- 1Department of General Surgery Thyroid Specialty, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Department of Urology, Xiangya Hospital, Central South University, Changsha, China
Background: The prevalence of thyroid carcinoma (TC) and Hashimoto’s thyroiditis (HT) has been increasing dramatically over the past decades. We investigated the relationship between HT and TC.
Methods: We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for carrying out and reporting this meta-analysis. The literature from January 1, 2010 to December 31, 2020, regardless of region and publication type, was searched comprehensively in PubMed, Embase, Web of Science, and Cochrane Library databases. After careful selection and data extraction, the pooled odds ratio of various clinical characteristics in 39 studies were calculated. Publication bias was analyzed using funnel plots.
Results: Meta-analysis of 39 original research articles showed HT to be a risk factor of TC (pooled odds ratio = 1.71; 95% confidence interval, 1.57–1.80; p < 0.00001) and papillary thyroid carcinoma (1.67, 1.51–1.85, <0.00001). Patients with papillary thyroid carcinoma (PTC) combined with HT were more likely to have multifocal carcinomas. The prevalence of an extrathyroidal extension, metastasis, BRAFV600E mutation, and recurrence was significantly lower in patients with PTC combined with HT.
Conclusions: HT is a “double-edged sword” in TC patients. HT increases the risk of TC and PTC but is a protective factor against PTC progression.
1 Introduction
Thyroid carcinoma (TC) is the most common malignant disease of the endocrine system (1, 2). The overall survival from TC is high, and the prognosis is good, especially for papillary thyroid carcinoma (PTC). However, in recent decades, TC incidence has been increasing dramatically (3). The incidence of autoimmune thyroid diseases, especially Hashimoto’s thyroiditis (HT), has also increased in recent decades (4). About one-third of PTC patients also have HT, and the number of people with PTC combined with HT is increasing (5, 6). The underlying reason for this increased incidence may be improved diagnostic tools (e.g., fine-needle aspiration, high-resolution ultrasound, and detection of thyroid gland-specific antibodies). About 20%–30% of HT patients will eventually experience hypothyroidism, and an increased serum level of thyroid-stimulating hormone (TSH) may promote TC occurrence (5, 6). In addition, exposure to adverse environmental factors can make people more vulnerable to thyroiditis (7, 8).
In 1893, Rudolf Virchow was the first to propose a link between chronic inflammation and cancer development. In the next century, his hypothesis was confirmed in several human diseases (9, 10). The most convincing evidence for his hypothesis was (i) the link between chronic inflammatory diseases of the intestine (Crohn’s disease and ulcerative proctocolitis) and colon adenocarcinoma, (ii) chronic infection with the hepatitis B virus or hepatitis C virus and liver cancer, and (iii) chronic gastritis and gastric cancer caused by Helicobacter pylori infection. Similarly, it has been postulated that having HT (as the most common type of thyroiditis) also carries an increased risk of TC. Many scholars have investigated this hypothesis, but most studies have been influenced by selection biases and imprecise indicators (11). Hence, studies have reached controversial (or even contrary) conclusions. Some studies have shown that HT may be a tumor-promoting factor (12). Other studies have reported HT to not have a relationship with the higher incidence of TC (13). HT has different influences on PTC in several aspects: certain clinical manifestations, sex, lymph-node metastasis, BRAFV600E mutation, and recurrence (14–18). These factors affect the aggressiveness, treatment effect, and prognosis of PTC directly. Analyses of original studies and meta-analysis that had controversial conclusions revealed that biases usually arose because the (i) diagnostic criteria for HT were not uniform (B-ultrasound, antibodies, or pathology), (ii) the number of original research was limited, and (iii) the definition of the indicators used was not clear, such as lymph-node metastasis (metastasis of central lymph nodes or metastasis of lateral lymph nodes, or both).
Taking into account the limitations of such studies, an updated systematic review and meta-analysis is necessary. To better understand the impact of HT on PTC progression, we undertook a meta-analysis through a comprehensive search of the literature.
2 Methods
2.1 Registration
We registered (CRD42021265538) a systematic review entitled “The relationship between Hashimoto’s thyroiditis and thyroid carcinoma: a meta-analysis” in PROSPERO. We have been updating information in that systematic review.
2.2 Search Strategy
We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for carrying out and reporting this meta-analysis (19). The MOOSE checklist is shown in Supplementary Table S1. The literature from January 1, 2010 to December 31, 2020, regardless of region and publication type, was searched comprehensively in PubMed, Embase, Web of Science, and Cochrane Library databases. We used the following medical subject headings (MeSH) terms: “Thyroid neoplasms” and “Hashimoto disease”. We combined the MeSH terms and entry words when constructing searches. In addition, we also use the related article function to expand the search scope. If multiple original studies involving the same population were published, we selected the latest and most comprehensive research. Exported citations were managed, and duplicate data were deleted, using EndNote™ (https://endnote.com/).
2.3 Inclusion and Exclusion Criteria
The inclusion criteria were the following: (i) the study type must be prospective, retrospective, randomized controlled trial, or case–control; (ii) the research focus was HT and thyroid nodules, TC, or PTC; (iii) patients must be adults (≥18 years); and (iv) HT, TC, or PTC was diagnosed by pathology.
The exclusion criteria were the following: (i) the study design was not a prospective, retrospective, or randomized controlled trial; (ii) those that were editorials, letters, comments, case reports, or laboratory-animal research; and (iii) data were incomplete, and extracting the data needed in the meta-analysis was not possible.
According to these criteria, two researchers (JX and LM) conducted a preliminary screening by reading the title and abstract of the article. For the articles remaining after the preliminary screening, JX and LM read the full text and followed the inclusion and exclusion criteria strictly for final screening of the article. This screening process was carried out independently by JX and LM. Disputes in the screening process were resolved through negotiation or help of a third author (KD).
2.4 Extraction and Quality Assessment of Data
JX and LM used identical standardized data-extraction tables. The latter included the characteristics of the original research (author, publication year, region, and research type) and clinical characteristics of thyroid nodules or TC related to HT (main results were the malignancy prevalence of thyroid nodules and lymph-node metastasis in PTC; secondary results were multifocality, extrathyroidal expansion, BRAFV600E mutation, recurrence, and distant metastasis). Disputes between JX and LM were resolved by KD. If the target data of the original study could not be obtained, then the corresponding author of the original study was contacted.
We used the Newcastle–Ottawa Scale (NOS) score to assess the quality of included studies (20). The NOS focuses on the choice of study subjects (four items), comparability between groups (two items) and measurement of results (three items, applicable to cohort studies), or exposure degree (three items, applicable to case–control studies). The NOS score of each study varied from 0 to 9 points (1 point for each item, 9 points in total). An original study with a NOS score ≥6 points was considered to be of “high quality.”
2.5 Statistical Analyses
The meta-analysis of all data was carried out using Revman 5.4 with Cochrane Training (https://training.cochrane.org/). The odds ratio (OR) and 95% confidence interval (95%CI) of each study were calculated. The χ2 test and I2 test were employed to evaluate heterogeneity. For the χ2 test, p < 0.10 was considered to denote significant heterogeneity. For the I2 test, heterogeneity types were divided into “none” (0%–25%), “mild” (25%–50%), “moderate” (50%–75%), and “severe” (75%–100%). If I2 > 50%, the random-effects model was chosen for the meta-analysis; otherwise, the fixed-effects model (FEM) was chosen. We drew funnel plots and observed their symmetry to identify publication bias, but this was mainly for meta-analysis containing ≥10 studies. If the number of included studies was too small, evaluation of the symmetry of the funnel chart was not possible. For sensitivity analysis, we observed changes in the overall results by eliminating individual studies one-by-one and utilizing different effect models.
3 Results
3.1 Literature Search
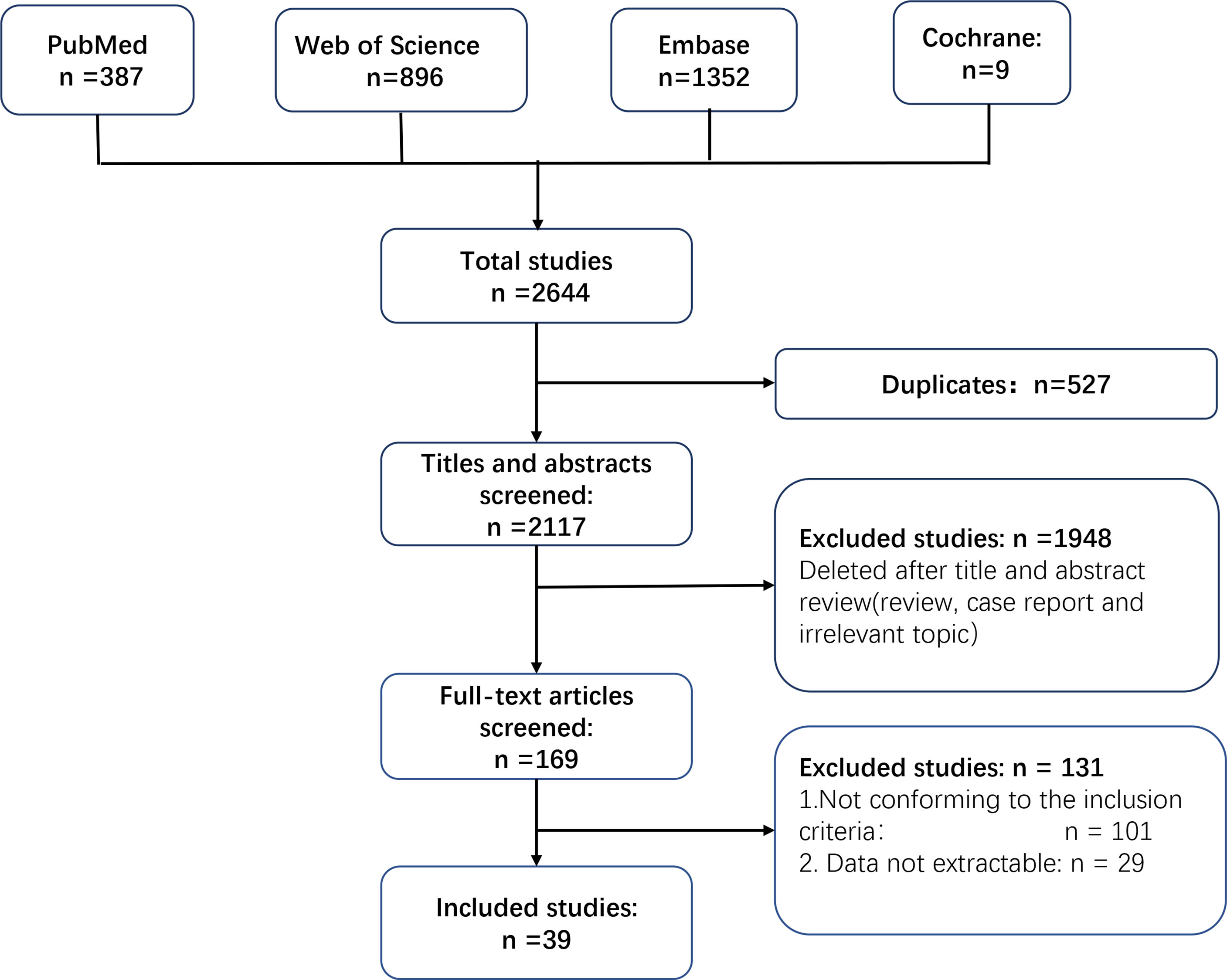
We identified 2,644 records in the last decade by searching PubMed, Embase, Web of Science, and Cochrane Library databases. A total of 527 studies were excluded because they contained duplicate data. A total of 1,948 studies were removed after screening of the title and abstract. JX and LM screened the full text of the remaining 169 studies independently. A total of 101 studies were excluded because they did not conform to the inclusion criteria, and an additional 29 studies were excluded because data could not be extracted. Finally, 39 studies were included for further analyses (14–16, 21–56). Figure 1 is a flowchart of how studies were chosen.
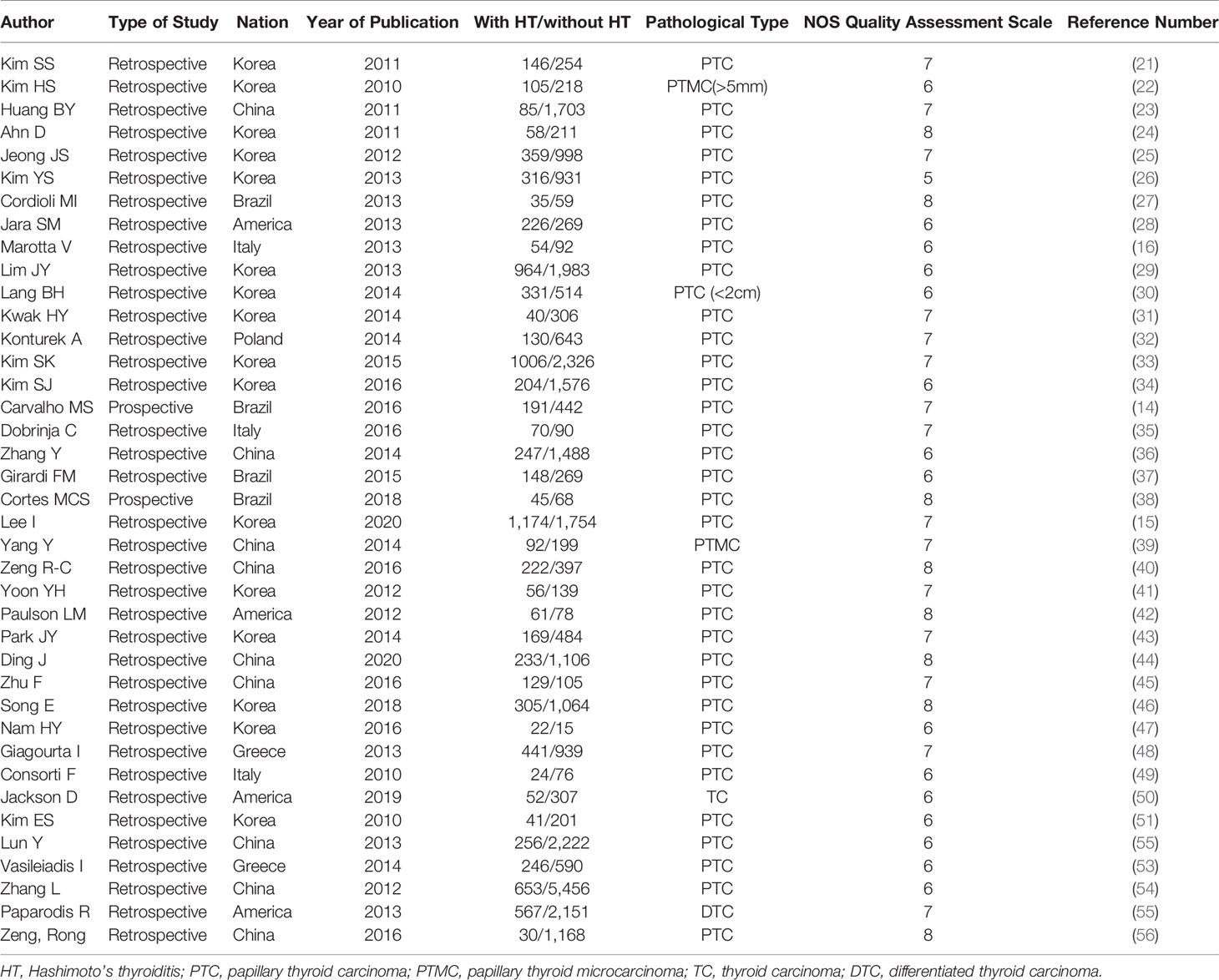
3.2 Characteristics and Methodological Quality of Included Studies
The characteristics and methodological quality of included studies are described in detail in Table 1. The included studies were retrospective except for the studies by Cortes and colleagues (38) and Carvalho and collaborators (14). According to the NOS score, studies were of high quality except for the study by Kim and colleagues (26), which received a NOS score of 5 points.
In the included studies, most scholars focused on HT and its relationship to PTC. However, a few studies simultaneously analyzed multiple pathological types of TC (PTC, follicular, medullary, and undifferentiated).
3.3 HT as a Risk Factor of TC and PTC
We wished to control for a confounding bias. Hence, we assessed if HT plays a part in multiple pathological types of TC (PTC, follicular, medullary, and undifferentiated) and whether HT is a risk factor in PTC patients (Figure 2).
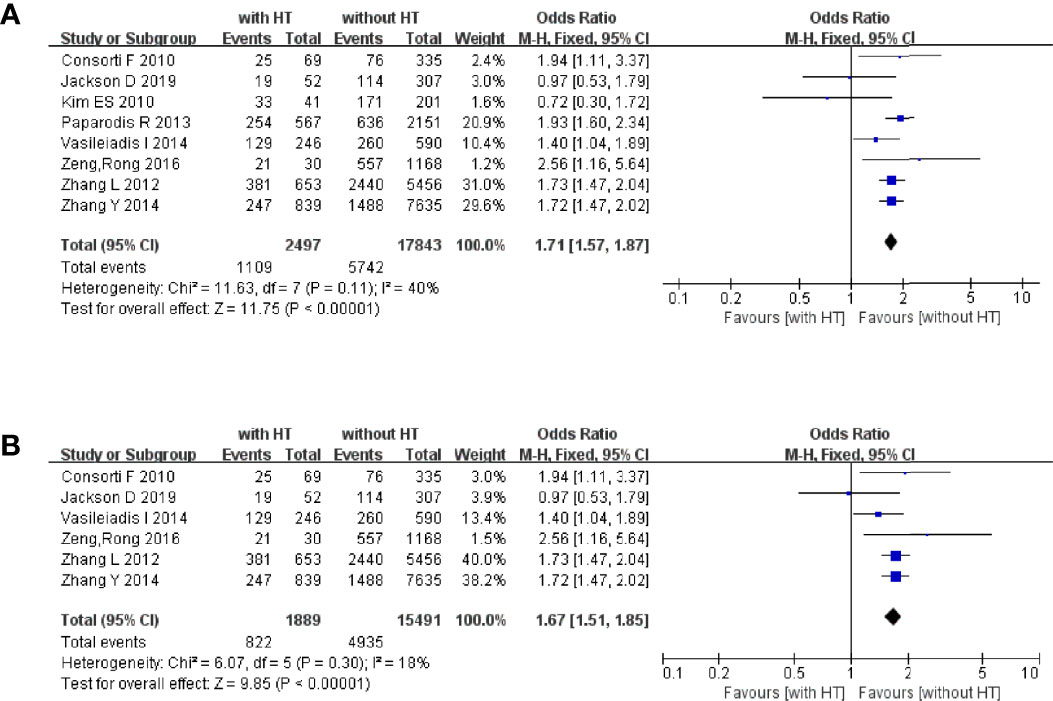
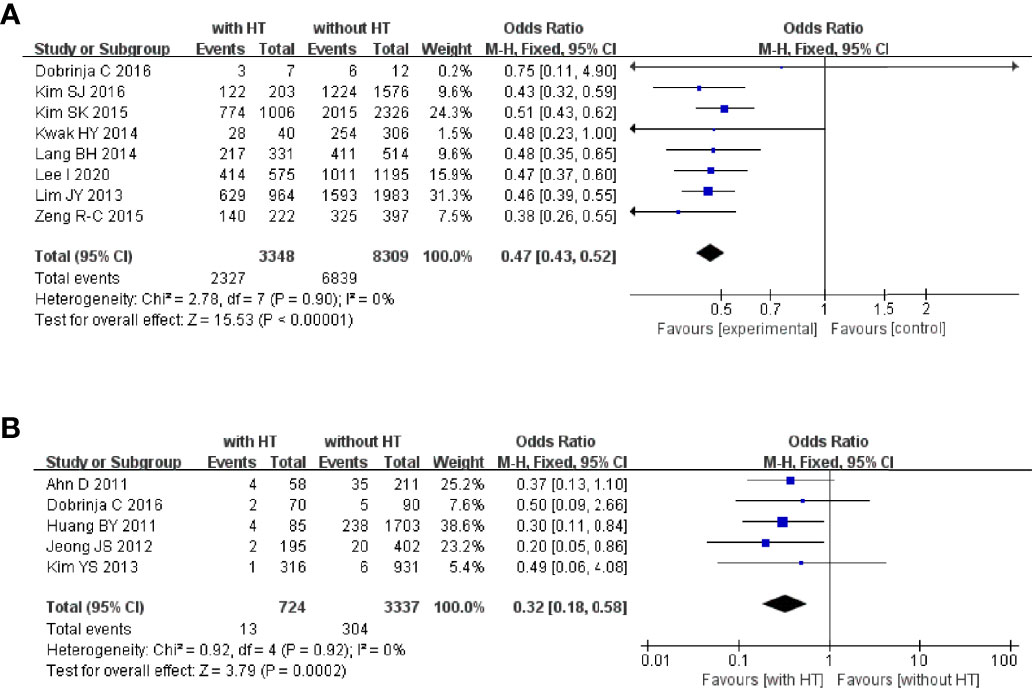
Figure 2 HT as a risk factor of TC and PTC: (A) forest plot of HT and TC and (B) forest plot of HT and PTC.
In 2,497 HT patients, 1,109 were finally diagnosed with TC regardless of the pathological type (Figure 2A). In 17,847 patients without HT, 5,742 were diagnosed with TC. The pooled OR was 1.71 (95%CI, 1.57–1.80; p < 0.00001, I2 = 40%, FEM), which provided evidence that HT is a risk factor for TC.
Six studies focused on the relationship between HT and PTC (Figure 2B). In 1,889 HT patients, 822 were diagnosed with PTC. In 15,491 patients without HT, 4,935 were diagnosed with PTC. The pooled OR was 1.67 (95%CI 1.51–1.85; p < 0.00001, I2 = 18%, FEM).
3.4 HT and Multiple Clinical Characteristics in PTC Patients
3.4.1 Multifocality and Extrathyroidal Extension
Nineteen studies reported the multifocality of PTC patients with or without HT. In PTC patients with HT, 2,018 of 5,793 patients had multifocal carcinomas. In PTC patients without HT, 4,443 of 14,880 patients had multifocal carcinomas (Figure 3A). The pooled OR was 1.17 (95%CI, 1.09–1.25; p < 0.00001, I2 = 0%, FEM). The funnel plot did not show an obvious publication bias (Figure 3B).
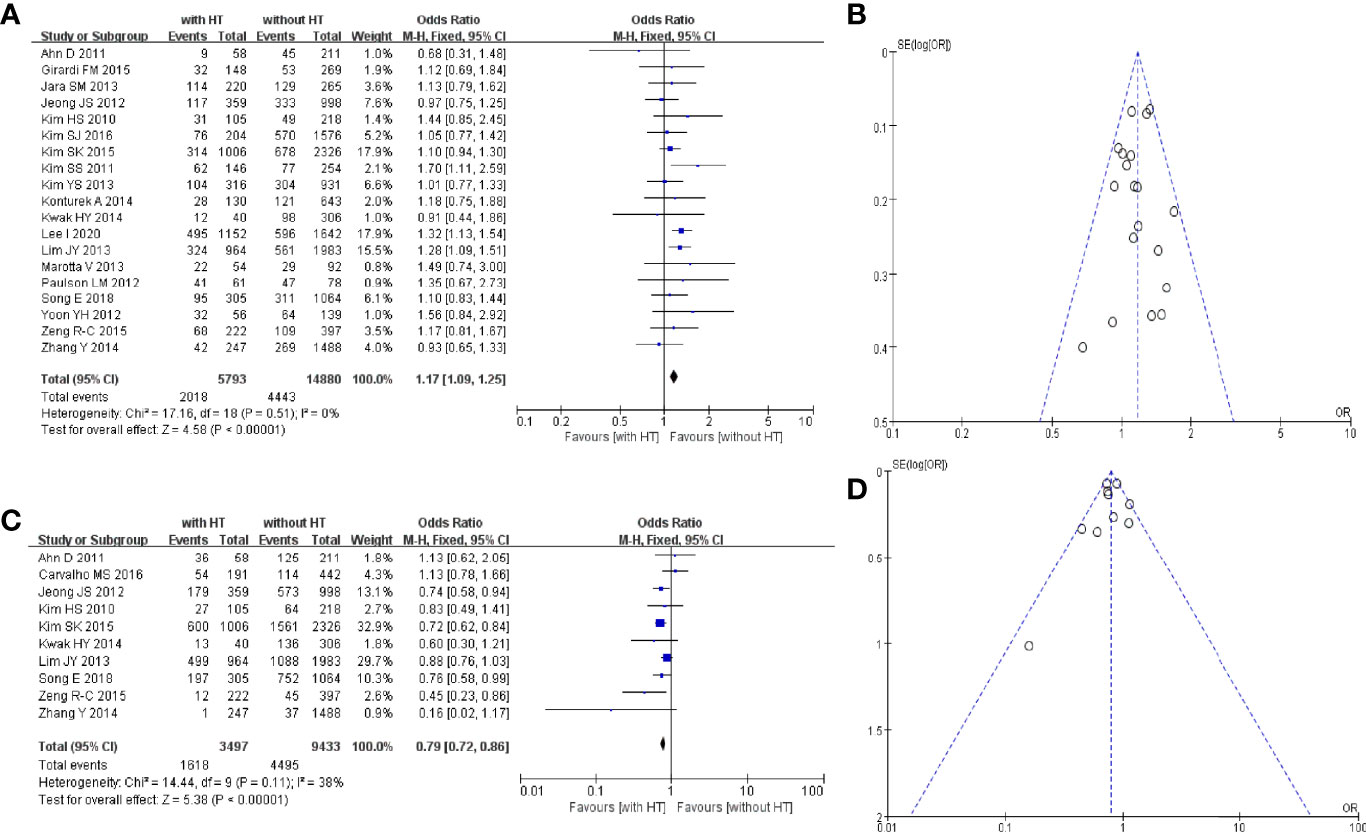
Figure 3 HT and multifocality of PTC patients: (A) forest plot and (B) funnel plot. HT and extrathyroidal extension of PTC patients: (C) forest plot and (D) funnel plot.
Ten studies reported the extrathyroidal extension of PTC patients with or without HT. In PTC patients with HT, 1,618 of 3,497 patients had an extrathyroidal extension. In PTC patients without HT, 4,495 of 9,433 patients had an extrathyroidal extension (Figure 3C). The pooled OR was 0.79 (95%CI, 0.72–0.86; p < 0.00001, I2 = 38%, FEM). The funnel plot did not show an obvious publication bias (Figure 3D).
3.4.2 Lymph-Node Metastasis and Distant Metastasis
Eleven studies reported metastasis of the central lymph nodes of PTC patients with or without HT. In PTC patients with HT, 1,784 of 3,847 patients had metastasis of the central lymph nodes. In PTC patients without HT, 4,835 of 9,685 patients had metastasis of the central lymph nodes (Figure 4A). The pooled OR was 0.80 (95%CI, 0.74–0.87; p < 0.00001, I2 = 37%, FEM). The funnel plot did not show an obvious publication bias (Figure 4B).
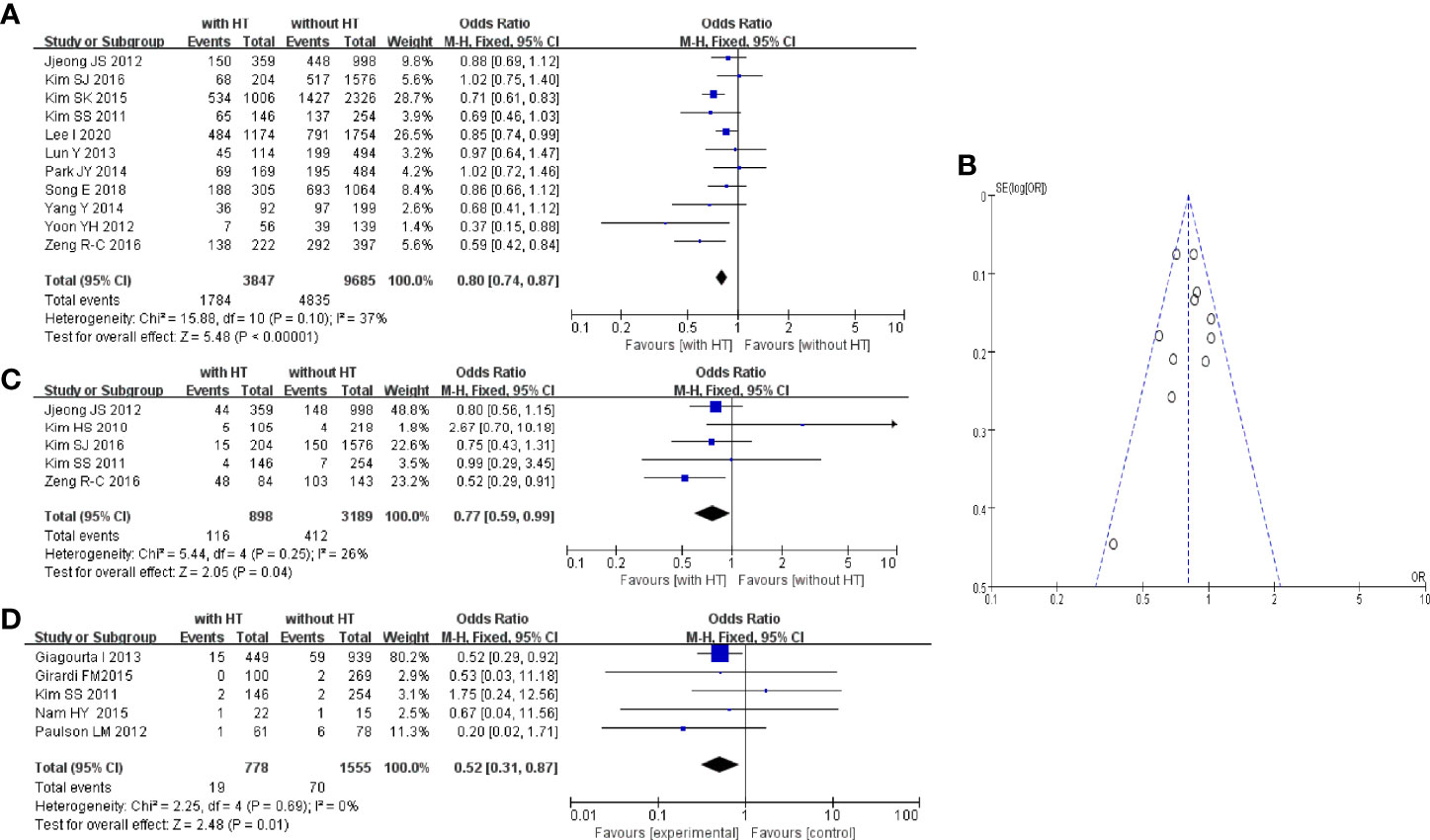
Figure 4 HT and central LN metastasis: (A) forest plot, (B) funnel plot; (C) Forest plot of HT and lateral LN metastasis; (D) forest plot of HT and distant metastasis.
Five studies reported metastasis of lateral lymph nodes of PTC patients with or without HT. In PTC patients with HT, 116 of 898 patients had metastasis of lateral lymph nodes. In PTC patients without HT, 412 of 3,189 patients had metastasis of lateral lymph nodes (Figure 4C). The pooled OR was 0.77 (95%CI, 0.59–0.99; p = 0.04, I2 = 26%, FEM).
Five studies reported distant metastasis in PTC patients with or without HT. In PTC patients with HT, 19 of 778 patients had distant metastasis. In PTC patients without HT, 70 of 1,555 patients had distant metastasis (Figure 4D). The pooled OR was 0.52 (95%CI, 0.31–0.87; p = 0.01, I2 = 0%, FEM).
3.4.3 BRAFV600E Mutation and Recurrence
Eight studies reported the BRAFV600E mutation in PTC patients with or without HT. In PTC patients with HT, 2,327 of 3,348 patients had the BRAFV600E mutation. In PTC patients without HT, 6,839 of 8,309 patients had the BRAFV600E (Figure 5A). The pooled OR was 0.47 (95%CI, 0.43–0.52; p < 0.00001, I2 = 0%, FEM).
Five studies reported the recurrence of PTC in patients with or without HT. In PTC patients with HT, 13 of 724 patients had tumor recurrence. In PTC patients without HT, 304 of 3,337 patients suffered tumor recurrence (Figure 5B). The pooled OR was 0.32 (95%CI, 0.18–0.58; p = 0.0002, I2 = 0%, FEM).
3.5 Sensitivity Analysis
Removal of the data of each study did not influence the pooled OR of the whole analysis. Transformation of the FEM or random-effects model did not influence the results. Hence, our analysis was reliable.
4 Discussion
The relationship between HT and TC was discussed first by Dailey in 1955 (57). Whether HT plays a part in the development and progression of TC has been controversial since then. We revealed a complicated relationship between HT and TC (especially PTC). HT appears to be a “double-edged sword” in TC. It is a potential risk factor in TC (especially PTC) patients. However, PTC patients with HT usually carry a better prognosis because they have a lower risk of extrathyroidal extension, BRAFV600E mutation, metastasis, or recurrence.
The relationship between autoimmune thyroid disease and TC has been controversial for decades. Giagurta and colleagues compared the prevalence of autoimmune thyroiditis between people with PTC and individuals with benign thyroid nodules over the past 16 years. They found that patients with thyroid nodules and autoimmune thyroiditis were not more likely to have malignant thyroid nodules than individuals without autoimmune thyroiditis (48). Del Rio and colleagues conducted a prospective cohort study of 9,851 patients who underwent assessment of thyroid nodules between 1995 and 2017 (13). They showed that, for people with HT, the risk of malignant thyroid nodules was increased significantly. Our meta-analysis showed that the risk of TC or PTC in thyroid nodules for people with HT was increased. Therefore, we concluded that HT is a risk factor of TC or PTC.
Three possible pathogenic mechanisms can explain the results of our meta-analysis. First, the inflammatory response creates a favorable environment for malignant transformation. The damage wrought by cytokines and growth factors to stromal cells leads to changes in stromal reactivity, which, in turn, can lead to the malignant transformation of epithelial cells (58). In addition, the infiltration of immune cells to the thyroid gland may promote abnormal repair of DNA, thereby inducing PTC (59). Second, TSH is not only an endogenous stimulator of thyroid-hormone production, it is also a growth factor for thyroid cells (60). An increased level of TSH in most HT patients stimulates follicular epithelial hyperplasia, which promotes PTC. Third, expression of some oncogenes, such as RET/PTC gene rearrangement (61) and p63 mutation (62), may be involved in the transformation from HT to PTC. In contrast, the BRAFV600E mutation, which is usually mutually exclusive with RET/PTC gene rearrangement (63), is more common in PTC without HT, a finding that is consistent with our meta-analysis.
Usually, TC (especially PTC) is considered to be less aggressive and to carry a better prognosis than that of a malignant tumor. However, the complicated extrathyroidal extension and various types of metastasis can be fatal in some circumstances. PTC shows a high tendency to spread to regional lymph nodes. The central region is the main region of lymph-node involvement in 20%–90% of PTC patients (64). Lymph-node metastasis is the main risk factor for PTC recurrence and is highly correlated with progression-free survival and overall survival in PTC patients (65, 66). Our meta-analysis showed PTC patients with HT to have a lower prevalence of lymph-node metastasis, distant metastasis, and recurrence. This conclusion is not consistent with the conclusions reached by Mao et al. (67) or Sun and colleagues (68). One reason for the different results could be a confounding bias (e.g., they did not distinguish lymph nodes from different areas).
According to our meta-analysis, PTC patients with HT had more favorable clinical characteristics and a better prognosis than PTC patients not suffering from HT. The latter is more common in young women. Thus, patients with HT are prone to be anxious and to undergo ultrasound of the thyroid gland frequently, so discovery of a malignant thyroid nodule at an early stage is likely. Multifocal carcinomas are independent risk factors for the TC prognosis. HT patients are more likely to have multifocal carcinomas, which leads to more aggressive and radical surgery, so their recurrence risk is lower than that of PTC patients without HT. Marotta and colleagues showed lymphocyte infiltration in HT patients to be a protective factor against PTC progression (16). Zhang et al. suggested that the BRAFV600E mutation can help to predict the prognosis of PTC (69). The BRAFV600E mutation is a marker of more aggressive behavior of PTC. In addition, the BRAFV600E mutation is less common in PTC patients with HT than in PTC patients without HT. The results of our meta-analysis and the studies stated above suggest that HT is a protective factor against PTC progression, but the mechanism of action merits further study.
Our meta-analysis explored the relationship between PTC and HT from occurrence to progression, but had two main limitations. First, the included studies were mainly retrospective: more prospective studies and real-world studies are needed to draw more accurate conclusions. Second, the included studies involved mainly Asian patients. More prospective cohort studies involving multiple ethnicities are needed to further clarify the relationship between HT and PTC.
5 Conclusions
HT is a double-edged sword in TC patients. HT increases the risk of TC and PTC but is a protective factor against PTC progression.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Second Xiangya Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JX and KD provided main effort in the procedure of meta-analysis and manuscript editing. LM, FY, and CG offered great help in the data extraction and data analysis. JH and CR gave many valuable suggestions to this article. CR also contributed to the manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China under Grant 2019YFE0190500.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge the librarians at the Libraries of Central South University for their efforts in obtaining primary resources for this meta-analysis. Additionally, we would like to acknowledge professors, colleagues, friends, and family members who assisted and gave encouragements in the writing procedure of this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.801925/full#supplementary-material
Abbreviations
HT, Hashimoto’s thyroiditis; TC, thyroid carcinoma; PTC, papillary thyroid carcinoma; TSH, thyroid-stimulating hormone
References
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
2. Londero SC, Krogdahl A, Bastholt L, Overgaard J, Pedersen HB, Hahn CH, et al. Papillary Thyroid Carcinoma in Denmark, 1996-2008: Outcome and Evaluation of Established Prognostic Scoring Systems in a Prospective National Cohort. Thyroid (2015) 25(1):78–84. doi: 10.1089/thy.2014.0294
3. Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid Cancer in Denmark 1943-2008, Before and After Iodine Supplementation. Int J Cancer (2012) 131(10):2360–6. doi: 10.1002/ijc.27497
4. Rizzo M, Rossi RT, Bonaffini O, Scisca C, Altavilla G, Calbo L, et al. Increased Annual Frequency of Hashimoto's Thyroiditis Between Years 1988 and 2007 at a Cytological Unit of Sicily. Ann Endocrinol (Paris) (2010) 71(6):525–34. doi: 10.1016/j.ando.2010.06.006
5. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune Thyroid Disorders. Autoimmun Rev (2015) 14(2):174–80. doi: 10.1016/j.autrev.2014.10.016
6. Caturegli P, De Remigis A, Rose NR. Hashimoto Thyroiditis: Clinical and Diagnostic Criteria. Autoimmun Rev (2014) 13(4-5):391–7. doi: 10.1016/j.autrev.2014.01.007
7. Latina A, Gullo D, Trimarchi F, Benvenga S. Hashimoto's Thyroiditis: Similar and Dissimilar Characteristics in Neighboring Areas. Possible Implications for the Epidemiology of Thyroid Cancer. PloS One (2013) 8(3):e55450. doi: 10.1371/journal.pone.0055450
8. Benvenga S, Guarneri F. Molecular Mimicry and Autoimmune Thyroid Disease. Rev Endocr Metab Disord (2016) 17(4):485–98. doi: 10.1007/s11154-016-9363-2
9. Fung J, Lai CL, Yuen MF. Hepatitis B and C Virus-Related Carcinogenesis. Clin Microbiol Infect (2009) 15(11):964–70. doi: 10.1111/j.1469-0691.2009.03035.x
10. Balkwill F, Mantovani A. Inflammation and Cancer: Back to Virchow? Lancet (2001) 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0
11. Guan H, de Morais NS, Stuart J, Ahmadi S, Marqusee E, Kim MI, et al. Discordance of Serological and Sonographic Markers for Hashimoto's Thyroiditis With Gold Standard Histopathology. Eur J Endocrinol (2019) 181(5):539–44. doi: 10.1530/EJE-19-0424
12. Silva de Morais N, Stuart J, Guan H, Wang Z, Cibas ES, Frates MC, et al. The Impact of Hashimoto Thyroiditis on Thyroid Nodule Cytology and Risk of Thyroid Cancer. J Endocr Soc (2019) 3(4):791–800. doi: 10.1210/js.2018-00427
13. Del Rio P, Montana Montana C, Cozzani F, Rossini M, Loderer T, Dall'Aglio E, et al. Is There a Correlation Between Thyroiditis and Thyroid Cancer? Endocrine (2019) 66(3):538–41. doi: 10.1007/s12020-019-01935-8
14. Carvalho MS, Rosario PW, Mourao GF, Calsolari MR. Chronic Lymphocytic Thyroiditis Does Not Influence the Risk of Recurrence in Patients With Papillary Thyroid Carcinoma and Excellent Response to Initial Therapy. Endocrine (2017) 55(3):954–8. doi: 10.1007/s12020-016-1185-1
15. Lee I, Kim HK, Soh EY, Lee J. The Association Between Chronic Lymphocytic Thyroiditis and the Progress of Papillary Thyroid Cancer. World J Surg (2020) 44(5):1506–13. doi: 10.1007/s00268-019-05337-9
16. Marotta V, Guerra A, Zatelli MC, Uberti ED, Di Stasi V, Faggiano A, et al. BRAF Mutation Positive Papillary Thyroid Carcinoma Is Less Advanced When Hashimoto's Thyroiditis Lymphocytic Infiltration Is Present. Clin Endocrinol (Oxf) (2013) 79(5):733–8. doi: 10.1111/cen.12194
17. Kim WW, Ha TK, Bae SK. Clinical Implications of the BRAF Mutation in Papillary Thyroid Carcinoma and Chronic Lymphocytic Thyroiditis. J Otolaryngol Head Neck Surg (2018) 47(1):4. doi: 10.1186/s40463-017-0247-6
18. Sakiz D, Sencar ME, Calapkulu M, Ozturk Unsal I, Aktas L, Ucan B, et al. The Effects of Chronic Lymphocytic Thyroiditis on Clinicopathologic Factors in Papillary Thyroid Cancer. Endocr Pract (2021) 27(12):1199–204. doi: 10.1016/j.eprac.2021.07.011
19. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
20. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
21. Kim SS, Lee BJ, Lee JC, Kim SJ, Jeon YK, Kim MR, et al. Coexistence of Hashimoto's Thyroiditis With Papillary Thyroid Carcinoma: The Influence of Lymph Node Metastasis. Head Neck (2011) 33(9):1272–7. doi: 10.1002/hed.21594
22. Kim HS, Choi YJ, Yun JS. Features of Papillary Thyroid Microcarcinoma in the Presence and Absence of Lymphocytic Thyroiditis. Endocr Pathol (2010) 21(3):149–53. doi: 10.1007/s12022-010-9124-9
23. Huang BY, Hseuh C, Chao TC, Lin KJ, Lin JD. Well-Differentiated Thyroid Carcinoma With Concomitant Hashimoto's Thyroiditis Present With Less Aggressive Clinical Stage and Low Recurrence. Endocr Pathol (2011) 22(3):144–9. doi: 10.1007/s12022-011-9164-9
24. Ahn D, Heo SJ, Park JH, Kim JH, Sohn JH, Park JY, et al. Clinical Relationship Between Hashimoto's Thyroiditis and Papillary Thyroid Cancer. Acta Oncol (2011) 50(8):1228–34. doi: 10.3109/0284186X.2011.602109
25. Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, et al. Coexistence of Chronic Lymphocytic Thyroiditis With Papillary Thyroid Carcinoma: Clinical Manifestation and Prognostic Outcome. J Korean Med Sci (2012) 27(8):883–9. doi: 10.3346/jkms.2012.27.8.883
26. Kim YS, Choi HJ, Kim ES. Papillary Thyroid Carcinoma With Thyroiditis: Lymph Node Metastasis, Complications. J Korean Surg Soc (2013) 85(1):20–4. doi: 10.4174/jkss.2013.85.1.20
27. Cordioli MI, Cury AN, Nascimento AO, Oliveira AK, Mello M, Saieg MA. Study of the Histological Profile of Papillary Thyroid Carcinomas Associated With Hashimoto's Thyroiditis. Arq Bras Endocrinol Metabol (2013) 57(6):445–9. doi: 10.1590/s0004-27302013000600006
28. Jara SM, Carson KA, Pai SI, Agrawal N, Richmon JD, Prescott JD, et al. The Relationship Between Chronic Lymphocytic Thyroiditis and Central Neck Lymph Node Metastasis in North American Patients With Papillary Thyroid Carcinoma. Surgery (2013) 154(6):1272–80; discussion 80-2. doi: 10.1016/j.surg.2013.07.021
29. Lim JY, Hong SW, Lee YS, Kim BW, Park CS, Chang HS, et al. Clinicopathologic Implications of the BRAF(V600E) Mutation in Papillary Thyroid Cancer: A Subgroup Analysis of 3130 Cases in a Single Center. Thyroid (2013) 23(11):1423–30. doi: 10.1089/thy.2013.0036
30. Lang BH, Chai YJ, Cowling BJ, Min HS, Lee KE, Youn YK. Is BRAFV600E Mutation a Marker for Central Nodal Metastasis in Small Papillary Thyroid Carcinoma? Endocr Relat Cancer (2014) 21(2):285–95. doi: 10.1530/ERC-13-0291
31. Kwak HY, Chae BJ, Eom YH, Hong YR, Seo JB, Lee SH, et al. Does Papillary Thyroid Carcinoma Have a Better Prognosis With or Without Hashimoto Thyroiditis? Int J Clin Oncol (2015) 20(3):463–73. doi: 10.1007/s10147-014-0754-7
32. Konturek A, Barczynski M, Nowak W, Wierzchowski W. Risk of Lymph Node Metastases in Multifocal Papillary Thyroid Cancer Associated With Hashimoto's Thyroiditis. Langenbecks Arch Surg (2014) 399(2):229–36. doi: 10.1007/s00423-013-1158-2
33. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Chronic Lymphocytic Thyroiditis and BRAF V600E in Papillary Thyroid Carcinoma. Endocr Relat Cancer (2016) 23(1):27–34. doi: 10.1530/ERC-15-0408
34. Kim SJ, Myong JP, Jee HG, Chai YJ, Choi JY, Min HS, et al. Combined Effect of Hashimoto's Thyroiditis and BRAF(V600E) Mutation Status on Aggressiveness in Papillary Thyroid Cancer. Head Neck (2016) 38(1):95–101. doi: 10.1002/hed.23854
35. Dobrinja C, Makovac P, Pastoricchio M, Cipolat Mis T, Bernardi S, Fabris B, et al. Coexistence of Chronic Lymphocytic Thyroiditis and Papillary Thyroid Carcinoma. Impact on Presentation, Management, and Outcome. Int J Surg (2016) 28(Suppl 1):S70–4. doi: 10.1016/j.ijsu.2015.12.059
36. Zhang Y, Dai J, Wu T, Yang N, Yin Z. The Study of the Coexistence of Hashimoto's Thyroiditis With Papillary Thyroid Carcinoma. J Cancer Res Clin Oncol (2014) 140(6):1021–6. doi: 10.1007/s00432-014-1629-z
37. Girardi FM, Barra MB, Zettler CG. Papillary Thyroid Carcinoma: Does the Association With Hashimoto's Thyroiditis Affect the Clinicopathological Characteristics of the Disease? Braz J Otorhinolaryngol (2015) 81(3):283–7. doi: 10.1016/j.bjorl.2014.04.006
38. Cortes MCS, Rosario PW, Mourao GF, Calsolari MR. Influence of Chronic Lymphocytic Thyroiditis on the Risk of Persistent and Recurrent Disease in Patients With Papillary Thyroid Carcinoma and Elevated Antithyroglobulin Antibodies After Initial Therapy. Braz J Otorhinolaryngol (2018) 84(4):448–52. doi: 10.1016/j.bjorl.2017.05.005
39. Yang Y, Chen C, Chen Z, Jiang J, Chen Y, Jin L, et al. Prediction of Central Compartment Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Clin Endocrinol (Oxf) (2014) 81(2):282–8. doi: 10.1111/cen.12417
40. Zeng R-C, Jin L-P, Chen E-D, Dong S-Y, Cai Y-F, Huang G-L, et al. Potential Relationship Between Hashimoto's Thyroiditis and BRAF(V600E) Mutation Status in Papillary Thyroid Cancer. Head Neck (2016) 38(Suppl 1):E1019–E25. doi: 10.1002/hed.24149
41. Yoon YH, Kim HJ, Lee JW, Kim JM, Koo BS. The Clinicopathologic Differences in Papillary Thyroid Carcinoma With or Without Co-Existing Chronic Lymphocytic Thyroiditis. Eur Arch Otorhinolaryngol (2012) 269(3):1013–7. doi: 10.1007/s00405-011-1732-6
42. Paulson LM, Shindo ML, Schuff KG. Role of Chronic Lymphocytic Thyroiditis in Central Node Metastasis of Papillary Thyroid Carcinoma. Otolaryngol Head Neck Surg (2012) 147(3):444–9. doi: 10.1177/0194599812445727
43. Park JY, Kim DW, Park HK, Ha TK, Jung SJ, Kim DH, et al. Comparison of T Stage, N Stage, Multifocality, and Bilaterality in Papillary Thyroid Carcinoma Patients According to the Presence of Coexisting Lymphocytic Thyroiditis. Endocr Res (2015) 40(3):151–5. doi: 10.3109/07435800.2014.977911
44. Ding J, Wu W, Fang J, Zhao J, Jiang L. Male Sex Is Associated With Aggressive Behaviour and Poor Prognosis in Chinese Papillary Thyroid Carcinoma. Sci Rep (2020) 10(1):4141. doi: 10.1038/s41598-020-60199-9
45. Zhu F, Shen YB, Li FQ, Fang Y, Hu L, Wu YJ. The Effects of Hashimoto Thyroiditis on Lymph Node Metastases in Unifocal and Multifocal Papillary Thyroid Carcinoma: A Retrospective Chinese Cohort Study. Med (Baltimore) (2016) 95(6):e2674. doi: 10.1097/MD.0000000000002674
46. Song E, Jeon MJ, Park S, Kim M, Oh HS, Song DE, et al. Influence of Coexistent Hashimoto's Thyroiditis on the Extent of Cervical Lymph Node Dissection and Prognosis in Papillary Thyroid Carcinoma. Clin Endocrinol (Oxf) (2018) 88(1):123–8. doi: 10.1111/cen.13475
47. Nam HY, Lee HY, Park GC. Impact of Co-Existent Thyroiditis on Clinical Outcome in Papillary Thyroid Carcinoma With High Preoperative Serum Antithyroglobulin Antibody: A Retrospective Cohort Study. Clin Otolaryngol (2016) 41(4):358–64. doi: 10.1111/coa.12520
48. Giagourta I, Evangelopoulou C, Papaioannou G, Kassi G, Zapanti E, Prokopiou M, et al. Autoimmune Thyroiditis in Benign and Malignant Thyroid Nodules: 16-Year Results. Head Neck (2014) 36(4):531–5. doi: 10.1002/hed.23331
49. Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of Malignancy From Thyroid Nodular Disease as an Element of Clinical Management of Patients With Hashimoto's Thyroiditis. Eur Surg Res (2010) 45(3-4):333–7. doi: 10.1159/000320954
50. Jackson D, Handelsman RS, Farra JC, Lew JI. Increased Incidental Thyroid Cancer in Patients With Subclinical Chronic Lymphocytic Thyroiditis. J Surg Res (2020) 245:115–8. doi: 10.1016/j.jss.2019.07.025
51. Kim ES, Lim DJ, Baek KH, Lee JM, Kim MK, Kwon HS, et al. Thyroglobulin Antibody Is Associated With Increased Cancer Risk in Thyroid Nodules. Thyroid (2010) 20(8):885–91. doi: 10.1089/thy.2009.0384
52. Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, et al. Hashimoto's Thyroiditis as a Risk Factor of Papillary Thyroid Cancer May Improve Cancer Prognosis. Otolaryngol–Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg (2013) 148(3):396–402. doi: 10.1177/0194599812472426
53. Vasileiadis I, Boutzios G, Charitoudis G, Koukoulioti E, Karatzas T. Thyroglobulin Antibodies Could be a Potential Predictive Marker for Papillary Thyroid Carcinoma. Ann Surg Oncol (2014) 21(8):2725–32. doi: 10.1245/s10434-014-3593-x
54. Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. The Clinical Features of Papillary Thyroid Cancer in Hashimoto's Thyroiditis Patients From an Area With a High Prevalence of Hashimoto's Disease. BMC Cancer (2012) 12:610. doi: 10.1186/1471-2407-12-610
55. Paparodis R, Imam S, Todorova-Koteva K, Staii A, Jaume JC. Hashimoto's Thyroiditis Pathology and Risk for Thyroid Cancer. Thyroid (2014) 24(7):1107–14. doi: 10.1089/thy.2013.0588
56. Zeng R, Shou T, Yang K-X, Shen T, Zhang J-P, Zuo R-X, et al. Papillary Thyroid Carcinoma Risk Factors in the Yunnan Plateau of Southwestern China. Ther Clin Risk Manag (2016) 12:1065–74. doi: 10.2147/TCRM.S105023
57. Dailey ME, Lindsay S, Skahen R. Relation of Thyroid Neoplasms to Hashimoto Disease of the Thyroid Gland. AMA Arch Surg (1955) 70(2):291–7. doi: 10.1001/archsurg.1955.01270080137023
58. Buyukasik O, Hasdemir AO, Yalcin E, Celep B, Sengul S, Yandakci K, et al. The Association Between Thyroid Malignancy and Chronic Lymphocytic Thyroiditis: Should It Alter the Surgical Approach? Endokrynol Pol (2011) 62(4):303–8.
59. Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto's Thyroiditis and Papillary Thyroid Carcinoma: Is There a Correlation? J Clin Endocrinol Metab (2013) 98(2):474–82. doi: 10.1210/jc.2012-2978
60. Nicolson NG, Brown TC, Korah R, Carling T. Immune Cell Infiltrate-Associated Dysregulation of DNA Repair Machinery may Predispose to Papillary Thyroid Carcinogenesis. Surgery (2020) 167(1):66–72. doi: 10.1016/j.surg.2019.02.024
61. McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and Thyroid Cancer Diagnosis: A Systematic Review and Dose-Response Meta-Analysis. J Clin Endocrinol Metab (2012) 97(8):2682–92. doi: 10.1210/jc.2012-1083
62. Wirtschafter A, Schmidt R, Rosen D, Kundu N, Santoro M, Fusco A, et al. Expression of the RET/PTC Fusion Gene as a Marker for Papillary Carcinoma in Hashimoto's Thyroiditis. Laryngoscope (1997) 107(1):95–100. doi: 10.1097/00005537-199701000-00019
63. Unger P, Ewart M, Wang BY, Gan L, Kohtz DS, Burstein DE. Expression of P63 in Papillary Thyroid Carcinoma and in Hashimoto's Thyroiditis: A Pathobiologic Link? Hum Pathol (2003) 34(8):764–9. doi: 10.1016/s0046-8177(03)00239-9
64. Kim KH, Suh KS, Kang DW, Kang DY. Mutations of the BRAF Gene in Papillary Thyroid Carcinoma and in Hashimoto's Thyroiditis. Pathol Int (2005) 55(9):540–5. doi: 10.1111/j.1440-1827.2005.01866.x
65. Kim SY, Kim BW, Pyo JY, Hong SW, Chang HS, Park CS. Macrometastasis in Papillary Thyroid Cancer Patients Is Associated With Higher Recurrence in Lateral Neck Nodes. World J Surg (2018) 42(1):123–9. doi: 10.1007/s00268-017-4158-5
66. Pelizzo MR, Boschin IM, Toniato A, Piotto A, Pagetta C, Gross MD, et al. Papillary Thyroid Carcinoma: 35-Year Outcome and Prognostic Factors in 1858 Patients. Clin Nucl Med (2007) 32(6):440–4. doi: 10.1097/RLU.0b013e31805375ca
67. Mao J, Zhang Q, Zhang H, Zheng K, Wang R, Wang G. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) (2020) 11:265. doi: 10.3389/fendo.2020.00265
68. Sun W, Lan X, Zhang H, Dong W, Wang Z, He L, et al. Risk Factors for Central Lymph Node Metastasis in CN0 Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. PloS One (2015) 10(10):e0139021. doi: 10.1371/journal.pone.0139021
Keywords: Hashimoto’s thyroiditis (HT), thyroid carcinoma (TC), meta-analysis, papillary thyroid carcinoma, BRAFV600E mutation
Citation: Xu J, Ding K, Mu L, Huang J, Ye F, Peng Y, Guo C and Ren C (2022) Hashimoto’s Thyroiditis: A “Double-Edged Sword” in Thyroid Carcinoma. Front. Endocrinol. 13:801925. doi: 10.3389/fendo.2022.801925
Received: 26 October 2021; Accepted: 18 January 2022;
Published: 24 February 2022.
Edited by:
Catherine Fiona Sinclair, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Laura Sterian Ward, State University of Campinas, BrazilChristoph Reiners, University Hospital Würzburg, Germany
Zhihong Wang, China Medical University, China
Copyright © 2022 Xu, Ding, Mu, Huang, Ye, Peng, Guo and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chutong Ren, MjIwNDEyMDYwMkBjc3UuZWR1LmNu
†These authors have contributed equally to this work
 Jiangyue Xu
Jiangyue Xu Ke Ding
Ke Ding Lan Mu
Lan Mu Jiangsheng Huang
Jiangsheng Huang Fei Ye
Fei Ye Yu Peng
Yu Peng Can Guo
Can Guo Chutong Ren
Chutong Ren