
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 22 April 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.869899
This article is part of the Research TopicTechnologies for DiabetesView all 16 articles
In developed countries, diabetes is the leading cause of chronic kidney disease (CKD) and accounts for 50% of incidence of end stage kidney disease. Despite declining prevalence of micro- and macrovascular complications, there are rising trends in renal replacement therapy in diabetes. Optimal glycemic control may reduce risk of progression of CKD and related death. However, assessing glycemic control in patients with advanced CKD and on dialysis (G4-5) can be challenging. Laboratory biomarkers, such as glycated haemoglobin (HbA1c), may be biased by abnormalities in blood haemoglobin, use of iron therapy and erythropoiesis-stimulating agents and chronic inflammation due to uraemia. Similarly, glycated albumin and fructosamine may be biased by abnormal protein turnover. Patients with advanced CKD exhibited heterogeneity in glycemic control ranging from severe insulin resistance to ‘burnt-out’ beta-cell function. They also had high risk of hypoglycaemia due to reduced renal gluconeogenesis, frequent use of insulin and dysregulation of counterregulatory hormones. Continuous glucose monitoring (CGM) systems measure glucose in interstitial fluid every few minutes and provide an alternative and more reliable method of glycemic assessment, including asymptomatic hypoglycaemia and hyperglycaemic excursions. Recent international guidelines recommended use of CGM-derived Glucose Management Index (GMI) in patients with advanced CKD although data are scarce in this population. Using CGM, patients with CKD were found to experience marked glycemic fluctuations with hypoglycemia due to loss of glucose and insulin during haemodialysis (HD) followed by hyperglycemia in the post-HD period. On the other hand, during peritoneal dialysis, patients may experience glycemic excursions with influx of glucose from dialysate solutions. These undesirable glucose exposure and variability may accelerate decline of residual renal function. Although CGM may improve the quality of glycemic monitoring and control in populations with CKD, further studies are needed to confirm the accuracy, optimal mode and frequency of CGM as well as their cost-effectiveness and user-acceptability in patients with advanced CKD and dialysis.
Diabetic kidney disease (DKD) is now the leading cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) in many countries. In 2014, DKD accounted for 50% of patients with ESKD in developed world (1). Data from the United States (US) suggested a slower decline in ESKD incidence compared with other diabetic complications including cardiovascular disease. The US Renal Registry reported a steady increase in incidence of ESKD due to diabetes up to 47% in 2017, compared with 15% in 1985 (2). In the Hong Kong Renal Registry, diabetes was the cause of ESKD in 50% of patients which had replaced glomerulonephritis as the leading cause of renal replacement therapy since 1998 (3).
Patients with diabetes and CKD have increased risk of morbidity and premature mortality than those without renal complications. In the Hong Kong Diabetes Register, patients with CKD had 63% higher risk in all-cause mortality than their non-CKD counterparts, after adjusting for factors such as age, body mass index (BMI), blood pressure and use of oral glucose lowering drugs (OGLDs) (4). Patients with CKD had high risk of cardiovascular events which accounted for 40-50% of mortality in those with estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73m2. This excess risk could not be explained by comorbid factors such as hypertension and dyslipidaemia (5) and might be attributed to additional factors such as vascular calcification, chronic inflammation and myocardial fibrosis (6). Patients with CKD are at increased risk and more vulnerable to hypoglyceamic episodes (4). In a cohort of over 30,000 US veterans with diabetes transitioning to dialysis, the frequency of hypoglycemia-related hospitalizations was associated with higher post-ESKD mortality in a dose-dependent manner (7).
Optimal glycemic control had been shown to delay progression of CKD and reduce death rate in diabetes. In the Diabetes Control and Complication Trial, 1441 patients with type 1 diabetes (T1D) were randomized to receive intensive or conventional insulin treatment. The risk of microalbuminuria was reduced by 34% in the intensive treatment group after at least four years of follow-up (8). The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial enrolled high risk patients with long duration of type 2 diabetes, (T2D), many of whom had prior history of complications. The in-trial reductions in the risk of ESKD was maintained during a total follow-up period of 9.9 years with a hazard ratio of 0.54 (29 events in the intensive treatment group and 53 events in the usual treatment group) (9). In a randomized controlled study of Japanese patients with 110 T2D lasting for 8 years, intensive insulin therapy reduced the rate of progression in nephropathy compared with conventional treatment (10). In the Dialysis Outcomes and Practice Pattern Study (DOPPS) including 9201 patients on dialysis with either T1D or T2D, there was a U-shaped relationship between HbA1c and all-cause mortality. Using HbA1c 7 – 8% as reference, there was 38% increased risk of mortality in patients with HbA1c ≥9% and 21% for those with HbA1c <7% (11). Based on the available evidence, The Kidney Disease Improving Global Outcome (KDIGO) 2020 guideline recommended an optimal HbA1c target range of 6.5-8.0% for patients with diabetes and CKD, with emphasis on individualization of targets taking age, comorbidities, life expectancy and hypoglycaemia risks into consideration (12).
Optimal glycemic management in patients with diabetes and CKD can be challenging, particularly in those with advanced CKD. Reasons include progressive decline in beta-cell function and increase in insulin resistance along with increased risk of severe hypoglycaemia and limited choices of OGLDs. Indeed, the heterogeneity in glycemic control amongst patients with CKD represents inter- and intra-individual variations amongst multiple interacting factors including insulin secretion, insulin resistance, renal clearance of insulin, renal gluconeogenesis and renal function. Increased insulin resistance in early CKD may be triggered by metabolic acidosis, uremic toxins, and chronic inflammation associated with reduced kidney function (13–16). With progression of CKD, the prolonged glucose-lowering effects of oral glucose lowering-drugs (OGLD) including insulin, together with reduced renal gluconeogenesis, shifts the balance towards increased risk of hypoglycaemia (17, 18). In patients with ESKD, around 30% had “burn-out diabetes” who required reduction or discontinuation of insulin treatment and OGLDs (18). In these patients, initiation of dialysis may remove uremic toxins with restoration of insulin sensitivity. Patients with “burnt-out diabetes’ often require only low-dose insulin treatment (19). On the other hand, the dialysis regimen and glucose content of dialysates can significantly influence day-to-day glucose profiles.
One of the greatest challenges in optimizing glycemic management is accurate assessment of glucose control. Conventional markers such as glycated haemoglobin (HbA1c),fructosamine or glycated albumin may be less reliable in in advanced CKD and ESKD. With the emergence of continuous glucose monitoring (CGM), this might be a helpful alternative in assessing and managing diabetes patients with advanced CKD and ESKD. The aim of this narrative review is to summarise current clinical evidence on the accuracy and utility of CGM in CKD patients. We have reviewed the literature on clinical reports, observational studies and clinical trials of use of CGM in CKD. Due to potential issues of sensor performance and the impact of dialysis regimens, we have devoted special attention to use of CGM in patients on haemodialysis and peritoneal dialysis, a challenging group who are prone to both hypoglycemic and hyperglycemic excursions.
The monitoring of glycemic status in patients with diabetes and CKD including ESKD is challenging. HbA1c, the gold standard as a laboratory glycemic marker, can be influenced by multiple factors in CKD. The formation of HbA1c is dependent on the intensity and duration of non-enzymatic interaction between blood glucose and hemoglobin. At any one time, patients may have a mixture of erythrocytes with different ages and varying degrees of exposure to glucose. Therefore, agents that alter erythropoiesis and lifespan of red blood cells will affect HbA1c. For example, HbA1c can be biased towards high values by iron or vitamin B12 deficiency due to reduced synthesis of red blood cells with increased relative amount of HbA1c. On the other hand, HbA1c can be biased towards low values by iron therapy and use of erythropoietin stimulating agents (ESA) with increased turnover of red blood cells (20, 21). The uremic environment in patients with advanced CKD can stimulate carbamylation of haemoglobin which may interfere with HbA1c assays using ion-exchange method, but this can be avoided by using other methods such as high-pressure liquid chromatography (22).
Alternative glycemic indicators such as glycated albumin (GA) and fructosamine have their own limitations in CKD. Extracellular GA is more susceptible to glycation than intracellular hemoglobin (23). Also, GA is unaffected by factors such as iron therapy and ESA frequently used in patients with CKD which can affect HbA1c (21). Due to the shorter half-life of albumin, GA reflects recent glycemic control lasting for 2-3 weeks. However, GA can be affected by albumin metabolism. In patients with low albumin state or increased protein turnover due to chronic inflammation, GA can be falsely low or high (24). In patients treated with peritoneal dialysis (PD) with increased protein loss, GA value may underestimate true glycaemia (25). Although GA can be corrected for serum albumin to reflect the true distribution (26), GA can be affected by oxidative and uremic environments, as well as reduced renal clearance of advanced glycation end products, resulting in positive bias (27).
Fructosamine are ketoamines formed by glycation of albumin and other less abundant serum proteins (28). Although this biomarker involves a wider spectrum of glycated proteins, fructosamine suffers similar bias as GA due to abnormal albumin metabolism and increased protein loss in patients with CKD. In patients with diabetes without CKD and normal serum albumin level, increased albuminuria was associated with low fructosamine value. Besides, fructosamine is sensitive to the fluctuation of serum levels of immunoglobulins and low-molecular-weight molecules (29). In patients with CKD, the uremic environment with altered immunoglobulin levels may affect fructosamine levels (30).
The introduction of continuous glucose monitoring (CGM) offers an alternative for more reliable and comprehensive glycemic evaluation in patients with CKD. Adherence to self-monitoring of blood glucose (SMBG) is often poor due to inconvenience of finger-pricking. In a survey conducted in China, only 40% of patients adhered to the recommended SMBG frequencies (31). Most commercially-available CGM devices are minimally-invasive by inserting a small filament into subcutaneous tissue for measurement of glucose in interstitial fluid. There is a dynamic equilibrium between interstitial glucose and blood glucose due to diffusion dependent on concentration gradient. The interstitial glucose is absorbed into the filament of the CGM device by capillary action. The concentration of interstitial glucose is determined by electrochemical reaction in the sensor (32). Minute-to-minute interstitial glucose readings are transmitted to and displayed in a mobile device, either a reader or smartphone app.
In general, CGM systems can be classified into three categories based on their principles of operation and clinical usage. For professional CGM devices, readings are principally used for glycemic assessment by health care professionals in clinical trial settings which may be blinded or unblinded to the user. Real-time CGM (rt-CGM) devices display readings to the user continuously and can incorporate hypoglycemic or hyperglycemic alerts and trend prediction. The intermittently-scanned or flash CGM devices display readings to user only when the user scans the transmitter (33). Real-time CGM and flash CGM are gaining popularity to facilitate self-monitoring in diabetes. In some countries, CGM devices are reimbursed or funded by public health systems for patients with T1D, including those on dialysis, and some patients with T2D receiving intensive insulin therapy (34).
The performance of CGM sensor is dependent on the enzymatic electrochemical reactions which may be subject to multiple interferences (Figure 1). In early CGM devices, interstitial glucose was detected by glucose oxidase-peroxidase method (36). This method continues to be used by some CGM systems due to the small size and rapid response time of the sensor. However, the electrodes often require pretreatment for attaching to the enzyme surface. Prolonged chemical reactions may pollute the surface of transducer and affect the electrochemical response (37). Both endogenous and exogenous substances may cause interference of the electrochemical sensing of the oxidase-peroxidase reaction.
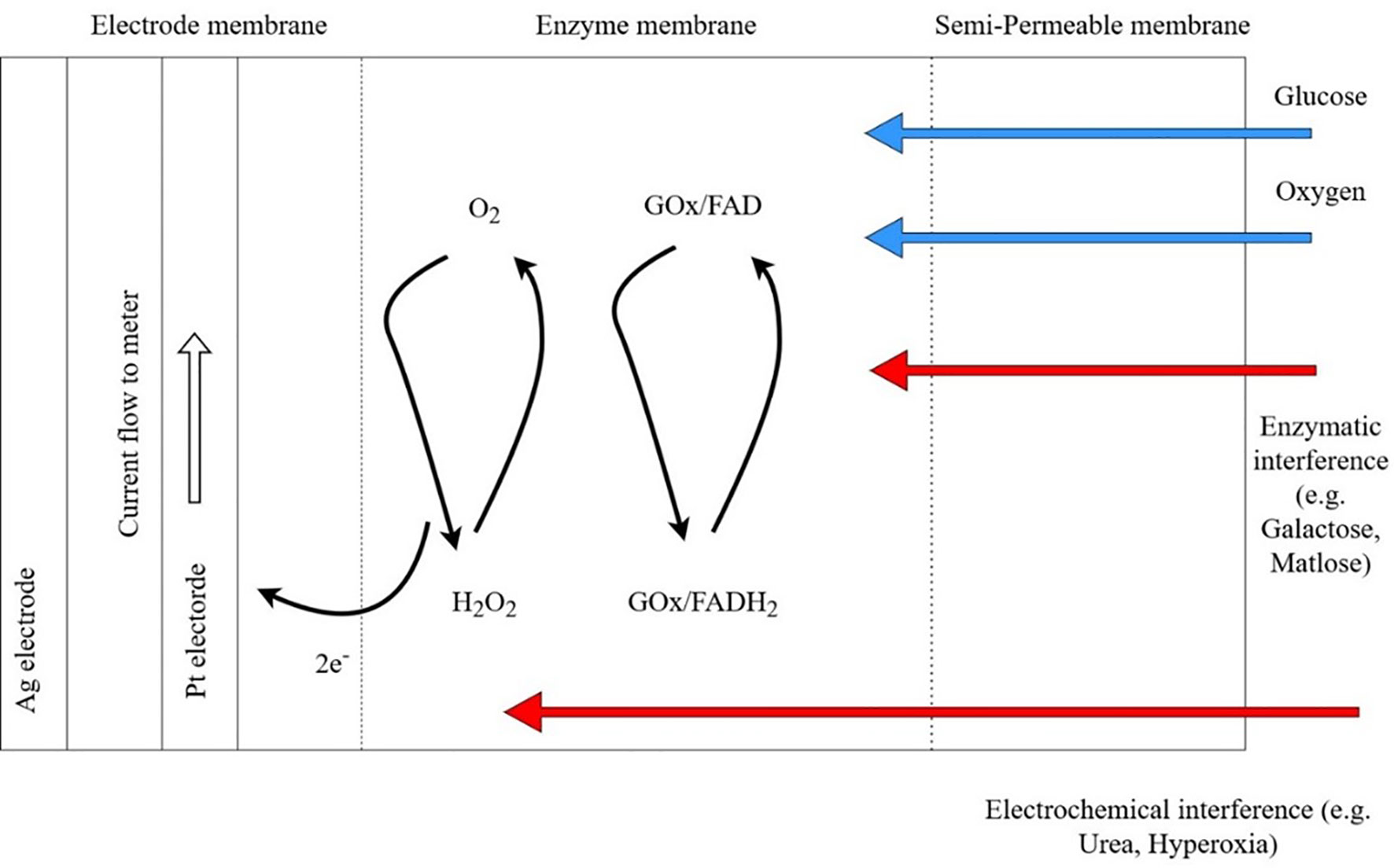
Figure 1 Potential enzymatic and electrochemical interference by substances commonly encountered in patients with chronic kidney disease using continuous glucose monitoring (CGM) systems. In the presence of oxygen (O2), energy in glucose (G) is gradually released in the form of electrons in a series of electrochemical chains catalyzed by Glucose oxidase (GO), an enzyme with Flavin Adenine Dinucleotide (FAD) as cofactor. Released electron is captured by the electrode membrane to generate electric current between platinum electrode and silver electrode. Platinum and silver are chosen for their excellent biocompatibility, electro-conductivity and non-toxicity. Blue arrows indicate normal substrate for electrochemical chain. Red arrows indicate potential interference of CGM sensors by for example galactose, maltose from peritoneal dialysis fluids. Enzymatic interference includes competitive inhibition on active site of GOx by inhibitors. Electrochemical interference includes interaction between the electrode and interfering chemicals that pass through the semi-permeable membrane. GOx, glucose oxidase; FAD, Flavin Adenine Dinucleotide; H2O2, hydrogen peroxide; Pt, platinum; Ag, Silver. Adapted from Boehm et al. (35).
In patients with advanced CKD, hypoxia or hyperoxia can give rise to false sensor glucose values by changing the oxygen concentration at the initiation of the glucose oxidase chain reaction (38). There had been reports on the effects of hematocrit in altering glucose readings of glucometers that use glucose-dehydrogenase or glucose-oxidase methods (39). Endogenous substances such as uric acid and uremia may affect sensor performance. Ogawa et al. demonstrated significant interference of uric acid, a reducing agent, on glucometers using glucose oxidase method comparing with laboratory glucose hexokinase reference (40) However, uric acid did not significantly interfere with sensor performance of a microdialysis-based CGM system (41). There are no dedicated studies evaluating the effect of pH on CGM sensor performance in ESKD. In critically ill patients, extreme pH <6.95 may affect the performance of point-of-care glucometers but not within pH range 6.97-7.84 (42). One study evaluated the effect of pH on the accuracy of CGM in a group pediatric intensive care patients and did not observe any significant effect (43). It is unknown whether fluid status might affect CGM performance in CKD patients due to lack of dedicated studies, however, a small study comparing hospitalized diabetes patients with and without congestive heart failure shown no differences in sensor accuracy (44).
Amongst exogenous substances, ascorbic acid, paracetamol, xylose, and ethanol have the potential to interfere with glucose oxidase sensors (45, 46) Other metabolites of icodextrin, such as maltose, also interfere with glucose dehydrogenase-based detectors using pyrroloquinoline quinone (GDH-PQQ) due to lack of selectivity on glucose (47). Use of GDH-PQQ glucometers can result in falsely elevated glucose readings in patients with PD using icodextrin dialysate. On the other hand, glucose-oxidase based capillary blood glucometers are mostly unaffected by icodextrin (35). Most commercially available CGM system use glucose-oxidase sensors although interference of CGM sensors by icodextrin had not been explored.
Performance of commercially available enzyme-based CGM systems have been validated in small numbers of patients on dialysis. For example, Yajima et al. evaluated accuracy of two CGM systems, the Freestyle Libre Pro and Medtronic iPro2™ with Enlite™ sensor versus capillary blood glucose in patients undergoing HD. For Freestyle Libre, 49% of readings fell within the Parkes Error Grid zone A and 51% in zone B. The Medtronic Ipro2™ sensor exhibited smaller deviations with 93% of readings within zone A and 6.3% in zone B which are regarded as clinically acceptable. Mean absolute relative difference (MARD) was 19.5% ± 13.2% for Freestyle Libre versus 8.1% ± 7.6% for Medtronic iPro2 (48). In a three-week study comparing the accuracy Freestyle Libre versus capillary blood glucose in 12 patients on haemodialysis, the MARD was found to be higher than people without ESKD (49). Only one study had evaluated the accuracy of Medtronic iPro2™ with Enlite™ sensor in 40 patients on PD. When compared with capillary blood glucose, MARD was 14%-19% (50). The accuracy of Dexcom sensors in haemodialysis is being investigated in ongoing trials (NCT04217161). Larger evaluation studies of sensor glucose against values measured by standard laboratory analyzers are needed in patients on different dialysis regimens.
Several studies analyzed the correlation between HbA1c, fructosamine, GA and average sensor glucose across different CKD stages (Table 2). In general, correlation between HbA1c and mean sensor glucose values tend to fall in CKD stage G4-5, in part confounded by differences in use of iron and ESA and blood haemoglobin. Lo and colleagues reported good correlation of mean CGM-glucose with HbA1c (r= 0.79) in patients with eGFR 30-59 ml/min/1.73m2 but fell (r=0.34) in participants (n=43) with eGFR below 30 ml/min/1.73m2 (51). In another study involving 25 patients with diabetes, the authors reported weak correlation (r=0.38) between mean CGM-glucose and HbA1c in patients with eGFR <30ml/min/1.73m2 (52).
Nathan et al. first estimated HbA1c by linearly regressing mean sensor glucose with HbA1c in intensively-treated patients with T1D in the Diabetes Control and Complication Trial (DCCT) (53). Bergenstal et al. later proposed the use of glucose management index (GMI) to reflect the relationship between CGM glucose and HbA1c (54). However, these equations were derived predominantly from T1D and T2D patients with normal renal function and the reliability of the current GMI equation is unknown in patients with CKD (55). In one cohort, Zelnick and colleagues reported similar correlations between GMI and HbA1c of 0.78 in patients with eGFR >30 ml/min/1.73m2 (n=80) and 0.76 in those with <30 ml/min/1.73m2 (n=24) (56). Nevertheless, the 2020 KDIGO guideline suggested GMI might be an alternative index for guiding treatment in patients with CKD G4-5 or dialysis where HbA1c had been shown to be less reliable (12). (Table 1).

Table 1 KDIGO 2020 recommendations on assessment of glycaemia in patients in chronic kidney disease (CKD) stages 1-4 (12).
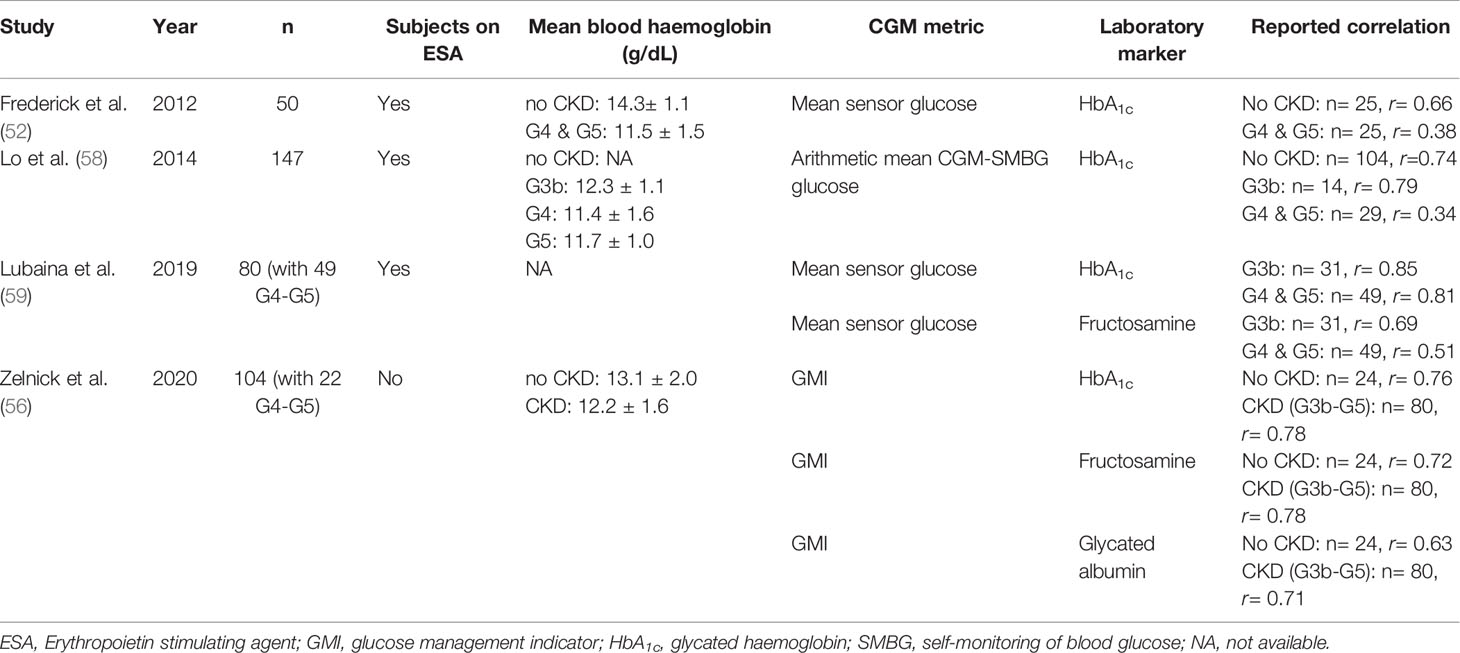
Table 2 Summary of studies assessing correlation between continuous glucose monitoring (CGM) metrics and glycemic markers in patients with chronic kidney disease (CKD).
Of equal if not greater importance is the use of time-in-ranges which describes the proportion of time the patient spent in hyperglycemia or hypoglycaemia range. In 2019, at the Advanced Technology and Treatment for Diabetes (ATTD) Conference, there was consensus on using a series of CGM-derived metrics as clinical targets for glycemic management. The recommended target in an adult patient with T2D and without complications was >70% Time in range (TIR, % time sensor glucose >3.9 and <10 mmol/L), <25% time in Time above range reflecting significant hyperglycemia (TAR, % time sensor glucose >10 mmol/L), <5% time below target suggesting hypoglycaemia (TBR, % time sensor glucose <3.9 mmol/L) with a Coefficient of Variation < 36% (%CV = SD (standard deviation) of sensor glucose/mean sensor glucose) (57). However, the validity of TIR targets and the prognostic values of CGM-derived metrics on complications and death need to be confirmed in clinical trials involving patients with advanced CKD and dialysis (12).
CGM systems provide comprehensive 24-hour profiles for assessment of relationships between glycemic variation, timing of dialysis regimens and insulin administration. In addition to the aforementioned CGM metrics, most CGM systems now provide standardized ambulatory glucose profiles (AGPs) which provide a graphical representation of 24-hour sensor glucose trends. Table 3 summarizes evaluation studies of CGM in patients on HD or PD.
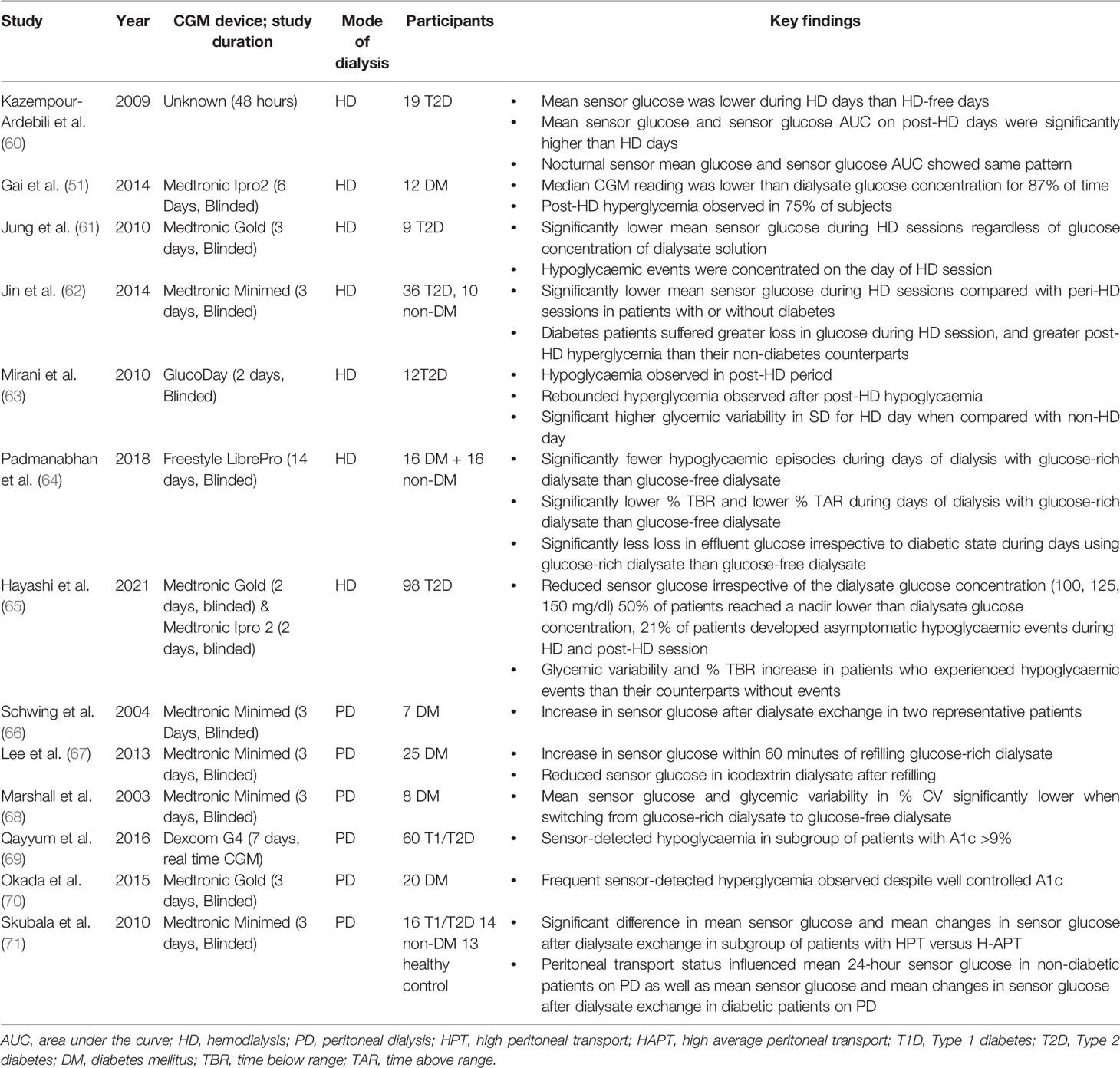
Table 3 Key Continuous Glucose Monitoring (CGM) studies in patients on hemodialysis or peritoneal dialysis.
In patients on HD, the composition of the dialysate and dialysis membrane both contribute to glycemic variability during HD and in the post-HD period. Differences in glucose profiles have also been reported between HD and non-HD days. The phenomenon of “glycemic disarray” in HD has been described, referring to the fall in glucose during HD followed by rebound hyperglycemia in the post-HD period (Figure 2). HD-induced hypoglycaemia is frequently observed. In early studies, Takahashi et al. demonstrated reduction in plasma glucose concentration from pre-dialyser site to post-dialyser site in patients under a dialysate of 5.55 mmol/L glucose. This reduction in serum glucose within the dialyzer might be induced by dialysate-stress triggered diffusion of plasma glucose into erythrocyte, as well as loss into the dialysate (72). In general, patients might lose around 15-30 g of glucose during HD session. In patients with ESKD, defective counter-regulatory effects, reduced renal gluconeogenesis and hypoglycaemia unawareness might result in frequent asymptomatic hypoglycaemic events. In 17 patients with T2D on HD, the mean sensor glucose was lower during the on-dialysis than the off-dialysis days (60). In 12 patients on dialysis, Gai et al. reported that the median CGM glucose level was below the concentration of dialysate of 5.55 mmol/L during most of the HD session (87% of time) (51). In 9 patients with T2D, Jung et al. reported significant reduction in mean sensor glucose during HD session, regardless of the glucose concentration of dialysate solution (5.55 – 11.1 mmol/L) with most of the hypoglycaemic events occurring on the day of HD (61). In 46 patients with ESKD with or without diabetes, Jin et al. reported a significant reduction in mean sensor glucose during HD session irrespective of the status of diabetes although patients with diabetes had greater glucose loss during HD session (62).
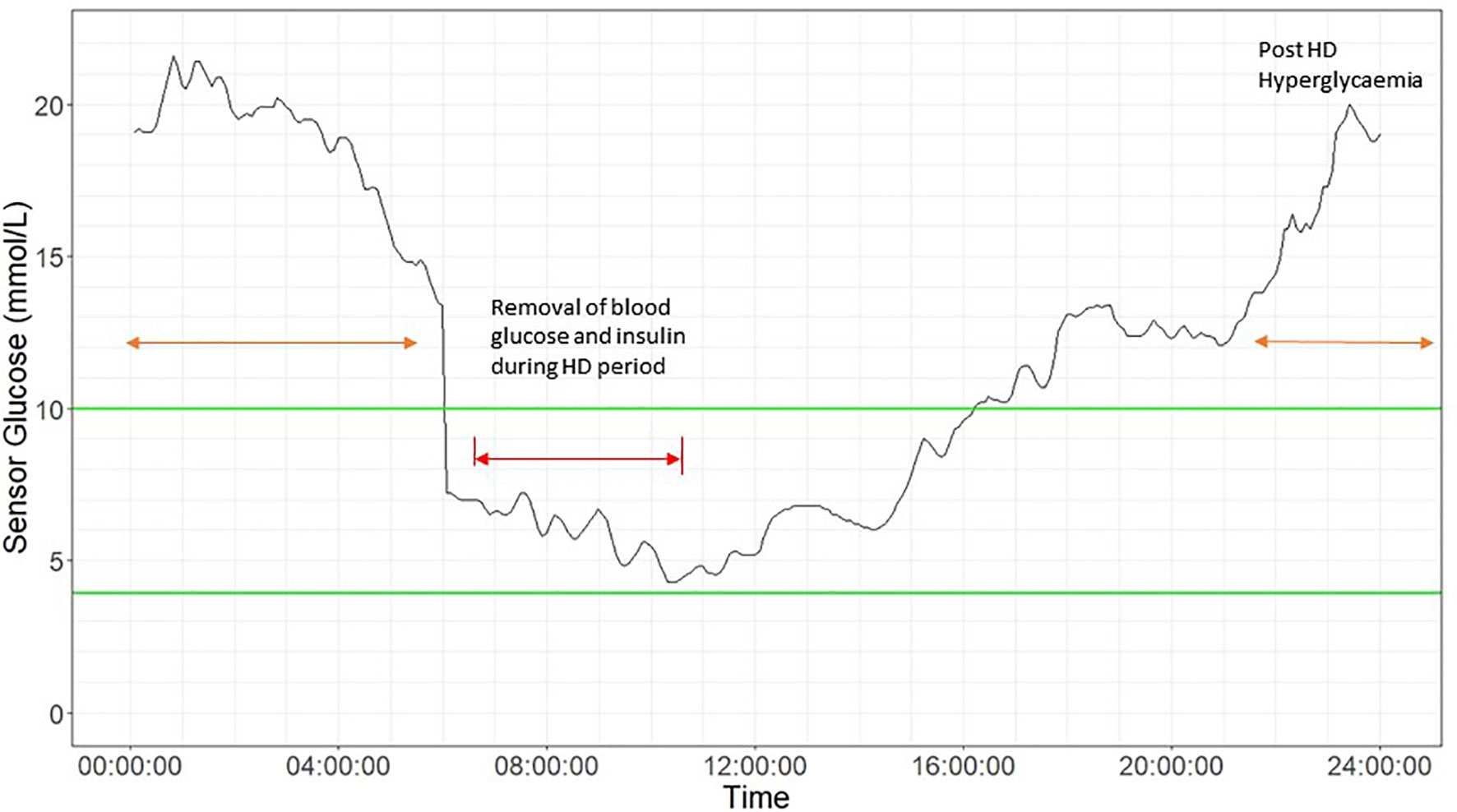
Figure 2 Glycemic disarray showing marked variability in patients during haemodialysis (HD) and post-HD period. 24-hour CGM glucose profile in a 58-year-old man with type 2 diabetes on HD using glucose- free dialysate. He was treated with insulin glargine 24 units in the morning and alogliptin 6.25mg daily with HbA1c of 8.2%. The HD period is indicated by red arrow, showing an acute drop in sensor glucose, followed by post HD-associated hyperglycemia (orange arrow) up to 20 mmol/l at midnight. Green lines indicates target range (3.9 mmol/L to 10 mmol/L).
In a recent study involving 98 Japanese patients with T2D on HD who had 2-day CGM, sensor glucose showed a sustained decline irrespective of dialysate glucose concentration with 50% of patients with diabetes reaching a glucose nadir lower than the dialysate concentration. In the whole group, 21% experienced HD-related hypoglycaemia <3.9 mmol/L either during the HD session or post-HD and before the next meal. There were no difference in terms of clinical characteristics (e.g. body mass index, duration of diabetes, insulin treatment) and traditional glycemic markers (e.g. HbA1c and GA), between patients with HD-related or post-HD hypoglycaemia and patients without hypoglycaemia. Despite an average HbA1c: 6.4% ± 1.2% for these T2D patients, asymptomatic HD-related hypoglycaemia was frequent and the HD-related hypoglycaemia was only captured by CGM (65).
Rebound hyperglycemia during the post-HD period may be related to choices of dialysate and dialysis membrane, which can influence plasma insulin concentrations during dialysis (73, 74). Insulin is readily removed from plasma by diffusion owing to its small molecular size and low protein-binding capacity. However, during HD, most of the insulin is removed via adsorption with dialysis membrane through electrostatic and hydrophobic interactions resulting in hyperglycemia in the post-HD period. The clearance of insulin by absorption depends on the type of dialysis membrane, with greatest absorption in polysulfone membrane and lowest absorption in polyester-polymer alloy (19). The counter-regulatory hormonal responses to HD-induced hypoglycaemia could increase insulin resistance and trigger post-HD hyperglycemia. Kazempour-Ardebili et al. demonstrated that nocturnal sensor glucose was significantly higher on the HD-day than HD-free day (60). This was also confirmed by other studies where time of HD-session was reported in the 24-hour CGM profile (51, 61, 63). Jin et al. confirmed post-HD hyperglycemia especially in patients with diabetes compared with their non-diabetic counterparts (62). Padmanabhan et al. evaluated the effects of different dialysate and dialysis membranes on glycemic control. In a study of 38 patients with and without diabetes, HD-induced hypoglycaemia and post-HD hyperglycemia occurred with the use of glucose-free dialysate but the fluctuation could be attenuated by using glucose-containing dialysate (64). Both HD-induced hypoglycaemia and post-HD hyperglycemia may contribute to heightened glycemic variability, increased oxidative stress and inflammation with worsening of clinical outcomes. By using CGM, these silent events may be detected early to inform treatment.
One of the determining factors of glycemic profile in patients with PD is the rate of peritoneal absorption of glucose, which is in turn affected by glucose concentration of dialysate, dwell time, and status of membrane transport (75). Ultrafiltration by peritoneal membrane is created by either crystalloid osmosis using a higher glucose concentration in the dialysate, or by colloid osmosis using large colloid agents like icodextrin (76). Icodextrin solution contains a mixture of glucose polymers which are slowly absorbed via lymphatics. Together with its osmotic effect, icodextrin leads to sustained ultrafiltration and is widely used as an alternative osmotic agent to dextrose especially in dialysate with long dwelling time (77). Early observational studies using CGM showed that patients with PD spent a large proportion of time in hyperglycemia (66). In a study of 20 patients with well-controlled T1D and T2D and mean HbA1c of 5.9% who were dialysed on glucose-containing dialysates, patients spent on average 33% time above 10 mmol/l and 1% time below 3.9 mmol/l (70). Lee et al. evaluated the impact of glucose influx from dialysate in 25 patients with diabetes on maintenance PD. In patients using glucose-based dialysate, the sensor glucose levels increased by 7-8 mg/dL within 1 hour of exchange using glucose-containing dialysate. The glycemic excursion was similar with 1.25% and 2.25% glucose solutions with larger increments observed with 3.86% glucose solutions (67). Figure 3 shows an example of CGM profile in a patient on continuous ambulatory peritoneal dialysis (CAPD).
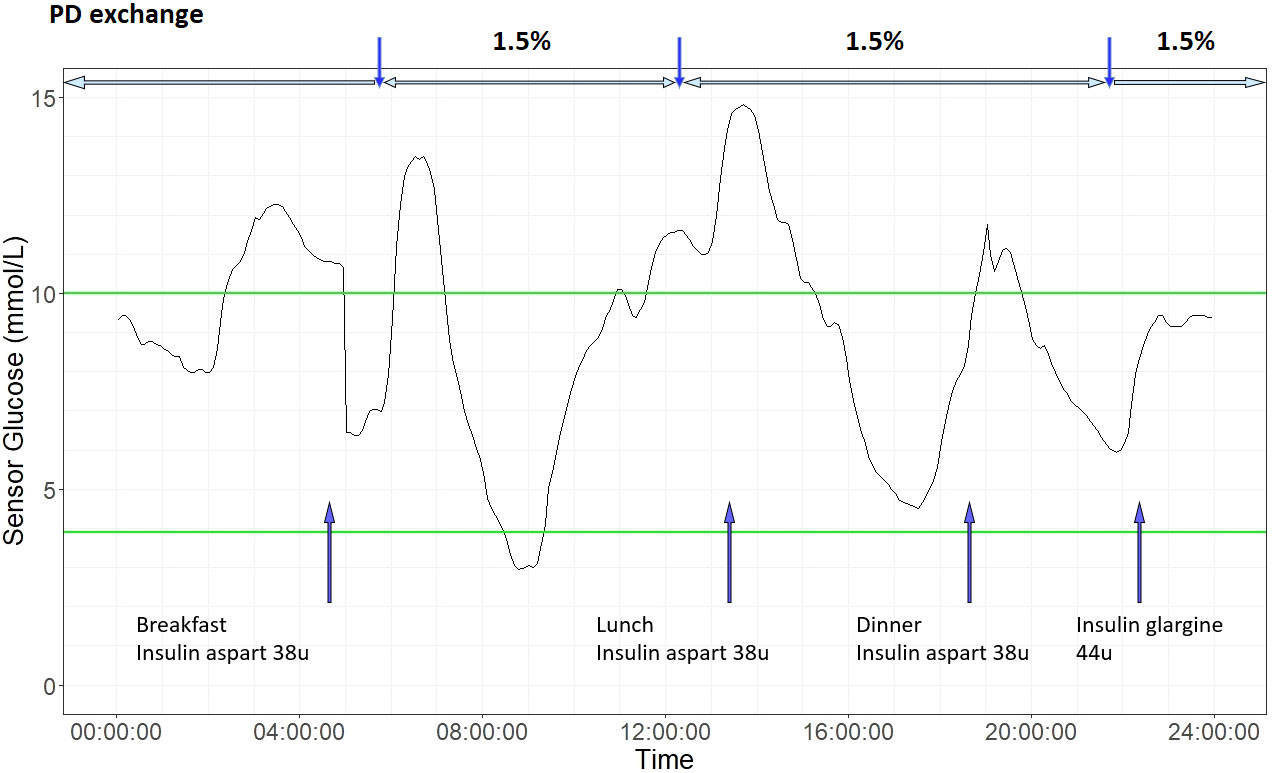
Figure 3 An illustrative 24-hour ambulatory glucose profile in a patient with type 2 diabetes on continuous ambulatory peritoneal dialysis (CAPD). He is on three 1.5% dextrose exchanges daily and basal-bolus insulin regimen. Laboratory measures of glycemic control were HbA1c 7.5% and Fructosamine 224 µmol/L. Based on CGM metrics, glucose management indicator (GMI) was 6.9%, coefficient variation (CV) 36.9%. Blue arrow on bottom indicates times of insulin injection and meal intake. Vertical blue arrow on top indicate PD exchange timing, and horizontal blue arrow on top indicate PD exchange period. Green lines indicates target CGM glucose range (3.9 mmol/L to 10 mmol/L).
Icodextrin is associated with stable or even decreases in CGM sensor glucose during PD dwells (67). Marshall et al. demonstrated the effect of switching dialysate on CGM profiles in 8 patients with PD. Switching from three 1.36% glucose exchanges and one 3.86% glucose exchange to two bags of 1.36% glucose exchange, one bag of amino acid exchange and one bag of icodextrin was associated with lower sensor glucose and glycemic variability (68). In a retrospective study of 60 patients with 95% of them receiving icodextrin dialysate, the CGM-detected time in hypoglycaemia was 5% which was often asymptomatic (69).
The diffusing capacity of the peritoneal membrane is another crucial factor in determining glycaemia. The exchange rate of serum-dialysate glucose is dependent on the osmotic pressure, as well as the transport status of peritoneal membrane. Osmotic gradient between dialysate and peritoneum is rapidly lost in patients with high transporter status due to rapid absorption of glucose from dialysate (78). As a result, these patients might have high risk of PD-related hyperglycemia. Skubala et al. demonstrated the effect of peritoneal transport status using CGM in 30 patients with and without diabetes. In their study, patients on 1.36% and 2.27% glucose dialysates had similar HbA1c, mean 24-hour CGM-glucose, mean post-PD glucose, and mean post-PD increment in glucose. However, mean post-PD glucose and mean post-PD increment in glucose was significantly different in patients with high peritoneal transport (HPT) and high average peritoneal transport (HAPT), even in nondiabetic individuals (71).
Another modifiable factor of CGM-glucose is the timing, route and dose of insulin administration in patients on PD. Subcutaneous basal bolus insulin regimen are effective regimens in patients with T1D or T2D on PD but require frequent self-monitoring (50). Intraperitoneal (IP) delivery of insulin can counteract the glucose absorption from dialysate. However, there are no standardised recommendations on initiation or titration of IP insulin for different dialysates (79). Dose adjustments are often based on infrequent fasting and post-meal capillary blood glucose with CGM having the potential to guide adjustment of insulin therapy in patients on PD.
In summary, patients with diabetes on HD or PD display distinct glycemic profiles and patterns which can be comprehensively assessed by CGM. Apart from patient factors (e.g. beta-cell function, PD transporter status), there are a number of modifiable treatment factors, such as choices of dialysate, dialysis regimen and doses/timing of insulin, where data from CGM can help optimize treatment.
Personal use of real-time (rt) or flash CGM devices may reduce hypoglycaemia and improve glycemic control in patients with diabetes without CKD. The benefits of CGM use in patients with T1D on improving glycemic control are now well-established. In the Randomized Controlled Trial Examining the Benefit of CGM Use for Adults with T1D on Insulin Injections (DIAMOND) trial (80), there was a significant HbA1c difference of -0.6% in favour of rt-CGM versus standard SMBG after 24 weeks of intervention in T1D patients on multiple daily injection (MDI). In another randomized study involving 161 patients with T1D treated with MDI, a similar significant difference of -0.43% in HbA1c in favor of rt-CGM versus standard SMBG was reported after 26-weeks of intervention and during 17-weeks of post-intervention washout period (80, 81). In an open-labelled randomized trial in adults with well-controlled T1D on MDI (REPLACE-BG trial), use of flash CGM without confirmatory SMBG was safe and reduced hypoglycaemia (82) with improved treatment satisfaction (83).
Several pilot and small-scale studies supported the potential beneficial effects of professional CGM in patients on HD or PD, whilst data on continuous personal use was limited. Most studies explored the use of blinded CGM for treatment titration. In a pilot-study, Képénékian al. used blinded CGM in 28 T2D patients on HD with suboptimal glycemic control for 54 hours at baseline and during a 3-month follow-up period. After 3 months of intervention, the CGM-adapted insulin regimen was associated with greater reduction in HbA1c without increasing symptomatic hypoglycaemia (84). The DIALYDIAB pilot study involved 15 patients with T1D or T2D and compared the effect of blinded-CGM SMBG using a two-period design. Use of blinded CGM triggered more frequent treatment adjustments compared with SMBG alone. This resulted in better glucose profile with significantly lower HbA1c and time above range without increasing hypoglycaemic episodes (85). There are also few studies in patients with PD where CGM was used to assess effects of structured education (50) or compare different glucose lowering drug regimens (86). These studies demonstrated the potential of CGM in promoting patient self-management and informing providers in treatment adjustment to improve glycemic control.
CGM systems have the potential to be combined with automated insulin delivery in closed-loop systems, also referred to as an ‘artificial pancreas”. A recent randomized trial evaluated a fully automated closed-loop system against standard insulin therapy in 26 patients with T2D on HD using a cross-over design (87). In this study, TIR was significantly higher (57.1% versus 42.5%) and time above and below range were lower (TAR: 42.6% versus 56.6%, TBR: 0.12% versus 0.17%) in the closed-loop phase. The mean sensor glucose was also significantly lower in the closed-loop versus control phase (10.1 mmol/L versus 11.6 mmol/L). Of note, the time spent in extreme hyperglycemia (defined as >20 mmol/L) was significantly lower during the closed-loop phase than the control phase (1.8% versus 6.7%). However, the system was given only for short-term use operated by healthcare professionals in a clinic setting rather than home use.
The ease of operation of personal CGM systems in patients with ESKD with multiple comorbidities need to be considered. Many patients with ESKD may have visual impairment due to retinopathy or cataracts, skin problems and cognitive issues that limit their ability to operate these devices. However, personal CGM with real-time alerts might benefit patients with ESKD on complex insulin regimens or vulnerability to hypoglycemia. Future research is required to investigate the utility and cost-effectiveness of personal CGM in patients with advanced CKD and dialysis.
There are some limitations in the use of CGM for patients under dialysis. Apart from potential sensor interference from endogenous and exogenous substances (46), accuracy of CGM is lower in the hypoglycemic range and under rapid changes in blood glucose values (88–90). False hypoglycaemic alerts may occur more frequently under these conditions, which may lead to unnecessary treatment. A confirmatory SMBG value is advisable for treatment decisions at these extreme glucose values. Additionally, repeated false positive alerts could lead to alarm fatigue and increase patient anxiety.
Optimal glycemic control will delay progression of CKD and improve clinical outcomes. HbA1c and alternative glycemic markers have limitations particularly in patients with advanced CKD. With the advent of CGM, it is now possible to monitor the glycemic status with better precision in patients with CKD. Professional CGM can inform health care professionals on glucose profiles not provided by HbA1c in patients with CKD to optimize treatment regimens. Real-time or flash CGM provide instant or timely feedback to users on impact of meals and treatment on glucose excursion. The inclusion of real-time alerts in CGM, displayed in smart devices, can provide early warnings against hyperglycemia and hypoglycaemia. This information may improve the safety of prescription of GLDs and insulin in these high-risk patients. Finally, the integration of these CGM system with fully automated closed loop insulin delivery systems offer the potential of more precise control.
The use of CGM in patients with ESKD has revealed distinct glycemic patterns during maintenance dialysis. Glycemic pattern in patients under HD are impacted by the glucose concentrations in dialysate and choices of dialysis membrane. Glucose-free dialysate is generally preferred due to lower cost and chance of bacterial infection. However, glucose-containing dialysate may reduce HD-related hypoglycaemia and post-HD hyperglycemia, especially in patients with diabetes. Health care professionals should consider providing glucose-containing dialysate, replenishing post-HD glucose loss by snacks, or adjusting insulin regimen to avoid HD-related glycemic excursion. Similar to HD, glycemic patterns in PD patients are impacted by diasylate glucose concentration and peritoneal membrane transport state. Health care professionals should consider glucose influx from glucose-rich dialysate and adjust insulin treatment to maintain a stable blood glucose. Although switch to glucose-free dialysates may theoretically reduce glucose influx, randomized trials suggested this might be associated with adverse outcomes (91). Pending further evidence, a careful adjustment of insulin and dialysate regimens in patients under PD may strike the balance between optimizing glycemic control and ultrafiltration. Future studies using CGM should be conducted to investigate whether the use of personal CGM with glycemic alerts will reduce hypoglycaemia and complications and improve long-term outcomes in patients with advanced CKD and dialysis.
JL and EC conceived the idea of the paper. JL researched and wrote the manuscript, JL, JN, EC, and JC critically revised the manuscript. All authors contributed to and approved the final manuscript.
EC has received institutional research support form Medtronic Diabetes and Powder Pharmaceuticals Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CKD, Chronic Kidney Disease; ESKD, End-Stage Kidney Disease; OGLD, Oral Glucose Lowering Drugs; eGFR, estimated Glomerulus Filtration Rate; KDIGO, Kidney Disease Improving Global Outcome; CGM, Continuous Glucose Monitoring; SMBG, Self-Monitoring Blood Glucose; PD, Peritoneal dialysis; HD, Haemodialysis; TAR, Target Above Range; TIR, Target In Range; TBR, Target Below Range; CV, Coefficient of Variation.
1. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic Kidney Disease: A Report From an Ada Consensus Conference. Diabetes Care (2014) 37(10):2864–83. doi: 10.2337/dc14-1296
2. Chan JCN, Lim L-L, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet Commission on Diabetes: Using Data to Transform Diabetes Care and Patient Lives. Lancet (2020) 396(10267):2019–82. doi: 10.1016/S0140-6736(20)32374-6
3. Leung CB, Cheung WL, Li PKT. Renal Registry in Hong Kong-The First 20 Years. Kidney Int Suppl (2011) (2015) 5(1):33–8. doi: 10.1038/kisup.2015.7
4. Kong AP, Yang X, Luk A, Cheung KK, Ma RC, So WY, et al. Hypoglycaemia, Chronic Kidney Disease and Death in Type 2 Diabetes: The Hong Kong Diabetes Registry. BMC Endocr Disord (2014) 14:48. doi: 10.1186/1472-6823-14-48
5. Vanholder R, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, et al. Uremic Toxicity: Present State of the Art. Int J Artif Organs (2001) 24(10):695–725. doi: 10.1177/039139880102401004
6. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease. Circulation (2021) 143(11):1157–72. doi: 10.1161/CIRCULATIONAHA.120.050686
7. Rhee CM, Kovesdy CP, You AS, Sim JJ, Soohoo M, Streja E, et al. Hypoglycemia-Related Hospitalizations and Mortality Among Patients With Diabetes Transitioning to Dialysis. Am J Kidney Dis (2018) 72(5):701–10. doi: 10.1053/j.ajkd.2018.04.022
8. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med (1993) 329(14):977–86. doi: 10.1056/nejm199309303291401
9. Wong MG, Perkovic V, Chalmers J, Woodward M, Li Q, Cooper ME, et al. Long-Term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: Advance-On. Diabetes Care (2016) 39(5):694–700. doi: 10.2337/dc15-2322
10. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive Insulin Therapy Prevents the Progression of Diabetic Microvascular Complications in Japanese Patients With Non-Insulin-Dependent Diabetes Mellitus: A Randomized Prospective 6-Year Study. Diabetes Res Clin Pract (1995) 28(2):103–17. doi: 10.1016/0168-8227(95)01064-k
11. Ramirez SPB, McCullough KP, Thumma JR, Nelson RG, Morgenstern H, Gillespie BW, et al. Hemoglobin a<Sub>1c</Sub> Levels and Mortality in the Diabetic Hemodialysis Population. Diabetes Care (2012) 35(12):2527. doi: 10.2337/dc12-0573
12. Navaneethan SD, Zoungas S, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, et al. Diabetes Management in Chronic Kidney Disease: Synopsis of the 2020 Kdigo Clinical Practice Guideline. Ann Internal Med (2020) 174:385–94. doi: 10.7326/M20-5938
13. McCaleb ML, Izzo MS, Lockwood DH. Characterization and Partial Purification of a Factor From Uremic Human Serum That Induces Insulin Resistance. J Clin Invest (1985) 75(2):391–6. doi: 10.1172/JCI111712
14. Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC, et al. Evidence for Anti-Inflammatory Effects of Exercise in Ckd. J Am Soc Nephrol (2014) 25(9):2121–30. doi: 10.1681/asn.2013070702
15. Roubicek T, Bartlova M, Krajickova J, Haluzikova D, Mraz M, Lacinova Z, et al. Increased Production of Proinflammatory Cytokines in Adipose Tissue of Patients With End-Stage Renal Disease. Nutrition (2009) 25(7-8):762–8. doi: 10.1016/j.nut.2008.12.012
16. Koppe L, Pelletier CC, Alix PM, Kalbacher E, Fouque D, Soulage CO, et al. Insulin Resistance in Chronic Kidney Disease: New Lessons From Experimental Models. Nephrol Dial Transplant (2014) 29(9):1666–74. doi: 10.1093/ndt/gft435
17. Rahhal MN, Gharaibeh NE, Rahimi L, Ismail-Beigi F. Disturbances in Insulin-Glucose Metabolism in Patients With Advanced Renal Disease With and Without Diabetes. J Clin Endocrinol Metab (2019) 104(11):4949–66. doi: 10.1210/jc.2019-00286
18. Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR. Glycemic Monitoring and Management in Advanced Chronic Kidney Disease. Endocrine Rev (2020) 41(5):756–74. doi: 10.1210/endrev/bnaa017
19. Abe M, Kalantar-Zadeh K. Haemodialysis-Induced Hypoglycaemia and Glycaemic Disarrays. Nat Rev Nephrol (2015) 11(5):302–13. doi: 10.1038/nrneph.2015.38
20. Speeckaert M, Van Biesen W, Delanghe J, Slingerland R, Wiecek A, Heaf J, et al. Are There Better Alternatives Than Haemoglobin A1c to Estimate Glycaemic Control in the Chronic Kidney Disease Population? Nephrol Dial Transplant (2014) 29(12):2167–77. doi: 10.1093/ndt/gfu006
21. Copur S, Onal EM, Afsar B, Ortiz A, van Raalte DH, Cherney DZ, et al. Diabetes Mellitus in Chronic Kidney Disease: Biomarkers Beyond Hba1c to Estimate Glycemic Control and Diabetes-Dependent Morbidity and Mortality. J Diabetes its Complicat (2020) 34(11):107707. doi: 10.1016/j.jdiacomp.2020.107707
22. Weykamp CW, Miedema K, de Haan T, Doelman CJA. Carbamylated Hemoglobin Interference in Glycohemoglobin Assays. Clin Chem (1999) 45(3):438–40. doi: 10.1093/clinchem/45.3.438
23. Garlick RL, Mazer JS. The Principal Site of Nonenzymatic Glycosylation of Human Serum Albumin In Vivo. J Biol Chem (1983) 258(10):6142–6. doi: 10.1016/S0021-9258(18)32384-6
24. Don BR, Kaysen G. Poor Nutritional Status and Inflammation: Serum Albumin: Relationship to Inflammation and Nutrition. Semin Dialysis (2004) 17(6):432–7. doi: 10.1111/j.0894-0959.2004.17603.x
25. Watanabe Y, Ohno Y, Inoue T, Takane H, Okada H, Suzuki H. Blood Glucose Levels in Peritoneal Dialysis Are Better Reflected by Hba1c Than by Glycated Albumin. Adv Perit Dial (2014) 30:75–82.
26. Fukami K, Shibata R, Nakayama H, Yamada K, Okuda S, Koga M. Serum Albumin-Adjusted Glycated Albumin Is a Better Indicator of Glycaemic Control in Diabetic Patients With End-Stage Renal Disease Not on Haemodialysis. Ann Clin Biochem (2015) 52(4):488–96. doi: 10.1177/0004563214568162
27. Thornalley PJ. Advanced Glycation End Products in Renal Failure. J Renal Nutr (2006) 16(3):178–84. doi: 10.1053/j.jrn.2006.04.012
28. Lloyd DR, Marples J. Serum Fructosamine Does Reflect Levels of Glycated Serum Albumin in Insulin-Dependent Diabetics. Ann Clin Biochem (1988) 25(4):432–4. doi: 10.1177/000456328802500421
29. Oimomi M, Masuta S, Sakai M, Ohara T, Hata F, Baba S. Influence of Serum Protein Levels on Serum Fructosamine Levels. Jpn J Med (1989) 28(3):312–5. doi: 10.2169/internalmedicine1962.28.312
30. Johnson RN, Metcalf PA, Baker JR. Relationship Between Albumin and Fructosamine Concentration in Diabetic and Non-Diabetic Sera. Clin Chim Acta (1987) 164(2):151–62. doi: 10.1016/0009-8981(87)90066-0
31. Yuan L, Guo X, Xiong Z, Lou Q, Shen L, Zhao F, et al. Self-Monitoring of Blood Glucose in Type 2 Diabetic Patients in China: Current Status and Influential Factors. Chin Med J (Engl) (2014) 127(2):201–7. doi: 103750/cma.j.issn.0366-6999.20131776
32. Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises. J Diabetes Sci Technol (2010) 4(6):1540–62. doi: 10.1177/193229681000400632
33. Kesavadev J, Ramachandran L, Krishnan G. Glucose Monitoring Technologies - Complementary or Competitive? Role of Continuous Glucose Monitoring Versus Flash Glucose Monitoring Versus Self-Monitoring of Blood Glucose. J Diabetol (2017) 8(3):61–7. doi: 10.4103/jod.jod_14_17
34. Graham C. Continuous Glucose Monitoring and Global Reimbursement: An Update. Diabetes Technol Ther (2017) 19(S3):S60–S6. doi: 10.1089/dia.2017.0096
35. Boehm R, Donovan J, Sheth D, Durfor A, Roberts J, Isayeva I. In Vitro Sugar Interference Testing With Amperometric Glucose Oxidase Sensors. J Diabetes Sci Technol (2019) 13(1):82–95. doi: 10.1177/1932296818791538
36. Rosevear JW, Pfaff KJ, Service FJ, Molnar GD, Ackerman E. Glucose Oxidase Method for Continuous Automated Blood Glucose Determination. Clin Chem (1969) 15(8):680–98. doi: 10.1093/clinchem/15.8.680
37. Rocchitta G, Spanu A, Babudieri S, Latte G, Madeddu G, Galleri G, et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors (Basel) (2016) 16(6):780. doi: 10.3390/s16060780
38. Tonyushkina K, Nichols JH. Glucose Meters: A Review of Technical Challenges to Obtaining Accurate Results. J Diabetes Sci Technol (2009) 3(4):971–80. doi: 10.1177/193229680900300446
39. Karon B S, Griesmann L, Scott R, Bryant SC, Dubois JA, Shirey TL, et al. Evaluation of the Impact of Hematocrit and Other Interference on the Accuracy of Hospital-Based Glucose Meters. Diabetes Technol Ther (2008) 10(2):111–20. doi: 10.1089/dia.2007.0257
40. Ogawa T, Murakawa M, Matsuda A, Kanozawa K, Kato H, Hasegawa H, et al. Endogenous Factors Modified by Hemodialysis May Interfere With the Accuracy of Blood Glucose-Measuring Device. Hemodialysis Int (2012) 16(2):266–73. doi: 10.1111/j.1542-4758.2011.00640.x
41. Lucarelli F, Ricci F, Caprio F, Valgimigli F, Scuffi C, Moscone D, et al. Glucomen Day Continuous Glucose Monitoring System: A Screening for Enzymatic and Electrochemical Interferents. J Diabetes Sci Technol (2012) 6(5):1172–81. doi: 10.1177/193229681200600522
42. Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose Measurement: Confounding Issues in Setting Targets for Inpatient Management. Diabetes Care (2007) 30(2):403–9. doi: 10.2337/dc06-1679
43. Marics G, Koncz L, Eitler K, Vatai B, Szénási B, Zakariás D, et al. Effects of Ph, Lactate, Hematocrit and Potassium Level on the Accuracy of Continuous Glucose Monitoring (Cgm) in Pediatric Intensive Care Unit. Ital J Pediatr (2015) 41(1). doi: 10.1186/s13052-015-0122-x
44. Dungan K, Graessle K, Sagrilla C. The Effect of Congestive Heart Failure on Sensor Accuracy Among Hospitalized Patients With Type 2 Diabetes. Diabetes Technol Ther (2013) 15(10):817–24. doi: 10.1089/dia.2013.0094
45. Ginsberg BH. Factors Affecting Blood Glucose Monitoring: Sources of Errors in Measurement. J Diabetes Sci Technol (2009) 3(4):903–13. doi: 10.1177/193229680900300438
46. Basu A, Slama MQ, Nicholson WT, Langman L, Peyser T, Carter R, et al. Continuous Glucose Monitor Interference With Commonly Prescribed Medications: A Pilot Study. J Diabetes Sci Technol (2017) 11(5):936–41. doi: 10.1177/1932296817697329
47. Floré KM, Delanghe JR. Analytical Interferences in Point-Of-Care Testing Glucometers by Icodextrin and Its Metabolites: An Overview. Perit Dial Int (2009) 29(4):377–83. doi: 10.1177/089686080902900403
48. Yajima T, Takahashi H, Yasuda K. Comparison of Interstitial Fluid Glucose Levels Obtained by Continuous Glucose Monitoring and Flash Glucose Monitoring in Patients With Type 2 Diabetes Mellitus Undergoing Hemodialysis. J Diabetes Sci Technol (2019) 14(6):1088–94. doi: 10.1177/1932296819882690
49. Hissa MRN, Hissa PNG, Guimarães SB, Hissa MN. Use of Continuous Glucose Monitoring System in Patients With Type 2 Mellitus Diabetic During Hemodialysis Treatment. Diabetol Metab Syndrome (2021) 13(1). doi: 10.1186/s13098-021-00722-8
50. Gómez AM, Vallejo S, Ardila F, Muñoz OM, Ruiz ÁJ, Sanabria M, et al. Impact of a Basal-Bolus Insulin Regimen on Metabolic Control and Risk of Hypoglycemia in Patients With Diabetes Undergoing Peritoneal Dialysis. J Diabetes Sci Technol (2018) 12(1):129–35. doi: 10.1177/1932296817730376
51. Gai M, Merlo I, Dellepiane S, Cantaluppi V, Leonardi G, Fop F, et al. Glycemic Pattern in Diabetic Patients on Hemodialysis: Continuous Glucose Monitoring (Cgm) Analysis. Blood Purif (2014) 38(1):68–73. doi: 10.1159/000362863
52. Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ. Assessment of Markers of Glycaemic Control in Diabetic Patients With Chronic Kidney Disease Using Continuous Glucose Monitoring. Nephrology (2012) 17(2):182–8. doi: 10.1111/j.1440-1797.2011.01517.x
53. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1c Assay Into Estimated Average Glucose Values. Diabetes Care (2008) 31(8):1473–8. doi: 10.2337/dc08-0545
54. Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose Management Indicator (Gmi): A New Term for Estimating A1c From Continuous Glucose Monitoring. Diabetes Care (2018) 41(11):2275–80. doi: 10.2337/dc18-1581
55. Herman WH. Do Race and Ethnicity Impact Hemoglobin A1c Independent of Glycemia? J Diabetes Sci Technol (2009) 3(4):656–60. doi: 10.1177/193229680900300406
56. Zelnick LR, Batacchi ZO, Ahmad I, Dighe A, Little RR, Trence DL, et al. Continuous Glucose Monitoring and Use of Alternative Markers to Assess Glycemia in Chronic Kidney Disease. Diabetes Care (2020) 43(10):2379. doi: 10.2337/dc20-0915
57. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care (2019) 42(8):1593–603. doi: 10.2337/dci19-0028
58. Lo C, Lui M, Ranasinha S, Teede HJ, Kerr PG, Polkinghorne KR, et al. Defining the Relationship Between Average Glucose and Hba1c in Patients With Type 2 Diabetes and Chronic Kidney Disease. Diabetes Res Clin Pract (2014) 104(1):84–91. doi: 10.1016/j.diabres.2014.01.020
59. Presswala L, Hong S, Harris Y, Romao I, Zhang M, Jhaveri KD, et al. Continuous Glucose Monitoring and Glycemic Control in Patients With Type 2 Diabetes Mellitus and Ckd. Kidney Med (2019) 1(5):281–7. doi: 10.1016/j.xkme.2019.07.006
60. Kazempour-Ardebili S, Lecamwasam VL, Dassanyake T, Frankel AH, Tam FWK, Dornhorst A, et al. Assessing Glycemic Control in Maintenance Hemodialysis Patients With Type 2 Diabetes. Diabetes Care (2009) 32(7):1137. doi: 10.2337/dc08-1688
61. Jung HS, Kim HI, Kim MJ, Yoon JW, Ahn HY, Cho YM, et al. Analysis of Hemodialysis-Associated Hypoglycemia in Patients With Type 2 Diabetes Using a Continuous Glucose Monitoring System. Diabetes Technol Ther (2010) 12(10):801–7. doi: 10.1089/dia.2010.0067
62. Jin YP, Su XF, Yin GP, Xu XH, Lou JZ, Chen JJ, et al. Blood Glucose Fluctuations in Hemodialysis Patients With End Stage Diabetic Nephropathy. J Diabetes its Complicat (2015) 29(3):395–9. doi: 10.1016/j.jdiacomp.2014.12.015
63. Mirani M, Berra C, Finazzi S, Calvetta A, Radaelli MG, Favareto F, et al. Inter-Day Glycemic Variability Assessed by Continuous Glucose Monitoring in Insulin-Treated Type 2 Diabetes Patients on Hemodialysis. Diabetes Technol Ther (2010) 12(10):749–53. doi: 10.1089/dia.2010.0052
64. Padmanabhan A, Velayudham B, Vijaykumar N, Allauddin S. Evaluation of Glycemic Status During the Days of Hemodialysis Using Dialysis Solutions With and Without Glucose. Saudi J Kidney Dis Transpl (2018) 29(5):1021–7. doi: 10.4103/1319-2442.243951
65. Hayashi A, Shimizu N, Suzuki A, Matoba K, Momozono A, Masaki T, et al. Hemodialysis-Related Glycemic Disarray Proven by Continuous Glucose Monitoring; Glycemic Markers and Hypoglycemia. Diabetes Care (2021) 44(7):1647. doi: 10.2337/dc21-0269
66. Schwing WD, Erhard P, Newman LN, Nodge MM, Czechanski BJ, Orlin SM, et al. Assessing 24-Hour Blood Glucose Patterns in Diabetic Paitents Treated by Peritoneal Dialysis. Adv Perit Dial (2004) 20:213–6.
67. Lee SY, Chen YC, Tsai IC, Yen CJ, Chueh SN, Chuang HF, et al. Glycosylated Hemoglobin and Albumin-Corrected Fructosamine Are Good Indicators for Glycemic Control in Peritoneal Dialysis Patients. PloS One (2013) 8(3):e57762. doi: 10.1371/journal.pone.0057762
68. Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW. Glycemic Control in Diabetic Capd Patients Assessed by Continuous Glucose Monitoring System (Cgms). Kidney Int (2003) 64(4):1480–6. doi: 10.1046/j.1523-1755.2003.00209.x
69. Qayyum A, Chowdhury TA, Oei EL, Fan SL. Use of Continuous Glucose Monitoring in Patients With Diabetes Mellitus on Peritoneal Dialysis: Correlation With Glycated Hemoglobin and Detection of High Incidence of Unaware Hypoglycemia. Blood Purif (2016) 41(1-3):18–24. doi: 10.1159/000439242
70. Okada E, Oishi D, Sakurada T, Yasuda T, Shibagaki Y. A Comparison Study of Glucose Fluctuation During Automated Peritoneal Dialysis and Continuous Ambulatory Peritoneal Dialysis. Adv Perit Dial (2015) 31:34–7.
71. Skubala A, Zywiec J, Zełobowska K, Gumprecht J, Grzeszczak W. Continuous Glucose Monitoring System in 72-Hour Glucose Profile Assessment in Patients With End-Stage Renal Disease on Maintenance Continuous Ambulatory Peritoneal Dialysis. Med Sci Monit (2010) 16(2):Cr75–83.
72. Takahashi A, Kubota T, Shibahara N, Terasaki J, Kagitani M, Ueda H, et al. The Mechanism of Hypoglycemia Caused by Hemodialysis. Clin Nephrol (2004) 62(5):362–8. doi: 10.5414/cnp62362
73. Abe M, Okada K, Maruyama T, Ikeda K, Kikuchi F, Kaizu K, et al. Comparison of the Effects of Polysulfone and Polyester-Polymer Alloy Dialyzers on Glycemic Control in Diabetic Patients Undergoing Hemodialysis. Clin Nephrol (2009) 71(5):514–20. doi: 10.5414/cnp71514
74. Abe M, Kikuchi F, Kaizu K, Matsumoto K. The Influence of Hemodialysis Membranes on the Plasma Insulin Level of Diabetic Patients on Maintenance Hemodialysis. Clin Nephrol (2008) 69(5):354–60. doi: 10.5414/cnp69354
75. Heimbürger O, Waniewski J, Werynski A, Lindholm B. A Quantitative Description of Solute and Fluid Transport During Peritoneal Dialysis. Kidney Int (1992) 41(5):1320–32. doi: 10.1038/ki.1992.196
76. Morelle J, Sow A, Fustin C-A, Fillée C, Garcia-Lopez E, Lindholm B, et al. Mechanisms of Crystalloid Versus Colloid Osmosis Across the Peritoneal Membrane. J Am Soc Nephrol (2018) 29(7):1875–86. doi: 10.1681/ASN.2017080828
77. Mistry CD, Mallick NP, Gokal R. Ultrafiltration With an Isosmotic Solution During Long Peritoneal Dialysis Exchanges. Lancet (1987) 2(8552):178–82. doi: 10.1016/s0140-6736(87)90764-1
78. Twardowski ZJ. Clinical Value of Standardized Equilibration Tests in Capd Patients. Blood Purif (1989) 7(2-3):95–108. doi: 10.1159/000169582
79. Beardsworth SF, Ahmad R, Terry E, Karim K. Intraperitonealinsulin: A Protocol for Administration During Capd and Review of Published Protocols. Peritoneal Dialysis Int (1988) 8(2):145–51. doi: 10.1177/089686088800800208
80. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The Diamond Randomized Clinical Trial. Jama (2017) 317(4):371–8. doi: 10.1001/jama.2016.19975
81. Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous Glucose Monitoring Vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The Gold Randomized Clinical Trial. Jama (2017) 317(4):379–87. doi: 10.1001/jama.2016.19976
82. Aleppo G, Ruedy KJ, Riddlesworth TD, Kruger DF, Peters AL, Hirsch I, et al. Replace-Bg: A Randomized Trial Comparing Continuous Glucose Monitoring With and Without Routine Blood Glucose Monitoring in Adults With Well-Controlled Type 1 Diabetes. Diabetes Care (2017) 40(4):538. doi: 10.2337/dc16-2482
83. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R, Bolinder J. Impact of Flash Glucose Monitoring on Hypoglycaemia in Adults With Type 1 Diabetes Managed With Multiple Daily Injection Therapy: A Pre-Specified Subgroup Analysis of the Impact Randomised Controlled Trial. Diabetologia (2018) 61(3):539–50. doi: 10.1007/s00125-017-4527-5
84. Képénékian L, Smagala A, Meyer L, Imhoff O, Alenabi F, Serb L, et al. Continuous Glucose Monitoring in Hemodialyzed Patients With Type 2 Diabetes: A Multicenter Pilot Study. Clin Nephrol (2014) 82(4):240–6. doi: 10.5414/cn108280
85. Joubert M, Fourmy C, Henri P, Ficheux M, Lobbedez T, Reznik Y. Effectiveness of Continuous Glucose Monitoring in Dialysis Patients With Diabetes: The Dialydiab Pilot Study. Diabetes Res Clin Pract (2015) 107(3):348–54. doi: 10.1016/j.diabres.2015.01.026
86. Bomholt T, Idorn T, Knop FK, Jørgensen MB, Ranjan AG, Resuli M, et al. The Glycemic Effect of Liraglutide Evaluated by Continuous Glucose Monitoring in Persons With Type 2 Diabetes Receiving Dialysis. Nephron (2021) 145(1):27–34. doi: 10.1159/000510613
87. Boughton CK, Tripyla A, Hartnell S, Daly A, Herzig D, Wilinska ME, et al. Fully Automated Closed-Loop Glucose Control Compared With Standard Insulin Therapy in Adults With Type 2 Diabetes Requiring Dialysis: An Open-Label, Randomized Crossover Trial. Nat Med (2021) 27(8):1471–6. doi: 10.1038/s41591-021-01453-z
88. Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of Hypoglycemia Diagnosis by Freestyle Libre Continuous Glucose Monitoring in Long-Term Care Home Residents With Diabetes. J Diabetes Sci Technol (2018) 12(3):727–8. doi: 10.1177/1932296817747887
89. Moser O, Eckstein ML, McCarthy O, Deere R, Pitt J, Williams DM, et al. Performance of the Freestyle Libre Flash Glucose Monitoring (Flash Gm) System in Individuals With Type 1 Diabetes: A Secondary Outcome Analysis of a Randomized Crossover Trial. Diabetes Obes Metab (2019) 21:2505–12. doi: 10.1111/dom.13835
90. Szadkowska A, Gawrecki A, Michalak A, Zozulińska-Ziółkiewicz D, Fendler W, Młynarski W. Flash Glucose Measurements in Children With Type 1 Diabetes in Real-Life Settings: To Trust or Not to Trust? Diabetes Technol Ther (2018) 20(1):17–24. doi: 10.1089/dia.2017.0287
Keywords: continuous glucose monitoring, end stage kidney disease (ESKD), dialysis, diabetes, type 2 (non-insulin-dependent) diabetes mellitus, diabetic kidney disease, diabetic nephropathy
Citation: Ling J, Ng JKC, Chan JCN and Chow E (2022) Use of Continuous Glucose Monitoring in the Assessment and Management of Patients With Diabetes and Chronic Kidney Disease. Front. Endocrinol. 13:869899. doi: 10.3389/fendo.2022.869899
Received: 05 February 2022; Accepted: 21 March 2022;
Published: 22 April 2022.
Edited by:
Giuseppina Salzano, University of Messina, ItalyReviewed by:
Yukihiro Fujita, Shiga University of Medical Science, JapanCopyright © 2022 Ling, Ng, Chan and Chow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elaine Chow, ZS5jaG93QGN1aGsuZWR1Lmhr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.