Nutrition Formulas on Glycemic and Cardiometabolic Endpoints in Patients with Type 2 Diabetes.
DSNFs vs. standard formulas: there are demonstrable benefits of DSNFs on acute glucose tolerance when compared with standard nutrition formulas (12, 14, 15).
In a randomized, controlled, double-blind, parallel-group study, 44 patients with T2D consumed 2 x 200 mL DSNF/day (n=22) or an isocaloric standard, fiber-containing control formula (n=22) for 4 weeks. Results showed that the four-hour postprandial glucose response was significantly lower after consumption of the DSNF, compared with the standard formula group, at baseline and remained significantly lower after the 4-week intervention period, suggesting superior PPG control by DSNF (12). There was high compliance (>90%) in both groups. Both products were equally well tolerated, with a low incidence and mild intensity of reported symptoms. This study reported no serious adverse events and no dropouts for product-related reasons (12).
A separate study examined the effect of DSNF serving size in patients with T2D and malnutrition (or at-risk for malnutrition) (14). In this double blind, crossover study, 20 patients randomly received the DSNF (1 x 200mL) and the standard nutritional supplement control (1 x 200mL) on two separate days (visit 1 and 2), separated by a washout of 3-14 days. After a second wash-out period after visit 2, 17/20 patients completed an open label third study day (visit 3) where patients consumed the DSNF in serving sizes of 100 mL (2 x 100mL), of which the first serving was at breakfast and the second serving was 2 hours later. The 4-hour postprandial glucose response was lower after intake of the DSNF (1 x 200mL) compared with the control (1 x 200mL), with no difference in the insulin response. Additionally, the 4-hour postprandial glucose and insulin response was significantly lower after oral intake of two half servings of DSNF (2 x 100mL) compared with one full serving of DSNF (1 x 200mL). All patients were reported to have consumed the DSNF or control within the allocated time (10mins) and were 100% compliant for each dosing during intake. Adverse events were reported, however, the authors concluded that none of them were considered to be related to consumption of the study products and did not qualify as a safety signal. These data suggest that the use of a DSNF is preferred for patients with T2D in need of nutritional support and that splitting oral intake of DSNF into two half servings 2 hours apart may provide additional advantages in blunting postprandial glycemic and insulinemic excursions (14).
Lastly, a study which looked at more specialized DSNFs containing isomaltulose and sucromalt in 16 patients with T2D found lower postprandial glucose, insulin, and gastric inhibitory polypeptide responses with higher glucagon-like peptide-1 (GLP-1) levels, and decreased subjective appetite responses compared with standard formula (15). Seven patients withdrew from the study (initial sample: 23 patients): four withdrew due to introduction of medical/lifestyle therapy and three were due to voluntary withdrawal. The study products were well-tolerated with no patients reporting nausea, dizziness or vomiting after consumption.
DSNFs vs. standard test meals: there are demonstrable advantages of DSNFs over standard test meals in acute clinical trials (16, 17).
In an open-label, randomized, crossover study, 40 patients with T2D were randomly assigned to receive either a DSNF or an isocaloric meal (cornflakes and milk) after a run-in period. The DSNF group showed decreased post-meal blood glucose and insulin excursion as compared with the isocaloric meal comparator group. The authors concluded that the DSNF was well-tolerated and may be a suitable option for patients with T2D in need of nutritional support (16).
In a separate study, two DSNFs were compared with oatmeal on glucose, insulin, GLP-1, free fatty acid (FFA) and triglyceride (TG) responses. Twenty-five patients with T2D were enrolled, of which 22 patients completed all study visits. The three drop-outs were indicated to be for personal reasons. Both DSNFs resulted in lower postprandial glucose response and higher postprandial GLP-1 responses, compared with oatmeal, with no differences in insulin, FFA, and TG levels (17).
A recent pilot study evaluated the impact of DSNF used twice daily (either at breakfast and afternoon snack, or at breakfast and pre-bed snack) by patients with T2D (18). After a baseline period where study participants consumed their habitual self-selected diets for six days, patients with T2D were randomized into three groups: (1) self-selected diet group consumed no study product; (2) DSNF breakfast/afternoon snack group consumed one DSNF as a breakfast meal replacement and a second as a mid-afternoon snack replacement; and (3) DSNF breakfast/pre-bed snack group consumed one DSNF as a breakfast meal replacement and a second as a pre-bed snack replacement. Glycemic response was assessed by continuous glucose monitoring. One-hundred and twenty-five patients were randomized of which 116 completed the study. A total of 81 patients who were evaluable (>80% adherent to study instructions) were analyzed (DSNF breakfast/afternoon snack group, N=24; DSNF breakfast/pre-bed snack group, N=25; self-selected diet group, n=32). Patients were well-compliant during the intervention phase (~6 days) consuming ~2 servings of DNSF per day. Overall, the DSNF breakfast/afternoon snack group demonstrated greater reductions in positive area under the curve (AUC) and adjusted peak value for blood glucose when compared to the self-selected diet group. Interestingly, the DSNF breakfast/afternoon snack group also had greater reductions in nocturnal glycemic variability, reported less cravings for starchy meals/sides, and exhibited increased confidence in selecting foods to control their diabetes when compared to baseline measurements prior to interventions. These data suggest that use of DNSFs to replace breakfast and as an afternoon snack can improve glycemic control and behavioral factors associated with management of T2D.
DNSFs in longer-term studies: sustainable reductions in glycemic control have also been observed with DSNF use (19, 20).
In a study conducted in Malaysia which implemented the transcultural diabetes nutrition algorithm (tDNA), patients with T2D were randomized to one of three interventions for 6 months: (1) tDNA with motivational interviewing, (2) tDNA with conventional counseling, or (3) usual care. The tDNA intervention consisted of a structured low-calorie meal plan, DSNFs, and increased physical activity. All patients were also followed up at 12 months. Patient retention rates were: 51/58 (88%) in the tDNA with motivational interviewing group, 40/57 (70%) in tDNA with conventional counseling group, and 98/115 (85%) in the usual care group. At 6 months, hemoglobin A1c (HbA1c) decreased on average by 1.1% in patients incorporating tDNA with motivational interviewing and 0.5% in patients incorporating tDNA with conventional counseling, with no significant changes in patients under usual care. Body weight also decreased significantly in patients incorporating tDNA with motivational interviewing (~7kg) and in patients incorporating tDNA with conventional counseling (~5kg), with no significant changes in patients under usual care. At 1 year, weight loss and reductions in HbA1c were only maintained in patients incorporating tDNA with motivational interviewing (19). In a follow-up study (24), eating self-efficacy was assessed using a locally validated Weight Efficacy Lifestyle (WEL) questionnaire. Eating self-efficacy is the confidence in an individual’s own ability to perform specific tasks to attain certain goals in challenging situations. Overall, eating self-efficacy improved in patients who maintained their weight loss and glycemic control following the tDNA approach utilizing DSNFs and the improvement was further enhanced with motivational interviewing.
In the large, multi-centre, Look AHEAD Trial (20, 25, 26), an intensive lifestyle intervention group, which incorporated various meal replacements including DSNFs, was compared to a diabetes support and education group. Although widespread significant comparative differences were seen in the first year, participants in the intensive lifestyle intervention group retained greater improvements than participants in the diabetes support and education group at 4 years in % weight loss (-6.15% vs. -0.88%, p < 0.001), HbA1c (-0.36% vs. -0.09%, p < 0.001), systolic blood pressure (-5.33 vs. -2.97 mmHg, p < 0.001), diastolic blood pressure (-2.92 vs. -2.48 mmHg, p=0.01), and high-density lipoprotein (HDL)-cholesterol (3.67 vs. 1.97 mg/dL, p < 0.001) (25). At year 8, clinically meaningful weight loss of ≥5% was still observed in 50.3% of patients in the intensive lifestyle intervention group (26).
Evidence from systematic reviews and meta-analyses: several systematic reviews and meta-analyses have also been conducted to examine the totality of the evidence in relation to the effectiveness of DSNFs.
In 2005, Elia et al. (21) identified 20 studies that compared the short- and long-term use of DSNFs with standard formulas on glycemic control in patients with type 1 diabetes or T2D. They found that DSNFs, when compared with standard formulas, resulted in significantly reduced postprandial rise in blood glucose, peak blood glucose concentration, and glucose AUC (by ~35%) with no significant effect on HDL, total cholesterol, or triglyceride concentrations. Reduced insulin requirement and fewer complications with DSNFs were also observed in individual studies when compared with standard nutritional formulas.
A separate systematic review and meta-analysis conducted by Ojo et al. (22) in 2019 focused on evaluating the effectiveness of DSNFs versus standard formulas on glycemic control in patients with T2D. Meta-analysis of 5 studies showed that DSNFs resulted in significantly lower fasting blood glucose levels (by 1.15 mmol [-2.07, -0.23]) and HbA1c levels (by 0.67% [-1.14, -0.21]) with no effect on total cholesterol, low-density lipoprotein cholesterol, and triglycerides when compared to standard formulas. They also found slightly greater HDL-c levels (by 0.09 mmol/L [0.00, 0.18]) in DSNFs groups compared with standard formula groups.
Lastly, a recent systematic review and meta-analysis conducted by Sanz-Paris et al. (23) compared the effects of DSNFs with a high content of monounsaturated fatty acids with standard formulas on outcomes of glycemic control, lipid metabolism, and tolerance. Eighteen studies involving 845 patients were included. This meta-analysis showed that DSNFs with a high content of monounsaturated fatty acids significantly decreased peak postprandial glucose, incremental glucose response, mean blood glucose, glucose variability, HbA1c, AUC plasma insulin, mean administered insulin dose, and mean blood total triglycerides, as well as significantly increased HDL-c (23). Analysis of gastrointestinal adverse events reported by authors of the included studies revealed no significant differences among treatments.
In 2010, the tDNA was developed as a tool to facilitate the delivery of nutrition therapy to patients with prediabetes and T2D in a variety of cultures and geographic locations. This included guidance on how many DSNFs patients should consume based on their body weight (underweight, normal weight or overweight) and level of glycemic control (uncontrolled or controlled) (Table 2). For example, patients with T2D and overweight/obesity are recommended to consume 2 to 3 DSNFs per day, as meal and/or snack replacements, as part of a reduced calorie meal plan. In contrast, patients with uncontrolled T2D (i.e., HbA1c> 7%) are recommended to consume 1 to 2 DSNFs per day, as meal and/or snack replacements, as part of a lower GI meal plan (27).
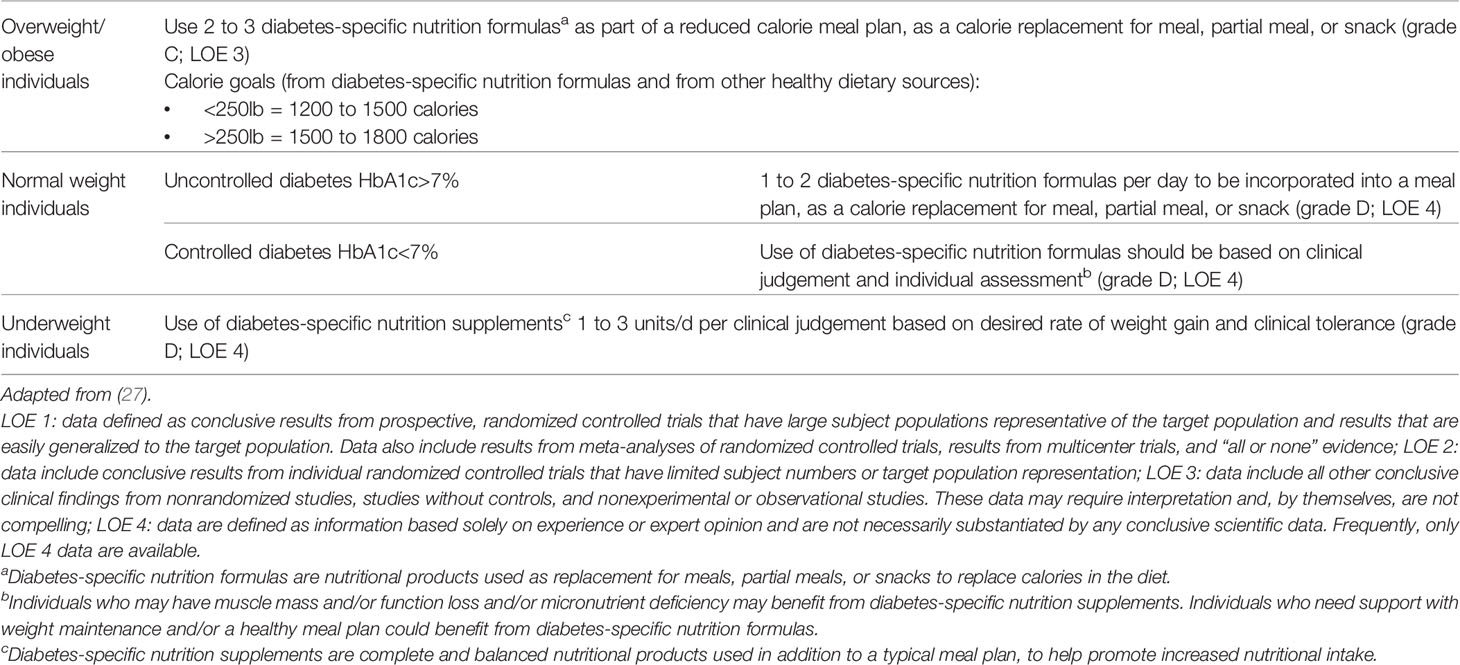
Table 2 Evidence-Based Algorithm for the use of Diabetes-Specific Nutrition Formulas in Prediabetes, Type 1 Diabetes and Type 2 Diabetes.
While the use of DSNFs in each population group are evidence-based, the specific dosing required has not been validated and therefore, expert opinion by international thought leaders was required to determine the number of unit doses for each clinical scenario. Implementation and adherence to the tDNA will also vary depending on cultural, psychological, ethnic, nutritional, and lifestyle factors, as well as the economic and social environment. A local task force may be required to detail the factors that need to be considered in the adaptation of the algorithm to specific regions, as was done in the Venezuelan adaptation of tDNA (28).
Despite the current evidence of the role of DSNFs in T2D, significant research, knowledge and practice gaps remain. There is a need for additional studies to elucidate the mechanism of action, along with larger randomized controlled trials, more relevant clinical studies that reflect real-world scenarios, and economic/cost studies to improve our certainty in the utility of DNSFs for patients with T2D. Additionally, knowledge gaps among various healthcare professionals (HCPs) such as, nurses, registered dietitians, and physicians, need to be addressed through education and training, while subsequently targeting practice gaps which enable HCPs in implementing DSNFs in the clinical setting (e.g., through the use of validated clinical practice algorithms).
The current evidence indicates that there is a role for DSNFs as meal/snack replacements in nutrition therapy for T2D. DNSFs have demonstrated reduced glycemic and insulin excursions when compared with standard formulas and some commonly consumed foods. These acute benefits have also translated into meaningful reductions in long-term glycemic control along with improvements in various cardiometabolic endpoints. The tDNA is an evidenced-based approach that can be used to facilitate the delivery of DSNFs to patients with T2D in the clinical setting.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
JN drafted the manuscript. JM critically reviewed the manuscript for important intellectual content. Both authors reviewed and approved the final manuscript.
This work was supported by the Toronto 3D Knowledge Synthesis and Clinical Trials foundation.
JN has worked as a clinical research coordinator at INQUIS Clinical Research. He has also received research support from Glycemia Consulting Inc. JM has received honoraria for lectures and program development from Abbott Nutrition and serves on the Advisory Boards of Aveta.Life, L-Nutra, and Twin Health.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of Cardiovascular Disease in Type 2 Diabetes: A Systematic Literature Review of Scientific Evidence From Across the World in 2007–2017. Cardiovasc Diabetology (2018) 17(1):83. doi: 10.1186/s12933-018-0728-6
2. Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular Disease in Europe — Epidemiological Update 2015. Eur Heart J (2015) 36(40):2696–705. doi: 10.1093/eurheartj/ehv428
3. Mensah GA, Roth GA, Sampson UK, Moran AE, Feigin VL, Forouzanfar MH, et al. Mortality From Cardiovascular Diseases in Sub-Saharan Africa, 1990-2013: A Systematic Analysis of Data From the Global Burden of Disease Study 2013. Cardiovasc J Afr (2015) 26(2 Suppl 1):S6–10. doi: 10.5830/CVJA-2015-036
4. International Diabetes Federation. IDF Diabetes Atlas. 10th. Brussels, Belgium (2021). Available at: https://www.diabetesatlas.org.
5. Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care (2019) 42(5):731–54. doi: 10.2337/dci19-0014
6. Sanz-Paris A, Álvarez Hernández J, Ballesteros-Pomar MD, Botella-Romero F, León-Sanz M, Martín-Palmero Á, et al. Evidence-Based Recommendations and Expert Consensus on Enteral Nutrition in the Adult Patient With Diabetes Mellitus or Hyperglycemia. Nutrition (2017) 41:58–67. doi: 10.1016/j.nut.2017.02.014
7. Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM - 2020 EXECUTIVE SUMMARY. Endocr Pract (2020) 26(1):107–39. doi: 10.4158/CS-2019-0472
8. Via M. The Malnutrition of Obesity: Micronutrient Deficiencies That Promote Diabetes. ISRN Endocrinol (2012) 2012:103472. doi: 10.5402/2012/103472
9. Mechanick JI, Marchetti A, Hegazi R, Hamdy O. Diabetes-Specific Nutrition Formulas in the Management of Patients With Diabetes and Cardiometabolic Risk. Nutrients (2020) 12(12):3616. doi: 10.3390/nu12123616
10. Buranapin S, Siangruangsang S, Chantapanich V, Hengjeerajarus N. The Comparative Study of Diabetic Specific Formula and Standard Formula on Postprandial Plasma Glucose Control in Type 2 DM Patients. J Med Assoc Thai (2014) 97(6):582–8.
11. Pohl M, Mayr P, Mertl-Roetzer M, Lauster F, Lerch M, Eriksen J, et al. Glycaemic Control in Type II Diabetic Tube-Fed Patients With a New Enteral Formula Low in Carbohydrates and High in Monounsaturated Fatty Acids: A Randomised Controlled Trial. Eur J Clin Nutr (2005) 59(11):1221–32. doi: 10.1038/sj.ejcn.1602232
12. Lansink M, van Laere KM, Vendrig L, Rutten GE. Lower Postprandial Glucose Responses at Baseline and After 4 Weeks Use of a Diabetes-Specific Formula in Diabetes Type 2 Patients. Diabetes Res Clin Pract (2011) 93(3):421–9. doi: 10.1016/j.diabres.2011.05.019
13. Vaisman N, Lansink M, Rouws CH, van Laere KM, Segal R, Niv E, et al. Tube Feeding With a Diabetes-Specific Feed for 12 Weeks Improves Glycaemic Control in Type 2 Diabetes Patients. Clin Nutr (2009) 28(5):549–55. doi: 10.1016/j.clnu.2009.05.004
14. Laksir H, Lansink M, Regueme SC, de Vogel-van den Bosch J, Pfeiffer AFH, Bourdel-Marchasson I. Glycaemic Response After Intake of a High Energy, High Protein, Diabetes-Specific Formula in Older Malnourished or at Risk of Malnutrition Type 2 Diabetes Patients. Clin Nutr (2018) 37(6 Pt A):2084–90. doi: 10.1016/j.clnu.2017.09.027
15. Angarita Dávila L, Bermúdez V, Aparicio D, Céspedes V, Escobar MC, Durán-Agüero S, et al. Effect of Oral Nutritional Supplements With Sucromalt and Isomaltulose Versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients With Type 2 Diabetes: A Randomised Cross-Over Study. Nutrients (2019) 11(7):1477. doi: 10.3390/nu11071477
16. Gulati S, Misra A, Nanda K, Pandey RM, Garg V, Ganguly S, et al. Efficacy and Tolerance of a Diabetes Specific Formula in Patients With Type 2 Diabetes Mellitus: An Open Label, Randomized, Crossover Study. Diabetes Metab Syndr (2015) 9(4):252–7. doi: 10.1016/j.dsx.2014.10.001
17. Mottalib A, Mohd-Yusof BN, Shehabeldin M, Pober DM, Mitri J, Hamdy O. Impact of Diabetes-Specific Nutritional Formulas Versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia. Nutrients (2016) 8(7):443. doi: 10.3390/nu8070443
18. Mustad VA, Hegazi RA, Hustead DS, Budiman ES, Rueda R, Maki K, et al. Use of a Diabetes-Specific Nutritional Shake to Replace a Daily Breakfast and Afternoon Snack Improves Glycemic Responses Assessed by Continuous Glucose Monitoring in People With Type 2 Diabetes: A Randomized Clinical Pilot Study. BMJ Open Diabetes Res Care (2020) 8(1):e001258. doi: 10.1136/bmjdrc-2020-001258
19. Chee WSS, Gilcharan Singh HK, Hamdy O, Mechanick JI, Lee VKM, Barua A, et al. Structured Lifestyle Intervention Based on a Trans-Cultural Diabetes-Specific Nutrition Algorithm (tDNA) in Individuals With Type 2 Diabetes: A Randomized Controlled Trial. BMJ Open Diabetes Res Care (2017) 5(1):e000384. doi: 10.1136/bmjdrc-2016-000384
20. Look AHEAD Research Group. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N Engl J Med (2013) 369(2):145–54. doi: 10.1056/NEJMoa1212914
21. Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral Nutritional Support and Use of Diabetes-Specific Formulas for Patients With Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care (2005) 28(9):2267–79. doi: 10.2337/diacare.28.9.2267
22. Ojo O, Weldon SM, Thompson T, Crockett R, Wang XH. The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients (2019) 11(8):1905. doi: 10.3390/nu11081905
23. Sanz-París A, Matía-Martín P, Martín-Palmero Á, Gómez-Candela C, Camprubi Robles M. Diabetes-Specific Formulas High in Monounsaturated Fatty Acids and Metabolic Outcomes in Patients With Diabetes or Hyperglycaemia. A Systematic Review and Meta-Analysis. Clin Nutr (2020) 39(11):3273–82. doi: 10.1016/j.clnu.2020.02.036
24. Gilcharan Singh HK, Chee WSS, Hamdy O, Mechanick JI, Lee VKM, Barua A, et al. Eating Self-Efficacy Changes in Individuals With Type 2 Diabetes Following a Structured Lifestyle Intervention Based on the Transcultural Diabetes Nutrition Algorithm (tDNA): A Secondary Analysis of a Randomized Controlled Trial. PloS One (2020) 15(11):e0242487. doi: 10.1371/journal.pone.0242487
25. Look Ahead Research Group. Long-Term Effects of a Lifestyle Intervention on Weight and Cardiovascular Risk Factors in Individuals With Type 2 Diabetes Mellitus: Four-Year Results of the Look AHEAD Trial. Arch Internal Med (2010) 170(17):1566–75. doi: 10.1001/archinternmed.2010.334
26. Look Ahead Research Group. Eight-Year Weight Losses With an Intensive Lifestyle Intervention: The Look AHEAD Study. Obes (Silver Spring) (2014) 22(1):5–13. doi: 10.1002/oby.20662
27. Mechanick JI, Marchetti AE, Apovian C, Benchimol AK, Bisschop PH, Bolio-Galvis A, et al. Diabetes-Specific Nutrition Algorithm: A Transcultural Program to Optimize Diabetes and Prediabetes Care. Curr Diabetes Rep (2012) 12(2):180–94. doi: 10.1007/s11892-012-0253-z
Keywords: meal replacement, glycemic response, glycemic control, diabetes, clinical practice
Citation: Noronha JC and Mechanick JI (2022) Is There a Role for Diabetes-Specific Nutrition Formulas as Meal Replacements in Type 2 Diabetes? Front. Endocrinol. 13:874968. doi: 10.3389/fendo.2022.874968
Received: 13 February 2022; Accepted: 29 March 2022;
Published: 29 April 2022.
Edited by:
Cyril WC Kendall, University of Toronto, CanadaReviewed by:
Carsten Dirksen, Hvidovre Hospital, DenmarkCopyright © 2022 Noronha and Mechanick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jarvis C. Noronha, ai5ub3JvbmhhQHVxY29ubmVjdC5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.