
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 14 April 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.831556
This article is part of the Research TopicDiabetes and Aging: Glycemic Control, Insulin Regulation, and the Subsequent EffectsView all 12 articles
 Antonella Pansini1,2†
Antonella Pansini1,2† Angela Lombardi3†
Angela Lombardi3† Maria Morgante1
Maria Morgante1 Salvatore Frullone1
Salvatore Frullone1 Anna Marro1
Anna Marro1 Mario Rizzo1,4
Mario Rizzo1,4 Giuseppe Martinelli5
Giuseppe Martinelli5 Eugenio Boccalone1
Eugenio Boccalone1 Antonio De Luca4
Antonio De Luca4 Gaetano Santulli3
Gaetano Santulli3 Pasquale Mone1,3*
Pasquale Mone1,3*Background: Frailty is a multidimensional condition typical of elders. Frail older adults have a high risk of functional decline, hospitalization, and mortality. Hypertension is one of the most common comorbidities in elders. Hyperglycemia (HG) is frequently observed in frail older adults, and represents an independent predictor of worst outcomes, with or without diabetes mellitus (DM). We aimed at investigating the impact of HG on physical impairment in frailty.
Methods: We studied consecutive older adults with frailty and hypertension at the ASL (local health unit of the Italian Ministry of Health) of Avellino, Italy, from March 2021 to September 2021. Exclusion criteria were: age <65 years, no frailty, no hypertension, left ventricular ejection fraction <25%, previous myocardial infarction, previous primary percutaneous coronary intervention and/or coronary artery bypass grafting. Blood glucose, Hb1Ac, and creatinine were measured in all patients. Physical frailty was assessed applying the Fried Criteria; we performed a 5-meter gait speed (5mGS) test in all patients.
Results: 149 frail hypertensive older adults were enrolled in the study, of which 82 had normoglycemia (NG), and 67 had HG. We observed a significantly slower 5mGS in the HG group compared to the NG group (0.52 ± 0.1 vs. 0.69 ± 0.06; p<0.001). Moreover, we found a strong and significant correlation between 5mGS and glycemia (r: 0.833; p<0.001). A multivariable linear regression analysis using 5mGS as a dependent variable revealed a significant independent association with glycemia (p<0.001) after adjusting for likely confounders.
Conclusions: HG drives physical impairment in frail hypertensive older adults independently of DM.
Frailty is a multidimensional condition typical of elders that determines physical decline. Frail older adults have a high risk of functional decline, hospitalization, and mortality (1–4). Hence, a careful geriatric evaluation is one of the best strategies to obtain an early diagnosis of physical impairment, and managing comorbidities and complications is fundamental to counteract it (5–11). Hypertension is one of the most common comorbidities in elders, affecting endothelial function, leading to oxidative stress, inflammation, and atherosclerosis (12–19).
Hyperglycemia (HG) is frequently observed in frail hypertensive older adults, and we and others have shown that it represents an independent predictor of worst outcomes, even if diabetes mellitus (DM) is not present (20–23). Indeed, HG drives inflammation and oxidative stress, leading to endothelial dysfunction, with a negative impact on frail patients (7, 24–28).
In this context, reaching and maintaining an optimal glycemic control may be crucial to reduce the incidence of functional decline and avoid complications (11, 29–32). On these grounds, we investigated the impact of HG on physical impairment in frail hypertensive older adults.
We studied consecutive older adults with frailty and hypertension at the ASL (local health unit of the Italian Ministry of Health) of Avellino and Caserta, Italy, from March 2021 to September 2021.
Inclusion criteria were: Age ≥65 years; frailty; primary hypertension. Exclusion criteria were: Age <65 years; absence of frailty; secondary hypertension or absence of hypertension; previous myocardial infarction, left ventricular ejection fraction <25%, and previous cardiac revascularization.
HG was defined as blood glucose level ≥140 mg/dL according to previous investigations that evaluated HG in complex patients, both diabetic and non-diabetic (33–37), and following ADA recommendations, which refer to this value for hospitalized patients (38) and/or subjects with impaired glucose tolerance (39).
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg on repeated measurements, or as a previously diagnosed hypertension (40). Blood samples to measure glycemia, HbA1c, hyperlipidemia, and creatinine were taken from all patients. The study was approved by the Campania Nord Ethical Committee. A written informed consent was signed by all patients.
A diagnosis of frailty status was made according to the Fried Criteria, as we previously reported (19, 41):
- Weight loss (unintentional loss ≥4.5 kg in the past year);
- Weakness (handgrip strength in the lowest 20% quintile at baseline, adjusted for sex and body mass index);
- Exhaustion (poor endurance and energy, self-reported);
- Slowness (walking speed under the lowest quintile adjusted for sex and height);
- Low physical activity level (lowest quintile of kilocalories of physical activity during the past week).
Frailty was diagnosed with at least 3 criteria out of 5.
A 5-meter gait speed (5mGS) test was performed in all patients, as we previously described (42). 5mGS was advocated as a reliable measure of physical capacity in frail patients with cardiovascular diseases (43). Indeed, this test evaluates lower extremity muscle function, neurological and cardiopulmonary capacity (44, 45).
Data are presented as mean ± SD or percentage. We developed a dispersion model using Pearson analysis to assess the correlation between glycemia and 5mGS. To explore the impact of comorbidities, we carried out a multivariable linear regression model with a 5mGS test as a dependent variable. All calculations were performed using the software Statistical Product and Service Solutions (SPSS) version 26.
We screened 189 frail hypertensive patients. Since 13 patients did not give their consent and 27 subjects did not meet inclusion criteria, 149 patients were enrolled in the study, of which 82 had normoglycemia (NG) and 67 had HG (Figure 1).
Patients were similar in age, BMI, sex distribution, and comorbidities (Table 1). We found a strong and significant correlation between 5mGS and glycemia (r: 0.833; 95% C.I.: -0.8766 to -0.7765; p<0.001) in all patients (Figure 2).
We observed a significantly slower 5mGS in the HG group compared to the NG group (0.52 ± 0.1 vs. 0.69 ± 0.06; p<0.001) (Figure 3). A multivariable linear regression analysis with 5mGS as a dependent variable (Table 2) confirmed the significant impact of glycemia (p<0.001) and revealed also an association with COPD (p: 0.043).
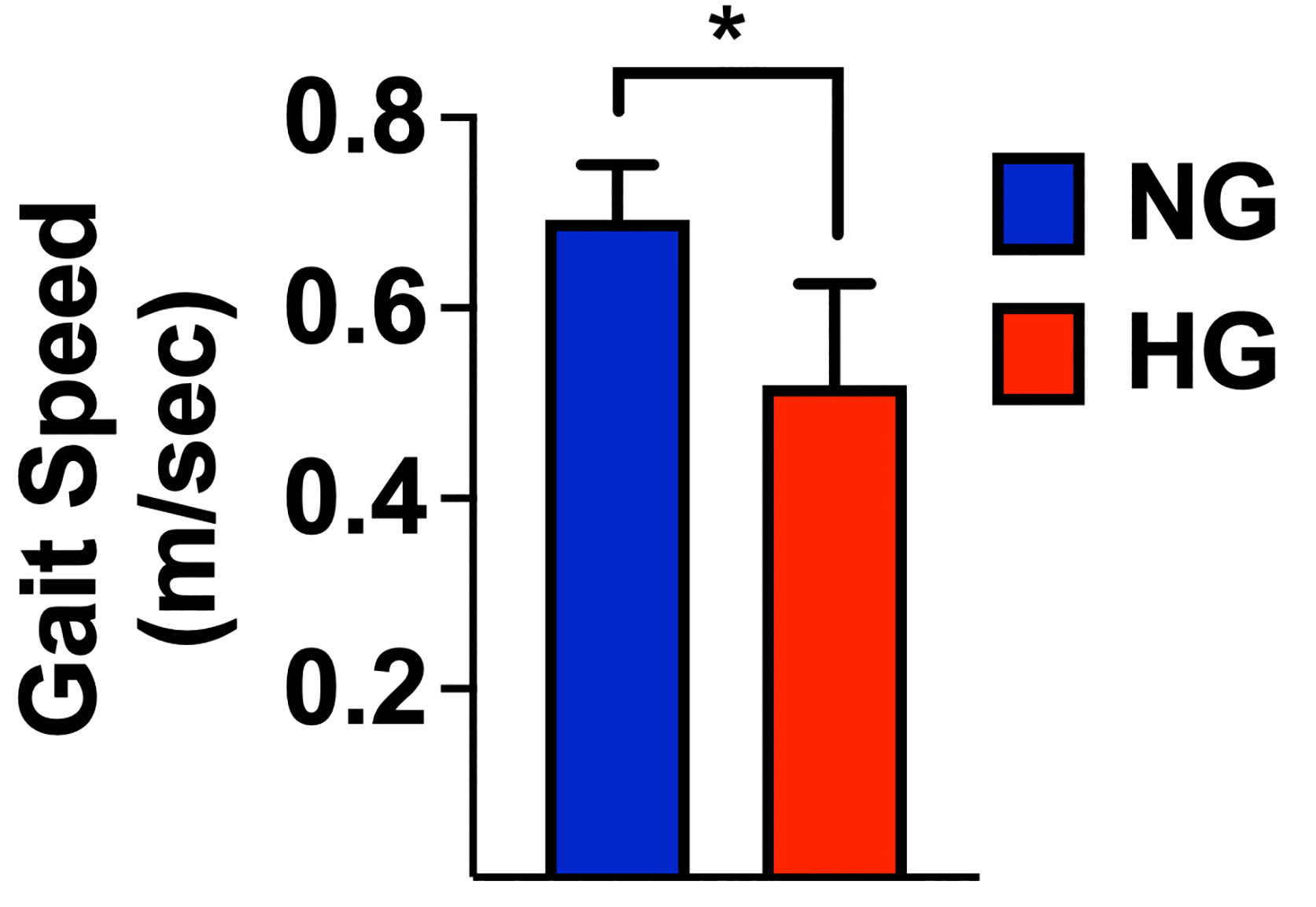
Figure 3 Gait speed measured in normoglycemic (NG) and hyperglycemic (HG) patients; mean±SD; *p < 0.001.
Our study indicates that frail hypertensive elders with HG have a significantly lower 5mGS compared to NG subjects. It is important to emphasize the fact that these results refer to a frail hypertensive population of older adults, in which physical performance affects functional decline, loss of independence, and cognitive impairment (30, 46).
Glucose levels may increase the risk of frailty in older adults without DM (31). It is interesting to observe that these findings are independent of a previous diagnosis of DM as well as from HbA1c values. In this scenario, HG drives physical impairment independently of DM and we speculate that glycemic control appears to be the best way to attempt to reverse physical impairment, with or without DM.
Our study does have some limitations. First, the study population is relatively small; second, there is no follow-up. Therefore, further studies are necessary to confirm our results, ideally in large randomized trials. We also reckon that a majority of our study population is represented by women; this finding is in agreement with the REPOSI Study on elderly people (47). Consistent with our observations, HG is associated with the development of frailty and lower extremity mobility limitations in older women (48, 49). Furthermore, a previous study had suggested to consider functionally independent women with osteoporosis and arthritis as a different cluster of frailty (50).
Taken together, our data indicate that HG drives physical impairment in frail and hypertensive older adults independently from DM and HbA1c values.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Campania Nord Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
AP, AL, GS, and PM designed the study, contributed to drafting the manuscript, approved its final version, and made the decision to submit and publish the manuscript. MM, MR, GM, ADL, and PM analyzed data, revised the manuscript’s intellectual content, and approved the final version. EB, SF, and AM acquired the data, revised the manuscript’s intellectual content, and approved the final version. PM is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of data analysis. All authors contributed to the article and approved the submitted version.
The Santulli’s Lab is supported in part by the National Institutes of Health (NIH: R01-HL146691, R01-DK123259, R01-HL159062, R01-DK033823, and T32-HL144456, to GS), by the Diabetes Action Foundation, by the Irma T. Hirschl and Monique Weill-Caulier Trusts (to GS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in Elderly People. Lancet (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
2. Cunha AIL, Veronese N, de Melo Borges S, Ricci NA. Frailty as a Predictor of Adverse Outcomes in Hospitalized Older Adults: A Systematic Review and Meta-Analysis. Ageing Res Rev (2017) 56:100960. doi: 10.1016/j.arr.2019.100960
3. Yannakoulia M, Ntanasi E, Anastasiou CA, Scarmeas N. Frailty and Nutrition: From Epidemiological and Clinical Evidence to Potential Mechanisms. Metabolism (2017) 68:64–76. doi: 10.1016/j.metabol.2016.12.005
4. Li CM, Lin CH, Li CI, Liu CS, Lin WY, Li TC, et al. Frailty Status Changes Are Associated With Healthcare Utilization and Subsequent Mortality in the Elderly Population. BMC Public Health (2021) 21:645. doi: 10.1186/s12889-021-10688-x
5. Ohman H, Savikko N, Strandberg TE, Pitkala KH. Effect of Physical Exercise on Cognitive Performance in Older Adults With Mild Cognitive Impairment or Dementia: A Systematic Review. Dement Geriatr Cognit Disord (2014) 38:347–65. doi: 10.1159/000365388
6. Siviero P, Limongi F, Noale M, Della Dora F, Martini A, Castiglione A, et al. The Prevalence of Frailty and Its Associated Factors in an Italian Institutionalized Older Population: Findings From the Cross-Sectional Alvise Cornaro Center Study. Aging Clin Exp Res (2021). doi: 10.1007/s40520-021-02020-9
7. Li G, Prior JC, Leslie WD, Thabane L, Papaioannou A, Josse RG, et al. Frailty and Risk of Fractures in Patients With Type 2 Diabetes. Diabetes Care (2019) 42:507–13. doi: 10.2337/dc18-1965
8. Rodriguez-Manas L, Laosa O, Vellas B, Paolisso G, Topinkova E, Oliva-Moreno J, et al. Effectiveness of a Multimodal Intervention in Functionally Impaired Older People With Type 2 Diabetes Mellitus. J Cachexia Sarcopenia Muscle (2019) 10:721–33. doi: 10.1002/jcsm.12432
9. Mone P, Izzo R, Marazzi G, Manzi MV, Gallo P, Campolongo G, et al. L-Arginine Enhances the Effects of Cardiac Rehabilitation on Physical Performance: New Insights for Managing Cardiovascular Patients During the COVID-19 Pandemic. J Pharmacol Exp Ther (2022) In press. doi: 10.1124/jpet.122.001149
10. Stringa N, Van Schoor NM, Milaneschi Y, Ikram MA, Del Panta V, Koolhaas CM, et al. Physical Activity as Moderator of the Association Between APOE and Cognitive Decline in Older Adults: Results From Three Longitudinal Cohort Studies. J Gerontol A Biol Sci Med Sci (2020) 75:1880–6. doi: 10.1093/gerona/glaa054
11. Tamura Y, Omura T, Toyoshima K, Araki A. Nutrition Management in Older Adults With Diabetes: A Review on the Importance of Shifting Prevention Strategies From Metabolic Syndrome to Frailty. Nutrients (2020) 12. doi: 10.3390/nu12113367
12. Wallace SM, Mceniery CM, Maki-Petaja KM, Booth AD, Cockcroft JR, Wilkinson IB. Isolated Systolic Hypertension Is Characterized by Increased Aortic Stiffness and Endothelial Dysfunction. Hypertension (2007) 50:228–33. doi: 10.1161/HYPERTENSIONAHA.107.089391
13. Santulli G, Trimarco B, Iaccarino G. G-Protein-Coupled Receptor Kinase 2 and Hypertension: Molecular Insights and Pathophysiological Mechanisms. High Blood Press Cardiovasc Prev (2013) 20:5–12. doi: 10.1007/s40292-013-0001-8
14. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of Dementia Over Three Decades in the Framingham Heart Study. N Engl J Med (2016) 374:523–32. doi: 10.1056/NEJMoa1504327
15. Ruan Q, D'onofrio G, Sancarlo D, Greco A, Lozupone M, Seripa D, et al. Emerging Biomarkers and Screening for Cognitive Frailty. Aging Clin Exp Res (2017) 29:1075–86. doi: 10.1007/s40520-017-0741-8
16. Varzideh F, Jankauskas SS, Kansakar U, Mone P, Gambardella J, Santulli G. Sortilin Drives Hypertension by Modulating Sphingolipid/Ceramide Homeostasis and by Triggering Oxidative Stress. J Clin Invest (2022) 132(3):e156624. doi: 10.1172/JCI156624
17. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of Frailty as a Moderator of the Relationship Between Neuropathology and Dementia in Alzheimer's Disease: A Cross-Sectional Analysis of Data From the Rush Memory and Aging Project. Lancet Neurol (2019) 18:177–84. doi: 10.1016/S1474-4422(18)30371-5
18. Huang ST, Tange C, Otsuka R, Nishita Y, Peng LN, Hsiao FY, et al. Subtypes of Physical Frailty and Their Long-Term Outcomes: A Longitudinal Cohort Study. J Cachexia Sarcopenia Muscle (2020) 11:1223–31. doi: 10.1002/jcsm.12577
19. Mone P, Gambardella J, Pansini A, De Donato A, Martinelli G, Boccalone E, et al. Cognitive Impairment in Frail Hypertensive Elderly Patients: Role of Hyperglycemia. Cells (2021) 10. doi: 10.3390/cells10082115
20. Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in Older Adults. Diabetes Care (2012) 35:2650–64. doi: 10.2337/dc12-1801
21. Perez-Tasigchana RF, Leon-Munoz LM, Lopez-Garcia E, Gutierrez-Fisac JL, Laclaustra M, Rodriguez-Artalejo F, et al. Metabolic Syndrome and Insulin Resistance Are Associated With Frailty in Older Adults: A Prospective Cohort Study. Age Ageing (2017) 46:807–12. doi: 10.1093/ageing/afx023
22. Tziomalos K, Dimitriou P, Bouziana SD, Spanou M, Kostaki S, Angelopoulou SM, et al. Stress Hyperglycemia and Acute Ischemic Stroke in-Hospital Outcome. Metabolism (2017) 67:99–105. doi: 10.1016/j.metabol.2016.11.011
23. Mone P, Gambardella J, Minicucci F, Lombardi A, Mauro C, Santulli G. Hyperglycemia Drives Stent Restenosis in STEMI Patients. Diabetes Care (2021) 44:e192–3. doi: 10.2337/dc21-0939
24. Wilson S, Mone P, Kansakar U, Jankauskas SS, Donkor K, Adebayo A, et al. Diabetes and Restenosis. Cardiovasc Diabetol (2022) 21:23. doi: 10.1186/s12933-022-01460-5
25. Jankauskas SS, Kansakar U, Varzideh F, Wilson S, Mone P, Lombardi A, et al. Heart Failure in Diabetes. Metabolism (2021) 125:154910. doi: 10.1016/j.metabol.2021.154910
26. Odegaard AO, Jacobs DR Jr., Sanchez OA, Goff DC Jr., Reiner AP, Gross MD. Oxidative Stress, Inflammation, Endothelial Dysfunction and Incidence of Type 2 Diabetes. Cardiovasc Diabetol (2016) 15:51. doi: 10.1186/s12933-016-0369-6
27. Clegg A, Hassan-Smith Z. Frailty and the Endocrine System. Lancet Diabetes Endocrinol (2018) 6:743–52. doi: 10.1016/S2213-8587(18)30110-4
28. Ida S, Kaneko R, Imataka K, Murata K. Relationship Between Frailty and Mortality, Hospitalization, and Cardiovascular Diseases in Diabetes: A Systematic Review and Meta-Analysis. Cardiovasc Diabetol (2019) 18:81. doi: 10.1186/s12933-019-0885-2
29. Zaslavsky O, Walker RL, Crane PK, Gray SL, Larson EB. Glucose Levels and Risk of Frailty. J Gerontol A Biol Sci Med Sci (2016) 71:1223–9. doi: 10.1093/gerona/glw024
30. Laiteerapong N, Karter AJ, Liu JY, Moffet HH, Sudore R, Schillinger D, et al. Correlates of Quality of Life in Older Adults With Diabetes: The Diabetes & Aging Study. Diabetes Care (2011) 34:1749–53. doi: 10.2337/dc10-2424
31. Santulli G. Tirzepatide Versus Semaglutide Once Weekly in Type 2 Diabetes. N Engl J Med (2022) 386(7):e17. doi: 10.1056/NEJMc2114590
32. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. Older Adults: Standards of Medical Care in Diabetes-2022. Diabetes Care (2022) 45:S195–207. doi: 10.2337/dc22-S013
33. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress Hyperglycaemia and Increased Risk of Death After Myocardial Infarction in Patients With and Without Diabetes: A Systematic Overview. Lancet (2000) 355:773–8. doi: 10.1016/S0140-6736(99)08415-9
34. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress Hyperglycemia and Prognosis of Stroke in Nondiabetic and Diabetic Patients: A Systematic Overview. Stroke (2001) 32:2426–32. doi: 10.1161/hs1001.096194
35. Falciglia M, Freyberg RW, Almenoff PL, D'alessio DA, Render ML. Hyperglycemia-Related Mortality in Critically Ill Patients Varies With Admission Diagnosis. Crit Care Med (2009) 37:3001–9. doi: 10.1097/CCM.0b013e3181b083f7
36. Soysal DE, Karakus V, Seren AR, Tatar E, Celik M, Hizar S. Evaluation of Transient Hyperglycemia in Non-Diabetic Patients With Febrile Neutropenia. Eur J Intern Med (2012) 23:342–6. doi: 10.1016/j.ejim.2011.12.010
37. Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of Hyperglycemia in Hospitalized Patients in Non-Critical Care Setting: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2012) 97:16–38. doi: 10.1210/jc.2011-2098
38. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2022. Diabetes Care (2022) 45:S244–53. doi: 10.2337/dc22-S016
39. American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care (2017) 40:S11–24. doi: 10.2337/dc17-S005
40. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
41. Mone P, Pansini A, Jankauskas SS, Varzideh F, Kansakar U, Lombardi A, et al. L-Arginine Improves Cognitive Impairment in Hypertensive Frail Older Adults. Front Cardiovasc Med (2022). in press.
42. Mone P, Gambardella J, Pansini A, Martinelli G, Minicucci F, Mauro C, et al. Cognitive Dysfunction Correlates With Physical Impairment in Frail Patients With Acute Myocardial Infarction. Aging Clin Exp Res (2021). doi: 10.1007/s40520-021-01897-w
43. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, et al. Frailty Assessment in the Cardiovascular Care of Older Adults. J Am Coll Cardiol (2014) 63:747–62. doi: 10.1016/j.jacc.2013.09.070
44. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait Speed and Survival in Older Adults. JAMA (2011) 305:50–8. doi: 10.1001/jama.2010.1923
45. Mone P, Lombardi A, Gambardella J, Pansini A, Macina G, Morgante M, et al. Empagliflozin Improves Cognitive Impairment in Frail Older Adults with Type 2 Diabetes and Heart Failure with Preserved Ejection Fraction. Diabetes Care. (2022) In press. doi: 10.2337/dc21-2434
46. Lee JS, Auyeung TW, Leung J, Kwok T, Leung PC, Woo J. Physical Frailty in Older Adults Is Associated With Metabolic and Atherosclerotic Risk Factors and Cognitive Impairment Independent of Muscle Mass. J Nutr Health Aging (2011) 15:857–62. doi: 10.1007/s12603-011-0134-1
47. Corrao S, Santalucia P, Argano C, Djade CD, Barone E, Tettamanti M, et al. Gender-Differences in Disease Distribution and Outcome in Hospitalized Elderly: Data From the REPOSI Study. Eur J Intern Med (2014) 25:617–23. doi: 10.1016/j.ejim.2014.06.027
48. Blaum CS, Xue QL, Tian J, Semba RD, Fried LP, Walston J. Is Hyperglycemia Associated With Frailty Status in Older Women? J Am Geriatr Soc (2009) 57:840–7. doi: 10.1111/j.1532-5415.2009.02196.x
49. Kalyani RR, Tian J, Xue QL, Walston J, Cappola AR, Fried LP, et al. Hyperglycemia and Incidence of Frailty and Lower Extremity Mobility Limitations in Older Women. J Am Geriatr Soc (2012) 60:1701–7. doi: 10.1111/j.1532-5415.2012.04099.x
Keywords: aging, blood glucose, cognitive impairment, COPD, diabetes, elderly, gait speed, MoCA score
Citation: Pansini A, Lombardi A, Morgante M, Frullone S, Marro A, Rizzo M, Martinelli G, Boccalone E, De Luca A, Santulli G and Mone P (2022) Hyperglycemia and Physical Impairment in Frail Hypertensive Older Adults. Front. Endocrinol. 13:831556. doi: 10.3389/fendo.2022.831556
Received: 10 December 2021; Accepted: 23 February 2022;
Published: 14 April 2022.
Edited by:
Helena Cristina Barbosa, State University of Campinas, BrazilReviewed by:
Silvano Piovan, State University of Maringá, BrazilCopyright © 2022 Pansini, Lombardi, Morgante, Frullone, Marro, Rizzo, Martinelli, Boccalone, De Luca, Santulli and Mone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pasquale Mone, cGFzcXVhbGUubW9uZUBlaW5zdGVpbm1lZC5vcmc=; ZHJwYXNxdWFsZS5tb25lQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.