- The Department of Health Medicine, Second Medical Center and National Clinical Research Center for Geriatric Diseases, Chinese People’s Liberation Army General Hospital, Beijing, China
Background: Several different criteria for subclinical hypothyroidism (SCH) have been used in the literature, but the performance of these criteria was unknown.
Objective: This retrospective study was to evaluate the diagnostic criteria for SCH.
Methods: Eligible participants were based on centration of thyroglobulin antibodies (TG-Ab), thyroid peroxidase antibodies (TPO-Ab), and five thyroid-related hormones including total thyroxine (TT4), total triiodothyronine (TT3), free thyroxine (FT4), free triiodothyronine (FT3), and thyroid-stimulating hormone (TSH). Euthyroid individuals were identified via specific criteria. Five different SCH diagnostic criteria were compared based on the distributions of those indicators. An appropriate TSH cut-off value was reconsidered.
Results: The study included 145,015 participants. The number of SCH cases diagnosed using criterion 5 was significantly different compared to the cases diagnosed using criteria 1-4 (P<0.05) and had the highest positive proportions of TG-Ab and TPO-Ab. Analysis of 60,515 subjects with normal other thyroid hormones revealed a median TSH concentration of 2.04 mIU/L, and the P2.5–P97.5 CI was 0.48-7.03 mIU/L. When the threshold for TSH elevation was elevated from ≥4.5 mIU/L to ≥6.50 mIU/L, the number of diagnosed SCH cases decreased from 7.30% to 2.09% and the proportions of positive TG-Ab and TPO-Ab increased from 23.69% and 24.07% to 33.75% and 35.06%, respectively (P<0.01).
Conclusions: Combination of an elevated TSH and normal TT3, TT4, FT3, and FT4 concentrations is a must for the diagnosis of SCH. A new TSH threshold should be identified for better patient monitoring and management, according to the real-world characteristics of TSH distribution in Chinese population.
Introduction
Subclinical hypothyroidism (SCH) is highly prevalent worldwide but remains challenging to diagnose. Individuals with SCH often do not have clinical symptoms and rarely seek medical care. However, several recent studies have shown that SCH is associated with coronary heart disease, hypertension, ischemic cerebrovascular disease, metabolic syndrome, osteoporosis, obstetric complications, and other diseases (1–3). Thus, attention has been drawn to identifying SCH, especially during physical examinations. It is generally accepted that SCH is characterized by elevated serum concentrations of thyroid-stimulating hormone (TSH) in the absence of clinical symptoms and thyroid hormone changes. However, due to technological limitations and the cost of screening, no consensus has been reached on how to diagnose SCH. For example, China’s Guidelines for the Diagnosis and Treatment of Adult Hypothyroidism define SCH as an endocrine syndrome associated with elevated concentrations of TSH but normal concentrations of serum total thyroxine (TT4) and serum free thyroxine (FT4) in the absence of obvious signs and symptoms (4). Other studies have defined SCH as a condition with elevated serum concentrations of TSH but normal concentrations of FT4 and free triiodothyronine (FT3) (5, 6). Another study has suggested diagnosing SCH based on elevated TSH concentrations and normal FT4 concentrations (7), while still other studies have recommended diagnosing SCH based on elevated TSH concentrations and normal serum concentrations of TT4 and serum total triiodothyronine (TT3) (8–11). Moreover, there are substantial differences in the reported cut-off values for identifying elevated TSH concentrations to diagnose SCH (12). These discrepancies have generated controversies regarding the diagnosis and clinical significance of SCH, and it is important to determine how to best diagnose SCH based on five to seven thyroid function indicators. This study aimed to compare different criteria for diagnosing SCH based on thyroid function indicators.
Materials and Methods
We evaluated individuals who underwent physical examinations in the Chinese People’s Liberation Army General Hospital between March 2014 and November 2019. Subjects were considered eligible if they were ≥18yrs old and had undergone testing to evaluate thyroid function indicators. However, subjects were excluded based on missing data regarding sex, age, and laboratory findings for at least one of the five thyroid function indicators. In order to find out the real-world distribution characteristics of TSH in population with normal TT3, TT4, FT3, FT4, TSH, TG-Ab, and TPO-Ab, a specific group was identified with strict criteria, which was selected by excluding pregnant women; individuals with definite thyroid-related diseases or a history of thyroid-related surgeries; individuals with a thyroid nodule diameter of ≥1.0 cm; and individuals with abnormal results of thyroid-associated hormones except TSH, and with abnormal thyroglobulin antibodies (TG-Ab) and thyroid peroxidase antibodies (TPO-Ab), or thyroid ultrasonography.
The retrospective study protocol was approved (S2017-003-02) by the Chinese People’s Liberation Army General Hospital ethics committee and complied with the principles of the Declaration of Helsinki and its contemporary amendments.
Data Collection
Data regarding age and sex were collected from the subjects’ medical records. Medical histories were collected via face-to-face interviews. The height, weight, and blood pressure were measured by well-trained nurses and doctors. The body mass index (BMI) was calculated as the weight divided by the height squared. All blood samples were collected after the subjects fasted for 8–12 hr. According to the quality control and testing standards set by the Clinical Laboratory Department of the Clinical Laboratory Department of the Chinese People’s Liberation Army General Hospital (13, 14), Fasting blood glucose, AST, triglyceride (TG), total cholesterol (TC), LDL-C and HDL-C levels were measured using a Roche C8000 automatic biochemical analyzer (Roche, Mannheim, Germany) with the corresponding reagents, calibrators, and quality control materials. The concentrations of TT4, TT3, FT4, FT3, TG-Ab, TPO-Ab, and TSH were determined using Roche Diagnostic reagents and the ACS:180 automatic chemiluminescence immunoassay system. The laboratory’s results had an intra-assay difference of <5% and an inter-assay difference of <10%. The normal reference ranges were 66.00–181.00 nmol/L for TT4, 1.30–3.10 nmol/L for TT3, 3.10–6.80 pmol/L for FT3, 12.00–22.00 pmol/L for FT4, <115.00 IU/mL for TG-Ab, <34.00 IU/mL for TPO-Ab, and 0.10–4.50 mIU/L for TSH.
Thyroid ultrasonography was performed by trained operators who were not aware of the laboratory test results. Thyroid size and morphology were evaluated using a high-resolution 7–13 MHz linear transducer, with the subject seated and their neck slightly extended.
Age Grouping
We divided the subjects into three age-based groups: young subjects (<40yrs), middle-aged subjects (40–59yrs), and elderly subjects (≥60yrs). For some analyses, we created the following age stratification: <20yrs, 20–29yrs, 30–39yrs, 40–49yrs, 50–59yrs, 60–69yrs, 70–79yrs, and ≥80yrs.
Statistical Analysis
Data were collected and analyzed using Stata software (version 11.0). Continuous data were reported as mean ± SD and categorical data were reported as number (%). The laboratory data for the thyroid-associated hormones were used to calculate the median value and confidence intervals (CIs) spanning the 5th to 95th percentiles (P5.0–P95 CIs) or the 2.5th to 97.5th percentiles (P2.5–P97.5 CIs). The variables were analyzed using the Kruskal-Wallis test, Wilcoxon rank-sum test, or chi-squared (χ2) test, as appropriate. Differences were considered statistically significant at P-values of <0.05.
Results
Demographic Characteristics
Between August 2013 and January 2018, a total of 150,035 subjects underwent physical examinations at the Chinese People’s Liberation Army General Hospital and were considered eligible. However, 5,020 subjects were excluded because they fulfilled the exclusion criteria. Therefore, the study ultimately evaluated 145,015 participants, including 90,011 male subjects (62.07%) and 55,004 female subjects (37.93%) with a mean age of 47.96 ± 9.72 yrs. The subjects were from 34 province-level administrative regions, which consisted of provinces, autonomous regions, directly controlled municipalities, and special administrative regions.
Distributions of the Serological Thyroid Function Indicators
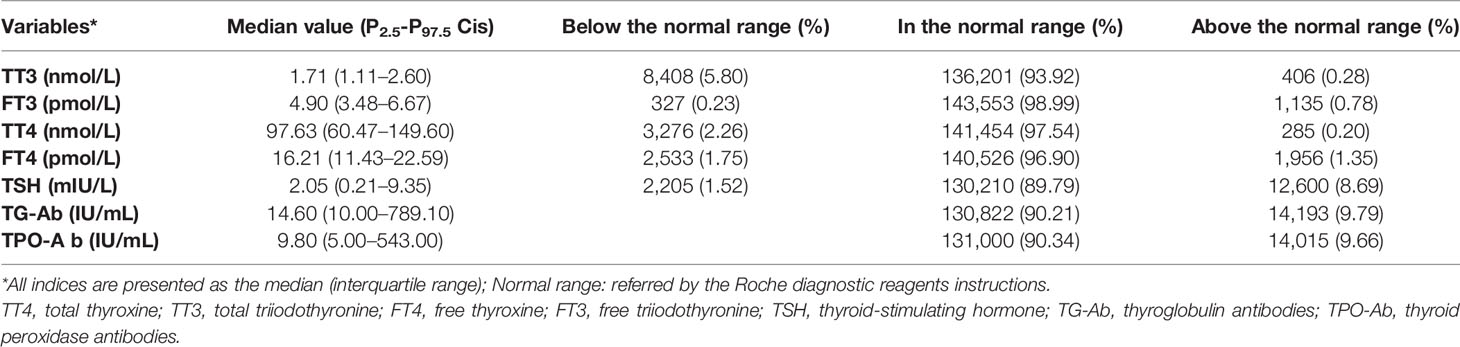
Normality tests indicated non-normal distributions for the concentrations of TT3, TT4, FT3, FT4, TSH, TG-Ab, and TPO-Ab. The median values and proportions of subjects with abnormal concentrations (relative to the reference ranges) were shown in Table 1. The highest abnormal rate was observed for TSH (10.21%), which was followed by TT3 (6.08%), FT4 (3.10%), TT4 (2.46%), and FT3 (1.01%). The median TSH concentration was 2.05 mIU/L, the P5.0–P95.0 CI was 0.78–5.42 mIU/L, and the P2.5–P97.5 CI was 0.21–9.35 mIU/L. TSH results above the normal reference value (>4.5 mIU/L) were observed for 8.69% of the subjects. There was no significant difference in the proportions of subjects who were TG-Ab-positive and TPO-Ab positive (χ2 = 0.61, P = 0.43).
Diagnosing SCH According to the Different Diagnostic Criteria
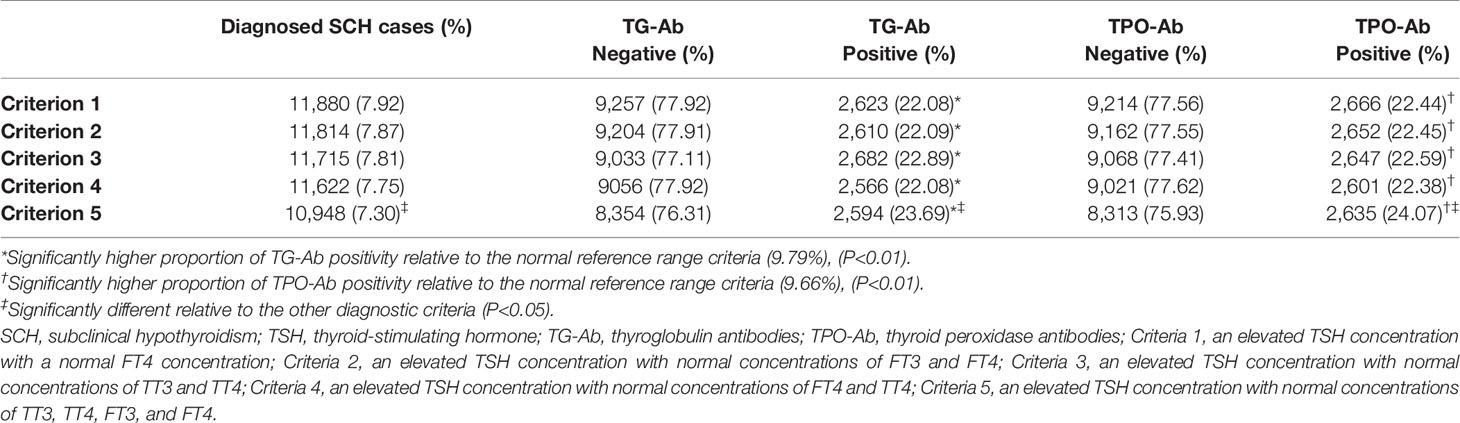
When an elevated TSH concentration was defined as ≥4.50 mIU/L, we compared the diagnosis of SCH based on five different criteria: (1) an elevated TSH concentration with a normal FT4 concentration, (2) an elevated TSH concentration with normal concentrations of FT3 and FT4, (3) an elevated TSH concentration with normal concentrations of TT3 and TT4, (4) an elevated TSH concentration with normal concentrations of FT4 and TT4, and (5) an elevated TSH concentration with normal concentrations of TT3, TT4, FT3, and FT4. The results of the SCH diagnoses using these criteria are summarized in Table 2. There were no significant differences in the numbers of SCH cases diagnosed using criteria 1–4 (P > 0.05), although a significantly different number of SCH cases was diagnosed using criterion 5 (vs. criteria 1–4, P < 0.05).
Relative to criterion 5, criterion 1 identified an additional 932 SCH cases, which included 35 cases with decreased FT3 concentrations, 31 cases with increased FT3 concentrations, 256 cases with decreased TT4 concentrations, 2 cases with increased TT4 concentrations, 778 cases with decreased TT3 concentrations, and 5 cases with increased TT3 concentrations. Relative to criterion 5, criterion 2 identified an additional 866 SCH cases, which included 747 cases with decreased TT3 concentrations, 3 cases with increased TT3 concentrations, 253 cases with decreased TT4 concentrations, and 2 cases with increased TT4 concentrations. Relative to criterion 5, criterion 3 identified an additional 767 SCH cases, which included 7 cases with decreased FT3 concentrations, 32 cases with increased FT3 concentrations, 690 cases with decreased FT4 concentrations, and 44 cases with increased FT4 concentrations. Relative to criterion 5, criterion 4 identified an additional 674 SCH cases, which included 33 cases with decreased FT3 concentrations, 30 cases with increased FT3 concentrations, 638 cases with decreased TT3 concentrations, and 3 cases with increased TT3 concentrations. Thus, using criteria 1–4 resulted in varying numbers of misdiagnosed SCH cases, relative to criterion 5 (i.e., normal TT3, TT4, FT3, and FT4 concentrations with elevated TSH concentrations). Regardless of the diagnostic criterion that was used, significantly higher proportions of TG-Ab positive and TPO-Ab positive individuals were observed among subjects who were diagnosed with SCH with criterion 5 (P < 0.01). Moreover, both of the positive proportions of TG-Ab and TPO-Ab in criteria 5 were significantly higher than those in criteria 1–4 (P < 0.001).
Identifying an Appropriate TSH Cut-Off Value
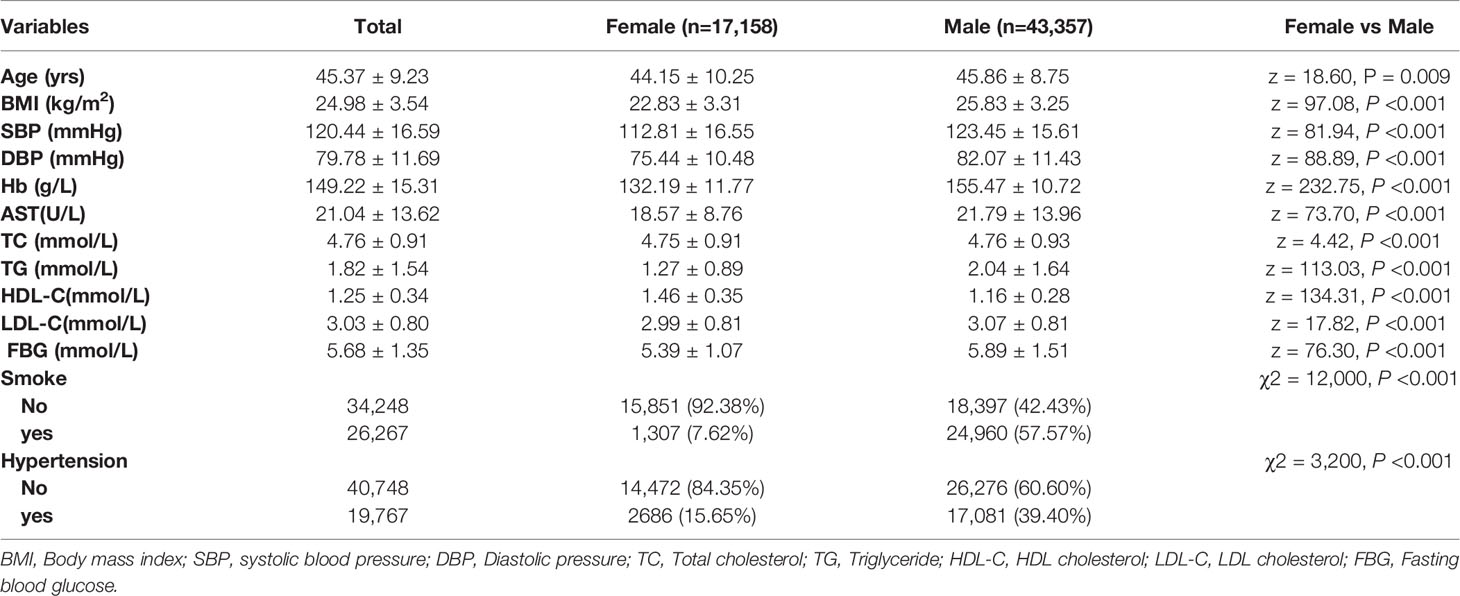
The strict criteria were fulfilled in 60,515 individuals, forming the specific group. Demographic characteristics of the individuals in this group were shown in Table 3. Those individuals showed normal concentrations of TT3, TT4, FT3, FT4, TG-Ab, TPO-Ab, and met other criteria, which included 43,357 males (71.65%) and 17,158 females (28.23%). The median TSH concentration was 2.04 mIU/L, the P5.0–P95.0 CI was 0.84–4.79 mIU/L, and the P2.5–P97.5 CI was 0.48-7.03 mIU/L in the special group. Thus, at least 5% of the 60,515 subjects had TSH concentrations that were ≥4.79 mIU/L. The Wilcoxon rank-sum test revealed a significant difference in TSH concentrations between male and female subjects (Z = 28.44, P < 0.001) (Table 4).

Table 4 Distributions of TSH concentrations in the different stratification in the specific group (n=60,515).
The TSH concentration distributions for each age group were shown in Table 4. The Kruskal-Wallis test revealed significant differences in the TSH concentrations across the different age stratification (χ2 = 62.42, P < 0.001). Furthermore, there were significant differences when the subjects were grouped as young subjects, middle-aged subjects, and elderly subjects (χ2 = 15.56, P < 0.001). The Wilcoxon rank-sum test also revealed significant differences between young and middle-aged subjects (Z=1.99, P=0.046), between young and elderly subjects (Z = 4.69, P < 0.001), and between middle-aged and elderly subjects (Z = 6.82, P < 0.001).
The P95th upper limits for TSH concentration were >4.50 mIU/L in all age groups, increased according to age, and reached 12.26 mIU/L for subjects who were ≥80yrs old. The P2.5–P97.5 CI values were 0.57–6.49 mIU/L for young subjects, 0.45–7.09 mIU/L for middle-aged subjects, and 0.43–8.96 mIU/L for elderly subjects. Thus, at least 2.5% of the 60,515 subjects with normal thyroid function and no thyroid-related diseases had TSH concentrations of ≥6.49 mIU/L.
There is general consensus that SCH patients with TSH concentrations of <10.00 mIU/L do not require clinical intervention (4, 15, 16), and that overdiagnosis could cause unnecessary psychological and economic burden. But the SCH patients with TSH concentrations of <10.00 mIU/L could develop clinical thyroid diseases every year, which means they could not be ignored and need monitoring. Therefore, although sex and age stratification were significant, we defined the TSH threshold for diagnosing SCH as 6.50 mIU/L as a clear, easily remembered, and practical cut-off value, according to the real TSH distribution in Chinese population, especially based on the Roche platform.
Re-Diagnosis According to the New TSH Cut-Off Value
The new cut-off value for elevated TSH concentrations was applied to diagnostic criteria 1–5 in order to re-diagnose SCH and the results were summarized in Table 5. There were still no statistically significant differences in the number of SCH cases diagnosed using criteria 1–4 (P > 0.05), although a significantly different number of SCH cases was diagnosed using criterion 5 (vs. criteria 1–4, P < 0.05). Similarly, both of the positive proportions of TG-Ab and TPO-Ab in criteria 5 were significantly higher than those in criteria 1–4 (P < 0.01). Furthermore, the number of SCH based on criterion 5 and the new TSH threshold (≥6.50 mIU/L) was significantly less than that a diagnosis was based on a frequently-used TSH threshold of ≥4.50 mIU/L (2.09% vs. 7.30%; χ2 = 4,600, P < 0.001). Moreover, relative to a diagnosis using a TSH threshold of ≥ 4.50 mIU/L, SCH cases diagnosed using criteria 1–5 and the new threshold (≥ 6.50 mIU/L) had significantly higher proportions of TG-Ab-positive and TPO-Ab-positive cases (P < 0.01).
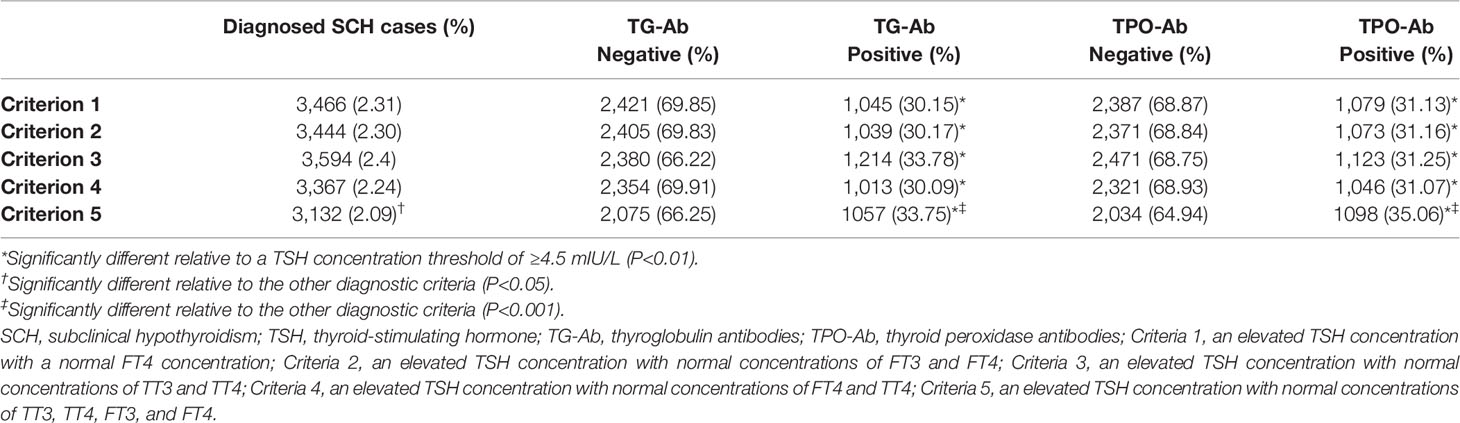
Table 5 Diagnosis of SCH based on the diagnostic criteria and TSH concentration threshold of ≥6.5 mIU/L.
Discussion
There is significant controversy regarding the diagnosis and management of SCH (17, 18). Although an increasing number of studies have demonstrated that SCH has adverse effects on various physical functions, there are inconsistent diagnostic criteria for SCH. This has generated confusion in and unnecessary burden on the medical community (19). Our study compared five commonly used criteria for diagnosing SCH and revealed that criteria 1–4 were basically equivalent in terms of the number of diagnosed SCH cases, in which included some cases that obviously did not meet the definition of SCH. However, a significantly different number of cases was diagnosed using criterion 5 and the positive proportions of TG-Ab and TPO-Ab were significantly higher than those in criteria 1–4 (P < 0.001). Our results indicate that criterion 5 was stricter and avoided misdiagnosis of SCH. Therefore, we recommend using criterion 5 in the clinical setting based on the Roche platform, despite it being associated with an increased cost of SCH screening.
Clinical laboratories typically use the reference ranges that are proposed by test manufacturers. However, those ranges are generally based on data from non-Chinese populations and there has been no comprehensive assessment of the five thyroid-associated hormones and their relationships with thyroid disease in a large cohort of healthy Chinese individuals (11). Our study revealed that at least 5% of the Chinese population with completely normal TT3, TT4, FT3, FT4, TG-Ab, TPO-Ab and no thyroid-related diseases would be expected to have TSH concentrations of ≥4.50 mIU/L (the upper limit of the normal reference range). Moreover, both the upper 95.0th and 97.5th percentile values for TSH concentration increased with age and reached 12.26 mIU/L among individuals who were ≥80 yrs. As the purpose of a clinical diagnosis is to guide careful monitoring or intervention for the patient, a diagnosis that does not prompt additional steps has limited clinical significance and may increase the economic and psychological burden on the patient. Most recent studies indicate that drug intervention is not necessary for patients with SCH until they have TSH concentrations of ≥10.00 mIU/L. Therefore, we set the TSH threshold to ≥6.50 mIU/L. In Korea, also in Asia, park WR, et al. (20) found that when SCH was more predictive of overt hypothyroidism, the cut off value was TSH > 7.45 µIU/ml and higher prevalence positive anti-thyroid peroxidase (anti-TPO Ab) and anti-thyroglobulin antibody (anti-Tg Ab). But their study enrolled only 197 patients.
Interestingly, a diagnosis of SCH based on criterion 5 and the new TSH threshold (≥6.50 mIU/L) was made in a significantly smaller proportion of the study population, relative to when the diagnosis was based on a threshold of ≥4.50 mIU/L. However, the higher TSH threshold identified SCH in a smaller proportion of subjects, these cases had significantly higher proportions of TG-Ab and TPO-Ab positivity, relative to cases that were identified using the lower threshold. Previous studies have also shown that the incidences of clinical hypothyroidism and SCH are significantly higher when subjects have high titers of TPO-Ab (20) or test positive for both TG-Ab and TPO-Ab (21). Therefore, given that individuals who were diagnosed with SCH using the new TSH threshold (≥6.50 mIU/L) are more likely to clinically progress, they are more suitable for clinical monitoring or intervention. In Huber’s study (22), it was considered that TSH≥6 was more predictive in patients with SCH. In another neighboring Asian country, a cohort study in Japan found that TSH level >8 mIU/L was a predictive value for development of overt hypothyroidism (23). While a study in South Korea, which is also located in East Asia, found that the TSH threshold of 6.86 is more suitable for the diagnosis of SCH (24). TSH could vary in different countries or ethnic groups (25, 26). Therefore, our results at TSH were slightly different from those of other studies.
One limitation of our study is that it was a single-center study of Chinese adults. Thus, the results cannot be generalized to other racial and ethnic groups. In addition, TSH secretions are theoretically sensitive to very small changes in serum FT4, which means that TSH changes could occur during early-stage hypothyroidism even before FT4 abnormalities are detectable (27). However, in practice, TSH concentrations are influenced by numerous factors, including age, race, sex (28), acute illness, renal insufficiency, medications (29), pregnancy (30), depression (31), and anorexia nervosa (32). Meanwhile, between-assay differences and variations in reference ranges can directly impact the diagnosis and management of subclinical hypothyroidism (33). Therefore, changes in the TSH concentrations for our study may not have always been directly related to changes in the concentrations of TT3, TT4, FT3, or FT4, and may have even exhibited inverse relationships. However, our study also had several strengths. First, we used broad inclusion criteria, without excluding patients with unrelated diseases or medications, which presumably allowed us to capture both healthy people and people with subclinical thyroid disease. Therefore, our study sample is likely representative. Our study also used an ultra-sensitive TSH measurement technique to minimize the risk of indirect changes in TSH concentrations.
In conclusion, our study revealed that the diagnosis of SCH can be made based on elevated TSH concentrations in the presence of normal TT3, TT4, FT3, and FT4 concentrations (criterion 5). Other commonly used simplified criteria (criteria 1–4) were associated with an increased risk of misdiagnosis, based on the Roche platform. We found that increasing the TSH threshold from ≥4.50 mIU/L to ≥6.50 mIU/L might improve the accuracy of SCH diagnosis and thus possibly guide better patient monitoring and management, which means a new TSH threshold should be identified according to the real TSH distribution characteristics in Chinese population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Chinese People’s Liberation Army General Hospital ethics committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QZ designed this study. Y-sZ wrote the original drafts. S-yD reviewed and edited the manuscript. YG acquired and analyzed the data. J-hW and FW wrote the review and prepared the tables. Y-sZ and S-yD contributed equally as co-first authors. All authors read and approved the final manuscript. We agree to the terms of the BioMed Central Copyright and License Agreement.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sgarbi JA, Matsumura LK, Kasamatsu TS, Ferreira SR, Maciel RMB. Subclinical Thyroid Dysfunctions Are Independent Risk Factors for Mortality in a 7.5-Year Follow-Up: The Japanese-Brazilian Thyroid Study. Eur J Endocrinol (2010) 162(3):569–77. doi: 10.1530/EJE-09-0845
2. Selmer C, Olesen JB, Hansen ML, Kappelgaard LM, Madsen JC, Hansen PR, et al. Subclinical and Overt Thyroid Dysfunction and Risk of All-Cause Mortality and Cardiovascular Events: A Large Population Study. J Clin Endocrinol Metab (2014) 99(7):2372–82. doi: 10.1210/jc.2013-4184
3. Shi HS, Kan ZQ, Liu YF, Li WW, Peng M, Yang TT. Efficacy and Safety of Thyroxine Therapy on Patients With Heart Failure and Subclinical Hypothyroidism: A Protocol for Systematic Review and Meta-Analysis. Med (Baltimore) (2021) 100(3):e23947. doi: 10.1097/MD.0000000000023947
4. Endocrinology Branch of the Chinese Medical Association. Guidelines for Diagnosis and Treatment of Hypothyroidism in Adults. Chin J Endocrinol Metab (2017) 33(2):167–80. doi: 10.3760/cma.j.issn.1000-6699.2017.02.018
5. Kim JS, Zhang YY, Chang YS, Ryu S, Guallar E, Shin YC, et al. Subclinical Hypothyroidism and Incident Depression in Young and Middle-Age Adults. J Clin Endocrinol Metab (2018) 103(5):1827–33. doi: 10.1210/jc.2017-01247
6. Mario FD, Pofi R, Gigante A, Rivoli L, Rosato E, Isidori AM, et al. Hypothyroidism and Nephrotic Syndrome: Why, When and How to Treat. Curr Vasc Pharmacol (2017) 15(5):398–403. doi: 10.2174/1570161115999170207114706
7. Redford C, Vaidya B. Subclinical Hypothyroidism: Should We Treat? Post Reprod Health (2017) 23(2):55–62. doi: 10.1177/2053369117705058
8. Tekle HA, Bobe TM, Tufa EG, Solomon FB. Age-Sex Disparities and Sub-Clinical Hypothyroidism Among Patients in Tikur Anbesa Specialized Hospital, Addis Ababa, Ethiopia. J Health Popul Nutr (2018) 37(1):18. doi: 10.1186/s41043-018-0149-x
9. Nie XM, Chen Y, Chen YC, Chen C, Han B, Li Q, et al. Lead and Cadmium Exposure, Higher Thyroid Antibodies and Thyroid Dysfunction in Chinese Women. Environ Pollut (2017) 230:320–8. doi: 10.1016/j.envpol.2017.06.052
10. Ghadhban BR, Abid FN. The Prevalence and Correlation Between Subclinical Hypothyroidism and Gall Stone Disease in Baghdad Teaching Hospital. Ann Med Surg (Lond) (2018) 37:7–10. doi: 10.1016/j.amsu.2018.11.017
11. Zou YT, Wang DC, Cheng XQ, Ma CC, Lin SB, Hu YY, et al. Reference Intervals for Thyroid-Associated Hormones and the Prevalence of Thyroid Diseases in the Chinese Population. Ann Lab Med (2021) 41(1):77–85. doi: 10.3343/alm.2021.41.1.77
12. Biondi B, Cappola AR, Cooper DS. Subclinical Hypothyroidism: A Review. JAMA (2019) 322(2):153–60. doi: 10.1001/jama.2019.9052
13. Zheng YS, Zhang LP, Zeng Q, Han CJ. Preliminary Analysis on Food Intolerances of 88,436 Healthy People to 14 Kinds of Foods. Br Food J (2019) 121(5):1010–9. doi: 10.1108/BFJ-07-2018-0482
14. Gong Y, Zeng Q, Yan Y, Han CJ, Zheng YS. Association Between Lifestyle and Gastroesophageal Reflux Disease Questionnaire Scores: A Cross-Sectional Study of 37442 Chinese Adults. Gastroenterol Res Pract (2019) 2019:5753813. doi: 10.1155/2019/5753813.
15. Morgan DJ, Dhruva SS, Coon ER, Wright SM, Korenstein D. 2019 Update on Medical Overuse: A Review. JAMA Intern Med (2019) 179(11):1568–74. doi: 10.1001/jamainternmed.2019.3842
16. Burgos N, Toloza FJK, Ospina NMS, Brito JP, Salloum RG, Hassett LC, et al. Clinical Outcomes After Discontinuation of Thyroid Hormone Replacement: A Systematic Review and Meta-Analysis. Thyroid (2020) 31(5):740–51. doi: 10.1089/thy.2020.0679
17. Feller M, Snel M, Moutzouri E, Bauer DC, Montmollin M, Aujesky D, et al. Association of Thyroid Hormone Therapy With Quality of Life and Thyroid-Related Symptoms in Patients With Subclinical Hypothyroidism: A Systematic Review and Meta-Analysis. JAMA (2018) 320(13):1349–59. doi: 10.1001/jama.2018.13770
18. Lamine F, Giorgi SD, Marino L, Michalaki M, Sykiotis GP. Subclinical Hypothyroidism: New Trials, Old Caveats. Hormones (Athens) (2018) 17(2):231–6. doi: 10.1007/s42000-018-0004-x
19. Bekkering GE, Agoritsas T, Lytvyn L, Heen AF, Feller M, Moutzouri E, et al. Thyroid Hormones Treatment for Subclinical Hypothyroidism: A Clinical Practice Guideline. BMJ (2019) 365:l2006. doi: 10.1136/bmj.l2006
20. Park WR, Oh TK, Jeon HJ. Prospective Observation of 5-Year Clinical Course of Subclinical Hypothyroidism in Korean Population. J Korean Med Sci 2013-11-01 (2013) 28(11):1622–6. doi: 10.3346/jkms.2013.28.11.1622
21. Shin DY, Kim EK, Lee EJ. Role of Ultrasonography in Outcome Prediction in Subclinical Hypothyroid Patients Treated With Levothyroxine. Endocr J (2010) 57:15–22. doi: 10.1507/endocrj.k09e-154
22. Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, et al. Prospective Study of the Spontaneous Course of Subclinical Hypothyroidism: Prognostic Value of Thyrotropin, Thyroid Reserve, and Thyroid Antibodies. J Clin Endocrinol Metab (2002) 87(7):3221–6. doi: 10.1210/jcem.87.7.8678
23. Imaizumi M, Sera N, Ueki I, Horie I, Ando T, Usa T, et al. Risk for Progression to Overt Hypothyroidism in an Elderly Japanese Population With Subclinical Hypothyroidism. Thyroid (2011) 21(11):1177–82. doi: 10.1089/thy.2010.0411
24. Kim WG, Kim WB, Woo G, Kim H, Cho Y, Kim TY, et al. Thyroid Stimulating Hormone Reference Range and Prevalence of Thyroid Dysfunction in the Korean Population: Korea National Health and Nutrition Examination Survey 2013 to 2015. Endocrinol Metab (Seoul) (2017) 32(1):106–14. doi: 10.3803/EnM.2017.32.1.106
25. Pérez-Campos Mayoral L, Hernández-Huerta MT, Mayoral-Andrade G, Pérez-Campos Mayoral E, Zenteno E, Martínez-Cruz R, et al. TSH Levels in Subclinical Hypothyroidism in the 97.5th Percentile of the Population. Int J Endocrinol (2020) 2020:2698627. doi: 10.1155/2020/2698627
26. Yoo WS, Chung HK. Subclinical Hypothyroidism: Prevalence, Health Impact, and Treatment Landscape. Endocrinol Metab (Seoul) (2021) 36(3):500–13. doi: 10.3803/EnM.2021.1066
27. Li YS, Teng D, Shan ZY, Teng XC, Guan HX, Yu XH, et al. Antithyroperoxidase and Antithyroglobulin Antibodies in a Five-Year Follow-Up Survey of Populations With Different Iodine Intakes. J Clin Endocrinol Metab (2008) 93(5):1751–7. doi: 10.1210/jc.2007-2368
28. Boucai L, Hollowell JG, Surks MI. An Approach for Development of Age-, Gender-, and Ethnicity-Specific Thyrotropin Reference Limits. Thyroid (2011) 21(1):5–11. doi: 10.1089/thy.2010.0092
29. Völzke H, Schmidt CO, John U, Wallaschofski H, Dörr M, Nauck M. Reference Levels for Serum Thyroid Function Tests of Diagnostic and Prognostic Significance. Horm Metab Res (2010) 42(11):809–14. doi: 10.1055/s-0030-1263121
30. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid (2011) 21(10):1081–125. doi: 10.1089/thy.2011.0087
31. Joffe RT, Sullivan TB. The Significance of an Isolated Elevated TSH Level in a Depressed Patient: A Clinical Commentary. Int J Psychiatry Med (2014) 48(3):167–73. doi: 10.2190/PM.48.3.b
32. Lawson EA, Klibanski A. Endocrine Abnormalities in Anorexia Nervosa. Nat Clin Pract Endocrinol Metab (2008) 4(7):407–14. doi: 10.1038/ncpendmet0872
Keywords: thyroid-associated hormones, hypothyroidism, subclinical hypothyroidism, diagnosis, criteria
Citation: Zheng Y-s, Dong S-y, Gong Y, Wang J-h, Wang F and Zeng Q (2022) Comparison of Five Different Criteria for Diagnosis of Subclinical Hypothyroidism in a Large-Scale Chinese Population. Front. Endocrinol. 13:820414. doi: 10.3389/fendo.2022.820414
Received: 23 November 2021; Accepted: 20 January 2022;
Published: 15 February 2022.
Edited by:
Daniel Elías-López, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoReviewed by:
Nayab Rizvi, University of the Punjab, PakistanCreswell John Eastman, Westmead Hospital, Australia
Copyright © 2022 Zheng, Dong, Gong, Wang, Wang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zeng, WnEzMDFAMTI2LmNvbQ==
†ORCID: Yan-song Zheng, orcid.org/0000-0002-3491-7672
Sheng-yong Dong, orcid.org/0000-0002-8395-4269
Yan Gong, orcid.org/0000-0002-2945-3228
Jia-hong Wang, orcid.org/0000-0002-0752-8241
Fei Wang, orcid.org/0000-0001-9776-4792
Qiang Zeng, orcid.org/0000-0002-4961-0171
‡These authors have contributed equally to this work and share first authorship
 Yan-song Zheng
Yan-song Zheng Sheng-yong Dong
Sheng-yong Dong Yan Gong
Yan Gong Jia-hong Wang
Jia-hong Wang Fei Wang
Fei Wang Qiang Zeng
Qiang Zeng