- 1The International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Shanghai Key Laboratory of Embryo Original Diseases, Shanghai, China
- 3School of Biomedical Engineering, Med-X Research Institute, Shanghai Jiao Tong University, Shanghai, China
Background: Primary ovarian insufficiency (POI) is gaining awareness as its prevalence increases and its effect on patients is extremely negative. To date, several therapies have been designed to treat POI, but the conclusions are conflicting, in part, due to inconsistent evaluation methods. Thus, we explore a multi-index of ovarian function assessment methods to evaluate the recovery of ovarian function after various therapies in order to evaluate effectiveness in a more comprehensive manner.
Aim: The purpose of this review is to assess the effectiveness of various therapies to recover ovarian function in patients with POI. The primary outcome measures were anti-Müllerian hormone (AMH) levels, follicle stimulating hormone (FSH) levels, and antral follicle count (AFC). The secondary outcomes included the change of mean ovarian volume, menstruation recovery, and pregnancy rate.
Methods: Our systematic searching including PubMed, Web of Science, Cochrane, and Embase databases was conducted to find all human clinical trial articles published from January 2000 to April 2021 and related to POI treatment, including the keywords: POI, AFC, and hormones. All prospective and retrospective studies exploring ovarian function recovery that include AFC, AMH levels, and FSH levels evolution throughout treatment were included. All patients included in the studies met the POI criteria described by the European Society for Human Reproductive Embryology (ESHRE) guideline.
Results: Six studies were selected based on the criteria: one randomized controlled trial and five observational studies. Among them, two studies focused on the intraovarian platelet-rich plasma (PRP) infusion treatment, two studies focused on dehydroepiandrosterone (DHEA) supplements, one study focused on hormone replacement therapy (HRT), and one study focused on autologous adipose-derived stromal cells (ADSCs) treatment. There was insufficient scientific evidence that any approach could help ovarian function recovery in patients with POI because the ovarian function markers in each study had inconsistent changes with 26 patients (6.2%) reporting spontaneous pregnancy.
Conclusion: Serum AMH levels, FSH levels, and AFC are sensitive indicators and reflect the evolution of ovarian function. Large randomized controlled trials are necessary, and the data on ovarian function should be collected comprehensively to evaluate the effectiveness of a variety of treatments.
Introduction
Primary ovarian insufficiency (POI) or premature ovarian insufficiency (POI) (1) is characterized by amenorrhea, elevated gonadotrophins, and decreased sex steroids, occurring in women under 40 years of age. POI is defined by the European Society of Human Reproduction and Embryology (ESHRE) guidelines as the presence of oligo/amenorrhea for at least 4 months and an elevated serum follicle stimulating hormone (FSH) level >25 IU/L on two occasions >4 weeks apart, with an onset before the age of 40 years (2). Over the past few decades, POI has become more common and has drawn more concern. The prevalence of POI occurs in about 1% of the population (2, 3). A recent meta-analysis showed that the prevalence of POI was as high as 3.7% (95% confidence interval: 3.1 - 4.3) (4). The consequences of POI range from the psychological damage associated with the diagnosis to the symptom burden (the loss of fertility), to the long-term consequences of decreased estrogen, including bone fragility and increased risk of life-long cardiovascular disease and neurocognitive disorders (5, 6). The causes of POI are variable, including genetic diseases (6, 7), microbial infections, viral infections (6), autoimmune diseases (8, 9), environmental toxins (10), and iatrogenic causes (2, 11). However, most cases of POI are idiopathic with unknown causes (2).
To date, there is still no effective therapy or intervention that can reliably improve residual ovarian function. Hormone replace therapy (HRT) is recommended for women with POI, both for symptom management and minimizing the burden from chronic disorders directly impacted by premature loss of estrogen (2, 12). For women who want to plan a family, assisted conception by in-vitro fertilization (IVF) using donor eggs could improve conception rates in women with POI. However, in many communities, this is not accepted either morally or socially (13). Furthermore, these strategies still could not restore ovarian function. Also, for patients not interested in future fertility, restoring ovarian function is an urgent need to improve their overall health condition. To date, most patients’ treatment studies have been focused on the achieved pregnancy (14, 15), but not the ovarian function recovery and the long-term improvement in ovarian function.
In women with POI, ovarian insufficiency can be semipermanent or intermittent and low ovarian function parameters are not always associated with the absence of follicles in the ovary (16). Indeed, Hubayter et al. had reported that 73% of patients with POI present antral follicles (17). Meanwhile, Kawamura et al. (18) found that up to 29.6% of patients with POI had follicular growth and developed preovulatory follicles within less than 6 months. However, studies have found that only 5–10% of women may become spontaneously pregnant after being diagnosed with POI (15).
Recently, antral follicle count (AFC), serum anti-Müllerian hormone (AMH) levels, and follicle stimulating hormone (FSH) levels have been used as valuable parameters of ovarian function (19) and also used as the marker for the prediction of POI (2, 20, 21). AMH expression is initiated in cuboidal granulosa cells of primary follicles as soon as primordial follicles develop, increases until the small antral follicles, and gradually diminish in subsequent stages of follicle development (20). AMH is only expressed in healthy follicles, but not in follicles that undergoing atresia (22). Also, AMH levels indirectly reflect ovarian function (23). Hansen et al. (24) compared forty-two healthy women’s ovarian primordial follicle number with AFC and hormone levels and found significant correlations between the primordial follicle count and AFC, AMH levels, and FSH levels. Rosen et al. (25) had investigated the relationship between AFC, AMH levels, and the age of Caucasian women (n=252) aged 25–45 years and found that AMH levels and AFC exhibited a significant correlation with age.
To date, there have been several clinical trials for patients with POI aimed at evaluating the recovery of ovarian function, such as intraovarian platelet-rich plasma (PRP) infusion and dehydroepiandrosterone (DHEA) supplements. Evaluating ovarian function by combining markers such as AFC, AMH levels, and FSH levels is necessary. Here, we review the literature in search of evidence for a real effect of such treatments to help recover ovarian function in women with POI.
Materials and Methods
Search Strategy
The systematic literature search was conducted in PubMed, Web of Science, Embase, and Cochrane databases covering the period January 2000 to July 2021. The systematic search used three keywords: POI (‘primary ovarian insufficiency’ or ‘premature ovarian insufficiency’ or POF (‘premature ovarian failure’) or ‘diminished ovarian reserve’ or ‘ovarian damage’), AFC (‘antral follicle count’), and hormone [AMH (‘Müllerian-inhibiting substance’ or ‘anti-Müllerian hormone’) or FSH (‘follicle stimulating hormone’)].
Eligibility Criteria
For this Systematic research, only full-length articles in English with clinical observations in humans were included. This review incorporates all articles concerning ovarian function variation in patients with POI that meet the ESHRE criteria after various treatments, including randomized controlled trials (RCTs) and prospective clinical trials. We used AMH levels, FSH levels, and AFC as evaluation indicators of ovarian function, and all of them were required in the included articles. The principal summary measure was AMH levels, FSH levels, and AFC dynamics during and after treatment.
Study Selection
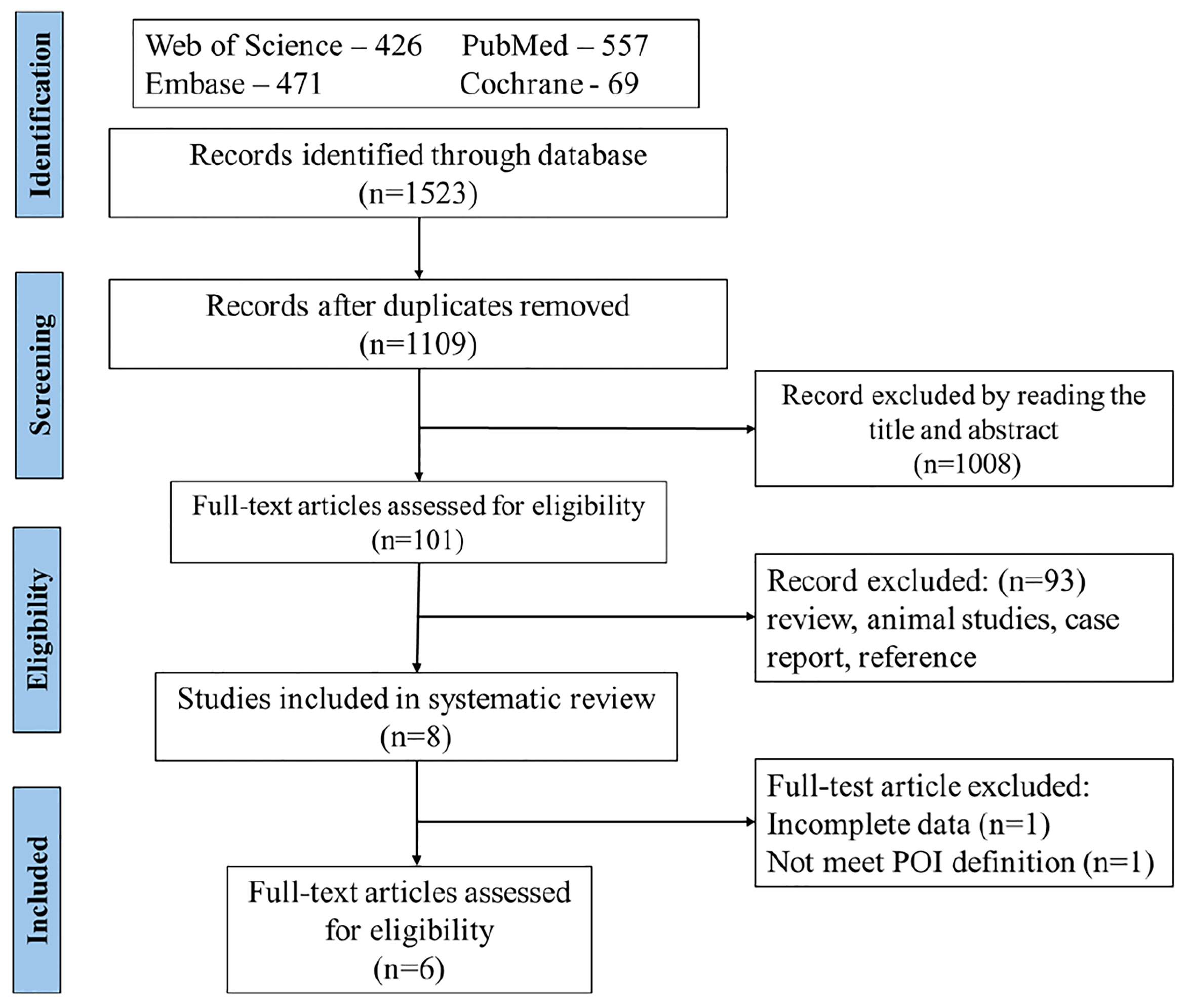
Titles and abstracts of the identified studies were examined and irrelevant studies were deleted. The full text of potentially relevant studies was retrieved and evaluated, and if relevant, included in the study. A total of 1,109 studies met the search criterion after duplicates were removed. During the initial evaluation, 1,008 records were excluded based on title, abstracts, or both. During the second phase of the selection process of the remaining 101 studies, 93 records were excluded because they were irrelevant (review, animal studies, case report, and reference). In the final selection process, two studies were excluded because of incomplete data (n=1) and one did not meet the POI definition of ESHRE guideline (n=1). Finally, six studies were included in this review. The flow chart for the search results of our systematic review is described in Figure 1.
Data Extraction
Data were collected using standard forms to document the characteristics of the study design, participants, intervention, comparisons, and main results. The data were extracted from the plots by WebPlotDigitizer (https://apps.automeris.io/wpd/index.zh_CN.html) if not given in the tables and texts.
Outcomes
The primary outcomes were the changes in ovarian function markers, including AFC, AMH levels, and FSH levels following the treatments. Other markers, such as menses recovery, mean ovarian volume (MOV), and spontaneous pregnancy were secondary outcomes.
Quality of Included Studies
The quality of the RCT evidence was judged to be moderate (26) according to the GRADE working group. The quality of evidence in observational studies was low.
Results
Description of Included Studies
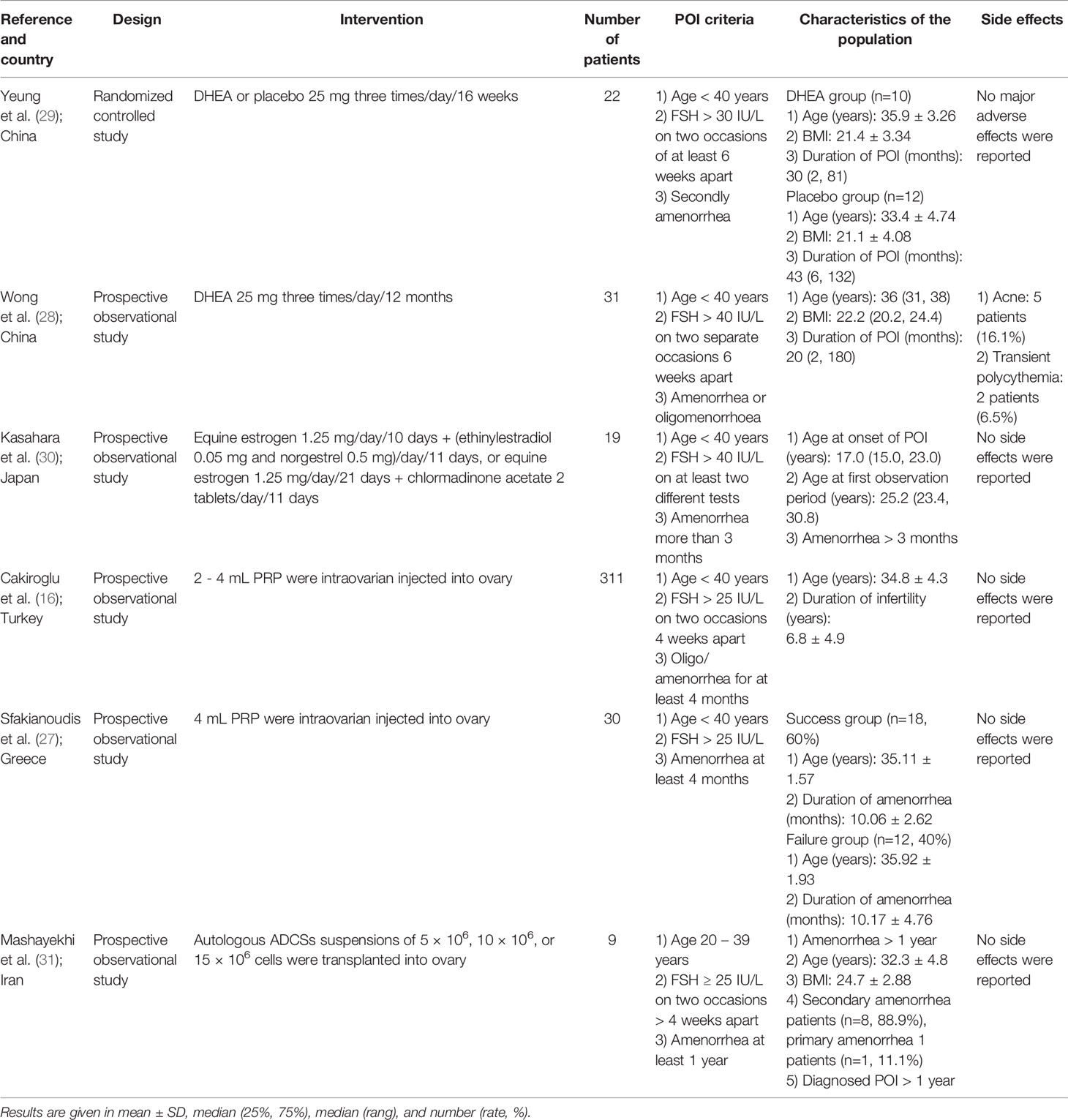
Six eligible studies were selected in this review, including one randomized controlled trial and five prospective observational cohort studies. Four strategies for ovarian function recovery were used, including PRP treatment (16, 27), DHEA supplement (28, 29), HRT treatment (30), and intraovarian transplantation of autologous adipose-derived mesenchymal stromal cells treatments (31). The sample size of the individual studies ranged from 9 to 311, with a total of 421 patients. All patients met the definition of the ESHRE guideline (2). There were four patients with a history of chemotherapy (n=4), and three with chromosomal abnormalities (n=3) in one study (30), and the remaining patients had normal karyotypes and no history of radiation and chemotherapy. The main characteristics of the six studies are shown in Tables 1, 2.
Ovarian Function Test Before Treatment
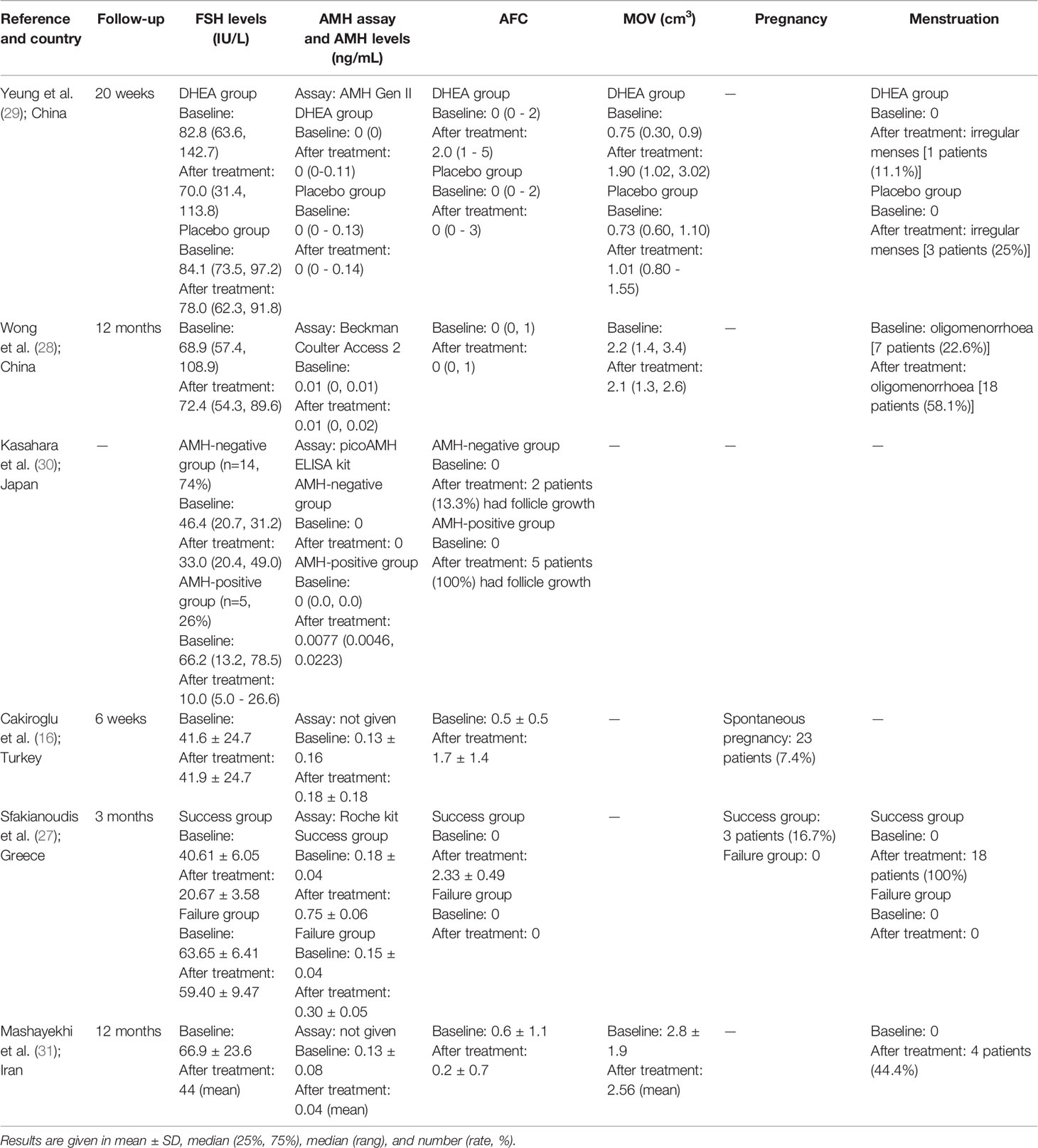
Participants in all 6 studies underwent ovarian function tests (included AFC, AMH levels, and FSH levels) before and after treatments. Before treatment, the mean (or median) AFC was similar across studies and ranged from 0 to 0.6. Mean (or median) serum AMH levels ranged from undetectable to 0.18 ng/mL. There was concern that the studies used four different AMH assays and in two studies the AMH assay methods were not reported. Before treatment, mean (or median) FSH levels varied significantly across the studies and ranged from 41.6 to 82.8 IU/L. Three studies had given the mean ovarian volume (MOV) data and the mean (or median) MOV before treatment varied across studies ranging from 0.75 to 2.8 cm3. Four studies had given the menstruation status before treatment and just 7 patients (22.6%) in one study reported oligomenorrhoea; all patients in other studies reported amenorrhoea.
Randomized Controlled Trial
As presented in Tables 1, 2, the RCT conducted by Yeung et al. (29) investigated the effect of DHEA on ovarian function in patients with POI. Twenty-two patients with unexplained POI were randomized and split into the DHEA group and placebo group. No significant changes were found in in AMH and FSH levels. AFC and ovarian volume of the DHEA group were significantly higher at the 12th week (2.00 range (1-5) vs. 1.00 range (0-2), P < 0.05) and the 20th week (median, 3.79 vs. 2.02 cm3, P < 0.05) after DHEA treatment compared to the placebo group. No significant difference in menses recovery was found between the two groups. This study found higher AFC in the DHEA group, while the other two markers (AMH levels and FSH levels) were not significantly changed.
Prospective Observational Cohort Studies
A prospective observational study of 31 Chinese women with POI conducted by Wong et al. (28) had evaluated the long-term effect of DHEA in patients with POI. All patients had a normal karyotype and 19 (61.3%) patients had used HRT previously and HRT was withheld for at least two months before DHEA treatment, 24 (77.4%) patients were amenorrhoeic, and 7 (22.6%) patients were oligomenorrhoeic. There was no significant difference in FSH levels, AMH levels, and AFC after the DHEA treatment. This study concluded that there was no significant improvement in ovarian function by long-term DHEA treatment.
Nineteen patients with POI were described in the prospective observational study by Kasahara et al. (30). Of them, 5 patients lacked definitive causes for POI, 4 had histories of chemotherapy, 3 had chromosomal abnormalities, 7 were positive for thyroid autoantibodies, and all participants were treated with HRT. Patients with one or more detectable AMH level cycles were defined as AMH-positive group, otherwise defined as AMH-negative group. Five patients (26.3%, AMH-positive group) had increased AMH levels, while another 14 patients (73.7%, AMH-negative group) remained undetectable AMH during treatment. The concentration of serum FSH levels decreased significantly during HRT treatment in all patients and AFC had increased in 7 patients (50%) during HRT treatment. This study had found that 26.3% of patients had increased AMH levels and AFC and decreased FSH levels during the HRT treatment, while other patients had a significant decrease in FSH levels and unchanged AMH levels and AFC.
Recent studies showed that intraovarian injection of autologous PRP could enhance ovarian folliculogenesis (32). Thus, intraovarian injection of autologous PRP was hypothesized to improve ovarian function for patients with POI. In a prospective observational cohort study by Cakiroglu et al. (16), 311 patients with POI received transvaginal PRP treatment. All patients had a normal karyotype, had at least one ovary, and had more than a one-year history of infertility. After PRP treatment, AFC and AMH levels were significantly increased, while FSH levels did not change. There were 186 (59.8%) patients with no follicles detected before PRP treatment and this number decreased to 87 (30.0%) after treatment. There were 23 (7.4%) patients who had spontaneous pregnancy after PRP treatment; 11 patients delivered and 5 were ongoing between 24th to 35th weeks of gestation.
Another prospective observational cohort study of PRP treatment was conducted by Sfakianoudis et al. (27). After transvaginal PRP treatment, 18 (60%) of women with POI presented with menstrual recovery and reduced FSH levels that failed to be re-classified as POI. These women were regarded as a success group. The other 12 (40%) patients were regarded as failure group. All patients in the success group had a statistically significant improvement in AMH levels, FSH levels, and AFC after PRP treatment. However, there was no significant change in AMH levels, FSH levels, and AFC in other patients. Thus, this study found that about 60% of patients had increased ovarian function during the PRP treatment.
Finally, in the non-randomized clinical trial, Mashayekhi et al. (31) used stem cell therapy to restore ovarian function. Nine patients with POI were split into three groups (every group had 3 patients) randomly assigned to receive either 5 × 106, 10 × 106, or 15 × 106 autologous adipose-derived stromal cells (ADSCs) transplanted into the ovary. All patients had a normal karyotype and none had FMR1 mutations. The concentration of FSH had decreased at the end of follow-up in all groups and there were no significant differences between the three groups. However, the AMH levels, AFC, and MOV were variable during the follow-up, and there were no significant changes after follow-up compared with the baseline, and there were no significant differences between the three groups. This trial found that the intraovarian embedding of ADCSs was associated with an inconsistent decline in FSH levels, but other ovarian function tests remained unchanged.
Discussion
It is an unpleasant experience for a woman who has been diagnosed with POI, which means the loss of ovarian hormone production and infertility. HRT is recommended for POI treatment of symptoms of low oestrogen (2), which may prevent diseases of the cardiovascular system and bone loss, but need long-term treatment and have other health risks (33). Oocyte donation is an established option for patients with POI (6). However, this choice is not accepted in many regions. Therefore, the ovarian function restoration approach is important for women with POI.
FSH levels are one of the most common indicators for ovarian function and ovarian response after treatment. Indeed, FSH is an important indicator for POI diagnostic (2). However, FSH levels would change with the menses cycle (1). Moreover, the increased oestrogen levels, by adding exogenous oestrogen, would reduce FSH levels through the hypothalamic-pituitary-gonadal axis. Thus, the reduction in FSH levels during HRT therapy does not represent the recovery of ovarian function (34). Therefore, monitoring FSH concentration could only indicate ovarian function recovery for treatments without exogenous oestrogen.
Different from FSH levels, serum AMH levels are largely constant within and across several menstrual cycles and unaffected by additional exogenous hormones (35, 36). AMH is a promising indicator for ovarian function and is widely used as an ovarian function marker in patients after chemotherapy or radiotherapy (37). In addition, although these studies had not found the AMH levels as a useful marker during or after POI treatment and one of the possible reasons might be the absence of a more sensitive AMH assay. Most patients with POI had undetectable baseline AMH levels and a small range change of AMH levels might not be achieved with the existing assay (30). Therefore, a more sensitive AMH assay technique should be developed. Also, international standardization of the AMH determination is required for comparing the AMH result between different studies.
This review has several limitations, including the limited number of articles, small sample sizes, variable treatment methods, and variable quality of the included studies. Notwithstanding, this review systematically analysed the efficiency of POI treatment methods combined with various indicators of ovarian function markers, including AMH levels, FSH levels, and AFC, which provide guidance for future studies.
Conclusion
POI may lead to long-term health complications due to the premature deprivation of ovarian sex hormones. With the rapid progress in the field of reproductive endocrinology, many potentially promising treatment modalities are already being explored. However, to date, there is still no effective method to restore ovarian function. It mainly lies in the lack of randomized clinical trials, large sample sizes, and unified indicators for ovarian function evaluation. It is recommended to combine multiple indexes to evaluate the effectiveness of future treatment modalities, such as AMH levels, FSH levels, and AFC. Among them, AMH is a sensitive ovarian function marker and an ultrasensitive AMH assay technique should be developed. In addition, large randomized controlled trials are necessary to comprehensively evaluate the improvement of ovarian function.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
XK and YT: literature search, screening, data extraction, data analysis and manuscription draft. DL, MJ, and HX: manuscription modification. All authors reviewed the final version of the manuscript and approve it for publication.
Funding
This work was funded by National Key Research and Development Program of China (No. 2018YFC1004800, 2018YFC1004802), the interdisciplinary program of Shanghai Jiao Tong University (YG2022ZD028 and YG2019QNA09), the National Natural Science Foundation of China (No. 81971334), and the Shanghai Municipal Council for Science and Technology (No. 20JC1412100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
POI, primary ovarian insufficiency; AMH, anti-Müllerian hormone; FSH, follicle stimulating hormone; AFC, antral follicle count; PRP, platelet-rich plasma; DHEA, dehydroepiandrosterone; HRT, hormone replacement therapy; ADSCs, adipose-derived stromal cells; IVF, in-vitro fertilization; MOV, mean ovarian volume.
References
1. De Vos M, Devroey P, Fauser BCJM. Primary Ovarian Insufficiency. Lancet (2010) 376:911–21. doi: 10.1016/s0140-6736(10)60355-8
2. The ESHRE Guideline on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: Management of Women With Premature Ovarian Insufficiency. Hum Reprod (2016) 31:926–37. doi: 10.1093/humrep/dew027
3. Jiao X, Zhang H, Ke H, Zhang J, Cheng L, Liu Y, et al. Premature Ovarian Insufficiency: Phenotypic Characterization Within Different Etiologies. J Clin Endocr Metab (2017) 102:2281–90. doi: 10.1210/jc.2016-3960
4. Golezar S, Tehrani FR, Khazaei S, Ebadi A, Keshavarz Z. The Global Prevalence of Primary Ovarian Insufficiency and Early Menopause: A Meta-Analysis. Climacteric (2019) 22:403–11. doi: 10.1080/13697137.2019.1574738
5. Wesevich V, Kellen AN, Pal L. Recent Advances in Understanding Primary Ovarian Insufficiency. F1000Research (2020) 9:1101. doi: 10.12688/f1000research.26423.1
6. Panay N, Anderson RA, Nappi RE, Vincent AJ, Vujovic S, Webber L, et al. Premature Ovarian Insufficiency: An International Menopause Society White Paper. Climacteric (2020) 23:426–46. doi: 10.1080/13697137.2020.1804547
7. Kasteren Y, Hundscheid RDL, Smits APT, Cremers FPM, Zonneveld Pv, Braat DDM. Familial Idiopathic Premature Ovarian Failure: An Overrated and Underestimated Genetic Disease? Hum Reprod (1999) 14:2455–9. doi: 10.1093/humrep/14.10.2455
8. Silva CA, Yamakami LYS, Aikawa NE, Araujo DB, Carvalho JF, Bonfa E. Autoimmune Primary Ovarian Insufficiency. Autoimmun Rev (2014) 13:427–30. doi: 10.1016/j.autrev.2014.01.003
9. Dixit H, Rao L, Padmalatha V, Raseswari T, Kapu AK, Panda B, et al. Genes Governing Premature Ovarian Failure. Reprod BioMed Online (2010) 20:724–40. doi: 10.1016/j.rbmo.2010.02.018
10. Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, et al. Environmental Pollutants, a Possible Etiology for Premature Ovarian Insufficiency: A Narrative Review of Animal and Human Data. Environ Health (2017) 16:37. doi: 10.1186/s12940-017-0242-4
11. Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced Ovarian Function in Long-Term Survivors of Radiation- and Chemotherapy-Treated Childhood Cancer. J Clin Endocr Metab (2003) 88:5307–14. doi: 10.1210/jc.2003-030352
12. Committee on Adolescent Health Care. Committee Opinion No. 605: Primary Ovarian Insufficiency in Adolescents and Young Women. Obstet Gynecol (2014) 124:193–7. doi: 10.1097/01.AOG.0000451757.51964.98
13. Ragab A, Goda H, Ragab A. Induction of Ovulation in Idiopathic Premature Ovarian Failure: A Randomized Double-Blind Trial. Reprod Biomed Online (2007) 15:215–9. doi: 10.1016/s1472-6483(10)60711-0
14. Fraison E, Crawford G, Casper G, Harris V, Ledger W. Pregnancy Following Diagnosis of Premature Ovarian Insufficiency: A Systematic Review. Reprod BioMed Online (2019) 39:467–76. doi: 10.1016/j.rbmo.2019.04.019
15. Kasteren Y, Schoemaker J. Premature Ovarian Failure: A Systematic Review on Therapeutic Interventions to Restore Ovarian Function and Achieve Pregnancy. Hum Reprod Update (1999) 5:483–92. doi: 10.1093/humupd/5.5.483
16. Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, et al. Effects of Intraovarian Injection of Autologous Platelet Rich Plasma on Ovarian Reserve and IVF Outcome Parameters in Women With Primary Ovarian Insufficiency. Aging (2020) 12:10211–22. doi: 10.18632/aging.103403
17. Hubayter ZR, Popat V, Vanderhoof VH, Ndubizu O, Johnson D, Mao E, et al. A Prospective Evaluation of Antral Follicle Function in Women With 46,XX Spontaneous Primary Ovarian Insufficiency. Fertil Steril (2010) 94:1769–74. doi: 10.1016/j.fertnstert.2009.10.023
18. Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo Signaling Disruption and Akt Stimulation of Ovarian Follicles for Infertility Treatment. Proc Natl Acad Sci U S A (2013) 110:17474–9. doi: 10.1073/pnas.1312830110
19. Venturella R, Lico D, Sarica A, Falbo MP, Gulletta E, Morelli M, et al. OvAge: A New Methodology to Quantify Ovarian Reserve Combining Clinical, Biochemical and 3D-Ultrasonographic Parameters. J Ovarian Res (2015) 8:21. doi: 10.1186/s13048-015-0149-z
20. Visser JA, Schipper I, Laven JS, Themmen AP. Anti-Müllerian Hormone: An Ovarian Reserve Marker in Primary Ovarian Insufficiency. Nat Rev Endocrinol (2012) 8:331–41. doi: 10.1038/nrendo.2011.224
21. Knauff EAH, Eijkemans MJC, Lambalk CB, ten Kate-Booij MJ, Hoek A, Beerendonk CCM, et al. Anti-Müllerian Hormone, Inhibin B, and Antral Follicle Count in Young Women With Ovarian Failure. J Clin Endocr Metab (2009) 94:786–92. doi: 10.1210/jc.2008-1818
22. Durlinger ALL, Visser JA, Themmen AP. Regulation of Ovarian Function: The Role of Anti-Müllerian Hormone. Reproduction (2002) 124:601–9. doi: 10.1530/rep.0.1240601. N.
23. Ruth KS, Soares ALG, Borges MC, Eliassen AH, Hankinson SE, Jones ME, et al. Genome-Wide Association Study of Anti-Müllerian Hormone Levels in Pre-Menopausal Women of Late Reproductive Age and Relationship With Genetic Determinants of Reproductive Lifespan. Hum Mol Genet (2019) 28:1392–401. doi: 10.1093/hmg/ddz015
24. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of Ovarian Reserve Tests With Histologically Determined Primordial Follicle Number. Fertil Steril (2011) 95:170–5. doi: 10.1016/j.fertnstert.2010.04.006
25. Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, et al. A Characterization of the Relationship of Ovarian Reserve Markers With Age. Fertil Steril (2012) 97:238–43. doi: 10.1016/j.fertnstert.2011.10.031
26. Robles A, Checa MA, Prat M, Carreras R. Medical Alternatives to Oocyte Donation in Women With Premature Ovarian Failure: A Systematic Review. Gynecol Endocrinol (2013) 29:632–7. doi: 10.3109/09513590.2013.797397
27. Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, et al. Reactivating Ovarian Function Through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. J Clin Med (2020) 9:1–25. doi: 10.3390/jcm9061809
28. Wong QHY, Yeung TWY, Yung SSF, Ko JKY, Li HWR, Ng EHY. The Effect of 12-Month Dehydroepiandrosterone Supplementation on the Menstrual Pattern, Ovarian Reserve Markers, and Safety Profile in Women With Premature Ovarian Insufficiency. J Assist Reprod Gen (2018) 35:1–6. doi: 10.1007/s10815-018-1152-2
29. Yeung TWY, Li RHW, Lee VCY, Ho PC, Ng EHY. A Randomized Double-Blinded Placebo-Controlled Trial on the Effect of Dehydroepiandrosterone for 16 Weeks on Ovarian Response Markers in Women With Primary Ovarian Insufficiency. J Clin Endocr Metab (2013) 98:380–8. doi: 10.1210/jc.2012-3071. Y.
30. Kasahara Y, Osuka S, Bayasula, Nakanishi N, Murase T, Nakamura T, et al. Very Low Levels of Serum Anti-Müllerian Hormone as a Possible Marker for Follicle Growth in Patients With Primary Ovarian Insufficiency Under Hormone Replacement Therapy. Reprod Sci (2021) 28:31–6. doi: 10.1007/s43032-020-00278-4
31. Mashayekhi M, Mirzadeh E, Chekini Z, Ahmadi F, Eftekhari-Yazdi P, Vesali S, et al. Evaluation of Safety, Feasibility and Efficacy of Intra-Ovarian Transplantation of Autologous Adipose Derived Mesenchymal Stromal Cells in Idiopathic Premature Ovarian Failure Patients: non-Randomized Clinical Trial, Phase I, First in Human. J Ovarian Res (2021) 14:5. doi: 10.1186/s13048-020-00743-3
32. Bakacak M, Bostanci MS, Inanc F, Yaylali A, Serin S, Attar R, et al. Protective Effect of Platelet Rich Plasma on Experimental Ischemia/Reperfusion Injury in Rat Ovary. Gynecol Obstet Invest (2016) 81:225–31. doi: 10.1159/000440617
33. Webber L, Anderson RA, Davies M, Janse F, Vermeulen N. HRT for Women With Premature Ovarian Insufficiency: A Comprehensive Review. Hum Reprod Open (2017) 2017:1–11. doi: 10.1093/hropen/hox007
34. Davies MC, Cartwright B. What is the Best Management Strategy for a 20-Year-Old Woman With Premature Ovarian Failure? Clin Endocrinol (2012) 77:182–6. doi: 10.1111/j.1365-2265.2012.04408.x
35. Hehenkamp WJK, Looman CWN, Themmen APN, de Jong FH, te Velde ER, Broekmans FJM. Anti-Müllerian Hormone Levels in the Spontaneous Menstrual Cycle do Not Show Substantial Fluctuation. J Clin Endocrinol Metab (2006) 91:4057–63. doi: 10.1210/jc.2006-0331
36. Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable Serum Levels of Anti-Müllerian Hormone During the Menstrual Cycle: A Prospective Study in Normo-Ovulatory Women. Hum Reprod (2007) 22:1837–40. doi: 10.1093/humrep/dem101
Keywords: antral follicle count, follicle-stimulating hormone, ovarian function, anti-Müllerian hormone, primary ovarian insufficiency
Citation: Kuang X, Tang Y, Xu H, Ji M and Lai D (2022) The Evaluation of Ovarian Function Recovery Following Treatment of Primary Ovarian Insufficiency: A Systematic Review. Front. Endocrinol. 13:855992. doi: 10.3389/fendo.2022.855992
Received: 16 January 2022; Accepted: 28 March 2022;
Published: 28 April 2022.
Edited by:
Lawrence Merle Nelson, Mary Elizabeth Conover Foundation, Inc., United StatesCopyright © 2022 Kuang, Tang, Xu, Ji and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Ji, amltaW4yMjY2MzFAMTYzLmNvbQ==; Dongmei Lai, bGFpZG9uZ21laUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Xiaojun Kuang
Xiaojun Kuang Yongzhe Tang
Yongzhe Tang Hong Xu3
Hong Xu3 Min Ji
Min Ji Dongmei Lai
Dongmei Lai