- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, South Korea
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, South Korea
- 3Pituitary Center, Seoul National University Hospital, Seoul, South Korea
Objective: Metabolic complications are common in patients with acromegaly. However, this occasionally does not improve post-surgery and may be related to postoperative weight gain. We aimed to investigate the postoperative weight change and factors associated with postoperative weight gain in patients with acromegaly.
Design and Methods: Overall, 113 consecutive patients with body weight records pre- and 3–6 months post-surgery between October 2009 and March 2021 were enrolled. Patients were divided into three groups: weight loss (weight decrease ≥3%), stable, and weight gain (weight increase ≥3%). Hormone status, metabolic comorbidities, and anthropometric parameters were compared between the groups.
Results: Among 113 patients, 29 (25.7%) and 26 (23.0%) patients lost and gained weight, respectively, post-surgery. There were no significant differences in baseline characteristics, including age at diagnosis, sex, body mass index, and growth hormone levels among the three groups. The prevalence of diabetes mellitus at diagnosis was significantly higher in the weight gain group than in the other groups. Patients with diabetes (n=22) had a 5.2-fold higher risk of postoperative weight gain than those with normal glucose tolerance (n=37) (P=0.006). In the diabetes mellitus group, the percentage lean mass decreased (-4.5 [-6.6–2.0]%, P=0.002), and the percentage fat mass significantly increased post-surgery (18.0 [4.6–36.6]%, P=0.003), whereas the normal glucose tolerance group did not show body composition changes post-surgery.
Conclusion: In patients with acromegaly, 23% experienced ≥3% weight gain post-surgery. Diabetes mellitus at diagnosis is a significant predictor of weight and fat gain post-surgery.
Introduction
Acromegaly is caused by excessive growth hormone (GH) secretion, mainly by pituitary adenomas (1). Under the influence of GH and insulin-like growth factor-1 (IGF-1), patients with acromegaly show a change in appearance and have several systemic complications such as cardiovascular, respiratory, and metabolic complications (1–5). Although insulin resistance and diabetes mellitus (DM) are associated with increased adiposity, patients with acromegaly exhibit increased insulin resistance despite a decrease in fat mass and increased muscle mass. As a putative mechanism, GH has a lipolytic effect by inducing the hydrolysis of triglycerides in adipose tissues, thereby increasing the production of free fatty acids and contributing to insulin resistance (6). In addition, GH itself reduces peripheral glucose uptake in the skeletal muscle and increases hepatic glucose production through gluconeogenesis, inhibiting pancreatic β-cell function and insulin secretion (7–9).
Therefore, it was expected that complications related to acromegaly would improve when GH levels normalized after surgery. Several studies have reported improvements in hypertension, cardiomyopathy, and DM after treatment (10–12). However, hypertension, DM, and obstructive sleep apnea did not improve after treatment in some patients (13, 14). The lack of improvement in postoperative metabolic complications may be related to weight gain after surgery (14). In this regard, several studies have reported that body weight and fat mass increase after acromegaly treatment (15–19). On the other hand, some studies have shown stable weight after treatment in patients with acromegaly (10).
Herein, we aimed to investigate the change in weight and body composition after surgery and elucidate predictors of postoperative weight gain to improve long-term outcomes in patients with acromegaly.
Materials and Methods
Study Patients
We included consecutive patients diagnosed with acromegaly based on oral glucose tolerance test (OGTT) and underwent surgery between October 2009 and March 2021 at the Seoul National University Hospital. The medical records of patients who underwent surgery between October 2009 and December 2013 (n=67) were reviewed retrospectively. From 2014, we analyzed the data of patients enrolled in the Seoul National University Pituitary Disease Cohort Study (ClinicalTrials.gov, protocol ID 1503-040-654) according to the pre-established protocol (n=100). We excluded patients who lost followed up within 3 months after surgery. Of the 167 patients with postoperative follow-up data, we further excluded patients with a history of previous pituitary surgery, radiotherapy to pituitary gland, or administration of somatostatin analogs before surgery (n=5), and those without weight information after surgery (n=49). In the final analysis, a total of 113 patients were included. Clinical information was obtained before and 3–6 months after surgery.
Weight gain was defined as an increase of ≥3% compared to before surgery, weight loss was defined as a decrease of ≥3%, and the stable weight was defined as weight change falling between these parameters. DM was defined as having an HbA1c level of ≥6.5% or use of antidiabetic drugs; prediabetes was defined as an HbA1c level of 5.7–6.5%, and normal glucose tolerance (NGT) was defined as HbA1c level of <5.7%. In addition, other comorbidities, such as hypertension, dyslipidemia, cardiovascular disease, stroke, osteoporosis, sleep apnea, arthralgia, and carpal tunnel syndrome, were defined based on medical records.
Anthropometric Measure/Body Composition Analysis
Anthropometric measurements were measured using bioelectrical impedance analysis (Inbody 970, Seoul, South Korea). Height (cm), body weight (kg), waist circumference (cm), hip circumference (cm), lean mass (kg), and fat mass (kg) were measured. In addition, body mass index (BMI) (kg/m2), waist-to-hip ratio, percentage lean mass (%), percentage fat mass (%), and visceral fat area (cm2) were calculated. The percentage lean mass (%) and percentage fat mass (%) are the ratios of lean mass and fat mass to body weight, respectively.
Hormone Assessment/Other Clinical Parameters
Hormone assessment was performed before surgery and 3–6 months after surgery. An OGTT was performed by measuring glucose and GH before, 1 and 2 h after administering 75 g glucose. Nadir GH levels were the lowest measured GH during the OGTT; IGF-1 levels were expressed as absolute values or the fold-change of the upper limit of the age- and sex-specific normal range (ULN). The age-specific reference ranges of IGF-1 were: 21–30 years, 232–385 ng/mL; 31–40 years, 177–382 ng/mL; 41–50 years, 124–290 ng/mL; 51–60 years, 71–263 ng/mL; 61–70 years, 94–269 ng/mL, 71–80 years, 76–160 ng/mL. Acromegaly was diagnosed based on the non-suppressed GH response on the OGTT and a high IGF-1 level above the age- and sex-specific reference range (20). Remission was defined as GH levels suppressed to <1 ng/mL during the OGTT, and IGF-1 within the age- and sex-specific reference range (21).
TSH (thyroid-stimulating hormone) deficiency was defined as a low or normal TSH level (reference rage, 0.4–4.1 μIU/mL) with low free T4 (<0.70 ng/dL). Adrenocorticotropic hormone (ACTH) deficiency was defined as a peak cortisol level <18 μg/dL after a short Synacthen test, or when ACTH levels were low or in the normal range (reference range, 10-65 pg/mL) with a low morning cortisol level (≤5 μg/dL). Normal menopause was defined as follicle-stimulating hormone (FSH) levels >30 mIU/mL with estradiol (E2) <50 pg/mL. FSH/luteinizing hormone (LH) deficiency was defined as testosterone or E2 levels were lower than normal with low or normal FSH and LH levels. Furthermore, central diabetes insipidus (CDI) was defined as requiring desmopressin administration.
Fasting plasma glucose, insulin, and total cholesterol levels were measured after fasting for more than 8 hours. In addition, 1-hour and 2-hour-postprandial glucose levels were measured 1 and 2 hours after OGTT, respectively. A homeostatic model assessment for insulin resistance (HOMA-IR) was obtained by dividing the product of fasting insulin (mIU/L) and fasting blood glucose (mg/dL) by 405 (22). Systolic and diastolic blood pressures were defined as the average of three blood pressures measured after hospitalization for hospitalized patients.
Maximal tumor size was defined as the largest size among width, length and height on T1-weighted contrast-enhanced magnetic resonance imaging.
Hormone Assay
GH was measured with an immunoradiometric assay kit (Izotop, Budapest, Hungry), and the intra- and inter-assay coefficient of variation (CVs) were 1.5–3.5% and 2.5–3.3%, respectively. IGF-1 was also measured with an immunoradiometric assay kit (Beckman Coulter, Brea, California, USA), the intra- and inter-assay CVs were <5.6% and <8.3%, respectively. The lowest detectable levels of GH and IGF-1 were 0.02 ng/mL and 4.55 ng/mL, respectively: we used the World Health Organization international standard measurement for GH (98/574) and IGF-1 (91/554). IGF-1 was expressed as a multiple of the upper limit of the normal. LH, FSH, E2, testosterone, prolactin, free T4, TSH, ACTH, and cortisol levels were measured between 8 and 10 am by radioimmunoassay and immunoradiometric assay.
Surgical Procedures
Details of the surgical procedures were similar to those in our previous report (23). The operation was initiated through the binostrial pure endonasal or transseptal route without resection of the turbinates or posterior septectomy with an endoscope. Extracapsular dissection was the standard procedure, and sharp dissection with microdissectors was performed in case without a definite pseudocapsule.
Statistical Analysis
Continuous variables with a normal distribution are expressed as mean and standard deviation, and variables with a non-normal distribution are expressed as median [interquartile range (IQR)]. Repeated-measures analysis of variance (ANOVA) and the Bonferroni multiple comparison test for post hoc analyses (the Friedman test and Dunn multiple comparison test for post hoc analyses were used to analyze non-normally distributed continuous variables) were used to compare patients according to weight change and glycemic status. Changes in clinical information before and after surgery were analyzed using paired t-test and Wilcoxon signed-rank test. The correlation between weight change and clinical information was analyzed using Pearson correlation analysis for parametric analysis and Spearman analysis for non-parametric analysis. The relationship between glycemic status and weight gain was analyzed using logistic regression analysis. Statistical significance was set at P <0.05. SPSS for Windows version 25.0 (IBM Co., Armonk, NY, USA) and Prism 5 for Windows version 5.03 (GraphPad Software, San Diego, CA, USA) were used for statistical analysis.
Ethical Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The trial protocol was approved by the Institutional Review Board (IRB) (No. H-2104-126-1213). The requirement for informed patient consent was waived by the IRB as this was a retrospective study, and analyses were performed using de-identified data.
Results
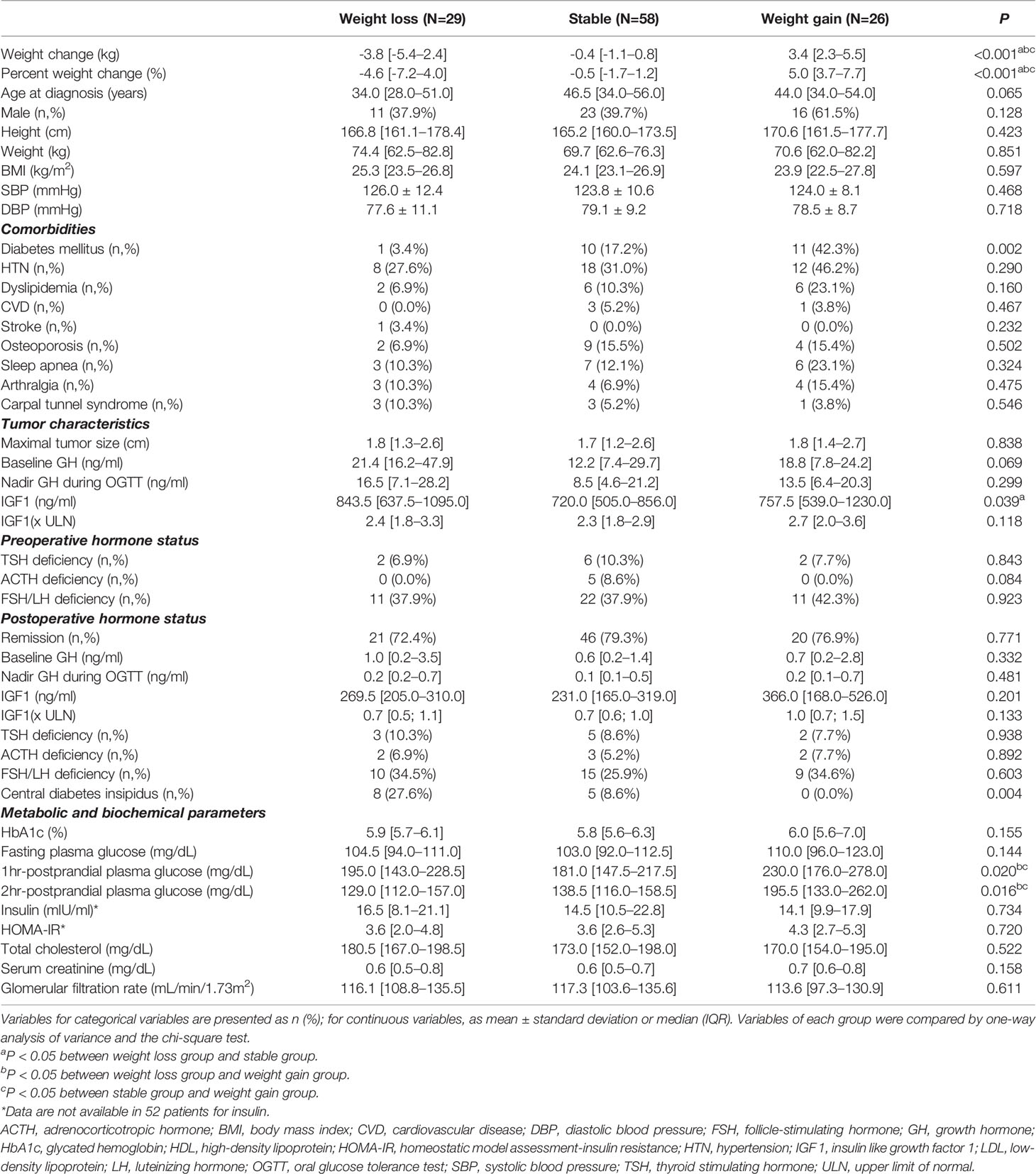
Baseline characteristics of the patients were compared by weight change: weight loss, stable weight, and weight gain (Table 1). There were no significant differences in age, sex, preoperative weight and BMI among the three groups. The prevalence of DM was highest in the weight gain group among the three groups (42.3%, 17.2%, and 3.4%, respectively, P=0.002). In contrast, the prevalence of other comorbidities, such as hypertension, dyslipidemia, cardiovascular disease, stroke, osteoporosis, sleep apnea, arthralgia, and carpal tunnel syndrome, did not differ among the three groups. There were no significant differences in tumor characteristics, including tumor size, GH, and IGF-1 levels (× ULN), among the three groups. However, the absolute level of IGF-1 (ng/mL) of the weight loss group was significantly higher than that of the stable group (843.5 ng/mL [637.5–1095.0 ng/mL] vs. 720.0 ng/mL [505.0–856.0 ng/mL], P=0.039). The remission rates and pre-and postoperative hormone status were similar among the three groups, and body weight change was not influenced by whether remission was achieved. More patients with CDI belonged to the weight loss group compared to the weight gain group (27.6% vs. 0.0%, P=0.004).
When the metabolic and biochemical parameters of the three groups were compared, HbA1c, fasting plasma glucose, and insulin resistance (HOMA-IR) were not significantly different between the three groups. However, 1 h- and 2 h-postprandial plasma glucose levels were significantly higher in the weight gain group than in the other two groups. Baseline body composition did not differ among the three groups (Supplemental Table 1).
We further conducted a correlation analysis between the percent weight change and clinical parameters (Figure 1 and Supplemental Table 2). Postoperative weight change was positively correlated with the age at diagnosis (r=0.25, P=0.023). However, there was no correlation between preoperative weight, BMI, GH levels, IGF-1 levels, and weight gain. In contrast, HbA1c (r=0.47, P<0.001), fasting plasma glucose (r=0.34, P=0.001), 1 h-postprandial plasma glucose (r=0.39, P<0.001), and 2 h-postprandial plasma glucose (r=0.44, P<0.001) were significantly correlated with postoperative weight change. However, HOMA-IR was not related to postoperative weight changes.

Figure 1 Correlation analysis between percent weight change and other parameters. The correlation between percent weight change and clinical parameters was analyzed by Pearson and Spearman correlation analysis. (A) Age at diagnosis, (B) baseline HbA1c, (C) FPG, (D) PP1, and (E) PP2 were significantly positively correlated with percentage weight change in acromegalic patients. FPG, fasting plasma glucose; PP1, 1hr-postprandial plasma glucose; PP2, 2hr-postprandial plasma glucose.
Since the presence of DM at baseline was the only significantly different variable among the three acromegaly groups according to weight change, we further investigated the weight and body composition parameters according to the glycemic status at baseline. The prevalence of prediabetes and DM was 47.8 and 19.5%, respectively. Weight gain after surgery was significantly higher in the DM group than the prediabetes and NGT groups (3.0% [-1.0%–7.2%], -0.9% [-4.0%–1.8%], and -1.2% [-2.7–1.7%], respectively), although there was no significant difference in preoperative body weight among the three groups (Figure 2). We further performed logistic regression analysis for postoperative weight gain according to the glycemic status (Table 2). The risk for postoperative weight gain was significantly higher in the DM group than in the NGT group after adjustment for age, IGF-1, and postoperative CDI (OR 7.65 [1.81–32.4]). Moreover, a 1% increase in HbA1c elevated the risk of postoperative weight gain by 1.6-fold.
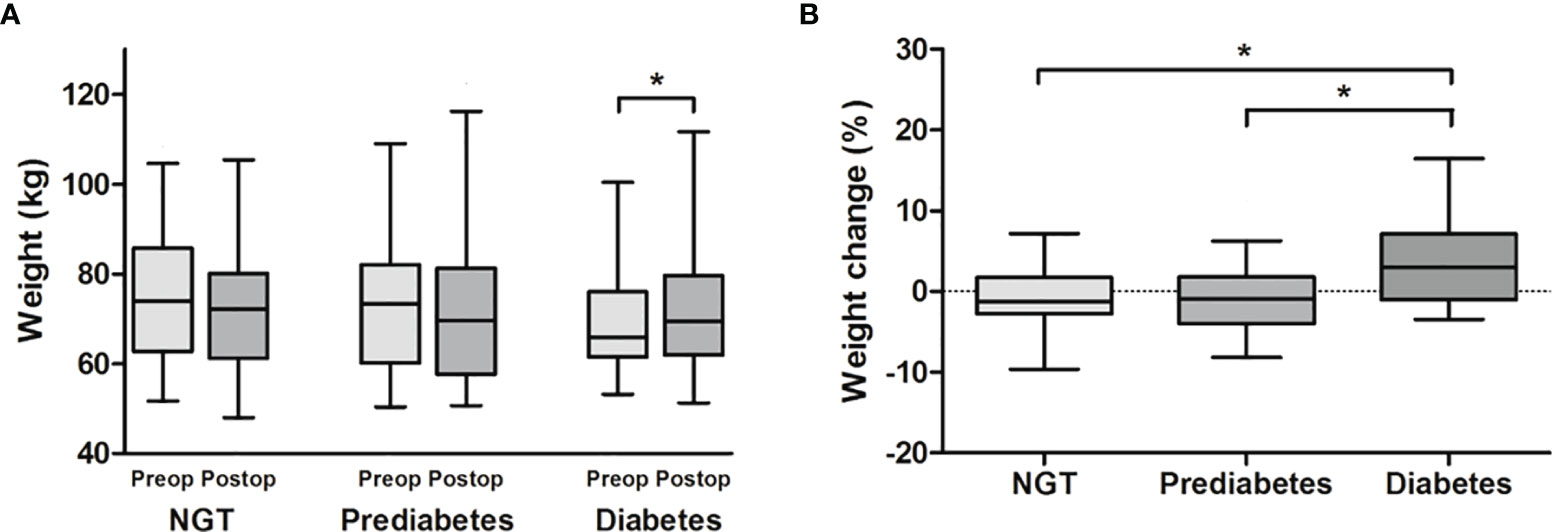
Figure 2 Comparison of (A) weight and (B) weight change according to the glycemic status at baseline. Acromegaly patients were divided into NGT, prediabetes, and diabetes groups. (A) Body weight before and after surgery was compared by paired t-test, and (B) postoperative weight change (%) were compared by one-way analysis of variance. There was a significant weight gain after surgery only in the diabetes group. Postoperative weight gain (%) was significantly greater in the diabetes group than in the NGT and prediabetes groups, respectively. *P < 0.05 between each group. NGT, normal glucose tolerance.
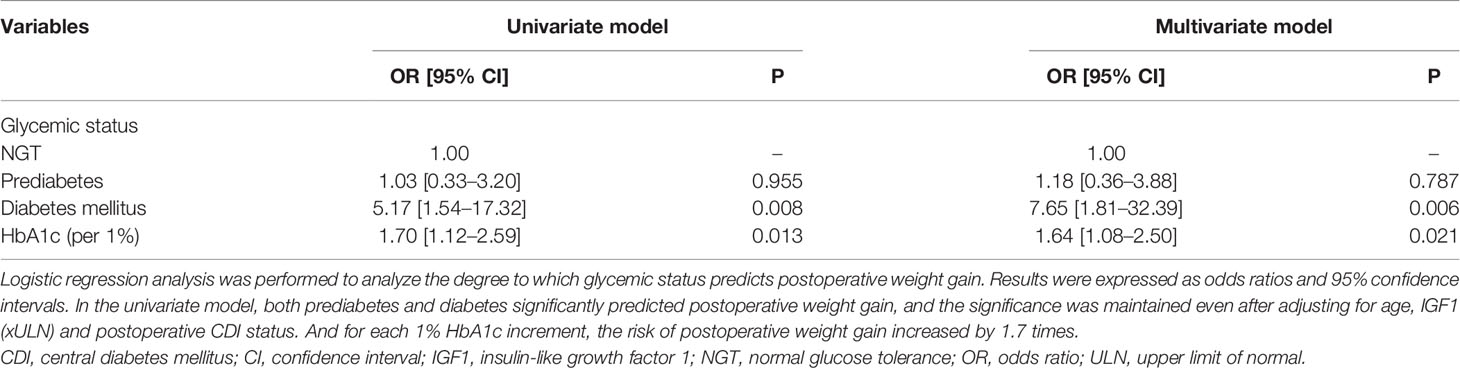
Table 2 Odds ratios of the prediabetes and diabetes group for risk of postoperative weight gain compared with the NGT group.
As shown in Table 3, baseline GH, nadir GH during OGTT, and IGF-1 in all three groups decreased significantly after surgery. In the prediabetes and DM groups, HbA1c and fasting plasma glucose levels significantly improved after surgery. When comparing the postoperative changes in body composition among the three groups (Table 4), waist circumference significantly increased only in the DM group; however, the waist-to-hip ratio increased in the prediabetes and DM groups. The lean mass and percentage lean mass decreased in the prediabetes and DM groups, while the fat mass, percentage fat mass, and visceral fat area significantly increased after surgery. However, the NGT group did not show any changes in body composition pre- and post-surgery.

Table 3 Change of metabolic parameters and body composition in patients with acromegaly according to the glycemic status at baseline.

Table 4 Postoperative change of body composition parameters in patients with acromegaly according to the glycemic status at baseline.
Discussion
In the present study, 23% of patients with acromegaly experienced a weight gain of ≥ 3% after surgery. The predictors for postoperative weight gain were DM at diagnosis and the absence of CDI. Further, the degree of weight gain after surgery was positively correlated with the baseline glycemic status in patients with acromegaly. Moreover, increased fat mass, i.e., visceral fat mass, contributed to postoperative weight gain in patients with prediabetes and DM despite decreased lean mass.
Previous studies have reported that biochemical control after surgery leads to increased fat mass and decreased muscle mass despite improved glycemic status and insulin resistance in patients with acromegaly (15, 16, 24). This study showed similar changes in body weight and composition postoperatively. The median weight change before and after surgery was 0.5% (-3.2 ~ 2.7%), and percentage fat mass and lean mass were 22.4 (10.9–43.6%) and -4.9% (-6.6% –2.1%), respectively, in all patients.
As an anabolic hormone, GH affects glucose and lipid metabolism, counteracting insulin action. GH increases free fatty acids in the blood by inducing lipolysis and lipid oxidation, which reduces glucose uptake in peripheral tissues (25). Therefore, subcutaneous and visceral fat mass is reduced, and intramuscular fat mass is increased in patients with active acromegaly (19, 26). Elevated free fatty acids also exhibit oxidative stress and proinflammatory effects (27). Chronic inflammation caused by free fatty acids can result in insulin resistance; this occurs by disrupting the insulin signaling pathway by degrading the phosphorylated insulin receptor substate-1 (28–30). Therefore, patients with acromegaly exhibit increased insulin resistance despite a decrease in fat mass and an increase in muscle mass. Surgical intervention for excessive GH, which lowers the elevated resting energy expenditure and lipolysis rates, may explain increases in weight and fat mass (17).
Some studies have identified that GH or IGF-1 levels or hypogonadism are correlated with fat and lean mass. However, this is inconsistent with our study findings (17, 24). The remission rate and postoperative hormone status, including nadir GH and IGF-1 levels and hypogonadism, were not different among the three groups in our study. Hence, other factors may contribute to weight gain after surgery in patients with acromegaly.
The present study shows that DM at diagnosis and glycemic status significantly contributed to changes in body composition after surgery. In addition, age at diagnosis was correlated with postoperative weight gain in our study. In acromegaly, insulin resistance due to the insulin-antagonistic effects of excess GH is compensated for by an increase in β-cell function and insulin secretion (7). However, prolonged disease duration may result in the exhaustion of β-cell secretory function and the development of overt DM in patients with acromegaly. We also observed that insulin levels were lower in patients with DM (Supplemental Table 3). Therefore, DM can persist in patients with acromegaly, even after surgical remission, due to the irreversible change in insulin secretion and sensitivity (13, 31). Hence, older patients with DM and acromegaly may exhibit the typical body composition phenotype of metabolic syndrome since ‘metabolic memory’ remains in patients with DM (32). Likewise, insulin resistance may persist even after normalizing GH in acromegalic patients with DM. In addition, hyperinsulinemia induced by insulin resistance may increase fat due to the anabolic effect of insulin (33).
Furthermore, an imbalance in adipokines in patients with DM may increase adiposity (34, 35). Ghrelin and leptin levels decrease, while adiponectin levels increase in patients with acromegaly, reflecting a decrease in healthy adipose tissue (15, 25, 36). After surgery, ghrelin levels increase due to GH normalization and improved insulin sensitivity, but leptin levels also increase, which is associated with the development of insulin resistance (15, 37). Increased adiponectin levels after surgery may be associated with an insulin-sensitizing effect in patients with acromegaly (38). Therefore, the imbalance of adipokines may be further altered in patients with DM and acromegaly, who may present with more significant weight gain after surgery (36).
On the other hand, it is controversial whether fat mass gain after disease control directly contributes to increased cardiovascular risk. Some reports have revealed no cardiovascular events after the treatment of acromegaly (21). However, other studies have noted that complications such as hypertension, DM, and obstructive sleep apnea did not improve after treating acromegaly (13, 14). Increased visceral and subcutaneous fat can offset the effect of improvement of metabolic complications caused by decreased GH in patients with acromegaly. Moreover, subcutaneous fat accumulation may aggravate facial and musculoskeletal disfigurement, which impairs quality of life and body image perception in patients with acromegaly (39).
This study has several limitations. As a retrospective study, there were missing values of evaluation items such as insulin and body composition. The number of study subjects was small, and the follow-up period was short. We used bioimpedance analysis rather than dual-energy X-ray absorptiometry to assess body composition. Bioimpedance analysis assumes a constant hydration status, but excess GH results in water retention and edema. Thus, skeletal muscle mass assessment may not be accurate, but fat mass assessment may be acceptable as it is not affected by water content (40). In addition, HbA1c and fasting plasma glucose levels were not significantly different among the three groups, although they were significantly related to the postoperative weight change. This might be attributed to the small number of subjects per group, i.e., the weight gain. Although it was not statistically significant, HbA1c and fasting plasma glucose levels were higher in the weight gain group. Antidiabetic medications can affect postoperative weight change. However, due to the low prevalence of DM and the use of antidiabetic medications in our study subjects (n=14), the effect of antidiabetic medications on body composition or insulin resistance could not be analyzed. Finally, although we assumed that the development of diabetes in acromegalic patients might be attributed to disease duration and individual responsiveness to GH as well as GH excess, we failed to explain the exact mechanism for more postoperative weight gain in patients with acromegaly and diabetes.
However, a strength of the present study is the identification of the high-risk group for weight gain after surgery in patients with acromegaly. Patients with acromegaly and DM have a greater postoperative weight gain and are prone to increasing fat mass, i.e., visceral fat. Although DM improved after disease control of acromegaly, there is a residual cardiovascular risk and impaired quality of life due to weight and fat gain after surgery. Accordingly, preoperative intensive glycemic control and postoperative weight control should be emphasized for patients with acromegaly and DM for better long-term prognosis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Seoul National University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception or design: JHK. Acquisition, analysis, or interpretation of data: HNJ and JHK. Drafting the work or revising: HNJ, YHK, and JHK. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the grant (No.: 2017R1D1A1B03031879 to JHK) from National Research Foundation by the Ministry of Science, ICT and Future Planning, Republic of Korea.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The biospecimens in this study were provided by the Biobank of Seoul National University Hospital, a member of the Korea Biobank Network. We also thank Serena Park for collecting data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.854931/full#supplementary-material
References
1. Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, et al. Acromegaly. Nat Rev Dis Primers (2019) 5:20. doi: 10.1038/s41572-019-0071-6
2. Pivonello R, Auriemma RS, Grasso LF, Pivonello C, Simeoli C, Patalano R, et al. Complications of Acromegaly: Cardiovascular, Respiratory and Metabolic Comorbidities. Pituitary (2017) 20:46–62. doi: 10.1007/s11102-017-0797-7
3. Cittadini A, Stromer H, Katz SE, Clark R, Moses AC, Morgan JP, et al. Differential Cardiac Effects of Growth Hormone and Insulin-Like Growth Factor-I in the Rat. A Combined In Vivo and In Vitro Evaluation. Circulation (1996) 93:800–9. doi: 10.1161/01.cir.93.4.800
4. Lu C, Schwartzbauer G, Sperling MA, Devaskar SU, Thamotharan S, Robbins PD, et al. Demonstration of Direct Effects of Growth Hormone on Neonatal Cardiomyocytes. J Biol Chem (2001) 276:22892–900. doi: 10.1074/jbc.M011647200
5. Feld S, Hirschberg R. Growth Hormone, the Insulin-Like Growth Factor System, and the Kidney. J Clin Endocrinol Metab (1996) 5:423–80. doi: 10.1210/edrv-17-5-423
6. Colao A, Ferone D, Marzullo P, Lombardi G. Systemic Complications of Acromegaly: Epidemiology, Pathogenesis, and Management. Endocr Rev (2004) 25:102–52. doi: 10.1210/er.2002-0022
7. Kasayama S, Otsuki M, Takagi M, Saito H, Sumitani S, Kouhara H, et al. Impaired Beta-Cell Function in the Presence of Reduced Insulin Sensitivity Determines Glucose Tolerance Status in Acromegalic Patients. Clin Endocrinol (Oxf) (2000) 52:549–55. doi: 10.1046/j.1365-2265.2000.00986.x
8. Moller N, Jorgensen JO. Effects of Growth Hormone on Glucose, Lipid, and Protein Metabolism in Human Subjects. Endocr Rev (2009) 30:152–77. doi: 10.1210/er.2008-0027
9. Frara S, Maffezzoni F, Mazziotti G, Giustina A. Current and Emerging Aspects of Diabetes Mellitus in Acromegaly. Trends Endocrinol Metab (2016) 27:470–83. doi: 10.1016/j.tem.2016.04.014
10. Jaffrain-Rea ML, Minniti G, Moroni C, Esposito V, Ferretti E, Santoro A, et al. Impact of Successful Transsphenoidal Surgery on Cardiovascular Risk Factors in Acromegaly. Eur J Endocrinol (2003) 148:193–201. doi: 10.1530/eje.0.1480193
11. Colao A, Cuocolo A, Marzullo P, Nicolai E, Ferone D, Della Morte AM, et al. Is the Acromegalic Cardiomyopathy Reversible? Effect of 5-Year Normalization of Growth Hormone and Insulin-Like Growth Factor I Levels on Cardiac Performance. J Clin Endocrinol Metab (2001) 86:1551–7. doi: 10.1210/jcem.86.4.7376
12. Ernaga Lorea A, Eguílaz Esparza N, Ollero García-Agulló MD, García Mouriz M, Iriarte Beroiz A, Rodríguez Erdozain R. Glucose Metabolism Before and After Treatment in Patients With Acromegaly. Endocrinología Diabetes y Nutrición (English ed) (2017) 64:363–8. doi: 10.1016/j.endinu.2017.05.010
13. Gonzalez B, Vargas G, de Los Monteros ALE, Mendoza V, Mercado M. Persistence of Diabetes and Hypertension After Multimodal Treatment of Acromegaly. J Clin Endocrinol Metab (2018) 103:2369–75. doi: 10.1210/jc.2018-00325
14. Annamalai AK, Webb A, Kandasamy N, Elkhawad M, Moir S, Khan F, et al. A Comprehensive Study of Clinical, Biochemical, Radiological, Vascular, Cardiac, and Sleep Parameters in an Unselected Cohort of Patients With Acromegaly Undergoing Presurgical Somatostatin Receptor Ligand Therapy. J Clin Endocrinol Metab (2013) 98:1040–50. doi: 10.1210/jc.2012-3072
15. Reyes-Vidal C, Fernandez JC, Bruce JN, Crisman C, Conwell IM, Kostadinov J, et al. Prospective Study of Surgical Treatment of Acromegaly: Effects on Ghrelin, Weight, Adiposity, and Markers of CV Risk. J Clin Endocrinol Metab (2014) 99:4124–32. doi: 10.1210/jc.2014-2259
16. Ronchi CL, Varca V, Beck-Peccoz P, Orsi E, Donadio F, Baccarelli A, et al. Comparison Between Six-Year Therapy With Long-Acting Somatostatin Analogs and Successful Surgery in Acromegaly: Effects on Cardiovascular Risk Factors. J Clin Endocrinol Metab (2006) 91:121–8. doi: 10.1210/jc.2005-1704
17. Reyes-Vidal CM, Mojahed H, Shen W, Jin Z, Arias-Mendoza F, Fernandez JC, et al. Adipose Tissue Redistribution and Ectopic Lipid Deposition in Active Acromegaly and Effects of Surgical Treatment. J Clin Endocrinol Metab (2015) 100:2946–55. doi: 10.1210/jc.2015-1917
18. Jorgensen JO, Moller L, Krag M, Billestrup N, Christiansen JS. Effects of Growth Hormone on Glucose and Fat Metabolism in Human Subjects. Endocrinol Metab Clin North Am (2007) 36:75–87. doi: 10.1016/j.ecl.2006.11.005
19. Brummer RJ, Lönn L, Kvist H, Grangård U, Bengtsson BA, Sjöström L. Adipose Tissue and Muscle Volume Determination by Computed Tomography in Acromegaly, Before and 1 Year After Adenomectomy. Eur J Clin Invest (1993) 23:199–205. doi: 10.1111/j.1365-2362.1993.tb00762.x
20. Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, et al. A Consensus Statement on Acromegaly Therapeutic Outcomes. Nat Rev Endocrinol (2018) 14:552–61. doi: 10.1038/s41574-018-0058-5
21. Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, et al. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J Clin Endocrinol Metab (2020) 105:e937–46. doi: 10.1210/clinem/dgz096
22. Matthews DR, Hosker J, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia (1985) 28:412–9. doi: 10.1007/BF00280883
23. Kim JH, Hur KY, Lee JH, Lee JH, Se YB, Kim HI, et al. Outcome of Endoscopic Transsphenoidal Surgery for Acromegaly. World Neurosurg (2017) 104:272–8. doi: 10.1016/j.wneu.2017.04.141
24. Bredella MA, Schorr M, Dichtel LE, Gerweck AV, Young BJ, Woodmansee WW, et al. Body Composition and Ectopic Lipid Changes With Biochemical Control of Acromegaly. J Clin Endocrinol Metab (2017) 102:4218–25. doi: 10.1210/jc.2017-01210
25. Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The Effects of Growth Hormone on Adipose Tissue: Old Observations, New Mechanisms. Nat Rev Endocrinol (2020) 16:135–46. doi: 10.1038/s41574-019-0280-9
26. Freda PU, Shen W, Heymsfield SB, Reyes-Vidal CM, Geer EB, Bruce JN, et al. Lower Visceral and Subcutaneous But Higher Intermuscular Adipose Tissue Depots in Patients With Growth Hormone and Insulin-Like Growth Factor I Excess Due to Acromegaly. J Clin Endocrinol Metab (2008) 93:2334–43. doi: 10.1210/jc.2007-2780
27. Devjit Tripathy, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects. Diabetes (2003) 52:2882–7. doi: 10.2337/diabetes.52.12.2882
28. Sears B, Perry M. The Role of Fatty Acids in Insulin Resistance. Lipids Health Dis (2015) 14:121. doi: 10.1186/s12944-015-0123-1
29. Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin Effects in Muscle and Adipose Tissue. Diabetes Res Clin Pract (2011) 93:S52–9. doi: 10.1016/S0168-8227(11)70014-6
30. Russell-Jones D, Khan R. Insulin-Associated Weight Gain in Diabetes–Causes, Effects and Coping Strategies. Diabetes Obes Metab (2007) 9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x
31. Alexopoulou O, Bex M, Kamenicky P, Mvoula AB, Chanson P, Maiter D. Prevalence and Risk Factors of Impaired Glucose Tolerance and Diabetes Mellitus at Diagnosis of Acromegaly: A Study in 148 Patients. Pituitary (2014) 17:81–9. doi: 10.1007/s11102-013-0471-7
32. Miller RG, Orchard TJ. Understanding Metabolic Memory: A Tale of Two Studies. Diabetes (2020) 69:291–9. doi: 10.2337/db19-0514
33. Williams KJ, Wu X. Imbalanced Insulin Action in Chronic Over Nutrition: Clinical Harm, Molecular Mechanisms, and a Way Forward. Atherosclerosis (2016) 247:225–82. doi: 10.1016/j.atherosclerosis.2016.02.004
34. Kim WK, Bae KH, Lee SC, Oh KJ. The Latest Insights Into Adipokines in Diabetes. J Clin Med (2019) 8. doi: 10.3390/jcm8111874
35. Oh KJ, Lee DS, Kim WK, Han BS, Lee SC, Bae KH. Metabolic Adaptation in Obesity and Type II Diabetes: Myokines, Adipokines and Hepatokines. Int J Mol Sci (2016) 18. doi: 10.3390/ijms18010008
36. Olarescu NC, Bollerslev J. The Impact of Adipose Tissue on Insulin Resistance in Acromegaly. Trends Endocrinol Metab (2016) 27:226–37. doi: 10.1016/j.tem.2016.02.005
37. Andrade-Oliveira V, Camara NO, Moraes-Vieira PM. Adipokines as Drug Targets in Diabetes and Underlying Disturbances. J Diabetes Res (2015) 2015:681612. doi: 10.1155/2015/681612
38. Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin Serum Levels Correlate With Insulin Resistance in Type 2 Diabetic Patients. Saudi Pharm J (2015) 23:250–6. doi: 10.1016/j.jsps.2014.11.011
39. Sibeoni J, Manolios E, Verneuil L, Chanson P, Revah-Levy A. Patients’ Perspectives on Acromegaly Diagnostic Delay: A Qualitative Study. Eur J Endocrinol (2019) 180:339–52. doi: 10.1530/EJE-18-0925
Keywords: acromegaly, growth hormone, fat metabolism, insulin resistance, adipokine
Citation: Jang HN, Kim YH and Kim JH (2022) Diabetes Mellitus Predicts Weight Gain After Surgery in Patients With Acromegaly. Front. Endocrinol. 13:854931. doi: 10.3389/fendo.2022.854931
Received: 14 January 2022; Accepted: 09 February 2022;
Published: 09 March 2022.
Edited by:
Marek Bolanowski, Wroclaw Medical University, PolandReviewed by:
Gherardo Mazziotti, University of Milan, ItalyLuiz Augusto Casulari, University of Brasilia, Brazil
Copyright © 2022 Jang, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung Hee Kim, amhreGluZ2Z1QGdtYWlsLmNvbQ==; amhlZTFAc251LmFjLmty
 Han Na Jang1
Han Na Jang1 Jung Hee Kim
Jung Hee Kim