
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 07 April 2022
Sec. Bone Research
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.870277
This article is part of the Research Topic The Potential Effects and Mechanisms of Chinese Traditional Medicine on Bone Homeostasis and Remodeling View all 12 articles
 Bai-Ru Cheng1
Bai-Ru Cheng1 Rou-Yan Wu1
Rou-Yan Wu1 Qin-Yang Gao1†
Qin-Yang Gao1† Kai-Xin Jiang2†
Kai-Xin Jiang2† Shuang-Sang Li2‡
Shuang-Sang Li2‡ Shi-Hao Qi2‡
Shi-Hao Qi2‡ Ming-Yi Yuan2‡
Ming-Yi Yuan2‡ Jian-Ping Liu3*
Jian-Ping Liu3*Objective: To assess the benefit and harm of Chinese medicine Xianling Gubao (XLGB) capsule compared to conventional medication or placebo to inform clinical practice.
Methods: We included randomized controlled trials (RCTs) with Jadad score ≥3 of XLGB capsule compared to pharmaceutical medication, placebo, or no treatment for primary osteoporosis. We conducted searches in EMBASE, Cochrane CENTRAL, MEDLINE, China National Knowledge Infrastructure, VIP, Wanfang, and Chinese Biomedical Literature Database (Sino-Med) from their inception till November 13th, 2021. Study selection and data extraction were done by two authors independently. The methodological quality of the RCTs was assessed using Cochrane’s risk of bias tool. The effect size was presented as risk ratio (RR) or mean difference (MD) with their 95% confidence interval (CI).
Results: Our searches identified 2292 records and after exclusions, eight trials involving 846 participants were included. There was no statistically significant difference between conventional medications with or without XLGB on new fracture (RR: 0.50, 95% CI: [0.13, 1.87]). Quality of life by SF-36 questionnaire of XLGB plus calcium carbonate, vitamin D3, and calcitriol was improved than that of without XLGB (MD: 6.72 scores, 95% CI: [2.82, 10.62]). XLGB increased bone mineral density similarly as calcium carbonate plus vitamin D3 (MD: 0.21, 95% CI: [-0.16, 0.58]) or as alendronate sodium, calcium carbonate plus vitamin D3 (MD: 0.00, 95% CI: [-0.10, 0.10]), but it had no additional effect as an add-on treatment to conventional medications (MD: 0.13, 95% CI: [-0.12, 0.37]). XLGB relieved pain via visual analog scale more effectively when combined with medications (MD: -1.55 score, 95% CI: [-2.47, -0.63]). XLGB as monotherapy did not increase adverse events (RR: 0.63, 95% CI: [0.28, 1.41]), or as an add-on treatment (RR: 0.25, 95% CI: [0.03, 2.16]).
Conclusion: This systematic review shows that XLGB capsule appears to be safe and has a beneficial effect on the quality of life and pain relief when used alone or in combination with conventional medications in osteoporosis patients. Further large, rigorous trials are warranted to test its long-term benefit.
Osteoporosis is characterized by the deformation of bone’s microarchitecture and fragility of bones, resulting in the increment of fractures, especially in postmenopausal women. Its diagnosis criteria vary (1), resulting in a wide range of reported incidence, fractures are a great threat for osteoporosis patients since they not only cause pain or humpback but also impair dignity and quality of life. Therefore, reducing fractures has become the primary goal in osteoporosis control (2).
Current therapies include physical activity, nutrient supplements, antiresorptive drugs, and anabolic drugs, while pharmacologic treatments are the most recommended (3, 4). Exercise and balance programs such as Tai-Chi have been proved to improve coordination, reducing falling-downs, consequently lowering fracture rates (5). Lifestyle changes such as quitting cigarette smoking or reducing alcohol intake help increase bone mineral density (BMD) and reduce the risk of falls (6). Nutrient supplements calcium and vitamin D have a controversial effect preventing fractures (7), and it was found to increase cardiovascular events, especially myocardial infarction (8). Pharmacologic agents such as alendronate, zoledronic acid, and calcitonin aim at preventing bone resorption or stimulating bone formation and have been shown to improve BMD and reduce the risk of fractures. The recommended therapy for women with low BMD and a fracture risk or history includes all the means mentioned above, but in China, some patients would also seek help from traditional Chinese medicine (TCM).
Xianling Gubao (XLGB) capsule is the most recommended Chinese proprietary medicine for osteoporosis treatment. It was approved by the China Food and Drug Administration in 2002 (9). It contains Longspur epimedium (Yin Yang Huo), Radix dipsaci (Xuduan), Salvia miltiorrhiza (Danshen), Rhizoma anemarrhenae (Zhimu), Rehmannia glutinosa (Dihuang), and Psoralea corylifolia (Bu Gu Zhi). Based on network pharmacology and molecular docking, the identified components such as icariin, quercetin, and luteolin, may aim at WnT, TNF, MAPK, PI3K-Akt pathways, relating to targets including STAT3, MAPK14, JUN, IL-2, and EGFR, which are important in bone homeostasis (10). XLGB capsule was found to downregulate RANKL mRNA and upregulate osteoprotegerin mRNA, which combines with RANKL to reduce osteoclasts, finally inhibiting bone destruction (11). It was also reported that the combination of six typical absorbed constituents of XLGB capsule could promote MC3T3-E1 cells’ differentiation and mineralization (12). In TCM theory, most bone diseases pertain to the deficiency of the kidney and the blood stasis in meridians which causes pain, thus, this formula could nourish the liver and the kidney, promote blood circulation, and remove meridian obstruction, to strengthen the muscles and bones. There is an increasing number of clinical trials on XLGB capsule in China (13–16). Although most trials present positive findings, some of them are of low quality, lacking blinding or proper randomization methods. Therefore, we thought it necessary to do a systematic review of randomized trials with adequate quality to provide reliable evidence for the clinical use of XLGB capsule.
We retrieved publications using computerized searches by EMBASE, Cochrane CENTRAL, MEDLINE, China National Knowledge Infrastructure, Wanfang, VIP, and Chinese Biomedical Literature Database (Sino-Med), with no limit on inception date or language. The last search date was November 13th, 2021. The search strategy for MEDLINE (via PubMed) is listed in Appendix 1.
Study design: parallel-group, randomized clinical trials regardless of blinding in all languages. There was no limit on the number of participants. Studies with a Jadad score ≥3 were included [using the Jadad scale (17)].
Patients with primary osteoporosis, diagnosed according to any clearly defined criteria were included, regardless of age, gender, or ethnic origin. Those whose chief complaint was a recent fracture and those with secondary osteoporosis such as diabetes-induced, rheumatoid arthritis-induced, or corticosteroid-induced osteoporosis were excluded.
Intervention: Chinese proprietary medicine Xianling Gubao capsule with a minimum treatment duration of three months.
Control intervention could be no treatment, placebo, or conventional pharmaceutical medicine (such as alendronate, zoledronic acid, hormone replacement therapy, bisphosphonate, calcitonin, calcium, and vitamin D).
Co-intervention was allowed as long as the Xianling Gubao group received the same conventional pharmaceutical medicine as at least one comparison group and the only difference between them was the add-on Xianling Gubao capsule.
1. Number of individuals with new fractures; 2. Quality of life (QoL) is measured by a validated tool or scale.
1. Bone mineral density (BMD); detected by one of the following methods of examination: single-photon absorptiometry (SPA), dual photon absorptiometry (DPA), quantitative computed tomography (QCT), dual-energy X-ray absorptiometry (DXA), or peripheral dual-energy X-ray absorptiometry (pDXA); 2. Biochemical indicators: serum calcium (Ca), phosphorus (P), bone alkaline phosphatase (BALP), OC (osteocalcin), TRACP (tartrate-resistant acid phosphatase); 3. Pain, muscle fatigue, and limited mobility; 4. Number and types of adverse events.
Two authors (BR Cheng and RY Wu) independently screened the titles and abstracts of all records. We retrieved the full texts of potentially eligible studies for further identification. Any uncertainty or discrepancy was resolved by discussion with a third author (JP Liu).
Two authors (MY Yuan and SS Li) independently extracted data using a predesigned data form using Excel (version Microsoft Excel 2016). Extracted data were checked together, and any disagreements were resolved by discussion with SH Qi.
Since only RCTs were included, the risk of bias was assessed through Cochrane’s risk of bias Tool for Randomized Trials (RoB) (18). Two authors (QY Gao and KX Jiang) independently assessed the risk of bias. Disagreements were resolved by discussion with a third author (BR Cheng). The risk of bias was assessed through the following five domains: 1) bias arising from the randomization process; 2) bias due to deviations from intended interventions; 3) bias due to missing outcome data; 4) bias in the measurement of the outcome; 5) bias in the selection of the reported result.
We provided a narrative synthesis of the findings from the included studies and worked with the data within a meta-analysis, through Review Manager 5.3. Heterogeneity related to the results of the studies was assessed using both the chi-square test and the I² statistic. If data had been sufficiently homogenous (I2 <50%), we would pool the results using a fixed-effect model, with a mean difference (MD) for continuous outcomes (in our review referring to QoL, BMD, Ca, P, BALP, OC, TRACP, and VAS score) and risk ratio (RR) for dichotomous outcomes (referring to the number of individuals with new fractures and adverse events), and calculated 95% confidence interval (CI) and two-sided p values for each outcome. We provided summaries of effect estimates for each study by calculating RR or MD. We considered an I² value greater than 50% as high heterogeneity. If there had been high heterogeneity across included studies, we would use a random-effect model or only provide a narrative synthesis of the findings.
Database searches initially identified 2292 records published in English or Chinese. The last search date was November 13th, 2021. After removing duplications, 1512 articles were screened by their titles and abstracts. 130 reports were sought for retrieval of full text but we failed to find two of them, as they had no available resources online, and 128 out of 130 reports were assessed for eligibility. A flowchart (Figure 1) with the number of included studies at each step was established, including reasons for excluding studies. Eight trials were finally included in the qualitative and quantitative synthesis.
Table 1 presents the characteristics of the included studies, and five out of the eight studies were master’s or doctor’s thesis, and three were published studies in journals. The included eight studies involved 846 participants (ranging from 60 to 180). The conventional treatments in the control groups included calcium carbonate and vitamin D3, calcitriol, alendronate sodium, and carbocalcitonin, as well as the combination of some of them. The treatment duration ranged from three to twelve months.
Figures 2, 3 are summaries of each included studies’ risk of bias. Overall, all included studies were of unclear or high risks, which was mostly contributed by lack of randomization sequence concealment and absence of blinding process. All studies had a proper randomization process, but none reported blinding of participants, personnel, or outcome assessment except 1 triple-blinded study, using a placebo (26). Only one study reported randomization concealment using sealed envelopes (22). Subjective outcomes such as the VAS score and the QoL score could have been biased due to the lack of participant blinding, but objective outcomes like bioindicator levels are less likely to be biased.
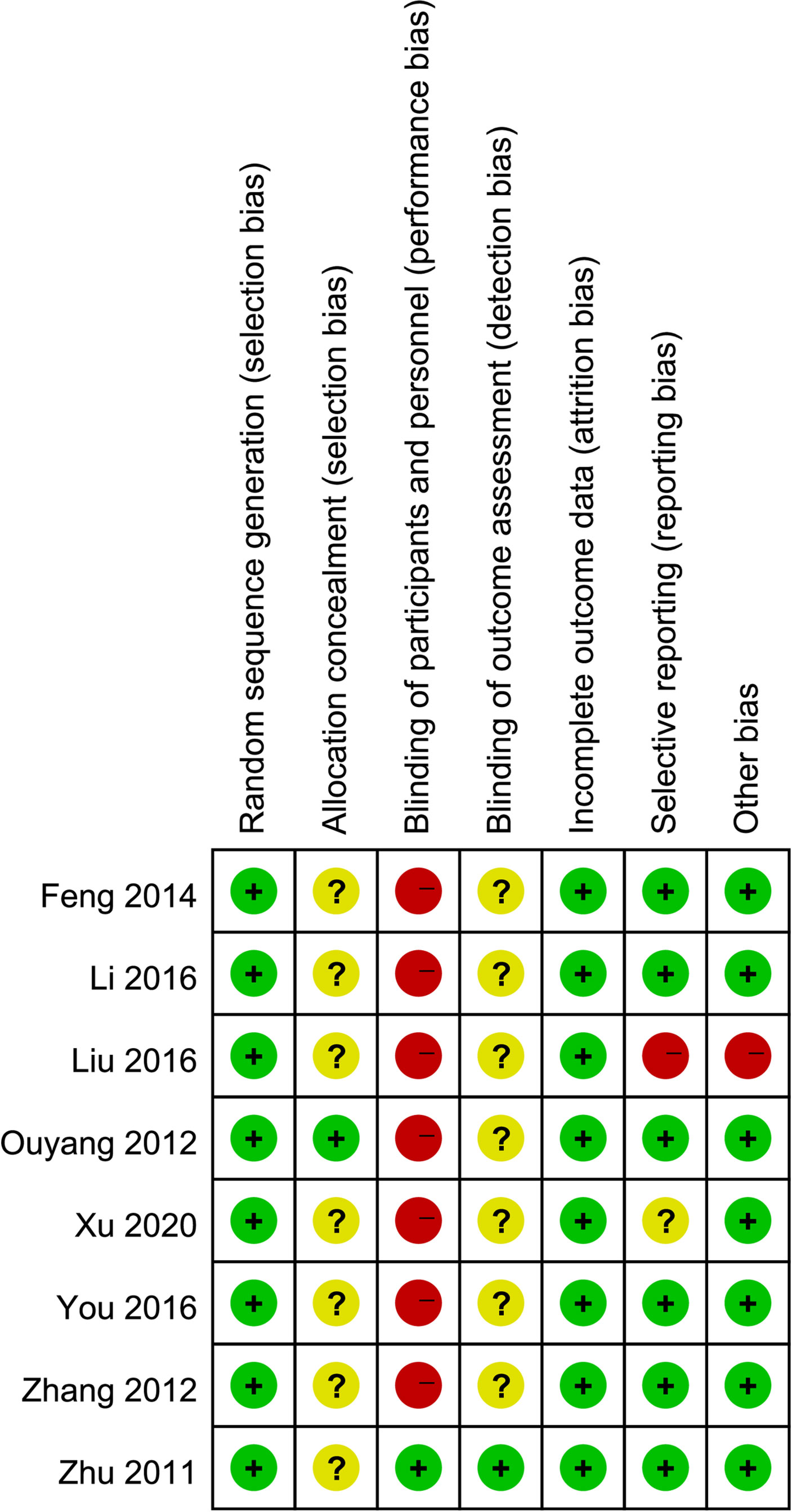
Figure 2 Risk of bias summary: a review of authors’ judgments about each risk of bias item for each included study.
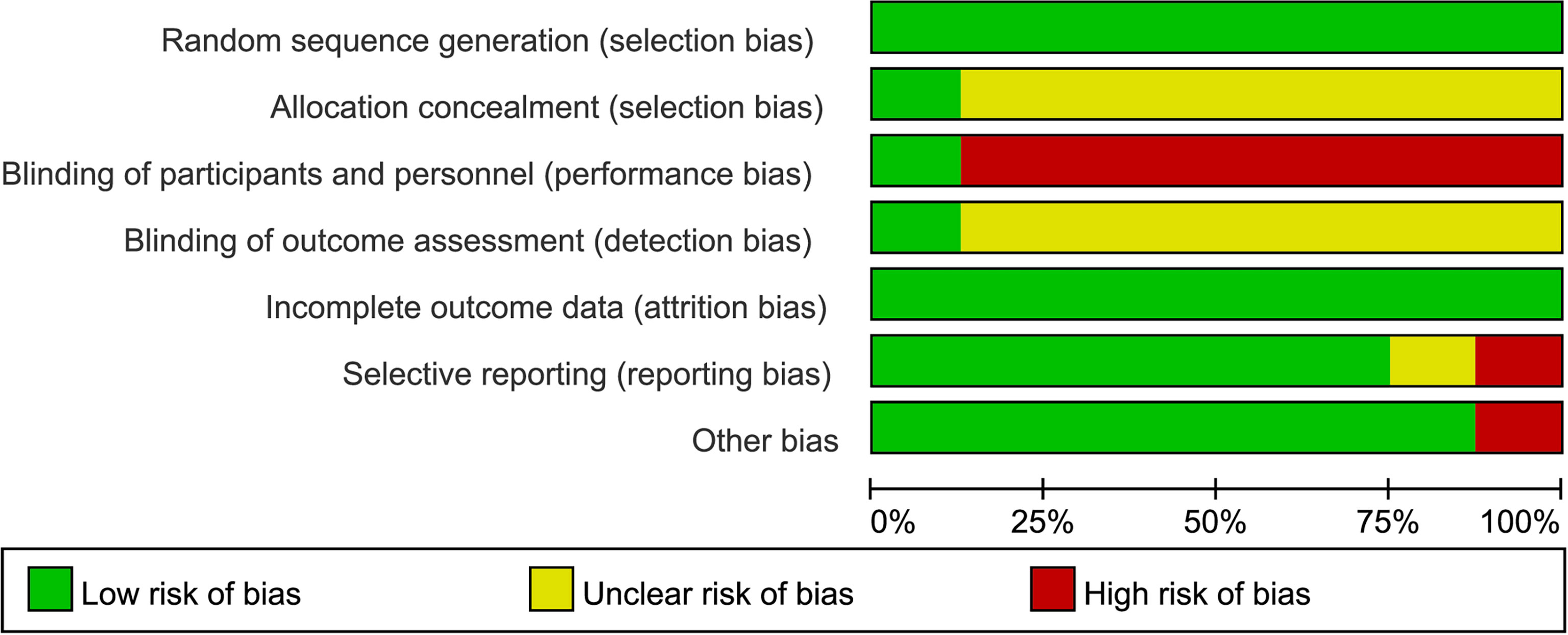
Figure 3 Risk of bias graph: A review of authors’ judgments about each risk of bias item presented as percentages across all included studies.
Only one trial reported this outcome (23). In the twelve-month follow-up after the six-month treatment, there was no significant difference between XLGB plus medications (carbocalcitonin, calcium carbonate, vitamin D3, and calcitriol) and medications on reported new fracture onset (3/42 versus 6/42; RR: 0.50, 95% CI: [0.13, 1.87]).
Only one trial used the SF-36 health survey questionnaire (eight domains with total scores of 100) to assess patients’ quality of life (17). It showed a significant difference between calcium carbonate, vitamin D3, and calcitriol with or without XLGB (MD: 6.72 scores, 95% CI: [2.82, 10.62]) (19).
Five trials reported BMD of the lumbar spine. Two reported BMD in T scores and one did not mention the place where the BMD was detected (21), making its result unable to be pooled. In two comparisons where the units were g/cm2 and T score, XLGB increased bone mineral density to a similar extent as calcium carbonate plus vitamin D3 (MD: 0.21, 95% CI: [-0.16, 0.58]), or as alendronate sodium, calcium carbonate plus vitamin D3 (MD: 0.00, 95% CI: [-0.10, 0.10]), but it had no additional effect as an add-on to conventional medications (MD: 0.13, 95% CI: [-0.12, 0.37]), though differences were seen another comparison (T score) of carbocalcitonin, calcium carbonate, vitamin D3, and calcitriol with or without XLGB (MD: 0.11, 95% CI: [0.09, 0.13]).
No comparison showed a difference between XLGB plus conventional treatment and the conventional treatment, but in one study (25), serum calcium and phosphorus levels in XLGB group were significantly lower than calcium carbonate plus vitamin D3 group (Table 2).
XLGB plus calcium carbonate and vitamin D3 resulted in a higher level of BALP than that of calcium carbonate and vitamin D3 (MD: 6.67 U/L, 95% CI: [1.88, 11.46]), but XLGB alone was no better than calcium carbonate plus vitamin D3 (MD: 6.79 U/L, 95% CI: [-23.99, 37.57]).
A higher osteocalcin level was observed in XLGB group compared to the calcium carbonate and vitamin D3 group. The combination of the two treatments showed no differences in osteocalcin than calcium carbonate and vitamin D3 alone (Table 2).
The results of the TRACP5b level were also conflicting. It was decreased in XLGB plus conventional treatment group compared to calcium carbonate, vitamin D3, plus calcitriol group but was higher in XLGB group compared to alendronate sodium plus calcium carbonate and vitamin D3 (Table 2).
No trials reported fatigue or limited mobility. VAS score (0-10 scale) was used by all studies to measure pain. XLGB plus conventional medications significantly relieved pain than conventional medications (MD: -1.55, 95% CI: [-2.47, -0.63]). Compared to alendronate sodium plus calcium carbonate and vitamin D3, XLGB alone did not show significant difference (MD: -0.12, 95% CI: [-0.82, 0.58]).
There was no significant difference in the overall rate of any adverse events between XLGB (both used alone and as add-on treatment) and conventional medication. No severe adverse events were reported. The reported adverse events in XLGB group included one case with headache, one losing appetite, one red flush, five gastrointestinal reactions, and one constipation. (Table 3).
Due to the small number of included trials, for primary outcomes–number of new fractures and quality of life–the results were not pooled, and XLGB did not significantly reduce fractures but improved quality of life. Table 2 presents the full results of all secondary outcomes. For bone mineral density, the direct comparison of XLGB to conventional treatments had insufficient data, but the effect of XLGB as an add-on treatment was not significant. For calcium, phosphorus, and osteocalcin levels, pooled results showed no differences in the conventional treatment with or without XLGB. It was shown that XLGB significantly reduced TRACP5b levels and VAS score, indicating that it could inhibit bone deformation and relieve pain. Table 3 shows safety outcomes, where XLGB did not result in more adverse events when added to conventional medications or compared to them.
This systematic review and meta-analysis investigated the efficacy and safety of XLGB capsule in patients with primary osteoporosis. Overall, XLGB capsule is a safe treatment that improved quality of life and reduces pain as an add-on treatment compared to conventional medications, including calcium carbonate, vitamin D3, calcitriol, alendronate sodium, and carbocalcitonin, or the combination of some of them, and XLGB plus conventional treatments had a better effect than conventional medications alone. Its effects on new fractures, bone mineral density, serum calcium, and serum phosphorous levels were not significant. The results of BALP, osteocalcin, and TRACP5b levels were controversial, which could be a result of insufficient data.
XLGB capsule is a Chinese proprietary medicine made of six herbs, making its components difficult to be fully analyzed. Based on network pharmacology and molecular docking, the identified components were cryptotanshinone, chryseriol, kaempferol, anhydroicaritin, quercetin, and luteolin, which might aim at WnT, TNF, MAPK, PI3K-Akt pathways, relating to targets including STAT3, MAPK14, JUN, IL-2, and EGFR (10). Among the mentioned molecules, flavonoids such as quercetin, luteolin, icariin are widely believed to be the main components. Quercetin, kaempferol, and icariin inhibit RANKL activation and OPG/RANK/RANKL is important in osteoclastic differentiation (27, 28). Luteolin stimulates osteoblast proliferation via Semaphorin 3A/Neuropilin-1/Plexin A1 pathway (29), and cryptotanshinone improves osteoblastic differentiation and reduces E2 to inhibit bone absorption (30, 31). Also, PI3K-Akt, TNF, and MAPK pathways have to do with osteoblastic differentiation, which promotes bone formation (32, 33). Furthermore, by analyzing the absorbed components in rats’ serum after intragastric administration by HPLC-MS/MS analysis, Yao et al. confirmed 15 components including sweroside, epimedin B, isopsoralen, asperosaponin VI, and neobavaisoflavone, which came from four herbs - Longspur epimedium, Radix dipsaci, Rhizoma anemarrhenae, and Psoralea corylifolia - in the formula (34). Sweroside interacts with membrane estrogen receptor-α and p38 pathway to promote osteoblastic differentiation and mineralization (35). Epimedin B downregulates PI3K-Akt, MAPK, and PPAR signaling pathways, which are mentioned above to result in bone destruction in mice (36) and have estrogen-like effects (37). Some other compounds like isopsoralen, asperosaponin VI, and neobavaisoflavone have also been proved to either enhance osteogenesis or inhibit osteoclast activation (38–42). Moreover, Wu found that the combination of six typical absorbed constituents of XLGB capsule could promote MC3T3-E1 cells’ differentiation and mineralization, which were not seen in any single-constituent groups, suggesting that there may be some unknown interactive mechanism of the combination (12). Moreover, Ren et al., found that XLGB capsule downregulated RANKL mRNA and upregulated the mRNA of osteoprotegerin, which combines with RANKL to reduce osteoclasts, finally inhibiting bone destruction (11). By analyzing and testing the components of XLGB capsule, we expect new phytomedicines which are more effective and with fewer adverse events.
One previous meta-analysis in Chinese reviewed the efficacy and safety of XLGB capsule in osteoporosis (43). Significant improvement in BMD, VAS, ALP, Ca, and BGP from XLGB was found, but it did not report quality of life or pain, and the difference in BMD was only slightly statistically significant that it may have limited clinical significance. Although the review specified primary osteoporosis, it included different osteoporosis. In addition, the review included randomized trials without any limitation to trial quality or treatment duration. Trials without proper randomization procedures may exaggerate the treatment effect. Compared to this review, our review has some strengths: 1) the first meta-analysis on this topic written in English and only included RCTs with adequate randomization, making the results more reliable; 2) our primary outcomes focusing on new fractures and quality of life, which are important to patients.
However, we do have some limitations: 1) the small number of included trials since we limited our inclusion criteria to those trials with moderate quality (Jadad score ≥3); 2) lack of placebo-controlled, double-blind trial, and add-on trials make blinding to participants or investigators not possible; 3) although we included trials with an adequate generation of allocation sequence, no trial reported allocation concealment, which may cause performance bias in outcome measurement such as VAS and quality of life; 4) small sample sizes (60-180 patients) of included trials may be underpowered for the effect estimates; and 5) the conventional medications used in control groups were diverse, including different combinations, so it was difficult to do subgroup analysis to investigate their efficacy respectively.
Clinicians should be aware that current evidence for XLGB capsule is limited due to small trials or a high risk of bias. Therefore, we suggest more solid evidence before the recommendation of its clinical use. We encourage placebo-controlled, double-blind trials with long follow up to test its efficacy and safety for primary osteoporosis. Only when we are confident of its efficacy would we suggest add-on trials of XLGB to current medications, especially paying attention to clinical outcomes such as the number of new fractures and quality of life.
This systematic review shows that XLGB capsule appears to be safe and may be used alone or with conventional treatments to improve osteoporosis patients’ quality of life and relieve pain. However, current evidence for its efficacy is limited especially for long-term outcomes such as new fractures.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
B-RC and J-PL conceived this review topic. B-RC, R-YW, and J-PL drafted the study protocol. B-RC and R-YW did database searches, removed duplications, screened titles, abstracts, and full texts of included papers. K-XJ and Q-YG assessed the risk of bias and extracted data. Outcome data were extracted by M-YY and S-SL, checked, and discussed with S-HQ. Data analyses were done by B-RC and discussed with all other members. Finally, the manuscript was revised by J-PL. All authors contributed to the article and approved the submitted version.
This review was supported by the National Natural Science Foundation project (No. 81830115), and J-PL was partially supported by the NCCIH grant (AT001293 with sub-award No. 020468C). The funders have no role in the review design, conduct, interpretation, and writing of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ 3rd. Trends in Fracture Incidence: A Population-Based Study Over 20 Years. J Bone Miner Res (2014) 29(3):581–9. doi: 10.1002/jbmr.2072
2. Black DM, Rosen CJ. Clinical PracticePostmenopausal Osteoporosis. N Engl J Med (2016) 374(3):254–62. doi: 10.1056/NEJMcp1513724
3. Qaseem A, Forciea MA, McLean RM, Denberg TD, Barry MJ, Cooke M, et al. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med (2017) 166(11):818–39. doi: 10.7326/M15-1361
4. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr Pract (2020) 26(Suppl 1):6–8. doi: 10.4158/GL-2020-0524SUPPL
5. Sinaki M. Exercise for Patients With Osteoporosis: Management of Vertebral Compression Fractures and Trunk Strengthening for Fall Prevention. PM R (2012) 4(11):882–8. doi: 10.1016/j.pmrj.2012.10.008
6. Zhu K, Prince RL. Lifestyle and Osteoporosis. Curr Osteoporos Rep (2015) 13(1):52–9. doi: 10.1007/s11914-014-0248-6
7. Bauer DC. Calcium Supplements and Fracture Prevention. New Engl J Med (2014) 370(4):387–8. doi: 10.1056/NEJMc1314100
8. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium Supplements With or Without Vitamin D and Risk of Cardiovascular Events: Reanalysis of the Women’s Health Initiative Limited Access Dataset and Meta-Analysis. BMJ (2011) 342:d2040. doi: 10.1136/bmj.d2040
9. Ma Y-Z, Wang Y-P, Liu Q, Li C-L. 2018 China Guideline for the Diagnosis and Treatment of Senile Osteoporosis. Chin J Gerontol (2019) 39(11):2557–75. doi: 10.3969/j.issn.1005-9202.2019.11.001
10. Guan J-L, Wang Q-Y, Liu P, Xi W-Q, Zhang Q-D, Guo W-S. Molecular Mechanism of Xianling Gubao Decoction in the Treatment of Osteoporosis Based on Network Pharmacology and Molecular Docking. J Liaoning Univ Tradit Chin Med (2021) 23(02):57–65. doi: 10.13194/j.issn.1673-842x.2021.02.014
11. Ren S-Y, Zhang Q-H, Yan X-Z. Effect of Xianling Gubao Capsules on Microstructure of Fractured Bone in Ovariectomized Rats and Regulation Mechanism of OPG/RANKL Signaling. Chin Tradit Patent Med (2021) 43(01):67–72. doi: 10.3969/j.issn.1001-1528.2021.01.013
12. Wu Q-C. Discovery of Bioactive Constituents From Absorbed Constituents of XLGB With Promoting Differentiation and Mineralization Activity on MC3T3-E1 Cells and Preliminary Study on Action Mechanism of Sweroside [Master’s Thesis]. Guangzhou, Guangdong, China: Jinan University (2019).
13. Lai Y-H, Shen J-L. Clinical Effect of Xianling Gubao Capsule Combined With Calcitriol in the Adjuvant Treatment of Osteoporosis. Chin J Clin Ration Drug Use (2021) 14(19):106–8. doi: 10.15887/j.cnki.13-1389/r.2021.19.041
14. Liu M-F, Yang F, Zeng L-J. Clinical Observation of Xianling Gubao Capsule in the Treatment of Postmenopausal Women With Osteoporosis. Yunnan J Tradit Chin Med Materia Med (2021) 42(06):37–40. doi: 10.16254/j.cnki.53-1120/r.2021.06.012
15. Ma J-T, Qv C, Fu W-B. Effects of Zoledronic Acid Combined With Xianling Gubao Capsule on Bone Metabolism and Carotid Intimal Thickness in Elderly Postmenopausal Osteoporosis Patients. J Med Theory Pract (2021) 34(24):4292–3. doi: 10.19381/j.issn.1001-7585.2021.24.029
16. Fu Y-F, Shi D-L. Effects of Xianling Gubao Capsule Combined With Zoledronic Acid Intravenous Drip and Calcium Carbonate D3 Tablet on Bone Mineral Density and Bone Turnover in Elderly Patients With Osteoporosis. Chin J Gerontol (2021) 41(22):5015–7. doi: 10.3969/j.issn.1005-9202.2021.22.043
17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin Trials (1996) 17(1):5–8. doi: 10.1016/0197-2456(95)00134-4
18. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
19. Feng M-M, Shi C-L, Gu P, Lv H, Zhang G-L, Liang Y-G, et al. Clinical Research of the Treatment of Primary Osteoporosis in Elder Patient With Combined Use of Xianlinggubao and Calcium. Tianjin Pharm (2014) 26(2):44–7.
20. Li W-J. The Clinical Study of the Effect of Jingu Capsule in OC and PINP Level of Senile Osteoporosis Patient [Master’s Thesis]. Guangzhou, Guangdong, China: Guangzhou University of Chinese Medicine (2016).
21. Liu B-Y, Bai R. Clinical Study of Xianling Gubao Capsule on Middle-Aged and Elderly Patients With Osteoporosis. Shaanxi J Tradit Chin Med (2016) 37(10):1364–5. doi: 10.3969/j.issn.1000-7369.2016.10.045
22. Ouyang J-J. Study of the Clinical Observation and Related Experiments on Treating Postmenopausal Osteoporosis of Kidney Yang Deficiency With Mild Moxibustion [Doctor’s Thesis]. Guangzhou, Guangdong, China: Guangzhou University of Chinese Medicine (2012).
23. Xu H-J. Clinical Observation on the Therapeutic Effect of Wenshenqianggu Pill on Senile Osteoporosis of Spleen Kidney Yang Deficiency Type [Master’s Thesis]. Lanzhou, Gansu, China: Gansu University of Chinese Medicine (2020).
24. You Z-Q. Clinical Observation of Bushen Juanbi Decoction in the Treatment of Postmenopausal Osteoporosis (Kidney Deficiency and Blood Stasis Syndrome) [Master’s Thesis]. Changsha, Hunan, China: Hunan University of Chinese Medicine (2016).
25. Zhang J. Clinical Study of Gushibao Capsule on Primary Osteoporosis [Master’s Thesis]. Shijiazhuang, Hebei, China: Hebei Medical University (2012).
26. Zhu HM, Qin L, Garnero P, Genant HK, Zhang G, Dai K, et al. The First Multicenter and Randomized Clinical Trial of Herbal Fufang for Treatment of Postmenopausal Osteoporosis. Osteoporos Int (2012) 23(4):1317–27. doi: 10.1007/s00198-011-1577-2
27. Huang J, Yuan L, Wang X, Zhang T-L, Wang K. Icaritin and its Glycosides Enhance Osteoblastic, But Suppress Osteoclastic, Differentiation and Activity In Vitro. Life Sci (2007) 81(10):832–40. doi: 10.1016/j.lfs.2007.07.015
28. Qi X-J, Chen G-M, Shi P-Y, Zhang Z-P, Fang C-S, Zheng S-J, et al. Analysis of Pharmacological Mechanism of XianlingGubao Capsule in Treating Osteoporosis Based on Network Pharmacology. Chin J Osteoporos (2020) 26(05):710–8+30. doi: 10.3969/j.issn.1006-7108.2020.05.017
29. Zheng L. Luteolin Stimulates Proliferation and Inhibits Late Differentiation of Primary Rat Calvarial Osteoblast Induced by High-Dose Dexamethasone via Sema3A/NRP1/Pleixin A1. Curr Pharm Biotechnol (2021) 22(11):1538–45. doi: 10.2174/1389201021666201216150442
30. Khosla S. New Insights Into Androgen and Estrogen Receptor Regulation of the Male Skeleton. J Bone Miner Res (2015) 30(7):1134–7. doi: 10.1002/jbmr.2529
31. Lee S-Y, Choi D-Y, Woo E-R. Inhibition of Osteoclast Differentiation by Tanshinones From the Root of Salvia Miltiorrhiza Bunge. Arch Pharm Res (2005) 28(8):909–13. doi: 10.1007/BF02973876
32. Huang RL, Yuan Y, Tu J, Zou GM, Li Q. Opposing TNF-α/IL-1β- and BMP-2-Activated MAPK Signaling Pathways Converge on Runx2 to Regulate BMP-2-Induced Osteoblastic Differentiation. Cell Death Dis (2014) 5:e1187. doi: 10.1038/cddis.2014.101
33. Zuo C, Zhao X, Shi Y, Wu W, Zhang N, Xu J, et al. TNF-α Inhibits SATB2 Expression and Osteoblast Differentiation Through NF-κb and MAPK Pathways. Oncotarget (2018) 9(4):4833–50. doi: 10.18632/oncotarget.23373
34. Yao Z-H, Dai Y, Geng J-L, Lin S-Y, Wu X-M, Yao X-S. HPLC-MS/MS Analysis of the Absorbed Blood Components in Serum After Intragastric Administration of Xianlinggubao to Rats. J Instrumental Anal (2013) 32(04):420–6. doi: 10.3969/j.issn.1004-4957.2013.04.005
35. Wu Q-C, Tang X-Y, Dai Z-Q, Dai Y, Xiao H-H, Yao X-S. Sweroside Promotes Osteoblastic Differentiation and Mineralization via Interaction of Membrane Estrogen Receptor-α and GPR30 Mediated P38 Signalling Pathway on MC3T3-E1 Cells. Phytomedicine (2020) 68:153146. doi: 10.1016/j.phymed.2019.153146
36. Diao X, Wang L, Zhou Y, Bi Y, Zhou K, Song L. The Mechanism of Epimedin B in Treating Osteoporosis as Revealed by RNA Sequencing-Based Analysis. Basic Clin Pharmacol Toxicol (2021) 129(6):450–61. doi: 10.1111/bcpt.13657
37. Xu F, Ding Y, Guo Y, Liu B, Kou Z, Xiao W, et al. Anti-Osteoporosis Effect of Epimedium via an Estrogen-Like Mechanism Based on a System-Level Approach. J Ethnopharmacol (2016) 177:148–60. doi: 10.1016/j.jep.2015.11.007
38. Ren Y, Song X, Tan L, Guo C, Wang M, Liu H, et al. A Review of the Pharmacological Properties of Psoralen. Front Pharmacol (2020) 11:571535. doi: 10.3389/fphar.2020.571535
39. Ge L, Cui Y, Cheng K, Han J. Isopsoralen Enhanced Osteogenesis by Targeting AhR/Erα. Molecules (2018) 23(10):2–7. doi: 10.3390/molecules23102600
40. Liu K, Liu Y, Xu Y, Nandakumar KS, Tan H, He C, et al. Asperosaponin VI Protects Against Bone Destructions in Collagen Induced Arthritis by Inhibiting Osteoclastogenesis. Phytomedicine (2019) 63:153006. doi: 10.1016/j.phymed.2019.153006
41. Weng Z-B, Gao Q-Q, Wang F, Zhao G-H, Yin F-Z, Cai B-C, et al. Positive Skeletal Effect of Two Ingredients of Psoralea Corylifolia L. @ on Estrogen Deficiency-Induced Osteoporosis and the Possible Mechanisms of Action. Mol Cell Endocrinol (2015) 417:103–13. doi: 10.1016/j.mce.2015.09.025
42. Chen H, Fang C, Zhi X, Song S, Gu Y, Chen X, et al. Neobavaisoflavone Inhibits Osteoclastogenesis Through Blocking RANKL Signalling-Mediated TRAF6 and C-Src Recruitment and NF-κb, MAPK and Akt Pathways. J Cell Mol Med (2020) 24(16):9067–84. doi: 10.1111/jcmm.15543
43. Wang G-Q, Liao X, Zhang Y-L, Xie Y-M. Systemic Evaluation and Meta-Analysis of Xianling Gubao Capsule in Treatment of Primary Osteoporosis in Randomized Controlled Trials. China J Chin Materia Med (2017) 42(15):2829–44. doi: 10.19540/j.cnki.cjcmm.20170705.007
Search strategy for MEDLINE (via PubMed):
1. bone diseases, metabolic[mh]
2. osteoporosis[mh]
3. bone density[all fields]
4. bone loss[all fields]
5. osteomalacia[tw]
6. osteodystrophy[tw]
7. osteopenia[tw]
8. bone mass[tw]
9. densitometry[mh]
10. fractures, bone[mh]
11. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
12. xianlinggubao[tw]
13. xianling gubao[tw]
14. XLGB[tw]
15. xian-ling-gu-bao[tw]
16. drugs, chinese herbal[mh]
17. herbal medicine[mh]
18. plants, medicinal[mh]
19. medicine, traditional[mh]
20. #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
21. randomized controlled trial[pt]
22. controlled clinical trial [pt]
23. randomized [tiab]
24. placebo [tiab]
25. clinical trials as topic [mesh:noexp]
26. randomly [tiab]
27. trial [ti]
28. #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27
29. animals [mh] NOT humans [mh]
30. #28 NOT #29
31. #11 AND #20 AND #30
Keywords: Xianling Gubao capsule, Chinese proprietary medicine, osteoporosis, quality of life, bone mineral density, systematic review, meta-analysis
Citation: Cheng B-R, Wu R-Y, Gao Q-Y, Jiang K-X, Li S-S, Qi S-H, Yuan M-Y and Liu J-P (2022) Chinese Proprietary Medicine Xianling Gubao Capsule for Osteoporosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Endocrinol. 13:870277. doi: 10.3389/fendo.2022.870277
Received: 06 February 2022; Accepted: 11 March 2022;
Published: 07 April 2022.
Edited by:
Jun Liu, Guangdong Provincial Academy of Chinese Medical Sciences, ChinaReviewed by:
Guoyan (Emily) Yang, Western Sydney University, AustraliaCopyright © 2022 Cheng, Wu, Gao, Jiang, Li, Qi, Yuan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Ping Liu, TGl1anBAYnVjbS5lZHUuY24=
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.