
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 18 February 2022
Sec. Bone Research
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.839885
This article is part of the Research Topic The Potential Effects and Mechanisms of Chinese Traditional Medicine on Bone Homeostasis and Remodeling View all 12 articles
Objective: The clinical efficacy of Xianling Gubao capsule (XLGB) and its combination therapy in the treatment of postmenopausal osteoporosis (PMOP) was systematically evaluated by frequency-based network meta-analysis.
Methods: We searched the China National Knowledge Infrastructure (CNKI), Wanfang, SinoMed, PubMed, Embase and Cochrane Library databases to identify clinical trials of XLGB for the treatment of PMOP from the establishment of each database to November 22, 2021. The quality of the included studies was evaluated by using the risk of bias assessment tool version 2.0 (Rob 2.0) recommended by Cochrane. Stata 14.0 was applied for statistical analysis of the data, and the surface under the cumulative ranking curve (SUCRA) was used to rank the intervention measures of each outcome index.
Results: This study included 22 clinical trials (including 19 RCTs and 3 non-RCTs) involving 12 drug therapies. According to the results of the network meta-analysis and SUCRA, the best three interventions for improving lumbar bone mineral density (BMD) are XLGB+BP+calcium (83.7%), XLGB+BP (68.5.7%) and XLGB+VD (67.1%). XLGB+calcium was the best combination regimen for improving femoral neck BMD and increasing bone Gla protein (BGP) and alkaline phosphatase (ALP) contents in serum. The SUCRA values of XLGB+calcium for improving the three outcome indicators were 68.0%, 59.5% and 82.1%, respectively.
Conclusions: The results of this network meta-analysis show that combined application of XLGB can effectively improve BMD and serum BGP and ALP compared to calcium alone, VD or BP. In the future, multicenter, large-sample and double-blind clinical RCTs should be carried out to supplement and verify the results of this study.
Postmenopausal osteoporosis (PMOP) is a systemic metabolic bone disease characterized by decreased bone mass, increased bone fragility and easy fracture (1). PMOP generally occurs within 5 to 10 years after menopause (2). Based on epidemiological statistics, there are approximately 27.6 million patients with osteoporosis over 50 years old in EU countries, including 22 million women, with a prevalence rate of 22.1% (3). The prevalence rate of osteoporosis in women is approximately three times that in men (3). The decline in ovarian function and the decrease in estrogen secretion in postmenopausal women can lead to an imbalance in bone turnover, a decrease in bone mass and an increase in bone fragility (4). A low BMI index, low milk intake, alcohol consumption, fracture history and smoking are risk factors for PMOP, and strengthening the management of the above controllable risk factors is beneficial to reduce PMOP complications (5). At present, the main drug used for the treatment of PMOP is a bone resorption inhibitor. Bisphosphonates, estrogens and other drugs recommended in clinical practice guidelines have good clinical efficacy, but their safety remains a concern (4, 6). Therefore, it is important to seek effective drugs while improving drug safety.
In traditional Chinese medicine, osteoporosis belongs to the category of “Guwei”, which is closely related to kidney deficiency: “The kidney stores essence and the main bone generates marrow” (7). Therefore, kidney nourishing is closely related to bone marrow regeneration. Xianling Gubao capsule (XLGB) is mainly used to clinically treat osteoporosis and osteoarthritis. XLGB includes Epimedium, Dipsacus, Psoralea, Rehmannia glutinosa, Salvia miltiorrhiza and Anemarrhena asphodeloides, which have the functions of nourishing the liver and kidney, activating blood circulation and dredging collaterals, and strengthening tendons and bones (7, 8). Kidney-tonifying herbs or compounds, such as XLGB, can regulate the proliferation and differentiation of osteoblasts and osteoclasts through a variety of signaling pathways to achieve a dynamic balance (8). XLGB is widely used in the treatment of PMOP in China, but compared with other types of drug regimens or combination therapy, an evidence-based foundation is lacking. Although XLGB is widely used in China, the comparison of efficacy and safety between XLGB and other drugs or combination therapies remains controversial (7, 8), and it is necessary to further evaluate efficacy between XLGB and other therapies. Therefore, this study used the frequency network meta-analysis method to compare the clinical efficacy of XLGB and other drug regimens for the treatment of PMOP to provide an evidence-based foundation for the rational clinical application of XLGB.
The inclusion criteria were as follows. 1) Participants: female postmenopausal patients with a clear diagnosis of primary osteoporosis and clear diagnostic criteria for osteoporosis in the study. 2) Interventions and comparisons: the experimental group was treated with XLGB alone or combined with XLGB on the basis of routine intervention measures in the control group; the control group was treated with no intervention, placebo or conventional Western medicine. 3) Outcome measure: the main outcome indicators were lumbar bone mineral density (BMD) and femoral neck BMD, and secondary outcome indicators were bone Gla protein (BGP) and alkaline phosphatase (ALP); all included studies involved at least one of the above evaluation indicators. 4) Study design: randomized controlled trials (RCTs) and nonrandomized controlled trials (non-RCTs). 5) To ensure the test efficiency of this study, the number of studies included in the intervention measures was ≥ 2.
The exclusion criteria were as follows: 1) animal experiments and case reports; 2) studies with incorrect or incomplete data; 3) studies with unobtainable full texts; and 4) repeated published literature.
We searched the China National Knowledge Infrastructure (CNKI), Wanfang, SinoMed, PubMed, Embase and Cochrane Library databases to identify clinical trials of XLGB for the treatment of PMOP. The retrieval time limit was from the establishment of each database to November 22, 2021. Each database was searched using subject words combined with free words. English search terms included “Xianlingguao”, “Xianling Gubao”, “XLGB”, “osteoporosis”, “postmenopausal osteoporosis”, “postmenopausal osteoporosis”, “primary osteoporosis”, and “PMOP”. The retrieval strategy for each database is shown in Supplementary Material 1.
Two researchers followed the inclusion and exclusion criteria to independently screen the literature and extract data; any disagreements were resolved by the corresponding author. The extracted contents included basic information, bias risk factors, and outcome index data.
Literature quality was evaluated using Cochrane’s recommended risk of bias assessment tool version 2.0 (Rob 2.0) (9). The evaluation tool assesses the risk of bias in five areas, including bias generated in the random process, bias deviating from the established intervention, bias of missing outcome data, bias of outcome measurement and bias of selective reporting of results.
The relative advantages and disadvantages of network meta-analysis can be evaluated by direct or indirect comparisons and the cumulative ranking probability. This study was based on the frequency framework and used Stata/MP 14.0 with the mvmeta and network packages (10–12). All indexes were analyzed using a random effect model. As the outcome indicators considered in this study were continuous variables, the mean difference (MD) was used as the effect value; the result is expressed with a 95% confidence interval (CI).
The size of the dot in the network diagram represents the sample size of the intervention, and the thickness of the line represents the amount of direct evidence between the two interventions. If there was a closed loop in the diagram, the inconsistency test was employed to evaluate consistency between the results of direct comparison and indirect comparison. If no closed loop formed, the consistency model was directly used for analysis. The surface under the cumulative ranking curve (SUCRA) was applied to rank the interventions of each outcome index, with a greater SUCRA value indicating a better the effect of the intervention. A “comparison correction” funnel plot was used to identify a small-sample effect between studies and publication bias in the studies included.
We preliminarily searched 1709 relevant studies, and 784 remained after deleting duplicate studies. After reading the titles and abstracts, we screened a total of 92 articles for full-text evaluation. A total of 22 clinical studies (13–34) were included after reading the full texts with reference to our inclusion and exclusion criteria. The process and results of the literature screening are shown in Figure 1.
A total of 22 studies (13–34) (including 19 RCTs and 3 non-RCTs) were included: 4 three-arm (21, 23, 26, 30) tests and 18 two-arm tests (13–20, 22, 24, 25, 27–29, 31–34). The total sample size was 2016 cases, including 924 cases in the treatment group and 1092 cases in the control group. The treatment course and follow-up time included in the study ranged from 1 to 12 months. Twelve medication measures were involved: calcium, XLGB, bisphosphonate (BP), XLGB+calcium, XLGB+BP, XLGB +BP+calcium, XLGB+vitamin D (VD), VD, BP+calcium, calcium+VD, estrogen+calcium, and XLGB+calcium+VD. The basic information of the included studies is shown in Table 1.
Nineteen studies (13, 15–18, 20–32, 34) reported specific methods of random assignment or a description of “random” assignment. In 18 studies (13, 15–17, 20–32, 34), intervention was according to the research protocol, whereas the specific basic treatment was not specified in the other 4 studies (14, 17, 19, 33). The outcome index data of all studies were complete and extractable. Because 3 studies (14, 15, 33) did not implement strict blinding methods, measurement bias may have been present. No selective reports were found. Overall, the quality of the literature included was general, and a more rigorous double-blind trial design is needed. The risk assessment of the included studies is shown in Figure 2.
1) Lumbar BMD (Table 2). Compared with calcium alone, XLGB+calcium significantly improved lumbar BMD (MD = 0.13, 95% CI [0.03, 0.22]). In addition, XLGB+VD showed more advantages in improving lumbar BMD compared with VD (MD = 0.09, 95% CI [0.04, 0.14]) or XLGB (MD = -0.09, 95% CI [-0.12, -0.06]) alone. Overall, the combined application of XLGB can improve the BMD of the lumbar spine compared with BP (MD = 0.10, 95% CI [0.07, 0.13]) or BP+calcium (MD = 0.13, 95% CI [0.08, 0.18]).
2) Femoral neck BMD (Table 3). Compared with VD (MD = 0.03, 95% CI [0.01, 0.06]) or XLGB (MD = -0.09, 95% CI [-0.11, -0.07]) alone, XLGB+VD significantly improved femoral neck the BMD. Compared with calcium alone (MD = 0.17, 95% CI [0.06, 0.29]) or BP+calcium (MD = 0.09, 95% CI [0.03, 0.15]), combined application of XLGB displayed a synergistic role in improving the BMD of the femoral neck.
3) ALP (Table 4). Compared with calcium alone (MD = 4.26, 95% CI [0.53, 7.98]), XLGB+calcium significantly increased the content of ALP in serum. XLGB+BP+calcium also increased serum ALP compared with BP alone (MD = 2.82, 95% CI [0.89, 4.75]). Nevertheless, XLGB+BP (MD = -2.55, 95% CI [-4.06, -1.04]) or XLGB+BP+calcium (MD = -15.70, 95% CI [-21.51, -9.89]) combination therapy did not necessarily increase the ALP content in serum compared with BP or calcium alone.
4) BGP (Table 5). Compared with calcium alone, XLGB+calcium increased the content of BGP in serum (MD = 3.75, 95% CI [0.59, 6.91]), and XLGB+BP+calcium reduced serum BGP compared with BP+calcium (MD = -24.97, 95% CI [-31.66, -18.28]).
This study included 12 interventions, 5 of which involved XLGB. The evidence network of the four outcome indicators is shown in Figure 3. The lines in the figure represent interventions with a direct comparison, the line thickness represents the number of studies, and the dot size represents the sample size of the intervention.
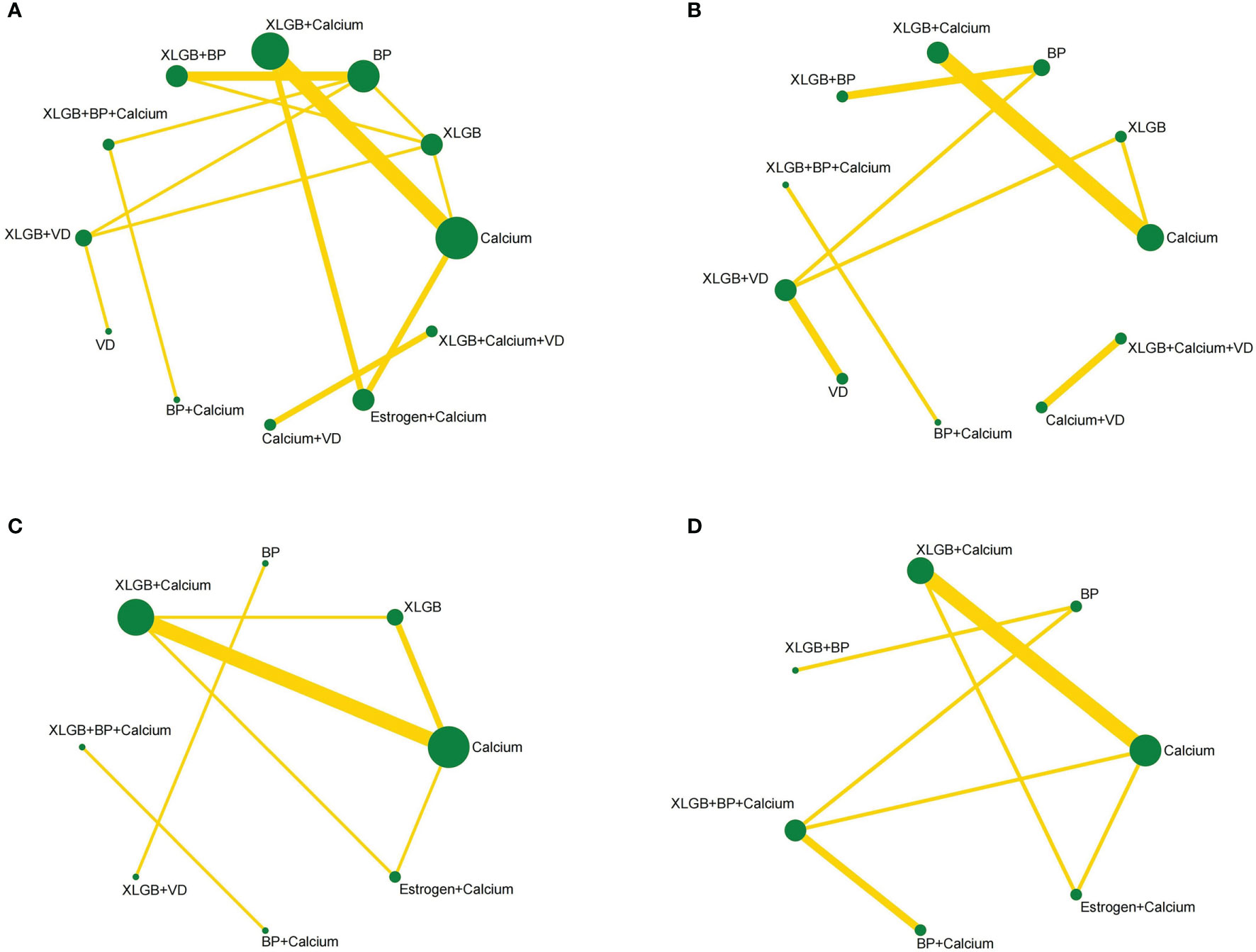
Figure 3 Network diagrams depicting direct evidence used in network meta-analysis. (A) Network diagram of lumbar BMD. (B) Network diagram of BMD of femoral neck. (C) Network diagram of BGP. (D) Network diagram of ALP.
The 12 interventions of lumbar BMD formed three closed loops. See Table 6 for the detection results of the characteristic intercirculation inconsistency factor (IF), 95% CI and intercirculation heterogeneity parameter t2 of lumbar BMD. The IF values of the three closed loops were 0.040, 0.085, and 0.127, and the lower limit of the 95% CIs was 0, which indicates that the consistency of each closed loop was good; that is, direct comparison and indirect comparison had little impact on the results of the whole network meta-analysis. The statistical results of the reticular meta-analysis of lumbar BMD were highly reliable. The eight interventions of ALP formed two closed loops. As shown in Table 6, the consistency of the two closed loops of ALP was good. The seven interventions of BGP formed a closed loop, with a large lower 95% CI limit (3.40), suggesting inconsistency. Therefore, the results of the network meta-analysis of BGP need to be interpreted carefully.
In the comparison of lumbar BMD, 45 pairwise comparisons were formed. The results of the network meta-analysis showed obvious advantages for XLGB+calcium in improving lumbar BMD compared with calcium alone (MD = 0.13, 95% CI [0.05, 0.20]), and the difference was statistically significant. In improving femoral neck BMD, 21 pairwise comparisons were formed. The results of the network meta-analysis showed that XLGB+calcium had more advantages than calcium alone (MD = 0.17, 95% CI [0.07, 0.27]). In terms of improving the contents of serum ALP and BGP, the results of the network meta-analysis did not suggest a pairwise comparison with a significant difference. The results of the network meta-analysis of the four outcome indicators concerned in this study are shown in Tables 7–10.
The best SUCRA probability rankings of the improvement effects of different interventions on the four outcome indicators are provided in Table 11. In terms of improving lumbar BMD, the best three interventions were XLGB+ BP+calcium (83.7%), XLGB+BP (68.5.7%) and XLGB+VD (67.1%). The three best interventions to improve femoral neck BMD were XLGB+calcium (68.0%), XLGB+BP (67.8%) and XLGB+VD (63.0%). The best interventions to increase the serum ALP content in were XLGB+calcium (59.5%), XLGB (52.6%) and calcium (47.8%). The best measures to improve the serum BGP content were XLGB+calcium (82.1%), estrogen+calcium (73.5%) and calcium (67.7%).
A funnel plot was drawn to evaluate a small-sample effect for the four outcome indicators in this study (Figure 4). Different colors in the funnel chart indicate different intervention measures. We found that most dots in the funnel chart of BMD of the lumbar spine and femoral neck were roughly symmetrically distributed on both sides of the vertical line with X = 0, which suggests no publication bias for these two outcome indicators. For ALP and BGP, a high degree of publication bias and a small sample effect were suggested.
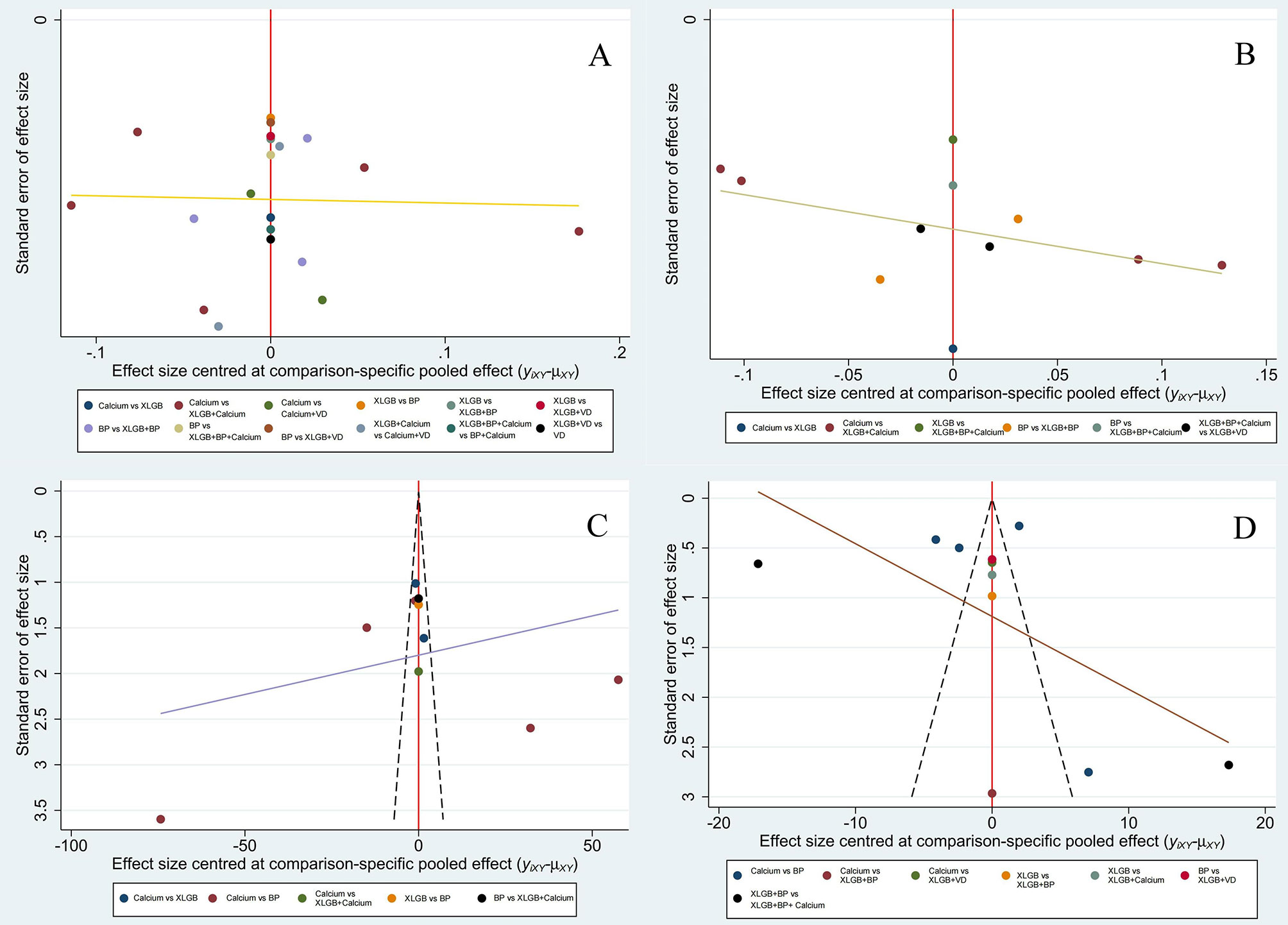
Figure 4 Comparison-adjusted funnel plot of lumbar BMD (A), BMD of the femoral neck (B), BGP (C) and ALP (D).
This study evaluated the efficacy of XLGB and other combined therapies for the treatment of PMOP by network meta-analysis. The results show that the combined application of XLGB can well treat PMOP. Osteoporosis is a systemic bone disease characterized by reduced bone mass and degeneration of bone microstructure, which leads to increased bone fragility and easy fracture (1). Among the types of osteoporosis, PMOP has a high incidence rate (3). XLGB is a Chinese patent medicine widely used for the treatment of osteoporosis (35–37). Due to the lack of comparisons between XLGB and other drug regimens in the treatment of PMOP, this study ranked the clinical efficacy of XLGB-related drug regimens in PMOP treatment with the help of network meta-analysis to provide an evidence-based foundation for its clinical use.
Traditional meta-analysis is a direct comparison between intervention measures, which can most intuitively compare differences in efficacy among drug regimens. In terms of improving lumbar BMD and femoral neck BMD (13, 14, 17, 18, 20–25, 27–34), combined application of XLGB can improve lumbar BMD and femoral neck BMD compared with calcium and VD alone, and XLGB+BP+calcium showed more advantages than BP alone. Furthermore, combined application of XLGB on the basis of BP+calcium had a good clinical effect on improving lumbar BMD. XLGB+calcium exhibited more advantages than calcium alone in increasing the content of serum BGP, and XLGB+BP+calcium had better clinical efficacy than BP alone (13–16, 20–22, 24, 28, 33). Moreover, XLGB showed better clinical efficacy than calcium in improving serum ALP content.
Network meta-analysis was used to examine differences among interventions through indirect comparisons. The results of this study show that XLGB+BP+calcium is most likely the optimal treatment for improving lumbar BMD but that XLGB+calcium is the best combination regimen for improving femoral neck BMD and increasing contents of serum BGP and ALP. Combining the results of this traditional meta-analysis and network meta-analysis, compared with the schemes of VD, BP or calcium alone, combined application of XLGB showed better clinical efficacy in improving BMD and serum BGP and ALP. In traditional Chinese medicine, orthopedic diseases such as osteoporosis are believed to be the manifestation of kidney deficiency. The kidney governs bone and generates marrow, which should be used to tonify the kidney and strengthen Yang (38, 39). Deficiency of the kidney and spleen is the root cause of the disease, and blood stasis is the promoting factor. Blood stasis further aggravates injury to the spleen and kidney and then accelerates the occurrence of osteoporosis (39). XLGB, which is mainly composed of Epimedium supplemented with Dipsacus, Salvia miltiorrhiza, Rehmannia glutinosa and Anemarrhena asphodeloides, has the effects of nourishing the liver and kidney, activating blood circulation and dredging collaterals, and strengthening tendons and bones (40). Previous studies have shown that XLGB combined with calcium or BP has a better clinical effect in the treatment of osteoporosis (7, 41), which is consistent with our conclusions. In addition, theoretical studies have shown that XLGB inhibits osteoclast activity and promotes osteoblast differentiation by regulating OPG/RANK/RANKL (42). Animal experiments have shown that XLGB increases the contents of Ca and P in rat serum, reduces the contents of ALP, OCN and DPD in serum, and increases the BMD of vertebrae as well as the femur and tibia (43). The above results provide a theoretical basis for using XLGB to treat PMOP.
This study has the following limitations. 1) Due to the lack of direct comparison evidence for each outcome index, it was not possible to rank the probability of individual interventions. 2) Most of the included studies were small-sample studies, reducing the statistical efficiency of tour analysis. None of the studies provided strict sample-size estimation standards, which may affect authenticity. 3) We also detected statistical heterogeneity and publication bias, and a pharmacoeconomic evaluation was not included. Furthermore, the great differences in the course of treatment and follow-up time included in the literature may also affect the credibility of the results. Therefore, the conclusions of this study need to be applied in combination with clinical practice. 4) Due to the limitations of this study, further animal or cell experiments are needed to verify the results to provide a more experimental or clinical basis.
The results of this network meta-analysis show that combined application of XLGB can effectively improve BMD and serum BGP and ALP compared to calcium, VD or BP alone. In the future, multicenter, large-sample and double-blind clinical RCTs need to be carried out to supplement and validate the results of this study.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
M-hL and J-lZ conceptualized the research question. M-hL and J-lZ participated in drafting and writing the review. N-jX, XX, W-xF, and J-lZ participated in the formulation of retrieval strategies, data acquisition, data analysis, and quality assessment. M-hL, XX, and J-lZ participated in the drawing of tables and figures. Z-pL and L-fZ participated in critical revision of the manuscript. All authors contributed to the research and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (No.82004383, No. 81974574), the National key research and development program (2021YFC1712804), the Project of Administration of Traditional Chinese Medicine of Guangdong Province (No.20201129), the Project of Guangdong Provincial Department of Finance (No. [2014]157, No. [2018]8), the Medical Science Research Foundation of Guangdong Province (No.A2020105, No.B2019091), the Science and Technology Planning Project of Guangzhou (No. 202102010273) and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No.YN2019ML08, YN2015MS15).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.839885/full#supplementary-material
1. Anastasilakis AD, Polyzos SA, Yavropoulou MP, Makras P. Combination and Sequential Treatment in Women With Postmenopausal Osteoporosis. Expert Opin Pharmaco (2020) 21(4):477–90. doi: 10.1080/14656566.2020.1717468
2. Ono Y, Miyakoshi N, Kasukawa Y, Akagawa M, Kimura R, Nagahata I, et al. Diagnosis of Presarcopenia Using Body Height and Arm Span for Postmenopausal Osteoporosis. Clin Interv Aging (2020) 15:357–61. doi: 10.2147/CIA.S231759
3. Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. Arch Osteoporosis (2013) 8:1–2. doi: 10.1007/s11657-013-0136-1
4. Black DM, Rosen CJ, Solomon CG. Postmenopausal Osteoporosis. N Engl J Med (2016) 374(3):254–62. doi: 10.1056/NEJMcp1513724
5. Migliaccio S, Francomano D, Romagnoli E, Marocco C, Fornari R, Resmini G, et al. Persistence With Denosumab Therapy in Women Affected by Osteoporosis With Fragility Fractures: A Multicenter Observational Real Practice Study in Italy. J Endocrinol Invest (2017) 40(12):1321–6. doi: 10.1007/s40618-017-0701-3
6. de Sire A, Baricich A, Renò F, Cisari C, Fusco N, Invernizzi M. Myostatin as a Potential Biomarker to Monitor Sarcopenia in Hip Fracture Patients Undergoing a Multidisciplinary Rehabilitation and Nutritional Treatment: A Preliminary Study. Aging Clin Exp Res (2020) 32(5):959–62. doi: 10.1007/s40520-019-01436-8
7. Chen J, Zheng J, Chen M, Lin S, Lin Z. The Efficacy and Safety of Chinese Herbal Medicine Xianling Gubao Capsule Combined With Alendronate in the Treatment of Primary Osteoporosis: A Systematic Review and Meta-Analysis of 20 Randomized Controlled Trials. Front Pharmacol (2021) 12:695832. doi: 10.3389/fphar.2021.695832
8. An J, Yang H, Zhang Q, Liu C, Zhao J, Zhang L, et al. Natural Products for Treatment of Osteoporosis: The Effects and Mechanisms on Promoting Osteoblast-Mediated Bone Formation. Life Sci (2016) 147:46–58. doi: 10.1016/j.lfs.2016.01.024
9. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
10. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults With Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet (2018) 391(10128):1357–66. doi: 10.1016/S0140-6736(17)32802-7
11. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G, B. Haibe-Kains B. Graphical Tools for Network Meta-Analysis in STATA. PloS One (2013) 8(10):e76654–4. doi: 10.1371/journal.pone.0076654
12. Marotta N, Demeco A, Moggio L, Marinaro C, Pino I, Barletta M, et al. Comparative Effectiveness of Breathing Exercises in Patients With Chronic Obstructive Pulmonary Disease. Complement Ther Clin Pract (2020) 41:101260. doi: 10.1016/j.ctcp.2020.101260
13. Zhu HM, Qin L, Garnero P, Genant HK, Zhang G, Dai K, et al. The First Multicenter and Randomized Clinical Trial of Herbal Fufang for Treatment of Postmenopausal Osteoporosis. Osteoporosis Int (2012) 23(4):1317–27. doi: 10.1007/s00198-011-1577-2
14. Zhang H, Wang Y, Nie H. Efficacy of Alendronate Sodium Combined With Xianling Gubao in the Treatment of Osteoporosis in Middle-Aged and Elderly Postmenopausal Patients. China Med Pharm (2021) 11(17):231–4. doi: 10.3969/j.issn.2095-0616.2021.17.063
15. Wu N, Chen J, Huang J, Huang J. Clinical Study of Fushanmei Combined With Xianlinggubao in the Treatment of Osteocalcin, Calcitonin and Bone Mineral Density in Postmenopausal Osteoporosis. Contemp Med (2008) 147(11):142–4.
16. Li L. Clinical Study on the Changes of Osteocalcin, Calcitonin and Bone Mineral Density of Fushanmei Combined With Xianlinggubao in the Treatment of Postmenopausal Osteoporosis. Oriental Diet Ther Health Care (2016) 9:230–1. doi: 10.3969/j.issn.1672-5018.2016.09.211
17. Ni G. Clinical Observation of Calcitriol Combined With Xianlinggubao in the Treatment of Postmenopausal Osteoporosis. J New Chin Med (2014) 46(02):113–5. doi: 10.13457/j.cnki.jncm.2014.02.032
18. Wang M. Clinical Study of Calcitriol Combined With Xianlinggubao in Treatment of Women With Osteoporosis. Baoding City: Hebei University (2014).
19. Lai F, Ma L, Liang X, Liu M, Luo Z, Li J. Clinical Observation on Jintiange Capsules in the Treatment of Postmenopausal Osteoporosis. Chin Med Modern Distance Educ China (2019) 17(15):63–5. doi: 10.3969/j.issn.1672-2779.2019.15.026
20. Dai Y, Shen L, Yang Y, Xie J, Zhou P. Effects of Migu Tablet on Bone Mineral Density, Serum Levels of Osteoprotegerin and Its Ligand and Bone Metabolic Markers in Patients With Postmenopausal Osteoporosis. Chin J Integrated Tradit Western Med (2007) 27(08):696–9. doi: 10.3321/j.issn:1003-5370.2007.08.009
21. Zhang X, Han J, Qian G, He M, Li Y. Effects of Xianling Gubao Capsule on Bone Mineral Density and Cytokines in Postmenopausal Osteaporofic Patients. Chin J Osteoporosis (2004) 10(01):99–102.
22. Wu W, Li D, Zhi X, Han M. Preventive and Therapeutic Effects of Xianling Gubao Capsules for Postmenopausal Osteoporosis. J Guangzhou Univ Tradit Chin Med (2005) 22(03):191–3. doi: 10.13359/j.cnki.gzxbtcm.2005.03.008
23. Xu M, Liu B, Huang C, Tang F, Lou Y, Liang Z, et al. Clinical Observation of Xianlinggubao Plus Alendronate on Postmenopausal Osteoporosis. J Liaoning Univ Tradit Chin Med (2009) 11(01):94–5. doi: 10.13194/j.jlunivtcm.2009.01.96.xum.106
24. Cai C, He G. Effects of Xianling Gubao Jiaonang on Lipid Metabolism, Bone Metabolism and Bone Density in Postmenopausal Osteoporosis Patients With Obesity. J Henan Med Coll (2021) 33(01):27–31. doi: 10.3969/j.issn.1008-9276.2021.01.007
25. Nie D, Peng M, Lin Z, Yang L. Clinical Research on Xianling Gubao Capsule and Rocalirol Tablet in Treating 35 Cases of Postmenopausal Osteoporosis Related Pain. Rehabil Med (2009) 19(03):37–8. doi: 10.13261/j.cnki.jfutcm.002170
26. Wu Z. Xianling Gubao Capsule Combined With Caltech D in the Treatment of Postmenopausal Osteoporosis. Strait Pharm J (2010) 22(12):159–60.
27. Wang Y, Sheng C. Alfacalcidol Capsules Combined With Xianling Gubao Capsules in Treatment of Postmenopausal Osteoporosis. Int Med Health Guidance News (2019) 25(07):1099–102. doi: 10.3760/cma.j.issn.1007-1245.2019.07.027
28. Li Y. Effect of Xianling Gubao Capsule Combined With Calcium Acetate Capsule on Post- Menopausal Women’s Osteoporosis. World J Complex Med (2020) 6(05):183–5. doi: 10.11966/j.issn.2095-994X.2020.06.05.61
29. Chen X, Zhu X, Lin W, Wang L, Yang S. Effect of Xianlinggubao Capsule in the Treatment of Postmenopausal Osteoporosis and its Effect on Osteoprotegerin, Receptor Activator of Nuclear Factor - κb Ligand. Chin J Clin Pharmacol (2015) 31(10):827–9+854. doi: 10.13699/j.cnki.1001-6821.2015.10.019
30. Shang Y, Liu Y, Li H. Clinical Observation of Xianling Gubao Capsule in the Treatment of Postmenopausal Osteoporosis. Chin J Integrated Tradit Western Med Intensive Crit Care (2007) 14(01):55.
31. Liu M, Yang F, Zeng L. Clinical Observation of Xianling Gubao Capsule in the Treatment of Postmenopausal Women With Osteoporosis. Yunnan J Tradit Chin Med Materia Med (2021) 42(06):37–40. doi: 10.16254/j.cnki.53-1120/r.2021.06.012
32. Song X. The Clinical Research Into Menopausal Osteoporosis Treated With Xianlinggubao Capsules. Henan Tradit Chin Med (2017) 37(04):686–8. doi: 10.16367/j.issn.1003-5028.2017.04.0244
33. Zhou J. Effects of Xianling Gubao Combined With Alendronate on Bone Metabolic Indexes, Bone Mineral Density and Symptoms of Bone Pain in Postmenopausal Osteoporosis. Chin J Gerontol (2020) 40(03):581–4. doi: 10.3969/j.issn.1005-9202.2020.03.041
34. Zhuang L. Xianlinggubao Combined With Fushanmei in the Treatment of Postmenopausal Osteoporosis. Zhejiang J Integrated Tradit Chin Western Med (2013) 23(07):558–60.
35. Wang X, He Y, Guo B, Tsang M, Tu F, Dai Y, et al. In Vivo Screening for Anti-Osteoporotic Fraction From Extract of Herbal Formula Xianlinggubao in Ovariectomized Mice. PloS One (2015) 10(2):e0118184. doi: 10.1371/journal.pone.0118184
36. Wu H, Zhong Q, Wang J, Wang M, Fang F, Xia Z, et al. Beneficial Effects and Toxicity Studies of Xian-Ling-Gu-Bao on Bone Metabolism in Ovariectomized Rats. Front Pharmacol (2017) 8:273. doi: 10.3389/fphar.2017.00273
37. Ai L, Yi W, Chen L, Wang H, Huang Q. Xian-Ling-Gu-Bao Protects Osteoporosis Through Promoting Osteoblast Differentiation by Targeting miR-100-5p/KDM6B/RUNX2 Axis. In Vitro Cell Dev Biol Anim (2021) 57(1):3–9. doi: 10.1007/s11626-020-00530-w
38. Chen YY, Hsue YT, Chang HH, Gee MJ. The Association Between Postmenopausal Osteoporosis and Kidney-Vacuity Syndrome in Traditional Chinese Medicine. Am J Chin Med (1999) 27(1):25–35. doi: 10.1142/S0192415X99000057
39. Liang J, Wang F, Huang J, Xu Y, Chen G. The Efficacy and Safety of Traditional Chinese Medicine Tonifying-Shen (Kidney) Principle for Primary Osteoporosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med (2020) 2020:1–21. doi: 10.1155/2020/5687421
40. Qin L, Zhang G, Hung W, Shi Y, Leung K, Yeung H, et al. Phytoestrogen-Rich Herb Formula “XLGB” Prevents OVX-Induced Deterioration of Musculoskeletal Tissues at the Hip in Old Rats. J Bone Miner Metab (2005) 23(S1):55–61. doi: 10.1007/BF03026324
41. Wang G, Liao X, Zhang Y, Xie Y. Systemic Evaluation and Meta-Analysis of Xianling Gubao Capsule in Treatment of Primary Osteoporosis in Randomized Controlled Trials. China J Chin Materia Med (2017) 42(15):2829–44. doi: 10.19540/j.cnki.cjcmm.20170705.007
42. Qi X, Chen G, Shi P, Zhang Z, Fang C, Zheng S, et al. Analysis of Pharmacological Mechanism of XianlingGubao Capsule in Treating Osteoporosis Based on Network Pharmacology. Chin J Osteoporosis (2020) 26(5):710–8. doi: 10.3969/j.issn.1006-7108.2020.05.017
Keywords: traditional Chinese medicine, efficacy, Xianling Gubao capsule, postmenopausal osteoporosis, network meta-analysis
Citation: Luo M-h, Zhao J-l, Xu N-j, Xiao X, Feng W-x, Li Z-p and Zeng L-f (2022) Comparative Efficacy of Xianling Gubao Capsules in Improving Bone Mineral Density in Postmenopausal Osteoporosis: A Network Meta-Analysis. Front. Endocrinol. 13:839885. doi: 10.3389/fendo.2022.839885
Received: 20 December 2021; Accepted: 24 January 2022;
Published: 18 February 2022.
Edited by:
Qi Wang, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Alessandro de Sire, University of Magna Graecia, ItalyCopyright © 2022 Luo, Zhao, Xu, Xiao, Feng, Li and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zi-ping Li, bHppcDAwOEAxNjMuY29t; Ling-feng Zeng, emVuZ2xmNjc3OEAxNjMuY29t
†ORCID: Jin-long Zhao, orcid.org/0000-0001-7079-1336
Ling-feng Zeng, orcid.org/0000-0001-8149-242X
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.