- 1Department of Pediatrics and Pediatric Endocrinology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
- 2Student Scientific Society, Department of Biophysics, Jagiellonian University Medical College, Kraków, Poland
- 3Department of Biochemistry, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Background: As Turner syndrome (TS) predisposes to obesity and metabolic disorders, and their complications, such as cardiovascular diseases, are the main causes of shortened life expectancy in patients with TS, new metabolic markers that could serve as early predictors of dysmetabolic state are sought.
Objective: Assessment of MMP-1 (matrix metalloproteinase-1), MMP-2 (matrix metalloproteinase-2), MMP-9 (matrix metallopeptidase-9), BDNF (brain-derived neurotrophic factor), GDNF (glial cell line-derived neurotrophic factor), and VEGF (vascular endothelial growth factor) before the onset of growth hormone (GH) therapy and then during GH treatment as well as markers assessment during GH medication in girls with TS to establish marker stability and repeatability, and the impact of GH on markers concentration.
Method: The concentrations of circulating MMP-1, MMP-2, MMP-9, BDNF, GDNF, and VEGF were measured in nine girls with TS before the onset of GH therapy and then after at least 3 months of treatment period. Subsequently, markers concentration was determined in 17 girls during GH medication, with the first determination after at least a 3-month treatment period. The patients’ clinical and biochemical phenotypes were determined by weight, height, BMI, total cholesterol, HDL cholesterol, triglycerides, and glucose concentration.
Results: Comparison of markers concentration revealed a significantly higher concentration of MMP-2 in patients undergoing GH treatment (132.1 ± 42.05) than before the onset of therapy (105.0 ± 45.5, p=0.045). The values of the first measurement of VEGF in girls with TS undergoing GH therapy were significantly higher than those during the second measurement (30.9 ± 33.4 vs. 12.5 ± 11.7, p=0.029). There were no statistically significant differences between the measurements of the remaining markers concentration at any stage of the analysis.
Conclusion: Increase in MMP-2 concentration is visible during GH therapy in comparison to the pre-GH period in girls with TS which demands confirmation in subsequent tests. The role of VEGF requires further studies in the context of carbohydrate-lipid disturbances in girls with TS and its association with GH treatment.
Introduction
Obesity is a major risk factor for many other metabolic disorders, and can also lead to the development of cardiovascular diseases and, consequently, to increased mortality (1). Metabolic syndrome (MetS) components occur more frequently in Turner syndrome (TS), taking into account the period of childhood (2). Moreover, heart defects are more common in TS than in the general population (3). All these factors lead to a shorter life expectancy in patients with TS (4). It is still uncertain whether higher cardiometabolic risks in TS are more a consequence of intrinsic factors or the result of modifiable metabolic risk factors connected with lifestyle. Therefore, the search for new markers as potential early predictors of the natural development of a dysmetabolic state seems to be reasonable. A better understanding of what affects markers expressions can help find more detailed targets of potential actions in the future.
Therefore, our work focuses on the assessment of concentrations of brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factors (GDNF), vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs) as potential early markers of dysmetabolic state. Our previous study was performed on 17 patients with TS undergoing growth hormone (GH) therapy (5). Subsequently, we re-examined the markers concentration before the onset of GH therapy to exclude its potential influence (6).
BDNF, a member of the nerve growth factor family, is also involved in weight regulation and energy expenditure by reducing appetite (7). The concentration of BDNF correlates negatively with body weight (8) and age (9); therefore, it is believed to be an anti-aging factor (6). BDNF affects glucose and lipid metabolism and is also considered an anorexigenic factor (10). Moreover, significantly lower plasma BDNF concentrations were observed in patients with MetS. It also appears that physical activity may alter the concentration of BDNF (11). These findings support the metabotropic deficit hypothesis (12, 13). Most likely, some BDNF genotypes resulting from BDNF gene polymorphism can be recognized as a potential factor in the development of obesity and insulin resistance (14, 15). Our previous studies revealed higher BDNF levels in girls with TS, both before the onset of GH therapy (5) as well as during treatment (6) compared to the control group. Interestingly, the assessment of BDNF concentration in adult TS patients by other authors confirms its higher concentration in patients with this syndrome (16).
GDNF is responsible for survival of neurons, protecting them from damage (17), and plays a neuroprotective role for catecholaminergic and sympathetic neurons, taking part in the regulation of intake and energy expenditure (18). Transgenic mice with overexpression of GDNF in glial cells are protected from obesity, glucose intolerance, insulin resistance, and hepatic steatosis caused by high-fat meals (19). Administration of GDNF can result in weight loss in humans (20). Mentioned reports suggest GDNF has a protective role against the development of major components of the metabolic syndrome. Like BDNF, GDNF signs in the suggested metabotropic hypothesis of metabolic syndrome.
VEGF is involved in embryogenesis, wound healing, tumor metastasis, rheumatoid arthritis, and formation of new blood vessels from pre-existing vessels (21), and appears to have a metabolic effect. Its concentration is lower in obese people, whose blood vessel development is insufficient. This results in hypoxia of adipocytes and local inflammation (22). Hypoxia leads to glucose intolerance (23), while ongoing low-grade inflammation causes insulin insensitivity (24), which is associated with metabolic syndrome. Transgenic mice with VEGF overexpression are protected against the development of obesity, as well as glucose intolerance and insulin resistance induced by diet, resulting from local hypoxia (25). However, data regarding VEGF levels and metabolic processes are inconclusive. One study revealed a positive correlation between the concentrations of circulating VEGF levels and BMI (26), and another study reported increased levels of VEGF in overweight and obese people (27), which contradicts the above-mentioned reports.
MMPs are a group of enzymes that degrade extracellular matrix components with activity influenced by growth factors, ongoing inflammation, or inhibitors of metalloproteinases (28). MMPs play an important role in angiogenesis and wound healing (29) and participate in the modulation of adipogenesis (30). MMP-1 seems to be involved in a process facilitating the development of adipose tissue, leading to obesity (31). Plasma concentrations of MMP-2 and MMP-9 are increased in patients with metabolic syndrome (32, 33). Moreover, MMP-2 and MMP-9 levels were elevated in patients with isolated systolic hypertension as compared to the control group (34, 35). In our previous study, MMP-1 was significantly higher in the study group, with a significant positive correlation with Z-score BMI (5), while studies before GH treatment revealed lower concentrations of MMP-2 in girls with TS compared to healthy girls with short stature (6).
Here, in the context of higher cardiometabolic risk in TS, we aimed to analyse the influence of GH therapy on discussed above markers and to monitor their stability and repeatability during GH therapy.
Patients and Methods
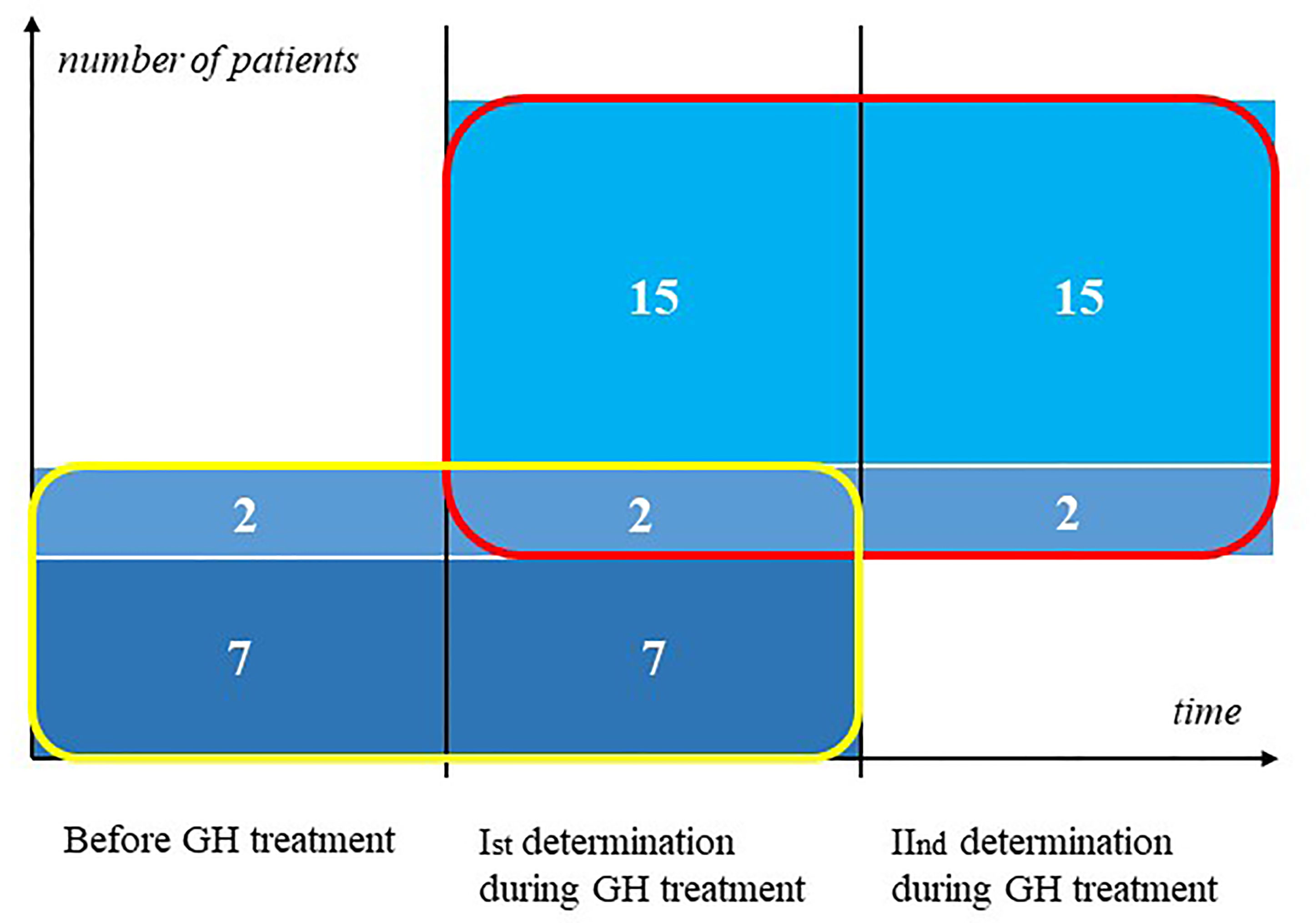
First, we determined markers concentration in nine girls with TS before the onset of GH therapy and then after at least a 3-month treatment period, before the end of the first year of therapy. Subsequently, we evaluated markers concentration in 17 girls during GH medication, the first measurement was performed after at least 3 months of treatment. The second measurement was performed after at least 3 months from the previous measurement (2 patients were assessed between 6 months to 1 year from the previous visit, 10 patients – between 1 to 2 years, and 5 patients had second determination after 2 years from first determination). 2 patients are included in both groups, they have a triple determination of markers. The other patients do not overlap (Figure 1). The diagnosis of Turner syndrome was confirmed by karyotyping with routine G-banding according to the recommendations of the American College of Medical Genetics.
Clinical Phenotype of Study Participants
The detailed anthropometric analysis was based on weight and height measurements, along with body mass index (BMI) calculation, using the standard formula of weight (kg) divided by height (m) squared. Weight was measured with a Seca scale with a precision of 100 g and height with a Harpenden stadiometer with a graduation of 0.1 cm. A BMI above the 97th percentile was classified as obese, while a BMI between the 90th and 97th percentiles as overweight based on the BMI chart for healthy girls (36). Based on the age, sex, BMI, and appropriate reference standard, the BMI Z-score was calculated using the international (International Obesity Task Force; IOTF) body mass index (BMI) cut-offs (37). The Tanner scale was used to assess puberty (38). Arterial pressure was determined in measurements with a sphygmomanometer or 24-hour monitoring of arterial pressure was performed and hypertension was diagnosed according to Blood Pressure Values Park’s Pediatric Cardiology for Practitioners (https://doctorlib.info/cardiology/).
Biochemical Phenotype of Study Participants
Morning fasting venous blood samples were collected to measure MMP-1, MMP-2, MMP-9, BDNF, GDNF, and VEGF. The concentrations of these markers were determined by sandwich ELISA using kits distributed by R&D systems.
Concentrations of total cholesterol (TCh), HDL cholesterol (HDL-chol), and triglycerides (TG) were analyzed enzymatically (Beckman Coulter, Brea, CA). An enzymatic test (hexokinase method) was used for the quantitative determination of glucose (Beckman Coulter). Insulin levels were determined using a chemiluminescence immunoassay using an IMMULITE 2000 analyzer. An oral glucose load test of 1.75 g/kg was performed (dose of 75 g was maximal), with the determination of glucose and insulin levels at two time points: 0’ and 120’. Fasting insulin-to-glucose ratio indices of the IR - the quotient of insulin concentration to fasting glucose >0.3 was considered as insulin resistance (39).
To assess the concentration of TG, HDL-chol, and fasting glucose in children aged 3–11 years, the results were categorized according to the IDEFICS study (40): HDL-chol <= 10th percentile or triglycerydes >= 90th percentile, fasting glucose >= 90th percentile. Hypertension was evaluated according to Blood Pressure Values Park’s Pediatric Cardiology for Practitioners and obesity was recognized while BMI was above 97 centile according to BMI charts for healthy girls (36).
For older children, IDF cut-off points were used (41): triglycerides >= 150 mg/dL, HDL- chol <40 mg/dl, glucose >=100 mg/dl; for recognition of hypertension and obesity we applied criteria as mentioned above.
MetS was recognized if a critical value was exceeded for three or more of these risk factor.
For technical reasons 2 out of 9 patients had no data on lipids and glucose before GH treatment.
Statistical Analysis
Data processing and statistical analyses were performed using Statistica 13 PL software. Statistical significance was set at P < 0.05. Normality was assessed using the Kolmogorov–Smirnov test. Variables with a normal distribution were analyzed using t-tests and reported as mean ± standard deviation (SD); those without normal distribution were analyzed using the Mann–Whitney U test and reported as median with interquartile range (IQR).
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Silesia (resolution number KNW/0022/KB1/162/15/16)). Informed consent was obtained from each participant aged over 16 years, a parent, or a legal guardian.
Results
Comparison Before and During GH Treatment
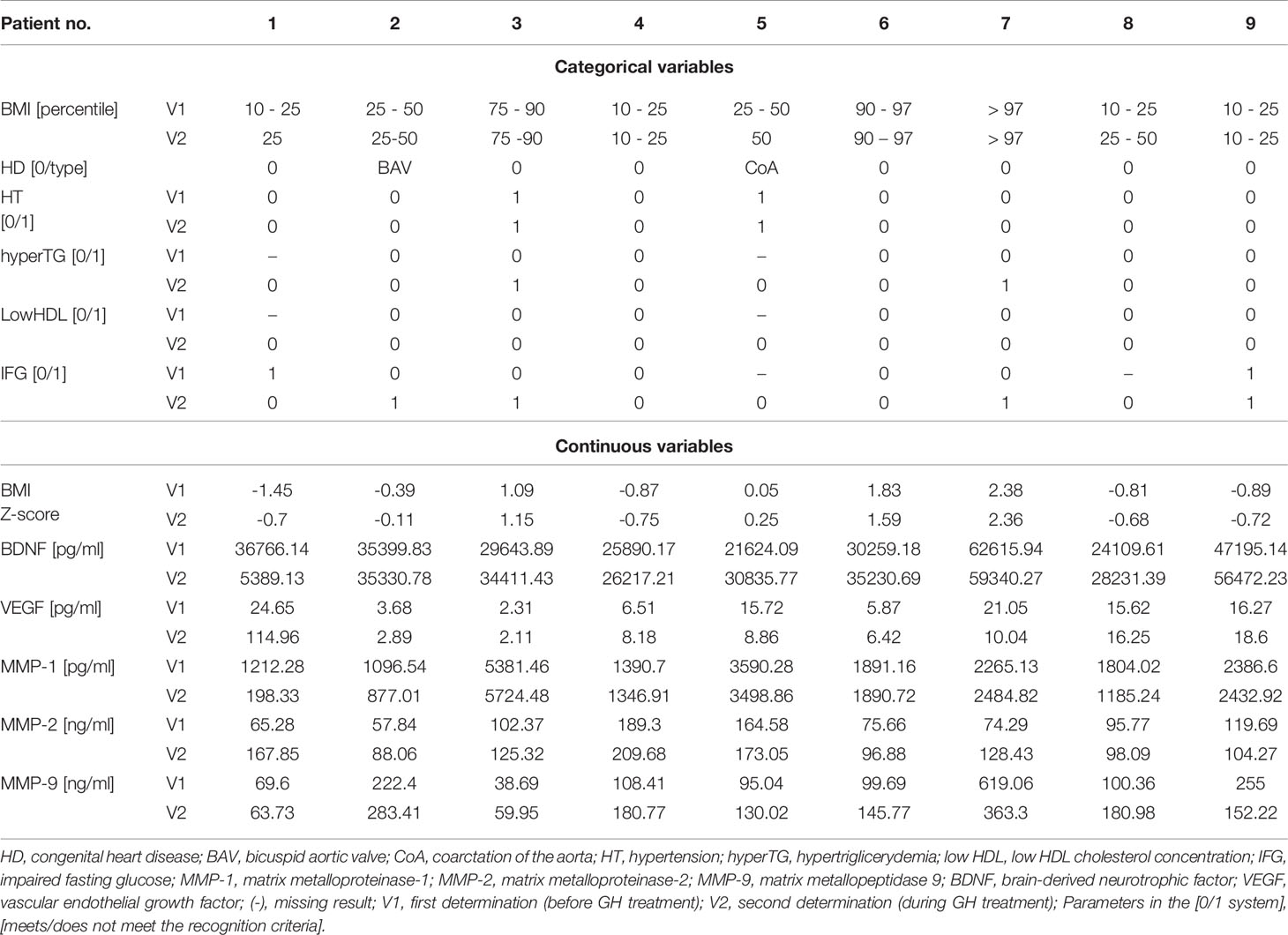
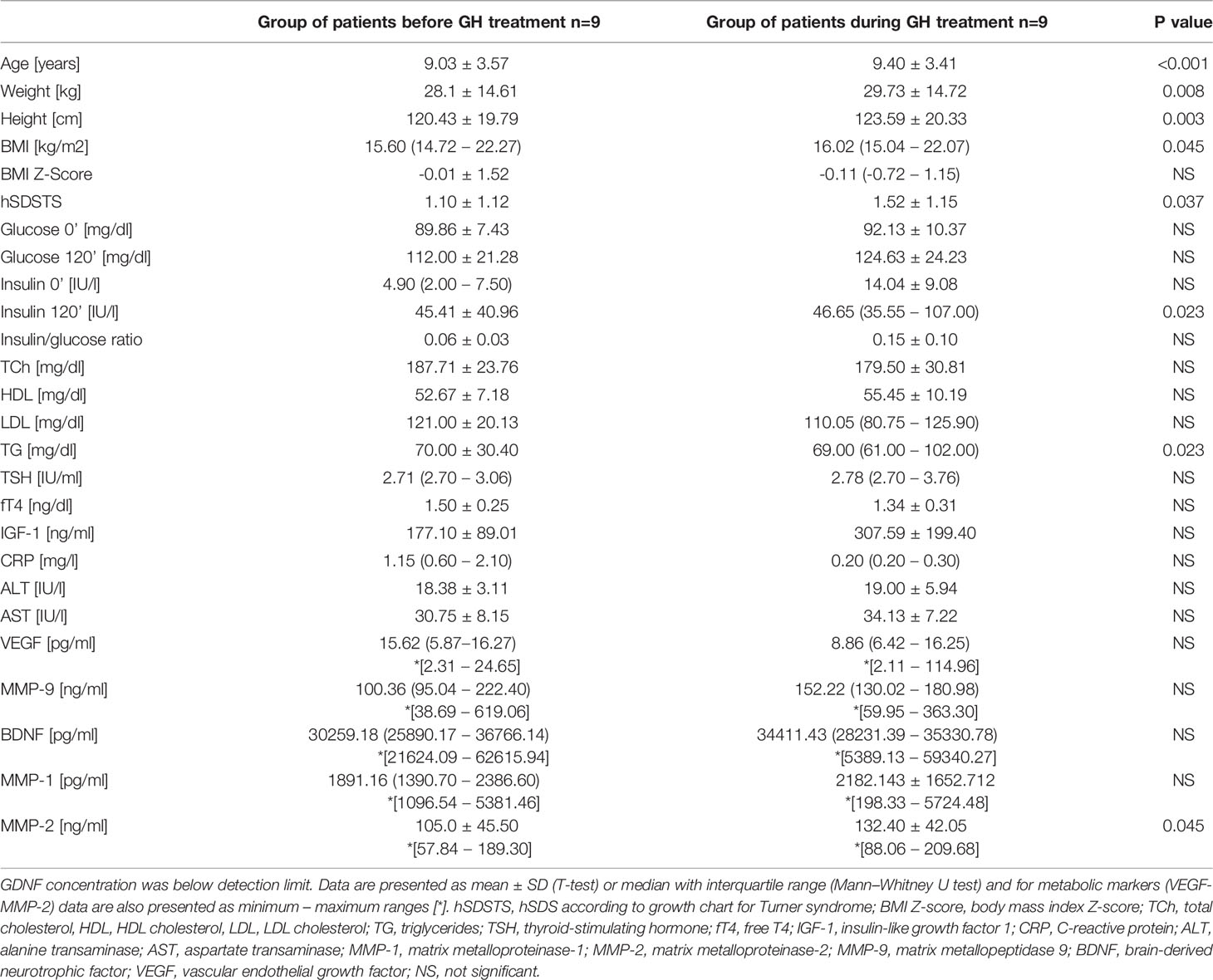
Comparison of the analyzed markers concentration revealed a significantly higher concentration of MMP-2 in patients undergoing GH treatment than before the onset of therapy (132.1 ± 42.05 vs. 105.0 ± 45.5 ng/ml, p=0.045). In almost all patients, the measurement during treatment showed a higher concentration of MMP-2. Two patients who had the highest MMP-2 values before starting treatment also had the highest levels during GH therapy. One of the girls had hypertension (Table 1 – Patients No. 4 and 5).
There were no statistically significant differences in the concentrations of the remaining markers before and during GH treatment. Detailed data are presented in Table 2.
It was noted that two clearly highest concentrations of MMP-1 before GH (5381.46 and 3590.28 pg/ml) belonged to two patients with elevated blood pressure values. They had the highest levels of MMP-1 during GH therapy as well (5724.48 and 3498.86 pg/ml). We observed development of metabolic disorders in one of these patients during GH treatment (Table 1 – Patient no.3).
In turn, the concentration of MMP-9 was clearly higher in one patient, both before GH and during therapy, compared to the rest. She was the only obese girl who qualified for the study, who additionally had impaired fasting blood glucose (IFG) on the second test (Table 1, Patient no.7).
Furthermore, remarkably higher BDNF concentrations were obtained in two patients. These were the same two patients with the highest values, both before and during the inclusion of GH. For the highest pre-GH result, it belonged to the only obese patient enrolled in the study (Table 1, Patient no.7), and the second belonged to girl with IFG (Table 1, Patients no.9). During treatment, the highest result was found in the obese patient who additionally revealed IFG (Table 1, Patient no.7), and the second to the same patient as the previously recognized IFG (Table 1, Patients no.9).
For VEGF concentrations, there were no potential associations with the presence of components of metabolic syndrome.
GDNF concentrations were below the limit of detection in all patients.
The clinical and biochemical characteristics of the participants presenting the frequency of the components of metabolic syndrome are presented in Table 3, all available clinical and biochemical parameters of patients are included in Table 2.
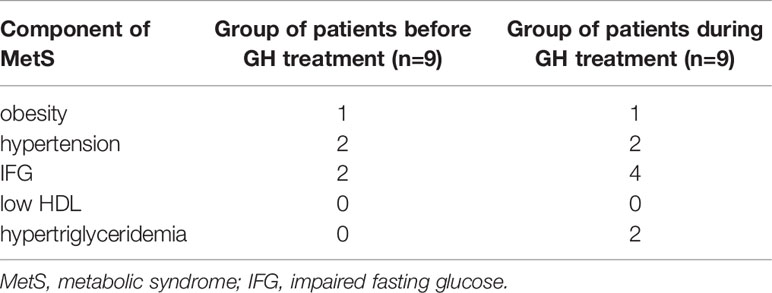
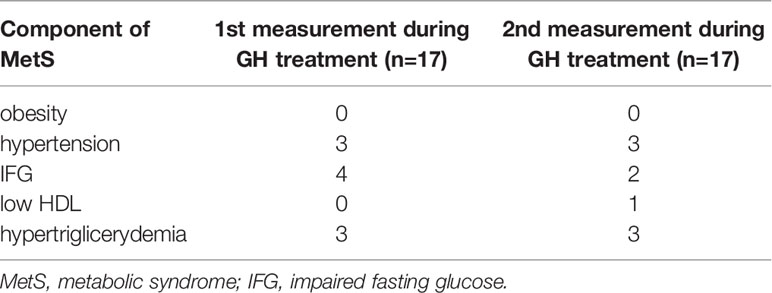
Table 3 Components of MetS in TS patients before and during GH treatment (2 out of 9 patients had no data on lipids and glucose before GH treatment).
Among the biochemical parameters, statistically significant differences were found between first and second determination of the concentration of triglycerides and insulin determined at 120 minute of the glucose load test. No statistically significant differences between the determinations were found between the other parameters. Detailed data is presented in Table 2. All patients had a fasting insulin/glucose ratio of <0.3. The concentration of TCh did not correlate with any of the metabolic markers in any of the analyzed groups.
According to the Tanner scale, two patients before the onset of GH therapy started puberty spontaneously (karyotype: 1st patient - 45,X[17]/46,XX [33] and 2nd patient – 45,X). The 3rd one with 45,X started induced puberty at the time of the second markers determination:Tanner stage = B2, no more advanced puberty characteristics. The onset of puberty was not recorded in any of the remaining participants. Heart defects were observed in two patients: coarctation of the aorta (1 patient) and bicuspid aortic valve (1 patient).
Comparison During GH Treatment
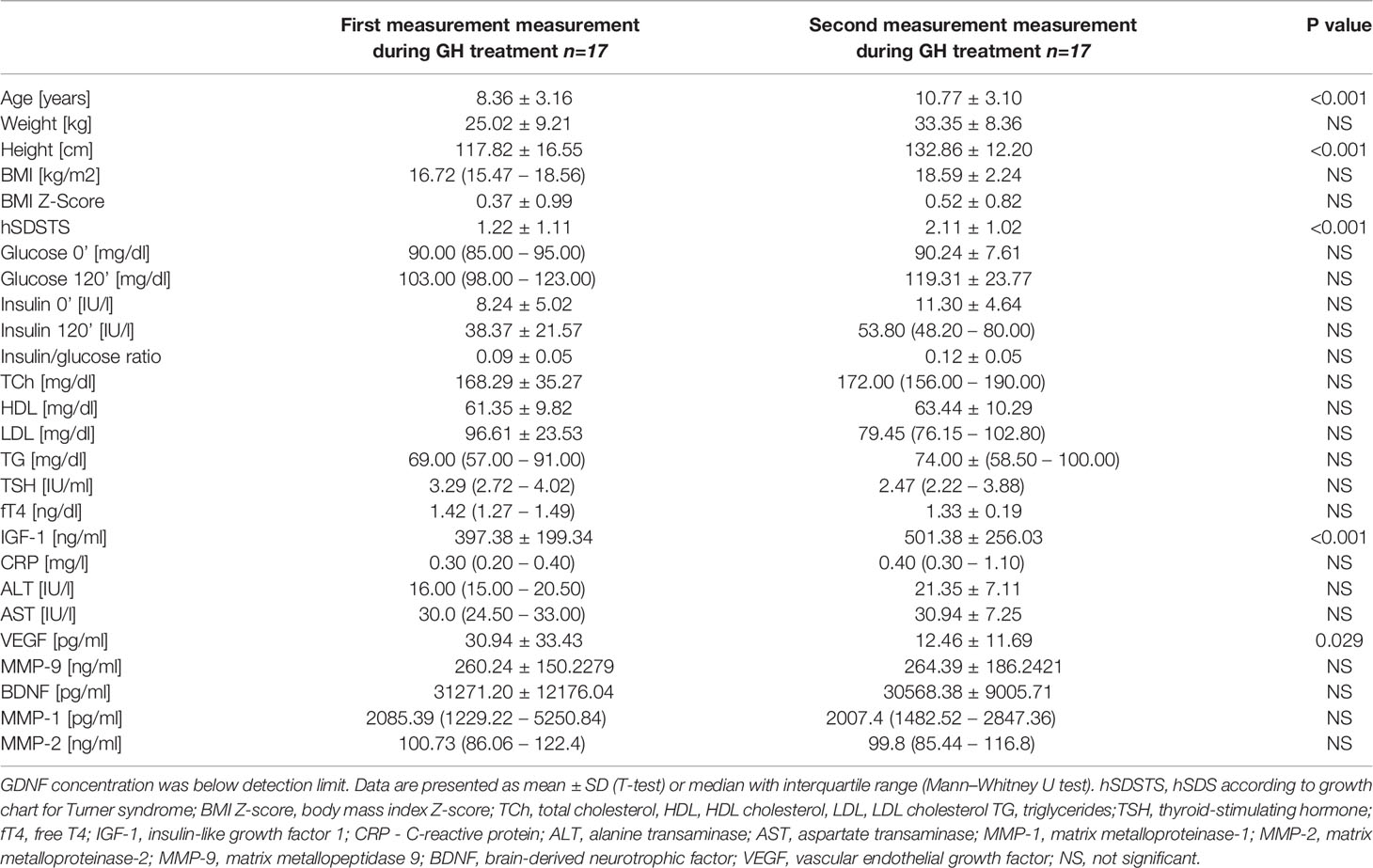
The only statistically significant difference was observed between the first and second VEGF measurements. The values of the first measurement were statistically significantly higher (30.9 ± 33.4 vs. 12.5 ± 11.7 pg/ml, p=0.029). There were no statistically significant differences between the measurements of the remaining markers concentration (Table 4). In the first evaluation (2 patients) and in the second evaluation (3 patients) had VEGF levels were below the detection limit.
In the case of MMP-1, we noted several clearly higher (above 6000.00 pg/ml), repeatable marker concentrations. Girl with highest level in the first and second determination had coexisting hypertension and during second determination she had elevated insulin level in 120 minute of oral glucose load test, however second highest level in the first and second measurement belonged to the girl without any metabolic syndrome components. The third highest measurement in first determination was found in girl with hypertriglicerydemia, however level of MMP-1 was not so high in second determination.
For the remaining markers, it was not noticed that the extreme values were related to the more frequent occurrence of metabolic disorders.
GDNF concentrations were below the limit of detection in all patients.
The clinical and biochemical characteristics of the participants presenting the frequency of the components of metabolic syndrome are presented in Table 5. All available clinical and biochemical parameters of patients are included in Table 4. Statistically significant differences were found in IGF-1 concentrations between the measurements, with an increase during the second measurement (p<0.001). All patients had a fasting insulin/glucose ratio of <0.3. The concentration of TCh did not correlate with any of the metabolic markers in any of the analyzed groups.
According to the Tanner scale, five patients at first (one - induced maturation with karyotype mos 45,X[85]/46,Xi(X)q10[15], four – spontaneous maturation with types of karyotype - 46,X, i(X)(q10); mos45,X[34]/46,X,r(X)(p22?q)?[16]; and 45,X in two patients) and eight study patients at the second markers determination started puberty (Tanner stage ≥ B2: additionally: one – induced maturation with karyotype 45,X and two spontaneous maturation - mos45,X[7]/46,XX[57] and mos 45,X[7]/46,XX[35]). Heart defects were observed in six patients: coarctation of the aorta (3 patients) and bicuspid aortic valve (3 patients).
Discussion
We observed that during GH treatment compared to the state before GH treatment, MMP-2 concentration was significantly higher, and we found that the highest values were reproducible in the same patients. It is known that MMP activity is considerably influenced by many secreted growth factors, as it could be an effect of GH (42–44).
Rendeva et al. conducted a study evaluating the concentrations of MMP-2, MMP-9, and VEGF in adults diagnosed with growth deficiency. However, in the case of MMP-2, the study was opposite to ours, as a reduction in the concentration of MMP-2 was observed under the influence of GH (45). Additionally, Rendeva et al. reported that MMP-2 concentration at baseline (before GH therapy) in the study group was higher than the concentration observed in the healthy control group (45), and lower levels of CRP were found in the control group. It is known that inflammation can induce the activation of MMPs (42, 43), as it could be one of the reasons for the high concentration in their study group. According to some reports, the concentration of MMPs increases with age (46), so it is difficult to clearly compare our results with those of Rendeva et al. Unfortunately, we do not have any reports from studies on MMP-2 levels in patients with TS, other than our two previous studies (5) (6), which suggested lower MMP-2 plasma concentration in girls with TS before GH treatment than in healthy girls with short stature.
It is known that MMP-2 plasma concentration can be increased in patients with isolated systolic hypertension (34, 35) and in patients with metabolic syndrome (32, 33). In our study group before GH medication, we had only two patients with hypertension, and none of the patients met the criteria for metabolic syndrome; hence, it is difficult to associate the concentration of this marker with the mentioned parameters.
Regarding the observed possible association of MMP-1 with hypertension among our results, it is worth noting that according to the literature, its expression is increased in human vascular smooth muscle cells exposed to angiotensin II, as has been described in cases of hypertension (47). However, it is not only one connection with metabolic syndrome components, as MMP-1 serum levels are associated with atherosclerotic total plaque burden (46), and its production can be intensified in macrophages during dysglycemia (48) or elevated in plasma in case of hypertriglyceridemia as well as in CRP increase (49). The network of connections identified to date was significant.
In turn, MMP-9 increased production in macprophages and smooth muscle cells was observed during dysglycemia (48) or dyslipidemia (46), and its plasma concentration is elevated in cases of hypertension (50) or obesity (51). It is worth recalling that in our study in the only obese patient before GH treatment, the concentration of MMP-9 was significantly higher than that in the remaining patients (3.5 times higher than the average in the untreated group and almost 2 times higher among GH-treated girls). In the second measurement, we additionally found an IFG in that girl. Perhaps such a high concentration is related to obesity or is an early indicator of carbohydrate disturbances.
All the described connections of MMPs with metabolic disorders suggest that they may serve as biomarkers and potential therapeutic targets for certain vascular disorders.
With respect to VEGF, we found a difference in the concentration of VEGF during the determinations under GH treatment. So far, we have not found any differences in our previous studies: there were no differences between the non-GH patients and healthy short stature girls (6), nor were there any differences in the pilot study where GH patients and healthy short stature girls serving as the control group were enrolled (5). Literature reports on the role of VEGF in TS concern the relationship of its overexpression with fetal hydrops and abnormal endocardial cushion development, and therefore, congenital heart defects. The authors also suggested secondary fibrosis of several organ systems as a consequence of excessive VEGF production (52). As mentioned, the role of VEGF is being studied in the context of carbohydrate-lipid disturbances. Despite the large number of reports on this theme, they are often indispensable or even contradictory. However, it seems that VEGF plays a role in the carbohydrate-lipid area, often intertwined in action from MMPs, enhancing their production (43, 53). As this factor also takes part in many processes, such as angiogenesis, embryogenesis, wound healing, inflammation, tumor metastasis, cardiovascular diseases, or rheumatoid arthritis (21), and its concentration can be influenced by significant familial correlations of its plasma concentration between genetically related individuals (54), it is difficult to confidently point out the reason for the difference between its concentration in girls during GH therapy. However, we want to note there are reports of a decline in VEGF associated with growth hormone treatment (45).
All the markers discussed so far have multiple functions that are intertwined in many areas. They all can have local as well as global effects on the body, are synthesized, among others, in smooth muscle cells or endothelium, and differences in their concentrations can be observed in plasma. It is known that GH, among its other actions, also exerts an effect on the endothelium (55, 56). Taking into account the beneficial effect of GH on endothelial dysfunction, the same effect on cardiovascular disorders (57), its use in TS seems to be particularly beneficial, where research confirms impaired function of the endothelium (56). Therefore, all observed changes during GH therapy should correspond to hypothetically favorable changes in markers concentration.
It remains to discuss the last group of markers analyzed in this study, that is, neurotrophic factors, of which BDNF seems to be more researched. We found no differences in BDNF concentrations during this study. However, in our two previous studies, we observed higher plasma levels of this factor in girls with TS than in healthy girls with short stature (5, 6). This result was also confirmed by Czyzyk et al. (16). Therefore, what leads to higher BDNF concentrations in TS? BDNF seems to be relevant to TS and, according to Farooqui et al., the gene encoding BDNF plays an important regulatory role in TS (58). Moreover, the relationship between BDNF and metabolic disorders cannot be ignored, taking into consideration the assumptions of the hypothesis of metabotropic deficit mentioned in the introduction (12, 13).
In turn, the GDNF concentration was below the limit of detection. The literature suggests that the plasma concentration of GDNF is also included in the metabotropic deficit hypothesis and does not necessarily reflect tissue synthesis (19).
It is difficult to say whether the occurrence of maturation features influenced the values of the determined markers due to the small size of the studied groups. It was not unequivocally found that the highest or the lowest values were found in patients with features of puberty.
We are aware of the limitations of our study as it was performed on a small number of patients. However, it is difficult and time-consuming to gather a sufficient number of patients with a rare syndrome. Despite this limitation, the study remains unique in the context of the analyzed group and selected metabolic markers.
Conclusion
Understanding the mechanisms of the impact of these markers on the development of metabolic syndrome components is an interesting area of research that seems to be even more important in TS, burdened with more frequent occurrence of metabolic syndrome and cardiovascular diseases. Additionally, the markers discussed here may serve as potential therapeutic targets for metabolic and cardiovascular disorders.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the Medical University of Silesia (resolution number KNW/0022/KB1/162/15/16). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
EB and AG designed the study, prepared the database, and wrote the manuscript. JGi monitored the patients and collected the samples for biochemical analysis. JGa analyzed the patient database and wrote the manuscript. MK-F and GH performed the laboratory analyses. TF collaborated in designing the work and performed the laboratory analyses. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all the patients and their families for participating in this study. We would like to thank Editage (www.editage.com) for English language editing.
References
1. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The Metabolic Syndrome. Endocr Rev (2008) 29:777–822. doi: 10.1210/er.2008-0024
2. Ostberg JE, Thomas EL, Hamilton G, Attar MJH, Bell JD, Conway GS. Excess Visceral and Hepatic Adipose Tissue in Turner Syndrome Determined by Magnetic Resonance Imaging: Estrogen Deficiency Associated With Hepatic Adipose Content. J Clin Endocrinol Metab (2005) 90:2631–5. doi: 10.1210/jc.2004-1939
3. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical Practice Guidelines for the Care of Girls and Women With Turner Syndrome: Proceedings From the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol (2017) 177:G1–G70. doi: 10.1530/EJE-17-0430
4. Sybert VP, McCauley E. Turner’s Syndrome. N Engl J Med (2004) 351:1227–38. doi: 10.1056/NEJMra030360
5. Błaszczyk E, Lorek M, Francuz T, Gieburowska J, Gawlik A. Selected Metabolic Markers in Girls With Turner Syndrome: A Pilot Study. Int J Endocrinol (2018) 2018:1–6. doi: 10.1155/2018/9715790
6. Błaszczyk E, Gawlik J, Gieburowska J, Tokarska A, Kimsa-Furdzik M, Hibner G, et al. Brain-Derived Neurotropic Factor, Vascular Endothelial Growth Factor and Matrix Metalloproteinases as Markers of Metabolic Status in Non-Growth Hormone-Treated Girls With Turner Syndrome. Front Endocrinol (Lausanne) (2021) 0:722199. doi: 10.3389/FENDO.2021.722199
7. Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-Induced Weight Loss. Exp Neurol (1995) 131:229–38. doi: 10.1016/0014-4886(95)90045-4
8. Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The Impact of Age, Weight and Gender on BDNF Levels in Human Platelets and Plasma. Neurobiol Aging (2005) 26:115–23. doi: 10.1016/j.neurobiolaging.2004.03.002
9. Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung H-D, Anders D, et al. Serum Neurotrophins–a Study on the Time Course and Influencing Factors in a Large Old Age Sample. Neurobiol Aging (2007) 28:1436–45. doi: 10.1016/j.neurobiolaging.2006.06.011
10. Chaldakov GN, Tonchev AB, Aloe L. NGF and BDNF: From Nerves to Adipose Tissue, From Neurokines to Metabokines. Riv Psichiatr (2009) 44(2):79–87.
11. Damirchi A, Tehrani BS, Alamdari KA, Babaei P. Influence of Aerobic Training and Detraining on Serum BDNF, Insulin Resistance, and Metabolic Risk Factors in Middle-Aged Men Diagnosed With Metabolic Syndrome. Clin J Sport Med (2014) 24:513–8. doi: 10.1097/JSM.0000000000000082
12. Hristova M, Aloe L, Rosmond R, Björntorp P, Blalock E, Besedovsky H, et al. Metabolic Syndrome – Neurotrophic Hypothesis. Med Hypotheses (2006) 66:545–9. doi: 10.1016/j.mehy.2005.08.055
13. Chaldakov GN, Fiore M, Hristova MG, Aloe L. Metabotrophic Potential of Neurotrophins: Implication in Obesity and Related Diseases? Med Sci Monit Int Med J Exp Clin Res (2003) 9:HY19–21.
14. Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-Wide Association Yields New Sequence Variants at Seven Loci That Associate With Measures of Obesity. Nat Genet (2009) 41:18–24. doi: 10.1038/ng.274
15. Bonaccorso S, Sodhi M, Li J, Bobo WV, Chen Y, Tumuklu M, et al. The Brain-Derived Neurotrophic Factor (BDNF) Val66Met Polymorphism Is Associated With Increased Body Mass Index and Insulin Resistance Measures in Bipolar Disorder and Schizophrenia. Bipolar Disord (2015) 17:528–35. doi: 10.1111/bdi.12294
16. Czyzyk A, Casarosa E, Luisi M, Podfigurna-Stopa A, Meczekalski B, Genazzani AR. Brain-Derived Neurotrophic Factor Plasma Levels in Patients With Turner Syndrome. Gynecol Endocrinol (2014) 30:245–9. doi: 10.3109/09513590.2013.871513
17. Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, et al. Mesencephalic Dopaminergic Neurons Protected by GDNF From Axotomy-Induced Degeneration in the Adult Brain. Nature (1995) 373:339–41. doi: 10.1038/373339a0
18. Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneo J. Absolute Requirement of GDNF for Adult Catecholaminergic Neuron Survival. Nat Neurosci (2008) 11:755–61. doi: 10.1038/nn.2136
19. Mwangi SM, Nezami BG, Obukwelu B, Anitha M, Marri S, Fu P, et al. Glial Cell Line-Derived Neurotrophic Factor Protects Against High-Fat Diet-Induced Obesity. Am J Physiol Gastrointest Liver Physiol (2014) 306:G515–25. doi: 10.1152/ajpgi.00364.2013
20. Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, et al. Randomized, Double-Blind Trial of Glial Cell Line-Derived Neurotrophic Factor (GDNF) in PD. Neurology (2003) 60:69–73. doi: 10.1212/WNL.60.1.69
21. Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of Vascular Endothelial Growth Factors. FEBS Lett (2006) 580:2879–87. doi: 10.1016/j.febslet.2006.03.087
22. Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, et al. Reduced Adipose Tissue Oxygenation in Human Obesity: Evidence for Rarefaction, Macrophage Chemotaxis, and Inflammation Without an Angiogenic Response. Diabetes (2009) 58:718–25. doi: 10.2337/db08-1098
23. Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, et al. Hypoxia Causes Glucose Intolerance in Humans. Am J Respir Crit Care Med (2004) 169(11):1231–7. doi: 10.1164/rccm200308-1200OC
24. Trayhurn P, Wood IS. Adipokines: Inflammation and the Pleiotropic Role of White Adipose Tissue. Br J Nutr (2004) 92:347. doi: 10.1079/BJN20041213
25. Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, et al. Adipose Tissue Overexpression of Vascular Endothelial Growth Factor Protects Against Diet-Induced Obesity and Insulin Resistance. Diabetes (2012) 61:1801. doi: 10.2337/db11-0832
26. Loebig M, Klement J, Schmoller A, Betz S, Heuck N, Schweiger U, et al. Evidence for a Relationship Between VEGF and BMI Independent of Insulin Sensitivity by Glucose Clamp Procedure in a Homogenous Group Healthy Young Men. PloS One (2010) 5:e12610. doi: 10.1371/journal.pone.0012610
27. Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic Factors are Elevated in Overweight and Obese Individuals. Int J Obes (2005) 29:1308–14. doi: 10.1038/sj.ijo.0802987
28. Nagase H, Visse R, Murphy G, Newby AC, Spinale FG, Shah PK, et al. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc Res (2006) 69:562–73. doi: 10.1016/j.cardiores.2005.12.002
29. Vu TH, Werb Z. Matrix Metalloproteinases: Effectors of Development and Normal Physiology. Genes Dev (2000) 14:2123–33. doi: 10.1101/gad.815400
30. Lijnen HR, Hedley A, Ogden C, Johnson C, Carroll M, Curtin L, et al. Angiogenesis and Obesity. Cardiovasc Res (2008) 78:286–93. doi: 10.1093/cvr/cvm007
31. Samuvel DJ, Jin J, Sundararaj KP, Li Y, Zhang X, Lopes-Virella MF, et al. TLR4 Activation and IL-6-Mediated Cross Talk Between Adipocytes and Mononuclear Cells Synergistically Stimulate MMP-1 Expression. Endocrinology (2011) 152:4662–71. doi: 10.1210/en.2011-1026
32. Hopps E, Lo Presti R, Montana M, Noto D, Averna MR, Caimi G. Gelatinases and Their Tissue Inhibitors in a Group of Subjects With Metabolic Syndrome. J Investig Med (2013) 61:978–83. doi: 10.231/JIM.0b013e318294e9da
33. Erman H, Gelisgen R, Cengiz M, Tabak O, Erdenen F, Uzun H. The Association of Vascular Endothelial Growth Factor, Metalloproteinases and Their Tissue Inhibitors With Cardiovascular Risk Factors in the Metabolic Syndrome That MMP-2 May Have a Role in the Increased Cardiovascular Risk of MetS Patients. Eur Rev Med Pharmacol Sci (2016) 20(6):1015–22.
34. Yasmin, Wallace S, McEniery CM, Dakham Z, Pusalkar P, Maki-Petaja K, et al. Matrix Metalloproteinase-9 (MMP-9), MMP-2, and Serum Elastase Activity Are Associated With Systolic Hypertension and Arterial Stiffness. Arterioscler Thromb Vasc Biol (2004) 25:372–8. doi: 10.1161/01.ATV.0000151373.33830.41
35. Belo VA, Luizon MR, Carneiro PC, Gomes VA, Lacchini R, Lanna CMM, et al. Effect of Metabolic Syndrome Risk Factors and MMP-2 Genetic Variations on Circulating MMP-2 Levels in Childhood Obesity. Mol Biol Rep (2013) 40:2697–704. doi: 10.1007/s11033-012-2356-7
36. Palczewska I. Siatki Centylowe do Oceny Rozwoju Somatycznego Dzieic I Młodzieży. Warszawa: Polish Institute of Mother’s and Child’s Health (1999). NZ.
37. Cole TJ, Lobstein T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr Obes (2012) 7:284–94. doi: 10.1111/j.2047-6310.2012.00064.x
38. Marshall WA, Tanner JM. Variations in Pattern of Pubertal Changes in Girls. Arch Dis Child (1969) 44:291–303. doi: 10.1136/adc.44.235.291
39. Cutfield WS, Jefferies CA, Jackson WE, Robinson EM, Hofman PL. Evaluation of HOMA and QUICKI as Measures of Insulin Sensitivity in Prepubertal Children. Pediatr Diabetes (2003) 4:119–25. doi: 10.1034/j.1399-5448.2003.t01-1-00022.x
40. Ahrens W, Moreno L, Mårild S, Molnár D, Siani A, De Henauw S, et al. Metabolic Syndrome in Young Children: Definitions and Results of the IDEFICS Study. Int J Obes (2014) 38:S4–S14. doi: 10.1038/ijo.2014.130
41. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The Metabolic Syndrome in Children and Adolescents. Lancet (2007) 369:2059–61. doi: 10.1016/S0140-6736(07)60958-1
42. Nagase H, Woessner JF. Matrix Metalloproteinases. J Biol Chem (1999) 274:21491–4. doi: 10.1074/jbc.274.31.21491
43. Wang X, Khalil RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol (2018) 81:241. doi: 10.1016/BS.APHA.2017.08.002
44. Verma RP, Hansch C. Matrix Metalloproteinases (MMPs): Chemical–biological Functions and (Q)SARs. Bioorg Med Chem (2007) 15:2223–68. doi: 10.1016/J.BMC.2007.01.011
45. Randeva HS, Lewandowski KC, Komorowski J, Murray RD, O’Callaghan CJ, Hillhouse EW, et al. Growth Hormone Replacement Decreases Plasma Levels of Matrix Metalloproteinases (2 and 9) and Vascular Endothelial Growth Factor in Growth Hormone–Deficient Individuals. Circulation (2004) 109:2405–10. doi: 10.1161/01.CIR.0000129763.51060.77
46. Wang M, Monticone RE, McGraw KR. Proinflammation, Profibrosis, and Arterial Aging. Aging Med (2020) 3:159. doi: 10.1002/AGM2.12099
47. Browatzki M, Larsen D, Pfeiffer CAH, Gehrke SG, Schmidt J, Kranzhöfer A, et al. Angiotensin II Stimulates Matrix Metalloproteinase Secretion in Human Vascular Smooth Muscle Cells via Nuclear Factor-κb and Activator Protein 1 in a Redox-Sensitive Manner. J Vasc Res (2005) 42:415–23. doi: 10.1159/000087451
48. Death AK, Fisher EJ, McGrath KCY, Yue DK. High Glucose Alters Matrix Metalloproteinase Expression in Two Key Vascular Cells: Potential Impact on Atherosclerosis in Diabetes. Atherosclerosis (2003) 168:263–9. doi: 10.1016/S0021-9150(03)00140-0
49. Lehrke M, Greif M, Broedl UC, Lebherz C, Laubender RP, Becker A, et al. MMP-1 Serum Levels Predict Coronary Atherosclerosis in Humans. Cardiovasc Diabetol (2009) 8:50. doi: 10.1186/1475-2840-8-50
50. Tayebjee MH, Nadar SK, MacFadyen RJ, Lip GYH. Tissue Inhibitor of Metalloproteinase-1 and Matrix Metalloproteinase-9 Levels in Patients With Hypertensionrelationship to Tissue Doppler Indices of Diastolic Relaxation. Am J Hypertens (2004) 17:770–4. doi: 10.1016/J.AMJHYPER.2004.06.023
51. Unal R, Yao-Borengasser A, Varma V, Rasouli N, Labbate C, Kern PA, et al. Matrix Metalloproteinase-9 Is Increased in Obese Subjects and Decreases in Response to Pioglitazone. J Clin Endocrinol Metab (2010) 95:2993. doi: 10.1210/JC.2009-2623
52. Brandenburg H, Steegers EAP, Gittenberger-de Groot AC, Yoneyama Y, Asakura H, Araki T, et al. Potential Involvement of Vascular Endothelial Growth Factor in Pathophysiology of Turner Syndrome. Med Hypotheses (2005) 65:300–4. doi: 10.1016/j.mehy.2005.02.030
53. Wang H, Keiser JA. Vascular Endothelial Growth Factor Upregulates the Expression of Matrix Metalloproteinases in Vascular Smooth Muscle Cells. Circ Res (1998) 83:832–40. doi: 10.1161/01.RES.83.8.832
54. Berrahmoune H, Herbeth B, Lamont JV, Masson C, Fitzgerald PS, Visvikis-Siest S. Heritability for Plasma VEGF Concentration in the Stanislas Family Study. Ann Hum Genet (2007) 71:54–63. doi: 10.1111/J.1469-1809.2006.00298.X
55. Evans M, Davies, Goodfellow, Rees, Scanlon. Endothelial Dysfunction in Hypopituitary Adults With Growth Hormone Deficiency. Clin Endocrinol (Oxf) (1999) 50:457–64. doi: 10.1046/J.1365-2265.1999.00671.X
56. O’Gorman CS, Syme C, Bradley T, Hamilton J, Mahmud FH. Impaired Endothelial Function in Pediatric Patients With Turner Syndrome and Healthy Controls: A Case-Control Study. Int J Pediatr Endocrinol (2012) 2012:5. doi: 10.1186/1687-9856-2012-5
57. Pfeifer M, Verhovec R, Zizek B, Prezelj J, Poredos P, Clayton RN. Growth Hormone (GH) Treatment Reverses Early Atherosclerotic Changes in GH-Deficient Adults. J Clin Endocrinol Metab (1999) 84:453–7. doi: 10.1210/JCEM.84.2.5456
Keywords: Turner syndrome, MMP-1 (matrix metalloproteinase-1), MMP-2 (matrix metalloproteinase-2), MMP-9 (matrix metalloproteinase-9), VEGF (vascular endothelial growth factor), GDNF (glial cell line-derived neurotrophic factor), BDNF (brain-derived neurotrophic factor)
Citation: Błaszczyk E, Gawlik J, Gieburowska J, Tokarska A, Kimsa-Furdzik M, Hibner G, Francuz T and Gawlik A (2022) Effect of Growth Hormone Treatment on the Concentration of Selected Metabolic Markers in Girls With Turner Syndrome. Front. Endocrinol. 13:818735. doi: 10.3389/fendo.2022.818735
Received: 19 November 2021; Accepted: 04 May 2022;
Published: 13 June 2022.
Edited by:
George Paltoglou, National and Kapodistrian University of Athens, GreeceReviewed by:
Andrzej Lewinski, Medical University of Lodz, PolandXiaoping Luo, Huazhong University of Science and Technology, China
Ekaterina Koledova, Merck, Germany
Copyright © 2022 Błaszczyk, Gawlik, Gieburowska, Tokarska, Kimsa-Furdzik, Hibner, Francuz and Gawlik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aneta Gawlik, agawlik@mp.pl
 Ewa Błaszczyk
Ewa Błaszczyk