
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 12 April 2022
Sec. Cardiovascular Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.855430
This article is part of the Research Topic Serum Uric Acid, Vascular Aging, and Endocrine Comorbidities View all 12 articles
Background: To determine the association between serum uric acid (SUA) and the retinal capillary plexus (RCP) using optical coherence tomography angiography (OCTA).
Methods: This cross-sectional study evaluated data from August 2019 to January 2020 from participants recruited from the Jidong community (Tangshan, Hebei, China). All participants completed detailed anthropometrical measurements, laboratory tests and comprehensive ophthalmic examinations. We assessed the vessel density in RCP using OCTA. We used multivariable analysis to evaluate the sex-specific association between SUA and RCP after adjusting for confounders.
Results: A total of 2730 participants were included in this study. The mean age of the participants was 44.0 ± 11.6 years, and 1463 (53.6%) were women. The multivariable βs and 95% confidence intervals (CIs) of superficial RCP vessel density in the second through fourth SUA quartiles compared with the lowest SUA quartiles were -0.27 (-0.56 – 0.03), -0.30 (-0.60 – 0.01), and -0.46 (-0.78 – -0.14) (P for trend = 0.007) in men.
Conclusions: Higher SUA levels were significantly associated with lower RCP vessel density in men. Our findings provide evidence for the detrimental effect of high SUA levels on the retinal microvasculature and imply the importance of modulating SUA to prevent the microvascular alternation especially for men.
Serum uric acid (SUA), a product of purine metabolism and its derivatives, has long been associated with various metabolic and cardiovascular diseases (1–4). People with high SUA have an approximately 30% higher rate of cardiovascular disease and all-cause mortality (5). Several studies suggested that higher uric acid level may cause microvascular alterations (6–8), though direct noninvasive in vivo observations of microcirculatory alterations are still lacking thus far. The retina is an ideal window for direct noninvasive in vivo observation of the microcirculation (9), through which systemic microvascular alterations in persons with high SUA can be indirectly observed. Additionally, sex differences were observed in many conditions about uric acid (10). Thus, it is important to exploring the sex-specific effect of SUA on the retinal microvasculature.
Previously, studies focused on the effect of SUA on retinal arterioles and venules (11, 12) but not on the subtle alteration of retinal capillaries due to the limitations of imaging technology. Recently, optical coherence tomography angiography (OCTA) technology has emerged as a promising new technology for better visualization and quantitative analysis of retinal capillaries (13). Previous studies have reported the wide use of OCTA not only in the assessment of many retinal diseases (14, 15) but also in systemic vascular diseases, such as hypertension (16), stroke (17), coronary artery disease (18), and chronic kidney disease (19). Numerous studies have shown that OCTA-derived vascular metrics, such as the retinal capillary plexus (RCP) vessel density, are useful to evaluate retinal capillary alternations and microvascular pathologic features (20), which may provide new insight into SUA-related effects on the microvasculature.
The relationship between uric acid and retinal microvascular alternations is still unclear. Additionally, close linkages of diabetic retinopathy and hypertensive retinopathy with SUA have been reported previously (21, 22). However, other previous epidemiological reports have failed to demonstrate these relationships (23, 24). Previously, only two relevant studies analyzed the association between higher SUA and retinal vasculature, focusing on retinal arterioles and venules (11, 12). Findings from these studies were inconsistent and contradictory. There is still a lack of studies on the subtle alterations of vessel density in the retinal capillary plexus. In view of this and considering the close linkage between SUA and sex, this community-based study aimed to investigate the sex-specific association of SUA with RCP using OCTA.
Our study was a substudy of the Jidong Eye Cohort Study (JECS). The data used for this study were obtained from JECS participants. Details of the JECS have been previously described (25). A total of 3377 participants were recruited from Jidong communities (Tangshan, Hebei, China) between August 2019 and January 2020. The following participants were removed from the analyzed sample due to the predetermined exclusion criteria: 31 individuals had missing SUA data; 113 individuals had ocular diseases; 163 individuals had suboptimal OCTA images; 11 individuals had low best corrected visual acuity (BCVA); 208 individuals had refraction above +2.00 or under -6.00 diopters; and 121 individuals had missing axial length data (Figure 1).
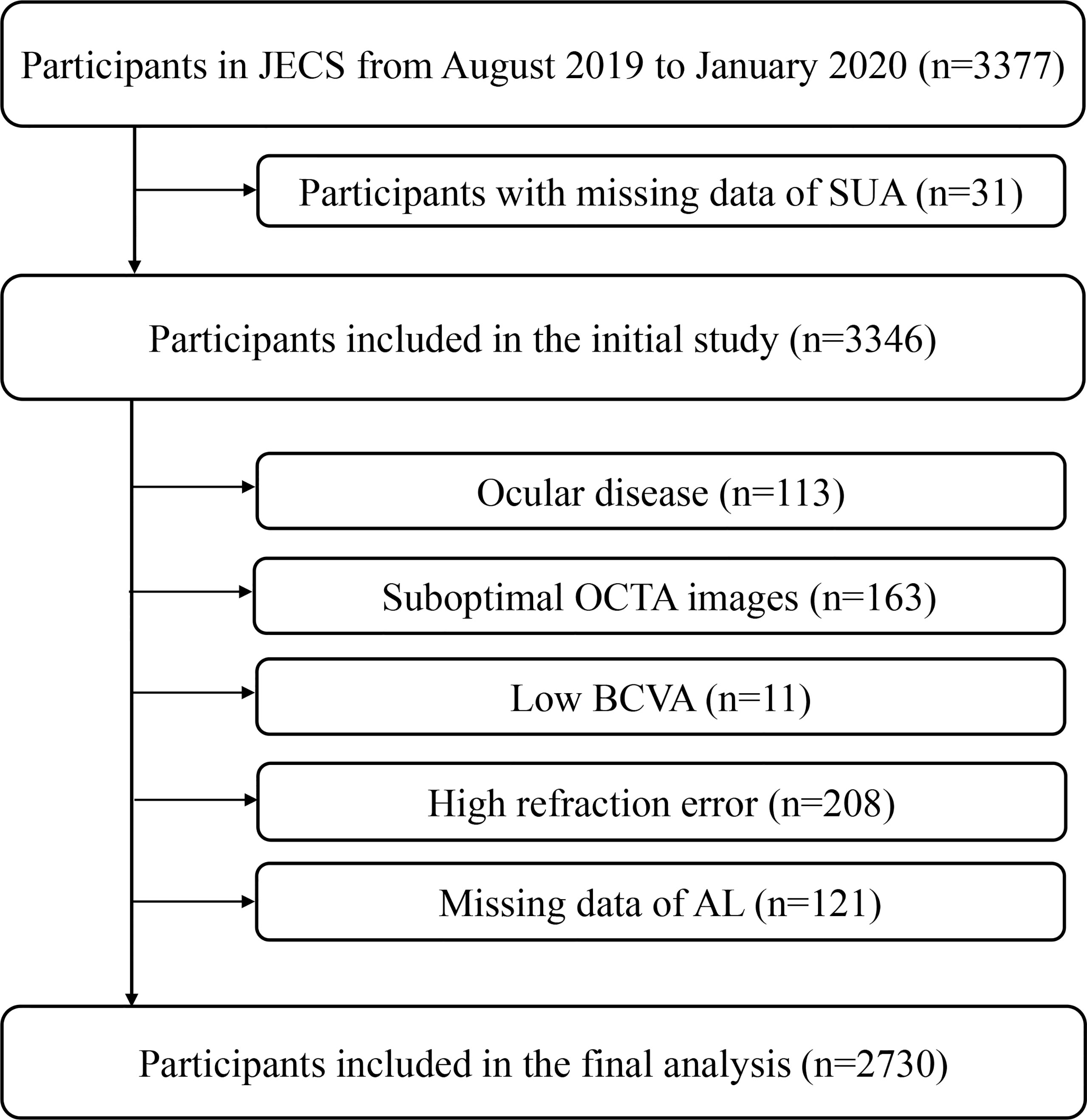
Figure 1 Flow Chart of the Study Participant. JECS, Jidong Eye Cohort Study; OCTA, optical coherence tomography angiography; BCVA, best corrected visual acuity; AL, axial length.
This study was approved by the Ethics Committee of the Staff Hospital of Jidong oil-field of Chinese National Petroleum (China national petroleum corporation Jidong oilfield branch staff hospital approval document of the medical ethics committee, 2018 YILUNZI 1) and followed the Declaration of Helsinki guidelines. We obtained written informed consent forms from all participants.
In this study, basic information on the participants was obtained using clinical examinations, laboratory tests, and standardized questionnaires for demographic characteristics, current smoking status, current alcohol consumption status, and medical history (26, 27). The average monthly income levels were divided into “< ¥3,000” and “≥ ¥3,000”. The education levels were categorized as “illiteracy, primary or middle school” and “college graduate or above”. In this study, diabetes was determined by either fasting blood glucose (FBG) ≥ 7.0 mmol/L, self-reported diabetes history, or current antidiabetic medication use; while hypertension was defined as either systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, self-reported hypertension history, or current use of antihypertensive medications. Dyslipidemia was defined as either low-density lipoprotein (LDL-C) ≥ 3.3 mmol/L, high-density lipoprotein (HDL-C) < 1.04 mmol/L, total cholesterol (TC) ≥ 5.17 mmol/L, triglyceride (TG) ≥ 1.7 mmol/L, current use of lipid-lowering medications, or self-reported history of dyslipidemia. We calculated the estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation and adjusted it by a coefficient of 1.1 for the Asian population (28, 29).
Fasting elbow venous blood samples were obtained in the morning after refraining from food and drinking for at least 8 hours and they were stored in vacuum tubes containing ethylenediaminetetraacetic acid. SUA levels were measured using an autoanalyzer (Hitachi, Tokyo, Japan) with the uricase-peroxidase method in the Central Laboratory of Jidong Oil-Field Hospital (25, 30). Participants in this study were stratified into quartiles based on sex-specific SUA levels (male, Q1: ≤ 335 μmol/L, Q2: 336 - 385 μmol/L, Q3: 386 - 443 μmol/L, Q4: ≥ 444 μmol/L; female, Q1: ≤ 244 μmol/L, Q2: 245 - 278 μmol/L, Q3: 279 - 322 μmol/L, Q4: ≥ 323 μmol/L). Hyperuricemia was defined as an SUA level ≥ 360 µmol/L (6.0 mg/dL) in women and ≥ 420 µmol/L (7.0 mg/dL) in men (31).
Participants in our study underwent a comprehensive eye examination including best-corrected visual acuity (BCVA) by a standard logarithmic visual acuity chart and refractive status by an autorefractometer (KR800; Topcon; Tokyo, Japan). Refraction was calculated as the spherical equivalent (spherical value and half of the cylindrical value). Measurements of axial length (AL) were obtained using a Lenstar 900 (Haag-Streit; Koeniz, Switzerland). Digital fundus photographs were obtained using a 45° nonmydriatic fundus camera (CR2AF; Canon; Tokyo, Japan). All examination results were reviewed by at least two ophthalmologists. Participants with ocular disease, low BCVA (worse than 0.5 logMAR), or high refraction error (spherical equivalent < -6.00 D/spherical equivalent > +2.00 D) were excluded from the analyses.
We obtained OCTA images using a spectral-domain OCTA device (RTVue XR Avanti with AngioVue; Optovue; Fremont, CA, USA). OCTA images were acquired using a 3 × 3 mm² scan with a scan density of 304×304 A-scans centered on the macula. The superficial (between the internal limiting membrane and inner plexiform layer) and deep (between the inner plexiform layer and outer plexiform layer) RCPs were automatically segmented according to the built-in software (AngioAnalytics, version 2017.1.0.155) (25). An algorithm incorporated into the device (optovue, Inc) automatically removes the 3D projection artifacts to reduce the motion artifacts and increase the image quality (32). Superficial and deep OCTA images were exported and then corrected using Bennett’s formula with AL magnification (33). The vessel density (the proportion of the flow signals) of the superficial and deep RCP was automatically generated by a custom algorithm operating on MATLAB (version 2017b; MathWorks, Inc.; Natick, MA, USA). Details of the processing and analysis methods have been previously described (33, 34). The processing algorithm based on a macula map was as follows: two concentric circles (0.6 and 2.5 mm diameters) divided into five quadrants (parafovea, superior, inferior, nasal, and temporal). In this report, the quadrant of the parafovea served as a representative of the average RCP due to the special structure of the foveal area, and the other quadrants were also presented.
Only OCTA scans with a signal strength index ≥ 7 were included for quantitative analysis and images with defects were excluded from the study. We used the measurement of the right eye if it was available, whereas the left eye was only analyzed in the absence of eligible scans for the right eye.
We expressed continuous variables as the means and standard deviations (SD) and categorical valuables as frequencies and percentages. We examined the differences between the different SUA quartile groups using a one-way ANCOVA test for normally distributed continuous variables and chi-square tests or Fisher’s exact test for categorical valuables.
We used multivariable generalized linear models to estimate the association between the SUA quartile and the vessel density in the RCP. We tested for trends by considering the SUA quartile as a continuous ordinal variable. We adjusted all multivariable generalized linear models for age, current drinking status, hypertension, diabetes, body mass index (BMI), TC, TG, HDL-C, LDL-C, and eGFR. Moreover, associations of a 1 standard deviation change in SUA and the presence of hyperuricemia with RCP vessel density were also tested, and we examined whether the associations between SUA and RCP differed by sex by including interaction terms in the adjusted models.
Associations were measured as βs and 95% confidence intervals (CIs). For all analyses, statistical significance was considered as a 2-tailed P value < 0.05. All statistical analyses were undertaken using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
A total of 2730 participants from Jidong communities were eventually included in the analyses. The mean age of the included participants was 44.0 ± 11.6 years, and there were 1463 (53.6%) women. Baseline characteristics by different quartiles of serum SUA levels are summarized in Table 1. Participants with higher SUA levels were younger (P = 0.006), more likely to be drinkers (p = 0.01), have a higher BMI (P < 0.001), have a lower eGFR (P < 0.001), and have more prevalent hypertension (P < 0.001) and dyslipidemia (P < 0.001).
Table 2 shows the RCP characteristics in different SUA quartile groups on the baseline survey. For the male participants stratified into quartiles, the vessel density of the superficial RCP decreased from Q1 to Q4 from 51.1% to 50.6% in the parafovea. For the vessel density in other quadrants of RCP, see Table 2. However, we did not observe a significant difference in deep RCP in men or superficial and deep RCP in women. Figure 2 shows the representative OCTA images of male participants in Q1 and Q4.
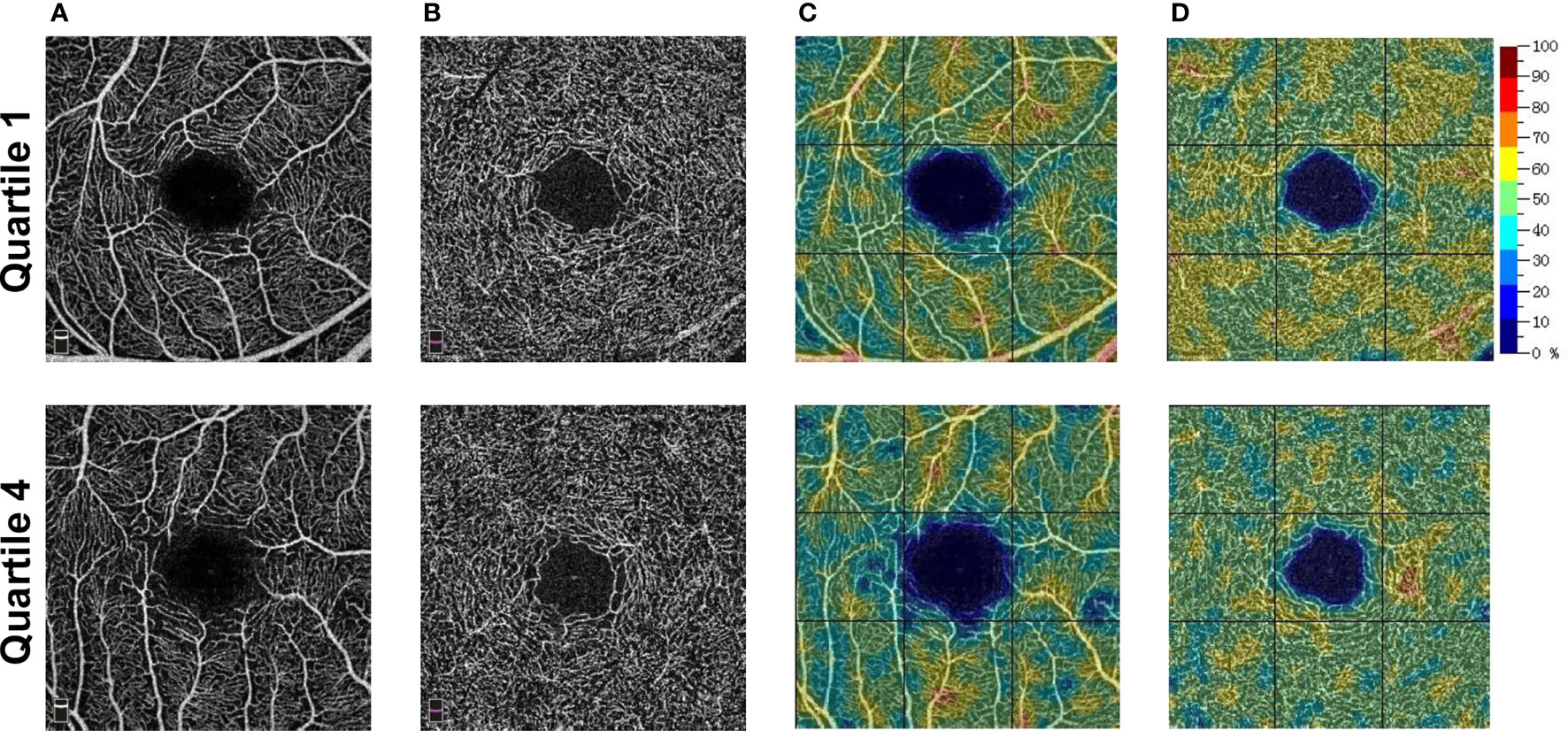
Figure 2 Representative OCTA images of male participants. (A) The superficial OCT angiograms. (B) The deep OCT angiograms. (C) The color-coded superficial RCP vessel density maps. (D) The color-coded deep RCP vessel density maps. Quartile 1, SUA level ≤335 umol/L; Quartile 4, SUA level ≥444 umol/L. OCTA, optical coherence tomography angiography; OCT, optical coherence tomography; SUA, serum uric acid; RCP, retinal capillary plexus.
We show the relationships between SUA quartile and RCP vessel density for men in Table 3. Male participants in the highest SUA quartile had a significant decrease in vessel density in the superficial RCP, even after adjusting for potential confounding factors. Compared to the lowest quartile, the multivariable βs and 95% Cis for the male participants in the second through fourth SUA quartiles were -0.27 (-0.56 – 0.03), -0.30 (-0.60 – 0.01), and -0.46 (-0.78 – -0.14) (P for trend = 0.007) in the parafovea. The βs in other quadrants of RCP are also shown. However, for deep RCP vessel density, we observed no significant association.
We show the relationships between SUA quartile and RCP vessel density for women in Table 4. No significant associations were found between SUA levels and RCP vessel density in women.
We observed a significant moderating effect of sex on the association between SUA and RCP. SUA levels were relatively more strongly associated with low superficial RCP vessel density (parafovea, interaction P=0.02) in men than in women (Table 5).
We show the adjusted associations between hyperuricemia and RCP in Table 6. After adjusting for the confounders mentioned above, we found significant associations between hyperuricemia and superficial RCP vessel density (parafovea: β = -0.24, 95% CI, -0.47 to -0.01) in men but not in women. However, no significant association was found between hyperuricemia and deep RCP in either sex. Moreover, associations between 1 standard deviation altered in SUA and the RCP vessel density were also tested, as shown in Table 7. We observed similar associations.
In this OCTA study, we evaluated the detrimental effect of high SUA levels on RCP in a relatively large community-based population. The multivariable generalized linear model analysis showed that the male participants with SUA levels in the highest quartile were significantly associated with lower RCP vessel density after adjusting for confounders.
Collectively, the studies to date have not shown a clear relationship between SUA and retinal vascular metrics (11, 12). In our study, we showed that higher SUA levels are associated with lower vessel density only in men. In supporting of our findings, YuanZhi et al. (11) previously showed an association between an elevated SUA and a smaller retinal arteriolar caliber and larger retinal venular caliber, and the associations were more pronounced in men than in women. However, a study in a Chinese coastal population showed that a higher SUA was associated with a larger retinal arteriolar caliber and larger retinal venular caliber only in women (12). This pattern of inconsistent findings may be explained by different SUA levels, different characteristics of the participants, and different imaging methods.
As reported in previous studies, the loss of blood flow or hypoperfusion in retinal vasculature leads to several ocular diseases, such as hypertensive retinopathy (22) and diabetic retinopathy (35, 36). Findings from our studies may help explain why people with higher SUA levels are more likely to develop ocular disorders and visual impairment (21, 37). However, it is important to point out that higher SUA levels are associated with only a small reduction in RCP vessel density, which may not reach clinical relevance, although it was statistically significant. Potentially, the reduced RCP in participants with higher SUA may be explained by the underlying physiological mechanism by which uric acid induces endothelial dysfunction (38, 39) and stimulates endothelial cell proliferation (40) by reducing nitric oxide and activating the renin-angiotensin system (41) and eventually leading to microvascular damage (42).
In our study, significant sex differences in SUA levels and their association with RCP vessel density were observed. We found that SUA levels were associated with RCP vessel density in men but not in women. It is important to point out that the man sex is a significant risk factor for high SUA levels, as man are up to four times more likely to be affected (43). Our findings suggest that retinal microvasculature in man are more possible to be affected by high SUA levels than women. Our study emphasized the importance of modulating SUA for men. The sex differences in this study may be attributed to menopause and estrogen (10), as we observed a significant fluctuation in SUA levels around the period of menopause in women. The studies mentioned above showed inconsistent findings (11, 12). In addition, a study in Japan reported an association between SUA levels and diabetic retinopathy only in men (44). Nevertheless, other studies previously showed that an increase in SUA can be more detrimental in women (10). The discrepancy may be attributed to age, SUA levels, hormone levels, and other cohort differences. Further research is still required.
Surprisingly, we observed that SUA levels were associated with vessel density only in the superficial RCP but not in the deep RCP. OCTA imaging in the deep retinal OCT angiogram, in contrast to superficial imaging, is more likely to be affected by motion artifacts, and the quantification of flow density in deep RCP is more challenging. Previous studies have shown that the repeatability of deep retinal OCT angiograms is weaker than that of superficial retinal OCT angiograms (45–47).
It has been well-documented that higher SUA levels may lead to hypertension (41). As a matter of fact, OCTA metrics may also be affected by hypertension (16) It is interesting to note that participants with higher SUA levels had more prevalent hypertension in our study. Findings from our studies may help explain why people with higher SUA levels are more likely to develop systemic microvascular disease (17, 18).
To our knowledge, this was the first study to identify a sex-specific association between SUA and RCP in a large community-based population using OCTA. The main strengths of this study include the use of detailed ophthalmic examination in a relatively large community-based study, adjustments for several potential confounding factors, a standardized quantitative system of retinal vascular plexus including magnification correction, and investigation using sensitivity analyses to ensure the robustness of the results.
However, several limitations in our study should also be discussed. First, we could not draw a causative association between SUA and RCP due to limitations of the cross-sectional nature of this study. There is no doubt that it is of great interest to explore the alterations of RCP in relation to high SUA levels over time, and it may be a future study objective as a follow-up to the JECS. Second, the study participants were all individuals from the Jidong community. This may not adequately represent other populations, and the applicability of our results to other racial/ethnic groups might be limited. More studies in other populations are required to further determine the effect of SUA on RCP. Finally, some potential confounders, such as hormone levels, cell factors that may affect SUA, retinal vasculature, and residual confounders, were not included in the analysis.
In conclusion, we found that higher SUA levels were associated with lower superficial RCP vessel density in men. Given that the retinal microvasculature is an ideal window to observe the alternations of the microcirculation, our findings help validate the detrimental effect of high SUA levels on the retinal microvasculature and imply the importance of modulating SUA to prevent the microvascular alternation especially for men.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Staff Hospital of Jidong oil-field of Chinese National Petroleum. The patients/participants provided their written informed consent to participate in this study.
ML, LC, JQ, and FL designed and conceptualized the study and interpreted the data. KY analyzed the data. KY, XZ, YX, BS, CL, KS, and YJ had a major role in the acquisition of data. KY drafted the manuscript. ML, LC, JQ, and FL revised the manuscript for intellectual content. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China, No. 81900903 (to ML); National Key R&D Program of China, No. 2020YFC2008200 (to FL) and 2019YFC0840708 (to LC); Zhejiang Provincial Natural Science Foundation of China, No. LY22H120007 (to LC); the Medical and Health Science and Technology Program of Zhejiang Province, No. 2017KY113 (to ML), No. 2018KY543 (to LC); Wenzhou Science and Technology Program, No. Y20210984 (to LC). The funding bodies played no role in the study design, collection, analysis, and interpretation of data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to the Jidong community for its collaboration, especially the dedicated participants and all research staff involved in the study. We thank Dr. Xianwei Wang who provided insightful methodological advice and wisdom throughout the study.
SUA, Serum uric acid; OCT, optical coherence tomography; OCTA, optical coherence tomography angiography; RCP, retinal capillary plexus; JECS, Jidong Eye Cohort Study; BCVA, low best corrected visual acuity; FBG, fasting blood glucose; LDL-C, low-density lipoprotein; HDL-C, high density lipoprotein; TC, total cholesterol; TG, triglyceride; eGFR, estimated glomerular filtration rate; AL, axial length; BMI, body mass index; CIs, confidence intervals.
1. Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Krämer BK, et al. Uric Acid and Cardiovascular Events: A Mendelian Randomization Study. JASN (2015) 26:2831–8. doi: 10.1681/ASN.2014070660
2. Sun H-L, Pei D, Lue K-H, Chen Y-L. Uric Acid Levels Can Predict Metabolic Syndrome and Hypertension in Adolescents: A 10-Year Longitudinal Study. PloS One (2015) 10:e0143786. doi: 10.1371/journal.pone.0143786
3. Verdoia M, Barbieri L, Schaffer A, Cassetti E, Nardin M, Bellomo G, et al. Impact of Diabetes on Uric Acid and its Relationship With the Extent of Coronary Artery Disease and Platelet Aggregation: A Single-Centre Cohort Study. Metabolism (2014) 63:640–6. doi: 10.1016/j.metabol.2014.01.010
4. Bos MJ, Koudstaal PJ, Hofman A, Witteman JCM, Breteler MMB. Uric Acid Is a Risk Factor for Myocardial Infarction and Stroke: The Rotterdam Study. Stroke (2006) 37:1503–7. doi: 10.1161/01.STR.0000221716.55088.d4
5. Choi HK, Curhan G. Independent Impact of Gout on Mortality and Risk for Coronary Heart Disease. Circulation (2007) 116:894–900. doi: 10.1161/CIRCULATIONAHA.107.703389
6. Gigante A, Barbano B, Barilaro G, Quarta S, Gasperini ML, Di Mario F, et al. Serum Uric Acid as a Marker of Microvascular Damage in Systemic Sclerosis Patients. Microvascular Res (2016) 106:39–43. doi: 10.1016/j.mvr.2016.03.007
7. Hayfron-Benjamin CF, van den Born B-J, Amoah AGB, Maitland-van der Zee AH, Meeks KAC, Beune EJAJ, et al. Associations of Serum Uric Acid Levels With Macrovascular and Renal Microvascular Dysfunction Among Individuals From Sub-Saharan Africa. JAMA Netw Open (2021) 4:e2128985. doi: 10.1001/jamanetworkopen.2021.28985
8. Prasad M, Matteson EL, Herrmann J, Gulati R, Rihal CS, Lerman LO, et al. Uric Acid Is Associated With Inflammation, Coronary Microvascular Dysfunction, and Adverse Outcomes in Postmenopausal Women. Hypertension (2017) 69:236–42. doi: 10.1161/HYPERTENSIONAHA.116.08436
9. MacGillivray TJ, Trucco E, Cameron JR, Dhillon B, Houston JG, van Beek EJR. Retinal Imaging as a Source of Biomarkers for Diagnosis, Characterization and Prognosis of Chronic Illness or Long-Term Conditions. BJR (2014) 87:20130832. doi: 10.1259/bjr.20130832
10. Halperin Kuhns VL, Woodward OM. Sex Differences in Urate Handling. IJMS (2020) 21:4269. doi: 10.3390/ijms21124269
11. YuanZhi Y. Hyperuricemia Accompanied With Changes in the Retinal Microcirculation in a Chinese High-Risk Population for Diabetes. BioMed Environ Sci (2011) 24(2):146–54. doi: 10.3967/0895-3988.2011.02.009
12. Li Q, Lin F, Gao Z, Huang F, Zhu P. Sex-Specific Association Between Serum Uric Acid and Retinal Microvessels. Med Sci Monit (2019) 25:9973–80. doi: 10.12659/MSM.919972
13. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical Coherence Tomography Angiography. Prog Retinal Eye Res (2018) 64:1–55. doi: 10.1016/j.preteyeres.2017.11.003
14. Tey KY, Teo K, Tan ACS, Devarajan K, Tan B, Tan J, et al. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Review of Current Applications. Eye Vis (2019) 6:37. doi: 10.1186/s40662-019-0160-3
15. Xu B, Chen J, Zhang S, Shen S, Lan X, Chen Z, et al. Association Between the Severity of Diabetic Retinopathy and Optical Coherence Tomography Angiography Metrics. Front Endocrinol (2021) 12:777552. doi: 10.3389/fendo.2021.777552
16. Peng Q, Hu Y, Huang M, Wu Y, Zhong P, Dong X, et al. Retinal Neurovascular Impairment in Patients With Essential Hypertension: An Optical Coherence Tomography Angiography Study. Invest Ophthalmol Vis Sci (2020) 61:42. doi: 10.1167/iovs.61.8.42
17. Liu B, Hu Y, Ma G, Xiao Y, Zhang B, Liang Y, et al. Reduced Retinal Microvascular Perfusion in Patients With Stroke Detected by Optical Coherence Tomography Angiography. Front Aging Neurosci (2021) 13:628336. doi: 10.3389/fnagi.2021.628336
18. Zhong P, Hu Y, Jiang L, Peng Q, Huang M, Li C, et al. Retinal Microvasculature Changes in Patients With Coronary Total Occlusion on Optical Coherence Tomography Angiography. Front Med (2021) 8:708491. doi: 10.3389/fmed.2021.708491
19. Zeng X, Hu Y, Chen Y, Lin Z, Liang Y, Liu B, et al. Retinal Neurovascular Impairment in Non-Diabetic and Non-Dialytic Chronic Kidney Disease Patients. Front Neurosci (2021) 15:703898. doi: 10.3389/fnins.2021.703898
20. Sun C, Ladores C, Hong J, Nguyen DQ, Chua J, Ting D, et al. Systemic Hypertension Associated Retinal Microvascular Changes can be Detected With Optical Coherence Tomography Angiography. Sci Rep (2020) 10:9580. doi: 10.1038/s41598-020-66736-w
21. Hu Y, Chan Z, Li C, Shi Y, She X, Gu C, et al. Higher Serum Uric Acid Levels Are Associated With an Increased Risk of Vision-Threatening Diabetic Retinopathy in Type 2 Diabetes Patients. Invest Ophthalmol Vis Sci (2021) 62:23. doi: 10.1167/iovs.62.4.23
22. Chen X, Meng Y, Li J, She H, Zhao L, Zhang J, et al. Serum Uric Acid Concentration is Associated With Hypertensive Retinopathy in Hypertensive Chinese Adults. BMC Ophthalmol (2017) 17:83. doi: 10.1186/s12886-017-0470-y
23. Wan H, Wang Y, Chen Y, Fang S, Zhang W, Xia F, et al. Different Associations Between Serum Urate and Diabetic Complications in Men and Postmenopausal Women. Diabetes Res Clin Pract (2020) 160:108005. doi: 10.1016/j.diabres.2020.108005
24. Xia Q, Zhang S-H, Yang S-M, Zhu X-L, Su S, Hu A-P, et al. Serum Uric Acid is Independently Associated With Diabetic Nephropathy But Not Diabetic Retinopathy in Patients With Type 2 Diabetes Mellitus. J Chin Med Assoc (2020) 83:350–6. doi: 10.1097/JCMA.0000000000000285
25. Yang K, Cui L, Zhang G, Wang X, Zhu X, Xiao Y, et al. The Jidong Eye Cohort Study: Objectives, Design, and Baseline Characteristics. Eye Vis (2020) 7:58. doi: 10.1186/s40662-020-00223-1
26. Song D-Y, Wang X-W, Wang S, Ge S-Q, Ding G-Y, Chen X-Y, et al. Jidong Cognitive Impairment Cohort Study: Objectives, Design, and Baseline Screening. Neural Regener Res (2020) 15:1111. doi: 10.4103/1673-5374.266070
27. Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, et al. Ideal Cardiovascular Health Metrics and the Risks of Ischemic and Intracerebral Hemorrhagic Stroke. Stroke (2013) 44:2451–6. doi: 10.1161/STROKEAHA.113.678839
28. Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, et al. GFR Estimating Equations in a Multiethnic Asian Population. Am J Kidney Dis (2011) 58:56–63. doi: 10.1053/j.ajkd.2011.02.393
29. Wang X, Wang Y, Patel UD, Barnhart HX, Li Z, Li H, et al. Comparison of Associations of Reduced Estimated Glomerular Filtration Rate With Stroke Outcomes Between Hypertension and No Hypertension. Stroke (2017) 48:1691–4. doi: 10.1161/STROKEAHA.117.016864
30. Tang Z, Chen X, Zhang W, Sun X, Hou Q, Li Y, et al. Association Between Gamma-Glutamyl Transferase and Mild Cognitive Impairment in Chinese Women. Front Aging Neurosci (2021) 13:630409. doi: 10.3389/fnagi.2021.630409
31. Ding X, Zhe F, Ni Z, HongLi L, Wang W, Wang N. Chinese Practice Guidelines for Diagnosis and Treatment of Hyperuricemia in Renal Disease (2017 Edition). Chin Med J Peking (2017) 97:1927–36. doi: 10.3760/cma.j.issn.0376-2491.2017.25.003
32. Kraus MF, Potsaid B, Mayer MA, Bock R, Baumann B, Liu JJ, et al. Motion Correction in Optical Coherence Tomography Volumes on a Per A-Scan Basis Using Orthogonal Scan Patterns. BioMed Opt Express (2012) 3:1182. doi: 10.1364/BOE.3.001182
33. Chen Q, Tan F, Wu Y, Zhuang X, Wu C, Zhou Y, et al. Characteristics of Retinal Structural and Microvascular Alterations in Early Type 2 Diabetic Patients. Invest Ophthalmol Vis Sci (2018) 59:2110. doi: 10.1167/iovs.17-23193
34. Shi C, Chen Y, Kwapong WR, Tong Q, Wu S, Zhou Y, et al. Characterization By Fractal Dimension Analysis Of The Retinal Capillary Network In Parkinson Disease. Retina (2020) 40:1483–91. doi: 10.1097/IAE.0000000000002641
35. Bjornstad P, Maahs DM, Rivard CJ, Pyle L, Rewers M, Johnson RJ, et al. Serum Uric Acid Predicts Vascular Complications in Adults With Type 1 Diabetes: The Coronary Artery Calcification in Type 1 Diabetes Study. Acta Diabetol (2014) 51:783–91. doi: 10.1007/s00592-014-0611-1
36. Liang C-C, Lin P-C, Lee M-Y, Chen S-C, Shin S-J, Hsiao P-J, et al. Association of Serum Uric Acid Concentration With Diabetic Retinopathy and Albuminuria in Taiwanese Patients With Type 2 Diabetes Mellitus. IJMS (2016) 17:1248. doi: 10.3390/ijms17081248
37. Melo LGN, Morales PH, Drummond KRG, Santos DC, Pizarro MH, Barros BSV, et al. Current Epidemiology of Diabetic Retinopathy in Patients With Type 1 Diabetes: A National Multicenter Study in Brazil. BMC Public Health (2018) 18:989. doi: 10.1186/s12889-018-5859-x
38. Kanellis J, Kang D-H. Uric Acid as a Mediator of Endothelial Dysfunction, Inflammation, and Vascular Disease. Semin Nephrol (2005) 25:39–42. doi: 10.1016/j.semnephrol.2004.09.007
39. Edwards NL. The Role of Hyperuricemia in Vascular Disorders. Curr Opin Rheumatol (2009) 21:132–7. doi: 10.1097/BOR.0b013e3283257b96
40. Kang D-H, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, et al. Uric Acid Causes Vascular Smooth Muscle Cell Proliferation by Entering CellsVia a Functional Urate Transporter. Am J Nephrol (2005) 25:425–33. doi: 10.1159/000087713
41. Mazzali M, Kanellis J, Han L, Feng L, Xia Y-Y, Chen Q, et al. Hyperuricemia Induces a Primary Renal Arteriolopathy in Rats by a Blood Pressure-Independent Mechanism. Am J Physiology-Renal Physiol (2002) 282:F991–7. doi: 10.1152/ajprenal.00283.2001
42. Serné EH, Gans ROB, ter Maaten JC, Tangelder G-J, Donker AJM, Stehouwer CDA. Impaired Skin Capillary Recruitment in Essential Hypertension Is Caused by Both Functional and Structural Capillary Rarefaction. Hypertension (2001) 38:238–42. doi: 10.1161/01.HYP.38.2.238
43. Dehlin M, Jacobsson L, Roddy E. Global Epidemiology of Gout: Prevalence, Incidence, Treatment Patterns and Risk Factors. Nat Rev Rheumatol (2020) 16:380–90. doi: 10.1038/s41584-020-0441-1
44. Kuwata H, Okamura S, Hayashino Y, Tsujii S, Ishii H. For the Diabetes Distress and Care Registry at Tenri Study Group. Serum Uric Acid Levels are Associated With Increased Risk of Newly Developed Diabetic Retinopathy Among Japanese Male Patients With Type 2 Diabetes: A Prospective Cohort Study (Diabetes Distress and Care Registry at Tenri [DDCRT 13]). Diabetes Metab Res Rev (2017) 33:e2905. doi: 10.1002/dmrr.2905
45. Spaide RF, Curcio CA. Evaluation of Segmentation of the Superficial and Deep Vascular Layers of the Retina by Optical Coherence Tomography Angiography Instruments in Normal Eyes. JAMA Ophthalmol (2017) 135:259. doi: 10.1001/jamaophthalmol.2016.5327
46. Al-Sheikh M, Ghasemi Falavarjani K, Akil H, Sadda SR. Impact of Image Quality on OCT Angiography Based Quantitative Measurements. Int J Retin Vitr (2017) 3:13. doi: 10.1186/s40942-017-0068-9
Keywords: serum uric acid, optical coherence tomography angiography, retinal capillary plexus, epidemiology, sex
Citation: Yang K, Li C, Shi K, Zhu X, Xiao Y, Su B, Ju Y, Lu F, Qu J, Cui L and Li M (2022) Association of Serum Uric Acid With Retinal Capillary Plexus. Front. Endocrinol. 13:855430. doi: 10.3389/fendo.2022.855430
Received: 15 January 2022; Accepted: 15 March 2022;
Published: 12 April 2022.
Edited by:
Xiong-Fei Pan, Sichuan University, ChinaCopyright © 2022 Yang, Li, Shi, Zhu, Xiao, Su, Ju, Lu, Qu, Cui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Li, bG1AZXllLmFjLmNu; Lele Cui, Y2xsQGV5ZS5hYy5jbg==; Jia Qu, amlhLnF1QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.