- 1Department of Geriatrics, Xuanwu Hospital Capital Medical University, National Clinical Research Center for Geriatric Diseases, Beijing, China
- 2Department of Cardiology, Xuanwu Hospital Capital Medical University, Beijing, China
Background: Although within the normal range, thyroid stimulating hormone (TSH) levels are associated with cardio-metabolic disorders and have an effect on the cardiovascular system. The aim of our study was to assess the prognostic value of normal TSH on long-term mortality in patients with ST-segment elevation myocardial infarction (STEMI).
Methods: Consecutive STEMI patients who had a TSH level within the normal range (0.55–4.78 μIU/ml) were enrolled from November 2013 to December 2018. Patients were stratified into three groups depending on the tertile of TSH level, and all-cause mortality and cardiac death were compared. TSH concentrations associated with risk of all-cause mortality were evaluated in a continuous scale (restricted cubic splines) and the Cox proportional hazards regression model.
Results: A total of 1,203 patients with STEMI were eligible for analysis. During a median follow-up of 39 months, patients in the 3rd tertile group had higher all-cause mortality (20.1% vs. 12.2% and 14.3%, p = 0.006) and cardiac death (15.4% vs. 7.7% and 12.3%, p = 0.001) as compared to the 1st and 2nd tertile groups. The Cox proportional hazards model showed that TSH was an independent predictor on long-term all-cause mortality (HR: 1.248, 95% CI: 1.046–1.490, p = 0.014). However, subgroup analysis indicated that TSH (HR: 1.313, 95% CI: 1.063–1.623, p = 0.012) was only significantly associated with long-term all-cause mortality in the patients without emergency reperfusion therapy. Restricted cubic spline analyses showed a linear relationship between TSH concentrations and all-cause mortality (P for non-linearity = 0.659).
Conclusions: A Higher TSH level - even in a normal range is associated with long-term mortality in patients with STEMI, proposing an additional indication to identify STEMI patients with poor prognosis.
Introduction
The mortality after ST-elevation myocardial infarction (STEMI) has dramatically decreased through reperfusion therapy and optimized antithrombotic treatment (1). However, it has always been a great challenge that STEMI patients are still at risk of major adverse cardiac events, even death after discharge. Therefore, current guidelines recommended some risk stratification models and biomarkers for identifying high-risk patients and more accurate secondary prevention (2–5).
Thyroid function exerts an important impact on the cardiovascular system. Overt and subclinical hyper- or hypothyroidism, manifested as low or high serum thyroid stimulating hormone (TSH) level, are associated with an increased risk of cardiovascular events and mortality (6–8). Interestingly, a previous study suggests that TSH levels in the upper part of the normal range are also positively associated with 30-day mortality in patients with coronary artery disease (CAD) after percutaneous coronary intervention (PCI) (9). So far, it is unclear whether TSH levels within the reference range have a predictive value on long-term prognosis in patients with STEMI. We therefore aimed to investigate the association of normal TSH levels with long-term mortality in patients following STEMI.
Methods
Study Population
In this retrospective study, 1,664 consecutive patients hospitalized for STEMI were enrolled in a single center from November 2013 to December 2018. Only patients with a TSH level within the reference range (0.55 to 4.78 μIU/ml) were eligible for analysis. Patients were divided into three groups according to the tertile of the TSH level (Figure 1). Exclusion criteria consist of 1) missing thyroid function test results (n = 76); 2) prior or current thyroid disease (including prior history, surgery, or drug therapy for thyroid disease) (n = 124); 3) abnormal thyroid status and TSH beyond the reference range (n = 397); and 4) receiving steroid and amiodarone before admission (n = 44). Clinical information was collected from questionnaires and electronic medical records. Patients were treated according to standard clinical guidelines (10). Reperfusion therapy was indicated in patients with symptoms of ischemia of ≦12 h duration and persistent ST-segment elevation. A primary PCI strategy was recommended over fibrinolysis within indicated timeframes. However, if timely PCI cannot be performed after STEMI diagnosis, fibrinolytic therapy is recommended with 12 h of symptom onset in patients without contraindications. Our study complied with the Declaration of Helsinki, and written information consents were obtained from all participants. The study was approved by the ethics committee of Xuanwu Hospital Capital Medical University.
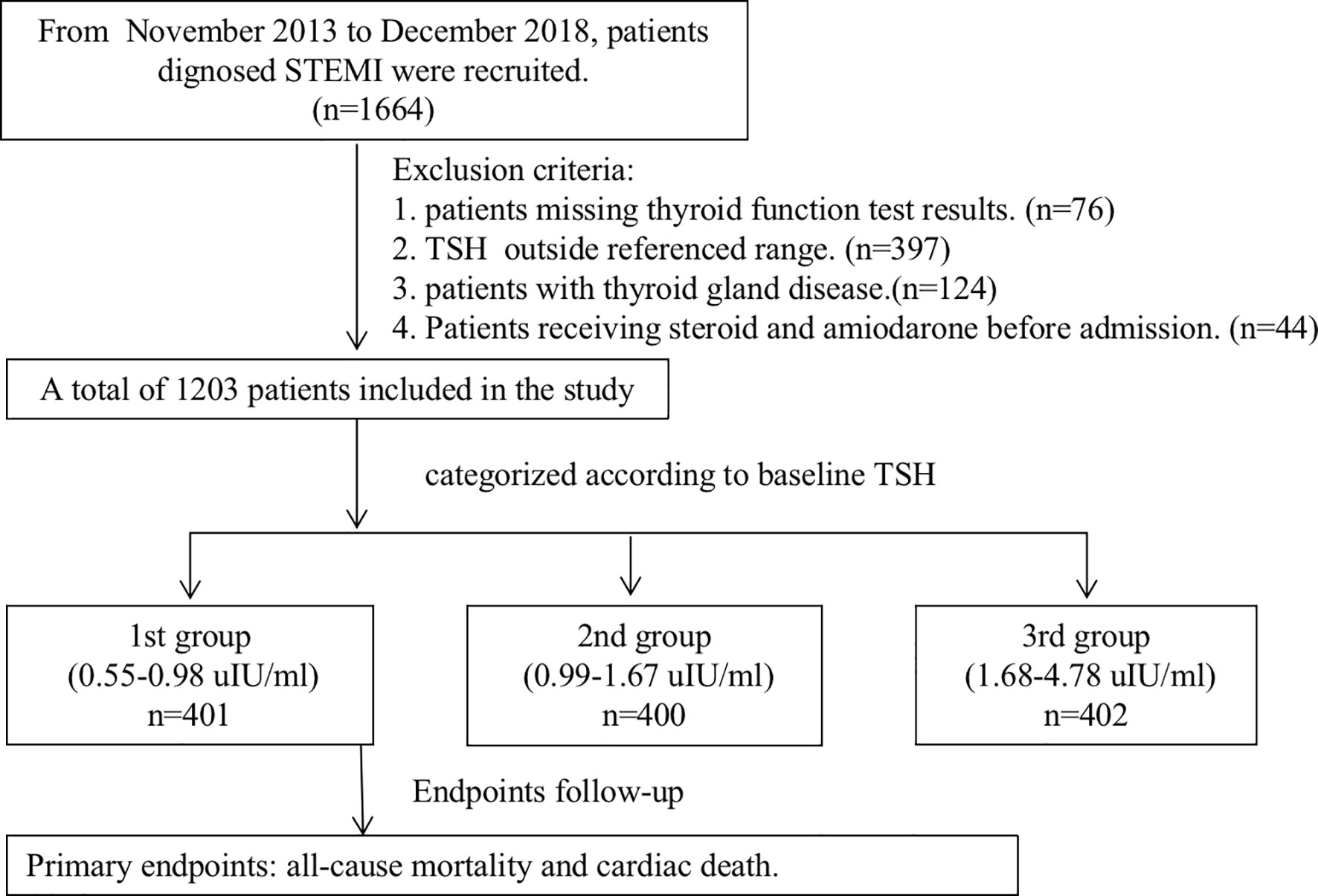
Figure 1 The study flowchart. STEMI, ST-segment elevation myocardial infarction; TSH, thyroid-stimulating hormone.
STEMI was defined as at least two of the three criteria: 1) presence of typical prolonged (≥20 min) chest pain; 2) significant ST-segment elevation (≧2 mm in at least 2 contiguous cordial leads, or ST-segment elevation ≧1 mm in at least 2 inferior leads) or new left bundle branch block; and 3) the elevation of myocardial biomarker (cTn or CK-MB) more than the 99th percentile or 2 folds of the normal value (11).
Thyroid Function Testing
Blood samples were collected within 24 h of hospital admission. The thyroid function test includes serum TSH, total triiodothyronine (TT3), total thyroxine (TT4), free triiodothyronine (FT3), and free thyroxine (FT4) levels. After coagulation at room temperature for 30 min, samples were centrifuged at 4,000 rpm for 5 min and serum was aspirated. They were measured by chemiluminescent immunoassay on Centaur (Siemens, Erlangen, Germany).
Follow-Up and Clinical Outcomes
Discharged patients were subsequently tracked via review of medical records and telephone interview at 6 and 12 months and then annually. The primary endpoint was all-cause mortality during follow-up. We further defined the death as cardiac and non-cardiac. Standard definitions were used for cardiac death (12).
Statistical Analysis
Continuous variables were presented as mean (± SD) when normally distributed and median (25th–75th percentile) when not normally distributed. Categorical variables were shown as frequencies and percentages. The Shapiro–Wilk normality test was performed to test the normality of the data. Mean values of continuous variables were compared using one-way analysis of variance or Kruskal–Wallis tests, as appropriate. Proportions for categorical variables were compared by the chi-square test or Fisher exact test. Bonferroni correction was used to correct multiple comparisons. Univariable and forward stepwise multivariable Cox regression analyses were performed to clarify the independent prognostic impact of TSH on the all-cause mortality. The proportional hazards assumption was confirmed through Schoenfeld residuals (Supplementary Files). Baseline variables that were considered clinically relevant or that showed a univariate relationship with outcome were entered into the multivariate Cox proportional-hazards regression model. Variables for inclusion were carefully chosen, given the number of events available, to ensure parsimony of the final model. The association between TSH concentration in the reference range and all-cause mortality was evaluated on a continuous scale with restricted cubic spline curves (13) based on Cox proportional hazards models with 3 knots at the 10th, 50th, and 90th percentiles of TSH. HR was reported with a corresponding 95% CI. A two-sided p value <0.05 was considered to indicate statistical significance. Statistical analyses and charts were performed using the SPSS 25 (IBM SPSS Statistics 25, IBM Corporation, Armonk, NY, USA) and the R package.
Results
Baseline Data
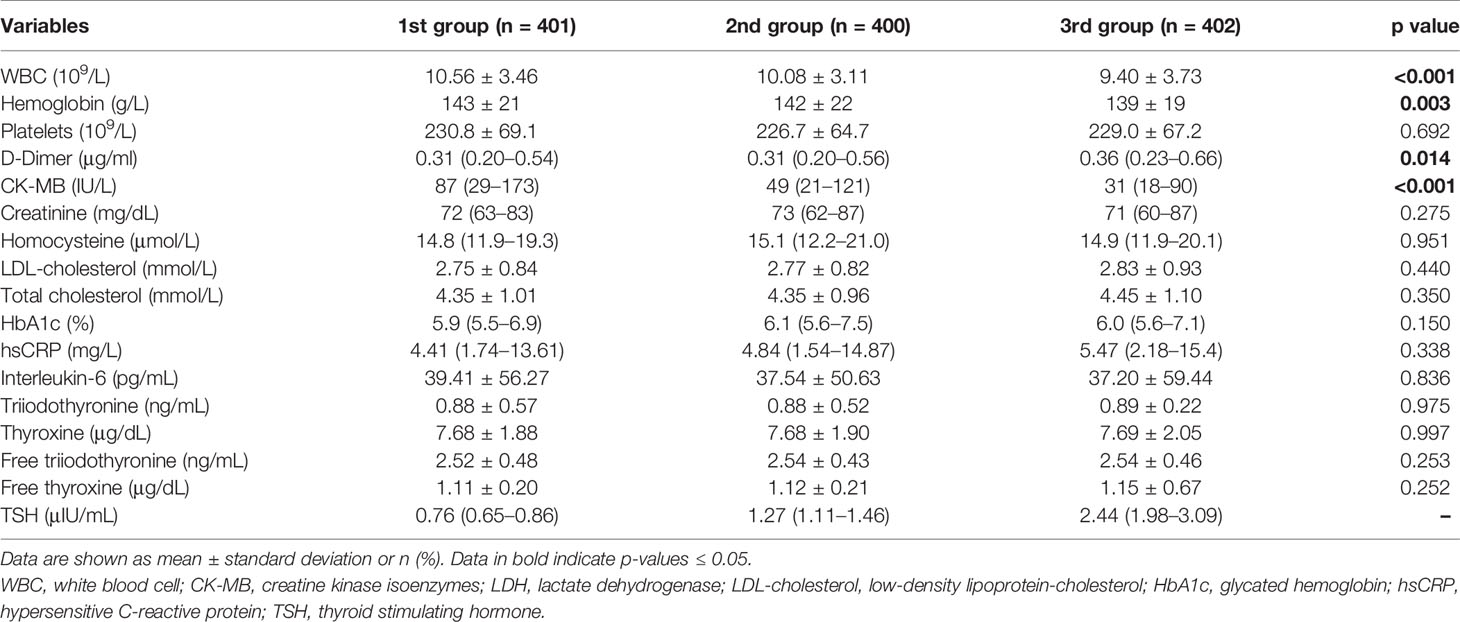
A total of 1,203 consecutive patients were enrolled and divided into three groups according to the tertile of the TSH level, which were as follows: 1st group (TSH level from 0.55 to 0.98 μIU/ml, n = 401), 2nd group (TSH level from 0.99 to 1.67 μIU/ml, n = 400), and 3rd group (TSH level from 1.68 to 4.78 μIU/ml, n = 402). Baseline demographic and clinical characteristics are shown in Table 1. Age, male, high-risk Global Registry of Acute Coronary Events (GRACE) score, smoking history, the degree of pain relief, and the time of symptom onset to admission over 12 h were significantly different in patients of various groups. There was no difference in the occurrence of in-hospital mortality among three groups (2.5%, 4.5%, and 5.5%, p = 0.099).
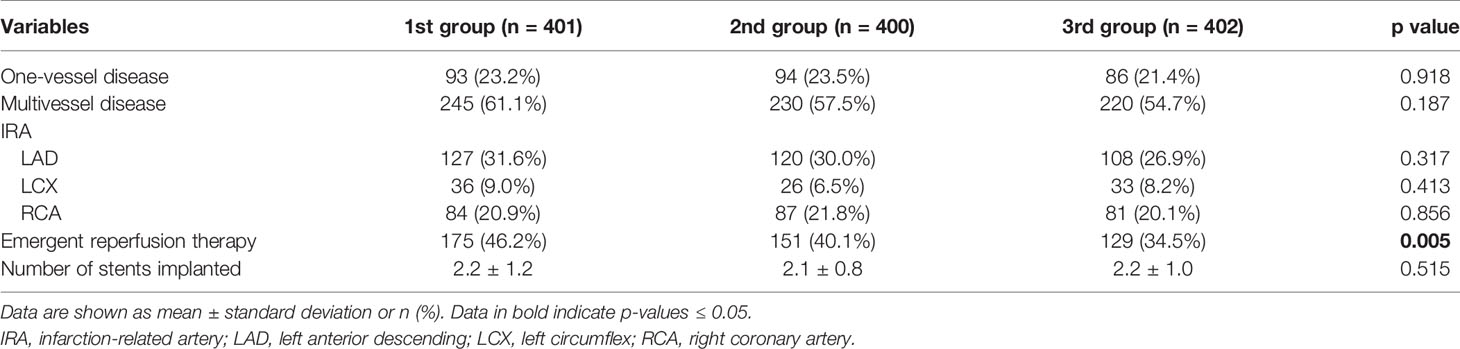
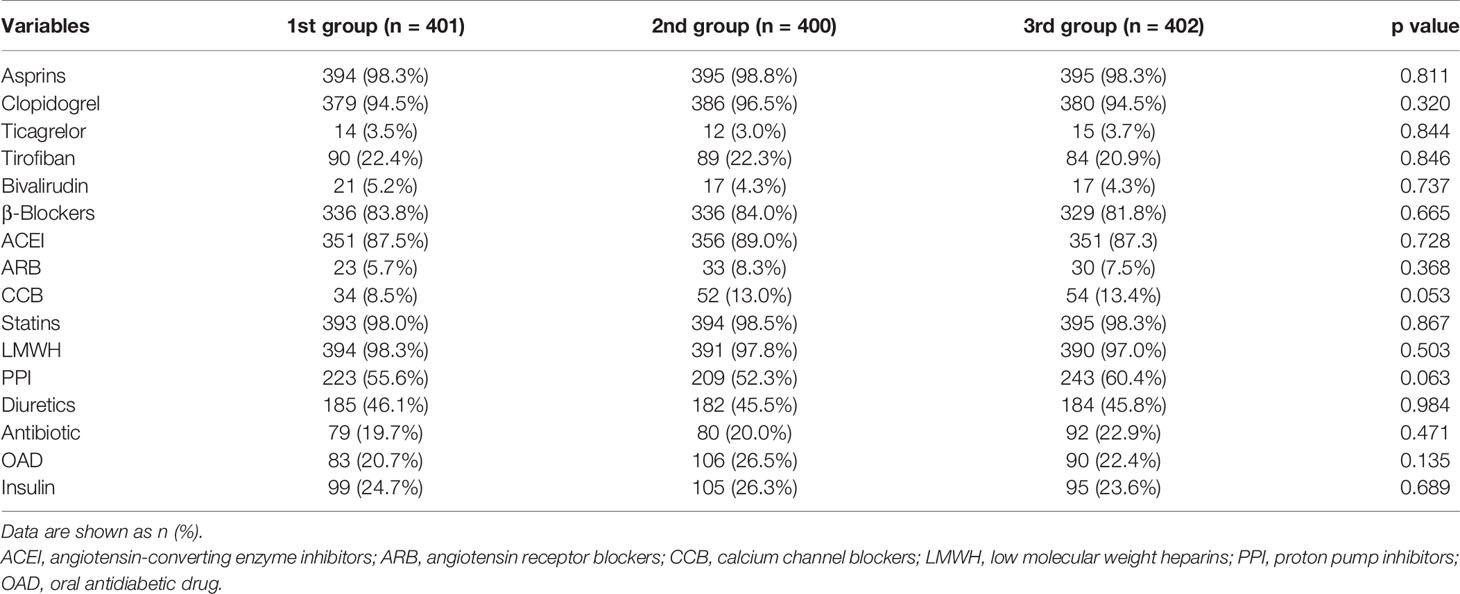
Laboratory tests of three groups are shown in Table 2. The significant differences were found among three groups in terms of white blood cell counts (WBC), hemoglobin, creatine kinase isoenzymes (CK-MB), and D-dimer. Angiographic and procedural characteristics among three groups are shown in Table 3. Fewer patients in the 3rd group received reperfusion therapy compared with those in the other two groups (34.5% vs. 46.2% and 40.1%, p = 0.005). Numbers of lesion vessels and infarction-related artery were similar among three groups. In Table 4, no difference in medication prescription in hospital and after discharge was found in three groups.
Clinical Outcomes
The median time of follow-up is 39 months, and the follow-up rate is 93.74%. The primary endpoint occurred in 187 patients: 49 (12.2%) patients in the 1st group, 57 (14.3%) patients in the 2nd group, and 81 (20.1%) patients in the 3rd group. Meanwhile, 62 (15.4%) patients in the 3rd group had higher cardiac death than 27 (6.7%) patients in the 1st group and 49 (12.3%) patients in the 2nd group (p < 0.001).
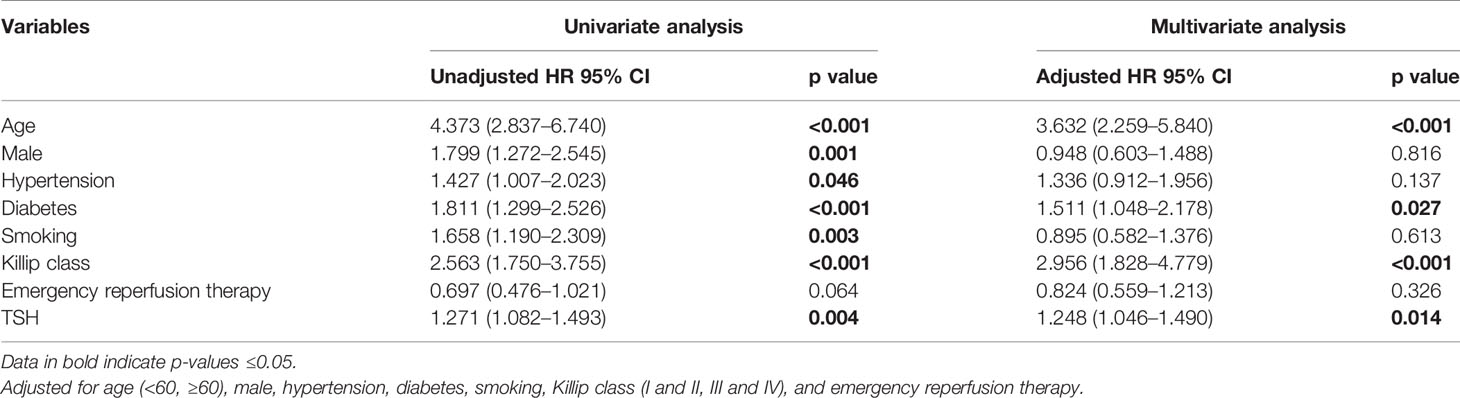
After adjusting the influence of other covariates incorporated in the multivariate Cox regression model (Table 5), we found that TSH was independently associated with the increased risk of all-cause mortality (HR: 1.248, 95% CI: 1.046–1.490, p = 0.014). Age, diabetes, Killip class, and hemoglobin retained statistical significance for all-cause mortality. All subjects were classified into two groups based on whether receiving emergency reperfusion therapy. After adjustment for confounding factors in a multivariate Cox regression analysis, hypertension (HR: 2.606, 95% CI: 1.225–5.541, p = 0.013) remained an independent predictor for patients with emergency reperfusion therapy, while age (HR: 5.360, 95% CI: 2.830–10.150, p < 0.001), diabetes (HR: 1.893, 95% CI: 1.219–2.938, p = 0.004), Killip class (HR: 2.760, 95% CI: 1.599–4.765, p < 0.001), and TSH level (HR: 1.313, 95% CI: 1.063–1.623, p = 0.012) retained statistical significance for patients without emergency reperfusion therapy (Table 6).
In Figure 2, we used a restricted cubic spline to flexibly model and visualize the relationship of TSH concentrations in the reference range with all-cause mortality in STEMI patients. Above 1.79 μIU/ml, TSH concentrations were positively associated with the risk of all-cause mortality (HR = 1.15, 95% CI: 1.002–1.322, P for non-linearity = 0.659).
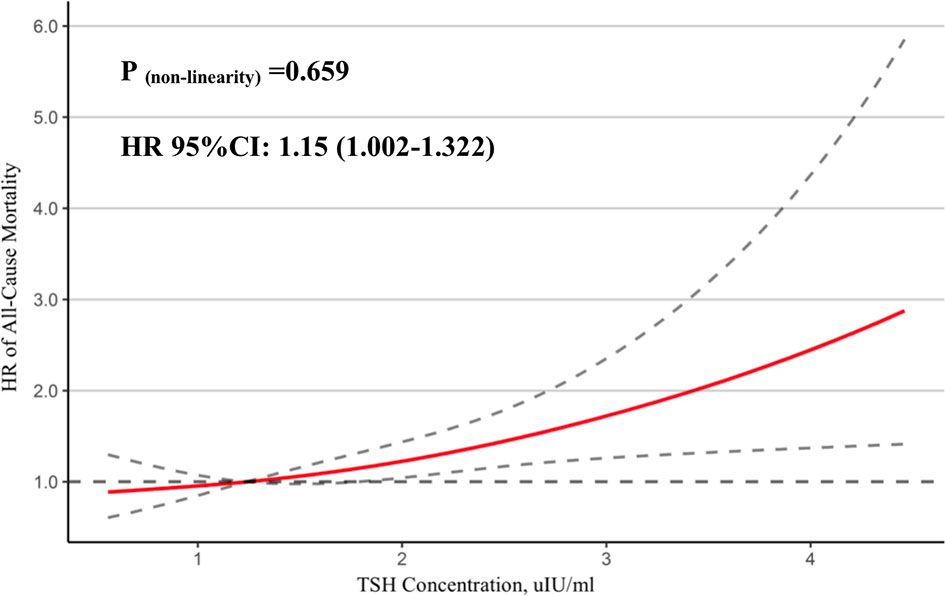
Figure 2 Association between TSH and mortality. A restricted cubic spline regression model was used to evaluate the relationship between normal TSH concentration and all-cause mortality in STEMI patients.
Discussion
The present study explored the relationship between TSH within the reference range and long-term mortality in patients with STEMI. The most important findings of the study can be summarized as the following: 1) in patients with STEMI, TSH in the highest tertile within the reference range was associated with a higher risk of long-term mortality, especially cardiac death, and 2) a high-normal TSH level at admission was an independent predictor of increased risk of mortality, especially in those without receiving reperfusion therapy.
The association between TSH level within the reference range and mortality has been reported in several longitudinal cohort studies. Ndrepepa et al. (9) enrolled 8,010 patients treated with PCI and found that the TSH level was associated with 30-day mortality but not with 30-day to 3-year mortality. Gürdoğan et al. (14) reported that the high-normal TSH group in euthyroid acute coronary syndrome (ACS) patients was an independent predictor for mortality during the 6-month follow-up. However, Åsvold et al. (15) analyzed 14 cohorts, including 55,412 patients with the TSH level within the reference range and no previously known thyroid or cardiovascular disease at baseline and concluded that TSH levels within the reference range were not associated with risk of cardiovascular events or CAD mortality. To our best knowledge, the current study is the first report demonstrating the relationship between normal TSH and long-term mortality in STEMI patients.
Taken together with our study and previous data, TSH may have a predictive value on patients with known CAD, who have more significant atherosclerosis and relatively higher risk of recurrent events than those without CAD. In our STEMI cohort, the impact of TSH changes was emphasized by disclosing a correlation of TSH with high-risk GRACE score, which represents an important clinical marker of worse prognosis in acute STEMI. Since in the 3rd TSH tertile group, there are more patients with time from symptom onset >12 h and fewer patients got primary PCI and fibrinolytic therapy, we conducted a subgroup analysis of reperfusion therapy. In subgroup analysis, the association between mortality and normal TSH level only persisted in STEMI patients without reperfusion therapy, who are at a higher risk of early complication and death. Consequently, TSH in the upper reference range can serve as an indicator for increased risk of long-term mortality in patients with STEMI.
The mechanism of association between TSH in the upper part of the reference range and increased risk of STEMI follow-up mortality is not clear. Some researchers reported that TSH levels in the upper part of the reference range are associated with endothelial dysfunction (16), unsteady systolic and diastolic blood pressure (17, 18), arterial stiffness (19), metabolic syndrome (20), and less favorable lipid levels (21). Meanwhile, studies have found that TSH can promote inflammation by stimulating adipose tissue pro-inflammatory cytokines (22), increase cardiometabolic disorders (23), affect heart remodeling after myocardial infarction (24), and increase higher thrombus burden (25). In addition, euthyroid sick syndrome, manifested normal TSH and decreased free T3 levels, are related to the increased mortality in both heart failure and acute myocardial infarction (26, 27). TSH in the upper part of the reference range may indicate a high-risk condition for STEMI patients, which should get more attention in clinical practice. In the current study, the contribution of these conditions in the relationship between normal TSH level and mortality remains unproven. Further investigation is needed to assess the mechanism of the normal TSH level in STEMI patients.
Delimitation of the reference range of TSH is controversial (28–30), and some people proposed to reduce the upper limit of reference to 2.5–3.0 mIU/l (31). Our study found that the TSH level was positively associated with the risk of all-cause mortality when above 1.79 μIU/ml. Inoue et al. (32) have reported that a U-curved association between TSH and the risk of all-cause mortality in the cohort study of 9,020 US adults and higher TSH levels (>1.96 mIU/l) were associated with increased risk of all-cause mortality. Gürdoğan et al. (14) reported that the high-normal TSH group (>1.60 μIU/ml) in ACS patients was an independent predictor for mortality during the 6-month follow-up. Evidence shows that although the serum TSH level reflects thyroid–pituitary feedback, it might not be efficient enough to show thyroid status in every organ (33). Based on this assumption, an appropriate pituitary FT4 level might not be suitable for the cardiovascular system and increases the incidence of CAD. Therefore, for CAD patients, the general reference range of TSH might not be reliable enough, and the continuous effect of TSH should be regarded as a risk factor. We look forward to more convincing studies to verify the relationship between TSH and mortality in STEMI patients, and to guide the division of TSH thresholds among different populations.
There are some limitations in our study. First, it is a retrospective and observational research with possible selection bias and confounding factors. Second, our TSH was measured by chemiluminescent immunoassay and the reference range is only response to this measurement. Moreover, TSH was monitored only once at admission; the relationship between TSH changes and mortality remained undefined without reexamination and regular monitoring. Thyroid function testing did not include thyroid autoantibodies, which was associated with clinical or subclinical thyroid diseases. Third, cardiac biomarkers in our study were not obtained at admission and thus might reflect the degree of cardiac injury insufficiently. Finally, the current study indicates that TSH is related to long-term mortality in STEMI patients, but the relevant mechanism was not explained thoroughly.
Conclusion
TSH in the upper part of the reference range is associated with an increased risk of long-term mortality in STEMI patients, especially in those without reperfusion therapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Xuanwu Hospital Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JL, LS, and KX contributed to the conception and design of the work. LS, XK, ZM, YZ, JS, NS, HZ and TZ contributed to the acquisition. JL, LS, ZM, HZ and KX drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was funded by the National Natural Science Foundations of China (grant number 81470491 and 82170347) and the Beijing Municipal Natural Science Foundation (grant number 7192078).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.806997/full#supplementary-material
Abbreviations
STEMI, ST-elevation myocardial infarction; TSH, thyroid stimulating hormone; CAD, coronary artery disease; PCI, percutaneous coronary intervention; TT3, total triiodothyronine; TT4, total thyroxine; FT3, free triiodothyronine; FT4, free thyroxine; GRACE, Global Registry of Acute Coronary Events; WBC, blood cell counts; CK-MB, creatine kinase isoenzymes; LDL, low-density lipoprotein; ACS, acute coronary syndrome.
References
1. Dégano IR, Salomaa V, Veronesi G, Ferriéres J, Kirchberger I, Laks T, et al. Twenty-Five-Year Trends in Myocardial Infarction Attack and Mortality Rates, and Case-Fatality, in Six European Populations. Heart (2015) 101(17):1413–21. doi: 10.1136/heartjnl-2014-307310
2. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation (2015 2016) 133(11):1135–47. doi: 10.1161/cir.0000000000000336
3. Zagidullin N, Motloch LJ, Gareeva D, Hamitova A, Lakman I, Krioni I, et al. Combining Novel Biomarkers for Risk Stratification of Two-Year Cardiovascular Mortality in Patients With ST-Elevation Myocardial Infarction. J Clin Med (2020) 9(2):550. doi: 10.3390/jcm9020550
4. Tymińska A, Kapłon-Cieślicka A, Ozierański K, Budnik M, Wancerz A, Sypień P, et al. Association of Galectin-3 and Soluble ST2 With in-Hospital and 1-Year Outcomes in Patients With ST-Segment Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. Pol Arch Intern Med (2019) 129(11):770–80. doi: 10.20452/pamw.15030
5. Sherwood MW, Morrow DA, Scirica BM, Jiang S, Bode C, Rifai N, et al. Early Dynamic Risk Stratification With Baseline Troponin Levels and 90-Minute ST-Segment Resolution to Predict 30-Day Cardiovascular Mortality in ST-Segment Elevation Myocardial Infarction: Analysis From CLopidogrel as Adjunctive ReperfusIon TherapY (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28. Am Heart J (2010) 159(6):964–71. doi: 10.1016/j.ahj.2010.03.005
6. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical Hypothyroidism and the Risk of Coronary Heart Disease and Mortality. JAMA (2010) 304(12):1365–74. doi: 10.1001/jama.2010.1361
7. Ning Y, Cheng YJ, Liu LJ, Sara JD, Cao ZY, Zheng WP, et al. What Is the Association of Hypothyroidism With Risks of Cardiovascular Events and Mortality? A Meta-Analysis of 55 Cohort Studies Involving 1,898,314 Participants. BMC Med (2017) 15(1):21. doi: 10.1186/s12916-017-0777-9
8. Liu YS, Wei M, Wang L, Liu G, Ma GP, Ono K, et al. The Impact of Subclinical Hypothyroidism on Long-Term Outcomes in Older Patients Undergoing Percutaneous Coronary Intervention. BMC Endocr Disord (2021) 21(1):43. doi: 10.1186/s12902-021-00702-z
9. Ndrepepa G, Braun S, Mayer K, Cassese S, Fusaro M, Byrne RA, et al. Prognostic Value of Thyroid-Stimulating Hormone Within Reference Range in Patients With Coronary Artery Disease. Metabolism (2015) 64(10):1308–15. doi: 10.1016/j.metabol.2015.07.009
10. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J (2017 2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
11. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third Universal Definition of Myocardial Infarction. J Am Coll Cardiol (2012) 60(16):1581–98. doi: 10.1016/j.jacc.2012.08.001
12. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol (2014 2015) 66(4):403–69. doi: 10.1016/j.jacc.2014.12.018
13. Desquilbet L, Mariotti F. Dose-Response Analyses Using Restricted Cubic Spline Functions in Public Health Research. Stat Med (2010) 29(9):1037–57. doi: 10.1002/sim.3841
14. Gürdoğan M, Altay S, Korkmaz S, Kaya Ç, Zeybey U, Ebik M, et al. The Effect of Thyroid Stimulating Hormone Level Within the Reference Range on In-Hospital and Short-Term Prognosis in Acute Coronary Syndrome Patients. Med (Kaunas) (2019) 55(5). doi: 10.3390/medicina55050175
15. Åsvold BO, Vatten LJ, Bjøro T, Bauer DC, Bremner A, Cappola AR, et al. Thyroid Function Within the Normal Range and Risk of Coronary Heart Disease: An Individual Participant Data Analysis of 14 Cohorts. JAMA Intern Med (2015) 175(6):1037–47. doi: 10.1001/jamainternmed.2015.0930
16. von Hafe M, Neves JS, Vale C, Borges-Canha M, Leite-Moreira A. The Impact of Thyroid Hormone Dysfunction on Ischemic Heart Disease. Endocr Connect (2019) 8(5):76–90. doi: 10.1530/ec-19-0096
17. Shimizu Y, Nabeshima-Kimura Y, Kawashiri SY, Noguchi Y, Nagata Y, Maeda T, et al. Associations Between Thyroid-Stimulating Hormone and Hypertension According to Thyroid Cyst Status in the General Population: A Cross-Sectional Study. Environ Health Prev Med (2020) 25(1):69. doi: 10.1186/s12199-020-00910-4
18. Onat A, Aydın M, Can G, Çelik E, Altay S, Karagöz A, et al. Normal Thyroid-Stimulating Hormone Levels, Autoimmune Activation, and Coronary Heart Disease Risk. Endocrine (2015) 48(1):218–26. doi: 10.1007/s12020-014-0269-z
19. Sakamaki K, Tsunekawa K, Ishiyama N, Kudo M, Ando K, Akuzawa M, et al. Association Between High Normal-Range Thyrotropin Concentration and Carotid Intima-Media Thickness in Euthyroid Premenopausal, Perimenopausal and Postmenopausal Women. Maturitas (2021) 144:29–36. doi: 10.1016/j.maturitas.2020.10.022
20. Nozarian Z, Abdollahi A, Mehrtash V, Nasiri Bonaki H. Upper Normal Limit of Thyroid-Stimulating Hormone and Metabolic Syndrome in Iranian Patients With Obesity. Iran J Pathol (2017) 12(1):88–93. doi: 10.30699/ijp.2017.24219
21. Luxia L, Jingfang L, Songbo F, Xulei T, Lihua M, Weiming S, et al. Correlation Between Serum TSH Levels Within Normal Range and Serum Lipid Profile. Horm Metab Res (2021) 53(1):32–40. doi: 10.1055/a-1191-7953
22. Gagnon A, Langille ML, Chaker S, Antunes TT, Durand J, Sorisky A. TSH Signaling Pathways That Regulate MCP-1 in Human Differentiated Adipocytes. Metabolism (2014) 63(6):812–21. doi: 10.1016/j.metabol.2014.02.015
23. Boggio A, Muzio F, Fiscella M, Sommariva D, Branchi A. Is Thyroid-Stimulating Hormone Within the Normal Reference Range a Risk Factor for Atherosclerosis in Women? Intern Emerg Med (2014) 9(1):51–7. doi: 10.1007/s11739-011-0743-z
24. Reindl M, Feistritzer HJ, Reinstadler SJ, Mueller L, Tiller C, Brenner C, et al. Thyroid-Stimulating Hormone and Adverse Left Ventricular Remodeling Following ST-Segment Elevation Myocardial Infarction. Eur Heart J Acute Cardiovasc Care (2019) 8(8):717–26. doi: 10.1177/2048872618770600
25. Viswanathan G, Balasubramaniam K, Hardy R, Marshall S, Zaman A, Razvi S. Blood Thrombogenicity Is Independently Associated With Serum TSH Levels in Post-non-ST Elevation Acute Coronary Syndrome. J Clin Endocrinol Metab (2014 2014) 99(6):1050–4. doi: 10.1210/jc.2013-3062
26. Özcan KS, Osmonov D, Toprak E, Güngör B, Tatlısu A, Ekmekçi A, et al. Sick Euthyroid Syndrome Is Associated With Poor Prognosis in Patients With ST Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Intervention. Cardiol J (2014) 21(3):238–44. doi: 10.5603/CJ.a2013.0108
27. Pingitore A, Landi P, Taddei MC, Ripoli A, L’abbate A, Iervasi G. Triiodothyronine Levels for Risk Stratification of Patients With Chronic Heart Failure. Am J Med (2005) 118:132–6. doi: 10.1016/j.amjmed.2004.07.052
28. Razvi S, Bhana S, Mrabeti S. Challenges in Interpreting Thyroid Stimulating Hormone Results in the Diagnosis of Thyroid Dysfunction. J Thyroid Res (2019) 4106816. doi: 10.1155/2019/4106816
29. Larisch R, Giacobino A, Eckl W, Wahl HG, Midgley JE, Hoermann R. Reference Range for Thyrotropin Post Hoc Assessment. Nuklearmedizin (2015) 54(3):112–7. doi: 10.3413/Nukmed-0671-14-06
30. Pérez-Campos Mayoral L, Hernández-Huerta MT, Mayoral-Andrade G, Pérez-Campos Mayoral E, Zenteno E, Martínez-Cruz R, et al. TSH Levels in Subclinical Hypothyroidism in the 97.5th Percentile of the Population. Int J Endocrinol (2020), 2698627. doi: 10.1155/2020/2698627
31. Wartofsky L, Dickey RA. The Evidence for a Narrower Thyrotropin Reference Range Is Compelling. J Clin Endocrinol Metab (2005) 90(9):5483–8. doi: 10.1210/jc.2005-0455
32. Inoue K, Ritz B, Brent GA, Ebrahimi R, Rhee CM, Leung AM. Association of Subclinical Hypothyroidism and Cardiovascular Disease With Mortality. JAMA Netw Open (2020) 3(2):e1920745. doi: 10.1001/jamanetworkopen
Keywords: thyroid stimulating hormone, ST-segment elevation myocardial infarction, mortality, prognosis, coronary artery disease
Citation: Sun L, Xiao K, Miao Z, Zhang Y, Si J, Shi N, Zhang H, Zhao T and Li J (2022) Prognostic Value of Normal Thyroid Stimulating Hormone in Long-Term Mortality in Patients With STEMI. Front. Endocrinol. 13:806997. doi: 10.3389/fendo.2022.806997
Received: 01 November 2021; Accepted: 20 January 2022;
Published: 22 February 2022.
Edited by:
Xiongfei Pan, Vanderbilt University Medical Center, United StatesReviewed by:
Tong Yan, The Third People’s Hospital of Chengdu, ChinaSheyu Li, Sichuan University, China
Copyright © 2022 Sun, Xiao, Miao, Zhang, Si, Shi, Zhang, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, c2hweGJiQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Lijie Sun
Lijie Sun Keling Xiao1†
Keling Xiao1† Jing Li
Jing Li