- 1Department of Clinical and Experimental Sciences, Endocrine and Metabolic Unit, University of Brescia, Azienda Socio Sanitaria Territoriale (ASST) Spedali Civili Brescia, Brescia, Italy
- 2Unit of Internal Medicine and Endocrinology, Istituto Clinico Scientifico (ICS) Maugeri Istituto di Ricovero e Cura a Carattere Scientifico (I.R.C.C.S.), Laboratory for Endocrine Disruptors, University of Pavia, Pavia, Italy
- 3Unit of Andrology and Reproductive Medicine, Department of Medicine, University of Padova, Padova, Italy
SARS-CoV-2 infection, responsible for the coronavirus disease 2019 (COVID-19), can impair any organ system including endocrine glands. However, hypothalamic–pituitary dysfunctions following SARS-CoV-2 infection remain largely unexplored. We described a case of hypothalamic amenorrhea following SARS-CoV-2 infection in a 36-year-old healthy woman. The diagnostic workup excluded all the causes of secondary amenorrhea, in agreement to the current guidelines, whereas the gonadotropin increase in response to GnRH analogue tests was suggestive for hypothalamic impairment. Therefore, since our patient did not present any organic cause of hypothalamic–pituitary disorder, we hypothesized that her hypothalamic deficiency may have been a consequence of SARS-CoV-2 infection. This assumption, besides on the temporal consecutio, is strengthened by the fact that SARS-CoV-2 infection can impair the hypothalamic circuits, altering the endocrine axes, given that angiotensin-converting enzyme 2 receptors have also been observed in the hypothalamus. We reviewed the literature regarding hypothalamic–pituitary dysfunction in patients with SARS-CoV-2 infection. No study has previously described female hypogonadotropic hypogonadism with secondary amenorrhea following COVID-19. We suggest clinicians focusing greater attention on this possible endocrine disorder.
Introduction
Coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a primarily respiratory system disease, but it can lead to systemic manifestations, including in the cardiovascular, neurological, and gastrointestinal systems (1). Moreover, COVID-19 can also affect the endocrine system, since the angiotensin-converting enzyme 2 (ACE2) receptor—which is responsible together with transmembrane serine protease 2 (TMPRSS2) for the entry of SARS-CoV-2 to the cells—is also expressed in endocrine glands (thyroid, testis, ovary, adrenal, and pituitary) (2, 3). In addition, this virus can impair the hypothalamic circuits, altering the endocrine axes, since ACE2 receptors have also been observed in the hypothalamus (4). However, the endocrine complications, especially hypothalamic–pituitary dysfunction of COVID-19, are poorly reported and remain largely unexplored (5).
Hypothalamic–Pituitary Dysfunction Following COVID-19: Review of Literatures
We present a case of hypothalamic amenorrhea following SARS-CoV-2 infection, and we reviewed the literature regarding hypothalamic–pituitary dysfunction of COVID-19 to evaluate if this possible dysfunction has already been reported.
Hypothalamic–pituitary dysfunctions following SARS-CoV-2 infection in adults have been described (Table 1) (6–29).
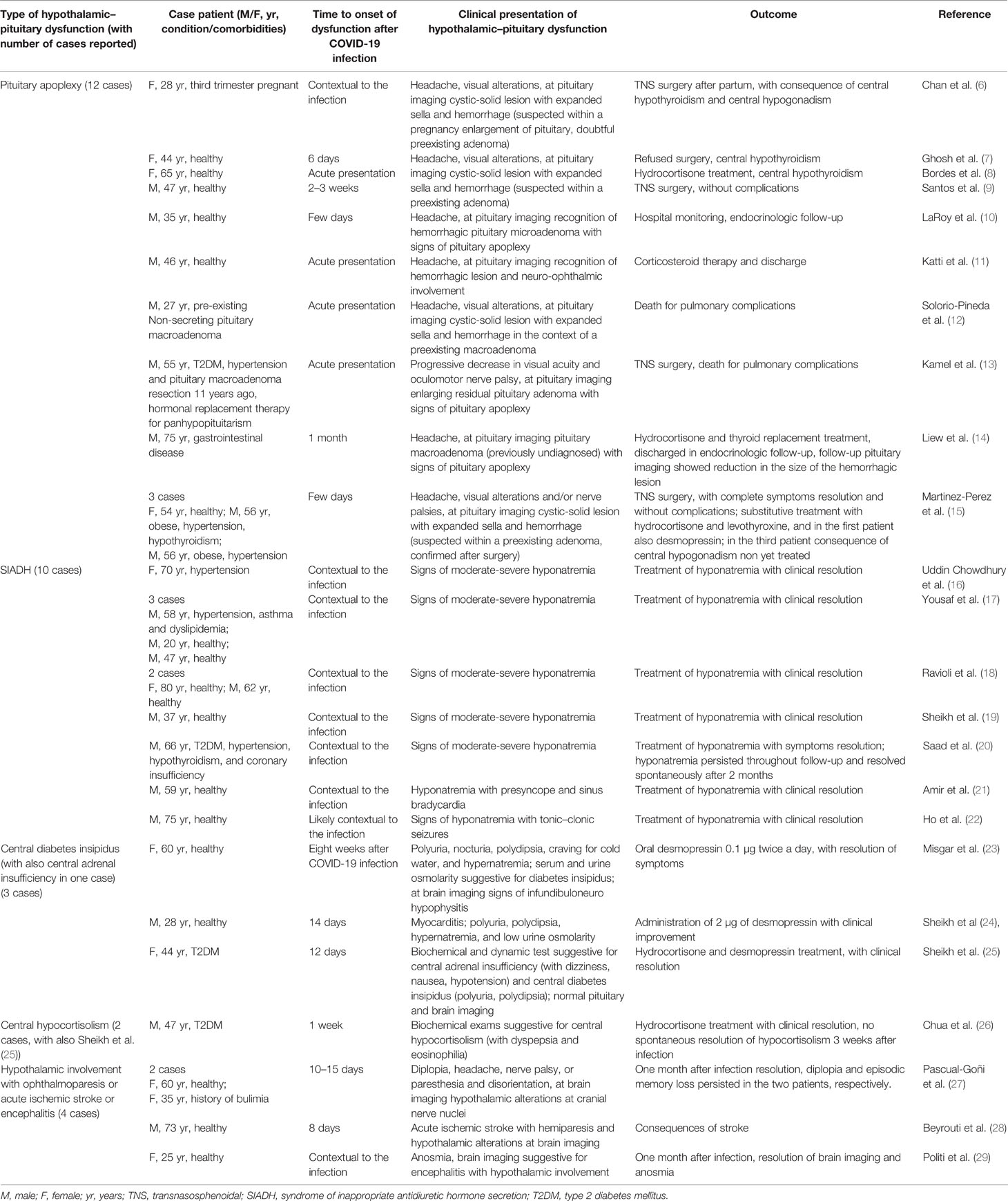
Table 1 Cases in literatures of hypothalamic–pituitary dysfunction after COVID-19 infection in adults.
The most frequently reported hypothalamic–pituitary alterations following COVID-19 are pituitary apoplexy and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) (5, 30).
Pituitary apoplexy is an acute syndrome due to a sudden vascular damage of the pituitary gland, with hemorrhagic infarction and ischemia, mainly in the context of a preexisting pituitary macroadenoma. Frara et al. have well described patients with pituitary diseases in which COVID-19 caused pituitary apoplexy, as a plausible precipitating risk factor (30). Moreover, Gaudino et al. recently reported hypothalamic–pituitary failure also in a child with SARS-CoV-2 infection and suprasellar tumor (31). Table 1 describes the clinical features and outcomes of cases of pituitary apoplexy in patients with SARS-CoV-2 infection.
On the other end, SIADH in COVID-19 seems due to several mechanisms related to systemic inflammation and pulmonary infection (17). In particular, a marked elevation of inflammatory cytokines can result in SIADH via two mechanisms. First, inflammatory cytokines (such as IL-6) can directly stimulate the no osmotic release of antidiuretic hormone (ADH) (32). Second, these cytokines can injure the lung tissue and alveolar cells, inducing SIADH via the hypoxic pulmonary vasoconstriction pathway (33). In patients with COVID-19, SIADH generally occurs with signs of moderate-severe hyponatremia (Table 1).
Few cases of central diabetes insipidus, central hypocortisolism, and rare findings of neurological symptoms with suspected radiological evidence of hypothalamic involvement have been described in patients with SARS-CoV-2 infection (Table 1).
Case Report
A 36-year-old woman came for evaluation at the Department of Clinical and Experimental Sciences, Endocrine and Metabolic Unit, ASST Spedali Civili Brescia, University of Brescia (Italy) in September 2021, reporting secondary amenorrhea for 6 months (last menstruation in March 8, 2021). Prior to March 2021, she reported regular menstrual cycles, one physiological pregnancy (in 2012), and no comorbidities or long-term therapy, and blood exams showed eugonadism (last assessment in December 2019). In March 2021, the patient presented symptoms including slight fever, myalgias, fatigue, sore throat, and hyposmia and was diagnosed with SARS-CoV-2 infection by nasopharyngeal reverse transcriptase polymerase reaction (positive swab on March 15, 2021). These symptoms were spontaneously resolved in 12 days without treatment or hospitalization (negative swab on April 15, 2021). She was vaccinated against SARS-CoV-2 in September 2021.
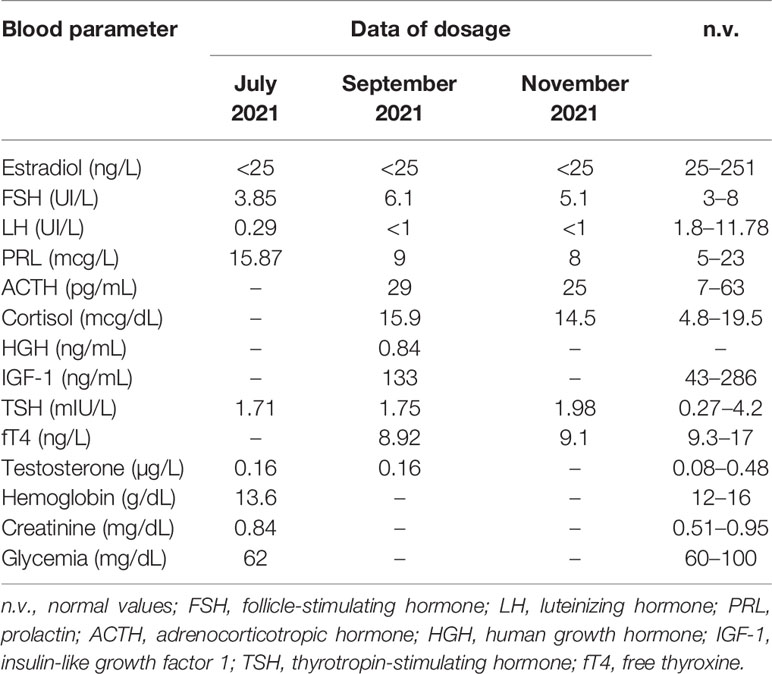
In July 2021, the patient underwent a gynecological evaluation: physical examination and pelvic ultrasound were normal, she presented no clinical and biochemical signs of hyperandrogenism (normal values of adrenal androgens), blood exams suggested hypogonadotropic hypogonadism (Table 2), and the medroxyprogesterone acetate (MAP) test was negative; pregnancy, polycystic ovary syndrome (PCOS), and gynecological causes of amenorrhea were excluded.
In August 2021, she underwent a dynamic enhanced MRI of the brain and pituitary region (given the finding of hypogonadotropic hypogonadism), showing a normal appearance of the sella turcica and regular dimensions of the adenohypophysis with uncertain millimetric (3 mm) pituitary microadenoma, and a normal appearance of the neurohypophysis, pituitary peduncle, median line structures, cavernous sinuses, optic chiasm, and supra- and subtentorial brain parenchyma. The FLAIR and DWI diffusive sequences of brain imaging did not report hypothalamic or other anormal findings.
At our endocrinological evaluation (September 2021), her temperature was 35.8°C, her pulse measured 72 beats per minute, and her blood pressure was 114/72 mmHg, weight 51 kg, height 1.58 m, and body mass index (BMI) 20.5 kg/m². She had no significant medical history, had no long-term therapy (no contraceptive pill or other drugs), and had never smoked. Besides amenorrhea, she presented no symptoms. Physical examination was normal. She reported no excessive physical training or significant fluctuations in body weight in recent years. The patient’s psychological state was carefully evaluated with particular care for stress related to the infection. The patient excluded any psychosocial state of stress.
On the suspicion of central amenorrhea, we carried out several blood tests (performed in the laboratory at our medical center): we excluded systemic disease (normal levels of inflammatory, coagulation, and hepatic markers), hemochromatosis (normal values of iron, ferritin, and transferrin saturation), celiac disease (negative anti-gliadin and anti-transglutaminase antibodies and no symptoms), overt primary thyroid disease, pituitary hypersecretion, adrenal axis deficit, and hyperprolactinemia, while we found low fT4 (free thyroxine) and confirmed hypogonadotropic hypogonadism, characterized in particular by low LH (luteinizing hormone) and estradiol with normal FSH (follicle-stimulating hormone) levels (Table 2).
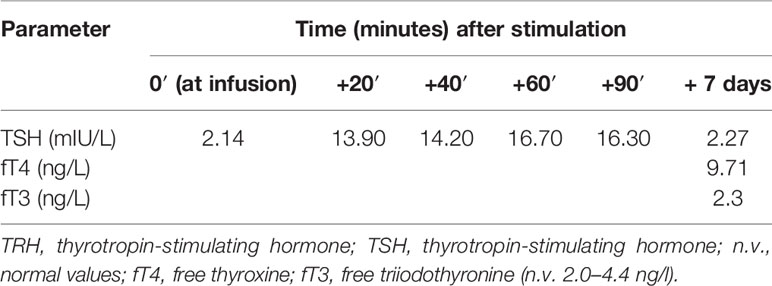
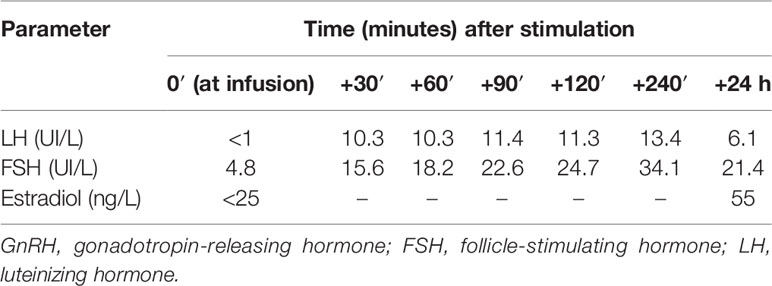
Then, on the suspicion of central amenorrhea and possible central hypothyroidism, we performed a TRH test (intravenous infusion of 200 mcg thyrotropin-stimulating hormone) and a GnRH (gonadotropin-releasing hormone) analogue test (with subcutaneous triptorelin 0.1 mcg), on September 30, 2021, and October 7, 2021, respectively. These tests indicated a delayed pituitary response to TRH and a gonadotropin increase to the GnRH analogue, suggesting a hypothalamic deficiency (Tables 3 and 4). In particular, LH and FSH were suppressed and normal, respectively, at baseline and both normally increased acutely and after 24 h from triptorelin injection. Seven days after the TRH test, she presented euthyroidism with normal values of TSH (thyrotropin-stimulating hormone), fT4, and fT3 (free triiodothyronine) (Table 3). Ten days after the GnRH analogue test, the patient experienced menstrual spotting for a few days only. On November 3, 2021 (Table 2), hypogonadotropic hypogonadism persisted (still characterized by low LH and estradiol and normal FSH values), without resumption of the menstrual cycle. We plan to reevaluate the patient to decide the diagnostic–therapeutic follow-up, based on her interest in bearing children.
Discussion
We described a case of hypothalamic amenorrhea following COVID-19.
Recently, Phelan and colleagues have described that the COVID-19 pandemic could affect female reproductive health, causing dysmenorrhea, irregular menstrual cycle, and premenstrual symptoms through psychological distress (34). Nevertheless, to the best of our knowledge, no study has described female hypogonadotropic hypogonadism with secondary amenorrhea following SARS-CoV-2 infection.
Secondary amenorrhea is defined as the cessation of previously regular menses for 3 months or previously irregular menses for 6 months (35). In our patient, a diagnostic workup excluded all the known causes of secondary amenorrhea, in agreement to the current indications (35, 36). She had no psychological distress or physical factors for causing functional hypothalamic amenorrhea.
To understand whether the disorder was due to a hypothalamic deficiency (GnRH deficiency) or pituitary disease (doubtful microadenoma of 3 mm), we performed a TRH test, given the finding of low fT4, and an GnRH analogue test, even if these tests are not suggested in the diagnostic workup for amenorrhea (35).
The delay of pituitary response to TRH was observed in our patient (37), but the gonadotropin increase in response to GnRH analogue tests was suggestive for hypothalamic impairment (38, 39). Therefore, since our patient did not present any organic cause of hypothalamic–pituitary disorder, we hypothesized that her hypothalamic deficiency may have been an apparent consequence of SARS-CoV-2 infection.
This assumption, besides on the temporal consecutio, is strengthened by the fact that SARS-CoV-2 infection can impair the hypothalamic circuits, altering the endocrine axes, given that ACE2 receptors—which are responsible for the cells of this virus entering the cells—have also been observed in the hypothalamus (4, 5) and with a low concentration also in pituitary tumors (40). Indeed, ACE2 mRNA expression in the hypothalamus has been reported in in vitro and animal studies and confirmed in an autoptic human study (30). An excessive inflammation-mediated process at the hypothalamic–pituitary level (resulting in impairment or lesions in areas of the brain including the hypothalamic circuits) (28, 29) and a direct hypothalamic damage (due to SARS-CoV-2 neuroinvasion through the nasopharyngeal epithelium via the olfactory nerve or blood/brain barrier) (4, 27) are the two possible mechanisms for the hypothalamic dysfunction due to COVID-19 (2, 41). Even if our hypothesis seems strengthened by the above evidence, we must consider as major limitation the impossibility to directly demonstrate the presence of virus in the hypothalamic tissue in vivo. Otherwise, it is also true that this limitation is frequently present in many reports regarding the interaction between SARS-CoV-2 infection and glands (11, 26, 42).
This dysfunction could unfortunately be of considerable significance, given the extent of the COVID-19 pandemic. Firstly, this hypothalamic alteration could be underdiagnosed, passing unnoticed in postmenopausal women due to the absence of secondary amenorrhea as a warning sign. In addition, and of note, we cannot exclude a possible impact of this condition on female fertility. In our patient, despite recovery from infection and a partial resumption of menstrual spotting after GnRH analogue stimulation, the hypothalamic amenorrhea does not yet appear reversible, to the extent that we must consider possible reproductive consequences.
Conclusion
We reported an unusual case of a young woman who developed hypothalamic amenorrhea following COVID-19. More cases are necessary to support the finding of hypothalamic amenorrhea as a complication of SARS-CoV-2 infection, also because it is difficult to directly demonstrate the presence of the virus in the hypothalamic tissue in vivo. Therefore, we suggest clinicians focusing greater attention and follow-up on possible existing or delayed endocrine disorders following COVID-19, in particular hypothalamic–pituitary dysfunction and female infertility. It would be desirable to consider and identify these possible complications and eventually direct the patient toward an adequate diagnostic–therapeutic management for female reproductive health.
Data Availability Statement
The raw data supporting the conclusions of this article have been made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Author Contributions
PF followed the patient, performed the literature review, wrote the first draft of the paper, and analyzed and critically discussed the results of the case report. VM followed the patient and wrote the first draft of the paper. AD wrote the first draft of the paper and analyzed and critically discussed the results of the case report. IP analyzed and critically discussed the results of the case report. MR analyzed and critically discussed the results of the case report. AF analyzed and critically discussed the results of the case report and the final version of the paper. CC followed the patient, coordinated the study, and analyzed and critically discussed the results of the case report and the final version of the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary Manifestations of COVID-19. Nat Med (2020) 26(7):1017–32. doi: 10.1038/s41591-020-0968-3
2. Pal R, Banerjee M. COVID-19 and the Endocrine System: Exploring the Unexplored. J Endocrinol Invest (2020) 43(7):1027–31. doi: 10.1007/s40618-020-01276-8
3. Marazuela M, Giustina A, Puig-Domingo M. Endocrine and Metabolic Aspects of the COVID-19 Pandemic. Rev Endocr Metab Disord (2020) 21(4):495–507. doi: 10.1007/s11154-020-09569-2
4. Mussa BM, Srivastava A, Verberne AJM. COVID-19 and Neurological Impairment: Hypothalamic Circuits and Beyond. Viruses (2021) 13(3):498. doi: 10.3390/v13030498
5. Frara S, Allora A, Castellino L, di Filippo L, Loli P, Giustina A. COVID-19 and the Pituitary. Pituitary (2021) 24(3):465–81. doi: 10.1007/s11102-021-01148-1
6. Chan JL, Gregory KD, Smithson SS, Naqvi M, Mamelak AN. Pituitary Apoplexy Associated With Acute COVID-19 Infection and Pregnancy. Pituitary (2020) 23(6):716–20. doi: 10.1007/s11102-020-01080-w
7. Ghosh R, Roy D, Mandal A, Dutta A, Naga D, Benito-León J. A Rare Case of SARS-CoV-2 Infection Associated With Pituitary Apoplexy Without Comorbidities. J Endocr Soc (2021) 5(3):bvaa203. doi: 10.1210/jendso/bvaa203
8. Bordes SJ, Phang-Lyn S, Najera E, Borghei-Razavi H, Adada B. Pituitary Apoplexy Attributed to COVID-19 Infection in the Absence of an Underlying Macroadenoma or Other Identifiable Cause. Cureus (2021) 13(2):e13315. doi: 10.7759/cureus.13315
9. Santos CDSE, Filho LMDC, Santos CAT, Neill JS, Vale HF, Kurnutala LN. Pituitary Tumor Resection in a Patient With SARS-CoV-2 (COVID-19) Infection. A Case Report and Suggested Airway Management Guidelines. Braz J Anesthesiol (2020) 70(2):165–70. doi: 10.1016/j.bjane.2020.05.003
10. LaRoy M, McGuire M. Pituitary Apoplexy in the Setting of COVID-19 Infection. Am J Emerg Med (2021) 47:329.e1–2. doi: 10.1016/j.ajem.2021.02.045
11. Katti V, Ramamurthy LB, Kanakpur S, Shet SD, Dhoot M. Neuro-Ophthalmic Presentation of COVID-19 Disease: A Case Report. Indian J Ophthalmol (2021) 69(4):992–4. doi: 10.4103/ijo.IJO_3321_20
12. Solorio-Pineda S, Almendárez-Sánchez CA, Tafur-Grandett AA, Ramos-Martínez GA, Huato-Reyes R, Ruiz-Flores MI, et al. Pituitary Macroadenoma Apoplexy in a Severe Acute Respiratory Syndrome-Coronavirus-2-Positive Testing: Causal or Casual? Surg Neurol Int (2020) 11:304. doi: 10.25259/SNI_305_2020
13. Kamel WA, Najibullah M, Saleh MS, Azab WA. Coronavirus Disease 2019 Infection and Pituitary Apoplexy: A Causal Relation or Just a Coincidence? A Case Report and Review of the Literature. Surg Neurol Int (2021) 12:317. doi: 10.25259/SNI_401_2021
14. Liew SY, Seese R, Shames A, Majumdar K. Apoplexy in a Previously Undiagnosed Pituitary Macroadenoma in the Setting of Recent COVID-19 Infection. BMJ Case Rep (2021) 14(7):e243607. doi: 10.1136/bcr-2021-243607
15. Martinez-Perez R, Kortz MW, Carroll BW, Duran D, Neill JS, Luzardo GD, et al. Coronavirus Disease 2019 and Pituitary Apoplexy: A Single-Center Case Series and Review of the Literature. World Neurosurg (2021) 152:e678–87. doi: 10.1016/j.wneu.2021.06.004
16. Uddin Chowdhury MR, Akter KS, Moula MM, Kabir MA, Bhuiyan SI, Das BC. COVID-19 Presented With Syndrome of Inappropriate ADH Secretion(SIADH): A Case Report From Bangladesh. Respir Med Case Rep (2020) 31:101290. doi: 10.1016/j.rmcr.2020.101290
17. Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-Associated SIADH: A Clue in the Times of Pandemic! Am J Physiol Endocrinol Metab (2020) 318(6):E882–5. doi: 10.1152/ajpendo.00178.2020
18. Ravioli S, Niebuhr N, Ruchti C, Pluess E, Stoeckli T, Lindner G. The Syndrome of Inappropriate Antidiuresis in COVID-19 Pneumonia: Report of Two Cases. Clin Kidney J (2020) 13(3):461–2. doi: 10.1093/ckj/sfaa080
19. Sheikh MM, Ahmad E, Jeelani HM, Riaz A, Muneeb A. COVID-19 Pneumonia: An Emerging Cause of Syndrome of Inappropriate Antidiuretic Hormone. Cureus (2020) 12(6):e8841. doi: 10.7759/cureus.8841
20. Saad G, Abdelkrim AB, El Abed YH, Tahri S, Gorchene A, Maaroufi A, et al. Disorders of Sodium Balance in COVID-19 Patients: Two Tunisian Patients Report. Pan Afr Med J (2021) 39:199. doi: 10.11604/pamj.2021.39.199.27626
21. Amir M, Renata A, Ratana LT. Symptomatic Sinus Bradycardia Due to Electrolyte Imbalances in Syndrome of Inappropriate Antidiuretic Hormone (SIADH) Related Covid-19: A Case Report. BMC Infect Dis (2021) 21(1):465. doi: 10.1186/s12879-021-06143-2
22. Ho KS, Narasimhan B, Kumar A, Flynn E, Salonia J, El-Hachem K, et al. [Syndrome of Inappropriate Antidiuretic Hormone as the Initial Presentation of COVID-19: A Novel Case Report]. Nefrol (Engl Ed) (2021) 41(2):219–20. doi: 10.1016/j.nefro.2020.05.004
23. Misgar RA, Rasool A, Wani AI, Bashir MI. Central Diabetes Insipidus (Infundibuloneuro Hypophysitis): A Late Complication of COVID-19 Infection. J Endocrinol Invest (2021) 44(12):2855–6. doi: 10.1007/s40618-021-01627-z
24. Sheikh AB, Javed N, Sheikh AAE, Upadhyay S, Shekhar R. Diabetes Insipidus and Concomitant Myocarditis: A Late Sequelae of COVID-19 Infection. J Investig Med High Impact Case Rep (2021) 9:2324709621999954. doi: 10.1177/2324709621999954
25. Sheikh AB, Javaid MA, Sheikh AAE, Shekhar R. Central Adrenal Insufficiency and Diabetes Insipidus as Potential Endocrine Manifestations of COVID-19 Infection: A Case Report. Pan Afr Med J (2021) 38:222. doi: 10.11604/pamj.2021.38.222.28243
26. Chua MWJ, Chua MPW. Delayed Onset of Central Hypocortisolism in a Patient Recovering From COVID-19. AACE Clin Case Rep (2021) 7(1):2–5. doi: 10.1016/j.aace.2020.11.001
27. Pascual-Goñi E, Fortea J, Martínez-Domeño A, Rabella N, Tecame M, Gómez-Oliva C, et al. COVID-19-Associated Ophthalmoparesis and Hypothalamic Involvement. Neurol Neuroimmunol Neuroinflamm 7(5) (2020) 7(5):e823. doi: 10.1212/NXI.0000000000000823
28. Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of Ischaemic Stroke Associated With COVID-19. J Neurol Neurosurg Psychiatry (2020) 91(8):889–91. doi: 10.1136/jnnp-2020-323586
29. Politi LS, Salsano E, Grimaldi M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol (2020) 77(8):1028–9. doi: 10.1001/jamaneurol.2020.2125
30. Frara S, Loli P, Allora A, Santini C, di Filippo L, Mortini P, et al. COVID-19 and Hypopituitarism. Rev Endocr Metab Disord (2021) 23(2):215–31. doi: 10.1007/s11154-021-09672-y
31. Gaudino R, Orlandi V, Cavarzere P, Chinello M, Antoniazzi F, Cesaro S, et al. Case Report: SARS-CoV-2 Infection in a Child With Suprasellar Tumor and Hypothalamic-Pituitary Failure. Front Endocrinol (Lausanne) (2021) 12:596654. doi: 10.3389/fendo.2021.596654
32. Park SJ, Shin JI. Inflammation and Hyponatremia: An Underrecognized Condition? Korean J Pediatr (2013) 56(12):519–22. doi: 10.3345/kjp.2013.56.12.519
33. Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-Mediated Inflammation in Acute Lung Injury. Cytokine Growth Factor Rev (2003) 14(6):523–35. doi: 10.1016/s1359-6101(03)00059-5
34. Phelan N, Behan LA, Owens L. The Impact of the COVID-19 Pandemic on Women's Reproductive Health. Front Endocrinol (Lausanne) (2021) 12:642755. doi: 10.3389/fendo.2021.642755
35. Klein DA, Paradise SL, Reeder RM. Amenorrhea: A Systematic Approach to Diagnosis and Management. Am Fam Physician (2019) 100(1):39–48.
36. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2017) 102(5):1413–39. doi: 10.1210/jc.2017-00131
37. Persani L, Brabant G, Dattani M, Bonomi M, Feldt-Rasmussen U, Fliers E, et al. European Thyroid Association (ETA) Guidelines on the Diagnosis and Management of Central Hypothyroidism. Eur Thyroid J (2018) 7(5):225–37. doi: 10.1159/000491388
38. Zimmer CA, Ehrmann DA, Rosenfield RL. Potential Diagnostic Utility of Intermittent Administration of Short-Acting Gonadotropin-Releasing Hormone Agonist in Gonadotropin Deficiency. Fertil Steril (2010) 94(7):2697–702. doi: 10.1016/j.fertnstert.2010.04.019
39. Mortimer CH, Besser GM, McNeilly AS, Marshall JC, Harsoulis P, Tunbridge WM, et al. Luteinizing Hormone and Follicle Stimulating Hormone-Releasing Hormone Test in Patients With Hypothalamic-Pituitary-Gonadal Dysfunction. Br Med J (1973) 4(5884):73–7. doi: 10.1136/bmj.4.5884.73
40. Gu WT, Zhou F, Xie WQ, Wang S, Yao H, Liu YT, et al. A Potential Impact of SARS-CoV-2 on Pituitary Glands and Pituitary Neuroendocrine Tumors. Endocrine (2021) 72(2):340–8. doi: 10.1007/s12020-021-02697-y
41. Oliviero A, de Castro F, Coperchini F, Chiovato L, Rotondi M. COVID-19 Pulmonary and Olfactory Dysfunctions: Is the Chemokine CXCL10 the Common Denominator? Neuroscientist (2021) 27(3):214–21. doi: 10.1177/1073858420939033
Keywords: hypothalamic amenorrhea, COVID-19, hypothalamic–pituitary dysfunction, female hypogonadotropic hypogonadism, central amenorrhea
Citation: Facondo P, Maltese V, Delbarba A, Pirola I, Rotondi M, Ferlin A and Cappelli C (2022) Case Report: Hypothalamic Amenorrhea Following COVID-19 Infection and Review of Literatures. Front. Endocrinol. 13:840749. doi: 10.3389/fendo.2022.840749
Received: 21 December 2021; Accepted: 02 May 2022;
Published: 10 June 2022.
Edited by:
Lucio Vilar, Federal University of Pernambuco, BrazilReviewed by:
Zhe Bao Wu, Shanghai Jiao Tong University, ChinaIoannis Ilias, General-Maternity District Hospital Helena Venizelou, Greece
Rahnuma Ahmad, Medical College for Women and Hospital, Bangladesh
Mainul Haque, National Defence University of Malaysia, Malaysia
Copyright © 2022 Facondo, Maltese, Delbarba, Pirola, Rotondi, Ferlin and Cappelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Cappelli, Y2FybG8uY2FwcGVsbGlAdW5pYnMuaXQ=
 Paolo Facondo
Paolo Facondo Virginia Maltese1
Virginia Maltese1 Andrea Delbarba
Andrea Delbarba Mario Rotondi
Mario Rotondi Alberto Ferlin
Alberto Ferlin Carlo Cappelli
Carlo Cappelli