- 1Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, Katowice, Poland
- 2Students’ Scientific Association by the Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, Katowice, Poland
Currently, there are about 150–200 million diabetic patients treated with insulin globally. The year 2021 is special because the 100th anniversary of the insulin discovery is being celebrated. It is a good occasion to sum up the insulin pen technology invention and improvement which are nowadays the leading mode of an insulin delivery. Even though so many years have passed, insulin is still administered subcutaneously, that is why devices to deliver it are of great importance. Insulin pens have evolved only through the last decades (the reusable, durable pens, and the disposable, prefilled pens) and modern smart insulin pens have been developed in the last few years, and both types of the devices compared to traditional syringes and vials are more convenient, discrete in use, have better dosing accuracy, and improve adherence. In this review, we will focus on the history of insulin pens and their improvement over the previous decades.
Introduction
The International Diabetes Federation (IDF) estimates that over 537 million people all over the world are currently struggling with diabetes mellitus (DM) (1) and there are about 150–200 million of them treated with insulin (2). The history of insulin dates back to the last century, when in 1921 Frederick Banting and Charles Best with the support of John Macleod and James Collip discovered insulin and thereby revolutionized the treatment of DM (3–5). The first injection of insulin on January 11, 1922, to a 14-year-old boy with the use of reusable glass-bodied syringes (6) started an entirely new era of diabetes management (4, 5) and led to the improvement of insulin delivery methods (3, 5). Even though insulin has been used for 100 years already, its administration remains subcutaneous where insulin pens which evolved only through the last four decades are the leading method of its delivery (about 60% of patients treated with insulin use insulin pens all over the world) (7–9). Insulin pen utility is not the same in different regions of the world. According to a report from the year 2008, insulin pens were used by only 15% of patients in the US, compared with 80%–90% in Europe, and it was suspected that it could be due to limited education regarding the benefits of insulin pens but also their higher price (10). The situation has changed in the next years where data from the year 2011 indicate that the number of patients initiating vial/syringe in the US decreased from 2005 to 2011 to approximately 30% while patients initiating pens increased to approximately 60% (11). According to a IQVIA® report for the period from June 2020 till June 2021 prepared for the purpose of this manuscript [data not published (12)], the usage of pens in US rose to 59% where in Europe it is comparably high and assessed to be 93.6%. Insulin pens have numerous advantages over traditional vial and syringe injections, among others easy use especially for patients with vision problems or manual dexterity, accuracy of delivering small doses of insulin, and discretion of use (13). It is worth noting that aspects of insulin administration may also contribute to the treatment outcomes even though the type of insulin and its efficacy and safety are the primary factors to consider. It is important to underline that each insulin-producing company has its own insulin pen dedicated to use with the produced insulin. It was proved in some studies that patients who use insulin pens are more adherent to the treatment regimen and have less hypoglycemic events compared to insulin vial users (14–18). Also, numerous studies report that patients’ preference for insulin pens exceeds that for vials or syringes (19–21) and portability of insulin pens improves patients’ convenience (22). However, it is important to note that the superiority of insulin pens in achieving and maintaining glycemic control has been questioned, and this question has not been resolved up to day (23). American and European guidelines underline the necessity of undertaking patient preference when selecting diabetes treatment especially when treatment is accoutered with pain due to injection (24). That is why recently a study assessing the patient perspective of injectable treatment among patients with type 2 diabetes (T2DM) has been performed and showed that there are some features of the injection device that patients choose more often which may help in future improvement of insulin pens (25). Development of insulin pens is parallel to the development of newer insulin formulation where insulin pen must adapt to changes to dosing and timing requirements like it is in case of modern ultra-long-acting insulin analogue icodec, administered once weekly, which is under development (26). This year, the discovery of insulin turns 100 years, and this provides an opportunity to reflect on its administration methods over the past years.
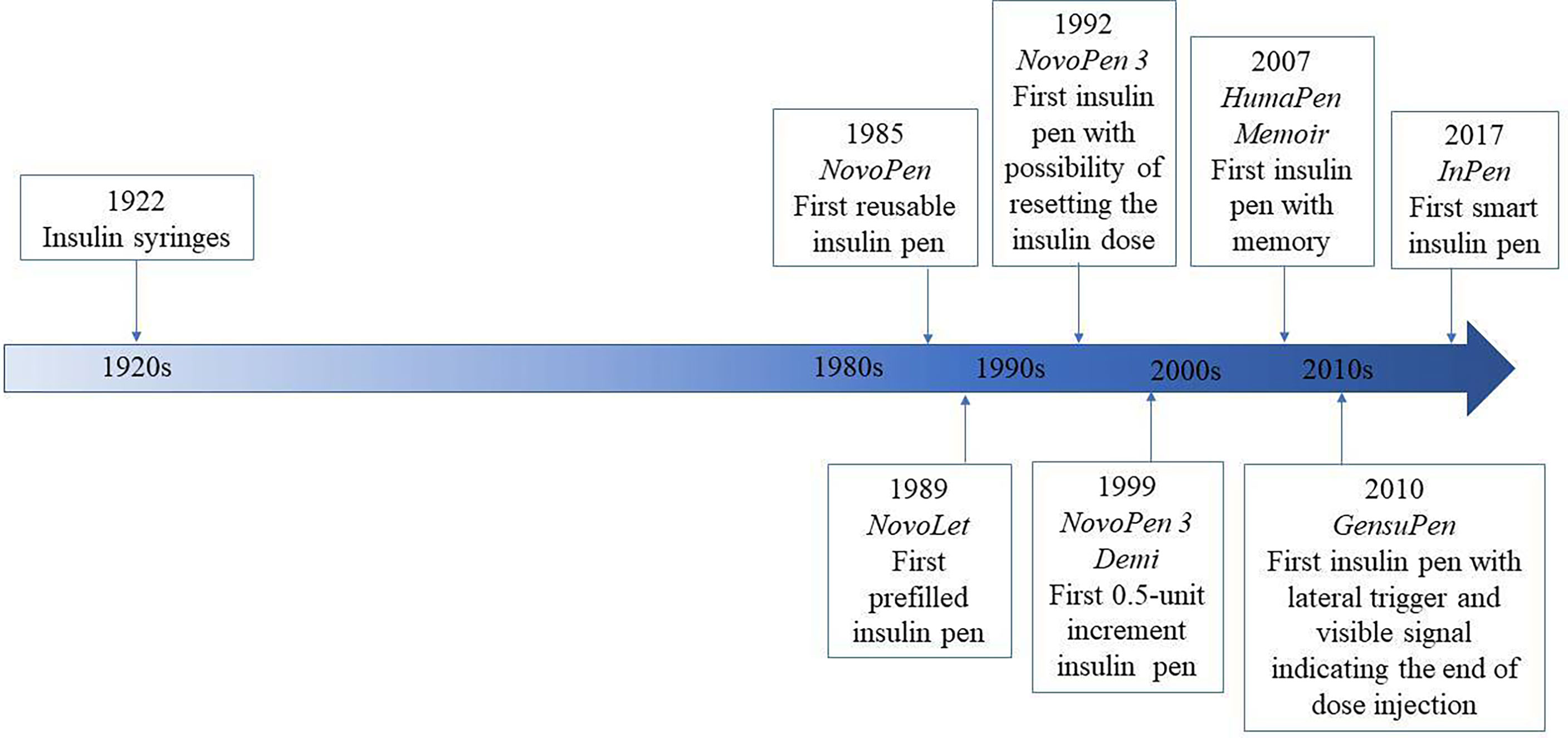
In this review, we will focus on the history of insulin pens and their improvement over the previous decades, starting from the first-generation insulin pens throughout modern smart insulin pens (Figure 1). It must be noticed that clinical trials in relation to the newest smart insulin pens and insulin pen caps are very limited to date, that is why information related to this new technology comes also from manufacturer websites and commercial data resources.
First-Generation Insulin Pens
The first insulin injections were made with large and heavy glass or metal syringes and reusable needles (4, 7, 24–26). Syringe was the only possible way of delivering insulin in clinical practice for the next several decades (4, 7, 27). This method of administration had several and serious disadvantages including poor dose accuracy, lack of social acceptance, and fear of injections (7, 27, 28). These inconveniences of the vial and syringe led to the manufacture of insulin pens. Majority of insulin pens are proprietary devices and are developed to work with specific insulin from the same manufacturer (29). Insulin pens are classified into two categories: being reusable (durable) or prefilled (disposable). The reusable insulin pen is loaded by the patient with replaceable insulin cartridges, and the prefilled insulin pen has the insulin reservoir cartridge already installed and the pen is discarded when the cartridge is empty. Both types of insulin pens can contain a maximum of 3 ml of insulin (30) and can deliver insulin in 0.5-, 1-, or 2-unit (U) increments up to 160 U with the use of a needle which has to be attached to the insulin pen.
Reusable Insulin Pens
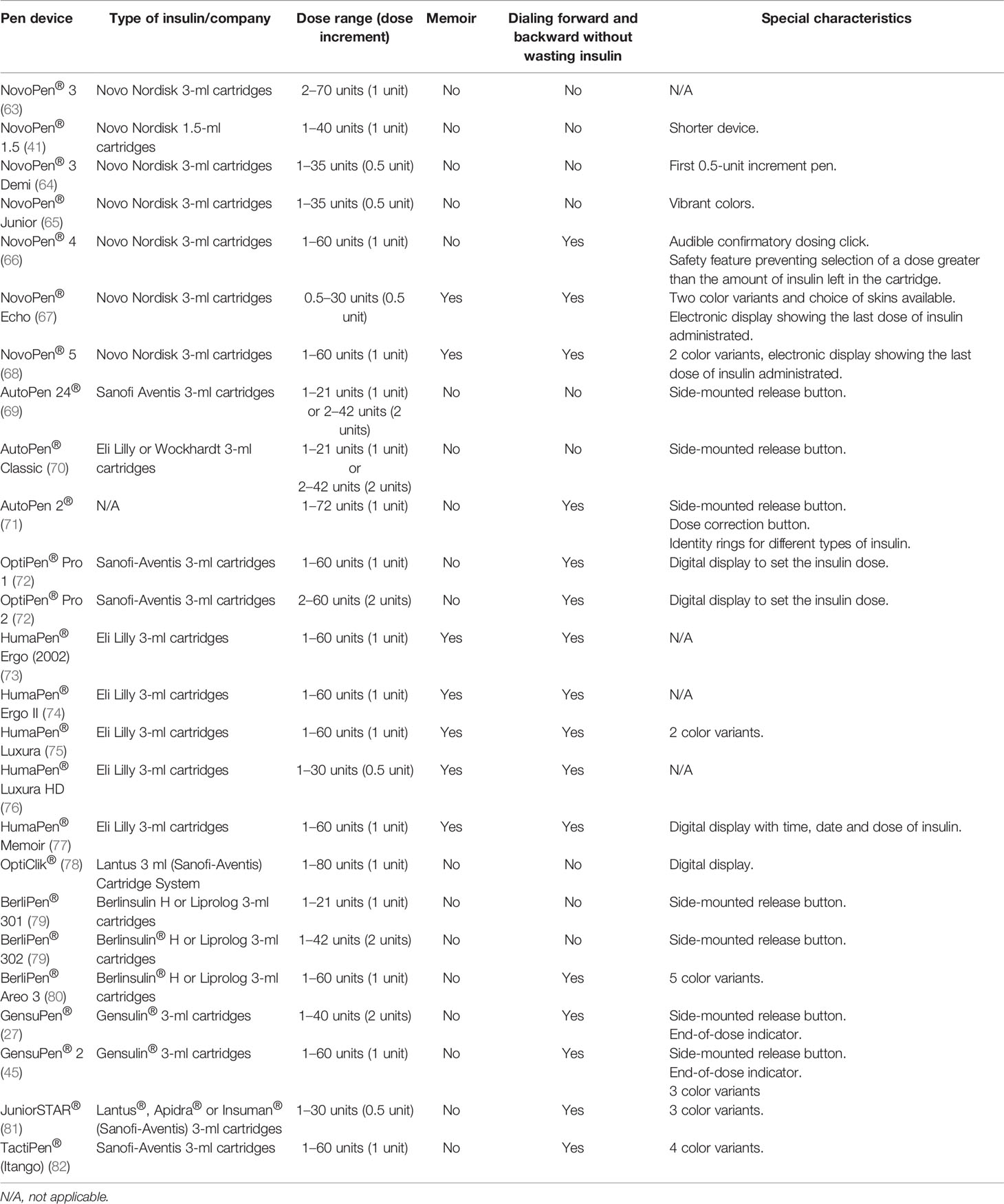
In 1985, Novo Nordisk has launched the first reusable insulin pen injector called NovoPen® to overcome barriers of the vial and syringe (31) and started a series of NovoPen® insulin injectors. The new device was a combination of the syringe and insulin vial in one mechanism, resembling a fountain pen (31). NovoPen® contained a disposable, replaceable 1.5-ml insulin cartridge connected with a single-use needle and one-unit incremental dosing (29, 30) which was ready to use whenever needed. This allowed patients to administer multiple, preprandial injections discreetly, and their daily schedule became more flexible (32–34). First studies related to insulin pen comprised only several patients in 1995 (31), but as the development of the devices has grown up, also the number of patients studied increased to several hundreds per study in 2002 (35) and up to several thousands in 2020 (27, 36). Initially, insulin cartridges dedicated to insulin pen contained short-acting insulin for numerous injections before meals and basal insulin was injected with conventional syringes (37). Soon after, in 1988 a new insulin pen NovoPen® 2 was presented to administer NPH and premixed insulins (38–40). Analogically as with short-acting insulins, majority of patients using the device to administer basal or mixed insulin preferred to continue the therapy with pens (38–40). In 1992, NovoPen® 3 was launched which had a maximum dose that could be administered at one time which increased to 70 U (from 36 U with NovoPen® 2) and the dialed doses could be reset without insulin waste. Soon after, in 1996 NovoPen® 1.5 was released which had a smaller insulin cartridge and was shorter in length, followed by NovoPen® 3 Demi to administer 0.5 U dose increments in 1999 and NovoPen® Junior in 2003 which was designed with vibrant colors and developed specifically for children with diabetes. In 2005, NovoPen® 4 was introduced which required reduced force to perform an injection, which had dose increments of 1.0 U and a maximum dose of 60 U (41). Moreover, NovoPen® 4 was reported as simpler to learn and easier to use for both insulin-naïve and currently using NovoPen® 3 patients (42). Following the release of NovoPen®s, other manufacturers have also introduced reusable insulin pens, including the HumaPen® range (Eli Lilly and Company, Indianapolis, IN, USA) and the OptiPen® Pro, OptiClik®, and ClikSTAR® pens (Sanofi, Bridgewater, NJ, USA) The inconvenience of the first insulin pens was no possibility of dialing backward without wasting insulin, but the thing changed with the introduction of NovoPen® 3 and HumaPen Ergo® (35, 41). This option translated to device acceptability in comparison with previous generations of insulin injectors and syringes (43). With time, the option of insulin-free dialing forward and backward became a prevailing way of setting the insulin doses. All mentioned insulin pens had the trigger placed at on the opposite site of the needle attach end, but there are also insulin pens with a side-mounted release button used for half-automatic insulin delivery, first developed in AutoPen (44), and this mechanism was also present later on in 2010 in GensuPen® and in 2017 in GensuPen® 2 insulin pens (27, 45). Such a mechanism ensured patients about proper insulin administration, simplified the way of injection, and was convenient for elderly patients (27). Moreover, it was proven that the GensuPen® 2 injector in comparison to NovoPen® 4 (Novo Nordisk, Bagsværd, Denmark) and HumaPen Ergo® (Eli Lilly, Indianapolis, IN) requires reduced force for insulin administration, especially at high doses of the drug (46).
In recent years, further improvement in insulin pen function has been made and there are several ones which possess the memory function of the last dose taken. In 2007, Eli Lilly released the world’s first digital insulin pen with memory function, namely, HumaPen Memoir (47). Soon after, in 2010, Novo Nordisk launched NovoPen® Echo (48), the first insulin pen with memory function and half-unit dosing feature. Most of the insulin pens available in the market have the feature to deliver insulin in 1-unit increments, and only a few deliver in half-units. 0.5-increment insulin pens are designed for patients who need small insulin doses, and the available ones are HumaPen Luxura HD, Humalog® Junior KwikPen®, NovoPen® Demi, Junior, Echo, JuniorSTAR®, and InPen™. Based on the trials’ outcomes, children, adolescents, and their parents appreciated both the memory function and simplicity of junior devices (49, 50).
Cited studies related to reusable insulin pens are summarized in Table 1, and the technical characteristics of reusable insulin pens are presented in Table 2.
Prefilled (Disposable) Insulin Pens
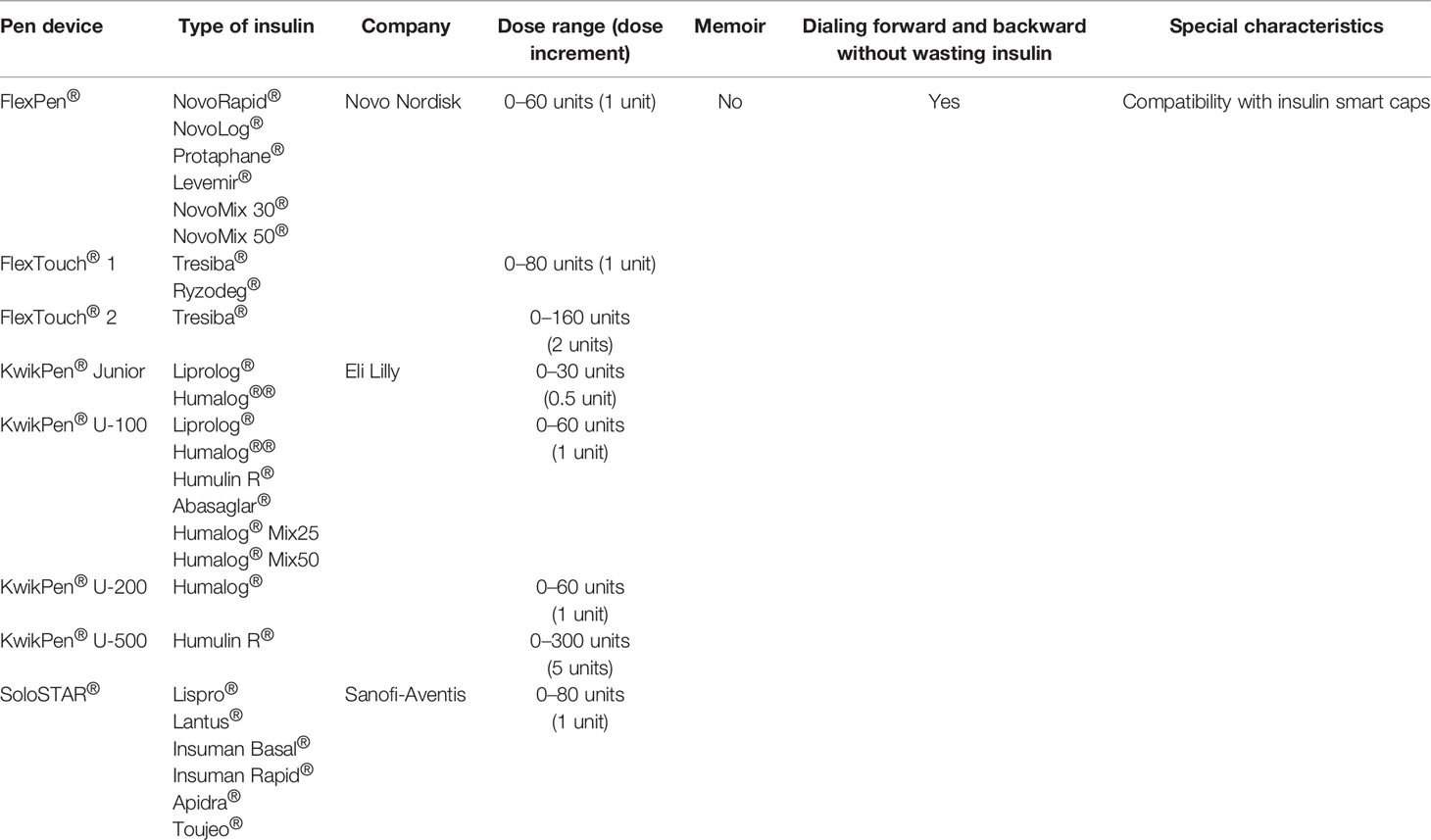
Prefilled (disposable) insulin pens, like reusable ones, are loaded with 3 ml (300 U) of insulin, and some of the patients find it easier to operate than the reusable insulin pens because there is no need to replace the cartridge (83). In 1989, Novo Nordisk launched the world’s first disposable, prefilled insulin pen namely NovoLet® (84) followed by FlexPen® introduced in 2001 (41) and Next Generation FlexPen (NGFP) in 2008 (85) and FlexTouch®, a reengineered version of the FlexPen® with a novel injection mechanism, in 2011 (86).
Other prefilled insulin pens include SoloSTAR® (Sanofi) launched in 2008, KwikPen® (Eli Lilly) launched in 2007 (87), and Junior KwikPen® launched in 2017, a half-unit insulin pen (88). Similarly to reusable insulin pens, prefilled ones when compared to vials and syringes were rated as much easier to handle, discreet in public use, confident in proper dose delivery, and preferred by majority of patients (with T1DM and T2DM), healthcare professionals (89–91), and patients’ caregivers (parents, relatives) (92). Moreover, both non-experienced healthcare practitioners and needle-naïve patients found the prefilled insulin pens much easier to teach and learn (93, 94).
For years, insulin pens were used with insulin 100 U/ml, but since the development of higher-concentration insulins, also new insulin pens for 200 and 300 U/ml have been manufactured and used since 2017, namely, Humalog® 200 U/ml KwikPen® (Eli Lilly) (95), Tresiba® 200 U/ml prefilled FlexTouch® (Novo Nordisk) (96), and Glargine U300 SoloSTAR® insulin pen (Sanofi-Aventis) (97). However, we must consider that disposable pens are less environment friendly and this is a globally growing importance nowadays (98). One can just imagine that if a patient is using approximately 40 units of insulin a day there is about 50 prefilled plastic pens thrown away every year and accounting for thousands of patients using insulin pens the number of insulin pens being thrown away per year is accounted in millions. Based just on a small study form Bosnia and Herzegovina published in 2020, it was predicted that only in this small country there were 3.2 million pens used and dispensed annually (99).
Cited studies related to prefilled insulin pens are summarized in Table 3, and the technical characteristics of prefilled pens are presented in Table 4.
Next-Generation Insulin Pens (Smart Insulin Pens) and Insulin Pen Caps
Nowadays, the way of health delivery is becoming more digital than ever before where face-to-face visits are often replaced by telephone or video contacts and continuous glucose monitoring or glucometer data can be revived through cloud-based data sharing technology which was very pronounced in the COVID-19 era. One of the key problems for patients with T1DM and T2DM treated with multiple daily insulin (MDI) is omitting or late insulin doses which has been found in the study which analyzed data from a continuous glucose monitoring system (CGM) (113). It was also described lately in the study with a Bluetooth®-enabled insulin pen cap that all of the patients taking part in the study missed the insulin doses and it could be intentionally missed because of inconvenience or eating pattern or just forgotten (113). It is important to note that it was also calculated already a years ago that omitting only two meal-related insulin doses per week is associated with a 0.4% increase in HbA1c value (114). Another problem with MDI is that patients rely on numeracy skills while deciding about the meal insulin dose, and it has been proven that these skills are many times not good enough which leads to errors in insulin dosing and to poor glycemic control (115–117). Because patients treated with MDI have to make their insulin dosing decisions without access to the amount and timing of previous insulin doses or residual active insulin, this can, on the other hand, cause overlapping of insulin boluses and put a patient at risk of hypoglycemia (118). That is why smart insulin pens and pen caps were and are being developed to overcome these barriers. Information coming from business research indicates that the smart insulin pen market value will significantly increase by the year 2027 in Latin America, the Middle East, and Africa (119) with the greatest market growth in Europe with a trend toward increased use of smart insulin pen market seen also in North America (120).
Smart Insulin Pens
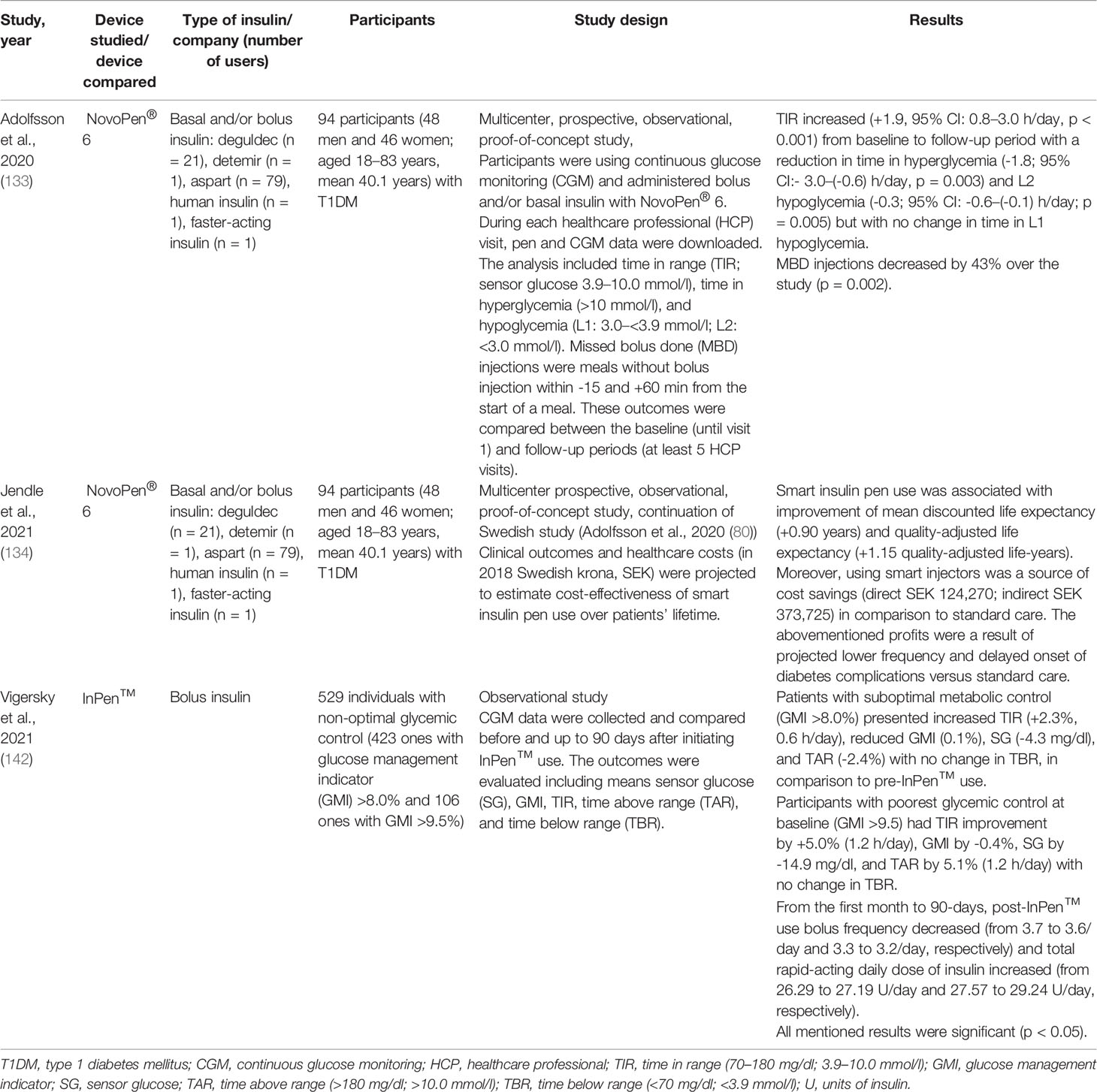
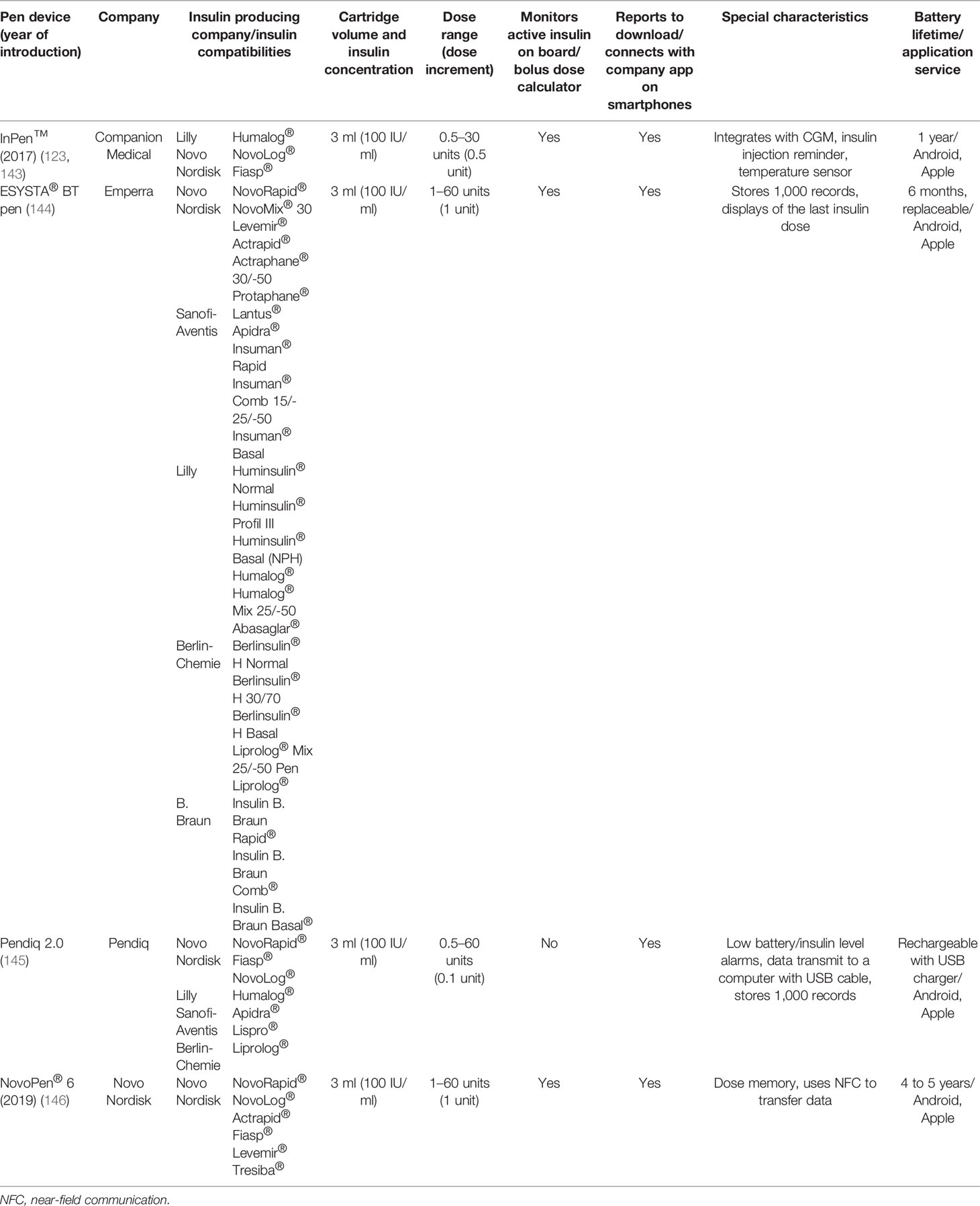
Smart insulin pens are digital, connected insulin pens which go beyond memory function and automatically transmit information about time and amount of insulin administered to the user’s mobile device and can remind about the insulin dose and help to calculate the bolus (7). The clinical data from the smart insulin pen are transferred wirelessly via Bluetooth® technology to an application (app) available for smartphones (7, 121, 122). Therefore, smart insulin pens require the use of an app to collect the data sent from the pen but eliminate the need for manual self-report logbooks (121). Thus, smart insulin pens can help to overcome the challenges that users of pen injectors have to deal with on a daily basis. Smart insulin pens are a relatively new invention, so it should come as no surprise that a few studies have been conducted in this field to date (121). In 2017, the world’s first US Food and Drug Administration (FDA)-approved insulin smart pen which uses Bluetooth® technology, namely, InPen™ (Companion Medical, San Diego, Ca, USA), was launched, and in November 2020 its new version was launched by Medtronic (123). This pen combines the insulin pen with a smartphone app which has the ability to record and store data of insulin injections and recommend doses, as well as display glycemia and related data on the paired smartphone app (124–126). InPen™ is designed for use with rapid-acting insulin U-100 Lilly Humalog® and Novo Nordisk NovoLog® (127). InPen™ is the first of its kind of smart insulin pen that allows to prepare reports for healthcare professionals, reminds about missed doses, and tracks insulin on board, but also alerts the user about an exposure of the device to abnormal (very high or very low) temperatures that may inactivate insulin (124–126, 128). What is likewise important, in InPen™ the dose can be increased or decreased in half-unit steps, and therefore the dose administered is very precise (128, 129). Later on, several new smart insulin pens emerged on the market, namely, ESYSTA® pens (Emperra), Pendiq 2.0 pens (Pendiq), and NovoPen® 6 (Novo Nordisk). It cannot escape the attention that insulin pen injectors may help not only patients but also diabetes care teams. They provide accurate information about missed doses as well as injection times in relation to meals and dose sizes, which is useful in making correct therapeutic decisions and giving personalized treatment plans (121, 130–132). The first study of clinical outcomes using a smart insulin pen was reported in 2020 (133). This investigation was conducted in Sweden and indicated that among patients with T1DM using smart insulin pens, clinical outcomes improved at lower costs compared to standard care. What is even more important, this research suggested that smart insulin pens have the potential to improve glycemic control and decrease glucose variability (133, 134).
Insulin Pen Caps
Insulin pen caps are another device which does not have a clear definition but displays the quantity of insulin in the pen and integrate the insulin-related information with a mobile app. Insulin pen caps are usually attached to the side or fit in the end of the pen.
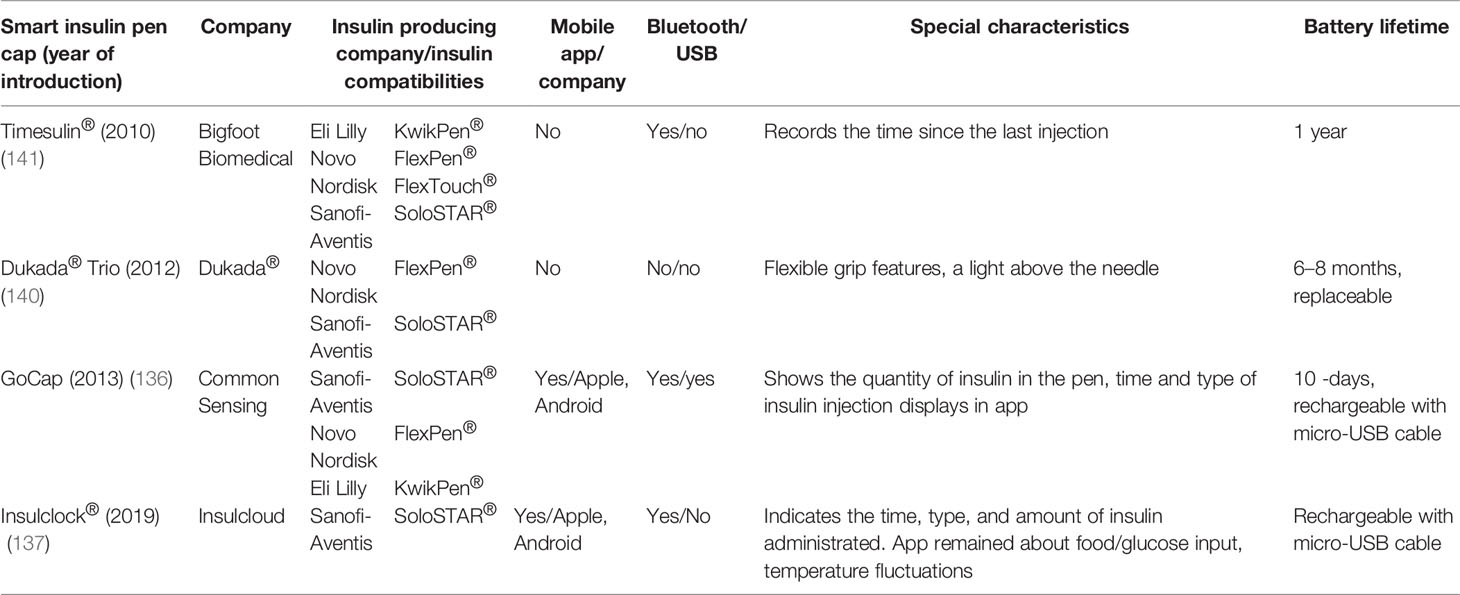
A first-of-its-kind smart pen cap for insulin pens (Bigfoot Unity™ Diabetes Management System) launched by Bigfoot Biomedical received FDA clearance in May 2021. This insulin pen cap is integrated with Abbott’s FreeStyle Libre 2 system and translated continuous monitored glucose data into on-demand insulin dose recommendations displayed on the pen cap screen. It is the first and only device which integrates a continuous glucose monitoring system (CGMS) to insulin dose recommendation (135).
Another smart cap integrated with a dedicated mobile app is GoCap (Common Sensing company) (136). The integration with the application helps calculate the meal or correct boluses, preventing overdosing by active insulin display (125, 136). Moreover, individual reminders allow to keep the schedule of basal insulin (136). Similarly, Insulclock® is an electronic device attached into the insulin pen and connected with a smartphone app and has an insulin reminder system to reduce insulin omissions (137); this device helps to improve glycemic control and reduce glycemic variability with improved adherence in a recent pilot, randomized study among T1DM (138) and among T2DM patients (139). Another two devices do not connect with any mobile app but present an interactive display (Timesulin®) or flash diode (Dukada® Trio), which define the time of last insulin injection (140, 141). The GoCap device received FDA approval (125). Clinical trials which compare different insulin pen caps are not available yet.
Cited studies related to smart insulin pens and their technical characteristics are summarized in Tables 5 and 6. As for the studies related to insulin pen caps and thier technical details the summery is provided in Tables 7 and 8, accordingly.
Conclusions
Insulin remains the primary medication in the treatment of T1DM and is often used therapy in T2DM. The methods and tools for insulin administration are various and have been constantly evolving for over the last 100 years. Insulin pens have changed the lives of millions of people who suffer from diabetes and now are the most widespread way of administering insulin. They are safe, simple to use, convenient, efficient, and less painful than conventional vials and syringes. An increasing number of modern, yet useful features may help to improve patients’ quality of life. Technology evolves to improve adherence and glycemic outcomes, optimize delivery, and reduce dosing errors. Studies performed up to date, summarized in this review, indicate that insulin pens came a long way from a very simple device produced in the year 1985 up till the newest insulin smart pens, and the further improvement is on the way.
Author Contributions
Conceptualization: MMas, KN, and JG. Writing—original draft preparation: MMas, KN, OJ, HK, MMac, and JG. Review and editing: MMas, KN, OJ, HK, and JG. Visualization: MMas, OJ, and KN. All authors contributed to the article and approved the submitted version.
Funding
There is no external funding for this review. Bioton S.A. company funded the publication fee only.
Conflict of Interest
MMas works for Bioton S.A. KN and JG received lecture honoraria from Bioton S.A., Eli Lilly, Sanofi Aventis, Novo Nordisk and Polfa Tarchomin. HK received lecture honoraria from Sanofi Aventis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MH declared a shared affiliation with the authors to the handling editor at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Diabetes Atlas. Brussels, Belgium. (2021) Available at: https://www.diabetesatlas.org (Accessed January 30, 2022).
2. Garg SK, Rewers AH, Akturk HK. Ever-Increasing Insulin-Requiring Patients Globally. Diabetes Technol Ther (2018) 20:21–4. doi: 10.1002/dmrr.2300
3. Roth J, Qureshi S, Whitford I, Vranic M, Kahn CR, Fantus IG, et al. Insulin’s Discovery: New Insights on Its Ninetieth Birthday. Diabetes Metab Res Rev (2014) 32):13–23. doi: 10.1002/dmrr
4. Vecchio I, Tornali C, Bragazzi NL, Martini M. The Discovery of Insulin: An Important Milestone in the History of Medicine. Front Endocrinol (Lausanne) (2018) 9:613. doi: 10.3389/fendo.2018.00613
5. Karamanou M. Milestones in the History of Diabetes Mellitus: The Main Contributors. World J Diabetes (2016) 7:1. doi: 10.4239/wjd.v7.i1.1
6. Quianzon CC, Cheikh I. History of Insulin. J Community Hosp Intern Med Perspect (2012) 2(2):10.3402/jchimp.v2i2.18701. doi: 10.3402/jchimp.v2i2.18701
7. Kesavadev J, Saboo B, Krishna MB, Krishnan G. Evolution of Insulin Delivery Devices: From Syringes, Pens, and Pumps to DIY Artificial Pancreas. Diabetes Ther (2020) 11:1251–69. doi: 10.1007/s13300-020-00831-z
8. Rex J, Jensen KH, Lawton SA. A Review of 20 Years’ Experience With the NovoPen® Family of Insulin Injection Devices. Clin Drug Investig (2006) 26:367–401. doi: 10.2165/00044011-200626070-00001
9. Perfetti R. Reusable and Disposable Insulin Pens for the Treatment of Diabetes: Understanding the Global Differences in User Preference and an Evaluation of Inpatient Insulin Pen Use. Diabetes Technol Ther (2010) 12:79–85. doi: 10.1089/dia.2009.0179
11. Perez-Nieves M, Jiang D, Eby E. Incidence, Prevalence, and Trend Analysis of the Use of Insulin Delivery Systems in the United States (2005 to 2011). Curr Med Res Opin (2015) 31:891–899. doi: 10.1185/03007995.2015.1020366
12. ATC A10C MAT/06/2021 (counting untis). Data Extract From IQVIA MIDAS 06/2021 (Europe Region Includes: Germany,UK, France, Italy, Spain, Poland, Romania, Czech, Hungary, Bulgaria, Slovakia, Croatia, Slovenia, Lithuania, Latvia, Estonia, Netherlands, Sweden. Warsaw: IQVIA (data not published) (2021).
13. Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, Efficacy, Acceptability of a Pre-Filled Insulin Pen in Diabetic Patients Over 60 Years Old. Diabetes Res Clin Pract (1995) 28:173–7. doi: 10.1016/0168-8227(95)01092-r
14. Cheen HHM, Lim SH, Huang MC, Bee YM, Wee HL. Adherence to Premixed Insulin in a Prefilled Pen Compared With a Vial/Syringe in People With Diabetes in Singapore. Clin Ther (2014) 36:1043–53. doi: 10.1016/j.clinthera.2014.05.009
15. Asche CV, Luo W, Aagren M. Differences in Rates of Hypoglycemia and Health Care Costs in Patients Treated With Insulin Aspart in Pens Versus Vials. Curr Med Res Opin (2013) 29:1287–96. doi: 10.1185/03007995.2013.825590
16. Slabaugh SL, Bouchard JR, Li Y, Baltz JC, Meah YA, Moretz DC. Characteristics Relating to Adherence and Persistence to Basal Insulin Regimens Among Elderly Insulin-Naïve Patients With Type 2 Diabetes: Pre-Filled Pens Versus Vials/Syringes. Adv Ther (2015) 32:1206–21. doi: 10.1007/s12325-015-0266-5
17. Buysman E, Conner C, Aagren M, Bouchard J, Liu F. Adherence and Persistence to a Regimen of Basal Insulin in a Pre-Filled Pen Compared to Vial/Syringe in Insulin-Naïve Patients With Type 2 Diabetes. Curr Med Res Opin (2011) 27:1709–17. doi: 10.1185/03007995.2011.598500
18. Grabner M, Chu J, Raparla S, Quimbo R, Zhou S, Conoshenti J. Clinical and Economic Outcomes Among Patients With Diabetes Mellitus Initiating Insulin Glargine Pen Versus Vial. Postgrad Med (2013) 125:204–13. doi: 10.3810/pgm.2013.05.2656
19. Molife C, Lee LJ, Shi L, Sawhney M, Lenox SM. Assessment of Patient-Reported Outcomes of Insulin Pen Devices Versus Conventional Vial and Syringe. Diabetes Technol Ther (2009) 11:529–38. doi: 10.1089/dia.2009.0007
20. Ahmann A, Szeinbach SL, Gill J, Traylor L, Garg SK. Comparing Patient Preferences and Healthcare Provider Recommendations With the Pen Versus Vial-and-Syringe Insulin Delivery in Patients With Type 2 Diabetes. Diabetes Technol Ther (2014) 16:76–83. doi: 10.1089/dia.2013.0172
21. Summers KH, Szeinbach SL, Lenox SM. Preference for Insulin Delivery Systems Among Current Insulin Users and Nonusers. Clin Ther (2004) 26:1498–505. doi: 10.1016/j.clinthera.2004.09.009
22. Brunton S. Insulin Delivery Systems: Reducing Barriers to Insulin Therapy and Advancing Diabetes Mellitus Treatment. Am J Med (2008) 121:35–41. doi: 10.1016/j.amjmed.2008.03.025
23. Lasalvia P, Barahona-Correa JE, Romero-Alvernia DM, Gil-Tamayo S, Castañeda-Cardona C, Bayona JG, et al. Pen Devices for Insulin Self-Administration Compared With Needle and Vial: Systematic Review of the Literature and Meta-Analysis. J Diabetes Sci Technol (2016) 10:959–66. doi: 10.1177/1932296816633721
24. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care (2018) 41:2669–701. doi: 10.2337/dci18-0033
25. Boye KS, Jordan JB, Malik RE, Currie BM, Matza LS. Patient Perceptions of and Preferences Between Characteristics of Injectable Diabetes Treatments. Diabetes Ther (2021) 12:2387–403. doi: 10.1007/s13300-021-01097-9
26. Eli Lilly and Company. ClinicalTrials.Gov. A Study of LY3209590 in Participants With Type 2 Diabetes Mellitus (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT03736785?term=NCT03.
27. Masierek M, Nabrdalik K, Kwiendacz H, Sawczyn T, Gumprecht J. A Multicenter, Prospective, Observational, Open-Label Study of the Safety and Comfort of Gensulin® Delivery Device Use in a Large Cohort of Adult and Elderly Patients With Type 2 Diabetes. Int J Environ Res Public Health (2020) 17:1–9. doi: 10.3390/ijerph17207587
28. Selam JL. Evolution of Diabetes Insulin Delivery Devices. J Diabetes Sci Technol (2010) 4:505–13. doi: 10.1177/193229681000400302
29. Pearson TL. Practical Aspects of Insulin Pen Devices. J Diabetes Sci Technol (2010) 4:522–31. doi: 10.1177/193229681000400304
30. Wright BM, Bellone JM, Mccoy EK. A Review of Insulin Pen Devices and Use in the Elderly Diabetic Population. Clin Med Insights Endocrinol Diabetes (2010) 3:53–63. doi: 10.4137/CMED.S5534
31. Berger AS, Saurbrey N, Kuhl C, Villumsen J. Clinical Experience With a New Device That Will Simplify Insulin Injection. Diabetes Care (1985) 8:73–6. doi: 10.2337/diacare.8.1.73
32. Jefferson G, Marteau M, Smith MA. A Multiple Injection Regimen Using an Insulin Injection Pen and Pre-Filled Cartridged Soluble Human Insulin in Adolescents With Diabetes. Diabet Med (1985) 2:493–5. doi: 10.1111/j.1464-5491.1985.tb00690.x
33. Dahl-Jorgensen K, Hanssen K, Mosand R, Sandvik L. The “Insulin Pen”: Comparison With Multiple Injection Treatment With Syringe. Pract Diabet (1986) 3:90–1. doi: 10.1002/pdi.1960030212
34. Murray DP, Keenan P, Gayer E, Salmon P, Tomkin GH, Drury MI, et al. A Randomized Trial of the Efficacy and Acceptability of a Pen Injector. Diabetes Med (1988) 5:750–4. doi: 10.1111/j.1464-5491.1988.tb01102.x
35. Ristic S, Bates PC, Martin JM, Llewelyn JA. Acceptability of a Reusable Insulin Pen, Humapen® Ergo, by Patients With Type 1 and Type 2 Diabetes. Curr Med Res Opin (2002) 18:68–71. doi: 10.1185/030079902125000327
36. Gorska-Ciebiada M, Masierek M, Ciebiada M. Improved Insulin Injection Technique, Treatment Satisfaction and Glycemic Control: Results From a Large Cohort Education Study. J Clin Transl Endocrinol (2020) 19:100217. doi: 10.1016/j.jcte.2020.100217
37. Fry A. Insulin Delivery Device Technology 2012: Where Are We After 90 Years? J Diabetes Sci Technol (2012) 6:947–53. doi: 10.1177/193229681200600428
38. Jørgensen JOL, Flyvbjerg A, Jørgensen JT, Sørensen HH, Johansen BR, Christiansen JS. NPH Insulin Administration by Means of a Pen Injector. Diabetes Med (1988) 5:574–6. doi: 10.1111/j.1464-5491.1988.tb01054.x
39. Engström LHK. Insulin Pen for Administration of Isophane Insulin. Pract Diabetes Int (1990) 7:162–4. doi: 10.1002/pdi.1960070408
40. Henderson MJ, Tindall H. Evaluation of Consumer Satisfaction and Quality of Life in Patients Changing to Novopen II. Pract Diabetes Int (1990) 7:206–8. doi: 10.1002/pdi.1960070506
41. Hyllested-Winge J, Jensen KH, Rex J. A Review of 25 Years’ Experience With the NovoPen Family of Insulin Pens in the Management of Diabetes Mellitus. Clin Drug Investig (2010) 30:643–74. doi: 10.2165/11584360-000000000-00000
42. Sommavilla B, Pietranera G. A Randomized, Open-Label, Comparative Crossover Handling Trial Between Two Durable Pens in Patients With Type 1 or 2 Diabetes Mellitus. Diabetes Technol Ther (2013) 15:1212–21. doi: 10.1089/dia.2013.1506
43. Stocks A, Perry SR, Brydon P. HumaPen Ergo®: A New 3.0ml Reusable Insulin Pen Evaluation of Patient Acceptability. Clin Drug Investig (2001) 21:319–24. doi: 10.2165/00044011-200121050-00001
44. Larbig M, Forst T, Forst S, Lorra B, Konig K, Fittkau T, et al. Evaluation of the Insulin Application System Autopen 24®. Pract Diabetes Int (2005) 22:364–366a. doi: 10.1002/pdi.877
45. Gensupen. Available at: https://bioton.com/en/patient/ids/medical-devices/gensupen/ (Accessed 12/10/2021).
46. Fendler W, Roman-Liu D, Tokarski T, Romanczuk R, Mlynarski W. Trigger Matters: An Ergonomy Analysis of Insulin Pens. Diabetes Technol Ther (2015) 17:171–6. doi: 10.1089/dia.2014.0177
47. Ignaut DA, Venekamp WJ. HumaPen Memoir: A Novel Insulin-Injecting Pen With a Dose-Memory Feature. Expert Rev Med Devices (2007) 4:793–802. doi: 10.1586/17434440.4.6.793
48. Hyllested-Winge J, Sparre T, Pedersen LK. NovoPen Echo(®) Insulin Delivery Device. Med Devices (Auckl) (2016) 9:11–8. doi: 10.2147/MDER.S59229
49. Olsen BS, Lilleøre SK, Korsholm CN, Kracht T. Novopen Echo® for the Delivery of Insulin: A Comparison of Usability, Functionality and Preference Among Pediatric Subjects, Their Parents, and Health Care Professionals. J Diabetes Sci Technol (2010) 4:1468–75. doi: 10.1177/193229681000400622
50. Klonoff D, Nayberg I, Rabbone I, Landgraf W, Domenger C, Danne T. Evaluation of the juniorSTAR® Half-Unit Insulin Pen in Young People With Type 1 Diabetes - User Perspectives. Eur Endocrinol (2013) 9:82–5. doi: 10.17925/ee.2013.09.02.82
51. Saurbrey N, Berger A, Kuhl C. The NovoPen - A Practical Tool for Simplifying Multiple Injection Insulin Therapy. Acta Paediatr Scand (1985) 74:64–5. doi: 10.1111/j.1651-2227.1985.tb10140.x
52. Walters DP, Smith PA, Marteau TM, Brimble A, Borthwick LJ. Experience With NovoPen, an Injection Device Using Cartridged Insulin, for Diabetic Patients. Diabetes Med (1985) 2:496–7. doi: 10.1111/j.1464-5491.1985.tb00691.x
53. Jensen T, Moller L, Andersen OO. Metabolic Control and Patient Accep- Tability of Multiple Insulin Injections Using NovoPen. Pract Diabetes (1986) 3:302–6. doi: 10.1002/pdi.1960030612
54. Saurbrey N, Arnold-Larsen S, Møller-Jensen B, Kühl C. Comparison of Continuous Subcutaneous Insulin Infusion With Multiple Insulin Injections Using the NovoPen. Diabetes Med (1988) 5:150–3. doi: 10.1111/j.1464-5491.1988.tb00962.x
55. Houtzagers CM, Berntzen PA, van der Stap H, Van Maarschalkerweerd WW, Lanting P, Boen-Tan I, et al. Efficacy and Acceptance of Two Intensified Conventional Insulin Therapy Regimens: A Long-Term Cross-Over Comparison. Diabetes Med (1989) 6:416–21. doi: 10.1111/j.1464-5491.1989.tb01196.x
56. Houtzagers CMGJ, Berntzen PA, van der Stap H, Van Maarschalkerweerd WWA, Heine RJ, van der Veen EA, et al. Multiple Daily Insulin Injections Improve Self-Confidence. Diabetes Med (1989) 6:512–9. doi: 10.1111/j.1464-5491.1989.tb01219.x
57. Tallroth G, Karlson B, Nilsson A, Agardh C-D. Influence of Intensified Insulin Regimen on Quality of Life and Metabolic Control in Insulin-Dependent Diabetes Mellitus. Diabetes Res Clin Pract (1994) 25:111–5. doi: 10.1016/0168-8227(94)90036-1
58. Tubiana-Rufi N, Levy-Marchal C, Mugnier E, Czernichow P. Long Term Feasibility of Multiple Daily Injections With Insulin Pens in Children and Adolescents With Diabetes. Eur J Pediatr (1989) 149:80–3. doi: 10.1007/BF01995851
59. Kadiri A, Chraibi A, Marouan F, Ababou MR, el Guermai N, Wadjinny A, et al. Comparison of NovoPen 3 and Syringes/Vials in the Acceptance of Insulin Therapy in NIDDM Patients With Secondary Failure to Oral Hypoglycaemic Agents. Diabetes Res Clin Pract (1998) 41:15–23. doi: 10.1016/S0168-8227(98)00055-2
60. Goksen D, Darcan S, Buyukinan M, Kurt E. Possible Problem With Optipen Pro-1: Should Diabetic Patients Contiune to Use This Product? Diabetes Care (2006) 29:1710. doi: 10.2337/dc06-0582
61. Venekamp WJRR, Kerr L, Dowsett SA, Johnson PA, Wimberley D, McKenzie C, et al. Functionality and Acceptability of a New Electronic Insulin Injection Pen With a Memory Feature. Curr Med Res Opin (2006) 22:315–25. doi: 10.1185/030079906X80477
62. Israël-Bultman H, Hyllested-Winge J, Kolaczynski M, Steindorf J, Garon J. Comparison of Preference for NovoPen® 4 With Previous Insulin Pen Treatments After 12 Weeks in Adult Patients With Type 1 and Type 2 Diabetes: A Multicenter Observational Study. Clin Ther (2011) 33:346–57. doi: 10.1016/j.clinthera.2011.04.001
63. Available at: https://www.nhsggc.org.uk/media/252760/novopen-3-instruction-manual.pdf (Accessed 12.10.2021).
64. Available at: https://www.manualslib.com/products/Novo-Nordisk-Novopen-3-Demi-8945258.html (Accessed 12.10.2021).
65. Available at: https://www.manualslib.com/manual/813555/Novo-Nordisk-Novopen-Junior.html (Accessed 12.10.2021).
66. Available at: https://www.diabeteswhatsnext.com/content/dam/patientengagementprogram/shared/content-pages/pdfs/NovoPen-4-generic-injection-guide.pdf (Accessed 12.10.2021).
67. Available at: https://www.diabeteswhatsnext.com/content/dam/patientengagementprogram/shared/content-pages/pdfs/NovoPen-Echo-generic-injection-guide.pdf (Accessed 12.10.2021).
68. Available at: https://www.diabeteswhatsnext.com/content/dam/patientengagementprogram/shared/content-pages/pdfs/NovoPen-5-generic-injection-guide.pdf (Accessed 12.10.2021).
69. Available at: https://www.owenmumford.com/sites/owen-mumford/files/owen-mumford/autopen/pdf/autopen-24.pdf (Accessed 12.10.2021).
70. Available at: https://www.owenmumford.com/sites/owen-mumford/files/owen-mumford/autopen/pdf/autopen-classic-1.pdf (Accessed 12.10.2021).
71. Available at: https://www.ompharmaservices.com/product-portfolio/autopen-2/ (Accessed 12.10.2021).
72. Available at: https://www.yumpu.com/la/document/read/19894662/optipenr-pro-1-sanofi (Accessed 12.10.2021).
73. Available at: https://pharmareview.files.wordpress.com/2011/07/humapenergocustomermanuale.pdf (Accessed 12.10.2021).
74. Available at: http://www.iabl.net/insert/Ergo-II-User-Manual.pdf (Accessed 12.10.2021).
75. Available at: https://old.sfda.gov.sa/ar/SURE_DrugList_Attachments/2873-human-EnUserGuide-Luxura%20Usermanual.pdf (Accessed 12.10.2021).
76. Available at: https://pi.lilly.com/us/HumaPen_Luxura_HD_um.pdf (Accessed 12.10.2021).
77. Available at: https://www.manualslib.com/products/Lilly-Humapen-Memoir-3818405.html (Accessed 12.10.2021).
78. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/21081s014lbl.pdf (Accessed 12.10.2021).
79. Available at: https://www.manualscat.com/en/berlin-chemie-berlipen-manual (Accessed 12.10.2021).
80. Available at: https://www.manualslib.de/manual/342255/Berlin-Chemie-Berlipen-Aero-2.html (Accessed 12.10.2021).
81. Available at: https://products.sanofi.ca/en/JuniorSTAR.pdf (Accessed 12.10.2021).
82. Available at: http://docplayer.net/164611538-I-pen-at-a-glance-manual-injection-auto-injection-reusable-disposable-hidden-needle-multiple-injections-variable-dosage-fixed-dosage.html (Accessed 12.10.2021).
83. McCoy EK, Wright BM. A Review of Insulin Pen Devices. Postgrad Med (2010) 122:81–8. doi: 10.3810/pgm.2010.05.2145
84. Available at: https://www.novonordisk.co.in/content/dam/Denmark/HQ/aboutus/documents/HistoryBook_UK.pdf (Accessed 12.10.2021).
85. Davis EM, Sexson EL, Spangler ML, Foral PA. An Evaluation of Prefilled Insulin Pens: A Focus on the Next Generation Flexpen ®. Med Devices Evid Res (2010) 3:41–50. doi: 10.2147/MDER.S11730
86. Wielandt JO, Niemeyer M, Hansen MR, Bucher D, Thomsen NB. FlexTouch: A Prefilled Insulin Pen With a Novel Injection Mechanism With Consistent High Accuracy at Low- (1 U), Medium- (40 U), and High- (80 U) Dose Settings. J Diabetes Sci Technol (2011) 5:1195–9. doi: 10.1177/193229681100500525
87. Ignaut DA, Opincar M, Lenox S. FlexPen and KwikPen Prefilled Insulin Devices: A Laboratory Evaluation of Ergonomic and Injection Force Characteristics. J Diabetes Sci Technol (2008) 2:533–7. doi: 10.1177/193229680800200327
88. Han DH. Humalog Junior KwikPen (2017). Available at: https://www.empr.com/home/news/humalog-junior-kwikpen-soon-to-be-available/.
89. Korytkowski M, Bell D, Jacobsen C, Suwannasari R. A Multicenter, Randomized, Open-Label, Comparative, Two-Period Crossover Trial of Preference, Efficacy, and Safety Profiles of a Prefilled, Disposable Pen and Conventional Vial/Syringe for Insulin Injection in Patients With Type 1 or 2 Diabetes Mellitus. Clin Ther (2003) 25:2836–48. doi: 10.1016/S0149-2918(03)80337-5
90. Ignaut DA, Schwartz SL, Sarwat S, Murphy HL. Comparative Device Assessments: Humalog KwikPen Compared With Vial and Syringe and FlexPen. Diabetes Educ (2009) 35:789–98. doi: 10.1177/0145721709340056
91. Campos C, Lajara R, Deluzio T. Usability and Preference Assessment of a New Prefilled Insulin Pen Versus Vial and Syringe in People With Diabetes, Physicians and Nurses. Diabetes Technol Ther (2014) 16:1837–46. doi: 10.1089/dia.2014.1506
92. Pfützner A, Bailey T, Campos C, Kahn D, Ambers E, Niemeyer M, et al. Accuracy and Preference Assessment of Prefilled Insulin Pen Versus Vial and Syringe With Diabetes Patients, Caregivers, and Healthcare Professionals. Curr Med Res Opin (2013) 29:475–81. doi: 10.1185/03007995.2013.775112
93. Asakura T, Seino H, Nakano R, Muto T, Toraishi K, Sako Y, et al. A Comparison of the Handling and Accuracy of Syringe and Vial Versus Prefilled Insulin Pen (FlexPen). Diabetes Technol Ther (2009) 11(10):657–61. doi: 10.1089/dia.2009.0006
94. Lajara R, Guerrero G, Thurman J. Healthcare Professional and Patient Perceptions of a New Prefilled Insulin Pen Versus Vial and Syringe. Expert Opin Drug Deliv (2012) 9:1181–96. doi: 10.1517/17425247.2012.721774
95. Healthworld.com. Eli Lilly Launches 200 U/mL Pre-Filled Insulin Pen. Econ Times (2017). Available at: https://health.economictimes.indiatimes.com/news/pharma/eli-lilly-launches-200u/ml-pre-filled-insulin-pen/57685481.
96. Gudiksen N, Hofstätter T, Rønn BB, Sparre T. FlexTouch: An Insulin Pen-Injector With a Low Activation Force Across Different Insulin Formulations, Needle Technologies, and Temperature Conditions. Diabetes Technol Ther (2017) 19:603–7. doi: 10.1089/dia.2017.0121
97. Pohlmeier H, Berard L, Brulle-Wohlhueter C, Wu J, Dahmen R, Nowotny I, et al. Ease of Use of the Insulin Glargine 300 U/mL Pen Injector in Insulin-Naïve People With Type 2 Diabetes. J Diabetes Sci Technol (2017) 11:263–9. doi: 10.1177/1932296816668877
98. Heinemann L, Krisiunas E. Diabetes Technology and Waste: A Complex Problem Piling Up! J Diabetes Sci Technol (2019) 13:815–6. doi: 10.1177/1932296819836395
99. Catic T, Gojak R, Djekic D. Disposal of Used Pens and Needles From Diabetes Patients Perspective. Mater Sociomed (2020) 32:267–70. doi: 10.5455/msm.2020.32.267-270
100. Niskanen L, Jensen LE, Råstam J, Nygaard-Pedersen L, Erichsen K, Vora JP. Randomized, Multinational, Open-Label, 2-Period, Crossover Comparison of Biphasic Insulin Aspart 30 and Biphasic Insulin Lispro 25 and Pen Devices in Adult Patients With Type 2 Diabetes Mellitus. Clin Ther (2004) 26:531–40. doi: 10.1016/S0149-2918(04)90055-0
101. Haak T, Edelman S, Walter C, Lecointre B, Spollett G. Comparison of Usability and Patient Preference for the New Disposable Insulin Device Solostar Versus Flexpen, Lilly Disposable Pen, and a Prototype Pen: An Open-Label Study. Clin Ther (2007) 29:650–60. doi: 10.1016/j.clinthera.2007.04.003
102. Asakura T, Jensen KH. Comparison of Intuitiveness, Ease of Use, and Preference in Two Insulin Pens. J Diabetes Sci Technol (2009) 3:312–9. doi: 10.1177/193229680900300212
103. Yakushiji F, Fujita H, Terayama Y, Yasuda M, Nagasawa K, Shimojo M, et al. The Best Insulin Injection Pen Device for Caregivers: Results of Injection Trials Using Five Insulin Injection Devices. Diabetes Technol Ther (2010) 12:143–8. doi: 10.1089/dia.2009.0110
104. Bailey T, Thurman J, Niemeyer M, Schmeisl G. Usability and Preference Evaluation of a Prefilled Insulin Pen With a Novel Injection Mechanism by People With Diabetes and Healthcare Professionals. Curr Med Res Opin (2011) 27:2043–52. doi: 10.1185/03007995.2011.616190
105. Hancu N, Czupryniak L, Genestin E, Sourij H. A Pan-European and Canadian Prospective Survey to Evaluate Patient Satisfaction With the SoloSTAR Insulin Injection Device in Type 1 and Type 2 Diabetes. J Diabetes Sci Technol (2011) 5:1224–34. doi: 10.1177/193229681100500531
106. Oyer D, Narendran P, Qvist M, Niemeyer M, Nadeau DA. Ease of Use and Preference of a New Versus Widely Available Prefilled Insulin Pen Assessed by People With Diabetes, Physicians and Nurses. Expert Opin Drug Deliv (2011) 8:1259–69. doi: 10.1517/17425247.2011.615830
107. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare Professional and Patient Assessment of a New Prefilled Insulin Pen Versus Two Widely Available Prefilled Insulin Pens for Ease of Use, Teaching and Learning. Curr Med Res Opin (2012) 28:3–13. doi: 10.1185/03007995.2011.644427
108. Pfützner A, Schipper C, Niemeyer M, Qvist M, Löffler A, Forst T, et al. Comparison of Patient Preference for Two Insulin Injection Pen Devices in Relation to Patient Dexterity Skills. J Diabetes Sci Technol (2012) 6:917–20. doi: 10.1177/193229681200600424
109. Schipper C, Musholt P, Niemeyer M, Qvist M, Löffler A, Forst T, et al. Patient Device Assessment Evaluation of Two Insulin Injection Devices in a Mixed Cohort of Insulin-Treated Patients With Type 1 or Type 2 Diabetes Mellitus. Curr Med Res Opin (2012) 28:1297–303. doi: 10.1185/03007995.2012.708325
110. Pfützner A, Forst T, Niemeyer M, Bailey T. Assessment for Ease of Use and Preference of a New Prefilled Insulin Pen (FlexTouch Degludec U100/U200) Versus the SoloSTAR Insulin Pen by Patients With Diabetes and Healthcare Professionals. Expert Opin Drug Deliv (2014) 11:1381–9. doi: 10.1517/17425247.2014.927438
111. Friedrichs A, Schmitz M, Kamlot S, Adler S. Dialing Torque Preferences of People With Diabetes When Using Insulin Pens: A Pilot Study. Diabetes Ther (2015) 6:85–93. doi: 10.1007/s13300-015-0097-z
112. Warren ML, Brod M, Håkan-Bloch J, Sparre T, Chaykin LB. Patient-Reported Outcomes From a Randomized, Crossover Trial Comparing a Pen Injector With Insulin Degludec Versus a Pen Injector With Insulin Glargine U100 in Patients With Type 2 Diabetes. Curr Med Res Opin (2019) 35:1623–9. doi: 10.1080/03007995.2019.1605769
113. Norlander LM, Anderson S, Levy CJ, Ekhlaspour L, Lam DW, Hsu LJ, et al. Late and Missed Meal Boluses With Multiple Daily Insulin Injections. Diabetes (2018) 67:992–P. doi: 10.2337/db18-992-P
114. Randløv J, Poulsen JU. How Much do Forgotten Insulin Injections Matter to Hemoglobin A1c in People With Diabetes? A Simulation Study. J Diabetes Sci Technol (2008) 2:229–35. doi: 10.1177/193229680800200209
115. Zaugg SD, Dogbey G, Collins K, Reynolds S, Batista C, Brannan G, et al. Diabetes Numeracy and Blood Glucose Control: Association With Type of Diabetes and Source of Care. Clin Diabetes (2014) 32:152–7. doi: 10.2337/diaclin.32.4.152
116. Marden S, Thomas PW, Sheppard ZA, Knott J, Lueddeke J, Kerr D. Poor Numeracy Skills Are Associated With Glycaemic Control in Type 1 Diabetes. Diabetes Med (2012) 29:662–9. doi: 10.1111/j.1464-5491.2011.03466.x
117. Cavanaugh K, Huizinga MM, Wallston KA, Gebretsadik T, Shintani A, Davis D, et al. Association of Numeracy and Diabetes Control. Ann Intern Med (2008) 148:737–46. doi: 10.7326/0003-4819-148-10-200805200-00006
118. Schmidt S, Nørgaard K. Bolus Calculators. J Diabetes Sci Technol (2014) 8:1035–41. doi: 10.1177/1932296814532906
119. Available at: https://www.researchandmarkets.com/research/4hklc2/lamea_smart?w=4 (Accessed 12.10.2021).
120. Available at: https://www.futuremarketinsights.com/reports/smart-insulin-pens-market (Accessed 12.10.2021).
121. Heinemann L, Schnell O, Gehr B, Schloot NC, Görgens SW, Görgen C. Digital Diabetes Management: A Literature Review of Smart Insulin Pens. J Diabetes Sci Technol (2021) 1932296820983863. doi: 10.1177/1932296820983863
122. Shah R, Patel M, Maahs D, Shah V. Insulin Delivery Methods: Past, Present and Future. Int J Pharm Investig (2016) 6:1. doi: 10.4103/2230-973x.176456
123. Available at: https://www.medscape.com/viewarticle/950892 (Accessed 12.10.2021).
124. Gildon BW. InPen Smart Insulin Pen System: Product Review and User Experience. Diabetes Spectr (2018) 31(4):354–8. doi: 10.2337/ds18-0011
125. Sangave NA, Aungst TD, Patel DK. Smart Connected Insulin Pens, Caps, and Attachments: A Review of the Future of Diabetes Technology. Diabetes Spectr (2019) 32:378–84. doi: 10.2337/ds18-0069
126. Sy SL, Munshi MM, Toschi E. Can Smart Pens Help Improve Diabetes Management? J Diabetes Sci Technol (2020):1932296820965600. doi: 10.1177/1932296820965600
127. PR Newswire. Companion Medical Announces U.S. Commercial Launch of Smart Insulin Pen System. Available at: https://www.prnewswire.com/news-releases/companion-medical-announces-us-commercial-launch-of-smart-insulin-pen-system-300571413.html (Accessed 12.10.2021).
128. Available at: https://www.medtronic.com/us-en/healthcare-professionals/therapies-procedures/diabetes/education/diabetes-digest/inpen-smart-insulin-pen.html (Accessed 12.10.2021).
129. Available at: https://InPen-Healthcare-Professional-Brochure-3.pdf (Accessed 12.10.2021).
130. Klonoff DC, Zhang JY, Shang T, Mehta C, Kerr D. Pharmacoadherence: An Opportunity for Digital Health to Inform the Third Dimension of Pharmacotherapy for Diabetes. J Diabetes Sci Technol (2020) 15:177–83. doi: 10.1177/1932296820973185
131. Warshaw H, Isaacs D, MacLeod J. The Reference Guide to Integrate Smart Insulin Pens Into Data-Driven Diabetes Care and Education Services. Diabetes Educ (2020) 46:3S–20S. doi: 10.1177/0145721720930183
132. Klonoff DC, Kerr D. Smart Pens Will Improve Insulin Therapy. J Diabetes Sci Technol (2018) 12:551–3. doi: 10.1177/1932296818759845
133. Adolfsson P, Hartvig NV, Kaas A, Møller JB, Hellman J. Increased Time in Range and Fewer Missed Bolus Injections After Introduction of a Smart Connected Insulin Pen. Diabetes Technol Ther (2020) 22:709–18. doi: 10.1089/dia.2019.0411
134. Jendle J, Ericsson Å, Gundgaard J, Møller JB, Valentine WJ, Hunt B. Smart Insulin Pens Are Associated With Improved Clinical Outcomes at Lower Cost Versus Standard-Of-Care Treatment of Type 1 Diabetes in Sweden: A Cost-Effectiveness Analysis. Diabetes Ther (2021) 12:373–88. doi: 10.1007/s13300-020-00980-1
135. Available at: https://www.bigfootbiomedical.com/about/press-room/press-releases/fda-clearance-bigfoot-unity (Accessed 12.10.2021).
136. Available at: https://www.manualslib.com/products/Gocap-1-1-8745018.html (Accessed 12.10.2021).
137. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Ruiz L. Insulclock: A Novel Insulin Delivery Optimization and Tracking System. Diabetes Technol Ther (2019) 21:209–14. doi: 10.1089/dia.2018.0361
138. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Cruz-Bravo M, María-Sanchez C, Poza G, et al. Efficacy of Insulclock in Patients With Poorly Controlled Type 1 Diabetes Mellitus: A Pilot, Randomized Clinical Trial. Diabetes Technol Ther (2020) 22:686–90. doi: 10.1089/dia.2019.0427
139. Galindo RJ, Ramos C, Cardona S, Vellanki P, Davis GM, Oladejo O, et al. Efficacy of a Smart Insulin Pen Cap for the Management of Patients With Uncontrolled Type 2 Diabetes: A Randomized Cross-Over Trial. J Diabetes Sci Technol (2021):19322968211033836. doi: 10.1177/19322968211033837
140. Available at: https://www.dukada.com/user-guide/ (Accessed 12.10.2021).
141. Available at: https://timesulin.com/how-to-use-it/ (Accessed 12.10.2021).
142. Available at: https://diabetes.diabetesjournals.org/content/70/Supplement_1/219-OR?utm_source=TrendMD&utm_medium=cpc&utm_campaign=Diabetes_TrendMD_0 (Accessed 12.10.2021).
143. Available at: https://www.companionmedical.com/guides/inpen-user-guide.pdf (Accessed 12.10.2021).
144. Available at: https://fccid.io/2AHMS-BTPEN1/User-Manual/15-ESYSTA-BT-Pen-B-and-W-UserMan-3201159 (Accessed 12.10.2021).
145. Available at: https://pendiq.com/wp-content/uploads/2017/05/Pendiq-2.0-English-User-Manual.pdf (Accessed 12.10.2021).
146. Available at: https://developer.novonordisk.com/content/dam/Global/AFFILIATE/digitalhealth-novonordisk-com/en_gb/documents/8-4260-00-004-1_cropped.pdf (Accessed 12.10.2021).
Keywords: insulin, pen, diabetes mellitus, prefilled pen, smart pen
Citation: Masierek M, Nabrdalik K, Janota O, Kwiendacz H, Macherski M and Gumprecht J (2022) The Review of Insulin Pens—Past, Present, and Look to the Future. Front. Endocrinol. 13:827484. doi: 10.3389/fendo.2022.827484
Received: 02 December 2021; Accepted: 02 February 2022;
Published: 08 March 2022.
Edited by:
Pierre De Meyts, Université Catholique de Louvain, BelgiumReviewed by:
Michał Holecki, Medical University of Silesia, PolandMariusz Dąbrowski, University of Rzeszow, Poland
Copyright © 2022 Masierek, Nabrdalik, Janota, Kwiendacz, Macherski and Gumprecht. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katarzyna Nabrdalik, knabrdalik@sum.edu.pl
 Małgorzata Masierek1
Małgorzata Masierek1