- 1Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Henan Key Laboratory of Reproduction and Genetics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Henan Provincial Obstetrical and Gynecological Diseases (Reproductive Medicine) Clinical Research Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: Polycystic ovary syndrome (PCOS) is a prevalent endocrine disease in reproductive women associated with poor pregnancy outcomes. In modern society, people pay more attention to the female factor, but it is uncertain whether sperm quality is another factor affecting pregnancy outcomes of patients with PCOS.
Method: The effect of sperm DNA fragmentation (SDF) on oocyte fertilization, embryonic development, and pregnancy outcomes for patients with PCOS who underwent in vitro fertilization (IVF) treatment was studied. A total of 141 PCOS patients and 332 control patients undergoing IVF treatment were recruited from January 2017 to December 2019. All female patients were designated into two groups according to the Rotterdam criteria. Each group was divided into two sets, DNA fragmentation index (DFI) ≤15% and DFI > 15%, on the basis of sperm DFI.
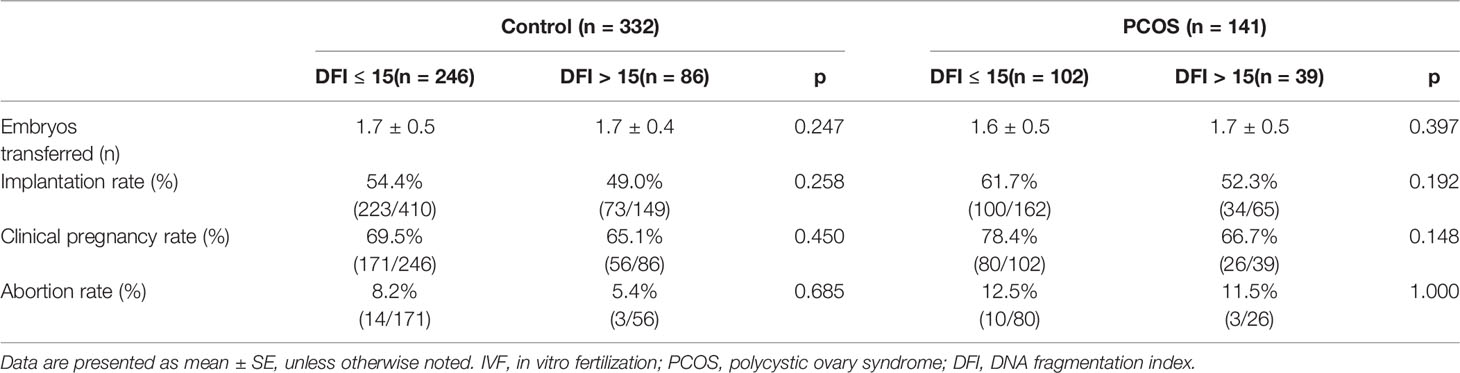
Result: There were no differences in basic clinical characteristics between couples with a sperm DFI ≤ 15% or DFI > 15%. For control patients, no differences were observed in IVF outcomes. However, for PCOS patients, although there were no differences in the fertilization (60.4% vs. 59.9%, p = 0.831), high-quality embryo (68.5% vs. 67.9% p = 0.832), clinical pregnancy (78.4% vs. 66.7%, p = 0.148), and abortion (12.5% vs. 11.5%, p = 1.000) rates, a significantly lower high-quality blastocyst formation rate (26.3% vs. 16.3%, p = 0.023) was observed for couples with a sperm DFI > 15% compared with a sperm DFI ≤ 15%.
Conclusion: For PCOS patients undergoing IVF, oocytes fertilized using sperm with higher DFI led to a lower high-quality blastocyst formation rate but had no influence on fertilization, high-quality embryo, clinical pregnancy, and miscarriage rates.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most widespread gynecological endocrine conditions in women of childbearing age and is generally considered to be an important public health problem (1, 2). In particular, PCOS is accompanied by infertility, menstrual absence or irregularity, hyperandrogenism and/or hyperandrogenemia, and obesity (3) and affects approximately 6%–15% of adult women (4, 5). During human ovarian follicular development, many primordial follicles are recruited as a group of growing follicles, and then a dominant follicle will ovulate. However, in patients with PCOS, ovarian hyperandrogenism and regulatory systems disrupt follicular development, which results in ovulation dysfunction (2, 6). Although increasing evidence shows that patients with PCOS produce more oocytes during treatment via assisted reproductive technology, clinical pregnancy outcomes remain disappointing (7–9). Studies have demonstrated in patients with PCOS that there are a lower fertilization rate and higher risk of early spontaneous miscarriage (10–12), associated with poor oocyte and embryo quality (13–16). A meta-analysis showed that women with PCOS had a significantly elevated risk of miscarriage and premature delivery (17) and that their babies had a significantly elevated risk to be treated at a neonatal intensive care unit (OR: 2.31; 95% CI: 1.25–4.26) and higher postpartum mortality (OR: 3.07; 95% CI: 1.03–9.21) (18).
The integrity of sperm DNA plays a major role in fertilization and the development of healthy offspring (19), so the sperm DNA fragmentation index (DFI) may be an important clinical indicator for figuring out the extent of sperm DNA damage and integrity (20). There is clear evidence that infertile men have a higher amount of sperm DFI than fertile men (21). Other studies pointed out that high sperm DFI was connected with decreased fertilization, high-quality embryo development, and conception rates and increased risk of abortion (22–25), as well as the risk of genetic diseases in offspring (21).
The genomic integrity of a zygote is indispensable for normal embryo development. Human sperm has a limited ability to repair DNA damage, so repairing sperm DNA damage is dependent on oocytes (26). Although an oocyte can repair DNA damage in a fertilizing spermatozoon, the capacity is limited. For example, studies showed that pregnancy outcomes were associated with the degree of DNA damage (27) and that oocytes could repair sperm DNA damage when it was less than 8% (28). For patients with PCOS, it remains uncertain whether oocytes have a disrupted ability to repair sperm DNA fragmentation (SDF). Therefore, this research observed whether the different extent of sperm DNA damage had an influence on embryonic development and clinical pregnancy outcomes for patients with PCOS undergoing in vitro fertilization (IVF) treatment.
Material and Methods
Patients and Experimental Design
This study enrolled a total of 473 infertile couples who underwent IVF for the first time at the Reproductive Medical Center of the First Affiliated Hospital of Zhengzhou University from January 2017 to December 2019. All couples had normal chromosome karyotypes and had cleavage-stage embryos or blastocysts for transfer. Male age was less than 45 years, and all male partners had recently not taken any drug that was harmful to the sperm. According to the Rotterdam criteria, all patients were divided into two groups, PCOS (n = 141) and control (n = 332), and each group was divided into two sets on the basis of sperm DFI: DFI ≤ 15% and DFI > 15%. The inclusion criteria of control patients in the study were women aged less than 35 years with a basal level of follicle-stimulating hormone (FSH) < 10 mIU/ml, 18.5 < body mass index (BMI) ≤ 24.9, and female infertility only due to fallopian tube dysfunction. Based on the 2003 Rotterdam criteria (29), PCOS patients should meet at least two of the three following characteristics: 1) oligo/anovulation, 2) clinical symptoms caused by high testosterone and/or hyperandrogenemia, and 3) ultrasonography showing more than 12 follicles with diameters of 2–9 mm in one or both ovaries and/or the volume of one ovarian >10 ml. The inclusion criteria of PCOS patients were as follows: 1) women were less than 35 years old, 2) female infertility was due to fallopian tube factor, and 3) a basal level of FSH < 10 mIU/ml.
Semen Analysis
All examinations were evaluated on the basis of the WHO laboratory manual (30). Semen samples were collected into sterile and non-poisonous containers by masturbation after 3 to 7 days of abstinence, and the samples were kept in a controlled environment to avoid changes that will affect sperm quality. After liquefaction for 30 min, sperm concentration and viability were examined in a Makler chamber (Sefi Medical Instruments, Haifa, Israel). In accordance with Kruger’s strict criteria, sperm morphology assessment was detected using H&E staining. After liquefaction, semen samples were prepared using two discontinuous density gradients (80%–40%), and the precipitate was collected after centrifugation. Then the samples were washed twice with IVF medium, and the final precipitant was suspended in a suitable volume of IVF medium.
Sperm DNA Fragmentation Index Detection
Semen was collected after 3 to 7 days of abstinence, and the sperm DFI was tested using sperm chromatin structure assay (SCSA) after liquefaction. The semen density was adjusted to (0.5–1.0) × 106/ml with TNT buffer, and the semen sample was incubated with acridine orange (AO; PH 6.0) solution. Then the sperm were analyzed by a flow cytometer (BD FACS Canto II, BD Biosciences, San Jose, CA, USA) and calculated by specific software to analyze the fluorescence signals. The double-stranded DNA emitted green fluorescence when binding to AO, while single-stranded DNA emitted red fluorescence. The number of sperm was not less than 5,000, and the DFI was calculated by measuring the percentage of sperm with red fluorescence in the total sperm number. At present, there are three major methodologies to test SDF, namely, the comet assay, the terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick end labeling (TUNEL) assay, and the SCSA. In comet assay, only 50–200 sperm cells per sample are measured by a fluorescence microscopic test, which lacks statistical robustness. Although the TUNEL assay can be done by flow cytometry, it also can be applied in bright field/fluorescence microscopy, which has much less statistical robustness. However, the SCSA is different from other tests, which is carried out under a normalized protocol (31) and has definite and clinically valuable cutoff levels for evaluating spermatogenic potential (32). In SCSA, 5,000–10,000 sperm cells can be analyzed within a few seconds, which can provide precise and repeatable statistical data. Although the variation of SCSA analysis among laboratories is relatively stable, the implementation rate of SCSA is lower because of an expensive flow cytometer.
Controlled Ovarian Stimulation and Laboratory Procedures
Patients undergoing IVF used an ultra-long protocol for promoting ovulation. On the second or third day of the menstrual cycle, the patients were injected intramuscularly with a gonadotropin-releasing hormone agonist (GnRH-a; triptorelin acetate) to reduce gonadotropin secretion in the pituitary gland. After 28–35 days, patients were assessed by color Doppler ultrasound inspection and routine blood examinations for FSH, luteinizing hormone, and estradiol levels. When the gonadotropin levels were at a low threshold, induction of ovulation was implemented, and the development of follicles was monitored by transvaginal ultrasound inspection. When the number of the mean diameter of dominant follicles with at least 17 mm was more than three, the usage of gonadotropin was ceased. Then the patients were injected intramuscularly with 10,000 IU of recombinant human chorionic gonadotropin (HCG, Livzon) to promote final follicle maturity. Oocytes were collected 35–37 h after injection.
Oocyte Fertilization and Embryo Culture
Fresh sperm were prepared using density gradient centrifugation in sperm-gradient 40% and 80% solutions, and the collected sperm underwent sperm swim-up technique to reach a final sperm concentration of 1 × 106/ml. Then sperm were cultured with mature oocytes for fertilization. Embryos were cultured in a comfortable atmosphere of 6% CO2 at 37°C. Then the physician decided to use two good-quality grade I or II embryos (using the Peter scoring system) on Day 3 or one blastocyst for transfer per patient based on embryo quality and the physical condition of the patients. The rate of fertilization was calculated by measuring the proportion of double pronuclear embryos (2PN) in total MII oocytes. The rate of high-quality embryos was defined as the number of embryos with grade I or II/the number of 2PN cleavage. The blastocysts were evaluated according to Gardner and Schoolcraft scoring system, and high-quality blastocysts were defined as those scoring ≥3 BB. The rate of high-quality blastocyst formation was defined as the number of high-quality blastocysts/total developed blastocysts.
Clinical Follow-Up
When the embryo was transferred at 14 days, the patients were tested for serum β-hCG levels. Serum β-hCG levels >50 IU/L were diagnosed as biochemical pregnancy. When the embryo transferred at 35 days, the patients were asked to get a transvaginal ultrasound. Detecting a normal fetal heart rate in the uterus was considered a clinical pregnancy. Abortion was defined as an embryo or fetus that did not survive.
Statistical Analysis
Statistical data were analyzed by SPSS 25.0 software. Variable data were shown as the mean ± SD (x ± s). The rates of early embryo development, clinical pregnancy, and abortion were contrasted using the χ2 test. p < 0.05 was defined as statistical significance.
Results
Relationships Between Sperm DNA Fragmentation and In Vitro Fertilization Outcomes for Women With Control and Polycystic Ovary Syndrome
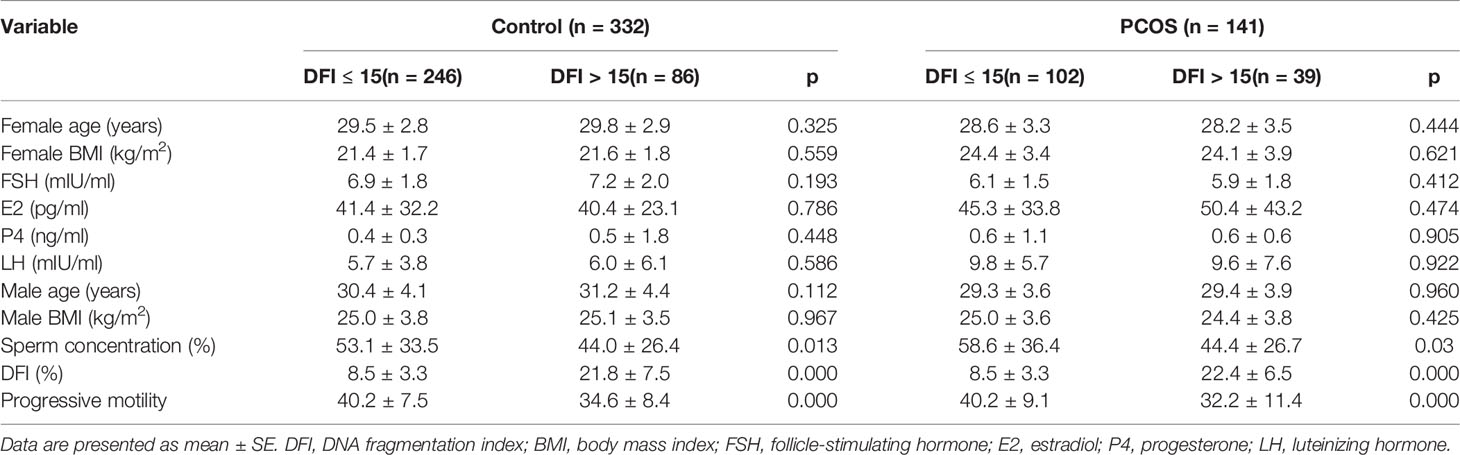
As shown in Table 1, the differences in basic clinical characteristics (age, BMI, and basal levels of hormone) between the couples with a sperm DFI ≤ 15% and a sperm DFI > 15% were not observed, but embryonic development differed between the two groups; all results are shown in Table 2.
We analyzed the relationship between sperm DFI and embryonic development in control and PCOS patients. Although there were no significant differences for the control couples undergoing IVF, for PCOS patients, a lower high-quality blastocyst rate (26.3% vs. 16.3%; p = 0.023) was observed for couples with a sperm DFI > 15% compared with a sperm DFI ≤ 15%. Surprisingly, no statistical differences between SDF and fertilization and high-quality embryo rates in the two groups were observed.
To further explore whether SDF had an impact on pregnancy outcomes, we analyzed the correlation between different damaged DNA and the rates of clinical pregnancy and abortion in two groups, as shown in Table 3. Surprisingly, no statistical differences regarding clinical pregnancy (78.4% vs. 66.7%, p = 0.148) and abortion (12.5% vs. 11.5%, p = 1.000) rates in two groups were observed.
Discussion
It is established that PCOS is a prevalent metabolic disease that negatively affects female reproduction, health, and quality of life (4). Most research has mainly focused on female infertility factors related to PCOS. However, poor pregnancy outcomes for PCOS women may be related not only to oocytes but also to sperm quality. Sperm DNA integrity plays an important role in precisely transmitting parental genetic material to the offspring (33, 34). Increasing evidence supported the view that high sperm DFI was associated with poor pregnancy outcomes, regardless of natural conception and fertility treatments (35, 36). A recent meta-analysis study pointed out that sperm DNA damage may adversely affect clinical gestation after patients underwent IVF and/or intracytoplasmic sperm injection (ICSI) treatment (37). The association between higher levels of sperm DFI and increased early abortion rate was observed in a retrospective cohort study (20), and a prospective observational study indicated that in ICSI cycles, elevated sperm DNA damage had a negative influence on pregnancy rates (38). However, few studies have explored the effect of sperm DFI on embryonic development and reproductive outcomes of patients with PCOS. Therefore, the current study investigated the effect of sperm DFI on embryonic development and fetation outcomes.
In this research, higher sperm DFI had no influence on the fertilization rates in patients with PCOS, which is consistent with previous reports that showed that sperm DNA is not involved in the fertilization process, even though spermatozoa with fragmented DNA could fuse with oocytes to begin the process of fertilization (33, 39–42). Before embryonic genes are activated, repair of paternal DNA is attributed to maternal RNA and proteins controlling DNA integrity (43, 44). Recent studies demonstrated that declining oocyte quality is positively correlated with diminished efficiency of DNA repair and negatively correlated with embryo development (23, 45). For patients with PCOS, the present study observed that oocytes fertilized with higher levels of SDF (>15%) resulted in a poor-quality blastocyst rate, which supports the work by Fatehi et al., which showed that oocytes fertilized with damaged sperm DNA exhibited normal 2–3 cleavage stage development but were blocked at the blastocyst stage (46). To a certain extent, oocytes can repair SDF, and good-quality oocytes can repair more damage (47). For example, a prospective observational study suggested that in donor cycles, an elevated sperm DFI had no influence on embryo development because of using the good-quality donated oocytes, which were sufficiently capable of repairing DNA damage (33). We speculated that oocytes from women with PCOS had a reduced ability to repair DNA damage, which led to lower high-quality blastocyst rates. Another study showed that in ICSI cycles in women of the advanced age group, the capacity of oocytes to repair sperm DNA damage was reduced, which led to lower high-quality Day 3 embryo implantation and conception rates and higher abortion rates (23). Conversely, the current findings showed no difference in clinical pregnancy and miscarriage rates for patients with PCOS using sperm with >15% DFI compared with ≤15% DFI. Our results show that elevated SDF had no effect on pregnancy outcomes in patients with PCOS because of the selection of the best embryos for transfer.
PCOS is a complex endocrine disorder, and sperm DNA damage in the current study may not be the only factor that influences embryonic development. Other factors intrinsic to patients with PCOS may have impacted negatively upon embryonic development. Also, in our study, semen was prepared using density gradient centrifugation to select optimal sperm fertilization. Therefore, one reason for no difference in clinical pregnancy and miscarriage rates between patients with PCOS using sperm with a DFI ≤ 15% and > 15% may be the preoptimized sperm. It is urgent to do more research to reveal the underlying mechanism by which increased sperm DNA damage is associated with reduced embryonic development for women with PCOS who undergo fertility treatments.
Although traditional semen analysis is important to evaluate male infertility (48), it does not fully reflect the fertility potential of sperm. Numerous studies have shown that about 15% of patients with normal semen parameters exhibit infertility (48, 49). Therefore, it is necessary to identify diagnostic measures to detect subtle sperm defects, such as chromosomal condensation defects or DNA strand breaks.
The study was limited by the number of patients in the PCOS group because a small sample size was more likely to produce research bias. Otherwise, as a retrospective study, there was no chance to detect the sperm DFI of the treated sperm sample. To further study whether higher sperm DFI can influence IVF outcomes of PCOS women, the sample size should be expanded, and it is better to evaluate the sperm DFI of treated sperm samples to exclude the influence during the pretreatment process in IVF cycles.
In conclusion, in the two groups, there was no difference between fertilization, high-quality embryo rates, and higher DFI (DFI > 15%). However, there was a significant statistical difference between the rate of good-quality blastocysts and higher DFI (26.3% vs. 16.3%, p = 0.023) for patients with PCOS undergoing IVF treatment. Surprisingly, clinical pregnancy and miscarriage rates were independent of SDF, because of the selection of the best embryos or blastocyst for transfer.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YS and QY made contributions to the study conception and design. QY and HW were responsible for the article drafting, data analysis, and interpretation. HW and HL took part in the result discussion. JZ, JX, YJ, and WC participated in data collection. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The study was supported by the National Key R&D Program of China 2019YFA0110900 and Key Program for International Science and Technology Cooperation Projects of China 81820108016 to YS and the National Natural Science Foundation of China 31970800 to QY.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the patients in the study from the Reproductive Medical Center of the First Affiliated Hospital of Zhengzhou University.
References
1. Hart R, Hickey M, Franks S. Definitions, Prevalence and Symptoms of Polycystic Ovaries and Polycystic Ovary Syndrome. Best Pract Res Clin Obstet Gynaecol (2004) 18(5):671–83. doi: 10.1016/j.bpobgyn.2004.05.001
2. Rotterdam EA-SPcwg. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum Reprod (2004) 19(1):41–7. doi: 10.1093/humrep/deh098
3. Escobar-Morreale HF, San Millan JL. Abdominal Adiposity and the Polycystic Ovary Syndrome. Trends Endocrinol Metab (2007) 18(7):266–72. doi: 10.1016/j.tem.2007.07.003
4. Aversa A, La Vignera S, Rago R, Gambineri A, Nappi RE, Calogero AE, et al. Fundamental Concepts and Novel Aspects of Polycystic Ovarian Syndrome: Expert Consensus Resolutions. Front Endocrinol (Lausanne) (2020) 11:516. doi: 10.3389/fendo.2020.00516
5. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions Statement: Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J Clin Endocrinol Metab (2006) 91(11):4237–45. doi: 10.1210/jc.2006-0178
6. Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound Examination of Polycystic Ovaries: Is It Worth Counting the Follicles? Hum Reprod (2003) 18(3):598–603. doi: 10.1093/humrep/deg115
7. Li Y, Wang L, Xu J, Niu W, Shi H, Hu L, et al. Higher Chromosomal Aberration Rate in Miscarried Conceptus From Polycystic Ovary Syndrome Women Undergoing Assisted Reproductive Treatment. Fertil Steril (2019) 111(5):936–43.e2. doi: 10.1016/j.fertnstert.2019.01.026
8. Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A Meta-Analysis of Outcomes of Conventional IVF in Women With Polycystic Ovary Syndrome. Hum Reprod Update (2006) 12(1):13–21. doi: 10.1093/humupd/dmi036
9. Nestler JE, Jakubowicz DJ. Lean Women With Polycystic Ovary Syndrome Respond to Insulin Reduction With Decreases in Ovarian P450c17 Alpha Activity and Serum Androgens. J Clin Endocrinol Metab (1997) 82(12):4075–9. doi: 10.1210/jcem.82.12.4431
10. Grundy SM. Hypertriglyceridemia, Atherogenic Dyslipidemia, and the Metabolic Syndrome. Am J Cardiol (1998) 81(4A):18B–25B. doi: 10.1016/S0002-9149(98)00033-2
11. Grundy SM. Obesity, Metabolic Syndrome, and Coronary Atherosclerosis. Circulation (2002) 105(23):2696–8. doi: 10.1161/01.CIR.0000020650.86137.84
12. Grundy SM. Hypertriglyceridemia, Insulin Resistance, and the Metabolic Syndrome. Am J Cardiol (1999) 83(9B):25F–9F. doi: 10.1016/S0002-9149(99)00211-8
13. Gupta D, Khan S, Islam M, Malik BH, Rutkofsky IH. Myo-Inositol’s Role in Assisted Reproductive Technology: Evidence for Improving the Quality of Oocytes and Embryos in Patients With Polycystic Ovary Syndrome. Cureus (2020) 12(5):e8079. doi: 10.7759/cureus.8079
14. Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN, Chaudhury K. Effect of Follicular Fluid Oxidative Stress on Meiotic Spindle Formation in Infertile Women With Polycystic Ovarian Syndrome. Gynecol Obstetric Invest (2010) 69(3):197–202. doi: 10.1159/000270900
15. Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of Association Between Polycystic Ovary Syndrome and Embryonic Aneuploidy. Fertil Steril (2007) 88(4):900–5. doi: 10.1016/j.fertnstert.2006.12.018
16. Qiao J, Feng HL. Extra- and Intra-Ovarian Factors in Polycystic Ovary Syndrome: Impact on Oocyte Maturation and Embryo Developmental Competence. Hum Reprod Update (2011) 17(1):17–33. doi: 10.1093/humupd/dmq032
17. Sha T, Wang X, Cheng W, Yan Y. A Meta-Analysis of Pregnancy-Related Outcomes and Complications in Women With Polycystic Ovary Syndrome Undergoing IVF. Reprod BioMed Online (2019) 39(2):281–93. doi: 10.1016/j.rbmo.2019.03.203
18. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A Meta-Analysis of Pregnancy Outcomes in Women With Polycystic Ovary Syndrome. Hum Reprod Update (2006) 12(6):673–83. doi: 10.1093/humupd/dml036
19. Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J Mens Health (2020) 38(4):412–71. doi: 10.5534/wjmh.200128
20. Yang H, Li G, Jin H, Guo Y, Sun Y. The Effect of Sperm DNA Fragmentation Index on Assisted Reproductive Technology Outcomes and Its Relationship With Semen Parameters and Lifestyle. Transl Androl Urol (2019) 8(4):356–65. doi: 10.21037/tau.2019.06.22
21. Zini A. Correlations Between Two Markers of Sperm DNA Integrity, DNA Denaturation and DNA Fragmentation, in Fertile and Infertile Men. Fertil Steril (2001) 75(4):674–7. doi: 10.1016/S0015-0282(00)01796-9
22. Lopes S, Sun J-G, Jurisicova A, Meriano J, Casper RF. Sperm Deoxyribonucleic Acid Fragmentation is Increased in Poor-Quality Semen Samples and Correlates With Failed Fertilization in Intracytoplasmic Sperm Injection 1. Fertil Steril (1998) 69(3):528–32. doi: 10.1016/S0015-0282(97)00536-0
23. Setti AS, Braga D, Provenza RR, Iaconelli A Jr., Borges E Jr. Oocyte Ability to Repair Sperm DNA Fragmentation: The Impact of Maternal Age on Intracytoplasmic Sperm Injection Outcomes. Fertil Steril (2021) 116(1):123–9. doi: 10.1016/j.fertnstert.2020.10.045
24. Sigman M. Significance of Sperm DNA Fragmentation and Paternal Age. Fertil Steril (2020) 114(2):262. doi: 10.1016/j.fertnstert.2020.05.011
25. Cissen M, Wely MV, Scholten I, Mansell S, Bruin JP, Mol BW, et al. Measuring Sperm Dna Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLos One (2016) 11(11):e0165125. doi: 10.1371/journal.pone.0165125
26. Ménézo Y, Dale B, Cohen M. DNA Damage and Repair in Human Oocytes and Embryos: A Review. Zygote (2010) 18(4):357–65. doi: 10.1017/S0967199410000286
27. Kollmann M, Martins WP, Lima ML, Craciunas L, Nastri CO, Richardson A, et al. Strategies for Improving Outcome of Assisted Reproduction in Women With Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Ultrasound Obstet Gynecol (2016) 48(6):709–18. doi: 10.1002/uog.15898
28. Ahmadi A, Ng SC. Fertilizing Ability of DNA-Damaged Spermatozoa. J Exp Zool (1999) 284(6):696–704. doi: 10.1002/(SICI)1097-010X(19991101)284:6<696::AID-JEZ11>3.0.CO;2-E
29. Wang F, Dai W, Yang XH, Guo YH, Sun YP. Analyses of Optimal Body Mass Index for Infertile Patients With Either Polycystic or Non-Polycystic Ovary Syndrome During Assisted Reproductive Treatment in China. Sci Rep (2016) 6:34538. doi: 10.1038/srep34538
30. WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Switzerland: WHO Press (2010). p. 223–5.
31. Evenson DP, Larson KL, Jost LK. Sperm Chromatin Structure Assay: Its Clinical Use for Detecting Sperm DNA Fragmentation in Male Infertility and Comparisons With Other Techniques. J Androl (2002) 23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x
32. Bungum M, Bungum L, Giwercman A. Sperm Chromatin Structure Assay (SCSA): A Tool in Diagnosis and Treatment of Infertility. Asian J Androl (2011) 13(1):69–75. doi: 10.1038/aja.2010.73
33. Antonouli S, Papatheodorou A, Panagiotidis Y, Petousis S, Prapas N, Nottola SA, et al. The Impact of Sperm DNA Fragmentation on ICSI Outcome in Cases of Donated Oocytes. Arch Gynecol Obstet (2019) 300(1):207–15. doi: 10.1007/s00404-019-05133-9
34. Fernandez-Diez C, Gonzalez-Rojo S, Lombo M, Herraez MP. Impact of Sperm DNA Damage and Oocyte-Repairing Capacity on Trout Development. Reproduction (2016) 152(1):57–67. doi: 10.1530/REP-16-0077
35. Agarwal A, Cho CL, Esteves SC. Should We Evaluate and Treat Sperm DNA Fragmentation? Curr Opin Obstet Gynecol (2016) 28(3):164–71. doi: 10.1097/GCO.0000000000000271
36. Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA Damage Measured by the Alkaline Comet Assay as an Independent Predictor of Male Infertility and In Vitro Fertilization Success. Fertil Steril (2011) 95(2):652–7. doi: 10.1016/j.fertnstert.2010.08.019
37. Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A Systematic Review and Meta-Analysis to Determine the Effect of Sperm DNA Damage on In Vitro Fertilization and Intracytoplasmic Sperm Injection Outcome. Asian J Androl (2017) 19(1):80–90. doi: 10.4103/1008-682X.182822
38. Siddhartha N, Reddy NS, Pandurangi M, Muthusamy T, Vembu R, Kasinathan K. The Effect of Sperm Dna Fragmentation Index on the Outcome of Intrauterine Insemination and Intracytoplasmic Sperm Injection. J Hum Reprod Sci (2019) 12(3):189–98. doi: 10.4103/jhrs.JHRS_22_19
39. Twigg JP, Irvine DS, Aitken RJ. Oxidative Damage to DNA in Human Spermatozoa Does Not Preclude Pronucleus Formation at Intracytoplasmic Sperm Injection. Hum Reprod (1998) 13(7):1864–71. doi: 10.1093/humrep/13.7.1864
40. Marchetti F, Bishop J, Gingerich J, Wyrobek AJ. Meiotic Interstrand DNA Damage Escapes Paternal Repair and Causes Chromosomal Aberrations in the Zygote by Maternal Misrepair. Sci Rep (2015) 5:7689. doi: 10.1038/srep07689
41. Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal Influence of Sperm DNA Integrity on Early Embryonic Development. Hum Reprod (2014) 29(11):2402–12. doi: 10.1093/humrep/deu228
42. Chen H, Liao SB, Cheung MP, Chow PH, Cheung AL, WS O. Effects of Sperm DNA Damage on the Levels of RAD51 and P53 Proteins in Zygotes and 2-Cell Embryos Sired by Golden Hamsters Without the Major Accessory Sex Glands. Free Radic Biol Med (2012) 53(4):885–92. doi: 10.1016/j.freeradbiomed.2012.06.007
43. Menezo Y Jr., Russo G, Tosti E, El Mouatassim S, Benkhalifa M. Expression Profile of Genes Coding for DNA Repair in Human Oocytes Using Pangenomic Microarrays, With a Special Focus on ROS Linked Decays. J Assist Reprod Genet (2007) 24(11):513–20. doi: 10.1007/s10815-007-9167-0
44. Jaroudi S, Kakourou G, Cawood S, Doshi A, Ranieri DM, Serhal P, et al. Expression Profiling of DNA Repair Genes in Human Oocytes and Blastocysts Using Microarrays. Hum Reprod (2009) 24(10):2649–55. doi: 10.1093/humrep/dep224
45. Horta F, Catt S, Ramachandran P, Vollenhoven B, Temple-Smith P. Female Ageing Affects the DNA Repair Capacity of Oocytes in IVF Using a Controlled Model of Sperm DNA Damage in Mice. Hum Reprod (2020) 35(3):529–44. doi: 10.1093/humrep/dez308
46. Fatehi AN, Bevers MM, Schoevers E, Roelen BA, Colenbrander B, Gadella BM. DNA Damage in Bovine Sperm Does Not Block Fertilization and Early Embryonic Development But Induces Apoptosis After the First Cleavages. J Androl (2006) 27(2):176–88. doi: 10.2164/jandrol.04152
47. Sakkas D, Urner F, Bianchi PG, Bizzaro D, Wagner I, Jaquenoud N, et al. Sperm Chromatin Anomalies Can Influence Decondensation After Intracytoplasmic Sperm Injection. Hum Reprod (1996) 11(4):837–43. doi: 10.1093/oxfordjournals.humrep.a019263
48. Agarwal A, Allamaneni SS. Sperm DNA Damage Assessment: A Test Whose Time has Come. Fertil Steril (2005) 84(4):850–3. doi: 10.1016/j.fertnstert.2005.03.080
Keywords: PCOS, sperm DNA fragmentation index (DFI), in vitro fertilization, embryonic development, pregnancy outcome
Citation: Wang H, Li H, Zhu J, Xu J, Jiang Y, Chen W, Sun Y and Yang Q (2022) The Effect of Sperm DNA Fragmentation on In Vitro Fertilization Outcomes for Women With Polycystic Ovary Syndrome. Front. Endocrinol. 13:822786. doi: 10.3389/fendo.2022.822786
Received: 26 November 2021; Accepted: 19 April 2022;
Published: 27 May 2022.
Edited by:
Giuseppe Ricci, University of Trieste, ItalyCopyright © 2022 Wang, Li, Zhu, Xu, Jiang, Chen, Sun and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingpu Sun, c3lwMjAwOEB2aXAuc2luYS5jb20=; Qingling Yang, cWluZ2xpbmc1MzFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Huan Wang
Huan Wang Hui Li
Hui Li Jing Zhu
Jing Zhu Jianmin Xu
Jianmin Xu Yuqing Jiang
Yuqing Jiang Wenhui Chen
Wenhui Chen Yingpu Sun
Yingpu Sun Qingling Yang
Qingling Yang