- 1Department of Pharmacy Practice, College of Pharmacy, Qassim University, Qassim, Saudi Arabia
- 2College of Pharmacy, University of Shaqra, Al Dawadmi, Saudi Arabia
- 3Department of Pharmacy Practice and Science, College of Pharmacy, University of Arizona, Tucson, AZ, United States
- 4Department of Pharmacy Practice, College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 5Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 6Pharmacoeconomics Research Unit, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 7Drug Sector, Saudi Food and Drug Authority, Riyadh, Saudi Arabia
Background: Previous reports suggest that the Coronavirus Disease-2019 (COVID-19) pandemic might have affected incidences of diabetic ketoacidosis (DKA) and new diagnoses of type 1 diabetes. This systematic review and meta-analysis aimed to estimate the risk of DKA, including severe DKA, during the COVID-19 pandemic versus the prior-to-COVID-19 period among pediatric patients with type 1 diabetes.
Methods: PubMed and EMBASE were searched for observational studies investigating the risk of DKA among pediatric patients with type 1 diabetes during the COVID-19 pandemic and the prior-to-COVID-19 period. A random meta-analysis model was performed to estimate the relative risk of DKA during the COVID-19 pandemic compared to before the pandemic. Subgroup analyses were conducted based on the type 1 diabetes status, established or newly diagnosed. In addition, sensitivity analysis was conducted for studies that reported results from adjusted analysis for potential confounders using fixed effect model.
Results: A total of 20 observational studies reported the risk of DKA, of which 18 reported the risk of severe DKA. The risks of DKA and severe DKA were 35% (RR 1.35, 95%CI 1.2-1.53, I2 = 71%) and 76% (RR 1.76, 95%CI 1.33-2.33, I2 = 44%) higher in the during-COVID-19 group compared to the prior-to-COVID-19 group, respectively. Among patients with newly diagnosed type 1 diabetes, the risk of DKA was 44% higher for the during-COVID-19 group compared to the prior-to-COVID-19 group (RR 1.44, 95%CI 1.26-1.65; I2 = 64%). Only two studies reported the risk of DKA among patients with established type 1 diabetes and the cumulative risk was not statistically significant. In the sensitivity analysis, four studies reported an adjusted odds ratio (aOR) of the risk of DKA during COVID-19 compared to the prior-to-COVID-19 period. The fixed estimate from the meta-analysis found an increase in the risk of DKA in the during-COVID-19 group compared to the prior-to-COVID-19 group (aOR 2.04, 95%CI 1.66-2.50).
Conclusions: This study showed that DKA risk, especially the risk of severe DKA, has increased significantly during the pandemic. Healthcare systems must be aware and prepared for such an increase in DKA cases and take all necessary measures to prevent future spikes during the pandemic.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=272775, identifier PROSPERO [CRD42021272775].
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening complication of diabetes that can occur at type 1 diabetes onset (1, 2). The International Society of Pediatric and Adolescent Diabetes defined DKA patients as having a blood glucose level greater than 200 mg/dl, a pH level under 7.3, and a bicarbonate level under 15 mmol/L. Severe DKA, however, is recognized by a decline in pH level to under 7.1 or potentially a bicarbonate level under 5 mmol/L (3). The incidence rate of DKA at type 1 diabetes onset ranged from 13 to 80%, requiring hospitalization in most cases and leading to the consumption of more healthcare resources (3–5). The recently reported prevalence of DKA among children with newly diagnosed type 1 diabetes was around 30%, with much variation in the prevalence between 13 countries in the study (6).
Patients with diabetes are at greater risk of infection relative to the general population, and the risk is higher for patients with type 1 diabetes compared to patients with type 2 diabetes (7). The Coronavirus Disease-2019 (COVID-19) pandemic has dramatically affected the lifestyle of patients and their access to healthcare services worldwide, including delayed diagnosis or management of chronic diseases, such as type 1 diabetes (8, 9). Moreover, several studies reported an increase in DKA and severe DKA cases among the pediatric population (10–17). Also, some studies reported a possible increase in type 1 diabetes cases during the pandemic (11, 12, 18). Therefore, we conducted a comprehensive systematic review and meta-analysis to estimate the risk of DKA, including severe DKA, among patients with type 1 diabetes prior to and during the COVID-19 pandemic.
Methods
Search Strategy and Databases
A systematic literature search was conducted by KSA, NHA, and NMB for observational studies using the PICO (population, intervention, comparison, outcome) framework (P: pediatric patients with type 1 diabetes, I: during the COVID-19 pandemic, C: prior to COVID-19, O: incidences of DKA). The electronic databases PubMed and EMBASE were searched from inception to December 28, 2021. The Elsevier Coronavirus Research Repository Hub was also searched for potentially eligible studies. The search strategy and keywords are available in Supplemental Table 1. This systematic review and meta-analysis was registered with PROSPERO (CRD42021272775), and the manuscript was prepared based on the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guideline (19).
Study Selection, Data Extraction, and Quality Assessment
All retrieved citations, after removing duplicates from the Rayyan software (20), were independently screened by the three investigators for eligibility; initially through titles and abstracts, then through a full-text review. Studies published as an abstract or in non-English language were excluded. Disagreements were resolved by consensus. Two investigators (KSA and NHA) extracted the following data into an Excel sheet: primary author’s last name, year of publication, location of the study, study period, key inclusion/exclusion criteria, number of patients with type 1 diabetes, study period, and number of patients with DKA and severe DKA. Two other investigators (ARA and OMA) checked the extracted data and independently performed the quality assessment of included articles using the Newcastle-Ottawa Scale (NOS) (21, 22). Disagreements were resolved by consensus.
Data Synthesis and Analysis
The primary outcome of the analysis estimated the risk of DKA among pediatric patients with type 1 diabetes in the during-COVID-19 pandemic group relative to the prior-to-COVID-19 group using the risk ratio (RR) with a 95% confidence interval (95% CI). The secondary outcome is the relative risk of severe DKA in the during-COVID-19 group versus the prior-to-COVID-19 group. We performed the meta-analysis with a random effect model using R version 4.0.4 and presented the results on a forest plot, including the heterogeneity I² statistics. Egger’s test was employed to assess the potential for publication bias. We performed subgroup analyses based on type 1 diabetes status (newly or established diagnoses) and sensitivity analyses for studies that reported adjusted point estimates (RR or OR) using the fixed effect model.
Results
Characteristics of Included Studies
The systematic search yielded 372 citations, and 20 studies met the inclusion criteria (Figure 1) (10–18, 24–34). These studies included 37,174 patients with type 1 diabetes in the prior-to-COVID-19 pandemic group and 27,812 patients in the during-COVID-19 group. Most of the studies were conducted in European countries from hospitals or tertiary care centers (Table 1).
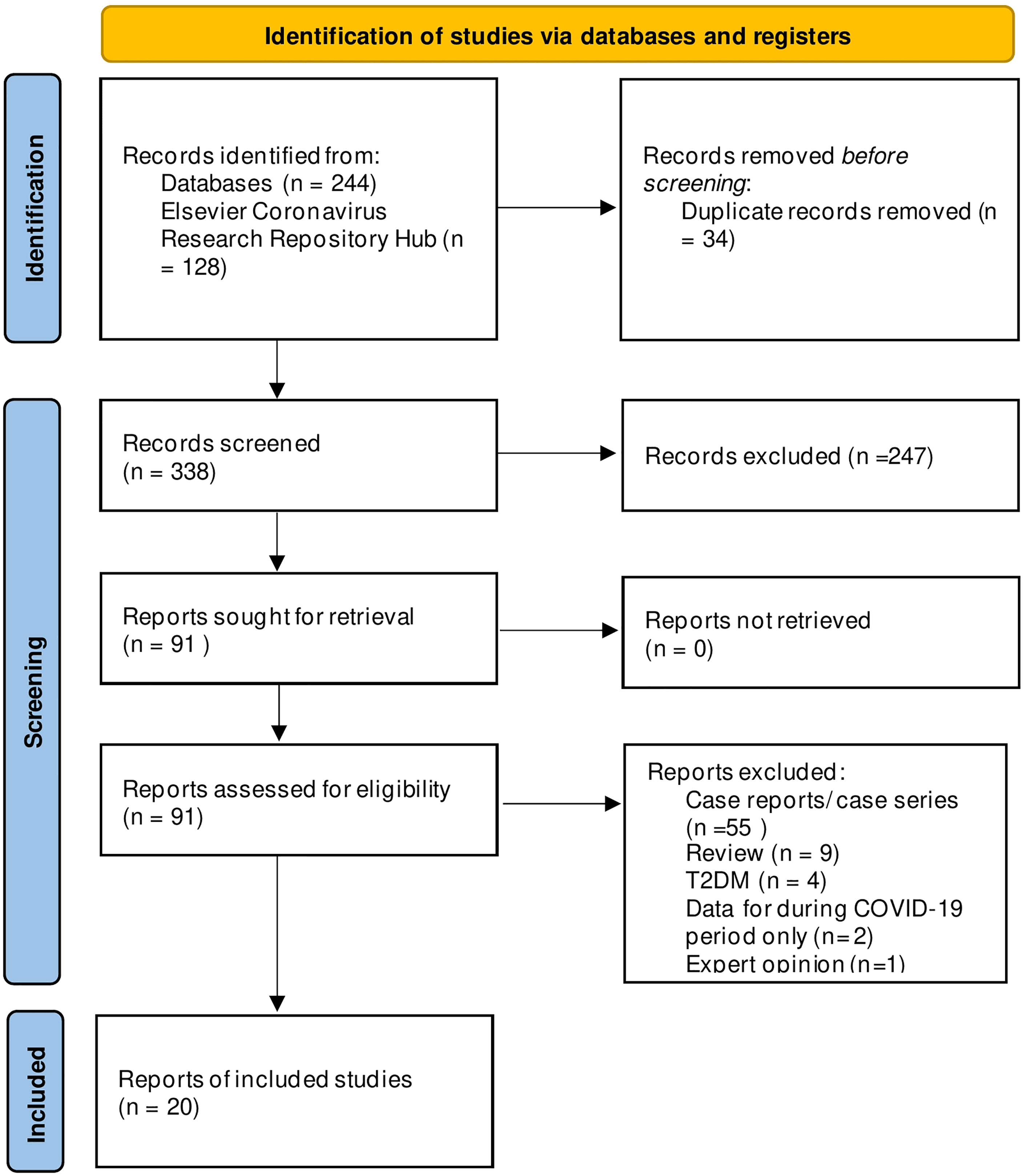
Figure 1 PRISMA Flowchart of included and excluded studies. Copyright statement: this PRISMA diagram contains public sector information licensed under the Open Government Licence v3.0. Adapted From: Moher et al. (23).
Risk of Bias Assessment
All the included studies had more than 7 points on the NOS scale, suggesting good (7–9) quality (Supplemental Table 2). However, only seven studies demonstrated a good quality on the comparability domain due to the adjustment for the baseline characteristics between the two groups (during-COVID-19 and prior-to-COVID-19).
Risk of DKA
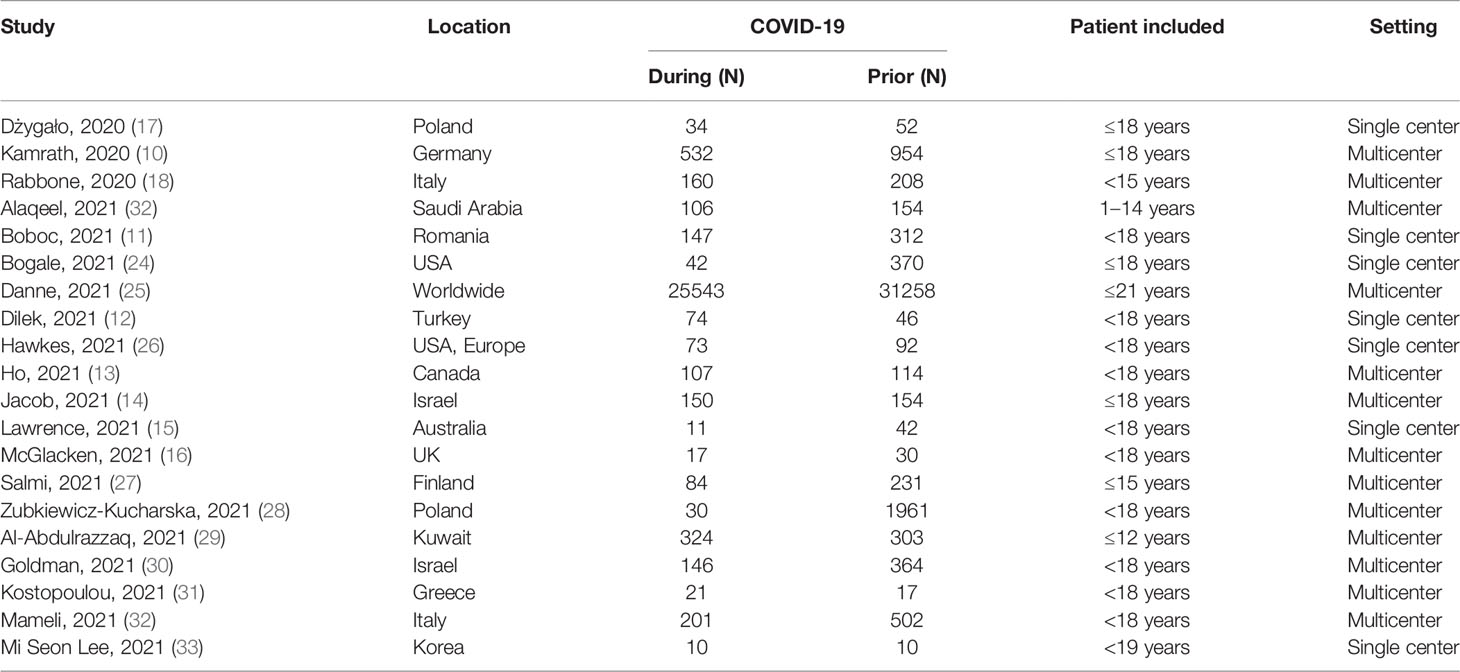
A total of 20 studies investigated the risk of DKA during COVID-19 compared to the prior-to-COVID-19 period (Figure 2). Seven studies have shown an increase in the risk of DKA during the pandemic. The cumulative risk of DKA was 35% higher for the during-COVID-19 group compared to the prior-to-COVID-19 group (RR 1.35, 95%CI 1.20-1.53) but with significant heterogeneity (I2 = 71%, p<0.01).
Risk of Sever DKA
Eighteen studies have investigated the risk of severe DKA during COVID-19 compared to the prior-to-COVID-19 period (Figure 3). Ten studies have shown an increase in the risk of severe DKA during the pandemic. The cumulative risk of severe DKA was 76% higher for the during-COVID-19 group compared to the prior-to-COVID-19 group (RR 1.76, 95%CI 1.33-2.33, I2 = 44%, p=0.03).
Subgroup Analysis
Risk of DKA Among Patients With Newly Diagnosed Type 1 Diabetes
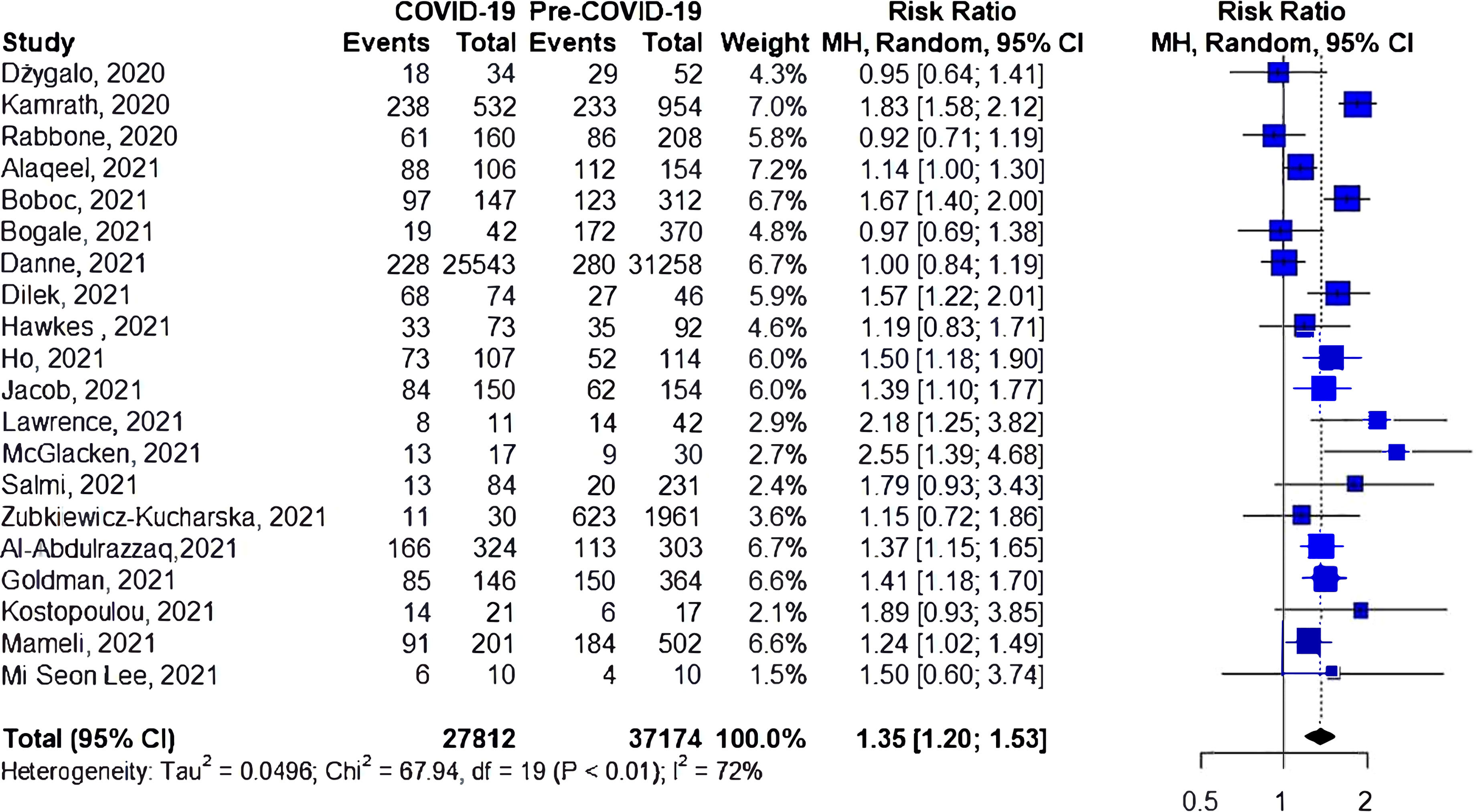
A total of 18 studies investigated the risk of DKA among patients with newly diagnosed type 1 diabetes during COVID-19 compared to the prior-to-COVID-19 period (Figure 4), with eleven reporting an increase in the risk of DKA during the pandemic. The cumulative risk showed a 44% increase in the risk of DKA for the during-COVID-19 compared to the prior-to-COVID-19 groups (RR 1.44, 95%CI 1.26-1.65), again with significant heterogeneity (I2 = 64%, p<0.01).
Risk of DKA Among Patients With Established Type 1 Diabetes
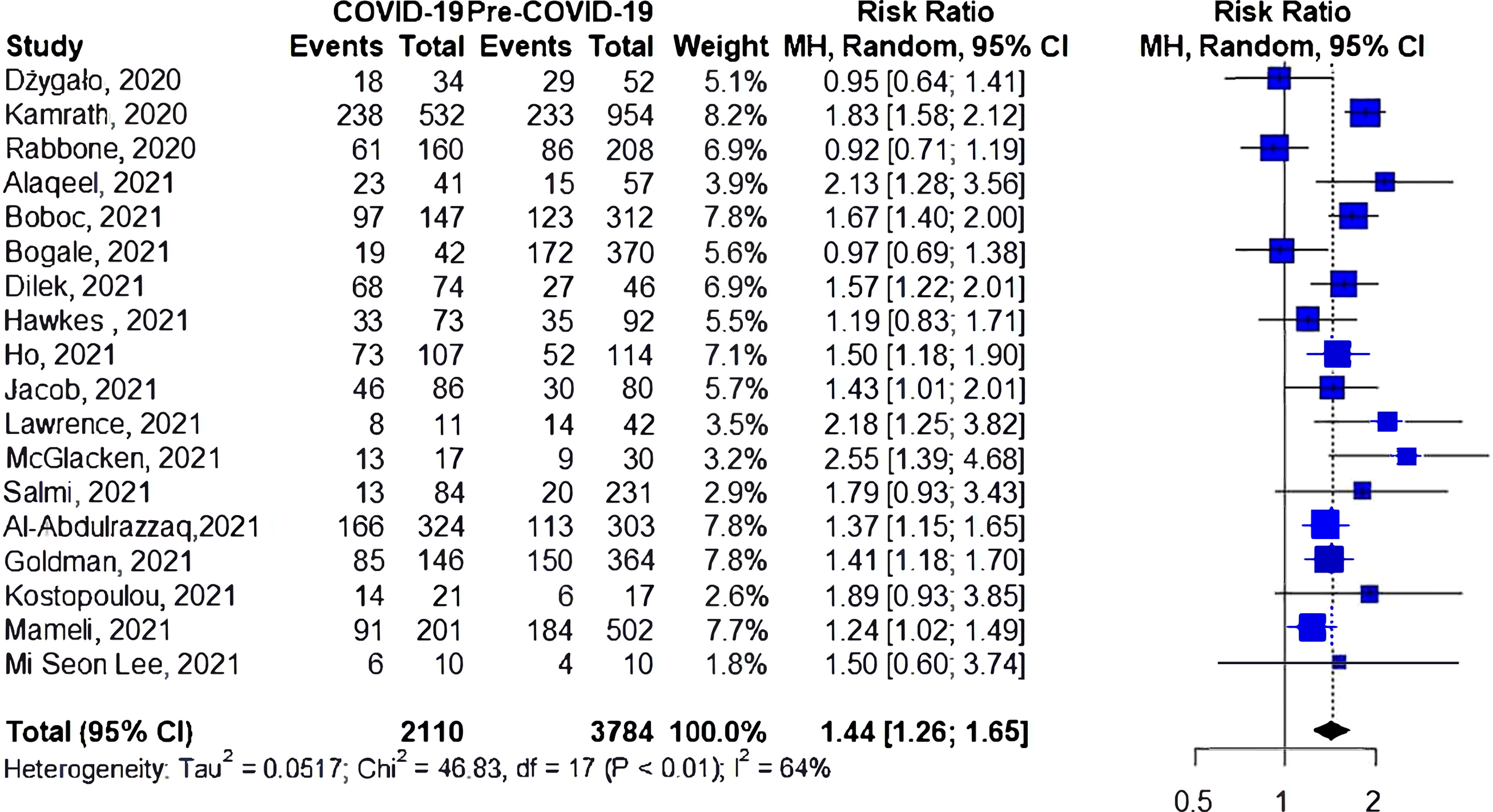
Two studies have reported the risk of DKA among patients with established type 1 diabetes (Figure 5); one of them found an increase in the risk of DKA during the pandemic. However, the cumulative risk of DKA was insignificant (RR 1.14, 95%CI 0.83-1.56, I2 = 76%, p=0.04).
Sensitivity Analysis
Four studies reported an adjusted odds ratio (aOR) of the risk of DKA during COVID-19 compared to the prior-to-COVID-19 period (Supplemental Figure 1). The fixed estimate from the meta-analysis showed an increase in the risk of DKA for the during-COVID-19 group compared to the prior-to-COVID-19 group (aOR 2.04, 95%CI 1.66-2.50). Conversely, three studies reported an adjusted relative risk (aRR) of DKA during COVID-19 compared to the prior-to-COVID-19 period (Supplemental Figure 2). The fixed estimate from the meta-analysis showed an increase in the risk of DKA for the during-COVID-19 group compared to the prior-to-COVID-19 group (aRR 1.27, 95%CI 1.18-1.36).
Publication Bias
The publication bias test was performed for all outcomes except for the subgroup analysis of the established type 1 diabetes cohort, which only included two studies. The results of Eager’s test did not show potential for publication bias.
Discussion
This meta-analysis aimed to estimate the risk of DKA, including severe DKA, among pediatric patients with type 1 diabetes during COVID-19 pandemic compared to prior-to-COVID-19. The cumulative risks of DKA and severe DKA were 35% and 76% higher, respectively, for the during-COVID-19 period compared to prior to COVID-19. The cumulative risk of DKA among patients with newly diagnosed type 1 diabetes showed a 44% increased risk of DKA for the during-COVID-19 compared to the prior-to-COVID-19 groups.
Several hypotheses were raised to explain the increase in incidences of DKA and severe DKA during the pandemic. A delay in seeking medical attention was suggested by several studies (10, 35, 36). Such a delay was attributed to fear of infection, cancellation of several medical services, or the closing of some centers due to an increase in infections among medical staff and admitted patients (10, 35, 37–39). For those newly diagnosed with type 1 diabetes presenting with DKA, it is hypothesized that delay in seeking medical help will be preceded by a longer duration of type 1 diabetes symptoms. However, several studies have reported that the duration of symptoms during the pandemic were comparable to duration prior to COVID-19 (11, 17, 28, 40). This finding suggests that the delay in diagnosing type 1 diabetes is not the only cause for an increased risk of DKA. This result might also be supported by the reported no-change or unexpected lower HbA1c levels during the pandemic (25, 28). That said, a limitation does exist regarding the duration of symptoms given that the information provided in the studies depends on parents’ ability to recall those symptoms.
Most of the studies included in this meta-analysis investigated those who were newly diagnosed with type 1 diabetes, which makes it unlikely that worsening glycemic control during the pandemic led to the increase in DKA cases. Only two studies have reported DKA cases among patients with a history of type 1 diabetes. Jacob et al. reported a statistically significant increase in DKA during the pandemic (14), while Alaqeel et al. showed no difference in DKA cases in those with preexisting type 1 diabetes (34). Although some studies have reported a decrease in physical activities during the lockdown period (25, 41), many studies have observed good glycemic control during the lockdown period, which might be a result of close parental supervision during the lockdown or the increased use of diabetes-related technology in some countries (25, 42–45).
Another possible factor could be an increase in the incidence of type 1 diabetes among children during the pandemic. However, most of the studies investigating this idea did not report a significant increase in the incidence of type 1 diabetes during the pandemic. Boboc et al. reported a 30% overall increase in incidences of type 1 diabetes between March 2020 and February 2021 (11). Interestingly, incidences were found to be lower during the early weeks of the pandemic (March to April 2020). Rabbone et al. also reported a decrease in new cases of type 1 diabetes during the pandemic from February 2020 to April 2020 (18). Such an early decrease in incidences of type 1 diabetes during the pandemic might be attributed to lower overall exposure to viral infections during the lockdown period, as viral infections are known risk factor for developing type 1 diabetes (46–48). Moreover, it is also reported that new type 1 diabetes cases are usually higher in the winter season compared to summer (49–51). Thus, it is unlikely that the seasonality of type 1 diabetes is the reason for the decrease in type 1 diabetes incidences reported by those studies (11, 18). Overall, the results regarding the changes in the incidence of type 1 diabetes during the pandemic are inconclusive, making larger studies with a longer duration warranted.
COVID-19 infection has resulted in several complications. Some reports suggest that the virus might be able to affect the pancreas, resulting in a dysregulation of glucose metabolism (52, 53). A UK study suggested a possible link between COVID-19 infection and new-onset of type 1 diabetes or severe DKA (40). However, this study was limited by its small sample size. Conversely, a German study showed that COVID-19 infection did not increase type 1 diabetes cases when there was no evidence of autoimmunity. Importantly, this study only covered the first wave of the pandemic in Germany (approximately four months period) (54). Thus, possible links cannot be entirely ruled out, but larger studies are needed to confirm them. Unfortunately, among the studies included in this meta-analysis, only a small number of patients had COVID-19, and data regarding prior exposure to the virus are limited, making it difficult to attribute such an increase in DKA cases to current or prior COVID-19 infection.
Regardless of the actual cause of increased DKA during the pandemic, DKA remains to be a serious life-threatening complication that must be prevented. Early detection of type 1 diabetes symptoms and early diagnoses is key in preventing DKA. Overall, incidences of DKA at type 1 diabetes diagnosis is a challenge (2, 6, 55, 56), despite the pandemic. Missed diagnoses of type 1 diabetes by healthcare providers have been documented (56, 57). Children diagnosed with type 1 diabetes were more likely to have visited their primary care providers within the 30 days prior to diagnosis (57), which indicates missed opportunities for early detection of type 1 diabetes symptoms.
Efforts should be directed toward increasing awareness of healthcare providers, patients, and families regarding the symptoms of type 1 diabetes. Prevention campaigns have proven useful in reducing the prevalence rate of DKA in newly diagnosed children with type 1 diabetes (58–60). Cherubini et al. study suggested several actions that can optimize awareness campaigns such as targeting families of children under the age of 15 years, smaller geographic areas and family pediatricians in addition to utilizing innovative communication tools (61). Although public health campaigns might be challenging during pandemics given the public is receiving large amount of health information, they could be useful as a public health tool during such pandemic periods when DKA is on the rise.
In the past few years there have been significant improvements in identifying those with high risk for developing type 1 diabetes. Moreover, screening for type 1 diabetes through islet autoantibodies and genetic testing is currently reserved for research (62). However, one main advantage of such screening at the population level would be the reduction of DKA at type 1 diabetes onset (63). This was observed in two studies were significant reductions in DKA frequency was noted (64, 65). However, the cost-effectiveness of screening needs to be assessed. Given the significant increase in DKA and severe DKA cases observed during the COVID-19 pandemic, screening for type 1 diabetes during or right after such pandemics might be considered as a potential tool to aid preventing DKA cases in the future.
This study is not without limitations. All the included studies are observational and had some degree of heterogeneity. Seven studies demonstrated a good quality in the comparability domain. Differences in inclusion criteria, analyzed periods, and number of subjects included might have played a role in the observed heterogeneity. Few studies presented an adjustment for potential confounders and included them in the sensitivity analysis. Only two studies reported DKA cases for those with a history of type 1 diabetes, which made it impossible to assess if poor glycemic control might have played a role in increased DKA cases. Besides that, the mean duration of type 1 diabetes was only reported in one study. Insufficient data were reported regarding COVID-19 infection status among the included studies which made it impractical to perform a subgroup analysis for those with positive COVID-19 infection at type 1 diabetes diagnosis.
In conclusion, the results of this meta-analysis showed a statistically significant increase in DKA and severe DKA risk among pediatrics during the pandemic in comparison to prior to the pandemic period. Such an increase might be attributed to several factors that might differ in magnitude from one country to another. Healthcare systems must be aware and prepared for such an increase in the risk of DKA or severe DKA during similar pandemic conditions. Timely access to healthcare, an increase in public and healthcare providers’ awareness of type 1 diabetes symptoms through public health educational and screening campaigns, and proper diabetes management during pandemics or similar situations remain important and key to avoiding similar spikes in incidences of DKA or severe DKA in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
OMA and ARA contributed to the conception and design of this study. KSA, NHA, and NMB conducted the literature search. KSA and NaA performed the data extraction. ARA did the statistical analysis and wrote the methods section with inputs from MA, OAA, and OMA. All authors contributed to writing the manuscript. All authors revised and approved the final version of the manuscript. OMA is the guarantor of this work.
Funding
This work was supported by the Researcher Supporting Project number (RSP-2021/77), King Saud University, Riyadh, Saudi Arabia. The funding agency had no role in designing the study, conducting the analysis, interpreting the data or writing the manuscript.
Author Disclaimer
The contents of this manuscript are solely the authors’ views and may not be understood or quoted as being made on behalf of or reflecting the position of the Saudi Food and Drug Authority.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to extend their appreciation to King Saud University for funding this work through the Researcher Supporting Project (RSP-2021/77), King Saud University, Riyadh, Saudi Arabia.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.856958/full#supplementary-material
References
1. Duca LM, Wang B, Rewers M, Rewers A. Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes Predicts Poor Long-Term Glycemic Control. Diabetes Care (2017) 40(9):1249–55. doi: 10.2337/dc17-0558
2. Rewers A, Klingensmith G, Davis C, Petitti DB, Pihoker C, Rodriguez B, et al. Presence of Diabetic Ketoacidosis at Diagnosis of Diabetes Mellitus in Youth: The Search for Diabetes in Youth Study. Pediatrics (2008) 121(5):e1258–66. doi: 10.1542/peds.2007-1105
3. Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic Ketoacidosis. Nat Rev Dis Prim (2020) 6(1):1–20. doi: 10.1038/s41572-020-0165-1
4. Umpierrez GE, Kitabchi AE. Diabetic Ketoacidosis: Risk Factors and Management Strategies. Treat Endocrinol (2003) 2(2):95–108. doi: 10.2165/00024677-200302020-00003
5. Saydah SH, Shrestha SS, Zhang P, Zhou X, Imperatore G. Medical Costs Among Youth Younger Than 20 Years of Age With and Without Diabetic Ketoacidosis at the Time of Diabetes Diagnosis. Diabetes Care (2019) 42(12):2256–61. doi: 10.2337/DC19-1041
6. Cherubini V, Grimsmann JM, Åkesson K, Birkebæk NH, Cinek O, Dovč K, et al. Temporal Trends in Diabetic Ketoacidosis at Diagnosis of Paediatric Type 1 Diabetes Between 2006 and 2016: Results From 13 Countries in Three Continents. Diabetologia (2020) 63(8):1530–41. doi: 10.1007/s00125-020-05152-1
7. Carey IM, Critchley JA, Dewilde S, Harris T, Hosking FJ, Cook DG. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care (2018) 41(3):513–21. doi: 10.2337/dc17-2131
8. Bauer J, Brüggmann D, Klingelhöfer D, Maier W, Schwettmann L, Weiss DJ, et al. Access to Intensive Care in 14 European Countries: A Spatial Analysis of Intensive Care Need and Capacity in the Light of COVID-19. Intensive Care Med (2020) 46(11):2026–34. doi: 10.1007/s00134-020-06229-6
9. Elbarbary NS, dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE. COVID-19 Outbreak and Pediatric Diabetes: Perceptions of Health Care Professionals Worldwide. Pediatr Diabetes (2020) 21(7):1083–92. doi: 10.1111/pedi.13084
10. Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in Children and Adolescents With Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA - J Am Med Assoc (2020) 324(8):801–4. doi: 10.1001/jama.2020.13445
11. Boboc AA, Novac CN, Ilie MT, Ieșanu MI, Galoș F, Bălgrădean M, et al. The Impact of Sars-Cov-2 Pandemic on the New Cases of T1dm in Children. A Single-Centre Cohort Study. J Pers Med (2021) 11(6):551. doi: 10.3390/jpm11060551
12. Dilek SÖ, Gürbüz F, Turan I, Celiloǧlu C, Yüksel B. Changes in the Presentation of Newly Diagnosed Type 1 Diabetes in Children During the COVID-19 Pandemic in a Tertiary Center in Southern Turkey. J Pediatr Endocrinol Metab (2021) 34(10):1303–9. doi: 10.1515/jpem-2021-0287
13. Ho J, Rosolowsky E, Pacaud D, Huang C, Lemay JA, Brockman N, et al. Diabetic Ketoacidosis at Type 1 Diabetes Diagnosis in Children During the COVID-19 Pandemic. Pediatr Diabetes (2021) 22(4):552–7. doi: 10.1111/pedi.13205
14. Jacob R, Weiser G, Krupik D, Takagi D, Peled S, Pines N, et al. Diabetic Ketoacidosis at Emergency Department Presentation During the First Months of the SARS-CoV-2 Pandemic in Israel: A Multicenter Cross-Sectional Study. Diabetes Ther (2021) 12(5):1569–74. doi: 10.1007/s13300-021-01049-3
15. Lawrence C, Seckold R, Smart C, King BR, Howley P, Feltrin R, et al. Increased Paediatric Presentations of Severe Diabetic Ketoacidosis in an Australian Tertiary Centre During the COVID-19 Pandemic. Diabetes Med (2021) 38(1):e14417. doi: 10.1111/dme.14417
16. McGlacken-Byrne SM, Drew SEV, Turner K, Peters C, Amin R. The SARS-CoV-2 Pandemic Is Associated With Increased Severity of Presentation of Childhood Onset Type 1 Diabetes Mellitus: A Multi-Centre Study of the First COVID-19 Wave. Diabetes Med (2021) 38(9):e1464. doi: 10.1111/dme.14640
17. Dżygało K, Nowaczyk J, Szwilling A, Kowalska A. Increased Frequency of Severe Diabetic Ketoacidosis at Type 1 Diabetes Onset Among Children During COVID-19 Pandemic Lockdown: An Observational Cohort Study. Pediatr Endocrinol Diabetes Metab (2020) 26(4):167–75. doi: 10.5114/pedm.2020.101003
18. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A. Has Covid-19 Delayed the Diagnosis and Worsened the Presentation of Type 1 Diabetes in Children? Diabetes Care (2020) 43(11):2870–2. doi: 10.2337/dc20-1321
19. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. J Am Med Assoc (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
20. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-A Web and Mobile App for Systematic Reviews. Syst Rev (2016) 5(1):1–10. doi: 10.1186/s13643-016-0384-4
21. Wells G, Shea B, O’Connell D, et al. Ottawa Hospital Research Institute (2021). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed December 1, 2021).
22. Quigley JM, Thompson JC, Halfpenny NJ, Scott DA. Critical Appraisal of Nonrandomized Studies-A Review of Recommended and Commonly Used Tools. J Eval Clin Pract (2019) 25(1):44–52. doi: 10.1111/JEP.12889
23. Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med (2009) 6(6):e1000097. doi: 10.1371/journal.pmed1000097
24. Bogale KT, Urban V, Schaefer E, Bangalore Krishna K. The Impact of COVID-19 Pandemic on Prevalence of Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes: A Single-Centre Study in Central Pennsylvania. Endocrinol Diabetes Metab (2021) 4(3):e00235. doi: 10.1002/edm2.235
25. Danne T, Lanzinger S, De Bock M, Rhodes ET, Alonso GT, Barat P, et al. A Worldwide Perspective on COVID-19 and Diabetes Management in 22,820 Children From the SWEET Project: Diabetic Ketoacidosis Rates Increase and Glycemic Control Is Maintained. Diabetes Technol Ther (2021) 23(9):632–41. doi: 10.1089/dia.2021.0110
26. Hawkes CP, Willi SM. A Trend Towards an Early Increase in Ketoacidosis at Presentation of Paediatric Type 1 Diabetes During the Coronavirus-2019 Pandemic. Diabetes Med (2021) 38(4):e14461. doi: 10.1111/dme.14461
27. Salmi H, Heinonen S, Hästbacka J, Lääperi M, Rautiainen P, Miettinen PJ, et al. New-Onset Type 1 Diabetes in Finnish Children During the COVID-19 Pandemic. Arch Dis Child (2021) 0:1–6. doi: 10.1136/archdischild-2020-321220
28. Zubkiewicz-Kucharska A, Seifert M, Stępkowski M, Noczyńska A. Diagnosis of Type 1 Diabetes During the SARS-CoV-2 Pandemic: Does Lockdown Affect the Incidence and Clinical Status of Patients? Adv Clin Exp Med (2021) 30(2):127–34. doi: 10.17219/ACEM/130359
29. Al-Abdulrazzaq D, Alkandari A, Alhusaini F, Alenazi N, Gujral UP, Narayan KMV, et al. Higher Rates of Diabetic Ketoacidosis and Admission to the Paediatric Intensive Care Unit Among Newly Diagnosed Children With Type 1 Diabetes in Kuwait During the COVID-19 Pandemic. Diabetes Metab Res Rev (2021) 22:e3506. doi: 10.1002/DMRR.3506
30. Goldman S, Pinhas-Hamiel O, Weinberg A, Auerbach A, German A, Haim A, et al. Alarming Increase in Ketoacidosis in Children and Adolescents With Newly Diagnosed Type 1 Diabetes During the First Wave of the COVID-19 Pandemic in Israel. Pediatr Diabetes (2021) 23(1):10–18. doi: 10.1111/PEDI.13296
31. Kostopoulou E, Eliopoulou MI, Rojas Gil AP, Chrysis D. Impact of COVID-19 on New-Onset Type 1 Diabetes Mellitus - A One-Year Prospective Study. Eur Rev Med Pharmacol Sci (2021) 25(19):5928–35. doi: 10.26355/EURREV_202110_26869
32. Mameli C, Scaramuzza A, Macedoni M, Marano G, Frontino G, Luconi E, et al. Type 1 Diabetes Onset in Lombardy Region, Italy, During the COVID-19 Pandemic: The Double-Wave Occurrence. EClinicalMedicine (2021) 39:101067. doi: 10.1016/J.ECLINM.2021.101067
33. Lee MS, Lee R, Ko CW, Moon JE. Increase in Blood Glucose Level and Incidence of Diabetic Ketoacidosis in Children With Type 1 Diabetes Mellitus in the Daegu-Gyeongbuk Area During the Coronavirus Disease 2019 (COVID-19) Pandemic. Yeungnam Univ J Med (2021) 39(1):46–52. doi: 10.12701/YUJM.2021.01221
34. Alaqeel A, Aljuraibah F, Alsuhaibani M, Huneif M, Alsaheel A, Dubayee MA, et al. The Impact of COVID-19 Pandemic Lockdown on the Incidence of New-Onset Type 1 Diabetes and Ketoacidosis Among Saudi Children. Front Endocrinol (Lausanne) (2021) 12:669302. doi: 10.3389/fendo.2021.669302
35. Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed Access or Provision of Care in Italy Resulting From Fear of COVID-19. Lancet Child Adolesc Heal (2020) 4(5):e10–1. doi: 10.1016/S2352-4642(20)30108-5
36. Cherubini V, Gohil A, Addala A, Zanfardino A, Iafusco D, Hannon T, et al. Unintended Consequences of Coronavirus Disease-2019: Remember General Pediatrics. J Pediatr (2020) 223:197–8. doi: 10.1016/j.jpeds.2020.05.004
37. Baum A, Schwartz MD. Admissions to Veterans Affairs Hospitals for Emergency Conditions During the COVID-19 Pandemic. JAMA - J Am Med Assoc (2020) 324(1):96–9. doi: 10.1001/jama.2020.9972
38. Lynn RM, Avis JL, Lenton S, Amin-Chowdhury Z, Ladhani SN. Delayed Access to Care and Late Presentations in Children During the COVID-19 Pandemic: A Snapshot Survey of 4075 Paediatricians in the UK and Ireland. Arch Dis Child (2021) 106(2):e8. doi: 10.1136/archdischild-2020-319848
39. Roland D, Harwood R, Bishop N, Hargreaves D, Patel S, Sinha I. Children’s Emergency Presentations During the COVID-19 Pandemic. J Clean Prod (2020) 4(8):e32. doi: 10.1016/S2352-4642(20)30206-6
40. Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, et al. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the U.K. Diabetes Care (2020) 43(11):e170–1. doi: 10.2337/dc20-1551
41. Dunton GF, Do B, Wang SD. Early Effects of the COVID-19 Pandemic on Physical Activity and Sedentary Behavior in Children Living in the U.S. BMC Public Health (2020) 20(1):1–13. doi: 10.1186/s12889-020-09429-3
42. Fernández E, Cortazar A, Bellido V. Impact of COVID-19 Lockdown on Glycemic Control in Patients With Type 1 Diabetes. Diabetes Res Clin Pract (2020) 166:108348. doi: 10.1016/j.diabres.2020.108348
43. Christoforidis A, Kavoura E, Nemtsa A, Pappa K, Dimitriadou M. Coronavirus Lockdown Effect on Type 1 Diabetes Management on Children Wearing Insulin Pump Equipped With Continuous Glucose Monitoring System. Diabetes Res Clin Pract (2020) 166:108307. doi: 10.1016/j.diabres.2020.108307
44. Capaldo B, Annuzzi G, Creanza A, Giglio C, De Angelis R, Lupoli R, et al. Blood Glucose Control During Lockdown for COVID-19: Cgm Metrics in Italian Adults With Type 1 Diabetes. Diabetes Care (2020) 43(8):e88–9. doi: 10.2337/dc20-1127
45. Minuto N, Bassi M, Montobbio C, Vinci F, Mercuri C, Perri FN, et al. The Effect of Lockdown and Physical Activity on Glycemic Control in Italian Children and Young Patients With Type 1 Diabetes. Front Endocrinol (Lausanne) (2021) 12:690222. doi: 10.3389/fendo.2021.690222
46. Wagenknecht LE, Roseman JM, Herman WH. Increased Incidence of Insulin-Dependent Diabetes Mellitus Following an Epidemic of Coxsackievirus B5. Am J Epidemiol (1991) 133(10):1024–31. doi: 10.1093/oxfordjournals.aje.a115811
47. Von Herrath M. Can We Learn From Viruses How to Prevent Type 1 Diabetes? The Role of Viral Infections in the Pathogenesis of Type 1 Diabetes and the Development of Novel Combination Therapies. Diabetes (2009) 58(1):2–11. doi: 10.2337/db08-9027
48. van der Werf N, Kroese FGM, Rozing J, Hillebrands JL. Viral Infections as Potential Triggers of Type 1 Diabetes. Diabetes Metab Res Rev (2007) 23(3):169–83. doi: 10.1002/dmrr.695
49. Szypowska A, Ramotowska A, Wysocka-Mincewicz M, Mazur A, Lisowicz L, Beń-Skowronek I, et al. Seasonal Variation in Month of Diagnosis of Polish Children With Type 1 Diabetes - A Multicenter Study. Exp Clin Endocrinol Diabetes (2019) 127(5):331–5. doi: 10.1055/s-0043-125321
50. Kostopoulou E, Papachatzi E, Skiadopoulos S, Rojas Gil AP, Dimitriou G, Spiliotis BE, et al. Seasonal Variation and Epidemiological Parameters in Children From Greece With Type 1 Diabetes Mellitus (T1DM). Pediatr Res (2021) 89(3):574–8. doi: 10.1038/s41390-020-0899-1
51. Nishioka Y, Noda T, Okada S, Myojin T, Kubo S, Higashino T, et al. Incidence and Seasonality of Type 1 Diabetes: A Population-Based 3-Year Cohort Study Using the National Database in Japan. BMJ Open Diabetes Res Care (2020) 8(1):e001262. doi: 10.1136/bmjdrc-2020-001262
52. Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-Onset Diabetes in Covid-19. N Engl J Med (2020) 383(8):789–90. doi: 10.1056/nejmc2018688
53. Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, et al. SARS-CoV-2 Infects and Replicates in Cells of the Human Endocrine and Exocrine Pancreas. Nat Metab (2021) 3(2):149–65. doi: 10.1038/s42255-021-00347-1
54. Kamrath C, Rosenbauer J, Tittel SR, Warncke K, Hirtz R, Denzer C, et al. Frequency of Autoantibody-Negative Type 1 Diabetes in Children, Adolescents, and Young Adults During the First Wave of the COVID-19 Pandemic in Germany. Diabetes Care (2021) 44(7):1540–6. doi: 10.2337/dc20-2791
55. Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic Ketoacidosis and the Hyperglycemic Hyperosmolar State. Pediatr Diabetes (2018) 19:155–77. doi: 10.1111/pedi.12701
56. Bui H, To T, Stein R, Fung K, Daneman D. Is Diabetic Ketoacidosis at Disease Onset a Result of Missed Diagnosis? J Pediatr (2010) 156(3):472–7. doi: 10.1016/j.jpeds.2009.10.001
57. Townson J, Cannings-John R, Francis N, Thayer D, Gregory JW. Presentation to Primary Care During the Prodrome of Type 1 Diabetes in Childhood: A Case-Control Study Using Record Data Linkage. Pediatr Diabetes (2019) 20(3):330–8. doi: 10.1111/PEDI.12829
58. Holder M, Ehehalt S. Significant Reduction of Ketoacidosis at Diabetes Onset in Children and Adolescents With Type 1 Diabetes-The Stuttgart Diabetes Awareness Campaign, Germany. Pediatr Diabetes (2020) 21(7):1227–31. doi: 10.1111/PEDI.13064
59. Vanelli M, Costi G, Chiari G, Giacalone T, Ghizzoni L, Chiarelli F. Effectiveness of a Prevention Program for Diabetic Ketoacidosis in Children. An 8-Year Study in Schools and Private Practices. Diabetes Care (1999) 22(1):7–9. doi: 10.2337/DIACARE.22.1.7
60. King BR, Howard NJ, Verge CF, Jack MM, Govind N, Jameson K, et al. A Diabetes Awareness Campaign Prevents Diabetic Ketoacidosis in Children at Their Initial Presentation With Type 1 Diabetes. Pediatr Diabetes (2012) 13(8):647–51. doi: 10.1111/J.1399-5448.2012.00896.X
61. Cherubini V, Marino M, Carle F, Zagaroli L, Bowers R, Gesuita R. Effectiveness of Ketoacidosis Prevention Campaigns at Diagnosis of Type 1 Diabetes in Children: A Systematic Review and Meta-Analysis. Diabetes Res Clin Pract (2021) 175:108838. doi: 10.1016/J.DIABRES.2021.108838
62. Committee ADAPP. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care (2022) 45(Supplement_1):S17–38. doi: 10.2337/DC22-S002
63. Besser REJ, Ng SM, Gregory JW, Dayan CM, Randell T, Barrett T. General Population Screening for Childhood Type 1 Diabetes: Is it Time for a UK Strategy? Arch Dis Child (2021) 5:archdischild-2021-321864. doi: 10.1136/ARCHDISCHILD-2021-321864
64. Larsson HE, Vehik K, Bell R, Dabelea D, Dolan L, Pihoker C, et al. Reduced Prevalence of Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes in Young Children Participating in Longitudinal Follow-Up. Diabetes Care (2011) 34(11):2347–52. doi: 10.2337/DC11-1026
Keywords: COVID-19, diabetic ketoacidosis, incidence, meta-analysis, pediatrics, systematic review, type 1 diabetes
Citation: Alfayez OM, Aldmasi KS, Alruwais NH, Bin Awad NM, Al Yami MS, Almohammed OA and Almutairi AR (2022) Incidence of Diabetic Ketoacidosis Among Pediatrics With Type 1 Diabetes Prior to and During COVID-19 Pandemic: A Meta-Analysis of Observational Studies. Front. Endocrinol. 13:856958. doi: 10.3389/fendo.2022.856958
Received: 17 January 2022; Accepted: 08 February 2022;
Published: 09 March 2022.
Edited by:
Andrea Enzo Scaramuzza, Istituti Ospitalieri di Cremona, ItalyReviewed by:
Valentino Cherubini, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyIvana Rabbone, University of Eastern Piedmont, Italy
Lorenzo Iughetti, University of Modena and Reggio Emilia, Italy
Copyright © 2022 Alfayez, Aldmasi, Alruwais, Bin Awad, Al Yami, Almohammed and Almutairi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osamah M. Alfayez, b2FsZmF5ZXpAcXUuZWR1LnNh
 Osamah M. Alfayez
Osamah M. Alfayez Kholood S. Aldmasi
Kholood S. Aldmasi Nada H. Alruwais
Nada H. Alruwais Nouf M. Bin Awad
Nouf M. Bin Awad Majed S. Al Yami
Majed S. Al Yami Omar A. Almohammed
Omar A. Almohammed Abdulaali R. Almutairi
Abdulaali R. Almutairi