
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 08 March 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.844040
 Chong Boon Teo1†
Chong Boon Teo1† Pek Yan Tan1†
Pek Yan Tan1† Shan Xian Lee2
Shan Xian Lee2 Joan Khoo3
Joan Khoo3 Jun Guan Tan4
Jun Guan Tan4 Su Fen Ang5
Su Fen Ang5 Sze Hwa Tan6
Sze Hwa Tan6 Tunn Lin Tay3
Tunn Lin Tay3 Eberta Tan3
Eberta Tan3 Su Chi Lim5,7
Su Chi Lim5,7 Bernhard O. Boehm8,9,10
Bernhard O. Boehm8,9,10 Wann Jia Loh3*
Wann Jia Loh3*The management of diabetes mellitus in an insulin-dependent patient is challenging in the setting of concomitant antibody-mediated-insulin hypersensitivity. We report a case of a 62-year-old woman with pre-existing type 2 diabetes mellitus of 10 years duration who developed type 3 hypersensitivity reaction to insulin analogue detemir, and subsequently, severe diabetic ketoacidosis (DKA). She was C-peptide negative and was diagnosed with insulin-dependent diabetes. Despite increasing dose adjustments, insulin-meal matching, and compliance with insulin, she experienced episodes of unexpected hyperglycaemia and hypoglycaemia. The development of rash after detemir initiation and rapid progression to DKA suggests an aberrant immune response leading to the insulin allergy and antibody-induced interference with insulin analogues. Glycaemic control in the patient initially improved after being started on subcutaneous insulin infusion pump with reduced insulin requirements. However, after a year on pump therapy, localised insulin hypersensitivity reactions started, and glycaemic control gradually deteriorated.
Insulin therapy is necessary in the management of insulin deficient diabetes mellitus, but exogenous insulin can cause severe complications apart from hypoglycaemia. These lesser known but clinically meaningful complications include insulin allergy (1), immunological insulin resistance (2) and lipoatrophy (3). Prior to the advent of purification techniques, the use of insulin derived from porcine (which differs from human insulin in one carboxy-terminal amino acid of the B-chain, where alanine substitutes for threonine) and bovine (which differs from human insulin which has threonine and isoleucine whereas bovine insulin has alanine and valine at positions 8 and 10) led to frequent hypersensitivity reactions (1). True insulin allergy is currently an uncommon phenomenon with an estimated prevalence of <0.1-1% with a wide range of symptoms ranging from localised reactions to anaphylaxis (1, 4–6). We present here an unusual case of an adult female who shortly after insulin detemir (Levemir) initiation, developed type III hypersensitivity reaction, and was admitted for severe diabetic ketoacidosis (DKA) 1 week after stopping detemir. The management was challenging because of high insulin requirements and cutaneous hypersensitivity reactions to insulin. We also performed a literature review of patients with insulin-dependent diabetes with insulin allergy to inform our management of this rare condition.
A 62-year-old female of Chinese ethnicity with a 10 years’ history of type 2 diabetes mellitus (T2DM) presented to the emergency department with a few days history of polyuria and polydipsia, and was diagnosed with DKA. In the first 7 years of her T2DM history, her glycaemic control was satisfactory (HbA1c of 6.6-7.6%) on combination regimen of metformin and glipizide. In the recent 3 years, her glycaemic control gradually worsened to a peak HbA1c of 10% but this was controlled by intensifying diet compliance and increasing metformin and glipizide doses to maintain a HbA1c of 8-8.5% (Figure 1). Her general practitioner added insulin detemir as a basal insulin supplement 3 weeks prior to admission, following which she experienced pruritic erythematous rashes at the injection site within a day. She stopped the insulin injections after 2 weeks in view of intolerable localised rashes with minimal response to antihistamines and presented 1 week later with severe DKA. Her other past medical history included hypertension, hyperlipidaemia, and obesity (weight 70kg; BMI 27.5kg/m2). She did not have any known drug allergies, past medical history or family history of allergy and autoimmune disorders. Her mother had T2DM at old age.

Figure 1 Glycaemic control in this patient, reflected as HbA1c trend, and continuous glucose monitoring (CGMS) before (A) and after (B) insulin pump. Time on X axis is presented as months from presentation to hospital with severe DKA and insulin (detemir) allergy.
On clinical examination during her admission, there were extensive erythematous urticarial rashes at insulin injection sites on the abdomen (Figure 2A). Blood tests showed moderate DKA (hyperglycaemia of 24.7 mmol/L, ketonemia of 6.5 mmol/L, acidosis of pH 7.126 and serum venous bicarbonate of 6 mmol/L). Her serum glucose level was controlled at 10-14mmol/L and DKA resolved while on intravenous infusion of regular insulin (actrapid) without hypersensitivity reactions. Prior to converting fully to subcutaneous insulin, low doses of insulin analogues glulisine, glargine and aspart were injected subcutaneously to monitor for hypersensitivity; after a few hours, she developed localised erythematous rash to subcutaneous insulin glulisine (Figure 2B), which resolved after a few days, but not to insulin glargine and insulin aspart. The patient was discharged from hospital with multiple daily injection (MDI) therapy at 38 units of glargine once daily and 6-8 units of aspart pre-meals (preliminary estimation ICR 1: 6-8g), total daily dose [TDD] of 1 unit/kg/day, capillary glucose readings 5-18mmol/L). She was closely followed up at outpatient for insulin titration, however, her glycaemic control worsened despite compliance and increasing doses of basal and prandial insulin (Figure 1). Although she was now C-peptide negative, metformin was continued to reduce insulin resistance.
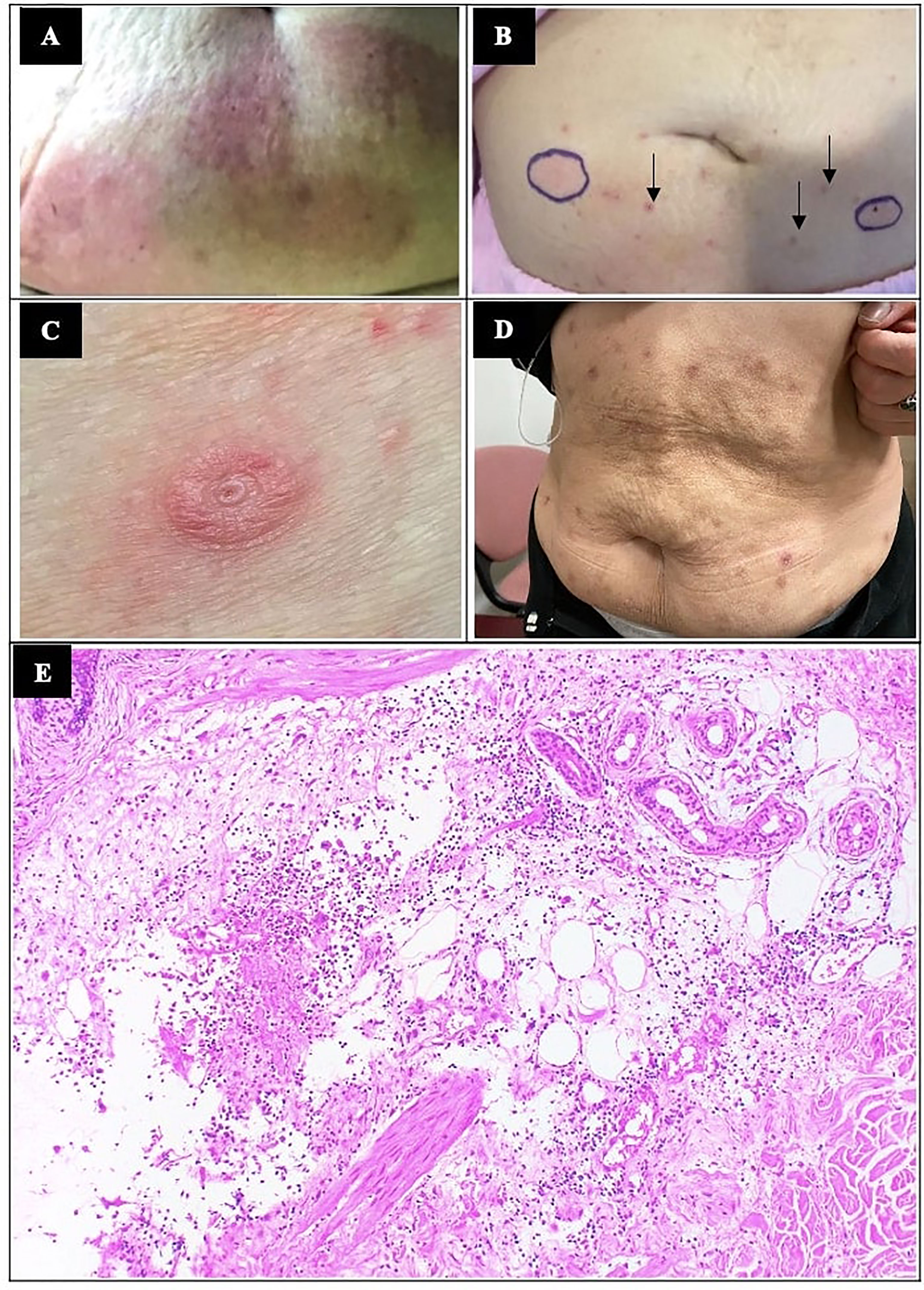
Figure 2 Localised cutaneous hypersensitivity reactions to insulininjections. (A) Localised erythematous rashes at sites of insulin detemir injections at initial presentation. (B) Localised erythematous skin reactions occurred after test doses of glulisine, circled in blue. A few of the resolving rashes (small arrows) at previous insulin detemir sites at 3 days after hospital admission are shown. (C) Localised cutaneous reaction at insulin administration sites when patient was on insulin aspart (novorapid) subcutaneous insulin pump is shown here. Patch testing to consumables of insulin pump were negative. (D) Increasingly altered cosmesis of her abdominal skin from repeated localised allergic reactions to insulin were observed after 4 years of insulin therapy. (E) Histology by haematoxylin and eosin staining (x100 magnification) showed dermal oedema and lymphohistiocytic perivascular inflammation suggestive of hypersensitivity reaction.
During her DKA, her full blood count, kidney function, liver function test, thyroid function test, lactate level, and procalcitonin level were all normal. There was no eosinophilia despite the allergic skin reactions and no evidence of infection with normal monocyte and leukocyte counts. Her HbA1c was 8.5%. Her vitamin levels such as folate, B12 and zinc levels were normal, except for vitamin D which was mildly deficient at 22.2 ug/L. Her retinal photo and urine albuminuria tests were normal. Apart from the insulin allergy to detemir, there were no clear precipitants to DKA such as infection, myocardial infarction, or pancreatitis. In view of the acute onset of complete insulin deficiency, evident by undetectable C-peptide levels, testing for pancreatic autoantibodies was performed. Her pancreatic autoantibodies were positive for islet cell antibody (anti-ICA) using indirect immunofluorescence against monkey pancreas, but negative for glutamic acid decarboxylase antibody (anti-GAD), IA2 and zinc transporter 8 antibody. Her C-peptide levels were repeatedly undetectable (<27pmol/L) when performed at presentation, few months after, and 2 years later, consistent with the diagnosis of complete insulin deficiency. Repeat anti-ICA and anti-GAD test were negative 2 years later.
Despite compliance and frequent insulin titrations, her glycemic control gradually deteriorated with unexpected hyperglycaemia and hypoglycaemia in the next 1 year warranting further investigations. CT pancreas was normal showing a pancreas of normal volume and with no structural lesions or inflammatory changes. She did not have any other autoimmune conditions such as Addison’s disease, Hashimoto thyroiditis (thyroid peroxidase antibody was negative and thyroid function test normal) or pernicious anaemia. She did not have clinical signs to suggest lipodystrophy syndrome or Cushing syndrome as a cause of insulin resistance.
Serologic tests for antibody towards insulin yielded positive results for antibody specific for insulin (IgG and IgM) with antibody titre raised at 0.20nmol/L (reference range <0.02nmol/L) while IgE antibody to insulin was absent (analysed at Mayo Medical Laboratories, USA). Measurement of insulin levels after polyethylene glycol (PEG) testing showed reduced free insulin levels; 47% recovery while on glargine (Lantus) alone (pre-PEG insulin levels: 10.44mIU/L), and 36% recovery on aspart (Novorapid) insulin infusion (pre-PEG insulin levels:4.25mIU/L), suggesting impaired free-insulin level likely due to presence of antibodies binding to insulin.
When her rashes recurred after one year on insulin pump, skin biopsy was performed at the site of cutaneous reactions to insulin. Histology showed dermal oedema and a moderate amount of lymphohistiocytic infiltrate admixed with some eosinophils, acute inflammatory yield and fibrinous exudates around the superficial dermal vessels and hair follicles (Figure 2E), in keeping with our clinical diagnosis of insulin allergy. The onset of few hours after injection of insulin aspart pointed towards type III hypersensitivity. As the localised insulin reactions recurred also on insulin pump treatment, patch testing to the additive and consumables of insulin were performed. The patch tests were unremarkable to cresol, adhesive, plastic needle, and plastic cannula. The patient was also patch tested (Chemotechnique Diagnostics, Vellinge, Sweden) to the international standard series (including allergens such as nickel and cobalt), cosmetic series (including allergens such as 2-tert-butyl-4-methoxyphenol, 2,6-ditert-butyl-4-cresol and p-chloro-m-cresol) and methacrylate series with negative results for all.
Management of glycaemic control was challenging and required constant insulin titration upwards; at 1 year later her insulin carbohydrate ratio (ICR) was 1:2 and basal insulin glargine was 0.6-0.7 units/kg/day with split dosing. Her glycaemic control remained suboptimal with hyperglycaemia (especially post-prandial), high glycemic variability, and raised HbA1c ≈10% despite full adherence to MDI (Figure 1). Her TDD reached a peak of 2 units/kg/day when she consumed a moderate carbohydrate intake (100-200g/day). To improve post-prandial hyperglycaemia despite high ICR, she self-restricted her carbohydrate intake to 30g carbohydrate per meal (ICR 1:2) at 3 meals a day with similar diet plans daily. Despite efforts to adjust ICR, her glucose readings fluctuated widely even with the same carbohydrate portion, activity level and insulin dose.
Continuous subcutaneous insulin infusion (CSII) with aspart was started in view of persistent suboptimal glycemic control. This improved her glycaemic control in terms of glycaemic variability, postprandial hyperglycaemia, and improved HbA1c to ≈7% (Figure 1), reduction of ICR to 1:3 and reduction of total basal insulin dose to 0.4-0.5 units/kg/day. The low glucose suspend safety feature of Minimed Medtronic pump 640G® was helpful to minimise hypoglycaemia however, the patient did not use the feature frequently because she did not like the additional attachment and cost of continuous glucose monitoring. At 1 year on insulin pump therapy, erythematous pruritic insulin injection reactions recurred and progressively worsened (Figure 2C). She was switched from insulin aspart to lispro or regular insulin (actrapid) on different occasions with similar hypersensitivity reactions and no improvement of clinical outcome. At 2-3 years after insulin pump initiation, she continued to experience tolerable cutaneous reactions at the sites of insulin administration resulting in an increasingly altered cosmesis of the abdominal skin (Figure 2D) and basal insulin requirement increased to 0.5-0.6 units/kg/day. When the patient switched back from insulin pump to MDI therapy with insulin glargine and aspart, there were still erythematous localised reactions. Her rashes remained tolerable with infrequent antihistamine use. Blood tests for full blood count and eosinophil levels were consistently normal throughout the years. Topical and oral steroids were considered but not used in this case due to lack of strong evidence for benefits of steroids while weighing against its side effects (7, 8).
This case is unique because the timeline of events suggests that insulin detemir triggered the event of insulin allergy. This was followed by rapid onset of complete insulin deficiency, and worsening insulin resistance. Exogenous insulin causing allergy is uncommon (1, 5) and immunological insulin resistance is rare (2). The very rapid-onset of DKA within few days of symptomatic hyperglycaemia with undetectable C-peptide levels and mostly negative for pancreatic autoantibodies, presents much similarity to a fulminant case of type 1 diabetes (9, 10). However, her pre-existing diagnosis of T2DM was not in line with the current definition of “fulminant type 1 diabetes” (10). To our knowledge, this is the first case report of insulin-dependent DM presenting with DKA which occurred after exposure to an exogenous insulin (detemir), and another rare report of a case of exogenous insulin allergy with concomitant immunological form of insulin resistance (11).
Detemir is an insulin analogue which differ from native insulin by the deletion of amino acid threonine B30 and addition of the saturated long-chain fatty acid myristic acid residue at B29 (12). Although all insulin analogues resemble human insulin, insulin analogues can cause immunogenicity leading to antibodies against insulin (2, 3) and insulin allergy (1, 5, 6, 11, 13). Among the long-acting insulin analogues, there are more reports of insulin detemir causing severe allergy (5, 6, 12), raising the question whether detemir is particularly immunogenic. Her clinical presentation did not fit into the usual DM classifications including T1DM, Latent Autoimmune Diabetes in Adult (LADA) and fulminant type 1 diabetes. She did not have ketosis-prone diabetes as her beta-cell failure was permanent. Whilst underlying aetiology of these conditions remain unclear, pathological immune reactions leading to pancreatic destruction have been postulated in T1DM and fulminant type 1 diabetes (9, 14), as well as checkpoint inhibitor-induced IDDM (15). Antibody testing is less diagnostic in Asian populations due to the lower prevalence of antibody-positivity in T1DM (14), while a minority of fulminant type 1 diabetes also have pancreatic autoantibodies (9). It is unclear what precipitated beta-cell failure in this patient.
Our patient possibly had immunological impaired insulin action and resistance leading to fluctuating hyperglycaemia and hypoglycaemia despite strict compliance to diet and insulin. Her insulin resistance could not be explained in total by her mild obesity. High-affinity antibodies towards insulin induce insulin resistance by causing low circulating free insulin levels whereas low-affinity antibodies cause hypoglycaemia due to delayed dissociation of the insulin from insulin-antibody complexes (2, 16). This phenomenon is referred as insulin antibody syndrome [IAS] when caused by non-insulin (e.g., methimazole) (17) and exogenous insulin antibody syndrome [EIAS]) when caused by exogenous insulin (2). The raised total antibody of IgG and IgM towards insulin and reduced free insulin level (post-PEG) (2, 16), supports our postulation. However, we are unable to prove the mechanisms because mechanistic and reaction kinetic studies to elucidate the affinity and capacity of the antibodies to insulin (16) were not available. Thus, it is debatable whether the insulin-antibody complex-induced resistance was at the subcutaneous level (i.e. subcutaneous insulin resistance syndrome [SIR]) (18) or systemic circulation level (EIAS) (2) or both, none of which is easily differentiable. The pathophysiology of SIR is also poorly understood; possible mechanisms include immune-mediated insulin resistance, enzymatic activity at subcutaneous sites, and insulin sequestration in adipose tissues (16, 18).
Insulin allergy is caused by type I (IgE-mediated), type III (antigen-antibody complexes) or type IV (delayed-type) hypersensitivity, of which type I is most commonly reported (1). The onset of symptoms for type I, III and IV occur within minutes, 2-6 hours, and 1-3 days later respectively. Detemir has been reported to cause type I, III and IV hypersensitivity reactions (1, 5, 12, 19). The raised antibodies towards insulin (IgG and IgM) are not specific for EIAS and could indicate type III hypersensitivity. Although skin prick testing to insulin is helpful, it is not always necessary for type III hypersensitivity, and there is concern of inducing serum sickness on re-introducing the allergenic insulin (1, 19). Hypersensitivity to insulin preparations including additives (e.g. zinc) and solvents (e.g. cresol) may mimic insulin allergy (1). In this case, the patient also had localised skin reactions to multiple various insulin formulations i.e. detemir, glulisine[apidra] (does not have zinc), aspart[novorapid], lispro[humalog] and regular insulin [actrapid]. Although these insulin formulations had cresol, she was patch tested negative for cresol. Other insulin-allergy mimics reported are due to allergens in insulin infusion devices (20), allergy to natural latex rubber antigens in insulin injection material (21) and faulty injection technique (22).
Avoiding insulin in the management of C-peptide negative subjects is not possible. Our literature review showed that insulin allergy in patients with T1DM have been reported in 16 cases (Table 1). Management of patients with T1DM with insulin allergy varies, with 7 cases switching the insulin type used (7, 8, 19, 25, 30–32), and 8 cases adopting the use of anti-histamines (7, 26–30, 32, 33). Where anti-histamines did not work or insulin therapy could not be switched, other measures are explored, with 5 cases adopting the use of insulin desensitisation with continuous subcutaneous insulin infusion (7, 11, 24, 28, 29), and 6 cases adopting the use of immunosuppression via steroids (7, 8, 23, 26, 29, 33), tacrolimus (30), rituximab (anti-CD20 monoclonal antibody) and IV immunoglobulin (11). Matheu et al. (29) in particular adopted both insulin desensitisation as well as immunosuppression strategies. The initial approach to management of insulin allergy was similar to approaches recommended by Heinzerling et al. (34) and Jacquier et al. (35), with switch to different insulin preparation and symptomatic therapy with anti-histamines being the first-line treatment before exploring other options such as inducing tolerance via continuous subcutaneous insulin infusion or specific immunotherapy with glucocorticoids. Other options that were successful after initial immunotherapy did not work or had complications include omalizumab, rituximab, mycophenolate mofetil, or colchicine and mercaptopurine (7, 8). However, use of immunomodulators is lacking strong clinical evidence and is associated with side effects e.g., mild hypersensitivity infusion reactions such as fever and chills, infections, and rituximab-associated progressive multifocal leukoencephalopathy (36). Leonet et al. attempted a vascularised whole pancreas transplant as last resort (30).
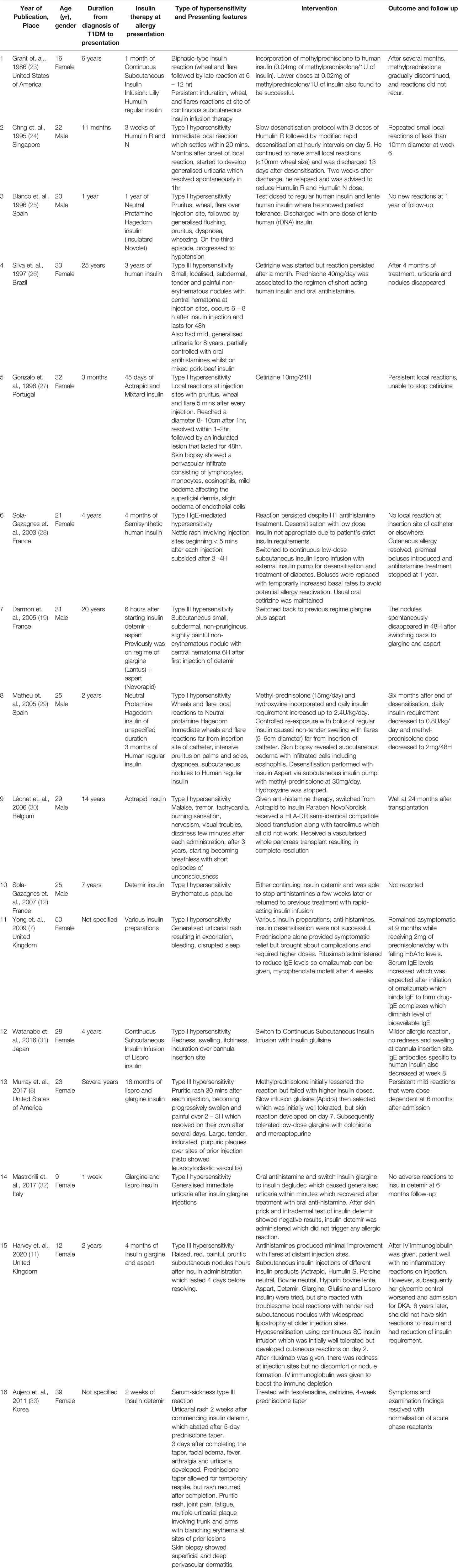
Table 1 Literature review of presentation and management of patients with type 1 diabetes mellitus with insulin allergy.
In conclusion, the workup and management of patients with insulin hypersensitivity is challenging. More studies are required to understand the pathophysiology of exogenous insulin-induced adverse reactions in order to develop safe and effective treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
WJL is the physician taking care of the patient, wrote the case report and was involved in the final approval of the paper. CBT and PYT drafted the case report and performed literature research. SXL was involved in care of and assessment of the patient’s dermatology condition. JK, SCL and BB were involved in the critical revision of the paper. JGT and SFA were involved in the laboratory analyses. SHT was involved in analyzing the histological slides. TLT and ET are the physicians involved in taking care of the patient. All authors contributed to the writing of the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ghazavi MK, Johnston GA. Insulin Allergy. Clin Dermatol (2011) 29(3):300–5. doi: 10.1016/j.clindermatol.2010.11.009
2. Hu X, Chen F. Exogenous Insulin Antibody Syndrome (EIAS): A Clinical Syndrome Associated With Insulin Antibodies Induced by Exogenous Insulin in Diabetic Patients. Endocr Connect (2018) 7(1):R47–55. doi: 10.1530/EC-17-0309
3. Saberi S, Esfandiari NH, MacEachern MP, Tan MH. Detemir Plus Aspart and Glulisine Induced Lipoatrophy: 2015 Literature Review and Report of a New Case. Clin Diabetes Endocrinol (2015) 1:10. doi: 10.1186/s40842-015-0013-5
4. Radermecker RP, Scheen AJ. Allergy Reactions to Insulin: Effects of Continuous Subcutaneous Insulin Infusion and Insulin Analogues. Diabetes Metab Res Rev (2007) 23(5):348–55. doi: 10.1002/dmrr.714
5. Bzowyckyj AS, Stahnke AM. Hypersensitivity Reactions to Human Insulin Analogs in Insulin-Naive Patients: A Systematic Review. Ther Adv Endocrinol Metab (2018) 9(2):53–65. doi: 10.1177/2042018817745484
6. Perez E, Gonzalez R, Martinez J, Iglesias J, Matheu V. Detemir Insulin-Induced Anaphylaxis. Ann Allergy Asthma Immunol (2009) 102(2):174–5. doi: 10.1016/S1081-1206(10)60255-4
7. Yong PF, Malik R, Arif S, Peakman M, Amiel S, Ibrahim MA, et al. Rituximab and Omalizumab in Severe, Refractory Insulin Allergy. N Engl J Med (2009) 360(10):1045–7. doi: 10.1056/NEJMc0808282
8. Murray BR, Jewell JR, Jackson KJ, Agboola O, Alexander BR, Sharma P. Type III Hypersensitivity Reaction to Subcutaneous Insulin Preparations in a Type 1 Diabetic. J Gen Intern Med (2017) 32(7):841–5. doi: 10.1007/s11606-017-4037-7
9. Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, et al. Fulminant Type 1 Diabetes: A Nationwide Survey in Japan. Diabetes Care (2003) 26(8):2345–52. doi: 10.2337/diacare.26.8.2345
10. World Health Organization. Classification of Diabetes Mellitus. Geneva: World Health Organization (2019). p. 36.
11. Harvey JN, Cronin M, Arkwright P. Insulin Hypersensitivity in Type 1 Diabetes: Investigation and Treatment With Immunodepletion. Pract Diabetes (2020) 37(2):59–61a. doi: 10.1002/pdi.2265
12. Sola-Gazagnes A, Pecquet C, M’Bemba J, Larger E, Slama G. Type I and Type IV Allergy to the Insulin Analogue Detemir. Lancet (2007) 369(9562):637–8. doi: 10.1016/S0140-6736(07)60301-8
13. Lindholm A, Jensen LB, Home PD, Raskin P, Boehm BO, Rastam J. Immune Responses to Insulin Aspart and Biphasic Insulin Aspart in People With Type 1 and Type 2 Diabetes. Diabetes Care (2002) 25(5):876–82. doi: 10.2337/diacare.25.5.876
14. Zimmet PZ, Rowley MJ, Mackay IR, Knowles WJ, Chen Q-Y, Chapman LH, et al. The Ethnic Distribution of Antibodies to Glutamic Acid Decarboxylase: Presence and Levels in Insulin-Dependent Diabetes Mellitus in Europid and Asian Subjects. J Diabetes Complications (1993) 7(1):1–7. doi: 10.1016/1056-8727(93)90016-R
15. Perdigoto AL, Quandt Z, Anderson M, Herold KC. Checkpoint Inhibitor-Induced Insulin-Dependent Diabetes: An Emerging Syndrome. Lancet Diabetes Endocrinol (2019) 7(6):421–3. doi: 10.1016/S2213-8587(19)30072-5
16. Jassam N, Amin N, Holland P, Semple RK, Halsall DJ, Wark G, et al. Analytical and Clinical Challenges in a Patient With Concurrent Type 1 Diabetes, Subcutaneous Insulin Resistance and Insulin Autoimmune Syndrome. Endocrinol Diabetes Metab Case Rep (2014) 2014:130086. doi: 10.1530/EDM-13-0086
17. Uchigata Y, Hirata Y, Iwamoto Y. Drug-Induced Insulin Autoimmune Syndrome. Diabetes Res Clin Pract (2009) 83(1):e19–20. doi: 10.1016/j.diabres.2008.10.015
18. Paulsen EP, Courtney JW 3rd, Duckworth WC. Insulin Resistance Caused by Massive Degradation of Subcutaneous Insulin. Diabetes (1979) 28(7):640–5. doi: 10.2337/diabetes.28.7.640
19. Darmon P, Castera V, Koeppel MC, Petitjean C, Dutour A. Type III Allergy to Insulin Detemir. Diabetes Care (2005) 28(12):2980. doi: 10.2337/diacare.28.12.2980
20. Herman A, de Montjoye L, Tromme I, Goossens A, Baeck M. Allergic Contact Dermatitis Caused by Medical Devices for Diabetes Patients: A Review. Contact Dermatitis (2018) 79(6):331–5. doi: 10.1111/cod.13120
21. Towse A, O’Brien M, Twarog FJ, Braimon J, Moses AC. Local Reaction Secondary to Insulin Injection. A Potential Role for Latex Antigens in Insulin Vials and Syringes. Diabetes Care (1995) 18(8):1195–7. doi: 10.2337/diacare.18.8.1195
22. Chakraborty PP, Biswas SN, Patra S. Faulty Injection Technique: A Preventable But Often Overlooked Factor in Insulin Allergy. Diabetes Ther (2016) 7(1):163–7. doi: 10.1007/s13300-016-0151-5
23. Grant W, deShazo RD, Frentz J. Use of Low-Dose Continuous Corticosteroid Infusion to Facilitate Insulin Pump Use in Local Insulin Hypersensitivity. Diabetes Care (1986) 9(3):318–9. doi: 10.2337/diacare.9.3.318
24. Chng HH, Leong KP, Loh KC. Primary Systemic Allergy to Human Insulin: Recurrence of Generalized Urticaria After Successful Desensitization. Allergy (1995) 50(12):984–7. doi: 10.1111/j.1398-9995.1995.tb02512.x
25. Blanco C, Castillo R, Quiralte J, Delgado J, Garcia I, de Pablos P, et al. Anaphylaxis to Subcutaneous Neutral Protamine Hagedorn Insulin With Simultaneous Sensitization to Protamine and Insulin. Allergy (1996) 51(6):421–4. doi: 10.1111/j.1398-9995.1996.tb00153.x
26. Silva ME, Mendes MJ, Ursich MJ, Rocha DM, Brito AH, Fukui RT, et al. Human Insulin Allergy-Immediate and Late Type III Reactions in a Long-Standing IDDM Patient. Diabetes Res Clin Pract (1997) 36(2):67–70. doi: 10.1016/S0168-8227(97)00031-4
27. Gonzalo MA, De Argila D, Revenga F, Garcia JM, Diaz J, Morales F. Cutaneous Allergy to Human (Recombinant DNA) Insulin. Allergy (1998) 53(1):106–7. doi: 10.1111/j.1398-9995.1998.tb03786.x
28. Sola-Gazagnes A, Pecquet C, Radermecker R, Pietri L, Elgrably F, Slama G, et al. Successful Treatment of Insulin Allergy in a Type 1 Diabetic Patient by Means of Constant Subcutaneous Pump Infusion of Insulin. Diabetes Care (2003) 26(10):2961–2. doi: 10.2337/diacare.26.10.2961
29. Matheu V, Perez E, Hernandez M, Diaz E, Darias R, Gonzalez A, et al. Insulin Allergy and Resistance Successfully Treated by Desensitisation With Aspart Insulin. Clin Mol Allergy (2005) 3:16. doi: 10.1186/1476-7961-3-16
30. Leonet J, Malaise J, Goffin E, Lefebvre C, Tennstedt D, Vandeleene B, et al. Solitary Pancreas Transplantation for Life-Threatening Allergy to Human Insulin. Transpl Int (2006) 19(6):474–7. doi: 10.1111/j.1432-2277.2006.00282.x
31. Watanabe K, Kusunoki Y, Katsuno T, Nakae R, Matsuo T, Ochi F, et al. A Case of Type 1 Diabetes Mellitus With Which Localized Insulin Allergy was Markedly Alleviated by Switching to Insulin Glulisine. Acta Diabetol (2016) 53(5):845–8. doi: 10.1007/s00592-016-0841-5
32. Mastrorilli C, Rizzuti L, Cangelosi AM, Iovane B, Chiari G, Caffarelli C. Long-Acting Insulin Allergy in a Diabetic Child. Int J Immunopathol Pharmacol (2017) 30(2):174–7. doi: 10.1177/0394632017700431
33. Aujero MP, Brooks S, Li N, Venna S. Severe Serum Sickness-Like Type III Reaction to Insulin Detemir. J Am Acad Dermatol (2011) 64(6):e127–8. doi: 10.1016/j.jaad.2010.11.028
34. Heinzerling L, Raile K, Rochlitz H, Zuberbier T, Worm M. Insulin Allergy: Clinical Manifestations and Management Strategies. Allergy (2008) 63(2):148–55. doi: 10.1111/j.1398-9995.2007.01567.x
35. Jacquier J, Chik CL, Senior PA. A Practical, Clinical Approach to the Assessment and Management of Suspected Insulin Allergy. Diabetes Med (2013) 30(8):977–85. doi: 10.1111/dme.12194
Keywords: insulin allergy, insulin hypersensitivity, insulin, insulin-dependent diabetes, diabetic ketoacidosis
Citation: Teo CB, Tan PY, Lee SX, Khoo J, Tan JG, Ang SF, Tan SH, Tay TL, Tan E, Lim SC, Boehm BO and Loh WJ (2022) Insulin Allergy to Detemir Followed by Rapid Onset of Diabetic Ketoacidosis: A Case Report and Literature Review. Front. Endocrinol. 13:844040. doi: 10.3389/fendo.2022.844040
Received: 27 December 2021; Accepted: 08 February 2022;
Published: 08 March 2022.
Edited by:
Hans Ulrich Häring, University of Tübingen, GermanyReviewed by:
Ayse Nur Torun, Near East University, CyprusCopyright © 2022 Teo, Tan, Lee, Khoo, Tan, Ang, Tan, Tay, Tan, Lim, Boehm and Loh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wann Jia Loh, bG9oLndhbm4uamlhQHNpbmdoZWFsdGguY29tLnNn
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.