- National Clinical Research Center for Metabolic Diseases, Key Laboratory of Diabetes Immunology (Central South University), Ministry of Education, and Department of Metabolism and Endocrinology, The Second Xiangya Hospital of Central South University, Changsha, China
Background and Aims: The NLRP3 gene is reportedly associated with several autoimmune diseases. However, in the Chinese Han population, whether NLRP3 polymorphisms are associated with type 1 diabetes (T1D) is unclear. Therefore, this study examined the associations of rs3806265 and rs4612666 of the NLRP3 gene with T1D susceptibility and the clinical characteristics of Chinese Han T1D patients.
Methods: In total, 510 classic T1D patients and 531 healthy controls from the Chinese Han population were recruited for a case-control study. rs3806265 and rs4612666 of the NLRP3 gene were genotyped by MassARRAY. Logistic regression analysis and the chi-square test were used to compare the distributions of the alleles and genotypes of rs3806265 and rs4612666. The relationships between rs3806265 and rs4612666 and the clinical characteristics of T1D patients were analyzed by Kruskal-Wallis one-way ANOVA. Student’s t test was used to analyze normally distributed data. Bonferroni correction was used for multiple comparisons.
Results: 1) rs3806265 was associated with glutamic acid decarboxylase antibody (GADA) titers (P = 0.02), and patients with the CC genotype had higher GADA titers than patients with the TT genotype. 2) rs4612666 was also associated with GADA titers (P=0.041). Compared with patients with the CC genotype, patients with the TT genotype had higher GADA titers. 3) rs3806265 and rs4612666 of the NLRP3 gene were not significantly associated with T1D susceptibility under different genetic models.
Conclusion: rs3806265 and rs4612666 of the NLRP3 gene were significantly associated with GADA titers in Chinese Han T1D patients.
Introduction
The NLRP3 inflammasome is a kind of polyprotein complex composed of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase-1 (1). The NLRP3 inflammasome can induce the maturation and secretion of pro-inflammatory factors such as IL-1β and IL18, leading to the inflammatory response (2). Because of the important role of the NLRP3 inflammasome, it is considered an effective target for the regulation of the occurrence and development of various kinds of autoimmune and inflammatory diseases (3).
T1D is an organ-specific autoimmune disease in which islet β cells are destroyed by autoreactive T cells, resulting in absolute insulin deficiency (4). On one hand, the loss of insulin results in excessive endogenous glucose production and a decrease in glucose uptake, thus causing hyperglycemia. On the other hand, the loss of insulin causes an increase in lipolysis, fatty acid oxidation and the excessive accumulation of ketones, leading to diabetic ketoacidosis.
Although the etiology of T1D is not yet fully understood, it is considered a complex inherited disease caused by genetic and environmental factors (5, 6). Genetic susceptibility is very important for the development of T1D (7–10). Studies have reported that in Caucasians, the risk of siblings all suffering from T1D is 6%, which is 15 times the risk in the general population (11). The consistency with which monozygotic twins are both affected by T1D (30–40%) is higher than that for dizygotic twins (6–8%) (11).
The NLRP3 gene, which is located on chromosome 1q44, is expressed in various types of cells, such as B cells, epithelial cells, and osteoblasts (12). The roles of the NLRP3 variants rs3806265 and rs4612666 in autoimmune and inflammatory diseases have been well researched. rs3806265 was reported to be associated with psoriatic juvenile idiopathic arthritis (13), recurrent aphthous stomatitis (14), inflammatory bowel disease (15) and so on, while rs4612666 was associated with periodontitis (16), food-induced anaphylaxis and aspirin-induced asthma (AIA) (17). Furthermore, rs4612666 is a functional variant that results in the enhanced stability of NLRP3 mRNA and an increase in the activity of NLRP3, which subsequently leads to a series of inflammatory responses (17).
Many studies have shown that the NLRP3 polymorphisms mentioned above are associated with several autoimmune diseases. With regard to T1D, it has been reported that there is an association between rs10754558 of the NLRP3 gene and T1D in the population of northeast Brazil (18). However, there have been no reports about whether the NLRP3 gene is correlated with T1D in the Chinese Han population. Given the roles played by rs3806265 and rs4612666 in autoimmune and inflammatory diseases and the fact that they have not been examined in relation to T1D in the Chinese Han population, we hypothesized that a relationship exists between these two SNPs and T1D in the Chinese Han population. In the present study, we further elucidated the etiology of T1D by investigating the associations of the two aforementioned SNPs in the NLRP3 gene with T1D in the Chinese Han population. This is the first report about the genetic association between NLRP3 polymorphisms (rs3806265 and rs4612666) and T1D.
Materials and Methods
Study Population
Every procedure of the study was approved by the Ethics Committee of Second Xiangya Hospital and followed the Helsinki Declaration guidelines. All participants signed written informed consent forms after the purpose and methods of the research were explained.
To evaluate the associations between the two SNPs and T1D as well as the clinical characteristics of T1D patients, a case-control study was performed, and 510 T1D patients (mean age 22 years; 275 males and 235 females) and 531 healthy people (mean age 42 years; 273 males and 258 females) were recruited from a Chinese Han population. The peak incidence of T1D appeared close to puberty; therefore, individuals recruited for the control group were much older, indicating that they had a lower likelihood of developing T1D in the future. The T1D patients were included when they were diagnosed in the Department of Metabolism and Endocrinology in the Second Xiangya Hospital. The recruitment criteria for T1D patients were as follows: (1) fulfilled the criteria for the diagnosis of diabetes developed by the WHO in 1999; (2) free from complications and did not receive additional medication except insulin injections; and (3) positive serum detection of one of the islet autoantibodies at diagnosis, such as GADA, IA-2A and ZnT8A (19, 20). In addition, patients with other kinds of diabetes, such as LADA and autoimmune diseases, and other kinds of autoimmune diseases were excluded through clinical examination. The healthy controls were recruited from an epidemiological study and physical examinations in the Second Xiangya Hospital and had normal clinical examination findings and no symptoms or a history of diabetes or autoimmune diseases. The inclusion criteria for the healthy controls were (1) a fasting plasma glucose (FPG) level < 5.6 mmol/l and (2) a 2-h postprandial plasma glucose (PPG) level < 7.8 mmol/l according to the 75 g OGTT test. To reduce the selection bias, we strictly followed the inclusion and exclusion criteria when enrolling the subjects.
Clinical Measurements
Clinical measurements of the individuals were collected by well-trained investigators, including sex, weight, height, the age at T1D onset and some laboratory examination results, such as the FPG level, 2-h PPG level according to the OGTT test, fasting C-peptide (FCP) level and 2-h postprandial C-peptide (PCP) level. The FCP and 2-h PCP levels were detected by a chemiluminescence method (ADVIA Centaur XP Immunoassay System, Siemens, Germany) in the laboratory of the Second Xiangya Hospital, and the HbA1c level was detected by automated liquid chromatography (HLC-723G8, Tosoh, Japan). The radioligand binding assay was used in our laboratory to detect islet autoantibodies such as GADA, IA-2A and ZnT8A (21–23).
DNA Extraction
A GeneNode Genomic DNA Extraction kit (Genenode Biotech Co., Ltd., Beijing) was used to extract DNA from peripheral whole blood samples in accordance with the manufacturer’s instructions. After extraction, the DNA samples were stored at -80°C.
SNP Selection and Genotyping
We selected rs3806265 (chr1: 247423034(GRCh38.p12); T>A or T>C; Intron variant) and rs4612666 (chr1: 247435768 (GRCh38.p12); T>C; Intron variant) of the NLRP3 gene for analysis. These two SNPs were nominated using a widely used nomenclature system. We selected these two SNPs because they were previously demonstrated to be correlated with many autoimmune and inflammatory diseases, and rs4612666 was even shown to serve as a functional variant that influenced the process of inflammation. In addition, we prepared SNPs with minor allele frequencies (MAFs) >0.05 in Asian people. Those selected SNPs were located in different linkage regions.
MassARRAY was used for genotyping. ADS2.0 from Agena Bioscience was used to design the sequences of the primers for the two SNPs, which were then synthesized by BGI. Typer 4.0 software (Agena Bioscience Inc.) was used to collect genotyping data after clustering. A specificity of 0.90 and a sensitivity of 0.95 were set to cluster all loci. All genotyping assays were performed when the clinical information of the research subjects was unknown in the laboratory of BGI Genomics, BGI-Shenzhen, Shenzhen 518083, China.
Statistical Analysis
The statistical analyses were performed with SPSS 20.0 software, and Hardy–Weinberg equilibrium (HWE) was detected by online software (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). We used the median (interquartile range (IQR)) to represent the values of continuous variables. The frequency distributions of the alleles and genotypes of the two SNPs between patients and controls were compared by the chi-square test and logistic regression. The clinical characteristics of patients with different genotypes of the two SNPs were compared by the chi-square test and the Kruskal-Wallis H test. Student’s t test was used to analyze the normally distributed data. P<0.05 was considered statistically significant. Bonferroni correction was used for multiple comparisons. The sample size was determined by Quanto software (version 1.2) with the following parameters: an expected odds ratio (OR) of 1.3, minor allele frequencies of 0.44 for rs3806265 and 0.44 for rs4612666 from an online website (http://pubs.broadinstitute.org/mammals/haploreg/haploreg.php), a prevalence of 1% of T1D patients in China and a statistical power of 0.8.
Results
Calculation of the Study Size by Using Quanto Software
The sample size calculated by Quanto software was 920, which is lower than the number of subjects who we actually recruited, suggesting that the sample size in our research was sufficient to detect an association between the two SNPs and T1D.
Clinical and Biochemical Measurements of T1D Patients and Healthy Controls
The clinical and biochemical measurements of T1D patients and controls have been reported previously (24). There were differences between the cases and controls in many clinical characteristics. The sex distributions were not different between the cases and controls (275/235 vs. 273/258, p=0.418). T1D patients were younger (p<0.01) than the controls, and the FPG (p<0.001) and 2-h PPG (p<0.001) levels were both higher in the cases than in the controls.
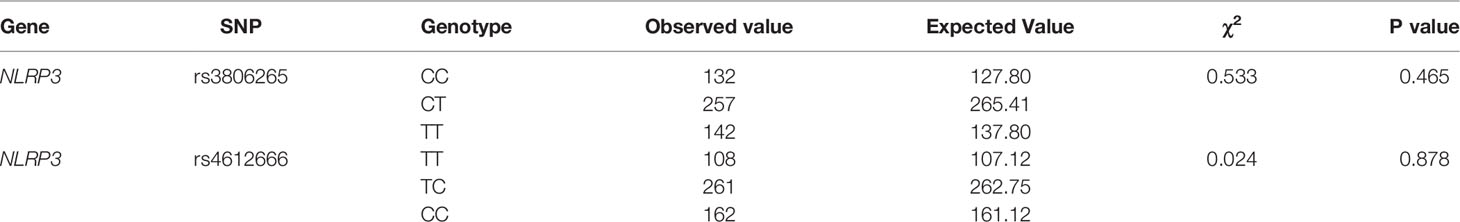
Detection of Hardy-Weinberg Equilibrium
The genotype frequencies of the two SNPs of the NLRP3 gene were in Hardy-Weinberg equilibrium (p>0.05), which means that the samples that we selected were representative. The results are shown in Table 1.
Frequency Distributions of the Alleles and Genotypes of rs3806265 and rs4612666
The results are shown in Table 2. A total of 508 of the 510 cases of rs3806265 and 506 of the 510 cases of rs4612666 and 531 healthy controls were genotyped. No significant differences were found in the frequency distributions of the alleles and genotypes of the two SNPs in the cases and controls.
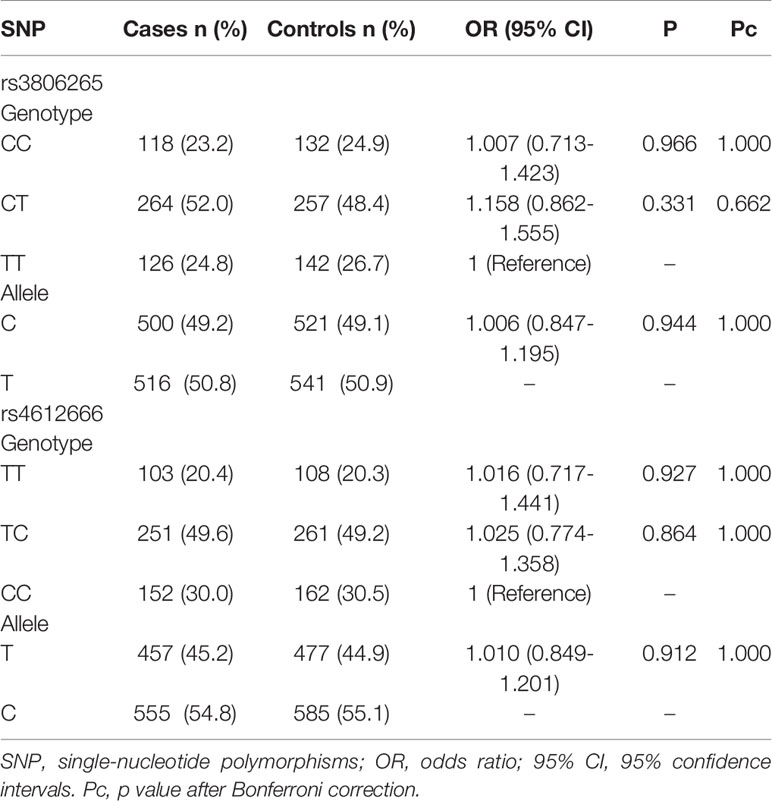
Table 2 Frequencies of the genotypes and alleles of rs3806265 and rs4612666 between cases and controls.
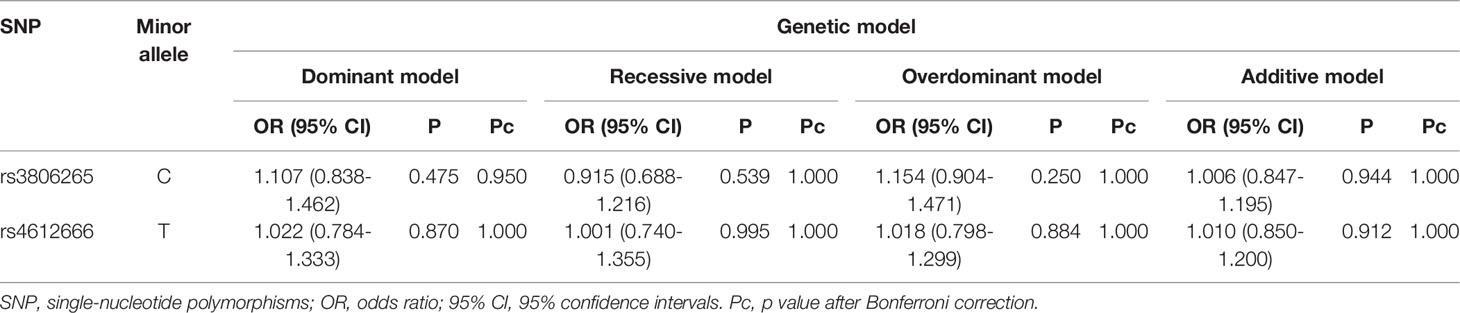
Associations Between the Two SNPs and Susceptibility to T1D Under Different Genetic Models
The results showed that rs3806265 and rs4612666 were not correlated with susceptibility to T1D under different genetic models. The results are shown in Table 3.
Associations Between the Two SNPs and the Clinical Characteristics of T1D Patients
We performed a genotype-phenotype association analysis combining basic information such as age, sex and duration of the disease and biochemical measurements such as HbA1c and FCP level and the titer and positivity rate of islet autoantibodies with different genotypes of the two SNPs. The data are shown in Tables 4, 5. rs3806265 was significantly associated with the titer of GADA (P = 0.02), and patients with the CC genotype had higher GADA titers than patients with the TT genotype. rs4612666 was also associated with the GADA titer (P=0.041). Compared with patients with the CC genotype, patients with the TT genotype had higher GADA titers.
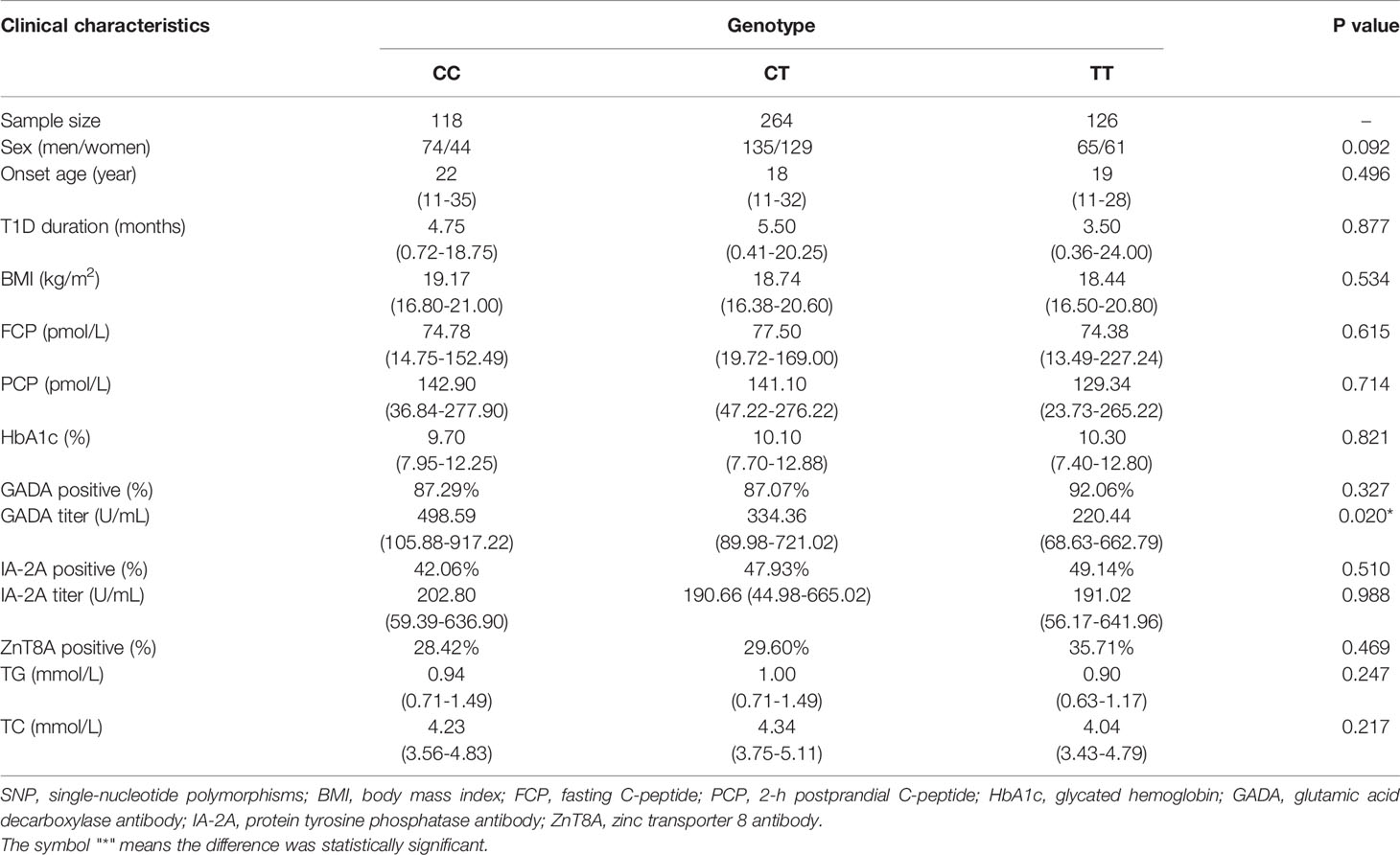
Table 4 Clinical and biochemical characteristics of T1D patients with different genotypes of rs3806265 of the NLRP3 gene.
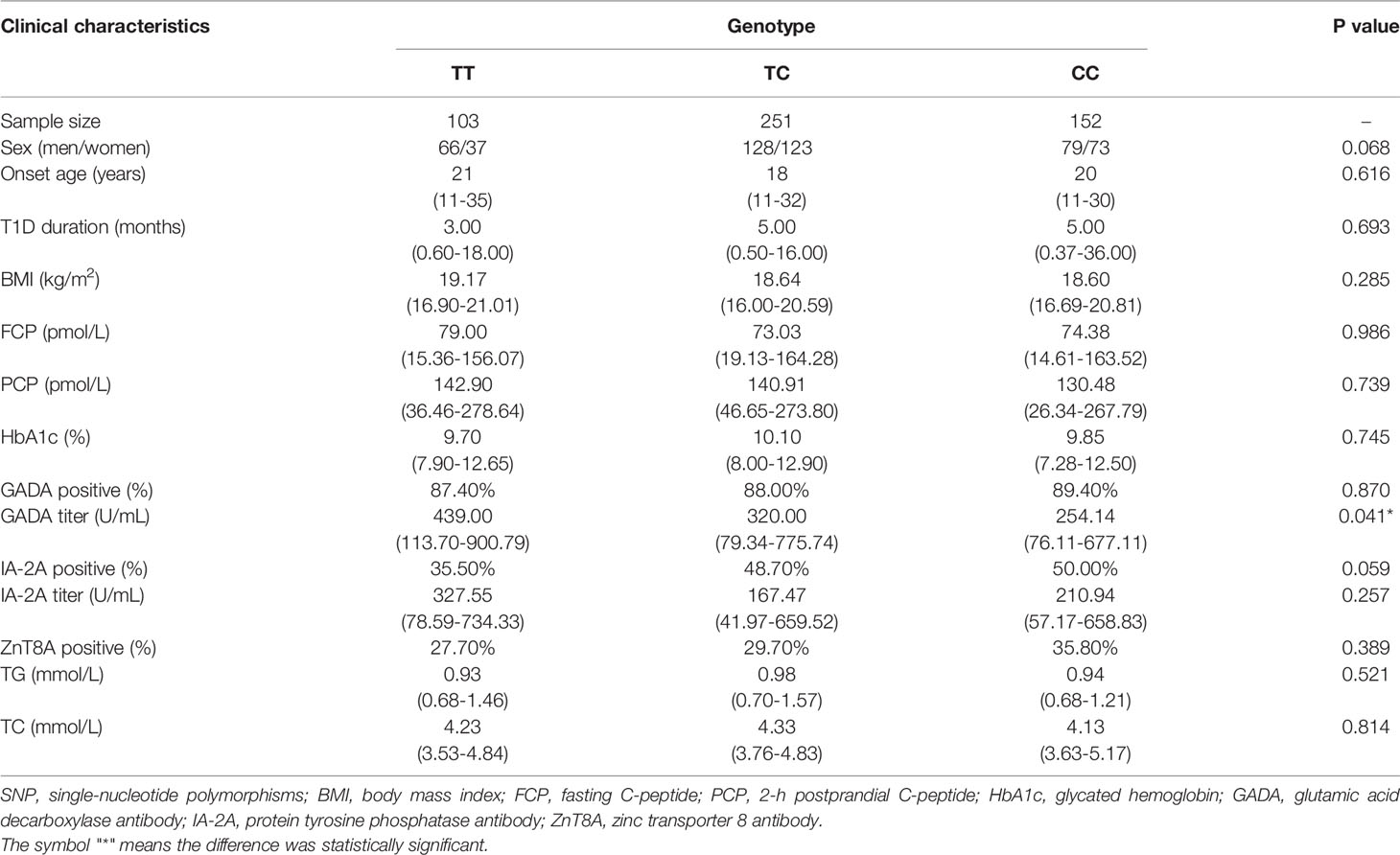
Table 5 Clinical and biochemical characteristics of T1D patients with different genotypes of rs4612666 of the NLRP3 gene.
Discussion
NLRP3, a key protein in the NLRP3 inflammasome, is essential for innate and acquired immunity. Therefore, some authors have proposed that NLRP3 plays a crucial role in the maintenance of tolerance conferring protection from autoimmune diseases (25, 26). NLRP3 mutations have been reported to lead to many autoimmune disorders (27, 28), but only a few disease-causing variants are associated with T1D (18).
T1D is an autoimmune disease characterized by impaired β cell function caused by autoreactive T cells. The positivity rate for the detection of islet autoantibodies in T1D patients is significantly higher than the rates in patients with other kinds of diabetes and healthy people. GADA, one of the main types of islet autoantibodies in T1D, is a marker of immunity in the early stage of the disease. Furthermore, a high titer of GADA strongly predicts the development of thyroid autoimmunity in T1D, suggesting that an association exists between a high titer of GADA and severe autoimmunity. Several studies also pointed out that a high titer of GADA was associated with progressive β cell failure in patients with slowly progressing T1D (29).
To date, this study is the first to clarify the associations between these two SNPs (rs3806265 and rs4612666) of the NLRP3 gene and T1D in a Chinese Han population. No association of the NLRP3 gene with susceptibility to T1D was found. However, significant differences were found between the two SNPs of the NLRP3 gene with regard to the GADA titer in T1D patients. Due to the limited number of our samples, further validation in a larger population is required as well as in other countries and ethnic groups. The lack of associations between the selected SNPs and susceptibility to T1D might be partly explained by the fact that two SNPs may not be fully representative of the entire gene. In addition, T1D is a multigenic disease; therefore, the effect of a single gene might be small. Furthermore, as a multifactorial disease, T1D is also caused by environmental factors. In future studies, we may consider involving larger sample sizes and more SNPs of the NLRP3 gene. Regarding the associations between the two selected SNPs and clinical characteristics of patients with T1D, both rs3806265 and rs4612666 of the NLRP3 gene were associated with the GADA titer. T1D patients with the CC genotype of rs3806265 had a higher GADA titer than patients with the TT genotype, while patients with the TT genotype of rs4612666 had a higher GADA titer than those with the CC genotype. Patients with different genotypes of the two SNPs had different GADA titers, which may indicate that residual β cell function and disease progression would also be different. We speculate that the two SNPs may influence the activation of the NLRP3 inflammasome therefore the process of cytokines factors production by NLRP3 inflammasome. The cytokines such as IL-18 and IL-1β, can enhance T and B lymphocyte responses and may play a role in the production of GADA. Functional experiments will be needed to explore the mechanism by which rs3806265 and rs4612666 affect the GADA titers of T1D patients. The GADA titer reflects the severity of cellular immune destruction and can predict the risk of β cell failure as well as the progression of T1D (30). The correlation we found in this study between the two SNPs and the GADA titer can be applied to the clinical treatment and prognosis of T1D in the future. Studies from our group have shown that GADA titers are associated with the loss of β-cell function (31), the rate of the loss of β-cell function in LADA patients with high GADA titers was significantly faster than in patients with low GADA titers. Early insulin therapy can delay the apoptosis of βcell and protect the function of the remaining βcells (32). In this research we found there was a relationship between the two SNPs of NLRP3 gene and GADA titers, therefore we suggest early insulin therapy for patients carrying the genotype associated with a high titer of GADA. Besides, studies from our group have shown that high titers of GADA is a strong predictor of the development of thyroid autoimmunity in patients with type 1 diabetes and latent autoimmune diabetes in adults (33), therefore clinicians should also focus more on islet function and glycemic control as well as the appearance of other autoimmune diseases in patients carrying the genotype associated with high titers of GADA.
In conclusion, this case-control study including 510 cases and 531 controls demonstrated that there are associations between rs3806265 and rs4612666 of the NLRP3 gene and the GADA titers in T1D patients. These findings have deepened our understanding of the pathophysiology of T1D, and the differences in the GADA titers in patients with different genotypes of the two SNPs will help us predict the residual β cell function as well as disease progression of T1D patients, thus providing new directions for disease prevention and treatment in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Second Xiangya Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Conception and design: ZX. Administrative support: ZZ. Provision of study materials or patients: ZZ, ZX, XL, YaX, GH, XS, JL, SL, and YiX. Collection and assembly of data: ZX, XS, and LX. Data analysis and interpretation: ZX, XS, and LX. Manuscript writing: LX and XS. Final approval of manuscript: All authors. All authors are in agreement with the content and the submission of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82070813,81873634, 81400783), the National Key R&D Program of China (2016YFC1305000, 2016YFC1305001, 2018YFC1315603), the Science and Technology Major Project of Hunan Province (2017SK1020), and the Hunan Natural Science Funds for Distinguished Young Scholars (Grant No.2020JJ2053).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our sincere appreciation to all the staff of the Department of Metabolism and Endocrinology of Second Xiangya Hospital for collecting the samples.
References
1. Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and Autoimmunity. Trends Mol Med (2011) 17(2):57–64. doi: 10.1016/j.molmed.2010.11.001
2. De Nardo D, Latz E. NLRP3 Inflammasomes Link Inflammation and Metabolic Disease. Trends Immunol (2011) 32(8):373–9. doi: 10.1016/j.it.2011.05.004
3. Zhang C, Boini KM, Xia M, Albais JM, Li X, Liu Q, et al. Activation of Nod-Like Receptor Protein 3 Inflammasomes Turns on Podocyte Injury and Glomerular Sclerosis in Hyperhomocysteinemia. Hypertension (2012) 60(1):154–62. doi: 10.1161/HYPERTENSIONAHA.111.189688
4. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 Diabetes. Lancet (2014) 383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7
5. Bluestone JA, Herold K, Eisenbarth G. Genetics, Pathogenesis and Clinical Interventions in Type 1 Diabetes. Nat (2010) 464(7293):1293–300. doi: 10.1038/nature08933
6. Xie Z, Huang G, Wang Z, Luo S, Zheng P, Zhou Z. Epigenetic Regulation of Toll-Like Receptors and Its Roles in Type 1 Diabetes. J Mol Med (Berl) (2018) 96(8):741–51. doi: 10.1007/s00109-018-1660-7
7. Luo S, Lin J, Xie Z, Xiang Y, Zheng P, Huang G, et al. HLA Genetic Discrepancy Between Latent Autoimmune Diabetes in Adults and Type 1 Diabetes: LADA China Study No. 6. J Clin Endocrinol Metab (2016) 101(4):1693–700. doi: 10.1210/jc.2015-3771
8. Xie Z, Chang C, Zhou Z. Molecular Mechanisms in Autoimmune Type 1 Diabetes: A Critical Review. Clin Rev Allergy Immunol (2014) 47(2):174–92. doi: 10.1007/s12016-014-8422-2
9. Pang H, Luo S, Huang G, Xia Y, Xie Z, Zhou Z. Advances in Knowledge of Candidate Genes Acting at the Beta-Cell Level in the Pathogenesis of T1DM. Front Endocrinol (Lausanne) (2020) 11:119. doi: 10.3389/fendo.2020.00119
10. Xu L, Sun X, Xia Y, Luo S, Lin J, Xiao Y, et al. Polymorphisms of the NLRC4 Gene are Associated With the Onset Age, Positive Rate of GADA and 2-H Postprandial C-Peptide in Patients With Type 1 Diabetes. Diabetes Metab Syndr Obes (2020) 13:811–8. doi: 10.2147/DMSO.S244882
11. Risch N. Assessing the Role of HLA-Linked and Unlinked Determinants of Disease. Am J Hum Genet (1987) 40(1):1–14.
12. Yang CS, Shin DM, Jo EK. The Role of NLR-Related Protein 3 Inflammasome in Host Defense and Inflammatory Diseases. Int Neurourol J (2012) 16(1):2–12. doi: 10.5213/inj.2012.16.1.2
13. Imani D, Azimi A, Salehi Z, Rezaei N, Emmanejad R, Sadr M, et al. Association of Nod-Like Receptor Protein-3 Single Nucleotide Gene Polymorphisms and Expression With the Susceptibility to Relapsing-Remitting Multiple Sclerosis. Int J Immunogenet (2018) 45(6):329–36. doi: 10.1111/iji.12401
14. Slezakova S, Borilova Linhartova P, Masopustova L, Bartova J, Petanova J, Kuklinek P, et al. Association of the NOD-Like Receptor 3 (NLRP3) Gene Variability With Recurrent Aphthous Stomatitis in the Czech Population. J Oral Pathol Med (2018) 47(4):434–9. doi: 10.1111/jop.12694
15. Hanaei S, Sadr M, Rezaei A, Shahkarami S, Ebrahimi Daryani N, Bidoki AZ, et al. Association of NLRP3 Single Nucleotide Polymorphisms With Ulcerative Colitis: A Case-Control Study. Clin Res Hepatol Gastroenterol (2018) 42(3):269–75. doi: 10.1016/j.clinre.2017.09.003
16. de Alencar JB, Zacarias JMV, Tsuneto PY, Souza VH, Silva COE, Visentainer JEL, et al. Influence of Inflammasome NLRP3, and IL1B and IL2 Gene Polymorphisms in Periodontitis Susceptibility. PloS One (2020) 15(1):e0227905. doi: 10.1371/journal.pone.0227905
17. Hitomi Y, Ebisawa M, Tomikawa M, Imai T, Komata T, Hirota T, et al. Associations of Functional NLRP3 Polymorphisms With Susceptibility to Food-Induced Anaphylaxis and Aspirin-Induced Asthma. J Allergy Clin Immunol (2009) 124(4):779–85.e776. doi: 10.1016/j.jaci.2009.07.044
18. Pontillo A, Brandao L, Guimaraes R, Segat L, Araujo J, Crovella S. Two SNPs in NLRP3 Gene Are Involved in the Predisposition to Type-1 Diabetes and Celiac Disease in a Pediatric Population From Northeast Brazil. Autoimmun (2010) 43(8):583–9. doi: 10.3109/08916930903540432
19. Yang L, Luo S, Huang G, Peng J, Li X, Yan X, et al. The Diagnostic Value of Zinc Transporter 8 Autoantibody (ZnT8A) for Type 1 Diabetes in Chinese. Diabetes Metab Res Rev (2010) 26(7):579–84. doi: 10.1002/dmrr.1128
20. Yi B, Huang G, Zhou ZG. Current and Future Clinical Applications of Zinc Transporter-8 in Type 1 Diabetes Mellitus. Chin Med J (Engl) (2015) 128(17):2387–94. doi: 10.4103/0366-6999.163389
21. Jin P, Huang G, Lin J, Luo S, Zhou Z. Epitope Analysis of GAD65 Autoantibodies in Adult-Onset Type 1 Diabetes and Latent Autoimmune Diabetes in Adults With Thyroid Autoimmunity. Acta Diabetol (2011) 48(2):149–55. doi: 10.1007/s00592-010-0250-0
22. Jin P, Xiang B, Huang G, Zhou Z. The Association of Cytotoxic T-Lymphocyte Antigen-4 + 49A/G and CT60 Polymorphisms With Type 1 Diabetes and Latent Autoimmune Diabetes in Chinese Adults. J Endocrinol Invest (2015) 38(2):149–54. doi: 10.1007/s40618-014-0162-x
23. Xiang Y, Huang G, Shan Z, Pan L, Luo S, Yang L, et al. Glutamic Acid Decarboxylase Autoantibodies are Dominant But Insufficient to Identify Most Chinese With Adult-Onset Non-Insulin Requiring Autoimmune Diabetes: LADA China Study 5. Acta Diabetol (2015) 52(6):1121–7. doi: 10.1007/s00592-015-0799-8
24. Sun X, Xia Y, Liu Y, Wang Y, Luo S, Lin J, et al. Polymorphisms in NLRP1 Gene Are Associated With Type 1 Diabetes. J Diabetes Res (2019) 2019:7405120. doi: 10.1155/2019/7405120
25. O'Sullivan BJ, Thomas HE, Pai S, Santamaria P, Iwakura Y, Steptoe RJ, et al. IL-1 Beta Breaks Tolerance Through Expansion of CD25+ Effector T Cells. J Immunol (2006) 176(12):7278–87. doi: 10.4049/jimmunol.176.12.7278
26. Mollah ZU, Pai S, Moore C, O'Sullivan BJ, Harrison MJ, Peng J, et al. Abnormal NF-Kappa B Function Characterizes Human Type 1 Diabetes Dendritic Cells and Monocytes. J Immunol (2008) 180(5):3166–75. doi: 10.4049/jimmunol.180.5.3166
27. Wang LF, Ding YJ, Zhao Q, Zhang XL. Investigation on the Association Between NLRP3 Gene Polymorphisms and Susceptibility to Primary Gout. Genet Mol Res (2015) 14(4):16410–4. doi: 10.4238/2015.December.9.10
28. Bidoki AZ, Harsini S, Sadr M, Soltani S, Mohammadzadeh M, Najafi S, et al. NLRP3 Gene Polymorphisms in Iranian Patients With Recurrent Aphthous Stomatitis. J Oral Pathol Med (2016) 45(2):136–40. doi: 10.1111/jop.12332
29. Tanaka S, Okubo M, Nagasawa K, Takizawa S, Ichijo M, Ichijo S, et al. Predictive Value of Titer of GAD Antibodies for Further Progression of Beta Cell Dysfunction in Slowly Progressive Insulin-Dependent (Type 1) Diabetes (SPIDDM). Diabetol Int (2016) 7(1):42–52. doi: 10.1007/s13340-015-0211-5
30. Wang X, Yang L, Cheng Y, Liang H, Hu J, Zheng P, et al. Downregulation of T-Cell Transcription Factors in Adult Latent Autoimmune Diabetes With High-Titer Glutamic Acid Decaroxylase Antibody. Diabetes Ther (2019) 10(3):917–27. doi: 10.1007/s13300-019-0594-6
31. Liu L, Li X, Xiang Y, Huang G, Lin J, Yang L, et al. Latent Autoimmune Diabetes in Adults With Low-Titer GAD Antibodies: Similar Disease Progression With Type 2 Diabetes: A Nationwide, Multicenter Prospective Study (LADA China Study 3). Diabetes Care (2015) 38(1):16–21. doi: 10.2337/dc14-1770
32. Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of Intensive Insulin Therapy on Beta-Cell Function and Glycaemic Control in Patients With Newly Diagnosed Type 2 Diabetes: A Multicentre Randomised Parallel-Group Trial. Lancet (2008) 371(9626):1753–60. doi: 10.1016/S0140-6736(08)60762-X
33. Jin P, Huang G, Lin J, Yang L, Xiang B, Zhou W, et al. High Titre of Antiglutamic Acid Decarboxylase Autoantibody Is a Strong Predictor of the Development of Thyroid Autoimmunity in Patients With Type 1 Diabetes and Latent Autoimmune Diabetes in Adults. Clin Endocrinol (Oxf) (2011) 74(5):587–92. doi: 10.1111/j.1365-2265.2011.03976.x
Keywords: inflammasome, single nucleotide polymorphism, type 1 diabetes, correlation analysis, glutamic acid decarboxylase antibody
Citation: Sun X, Xu L, Xia Y, Luo S, Lin J, Xiao Y, Huang G, Li X, Xie Z and Zhou Z (2022) rs3806265 and rs4612666 of the NLRP3 Gene Are Associated With the Titer of Glutamic Acid Decarboxylase Antibody in Type 1 Diabetes. Front. Endocrinol. 13:835054. doi: 10.3389/fendo.2022.835054
Received: 28 January 2022; Accepted: 15 March 2022;
Published: 21 April 2022.
Edited by:
Weiping Han, Singapore Bioimaging Consortium (A*STAR), SingaporeReviewed by:
Jon D. Piganelli, University of Pittsburgh, United StatesCong-Yi Wang, Tongji Medical College, China
Copyright © 2022 Sun, Xu, Xia, Luo, Lin, Xiao, Huang, Li, Xie and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguang Zhou, emhvdXpoaWd1YW5nQGNzdS5lZHUuY24=; Zhiguo Xie, eGllemhpZ3VvQGNzdS5lZHUuY24=
†These authors have contributed equally to this work
 Xiaoxiao Sun†
Xiaoxiao Sun† Ying Xia
Ying Xia Shuoming Luo
Shuoming Luo Gan Huang
Gan Huang Xia Li
Xia Li Zhiguo Xie
Zhiguo Xie Zhiguang Zhou
Zhiguang Zhou