- 1Department of Obstetrics and Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 2Department of Medical Statistics and Epidemiology, Sun Yat-sen University, Guangzhou, China
- 3School of Public Health, Harvard University, Boston, MA, United States
- 4Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH), Bethesda, MD, United States
Objective: Since Asians are particularly vulnerable to the risk of gestational diabetes mellitus (GDM), the lifecourse health implications of which are far beyond pregnancy, we aimed to summarize the literature to understand the research gaps on current GDM research among Asians.
Methods: We systematically searched the articles in PubMed, Web of Science, Embase, and Scopus by 30 June 2021 with keywords applied on three topics, namely “GDM prevalence in Asians”, “GDM and maternal health outcomes in Asians”, and “GDM and offspring health outcomes in Asians”.
Results: We observed that Asian women (natives and immigrants) are at the highest risk of developing GDM and subsequent progression to type 2 diabetes among all populations. Children born to GDM-complicated pregnancies had a higher risk of macrosomia and congenital anomalies (i.e. heart, kidney and urinary tract) at birth and greater adiposity later in life.
Conclusion: This review summarized various determinants underlying the conversion between GDM and long-term health outcomes in Asian women, and it might shed light on efforts to prevent GDM and improve the lifecourse health in Asians from a public health perspective.
Systematic Review Registration: Prospero, CRD42021286075.
Introduction
Diabetes is a significant cause of morbidity, mortality, and healthcare costs worldwide (1). The global age-adjusted comparative prevalence of diabetes among adults between 20-79 years of age was estimated at 8.3% (463 million) in 2019 (2), including 223 million women living with diabetes. And it is projected to reach 700 million people and 343 million women alone in 2045, respectively (2). Diabetes in pregnancy is similarly increasing in prevalence, with concerning consequences for both mother and offspring (3). Approximately 1 in 6 live births is affected by diabetes in pregnancy, 84% of which are diagnosed as gestational diabetes mellitus (GDM) (2, 4).
GDM is defined as glucose intolerance with the first onset or recognition during pregnancy (2, 4). Women with GDM have higher risks of cardiometabolic disorders during pregnancy and later in life (5). At the same time, offspring born to women with a history of GDM also encounter increased risks of developing obesity and other cardiometabolic disorders later in life (6, 7). The documented prevalence of GDM varies substantially worldwide, ranging from 1% to >30% (3), while compelling evidence has shown Asians share a high prevalence (i.e., Middle East: 8.8-20.0%; South-East Asia: 9.6-18.3%; Western Pacific: 4.5-20.3%) (3) regardless of the racial/ethnic differences in body mass index (BMI).
A meta-analysis found a more than sevenfold increased risk of T2DM in women with GDM after index pregnancy, compared with women with normoglycaemic pregnancies (8). Data on risk factors—particularly modifiable risk factors that may inform effective intervention strategies are relatively more collected in the Western population (e.g., North America, Europe, and Oceania) than the Asian population (3, 8–10). Research reporting a full spectrum of long-term health outcomes among both mothers and offspring following pregnancies complicated by GDM also mainly stemmed from the Western population (11). Furthermore, GDM studies have not been comprehensively reviewed on Asian immigrants exclusively, given that an increasing number of Asian migrants live in Western countries for a long-term residency (12). Due to the different environmental exposures such as socioeconomic transitions, lifestyle adaptations, cultural assimilation hardship, and health disparities9,10, there might be exceptionally high attributable risks on GDM development for Asian immigrants compared with Native Asians.
This review sought to summarize the literature to understand research gaps and develop future research directions on Asian women with GDM from a population health perspective. Thus, our review serves the objectives to 1) comprehensively examine the epidemiology of GDM, its risk factors, and health consequences; and 2) identify areas for future research for public health interventions to prevent GDM and its health consequences.
Methods
Search Strategy and Selection Criteria
We conducted the systematic review according to PRISMA for systematic review protocols. References for this review were identified through searches of Pubmed, Web of Science, Embase, and Scopus for articles published until 30 June 2021. We included three topics in our review, namely “Topic 1—GDM prevalence in Asians”, “Topic 2—GDM and maternal health outcomes in Asians”, and “Topic 3—GDM and offspring health outcomes in Asians”. Search terms included “prevalence”, “incidence”, “gestational diabetes mellitus”, “gestational diabetes” and “diabetes in pregnancy” in combination with the terms “Asia”, “Asians” and “Asian countries” in Topic 1. Search terms included “gestational diabetes mellitus”, “gestational diabetes” and “diabetes in pregnancy” in combination with the terms “Type 2 diabetes”, “prediabetes”, “glucose intolerance”, “abnormal glucose”, “hypertension”, “high blood pressure”, “cardiovascular disease”, “kidney disease”, “cancer”, “liver dysfunction”, “non-alcoholic fatty liver disease” and “health outcomes” and also in combination with the terms “After delivery” and “postpartum” in Topic 2. Search terms included “gestational diabetes mellitus”, “gestational diabetes”, “diabetes in pregnancy” and in combination with terms “cardio-metabolic outcome”, “cognitive outcome”, “congenital disease”, “adiposity”, “hypertension”, “health outcome”, “neuro-cognitive outcome”, “obesity”, “diabetes”, “cardiovascular disease”, “kidney disease” and “cancer” and also in combination with “child” and “offspring” in Topic 3. Articles resulting from these searches and relevant references cited in those articles were reviewed, among which reporting non-Asian human subjects or without full-text available were excluded. Flow charts for literature searching on each topic are shown in Supplementary Figures 1–3. The Prospero registration number for this systematic review is registered as CRD42021286075.
Data Screening & Assessments
Double literature screening was conducted during the literature searching phase by two investigators (H L & L-J L). Furthermore, one investigator (A C) performed the quality assessments for all papers based on the Newcastle–Ottawa Scale Criteria (NOSC), and the other investigators (L-J L) verified the findingsindependently. The maximum score of 9 points in the Newcastle–Ottawa Scale is distributed in three aspects, namely selection of study groups (four points), comparability of groups (two points), and ascertainment of exposure and outcomes (three points) for case–control and cohort studies (13). We used the points to further categorize the publication quality with low risk of bias (between 7-9 points), high risk of bias (between 4-6 points), and very high risk of bias (between 0-3 points) (Supplementary Tables 1, 2).
Results
Prevalence of GDM by Geography
Overview
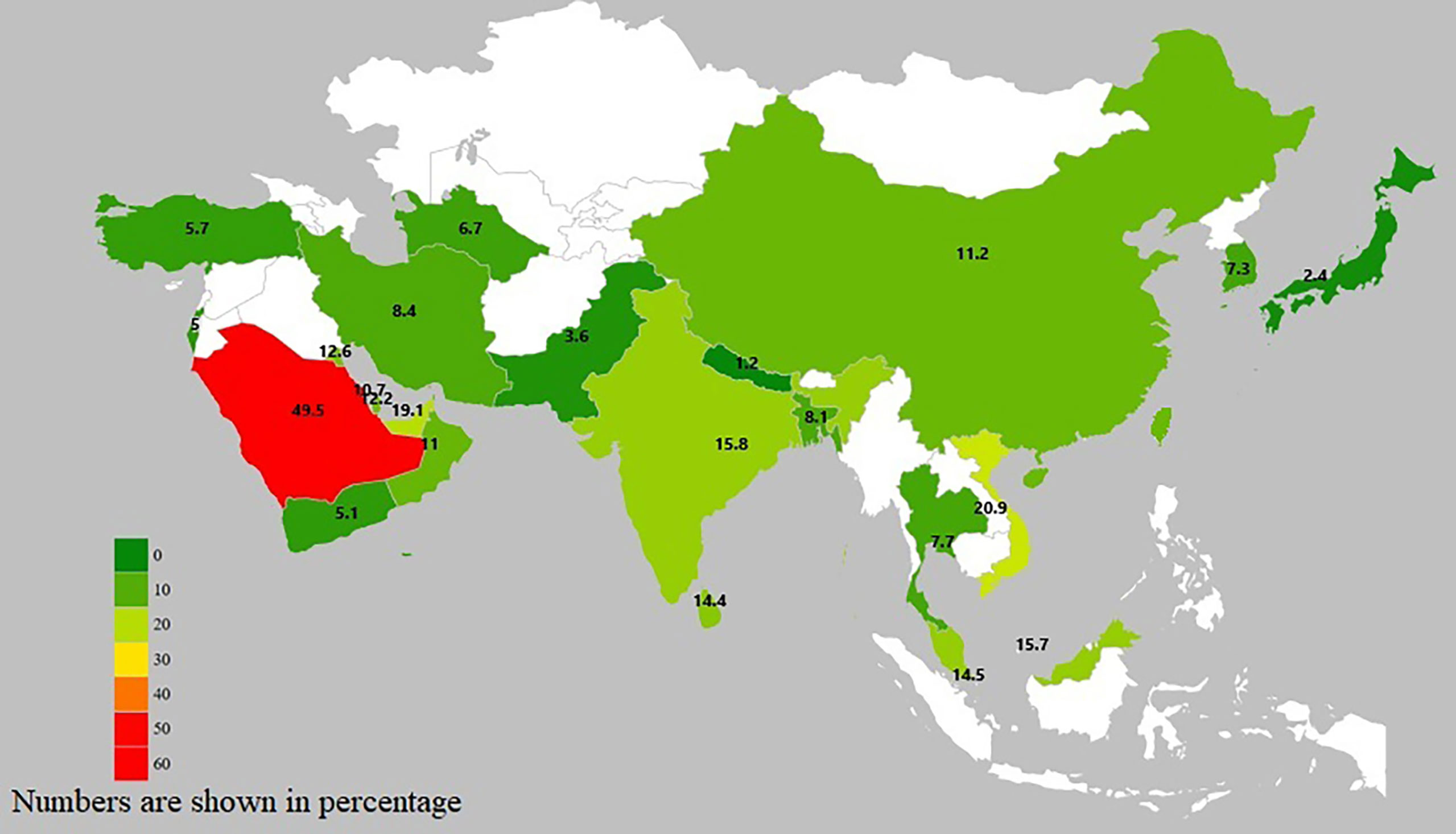
GDM prevalence in Asian countries ranges widely from 1.2 to 49.5%, largely accounting for differences in diagnostic criteria, sample size and population source (e.g., hospital-based, community-based) (Figure 1 and Supplementary Table 3).
Guideline-Specific Prevalence of GDM
The prevalence of GDM varied substantially across Asian countries using different guidelines (Figure 2). We identified 29 GDM diagnostic criteria (Supplementary Table 4), among which the International Association of Diabetes and Pregnancy Study Groups (IADPSG) (14), World Health Organization (WHO) (15), Carpenter-Coustan (16), and American College of Obstetricians and Gynecologists (ACOG) (17) criteria were commonly used. Some countries adopted international guidelines as their national guidelines [e.g., China MOH guidelines (18), Malaysia MOH guidelines (19)], while some countries defined their own [e.g., Japan [Japan Diabetes Society] (20), India [Diabetes in Pregnancy Study group of India; DIPSI] (21), Turkmenistan (22), Oman (23)]. As the majority (123 out of 147) of included studies were published since 2010, we were not able to tease out whether the increment in GDM prevalence over the years in Asians is due to emerging evidence or new adoption of universal screening [i.e., IADPSG (14)].
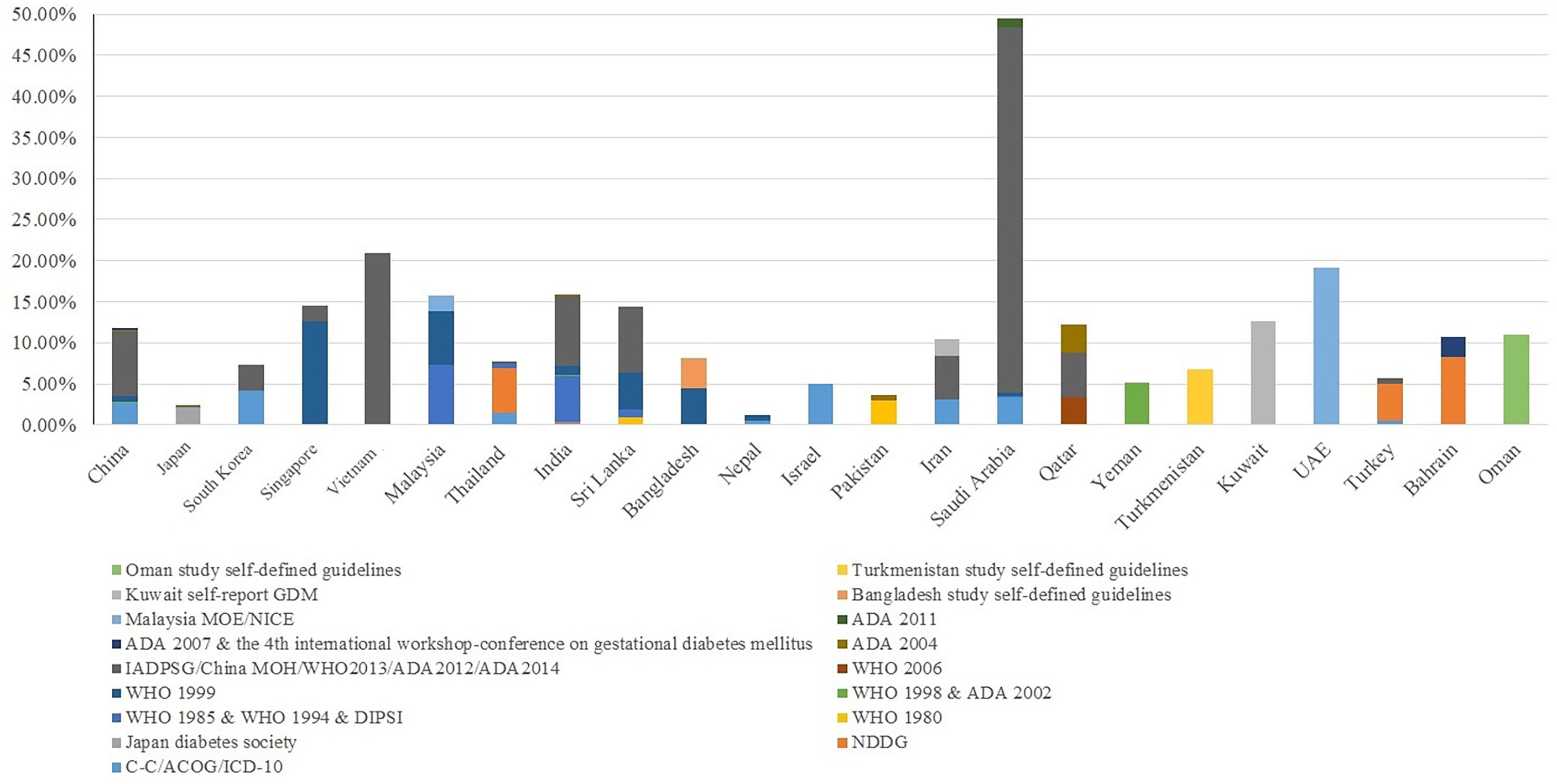
Figure 2 Country-specific prevalence of GDM in Asian studies. Due to the homogeneity of Chinese population residing in China, Taiwan and Hong Kong, we reported the country-specific prevalence of these three regions as a whole.
We included studies using either one-step or two-step diagnostic guidelines, the latter of which performed a 1-h 50-g glucose challenge test (GCT) glucose challenging test (GCT) additionally during 24-28 weeks of gestation, with a whole blood glucose threshold of 7.2 mmol/l (130 mg/dl). In general, we observed a link between adopting any one-step diagnostic guidelines (e.g., the IADPSG guidelines, the WHO 1999 guidelines) and higher GDM prevalence among Asian studies. For example, countries exclusively using (e.g., Singapore, UAE) or primarily using (e.g., China, Saudi Arabia, India) a one-step diagnostic approach reported an overall GDM prevalence above 10%. In contrast, countries exclusively using (e.g., Pakistan, South Korea) or primarily using (e.g., Thailand, Turkey, Japan) a two-step diagnostic approach reported an overall GDM prevalence below 10% (Figure 3).
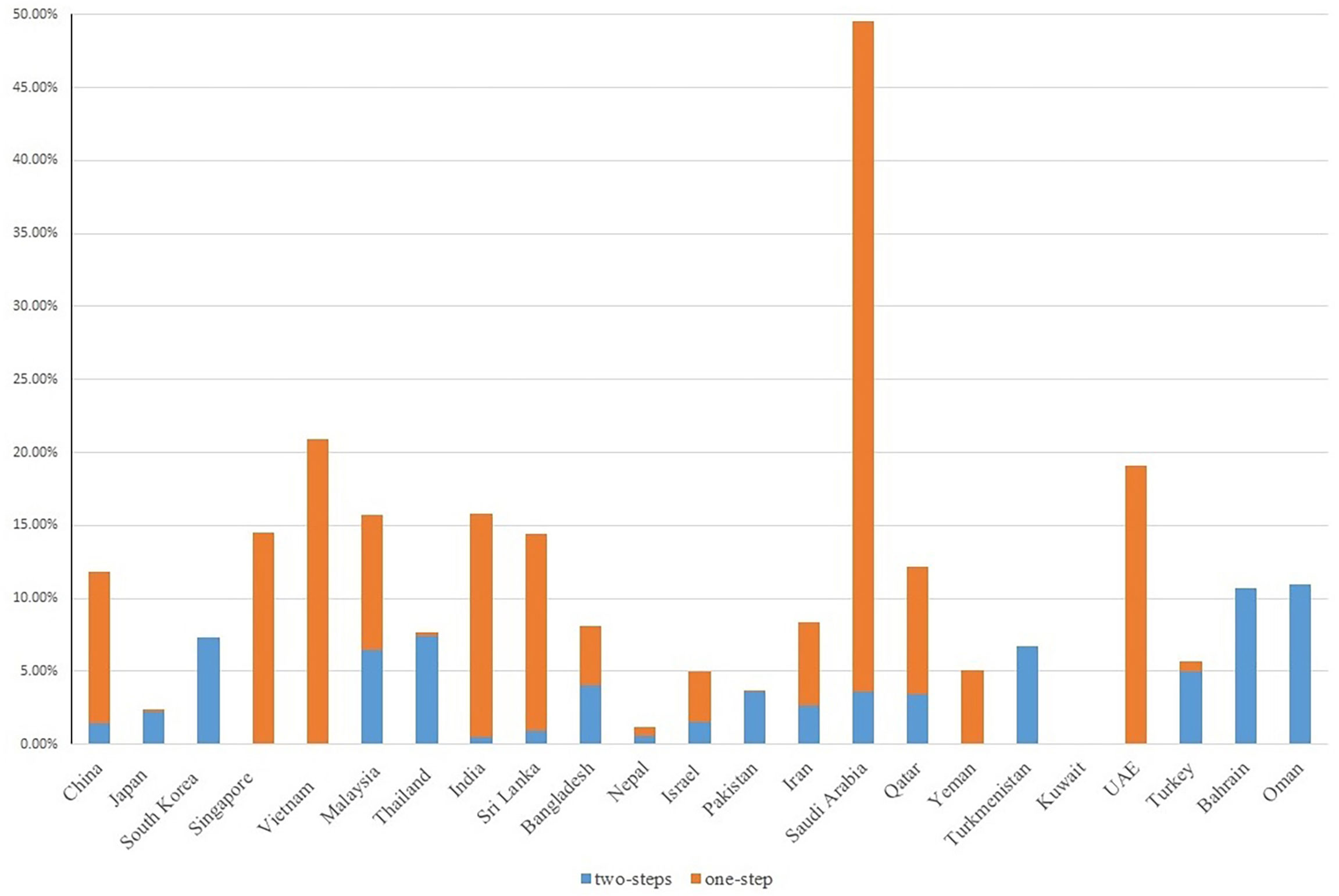
Figure 3 GDM screening steps with GDM prevalence in Asian studies. Due to the homogeneity of Chinese population residing in China, Taiwan and Hong Kong, we reported the country-specific prevalence of these three regions as a whole.
Prevalence of GDM in Asian Migrants
Twenty-eight studies reported GDM prevalence among Asian migrants in Europe, Oceania, and North America, with sample sizes ranging from 1,491 to 10,823,924 participants. Overall GDM prevalence among Asian migrants is comparable to the Native Asian population. However, the prevalence of GDM was generally higher in Asian immigrants (0.18%-24.2%) than non-Hispanic White (NHW) (0.02%-7.0%) living in the same country, regardless of GDM diagnostic guidelines used (Supplementary Table 5). Among Asian immigrants in UK and Norway, South, East, and West Asian immigrants, as a whole, had doubled the odds for GDM than NHW (24, 25). Interestingly, length of immigration and birth countries seemed to relate to GDM prevalence. For instance, Danish-Chinese migrants with a longer stay (≥ 10 years) had a 62% higher odds of GDM onset than those with a shorter stay (≤ 5 years) (26). Also, foreign-born US-Indian migrants had a higher GDM prevalence than local-born US-Indian migrants (22.9% vs. 12.8%) (27).
Adverse Health Outcomes and Attributable Risk Factors Following an Index GDM-Complicated pregnancy
Overview
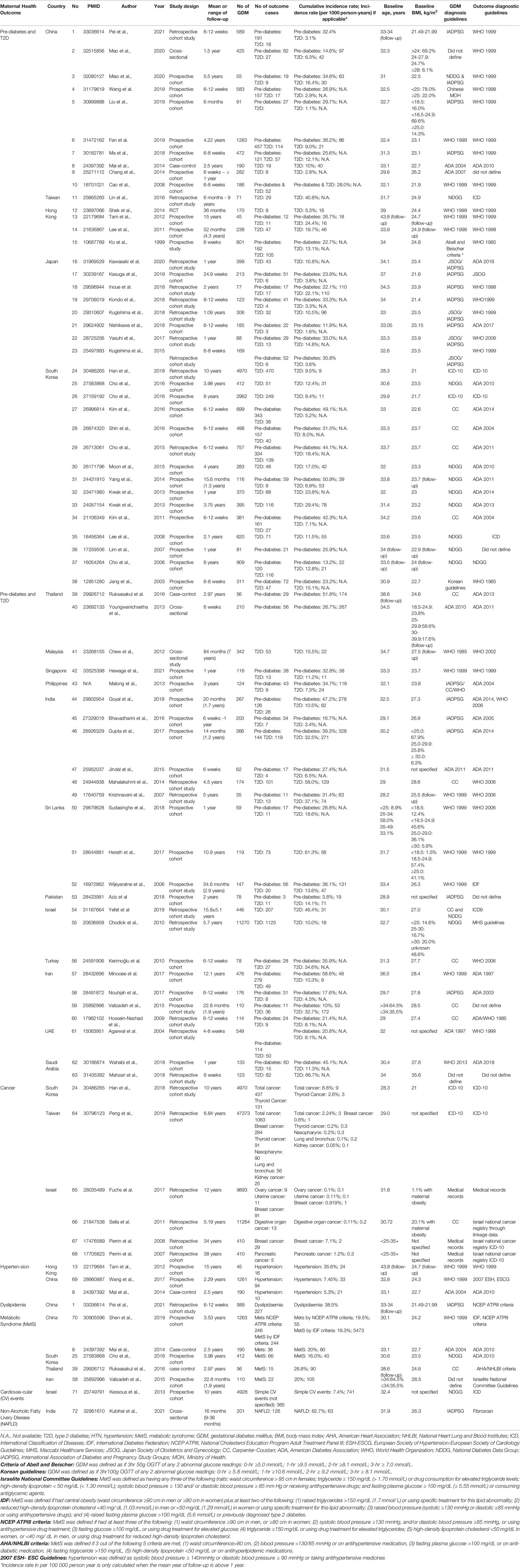
Overall, seventy-two studies, predominantly longitudinal cohorts on GDM and maternal postpartum health outcomes, were identified in Asian countries (Table 1 and Figure 4). Among them, prediabetes and T2D, cardiovascular disorders, cancer, and non-alcoholic fatty liver disease (NAFLD) were reported following index pregnancy complicated by GDM, with a mean or median follow-up from 4 weeks to 38 years after delivery. The majority of studies were reported from East Asia (42/72 studies, 58.3%), especially in the Chinese population. Two studies that reported postpartum T2D development in Asian immigrants were identified (Supplementary Table 6). Thirteen out of 74 included studies (18%) were assessed low in risk of bias, while the rest majority (80%) were either high or very high risk of bias (Supplementary Table 1).
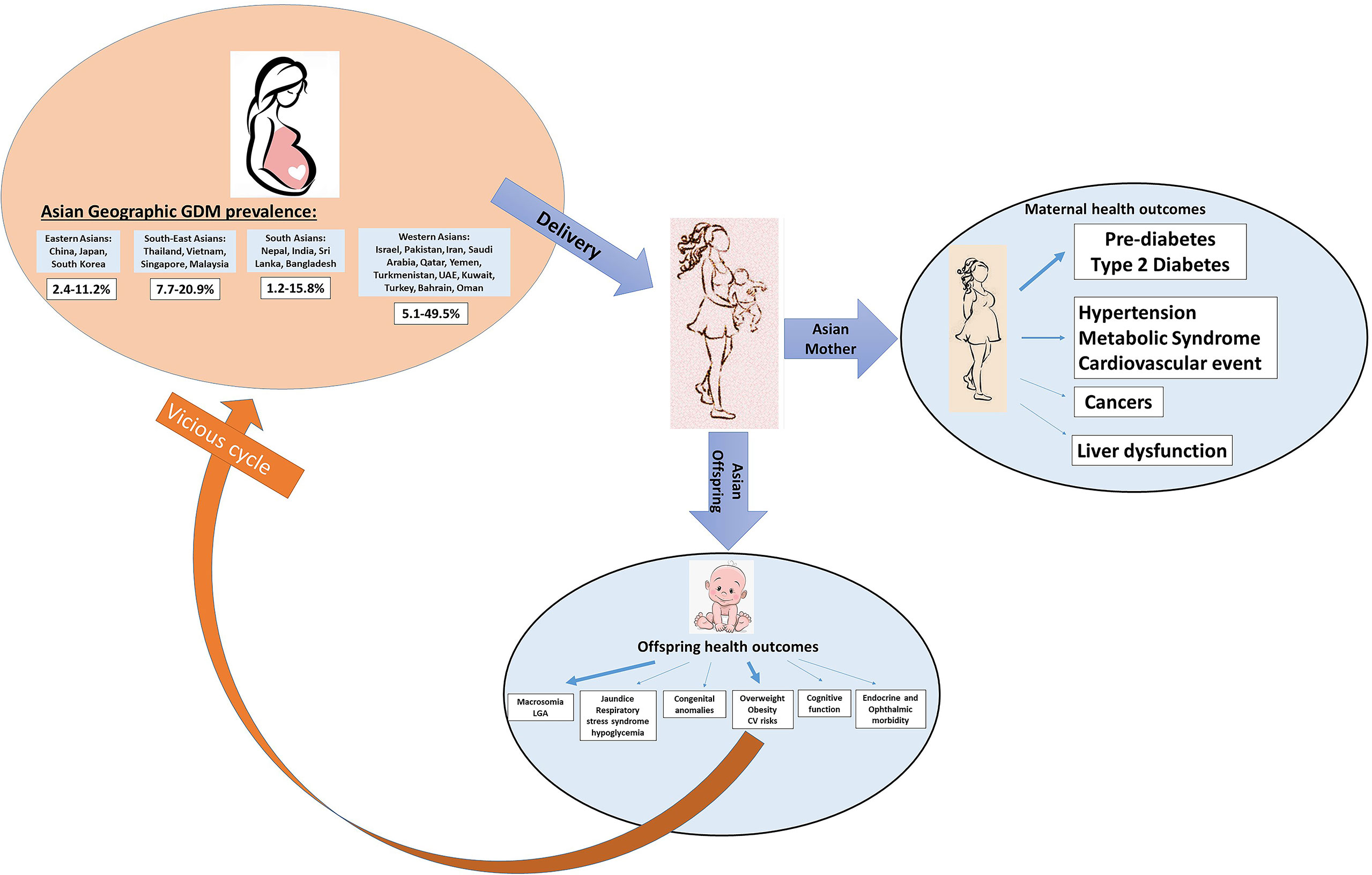
Figure 4 Schematic graphs of GDM leading transgenerational health outcomes in Asian studies. The arrow represents the found associations between GDM and different transgenerational outcomes. A thicker arrow indicates a higher number of studies reported on this topic.
Prediabetes and T2D
It is well-known that women with a history of GDM have a substantially increased risk of developing T2D than counterparts without such a history (8). A systematic review and meta-analysis on prospective studies with reasonable retention rates (mainly on European women) suggested that the conversion rate from GDM to T2D was seven folds increased among women GDM after index pregnancy, compared with those who had a normoglycaemic pregnancy (RR 7.43, 95% CI: 4.79-11.51) (8).
Sixty-three studies described the postpartum incidence rate of prediabetes and T2D among mothers diagnosed with GDM in Asia, with sample sizes ranging from 35 to 11 270 subjects, most of which defined prediabetes and T2D using the same guidelines [e.g., WHO 1999 (41) or ADA 2014 guidelines (42)] even though their GDM diagnostic criteria differed. We reported the percentage incidence (%) if prediabetes or T2D was recorded within one year from delivery (mostly between 6 and 12 weeks). Then we reported person-years incidence (per 1000 person-years) if prediabetes or T2D was recorded beyond one year from delivery (up to 15 years).
Within one year from delivery, the conversion rate varied significantly between studies from GDM to prediabetes (11.9%-49.1%) and from GDM to T2D (1.1%-66.7%), respectively. Beyond one year after delivery, the incidence rate from GDM to T2D was the highest in South Asia (47 – 271 per 1000 person-years), followed by East Asia (9 – 110 per 1000 person-years). We noted inconsistencies with study estimates within the same region. For instance, one study in Iran reported a much higher incidence T2D conversion rate than another study in Iran (172 vs. 9 per 1000 person-years) (35, 43). Potential reasons for inconsistencies in the conversion rates from GDM to T2D could be the variation in studied population characteristics, duration of follow-up, retention rate, and data collection quality.
As for Asian immigrants, we identified only two reports comparing Asian immigrants with non-Asian counterparts, one from Spain with one-year follow-up (44) and the other from the US with an average 7.6-year follow-up (45). Both studies suggested that prediabetes and T2D conversion rates were higher in South Asian migrants than native NHW [prediabetes: 43.3% vs. 28.5% (44); T2D: 55 vs. 40 per 1000 person-years (45)].
Existing data on risk factors of T2D among women with a history of GDM were firstly reported in the NHW population, such as greater pre-pregnancy BMI (8, 9), excessive weight gain (3), unhealthy dietary patterns (3), physical inactivity (3), and a short period of lactation (3, 10). In the Asian population, there are also quite a few at-risk pre-natal maternal characteristics recently added to this pond of evidence, such as family history of diabetes (43), a higher degree of consanguineous marraiges (43), higher pre-pregnancy BMI (29, 31, 32, 46), higher total cholesterol quartile at GDM diagnosis during the index pregnancy (47), younger age at delivery (<30 years) (46), and a short period of lactation (<6 months) (33). Post-natal risk such as missing medical assistance in the continuum of GDM care after delivery could be another risk for T2D progression among Asian mothers with a history of GDM (48).
Cardiovascular Disorders
Hypertension
A history of GDM was related to increased risk of hypertension (HTN) after the index pregnancy in some but not all studies. For instance, the US Nurses’ Health Study found an increased risk of postpartum HTN among women with a history of GDM (49). In contrast, a Dutch cohort suggested the risk of developing HTN was mainly significant among women with a history of hypertensive disorders during pregnancy (HDP) rather than GDM (50). Among the three studies identified in our review on GDM and subsequent hypertension risk (28, 30, 38), the Chinese Tianjin GDM prevention program reported a much higher incidence rate of HTN among women diagnosed with HDP and GDM than women with GDM alone (118 vs. 26 per 1000 person-years) (38), which partially agreed with the Dutch cohort.
The mechanisms underlying postpartum HTN in women with GDM remain un-elucidated. Insulin resistance may be a component of the underlying pathophysiology linking GDM with postpartum HTN, with or without HDP (51). As we know, obesity and excessive weight gain during pregnancy are associated with insulin resistance (38), inflammation and oxidation (52), all of which may lead to permanent vascular damage (51) and even irreversible peripheral vascular resistance. Due to the largely inadequate evidence, future research to investigate the role of antenatal and postpartum lifestyle (e.g., dietary patterns, physical activities) in the progression of HTN is warranted in Asians.
Cardiovascular Risks and Cardiovascular Diseases
Emerging evidence has led to the increasing recognition of the association between GDM and cardiovascular (CV) risks and CV events later in life (53). Previous studies in the Western population have identified a higher level of inflammatory (e.g., C-reactive protein) (54), vascular endothelial dysfunction (e.g., intimal medial thickness) (55), and a 2-7 times higher risk of coronary artery calcification or CVD after 12-15 years’ follow-up (56–58), among women with a history of GDM. In Asia, five studies reported metabolic syndrome in Asian women with a history of GDM, with an incidence rate ranging from 40 to 90 per 1000 person-years. One Chinese study reported postpartum dyslipidemia (38.5%) among women with a history of GDM (47), while the other Israelite study reported a 30-70% higher risk of developing CV events and CV hospitalization among women with a history of GDM, even after adjusting for pre-eclampsia and maternal obesity at index pregnance (39).
Thus far, only determinants for postpartum CVD risks and CV events were reported as family history of T2D (59) and postpartum development of T2D (58) in the western population. Even though postpartum CVD determinants among women with GDM have yet to be fully investigated, long-standing exposure to cardio-metabolic risks has been speculated in the GDM-CVD link.
Cancer
GDM was associated with 30-40% increased risks of breast cancer, thyroid cancer, stomach cancer, and liver cancer for all races and ethnicities in a recent meta-analysis (60). As in the Asian population alone, we identified six retrospective cohort studies (Taiwan, South Korea and Israel) using either national insurance or a medical database to investigate the association between GDM and various cancers. All of them reported higher incidences of breast cancer, thyroid cancer, pancreatic cancer, ovarian cancer, lung cancer, and kidney cancer among the Asian female population with a history of GDM after a median of 5-38 years of follow-up than those parous women without such a history. For example, the incidence rate of cancer among Israelite women with a history of GDM was reported in breast (2 per 1000 person year) (37) and ovary (1 per 100 person year) (36), respectively.
It has been well documented that T2D is associated with higher risks of all-cancer incidence (61), especially malignancies in the breast, pancreas, and liver in women (62, 63). Some evidence has alluded to the mitogenic effect while binding to the insulin-like growth factor-I receptor secondary to insulin resistance (64). Furthermore, hyperglycemia itself might promote carcinogenesis via increasing oxidative stress (65, 66). However, data regarding cancer risks associated with GDM are merely gathered in the Western population.
Liver Dysfunction
Liver dysfunction is a common cause of chronic liver disease that affects approximately one in four adults worldwide, which is characterized by liver steatosis (fat deposition), inflammation, and hepatocyte damage (67). Researchers have suggested a link between metabolic risks (i.e., obesity, hyperglycemia, hyperlipidemia, and insulin resistance) and hepatic fatty deposition and non-alcoholic fatty liver disease (NAFLD) in the past decades (68, 69). Notably, women with a history of GDM were found to have raised liver triglyceride (TG) levels, highlighting a potential link between GDM and liver dysfunction (70, 71). Despite the higher prevalence of postpartum liver fat (72), abnormal liver score (73) and even NAFLD (71, 74), such results were mostly gathered from the Western population. There is one study from South Asia (India) reported a 2.11-fold higher odds of NAFLD among women with GDM, compared with women without GDM. The researchers suggested that postpartum medical conditions such as overweight/obesity, metabolic syndrome, and prediabetes were risk factors for developing NAFLD, during a median of 16 months’ follow-up after delivery (40).
Adverse Health Outcomes of Offspring Born From Pregnancies Complicated by GDM
Overview
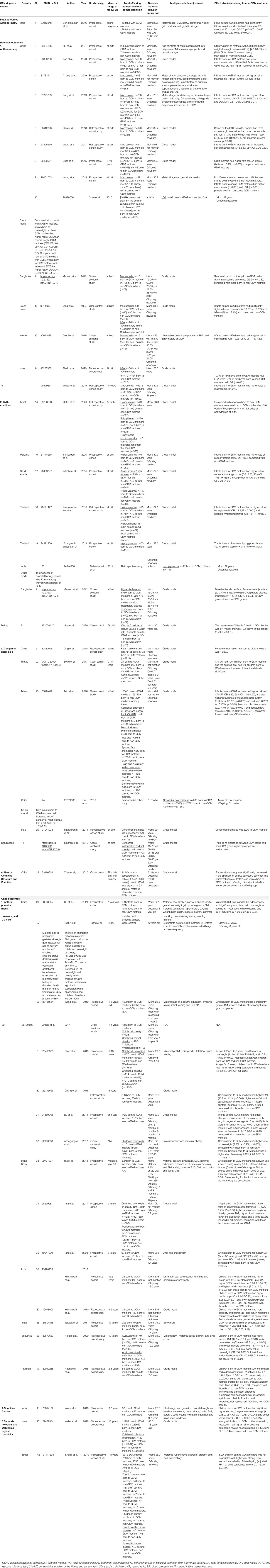
A body of evidence has implied that specific developmental programming in offspring is influenced by maternal hyperglycemia; in particular, epigenetic modification may be the key underlying mechanism (75, 76). Our review identified forty-two studies conducted on Native Asians (Table 2) and eight studies conducted on Asian immigrants (Supplementary Table 7) with up to 18 years’ follow-up, all of which were within the research scope of adverse health outcomes among offspring born to mothers with GDM. Offspring health outcomes, including fetal growth and neonatal anthropometric measures, were reported in Native Asians and Asian migrants, whereas offspring health outcomes, including congenital anomalies, neuro-cognitive function, and cardio-metabolic phenotypes, were only reported in Native Asians (Figure 4). None of these studies investigated risk factors underlying maternal GDM and the development of offspring health outcomes. Among 50 included studies in this topic, fourteen (28%) were assessed low in risk of bias, while the rest 72% were assessed either high or very high in risk of bias.
GDM and Fetal Growth
In-utero over nourishment can lead to fetal overgrowth, and such influence may predispose the offspring to obesity and T2D later in life if there is an obesogenic environment (84). A cohort in India reported an association between GDM and antenatal fetal growth at mid-late trimester (85). In this prospective cohort, fetuses of women with GDM had a thicker anterior abdominal wall while smaller femur length and biparietal diameter than fetuses of women without GDM. The researcher referred to this as “the thin-fat-phenotype” which represented a predisposition to T2D at birth (85).
Among Asian immigrants, one Norwegian study found that fetuses exposed to maternal GDM tended to be smaller in fetal weight at 24 weeks of gestation but thereafter grew faster until delivery, compared with fetuses not exposed to maternal with GDM (86). This trend was more prominent in South Asian women (86).
GDM and Neonatal Outcomes
Anthropometric Outcome At Birth
It is well-accepted that GDM is related to increased risk for macrosomia and large for gestational age (LGA) (6). We identified 14 papers that focused on this topic, with sample sizes ranging from 72 to 11 999 neonates. Among them, the majority reported consistent findings on either higher prevalence rates (11% to 40%) or higher risk ratios (2.0-2.7 times) of macrosomia or LGA among neonates born to GDM mothers, compared with their non-GDM counterparts, despite a couple reported otherwise. Interestingly, one study specifically looked at different combinations of glycemic abnormalities (fasting, 1-hour, and 2-hour glycemic levels) with macrosomia (77). The researchers found that women with three abnormal OGTT glycemic values had a much higher macrosomia rate in their offspring than those with two or one abnormal glycemic value (77). Such results—to some extent—suggested there might be remarkable neonatal outcomes specific to different GDM phenotypes (77).
Four studies reported neonatal birth size in Asian migrants equivocally. The US studies showed no differences in macrosomia rate between neonates born to NHW and Asian women with GDM (87, 88). In contrast, compared with the NHW counterparts, the Dutch study showed a lower macrosomia rate in offspring born to West Asian migrants (Turkish) (89) (18.6% vs. 22.6% [NHW]), while the Canadian study found that newborns born to South Asian female migrants had a greater skinfold thickness (11.7 vs 10.6 mm [NHW]; p=0.0001) (90).
Neonatal Health Ouctomes
Eight papers reporting other neonatal conditions were identified in our review, ranging from 72 to 10 543 in sample size. Neonatal disorders were listed as hypoglycemia, low Apgar score, hyperbilirubinemia/jaundice, polycythemia and respiratory distress syndrome. All studies consistently reported that neonates born to women with GDM were more susceptible to hypoglycemia, hyperbilirubinemia, respiratory distress syndrome and low Apgar score (<7 at 5 minutes), compared with those born to women without GDM.
Congenital Diseases
A total number of six studies reported findings on this topic, only half of which had specified the type of malformation as either congenital heart disease or congenital anomalies of the kidney and urinary tract (CAKUT). In general, evidence showed that neonates born to mothers with GDM tended to have a 2-3 times higher risk of developing congenital heart disease and CAKUT, especially more evident in male neonates (79). Despite the unclear pathophysiological mechanism, it has been speculated that serial maternal antenatal characteristics could affect embryonic development during the first trimester, such as pre-existing diabetes prior to pregnancy, overweight and obesity, and excessive weight gain during pregnancy (79, 91, 92).
Neuro-Cognitive Structure and Function
There is one case-control study investigated brain function in pre-term infants born to mother with GDM. In the first 33 days after delivery, the researchers used MRI image and discovered that infants born to mother with GDM tended to have multiple reduced fractional anisotropy in the brain, reflecting a microstructural white matter abnormalities compared with the infants born to mother without GDM (80).
GDM and Childhood Outcomes
Twenty studies on this topic were identified, with nearly half reported in China (n=8), then followed by India (n=4), Israel (n=3), Hong Kong (n=3), Pakistan (n=1), and Sri Lanka (n=1). Childhood outcomes spanned several traits and conditions, including adiposity and cadiometabolic outcomes, cognitive function, endocrinological and ophthalmological morbidity.
Anthropometry, Blood Pressure and Cardiometaboilc Outcomes
The majority of studies (17/20, 85.0%) reported consistent findings on long-term outcomes like childhood adiposity and cardio-metabolic risks. Overall, offspring born to women with GDM had higher BMI z-score, higher systolic blood pressure and diastolic blood pressure, higher childhood overweight and obesity rates, higher lipid profile levels, and higher insulin and insulin resistance levels, than those born to women without GDM. These studies involved small (n=164) to large (n=27 157) sample sizes of offspring with an average follow-up of 1-18 years among different ethnicities (Chinese, Indians, Sri Lankans and Israelite Jews).
In terms of cardiac function, we included one Pakistani study (93) and one Indian study (81) with small sample sizes of 136 and 236. Compared with their counterparts, offspring born to women with GDM had higher Carotid Intima-Media Thickness (cIMT), cardiac output and stroke volume, decreased mitral E/A ratio, and total peripheral resistance in early childhood and early adolescence, respectively.
Among Asian immigrants, two studies in the UK (94, 95) and one study in the US (96) with sample sizes ranging from 382 to 6 060 reported a consistent association between GDM and childhood obesity across all races and ethnic groups. The magnitude in such association between NHW women and Asian female immigrants was similar.
Neuro-Cognitive Outcomes
Hyperglycemia during pregnancy may affect fetal neurodevelopment and leave a significant impact on offspring cognition (97). Only one Indian study reported neurocognitive outcomes in the offspring at a mean 9.7 years of age (82). Children born to women with GDM had higher learning, long-term retrieval and storage, and better verbal ability than children born to women without GDM. The authors propose that the finding may be confounded by the strong correlation between GDM and higher social-economic status among this cohort (82).
Endocrinological and Ophthalmological Outcomes
Other childhood outcomes related to GDM include endocrine and ophthalmic morbidities. In two large-scale Israelite cohort studies where young adults (≤ 18 years) with a history of small-than-gestational age (SGA) conditions were recruited. One study showed no difference in the incidence of endocrine morbidity between young adults born to women with and without GDM (83). In contrast, the other study observed a higher prevalence of offspring ophthalmic inflammation (0.74% vs. 0.60%) and a 60% higher risk in ophthalmic-related hospitalization among young adults born to women with GDM and treated with medication (metformin, insulin) (78).
Discussion and Future Direction
Our review reinforces that, in general, Asians are at the highest risk of developing GDM and for subsequent progression to T2D among all populations. Yet, data among the Asian population on long-term health implications of GDM on women and offspring remain limited and are less in-depth than the Western population. In addition, studies in identifying attributable risk factors that may inform preventive strategies of long-term adverse health outcomes among women and their offspring are less comprehensive in Asians than in the Western population. Methodologically, inferences from existing published data are hindered by considerable heterogeneity in study designs, a high risk of bias (Supplementary Tables 1, 2), and standardized protocols for defining studies of Asians.
In order to address such critical knowledge gaps, future endeavors in the following aspects may be warranted to dissect the vicious circle of “diabetes begetting diabetes” and improve the health and well-being of this and future generations.
1. Conducting large scale well-designed cohort studies and/or consortium networks among Asians to investigate risk factors and etiology of GDM. A better understanding of GDM pathogenesis specific to Asian women shall further enhance our knowledge on the unique GDM characteristics among Asian women and develop more targeted and effective intervention approaches to prevent GDM and interrupt the transgenerational diabetic vicious cycle. However, such GDM heterogeneity-specific maternal health outcomes in Asians are still limited in scope, let alone other elements of the potential impact such as genetic factors and fetal sex. Future endeavors to establish parallel prospective pregnancy cohorts—with longitudinal data collection and comprehensive characterization of metabolic profiles through pregnancy in different Asian regions—are warranted to understand biological differences across Asian ethnicities, identify determinants and even develop prediction models for GDM onset and its phenotype-specific transgenerational health outcomes.
2. Conducting prospective cohort studies and/or intervention studies to follow up both GDM women and their offspring following the index pregnancy to identify factors that may mitigate the adverse impact of GDM on both women and their children. With the increasing awareness of the GDM burden and subsequent adverse health outcomes in Asian women and their offspring, a few large-scale ongoing pre-conception and pregnancy trials have focused on lifestyle intervention in Asia, such as Project SARAS in Mumbai (98) and the VINAVAC study in Vietnam (99). However, inferences from these two trials are inconsistent, which might be hindered by participants’ low compliance, including low uptake rate of OGTT, poor quality of data collection (e.g., physical examination, questionnaires administration) during research visits, and not quantitative constituents in the snack or freshly-prepared food given to the intervention group (98, 99). In terms of postpartum trials, substantial evidence in either lifestyle modifications (100) or pharmacological therapies (101–103) gathered from developed countries has shown promising results. However, intervention studies with customized approaches (e.g., diet recommendation, lifestyle modification) according to the Asian population are much fewer in scope than the Western population. Recently, there have been some improvements, including a few postpartum T2D prevention trials conducted in countries like China (100, 104), Singapore (105), Malaysia (106), and India (107), focusing on lifestyle modification, with a sample range between 77 and 1 414 and a length of follow-up up to 10 years. However, most of them are still ongoing, and only two trials reported more significant weight loss, reduction in waist circumference, and improved glucose tolerance during the 6-12 months’ postpartum period (104, 106).
3. Conducting studies of Health Disparities in GDM Care in Asian Populations across countries and continents. Even though developing countries in Asia (e.g., India) have shown increased life expectancy over the past several decades, health inequity is still a severe national issue as progress is uneven within each country (108). Furthermore, not all but a substantial proportion of Asian migrants in Western countries face socio-economical disadvantages such as access to health care and education (109). Among them, women seem to be more affected than men due to their vulnerability (109). Therefore, the fight against GDM and its harm to Asian mothers and children should account for existing health inequity and develop strategies to address health disparities.
4. Health Care System Improvement in Asia. Emerging evidence has pointed out that a portion of GDM cases was indeed overt diabetes that has not been identified before pregnancy, which ultimately drives the risk of maternal and offspring health outcomes even higher (110). For example, collecting information on pre-existing maternal diabetes or overt diabetes identification during early pregnancy in the Asian health care system is critical to screen for and even prevent offspring congenital abnormality or other adverse fetal and neonatal health outcomes. Ideally, GDM rates in the population could be reduced by individual and societal measures designed to promote healthy lifestyle changes, including optimal dietary intake and increased physical activity in the general population, focusing on the health and fitness of women of reproductive age.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
L-JL contributed to the review’s framework conceptualization, study, design, literature research, data collection, analysis and interpretation, and manuscript write-up; LH contributed to literature search, data collection and summary; DT contributed to data interpretation and manuscript editing; CZ contributed to the review’s framework conceptualization, study design, data interpretation and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
L-JL is funded by Singapore National Medical Research Council Clinician Science Award 2021 (NMRC CSAINV/002/2021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.840331/full#supplementary-material
Supplementary Figure 1 | Flow diagram of search strategy and selection of GDM prevalence in the Asian population including Native Asians and Asian migrants.
Supplementary Figure 2 | Flow diagram of search strategy and selection of GDM-related maternal postpartum health outcomes in the Asian population including Native Asians and Asian migrants.
Supplementary Figure 3 | Flow diagram of search strategy and selection of GDM-related offspring postpartum health outcomes in the Asian population including Native Asians and Asian migrants.
References
1. Collaboration NCDRF. Worldwide Trends in Diabetes Since 1980: A Pooled Analysis of 751 Population-Based Studies With 4.4 million participants. Lancet (2016) 387(10027):1513–30. doi: 10.1016/S0140-6736(16)00618-8
2. International Diabetes Federation. IDF Diabetes ATLAS 9th Edition 2019 (2019). Available at: https://wwwidforg/our-activities/care-prevention/gdm.
3. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational Diabetes Mellitus. Nat Rev Dis Primers (2019) 5(1):47. doi: 10.1038/s41572-019-0098-8
4. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res Clin Pract (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
5. Yogev Y, Xenakis EM, Langer O. The Association Between Preeclampsia and the Severity of Gestational Diabetes: The Impact of Glycemic Control. Am J Obstet Gynecol (2004) 191(5):1655–60. doi: 10.1016/j.ajog.2004.03.074
6. Group HSCR, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and Adverse Pregnancy Outcomes. N Engl J Med (2008) 358(19):1991–2002. doi: 10.1056/NEJMoa0707943
7. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High Prevalence of Type 2 Diabetes and Pre-Diabetes in Adult Offspring of Women With Gestational Diabetes Mellitus or Type 1 Diabetes: The Role of Intrauterine Hyperglycemia. Diabetes Care (2008) 31(2):340–6. doi: 10.2337/dc07-1596
8. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 Diabetes Mellitus After Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet (2009) 373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5
9. Bao W, Yeung E, Tobias DK, Hu FB, Vaag AA, Chavarro JE, et al. Long-Term Risk of Type 2 Diabetes Mellitus in Relation to BMI and Weight Change Among Women With a History of Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetologia (2015) 58(6):1212–9. doi: 10.1007/s00125-015-3537-4
10. Ley SH, Chavarro JE, Li M, Bao W, Hinkle SN, Wander PL, et al. Lactation Duration and Long-Term Risk for Incident Type 2 Diabetes in Women With a History of Gestational Diabetes Mellitus. Diabetes Care (2020) 43(4):793–8. doi: 10.2337/dc19-2237
11. Eyal S. Gestational Diabetes Mellitus: Long-Term Consequences for the Mother and Child Grand Challenge: How to Move on Towards Secondary Prevention? Front Clin Diabetes Healthc (2020) 1(546256). doi: 10.3389/fcdhc.2020.546256
12. World Migration Report 2020 (2020). Available at: https://publicationsiomint/system/files/pdf/wmr_2020pdf (Accessed on 24 Aug 2021).
13. Wells BS GA, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses . Available at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp (Accessed on 2 Nov 2021. 2013).
14. International Association of D, Pregnancy Study Groups Consensus P, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care (2010) 33(3):676–82. doi: 10.2337/dc09-1848
15. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications : Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization (1999).
16. Carpenter MW, Coustan DR. Criteria for Screening Tests for Gestational Diabetes. Am J Obstet Gynecol (1982) 144(7):768–73. doi: 10.1016/0002-9378(82)90349-0
17. Committee Opinion No. 504: Screening and Diagnosis of Gestational Diabetes Mellitus. Obstet Gynecol (2011) 118(3):751–3. doi: 10.1097/AOG.0b013e3182310cc3
18. Yang HX. Diagnostic Criteria for Gestational Diabetes Mellitus (WS 331-2011). Chin Med J (Engl) (2012) 125(7):1212–3. doi: 10.2337/dc10-0572
19. Malaysia MoH. Perinatal Care Manual 3rd Edition. Putrajaya, Malaysia: Division of Family Health Development, MOH (2013). p. 251.
20. Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. Diabetes Res Clin Pract (2002) 55(1):65–85. doi: 10.1016/S0168-8227(01)00365-5
21. Seshiah V, Das AK, Balaji V, Joshi SR, Parikh MN, Gupta S, et al. Gestational Diabetes Mellitus–Guidelines. J Assoc Physicians India (2006) 54:622–8.
22. Parhofer KG, Hasbargen U, Ulugberdiyewa A, Abdullayewa M, Melebayewa B, Annamuhammedov A, et al. Gestational Diabetes in Turkmenistan: Implementation of a Screening Program and First Results. Arch Gynecol Obstet (2014) 289(2):293–8. doi: 10.1007/s00404-013-2961-2
23. Abu-Heija AT, Al-Bash M, Mathew M. Gestational and Pregestational Diabetes Mellitus in Omani Women: Comparison of Obstetric and Perinatal Outcomes. Sultan Qaboos Univ Med J (2015) 15(4):e496–500. doi: 10.18295/squmj.2015.15.04.009
24. Khalil A, Rezende J, Akolekar R, Syngelaki A, Nicolaides KH. Maternal Racial Origin and Adverse Pregnancy Outcome: A Cohort Study. Ultrasound Obstet Gynecol (2013) 41(3):278–85. doi: 10.1002/uog.12313
25. Jenum AK, Morkrid K, Sletner L, Vangen S, Torper JL, Nakstad B, et al. Impact of Ethnicity on Gestational Diabetes Identified With the WHO and the Modified International Association of Diabetes and Pregnancy Study Groups Criteria: A Population-Based Cohort Study. Eur J Endocrinol (2012) 166(2):317–24. doi: 10.1530/EJE-11-0866
26. Kragelund Nielsen K, Andersen GS, Damm P, Andersen AN. Gestational Diabetes Risk in Migrants. A Nationwide, Register-Based Study of All Births in Denmark 2004 to 2015. J Clin Endocrinol Metab (2020) 105(3). doi: 10.1210/clinem/dgaa024
27. Janevic T, Zeitlin J, Egorova N, Balbierz A, Howell EA. The Role of Obesity in the Risk of Gestational Diabetes Among Immigrant and U.S.-Born Women in New York City. Ann Epidemiol (2018) 28(4):242–8. doi: 10.1016/j.annepidem.2018.02.006
28. Mai C, Wang B, Wen J, Lin X, Niu J. Lipoprotein-Associated Phospholipase A2 and AGEs Are Associated With Cardiovascular Risk Factors in Women With History of Gestational Diabetes Mellitus. Gynecol Endocrinol (2014) 30(3):241–4. doi: 10.3109/09513590.2013.871522
29. Shek NW, Ngai CS, Lee CP, Chan JY, Lao TT. Lifestyle Modifications in the Development of Diabetes Mellitus and Metabolic Syndrome in Chinese Women Who Had Gestational Diabetes Mellitus: A Randomized Interventional Trial. Arch Gynecol Obstet (2014) 289(2):319–27. doi: 10.1007/s00404-013-2971-0
30. Tam WH, Ma RC, Yang X, Ko GT, Lao TT, Chan MH, et al. Cardiometabolic Risk in Chinese Women With Prior Gestational Diabetes: A 15-Year Follow-Up Study. Gynecol Obstet Invest (2012) 73(2):168–76. doi: 10.1159/000329339
31. Kondo M, Nagao Y, Mahbub MH, Tanabe T, Tanizawa Y. Factors Predicting Early Postpartum Glucose Intolerance in Japanese Women With Gestational Diabetes Mellitus: Decision-Curve Analysis. Diabetes Med (2018) 35(8):1111–7. doi: 10.1111/dme.13657
32. Kugishima Y, Yasuhi I, Yamashita H, Fukuda M, Kuzume A, Sugimi S, et al. Risk Factors Associated With Abnormal Glucose Tolerance in the Early Postpartum Period Among Japanese Women With Gestational Diabetes. Int J Gynaecol Obstet (2015) 129(1):42–5. doi: 10.1016/j.ijgo.2014.09.030
33. Hewage SS, Koh XYH, Soh SE, Pang WW, Fok D, Cai S, et al. Breastfeeding Duration and Development of Dysglycemia in Women Who Had Gestational Diabetes Mellitus: Evidence From the GUSTO Cohort Study. Nutrients (2021) 13(2):408. doi: 10.3390/nu13020408
34. Malong CL, Sia-Atanacio A, Andag-Silva A, Cunanan E. Incidence of Postpartum Diabetes and Glucose Intolerance Among Filipino Patients With Gestational Diabetes Mellitus Seen at a Tertiary Hospital. J ASEAN Fed Endocr Soc (2013) 28(1):56
35. Valizadeh M, Alavi N, Mazloomzadeh S, Piri Z, Amirmoghadami H. The Risk Factors and Incidence of Type 2 Diabetes Mellitus and Metabolic Syndrome in Women With Previous Gestational Diabetes. Int J Endocrinol Metab (2015) 13(2):e21696. doi: 10.5812/ijem.21696
36. Fuchs O, Sheiner E, Meirovitz M, Davidson E, Sergienko R, Kessous R. The Association Between a History of Gestational Diabetes Mellitus and Future Risk for Female Malignancies. Arch Gynecol Obstet (2017) 295(3):731–6. doi: 10.1007/s00404-016-4275-7
37. Perrin MC, Terry MB, Kleinhaus K, Deutsch L, Yanetz R, Tiram E, et al. Gestational Diabetes and the Risk of Breast Cancer Among Women in the Jerusalem Perinatal Study. Breast Cancer Res Treat (2008) 108(1):129–35. doi: 10.1007/s10549-007-9585-9
38. Wang L, Leng J, Liu H, Zhang S, Wang J, Li W, et al. Association Between Hypertensive Disorders of Pregnancy and the Risk of Postpartum Hypertension: A Cohort Study in Women With Gestational Diabetes. J Hum Hypertens (2017) 31(11):725–30. doi: 10.1038/jhh.2017.46
39. Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An Association Between Gestational Diabetes Mellitus and Long-Term Maternal Cardiovascular Morbidity. Heart (2013) 99(15):1118–21. doi: 10.1136/heartjnl-2013-303945
40. Kubihal S, Gupta Y, Shalimar, Kandasamy D, Goyal A, Kalaivani M, et al. Prevalence of Non-Alcoholic Fatty Liver Disease and Factors Associated With it in Indian Women With a History of Gestational Diabetes Mellitus. J Diabetes Investig (2021) 12(5):877–85. doi: 10.1111/jdi.13411
41. World Health Organization. Definition DaCoDMaiCRoaWC. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Org (1999). Available at: https://appswhoint/iris/bitstream/handle/10665/66040/WHO_NCD_NCS_992pdf?sequence=1&isAllowed=y.
42. American Diabetes A. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care (2014) 37 Suppl 1:S81–90. doi: 10.2337/dc14-S081
43. Minooee S, Ramezani Tehrani F, Rahmati M, Mansournia MA, Azizi F. Diabetes Incidence and Influencing Factors in Women With and Without Gestational Diabetes Mellitus: A 15year Population-Based Follow-Up Cohort Study. Diabetes Res Clin Pract (2017) 128:24–31. doi: 10.1016/j.diabres.2017.04.003
44. Prados M, Flores-Le Roux JA, Benaiges D, Llaurado G, Chillaron JJ, Paya A, et al. Gestational Diabetes Mellitus in a Multiethnic Population in Spain: Incidence and Factors Associated to Impaired Glucose Tolerance One Year After Delivery. Endocrinol Diabetes Nutr (2019) 66(4):240–6. doi: 10.1016/j.endinu.2018.07.007
45. Mukerji G, Chiu M, Shah BR. Impact of Gestational Diabetes on the Risk of Diabetes Following Pregnancy Among Chinese and South Asian Women. Diabetologia (2012) 55(8):2148–53. doi: 10.1007/s00125-012-2549-6
46. Shen Y, Wang P, Wang L, Zhang S, Liu H, Li W, et al. Gestational Diabetes With Diabetes and Prediabetes Risks: A Large Observational Study. Eur J Endocrinol (2018) 179(1):51–8. doi: 10.1530/EJE-18-0130
47. Pei L, Xiao H, Lai F, Li Z, Li Z, Yue S, et al. Early Postpartum Dyslipidemia and Its Potential Predictors During Pregnancy in Women With a History of Gestational Diabetes Mellitus. Lipids Health Dis (2020) 19(1):220. doi: 10.1186/s12944-020-01398-1
48. Girgis CM, Gunton JE, Cheung NW. The Influence of Ethnicity on the Development of Type 2 Diabetes Mellitus in Women With Gestational Diabetes: A Prospective Study and Review of the Literature. ISRN Endocrinol (2012) 2012:341638. doi: 10.5402/2012/341638
49. Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased Risk of Hypertension After Gestational Diabetes Mellitus: Findings From a Large Prospective Cohort Study. Diabetes Care (2011) 34(7):1582–4. doi: 10.2337/dc11-0268
50. Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, et al. Earlier Age of Onset of Chronic Hypertension and Type 2 Diabetes Mellitus After a Hypertensive Disorder of Pregnancy or Gestational Diabetes Mellitus. Hypertension (2015) 66(6):1116–22. doi: 10.1161/HYPERTENSIONAHA.115.06005
51. Carpenter MW. Gestational Diabetes, Pregnancy Hypertension, and Late Vascular Disease. Diabetes Care (2007) 30 Suppl 2:S246–50. doi: 10.2337/dc07-s224
52. Li LJ, Ikram MK, Cheung CY, Lee YS, Lee LJ, Gluckman P, et al. Effect of Maternal Body Mass Index on the Retinal Microvasculature in Pregnancy. Obstet Gynecol (2012) 120(3):627–35. doi: 10.1097/AOG.0b013e3182639577
53. Burlina S, Dalfra MG, Chilelli NC, Lapolla A. Gestational Diabetes Mellitus and Future Cardiovascular Risk: An Update. Int J Endocrinol (2016) 2016:2070926. doi: 10.1155/2016/2070926
54. Di Cianni G, Lencioni C, Volpe L, Ghio A, Cuccuru I, Pellegrini G, et al. C-Reactive Protein and Metabolic Syndrome in Women With Previous Gestational Diabetes. Diabetes Metab Res Rev (2007) 23(2):135–40. doi: 10.1002/dmrr.661
55. Bo S, Valpreda S, Menato G, Bardelli C, Botto C, Gambino R, et al. Should We Consider Gestational Diabetes a Vascular Risk Factor? Atherosclerosis (2007) 194(2):e72–9. doi: 10.1016/j.atherosclerosis.2006.09.017
56. Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of History of Gestational Diabetes With Long-Term Cardiovascular Disease Risk in a Large Prospective Cohort of US Women. JAMA Intern Med (2017) 177(12):1735–42. doi: 10.1001/jamainternmed.2017.2790
57. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-Eclampsia and Risk of Cardiovascular Disease and Cancer in Later Life: Systematic Review and Meta-Analysis. BMJ (2007) 335(7627):974. doi: 10.1136/bmj.39335.385301.BE
58. Shah BR, Retnakaran R, Booth GL. Increased Risk of Cardiovascular Disease in Young Women Following Gestational Diabetes Mellitus. Diabetes Care (2008) 31(8):1668–9. doi: 10.2337/dc08-0706
59. Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, et al. Gestational Diabetes Mellitus Increases the Risk of Cardiovascular Disease in Women With a Family History of Type 2 Diabetes. Diabetes Care (2006) 29(9):2078–83. doi: 10.2337/dc05-2482
60. Wang Y, Yan P, Fu T, Yuan J, Yang G, Liu Y, et al. The Association Between Gestational Diabetes Mellitus and Cancer in Women: A Systematic Review and Meta-Analysis of Observational Studies. Diabetes Metab (2020) 46(6):461–71. doi: 10.1016/j.diabet.2020.02.003
61. Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly Increased Risk of Cancer in Patients With Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr Pract (2011) 17(4):616–28. doi: 10.4158/EP10357.RA
62. Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes Mellitus and Breast Cancer. Lancet Oncol (2005) 6(2):103–11. doi: 10.1016/S1470-2045(05)01736-5
63. Zhou XH, Qiao Q, Zethelius B, Pyorala K, Soderberg S, Pajak A, et al. Diabetes, Prediabetes and Cancer Mortality. Diabetologia (2010) 53(9):1867–76. doi: 10.1007/s00125-010-1796-7
64. Sciacca L, Cassarino MF, Genua M, Pandini G, Le Moli R, Squatrito S, et al. Insulin Analogues Differently Activate Insulin Receptor Isoforms and Post-Receptor Signalling. Diabetologia (2010) 53(8):1743–53. doi: 10.1007/s00125-010-1760-6
65. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting Serum Glucose Level and Cancer Risk in Korean Men and Women. JAMA (2005) 293(2):194–202. doi: 10.1001/jama.293.2.194
66. Stocks T, Rapp K, Bjorge T, Manjer J, Ulmer H, Selmer R, et al. Blood Glucose and Risk of Incident and Fatal Cancer in the Metabolic Syndrome and Cancer Project (Me-can): Analysis of Six Prospective Cohorts. PloS Med (2009) 6(12):e1000201. doi: 10.1371/journal.pmed.1000201
67. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431
68. Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine Aminotransferase, Gamma-Glutamyltransferase, and Incident Diabetes: The British Women's Heart and Health Study and Meta-Analysis. Diabetes Care (2009) 32(4):741–50. doi: 10.2337/dc08-1870
69. Ruhl CE, Everhart JE. Elevated Serum Alanine Aminotransferase and Gamma-Glutamyltransferase and Mortality in the United States Population. Gastroenterology (2009) 136(2):477–85 e11. doi: 10.1053/j.gastro.2008.10.052
70. Forbes S, Godsland IF, Taylor-Robinson SD, Bell JD, Thomas EL, Patel N, et al. A History of Previous Gestational Diabetes Mellitus Is Associated With Adverse Changes in Insulin Secretion and VLDL Metabolism Independently of Increased Intrahepatocellular Lipid. Diabetologia (2013) 56(9):2021–33. doi: 10.1007/s00125-013-2956-3
71. Ajmera VH, Gunderson EP, VanWagner LB, Lewis CE, Carr JJ, Terrault NA. Gestational Diabetes Mellitus Is Strongly Associated With Non-Alcoholic Fatty Liver Disease. Am J Gastroenterol (2016) 111(5):658–64. doi: 10.1038/ajg.2016.57
72. Mehmood S, Margolis M, Ye C, Maple-Brown L, Hanley AJ, Connelly PW, et al. Hepatic Fat and Glucose Tolerance in Women With Recent Gestational Diabetes. BMJ Open Diabetes Res Care (2018) 6(1):e000549. doi: 10.1136/bmjdrc-2018-000549
73. Donnelly SR, Hinkle SN, Rawal S, Grunnet LG, Chavarro JE, Vaag A, et al. Prospective Study of Gestational Diabetes and Fatty Liver Scores 9 to 16 Years After Pregnancy. J Diabetes (2019) 11(11):895–905. doi: 10.1111/1753-0407.12934
74. Forbes S, Taylor-Robinson SD, Patel N, Allan P, Walker BR, Johnston DG. Increased Prevalence of Non-Alcoholic Fatty Liver Disease in European Women With a History of Gestational Diabetes. Diabetologia (2011) 54(3):641–7. doi: 10.1007/s00125-010-2009-0
75. Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, Epigenetics and Gestational Diabetes: Consequences in Mother and Child. Epigenetics (2019) 14(3):215–35. doi: 10.1080/15592294.2019.1582277
76. Aiken CE, Ozanne SE. Transgenerational Developmental Programming. Hum Reprod Update (2014) 20(1):63–75. doi: 10.1093/humupd/dmt043
77. Ding TT, Xiang J, Luo BR, Hu J. Relationship Between the IADPSG-Criteria-Defined Abnormal Glucose Values and Adverse Pregnancy Outcomes Among Women Having Gestational Diabetes Mellitus: A Retrospective Cohort Study. Med (Baltimore) (2018) 97(43):e12920. doi: 10.1097/MD.0000000000012920
78. Walter E, Tsumi E, Wainstock T, Spiegel E, Sheiner E. Maternal Gestational Diabetes Mellitus: Is It Associated With Long-Term Pediatric Ophthalmic Morbidity of the Offspring? J Matern Fetal Neonatal Med (2019) 32(15):2529–38. doi: 10.1080/14767058.2018.1439918
79. Liu X, Liu G, Wang P, Huang Y, Liu E, Li D, et al. Prevalence of Congenital Heart Disease and Its Related Risk Indicators Among 90,796 Chinese Infants Aged Less Than 6 Months in Tianjin. Int J Epidemiol (2015) 44(3):884–93. doi: 10.1093/ije/dyv107
80. Xuan DS, Zhao X, Liu YC, Xing QN, Shang HL, Zhu PY, et al. Brain Development in Infants of Mothers With Gestational Diabetes Mellitus: A Diffusion Tensor Imaging Study. J Comput Assist Tomogr (2020) 44(6):947–52. doi: 10.1097/RCT.0000000000001110
81. Krishnaveni GV, Veena SR, Jones A, Srinivasan K, Osmond C, Karat SC, et al. Exposure to Maternal Gestational Diabetes Is Associated With Higher Cardiovascular Responses to Stress in Adolescent Indians. J Clin Endocrinol Metab (2015) 100(3):986–93. doi: 10.1210/jc.2014-3239
82. Veena SR, Krishnaveni GV, Srinivasan K, Kurpad AV, Muthayya S, Hill JC, et al. Childhood Cognitive Ability: Relationship to Gestational Diabetes Mellitus in India. Diabetologia (2010) 53(10):2134–8. doi: 10.1007/s00125-010-1847-0
83. Shorer DT, Wainstock T, Sheiner E, Landau D, Pariente G. Long-Term Endocrine Outcome of Small for Gestational Age Infants Born to Mothers With and Without Gestational Diabetes Mellitus. Gynecol Endocrinol (2019) 35(11):1003–9. doi: 10.1080/09513590.2019.1616174
84. Dabelea D, Crume T. Maternal Environment and the Transgenerational Cycle of Obesity and Diabetes. Diabetes (2011) 60(7):1849–55. doi: 10.2337/db11-0400
85. Venkataraman H, Ram U, Craik S, Arungunasekaran A, Seshadri S, Saravanan P. Increased Fetal Adiposity Prior to Diagnosis of Gestational Diabetes in South Asians: More Evidence for the 'Thin-Fat' Baby. Diabetologia (2017) 60(3):399–405. doi: 10.1007/s00125-016-4166-2
86. Sletner L, Jenum AK, Yajnik CS, Morkrid K, Nakstad B, Rognerud-Jensen OH, et al. Fetal Growth Trajectories in Pregnancies of European and South Asian Mothers With and Without Gestational Diabetes, a Population-Based Cohort Study. PloS One (2017) 12(3):e0172946. doi: 10.1371/journal.pone.0172946
87. Bowers K, Laughon SK, Kiely M, Brite J, Chen Z, Zhang C. Gestational Diabetes, Pre-Pregnancy Obesity and Pregnancy Weight Gain in Relation to Excess Fetal Growth: Variations by Race/Ethnicity. Diabetologia (2013) 56(6):1263–71. doi: 10.1007/s00125-013-2881-5
88. Mocarski M, Savitz DA. Ethnic Differences in the Association Between Gestational Diabetes and Pregnancy Outcome. Matern Child Health J (2012) 16(2):364–73. doi: 10.1007/s10995-011-0760-6
89. Kosman MW, Eskes SA, van Selst J, Birnie E, van Gemund N, Karsdorp VH, et al. Perinatal Outcomes in Gestational Diabetes in Relation to Ethnicity in the Netherlands. Neth J Med (2016) 74(1):22–9.
90. Anand SS, Gupta M, Teo KK, Schulze KM, Desai D, Abdalla N, et al. Causes and Consequences of Gestational Diabetes in South Asians Living in Canada: Results From a Prospective Cohort Study. CMAJ Open (2017) 5(3):E604–E11. doi: 10.9778/cmajo.20170027
91. Renkema KY, Verhaar MC, Knoers NV. Diabetes-Induced Congenital Anomalies of the Kidney and Urinary Tract (CAKUT): Nurture and Nature at Work? Am J Kidney Dis (2015) 65(5):644–6. doi: 10.1053/j.ajkd.2015.02.320
92. Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, et al. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus With Congenital Anomalies of the Newborn. Diabetes Care (2020) 43(12):2983–90. doi: 10.2337/dc20-0261
93. Hoodbhoy Z, Mohammed N, Aslam N, Fatima U, Ashiqali S, Rizvi A, et al. Is the Child at Risk? Cardiovascular Remodelling in Children Born to Diabetic Mothers. Cardiol Young (2019) 29(4):467–74. doi: 10.1017/S1047951119000040
94. West J, Santorelli G, Whincup PH, Smith L, Sattar NA, Cameron N, et al. Association of Maternal Exposures With Adiposity at Age 4/5 Years in White British and Pakistani Children: Findings From the Born in Bradford Study. Diabetologia (2018) 61(1):242–52. doi: 10.1007/s00125-017-4457-2
95. Fairley L, Santorelli G, Lawlor DA, Bryant M, Bhopal R, Petherick ES, et al. The Relationship Between Early Life Modifiable Risk Factors for Childhood Obesity, Ethnicity and Body Mass Index at Age 3 Years: Findings From the Born in Bradford Birth Cohort Study. BMC Obes (2015) 2:9. doi: 10.1186/s40608-015-0037-5
96. Faith MS, Hittner JB, Hurston SR, Yin J, Greenspan LC, Quesenberry CP Jr., et al. Association of Infant Temperament With Subsequent Obesity in Young Children of Mothers With Gestational Diabetes Mellitus. JAMA Pediatr (2019) 173(5):424–33. doi: 10.1001/jamapediatrics.2018.5199
97. Anderson JL, Waller DK, Canfield MA, Shaw GM, Watkins ML, Werler MM. Maternal Obesity, Gestational Diabetes, and Central Nervous System Birth Defects. Epidemiology (2005) 16(1):87–92. doi: 10.1097/01.ede.0000147122.97061.bb
98. Sahariah SA, Potdar RD, Gandhi M, Kehoe SH, Brown N, Sane H, et al. A Daily Snack Containing Leafy Green Vegetables, Fruit, and Milk Before and During Pregnancy Prevents Gestational Diabetes in a Randomized, Controlled Trial in Mumbai, India. J Nutr (2016) 146(7):1453S–60S. doi: 10.3945/jn.115.223461
99. Nga HT, Quyen PN, Chaffee BW, Diep Anh NT, Ngu T, King JC. Effect of a Nutrient-Rich, Food-Based Supplement Given to Rural Vietnamese Mothers Prior to and/or During Pregnancy on Birth Outcomes: A Randomized Controlled Trial. PloS One (2020) 15(5):e0232197. doi: 10.1371/journal.pone.0232197
100. Guo J, Tang Y, Wiley J, Whittemore R, Chen JL. Effectiveness of a Diabetes Prevention Program for Rural Women With Prior Gestational Diabetes Mellitus: Study Protocol of a Multi-Site Randomized Clinical Trial. BMC Public Health (2018) 18(1):809. doi: 10.1186/s12889-018-5725-x
101. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of Pancreatic Beta-Cell Function and Prevention of Type 2 Diabetes by Pharmacological Treatment of Insulin Resistance in High-Risk Hispanic Women. Diabetes (2002) 51(9):2796–803. doi: 10.2337/diabetes.51.9.2796
102. Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, et al. Effect of Pioglitazone on Pancreatic Beta-Cell Function and Diabetes Risk in Hispanic Women With Prior Gestational Diabetes. Diabetes (2006) 55(2):517–22. doi: 10.2337/diabetes.55.02.06.db05-1066
103. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the Incidence of Type 2 Diabetes With Lifestyle Intervention or Metformin. N Engl J Med (2002) 346(6):393–403. doi: 10.1056/NEJMoa012512
104. Liu H, Wang L, Zhang S, Leng J, Li N, Li W, et al. One-Year Weight Losses in the Tianjin Gestational Diabetes Mellitus Prevention Programme: A Randomized Clinical Trial. Diabetes Obes Metab (2018) 20(5):1246–55. doi: 10.1111/dom.13225
105. Lim K, Chi C, Chan SY, Lim SL, Ang SM, Yoong JS, et al. Smart Phone APP to Restore Optimal Weight (SPAROW): Protocol for a Randomised Controlled Trial for Women With Recent Gestational Diabetes. BMC Public Health (2019) 19(1):1287. doi: 10.1186/s12889-019-7691-3
106. Shyam S, Arshad F, Abdul Ghani R, Wahab NA, Safii NS, Nisak MY, et al. Low Glycaemic Index Diets Improve Glucose Tolerance and Body Weight in Women With Previous History of Gestational Diabetes: A Six Months Randomized Trial. Nutr J (2013) 12:68. doi: 10.1186/1475-2891-12-68
107. Gupta Y, Kapoor D, Josyula LK, Praveen D, Naheed A, Desai AK, et al. A Lifestyle Intervention Programme for the Prevention of Type 2 Diabetes Mellitus Among South Asian Women With Gestational Diabetes Mellitus [LIVING Study]: Protocol for a Randomized Trial. Diabetes Med (2019) 36(2):243–51. doi: 10.1111/dme.13850
108. The L. Taking Urgent Action on Health Inequities. Lancet (2020) 395(10225):659. doi: 10.1016/S0140-6736(20)30455-4
109. Watkinson RE, Sutton M, Turner AJ. Ethnic Inequalities in Health-Related Quality of Life Among Older Adults in England: Secondary Analysis of a National Cross-Sectional Survey. Lancet Public Health (2021) 6(3):e145–54. doi: 10.1016/S2468-2667(20)30287-5
Keywords: gestational diabetes mellitus, Asians, prevalence, diagnostic criteria, diagnostic guidelines, maternal health outcomes, offspring health outcomes
Citation: Li L-J, Huang L, Tobias DK and Zhang C (2022) Gestational Diabetes Mellitus Among Asians – A Systematic Review From a Population Health Perspective. Front. Endocrinol. 13:840331. doi: 10.3389/fendo.2022.840331
Received: 21 December 2021; Accepted: 11 April 2022;
Published: 16 June 2022.
Edited by:
Xiongfei Pan, West China Second University Hospital, Sichuan University, ChinaReviewed by:
Tai-Ho Hung, Taipei Chang Gung Memorial Hospital, TaiwanYeyi Zhu, Kaiser Permanente, United States
Copyright © 2022 Li, Huang, Tobias and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-Jun Li, b2JnbGxqQG51cy5lZHUuc2c=; Cuilin Zhang, b2JnY3pAbnVzLmVkdS5zZw==
 Ling-Jun Li
Ling-Jun Li Lihua Huang2
Lihua Huang2 Cuilin Zhang
Cuilin Zhang