- 1Department of Obstetrics and Gynaecology, Hospital Sultan Ismail, Johor Bahru, Malaysia
- 2Advanced Reproductive Centre, Universiti Kebangsaan Malaysia (UKM) Medical Centre, Kuala Lumpur, Malaysia
- 3Department of Obstetrics and Gynaecology, Universiti Kebangsaan Malaysia (UKM) Medical Centre, Kuala Lumpur, Malaysia
- 4Department of Public Health, Universiti Kebangsaan Malaysia (UKM) Medical Centre, Kuala Lumpur, Malaysia
The Polycystic Ovary Syndrome Questionnaire is a reliable instrument for measuring health-related quality of life. This study aimed to develop a Malay version of the Polycystic Ovary Syndrome Questionnaire and to evaluate the health-related impact of Malaysian women with polycystic ovary syndrome. The participants were women who were diagnosed with polycystic ovary syndrome using Rotterdam criteria in a gynecology clinic. Reliability was determined by internal consistency using Cronbach’s coefficient alpha and test–retest reliability using an intra-class correlation coefficient. Validity was assessed through convergent and discriminant validity. Examining the correlation between similar content of the Malay version of the Polycystic Ovary Syndrome Questionnaire and the SF-36 assessed the convergent validity. The discriminant validity was assessed using the known group comparison. Cronbach’s alpha coefficient was over 0.70 for the total scale and over 0.60 for each subscale. Known group comparison supported the discriminant validity. The Malay version of the Polycystic Ovary Syndrome Questionnaire differentiated between the subgroups of women who differed in polycystic ovary syndrome-specific symptoms. Convergent validity was consistent with the good positive correlation between related subscales of the two instruments. Polycystic ovary syndrome women in Malaysia scored the lowest for the weight (3.74) and infertility (3.41) domains, thereby indicating worse health status in these domains. Body hair (5.42) was the least troublesome for the local population. The Malay version of the Polycystic Ovary Syndrome Questionnaire is a reliable and valid tool for assessing the health-related quality of life among women in the local population. It can be used to objectively assess the quality of life among Malaysian women with polycystic ovary syndrome and evaluate their responsiveness to treatment modalities.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine condition that affects women, with an estimated prevalence of 10–15% (1–3). Due to its heterogeneity, PCOS may manifest as a broad-spectrum feature with varying severity. It is commonly associated with menstrual cycle disturbances, hirsutism, acne, alopecia, obesity, and fertility issues (4–7). The introduction of Rotterdam criteria, which consist of amenorrhea or oligomenorrhea, hyperandrogenism features, and sonographic evidence of polycystic ovaries, allowed the exclusion of other possible causes of menstrual disturbance and androgen excess. Women with PCOS may show profound general health implications, mainly those related to psychological wellbeing, and these are usually neglected by clinicians (8–10).
The symptoms that are typically associated with PCOS have led to a significant reduction in health-related quality of life (HRQoL) (11–14). The affected aspects of HRQoL differ individually, as each woman may present with different concerns related to PCOS. Adult women are mainly concerned about subfertility issues and hirsutism, whereas adolescents are more likely to worry about acne and weight gain (15–18). The development of a disease-specific questionnaire, such as the Polycystic Ovarian Syndrome Questionnaire (PCOSQ) by Cronin et al., has led to the proper assessment of specific issues affecting women with PCOS. The lowest score from each subscale reflected a poor quality of life (19).
The impact of PCOS on the quality of life varied among women from different populations and backgrounds worldwide despite its specificity. The PCOSQ was found to be reliable among Iranian and Arab populations, but it was lacking in the menstrual problem domain. In the UK and Chinese populations, all domains were valid, reliable, and culturally acceptable; however, it can be improved by adding the acne dimension (20–24). Therefore, a translation of the PCOSCQ is compulsory for adjusting the cultural and language differences in various countries.
The validation of the Malay version has not been conducted; hence, no data are available on the health-related QoL of the Malaysian population. The aim of this study was to demonstrate the reliability and validity of the Malay version of PCOSQ. Furthermore, this questionnaire would allow comparisons of results between different regions, thereby leading to the improvement of the HRQoL of women with PCOS.
Materials and Methods
Study Design
A prospective cohort study was conducted at the University Kebangsaan Malaysia Medical Centre (UKMMC) from June 2018 to June 2019. All women aged 18 to 45 years old who were diagnosed with PCOS following the Rotterdam criteria were recruited. Women with psychiatric illnesses were excluded.
Study Tools
The original PCOSQs were published in the Journal of Clinical Endocrinology and Metabolism by The Endocrine Society in 1998 (19). The permission to translate the original questionnaires was obtained through the Copyright Clearance Center. Two Malay translators from the Malaysian National Institute of Translations who were competent in English translated the PCOSQ into the Malay language independently. Following the forward translation, a discussion was held between the translators to develop a single Malay version. Afterward, two other translators who were both blinded from the original version performed a backward translation from Malay to English. The original English version and the translated Malay version were assessed, and no major discrepancy was identified. A pilot study was conducted on 20 women to assess their response and understanding of each question and to determine the duration of completing the questionnaire.
SF-36v2
The SF-36v2 is the latest version of SF36. It is a generic indicator of health status and has been applied to the assessment of outcomes of various health conditions in the general population. The instrument has been tested for validity and reliability. The SF-36v2 measured and included eight subscales as follows: physical functioning, role limitations due to physical problems, bodily pain, general health perception, social functioning, role limitations due to emotional problems, vitality, and mental health. The score for each subscale ranged from 0 to 100. A higher score indicated a better condition. A Malay version is available; it is deemed reliable and valid for use (Appendix 1).
Mal-PCOSQ
The original PCOS health-related quality of life questionnaire is in English; it was published by Cronin et al. (19) in 1998 in the USA. It includes 26 items in five domains, and it takes 10–15 min to complete. The domains are divided into emotions (8 items), body hair (5 items), weight (5 items), infertility (4 items), and menstrual problems (4 items). Each question is associated with a 7-point scale, in which 7 represents optimal function and 1 represents the poorest function. The analysis from this study found that this PCOSQ is easy to understand and use. It is recommended that investigators weigh the items equally and present the results as the mean score per item for each of the domains (Appendix 2).
Data Collection
Patients were given an information sheet regarding the study, and they provided their consent. The patients’ demographic data and the details of their menstrual and infertility history were obtained. Body mass index was calculated from the measurement of weight and height. Hirsutism is assessed clinically by using a pictorial scoring system of nine areas of the body following the modified Ferriman–Gallwey score. A score of 0 indicated no terminal hair, whereas a score of 4 indicated severe hirsutism. A total score of 7 or more signified hirsutism. Eligible women were asked to complete the sets of questionnaires that included SF-36v2 and Mal-PCOSQ while waiting to see the doctor. The collected questionnaires were checked for completeness.
Statistical Analysis
The reliability of the Mal-PCOSQ was assessed by internal consistency using Cronbach’s alpha. A value of >0.70 was considered acceptable. Discriminant validity was assessed using correlation analysis between Mal-PCSOQ and known group comparisons in body hair using the Ferriman–Gallwey score, menstrual irregularities, subfertility, and body mass index. To test for convergent validity, an estimation was calculated between Mal-PCOSQ and SF-36v2 by using Pearson’s correlation, as both are reliable tools for measuring the quality of life. It was hypothesized that a strong correlation exists between the emotional disturbance domains of the Mal-PCOSQ and the role of emotion and mental health domains of SF-36v2. A correlation value of >0.40 was considered good. For predictors of a low score, a logistic regression analysis will be used for categorical independent variables, whereas continuous data will be analyzed using linear regression. For descriptive analyses, the mean, standard deviation, minimum and maximum scores, and skewness were calculated. All statistical analyses of the study were conducted by SPSS 20.0 Windows (SPSS, Inc., USA), and the significance level was set at 0.05.
Ethical Considerations
Ethical approval was obtained from the Medical Research and Ethics Committee, Universiti Kebangsaan Malaysia Medical Center (FF-2018-020).
Results
Demographic Data
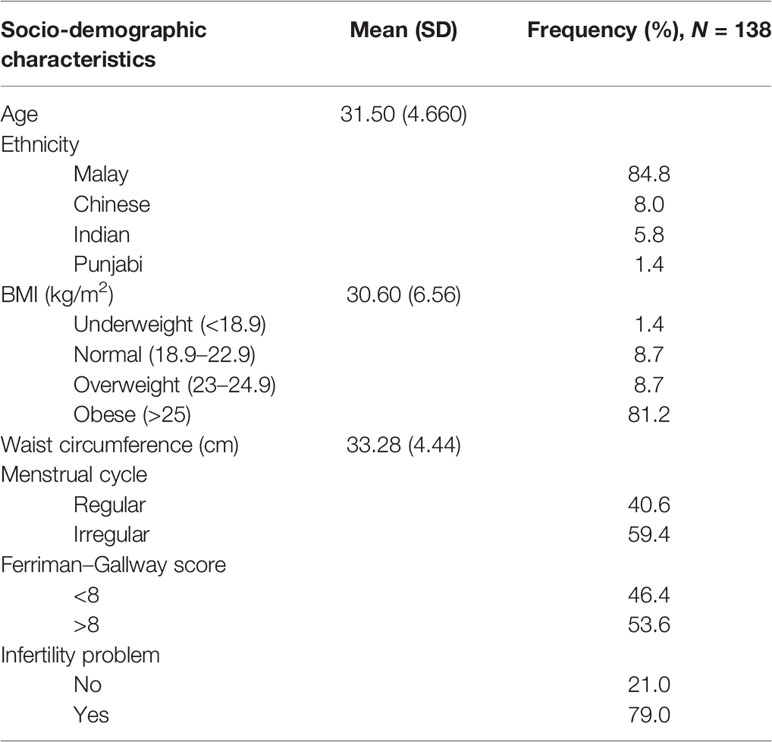
A total of 138 participants were recruited, and all completed the questionnaires with no missing data. The mean age of patients was 31.5 ± 4.6 years old. Most participants were Malay, followed by Chinese and Indian participants. Many were obese, with a mean body mass index of 30.6 ± 6.56 kg/m2 and a mean waist circumference of 33.28 ± 4.44 cm. About 59.7% of the participants had irregular cycles, and 53.6% had a Ferriman–Gallway score >8; 79% had issues with subfertility (Table 1).
Reliability
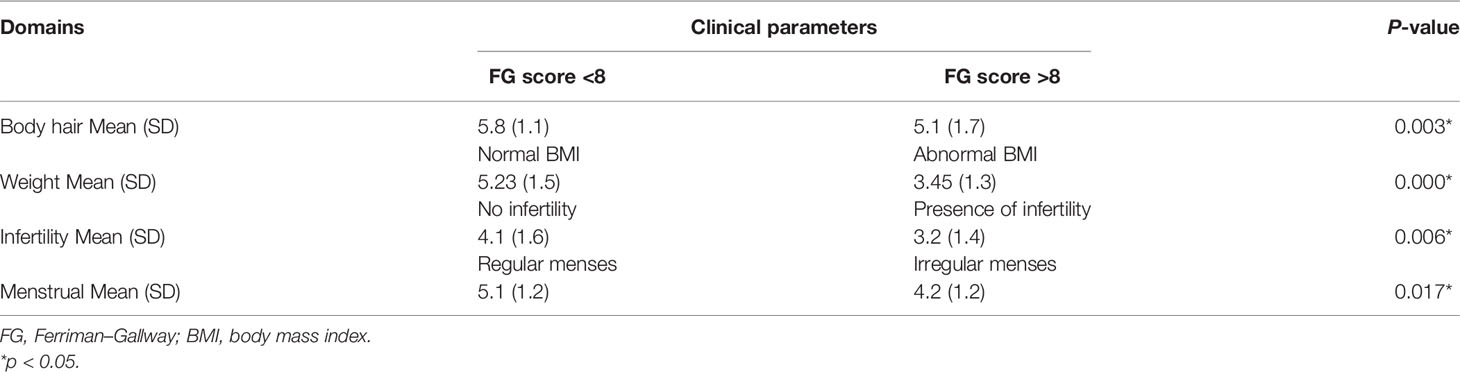
The internal reliability test is shown in Table 2. The Cronbach’s alpha was above 0.70; all domains had values above 0.80, except for the menstrual problem domain, which had a Cronbach’s alpha of 0.678. This implied satisfactory reproducibility.
Type of HRQoL Impairment
The distribution of scores for Mal-PCOSQ and SF-36 are listed in Table 3. The lowest scores for Mal-PCOSQ were reported in the weight (3.74) and infertility (3.41) domains, thereby indicating worse health in these domains. Body hair (5.42) was the least concerning issue. For SF-36v2, the mental component summary (44.83) and physical component summary (50.15) were both rated low. The highest mean score was seen in physical function (78.51), while vitality (55.75) and general health (57.42) had the lowest scores. The percentages of women who showed the lowest scores (i.e., floor effect) and the highest scores (i.e., ceiling effect) were low.
Validity
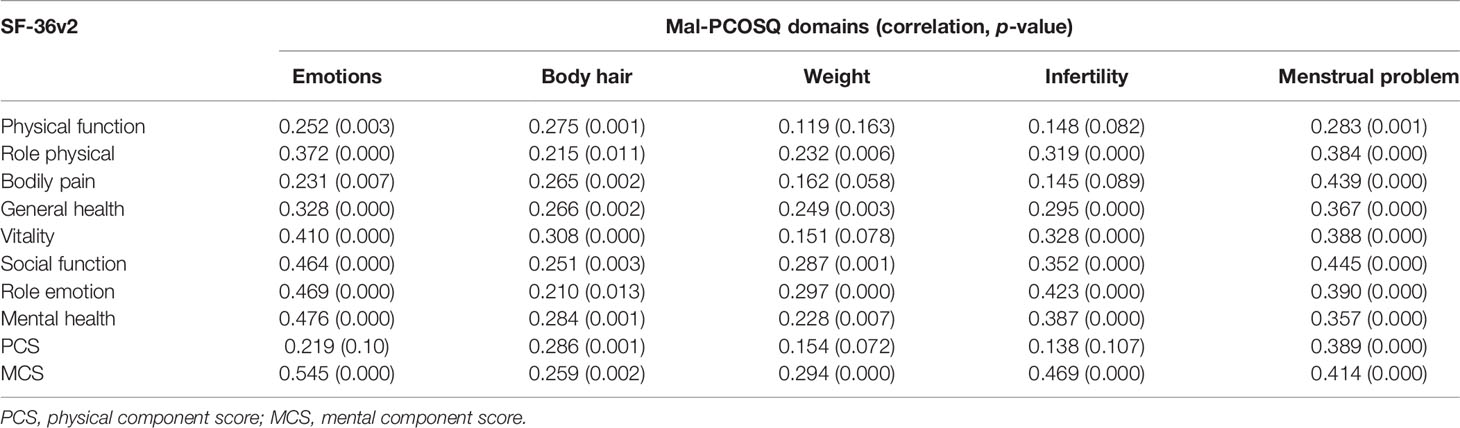
Discriminant validity was assessed using known group comparisons. Every domain in Mal-PCOSQ was able to determine women who differed in PCOS-specific symptoms, hence supporting the validity of Mal-PCOSQ (Table 4). For convergence validity testing, a good correlation was found between similar domains of the two instruments used, namely, Mal-PCOSQ and SF-36v2 (Table 5). In the Mal-PCOSQ, the emotional domain correlated well with role emotion (r = 0.469; p < 0.001), social function (r = 0.464; p < 0.01), mental health (r = 0.476; p < 0.01), and mental component summary (r = 0.545; p < 0.01) of SF-36v2. These correlations were stronger than the other domains, e.g., physical function (r = 0.252). In the Mal-PCOSQ, the menstrual problem domain correlated with the bodily pain domain in the SF-36v2 (r = 0.439; p < 0.01). In the Mal-PCOSQ, the infertility role domain correlated significantly with role emotion in the SF-36v2 (r = 0.423; p < 0.01), thereby indicating that infertility has a significant impact on the emotions of women with PCOS.
Prediction Factor for the Mal -PCOSQ Subscale
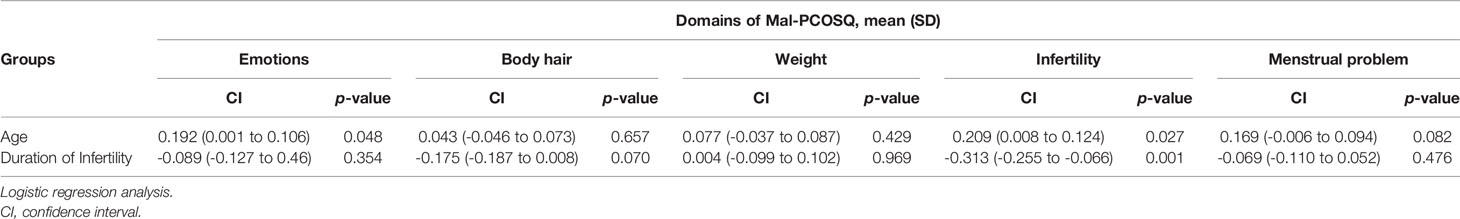
Age can predict the scores of the emotion subscale (=0.192, p = 0.048) and infertility subscale (=0.209, p = 0.027) of the MAL-PCOSQ, which means that, in our population, age is an important low score predictor factor. The duration of infertility is also a predictor of the scores of the infertility subscale of MAL-PCOSQ (=-0.313, p = 0.001). The longer the duration of infertility was, the lower the score of the infertility subscale was (Table 6).
Comparison of the HRQOL of Malaysian Women With PCOS With Those in Other Countries
Compared with women of other ethnic origins, the affected domains varied significantly (Figure 1). In UK populations (22), weight and infertility appeared to be the main concerns, whereas the Iranian (20), Chinese (24), and Malaysian populations’ scores for infertility were the lowest. The Asian populations were the least concerned about body hair, unlike the UK population. This proved our hypothesis that PCOS affects various ethnicities differently.
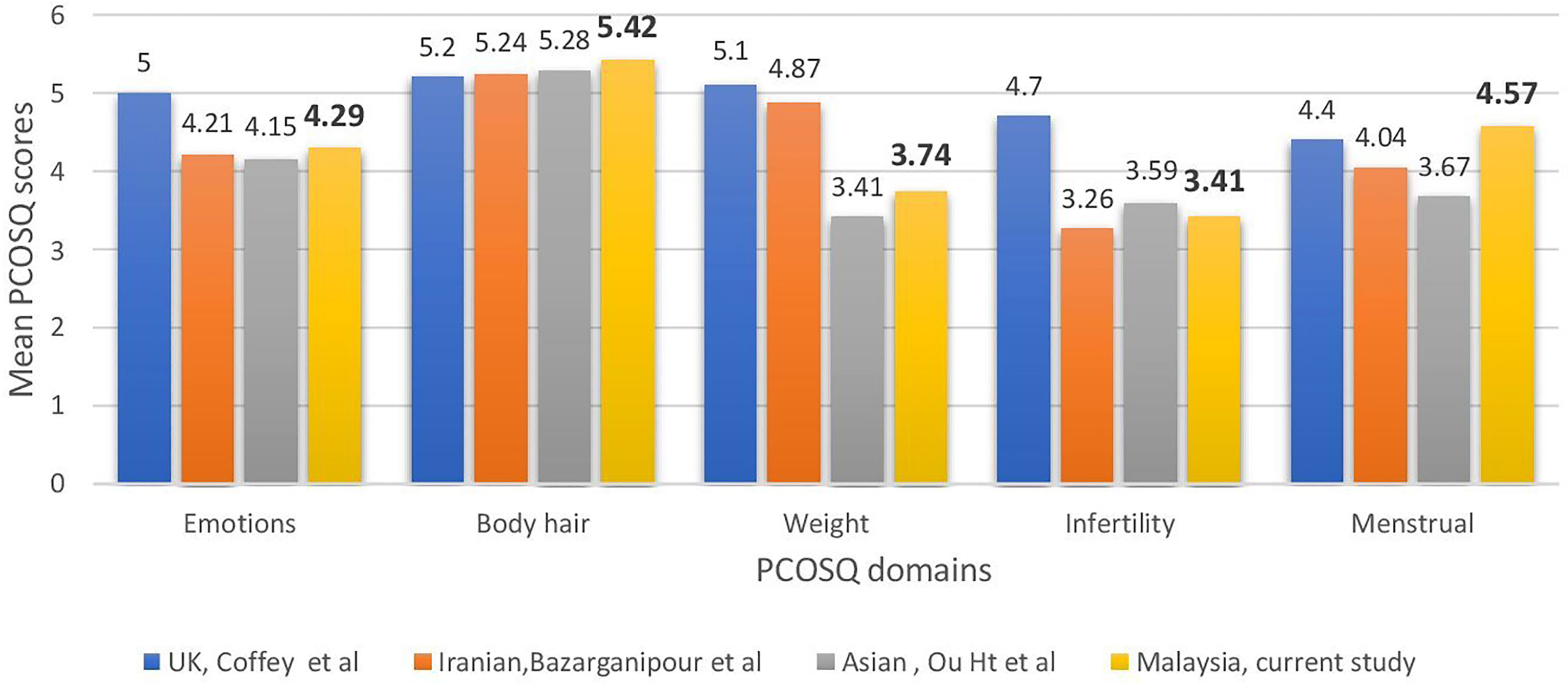
Figure 1 Comparisons across countries in health-related quality of life of polycystic ovary syndrome women measured using the Polycystic Ovarian Syndrome Questionnaire.
Discussion
The present study provided evidence of reliability and validity testing of Mal- PCOSQ. There was no dropout in our study, as it was applicable and relevant to the patients’ condition. None of the patients had difficulty understanding the questions in Malay, indicating that the Mal-PCOSQ could feasibly be used in the local population. All coefficients of the Mal-PCOSQ domains were analyzed using test– retest reliability analysis, which proved its good reliability with a Cronbach alpha score of >0.7 (average, 0.852). However, further modification regarding the menstrual problem domain is necessary as the alpha score was 0.678, which was in accordance with the previous studies (21, 22). Relocating certain non-specific questions, such as headache in the menstrual problem domain, was suggested to improve the questionnaire’s reliability.
The floor and ceiling effects were small in all subscales of the Mal-PCOSQ when compared with the SF-36v2. These findings indicated that the instrument was more specific towards certain areas pertaining to the disease or the condition itself, which was better than the generic instrument. The SF-36v2 questionnaire analysis also showed that both mental and physical score summaries were low, the former being 44.83 and the latter being 50.15. These results showed that our PCOS patients were significantly affected in terms of both mental and physical health, thereby supporting the findings of the Mal-PCOSQ and further enhancing its validity. Similar to the previous study by Coffey et al. (25), the convergent validity of the Mal-PCOSQ was proven by its correlation with similar domains in the SF-36v2 in which the “emotion” domain (Mal-PCOSQ) correlated well with the “role emotion” and “mental health” of the SF-36v2.
Known group comparisons, such as obesity, hirsutism, irregular menses, and subfertility, indicated that the Mal-PCOSQ was able to differentiate between subgroups with different clinical features, proving the discriminant validity of the instrument. A lower Mal-PCOSQ score was observed in women with PCOS with a higher Ferriman–Gallway score and body mass index and in those showing subfertility and irregular menses complications, in accordance with a previous study done by Chung et al. (23).
The present study also shows that Malaysian women with PCOS are affected by their condition, as evidenced by low scores in the infertility and weight domains. The local population was the least concerned about body hair. This was in contrast with the results of Cronin et al. (19), who found that the infertility and emotion subscales in the original PCOSQ had the highest impact scores among African-American women with PCOS. Huang et al. (24) showed that the burden of hirsutism on HRQoL among South Asians was less than that among Caucasians, with weight and infertility as the worst domains on the condition-specific questionnaire for both ethnic groups, according to the validated Chinese version of PCOSQ. The Iranian study (20) findings showed that Iranian patients were more affected by infertility and menstruation. The results of the present study were more similar to the study of Huang et al. (24), which included a similar Asian population. This finding proved the hypothesis that the impact of PCOS on quality of life varied among people of different ethnicities and backgrounds.
The Mal-PCOSQ can potentially be used in the diagnosis of the disease and during follow-ups to ascertain which component affects the patient the most and to assess the efficacy of the treatment provided. This will further improve patient care and outcome because each patient’s management will be tailored to their needs.
Conclusion
The Mal-PCOSQ is a reliable and valid instrument that can be potentially used to assess the effect of PCOS on the quality of life and to assess the efficacy of treatment in follow-up consultations.
Limitation of the Study
A limitation of this study is that it may not be representative of the Malaysian population as the participants were only recruited from one center. A further study can be done to include other centers to further investigate the strength of this questionnaire as well as to study the effect of PCOS on different populations, e.g., the indigenous group or adolescent group. A future study can also expand the domains of the Mal-PCOSQ by adding the acne subscale, which was proven to significantly affect the Chinese population.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research and Ethical Committee, Faculty of Medicine, University Kebangsaan Malaysia. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LLM, MAA, and CKT designed the study and performed data collection. AI and AAZ analyzed the data. AGNA wrote the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.848860/full#supplementary-material
References
1. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The Prevalence of Polycystic Ovary Syndrome in a Community Sample Assessed Under Contrasting Diagnostic Criteria. Hum Reprod (2010) 25(2):544–51. doi: 10.1093/humrep/dep399
2. Balen AH. Polycystic Ovary Syndrome (PCOS). Obstet Gynaecol (2017) 19:119–29. doi: 10.1111/tog.12345
3. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic Ovary Syndrome. Nat Rev Dis Primers (2016) 2:1–18. doi: 10.1038/nrdp.2016.57
4. Escobar-Morreale HF. Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat Rev Endocrinol (2018) 14:270. doi: 10.1038/nrendo.2018.24
5. Gilbert EW, Tay CT, Hiam DS, Teede HJ, Moran LJ. Comorbidities and Complications of Polycystic Ovary Syndrome: An Overview of Systematic Reviews. Clin Endocrinol (2018) 89:683–99. doi: 10.1111/cen.13828
6. Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, et al. Androgen Excess-Polycystic Ovary Syndrome Society: Position Statement on Depression, Anxiety, Quality of Life, and Eating Disorders in Polycystic Ovary Syndrome. Fertil Steril (2018) 109:888–99. doi: 10.1016/j.fertnstert.2018.01.038
7. Greenwood EA, Pasch LA, Cedars MI, Legro RS, Huddleston HG, Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network. Association Among Depression, Symptom Experience, and Quality of Life in Polycystic Ovary Syndrome. Am J Obstet Gynecol (2018) 219(3):279.e1–7. doi: 10.1016/j.ajog.2018.06.017
8. Cooney LG, Lee I, Sammel MD, Dokras A. High Prevalence of Moderate and Severe Depressive and Anxiety Symptoms in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum Reprod (2017) 32:1075–91. doi: 10.1093/humrep/dex044
9. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed Diagnosis and a Lack of Information Associated With Dissatisfaction in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2017) 102:604–12. doi: 10.1210/jc.2016-2963
10. Kazemi M, McBreairty LE, Chizen DR, Pierson RA, Chilibeck PD, Zello GA. A Pulse-Based Diet and the Therapeutic Lifestyle Changes Diet in Combination With Health Counseling and Exercise Improve Health-Related Quality of Life in Women With Polycystic Ovary Syndrome: Secondary Analysis of a Randomized Controlled Trial. J Psychosom Obstet Gynaecol (2020) 41(2):144–53. doi: 10.1080/0167482X.2019.1666820
11. Jones GL, Palep-Singh M, Ledger WL, Balen AH, Jenkinson C, Campbell MJ, et al. Do South Asian Women With PCOS Have Poorer Health-Related Quality of Life Than Caucasian Women With PCOS? A Comparative Cross-Sectional Study. Health Qual Life Outcomes (2010) 8:149. doi: 10.1186/1477-7525-8-149
12. Behboodi Moghadam Z, Fereidooni B, Saffari M, Montazeri A. Polycystic Ovary Syndrome and its Impact on Iranian Women’s Quality of Life: A Population-Based Study. BMC Women's Health (2018) 18:164. doi: 10.1186/s12905-018-0658-1
13. Böttcher B, Fessler S, Friedl F, Toth B, Walter MH, Wildt L, et al. Health-Related Quality of Life in Patients With Polycystic Ovary Syndrome: Validation of the German PCOSQ-G. Arch Gynecol Obstet (2018) 297:1027–35. doi: 10.1007/s00404-017-4623-2
14. Nasiri-Amiri F, Ramezani Tehrani F, Simbar M, Montazeri A, Mohammadpour RA. Health-Related Quality of Life Questionnaire for Polycystic Ovary Syndrome (PCOSQ-50): Development and Psychometric Properties. Qual Life Res (2016) 25:1791–801. doi: 10.1007/s11136-016-1232-7
15. Kaczmarek C, Haller DM, Yaron M. Health-Related Quality of Life in Adolescents and Young Adults With Polycystic Ovary Syndrome: A Systematic Review. J Pediatr Adolesc Gynecol (2016) 29:551–7. doi: 10.1016/j.jpag.2016.05.006
16. Jones GL, Hall JM, Lashen HL, Balen AH, Ledger WL. Health-Related Quality of Life Among Adolescents With Polycystic Ovary Syndrome. J Obstet Gynecol Neonatal Nurs (2011) 40:577–88. doi: 10.1111/j.1552-6909.2011.01279.x
17. Luo YY, Xu XL, Li XB. Psychometric Properties of the Chinese Version of the Modified Polycystic Ovary Syndrome Health-Related Quality-of-Life Questionnaire. Health Qual Life Outcomes (2020) 18(1):131. doi: 10.1186/s12955-020-01380-6
18. Rzońca E, Bień A, Wdowiak A, Szymański R, Iwanowicz-Palus G. Determinants of Quality of Life and Satisfaction With Life in Women With Polycystic Ovary Syndrome. Int J Environ Res Public Health (2018) 15:376. doi: 10.3390/ijerph15020376
19. Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, et al. Development of a Health-Related Quality-of-Life Questionnaire (PCOSQ) for Women With Polycystic Ovary Syndrome (PCOS). J Clin Endocrinol Metab (1998) 83:1976–87. doi: 10.1210/jcem.83.6.4990
20. Bazarganipour F, Ziaei S, Montazeri A, Foroozanfard F, Faghihzadeh S. Iranian Version of Modified Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire: Discriminant and Convergent Validity. Iran J Reprod Med (2013) 11:753.
21. Alghadeer S, Algarawi A, Abu-Rkybah F, Alshebly MM, Alruthia Y. The Translation and Validation of the Arabic Version of the Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (AR-PCOSQ). BMC Womens Health (2020) 20(1):244. doi: 10.1186/s12905-020-01108-0
22. Jones GL, Benes K, Clark TL, Denham R, Holder MG, Haynes TJ, et al. The Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ): A Validation. Hum Reprod (2004) 19(2):371–7. doi: 10.1093/humrep/deh048
23. Chung J, Kwan A, Kwok J, Chan S. Health-Related Quality-of-Life Questionnaire for Women With Polycystic Ovary Syndrome: A Chinese Translation and Validation Study. BJOG: Int J Obstet Gynaecol (2016) 123:1638–45. doi: 10.1111/1471-0528.14217
24. Ou HT, Wu MH, Lin CY, Chen PC. Development of Chinese Version of Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (Chi- PCOSQ). PLoS One (2015) 10:e0137772. doi: 10.1371/journal.pone.0137772
25. Coffey S, Bano G, Mason HD. Health-Related Quality of Life in Women With Polycystic Ovary Syndrome: A Comparison With the General Population Using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form- 36 (SF-36). Gynecol Endocrinol (2006) 22:80–6. doi: 10.1080/09513590600604541
Keywords: polycystic ovary syndrome, Polycystic Ovary Syndrome Questionnaire, Malay version PCOSQ, health-related quality of life, reliability, validity
Citation: Mei LL, Abu MA, Chew KT, Ismail A, Zainuddin AA and Nur Azurah AG (2022) The Reliability and Validity of the Malay Version of Polycystic Ovarian Syndrome Health-Related Quality of Life Questionnaire. Front. Endocrinol. 13:848860. doi: 10.3389/fendo.2022.848860
Received: 11 February 2022; Accepted: 30 March 2022;
Published: 26 May 2022.
Edited by:
Lawrence Merle Nelson, Mary Elizabeth Conover Foundation, Inc., United StatesReviewed by:
Luiz Augusto Casulari, University of Brasilia, BrazilMd Sayeed Akhtar, King Khalid University, Saudi Arabia
Copyright © 2022 Mei, Abu, Chew, Ismail, Zainuddin and Nur Azurah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Azrai Abu, YXpyYWlhYnUxOTgzQGdtYWlsLmNvbQ==
 Lim Leek Mei1
Lim Leek Mei1 Muhammad Azrai Abu
Muhammad Azrai Abu Kah Teik Chew
Kah Teik Chew Abdul Ghani Nur Azurah
Abdul Ghani Nur Azurah