- 1Department of Biology, The Pennsylvania State University, University Park, PA, United States
- 2Intercollege Graduate Degree Program in Ecology, The Pennsylvania State University, University Park, PA, United States
- 3The Department of Ecosystem Science and Management, The Pennsylvania State University, University Park, PA, United States
- 4Department of Biological Sciences, University of Alabama, Tuscaloosa, AL, United States
Colorful traits (i.e., ornaments) that signal quality have well-established relationships with individual condition and physiology. Furthermore, ornaments expressed in females may have indirect fitness effects in offspring via the prenatal physiology associated with, and social consequences of, these signaling traits. Here we examine the influence of prenatal maternal physiology and phenotype on condition-dependent signals of their offspring in adulthood. Specifically, we explore how prenatal maternal testosterone, corticosterone, and ornament color and size correlate with female and male offspring survival to adulthood and ornament quality in the lizard Sceloporus undulatus. Offspring of females with more saturated badges and high prenatal corticosterone were less likely to survive to maturity. Badge saturation and area were negatively correlated between mothers and their male offspring, and uncorrelated to those in female offspring. Maternal prenatal corticosterone was correlated negatively with badge saturation of male offspring in adulthood. Our results indicate that maternal ornamentation and prenatal concentrations of a stress-relevant hormone can lead to compounding fitness costs by reducing offspring survival to maturity and impairing expression of a signal of quality in surviving males. This mechanism may occur in concert with social costs of ornamentation in mothers. Intergenerational effects of female ornamentation and prenatal stress may be interdependent drivers of balancing selection and intralocus sexual conflict over signaling traits.
Introduction
Many animal species employ visually conspicuous traits in communication and signaling (1, 2). The expression and strength of such signals can be mediated by hormones, such as sex steroids (3) and glucocorticoids involved in stress responses (4). However, individual phenotype and fitness can also be influenced by maternal hormones during the prenatal phase (5). Evidence for maternal physiological effects on progeny are widespread and diverse, ranging from phenotypic expression (6) to hatching success and survival (7). In females, the prevalence and adaptive potential of sexual ornaments has received significant attention (8–12), but the maternal influence – either via genetic inheritance or prenatal effects – on adaptive expression of these traits in offspring remains unclear. Intergenerational associations between ornaments in offspring and maternal hormones and/or maternal inheritance might have important fitness consequences and could represent alternative ways in which female ornamentation can be evolutionarily relevant.
Maternal prenatal physiology can affect the fitness of progeny in various ways. A commonly proposed mechanism for these intergenerational responses is embryonic exposure in ovo or in utero to circulating maternal hormones that cross the amniotic barrier such as androgens (13, 14) and glucocorticoids (15). Recent work highlights the adaptive potential of prenatal effects within changing environments (16, 17), including behavioral and morphological changes in offspring that enhance survival (18). Maternal glucocorticoids may also impair offspring fitness by reducing their survival (19, 20) or egg hatching success (21). Thus, the generality of adaptive transgenerational effects is still debated (22, 23), and their range of influence requires further investigation. Condition-dependent signals such as sexual ornaments are often co-regulated by hormones with intergenerational potential (24), and thus constitute fitness-relevant traits that might be influenced by maternal effects.
Many species of the lizard family Phrynosomatidae exhibit conspicuous ventral coloration that functions in intraspecific communication (25, 26). In the eastern fence lizard Sceloporus undulatus, sexually mature males bear blue ventral and gular badges formed by iridophores and melanophores (Figure 1). Males with more saturated badges have higher body condition and immune response, indicating that color is an advantageous signal of superior quality to conspecifics (27). Females have badges that lack the melanin pigmentation seen in males and consequently show a fainter (less saturated) blue (28). The fitness consequences of ornamentation in female fence lizards is still ambiguous, with studies linking it to costs (25, 29) and benefits (30). Further, the size and saturation of these color patches are co-regulated by individual condition and physiology, with testosterone (T), dihydrotestosterone (DHT), and corticosterone (CORT) playing important roles (27, 31, 32). If badges of fence lizards are correlated between mothers and offspring, or if prenatal maternal physiology is relevant to trait development in offspring, then ornamentation in mothers could be associated with increased grand-parentage because male offspring with more vivid badges are more successful in attracting mates (33).

Figure 1 Above: eastern fence lizard in early adulthood in early adulthood and released after the conclusion of this study; below: representative photos of mature offspring used in this study. Left: female; center: male with highly saturated badges; right: male with low saturation badges. All lizards were approximately 323 days of age.
We hypothesized that maternal T and CORT during pregnancy would affect clutch survival and ornament quality in offspring. We monitored 120 fence lizard hatchlings from 12 mothers throughout early ontogeny (i.e., hatching to sexual maturity), and predicted that: 1) high maternal CORT would reduce offspring survival to sexual maturity while low maternal CORT and high maternal T would improve offspring ornament quality (color saturation and badge area) due to known intraindividual relationships between these hormones and traits (27), and 2) maternal ornament quality would be correlated with that of their offspring due to maternal genetic inheritance. Support for these predictions could indicate indirect fitness benefits of female ornamentation via maternal investment in their offspring, with maternal physiology and genetic inheritance as potential mechanisms maintaining secondary sexual characteristics in females.
Methods
Animal Collection and Housing
We collected gravid females from the St. Francis National Forest and private lands in Lee County, Arkansas (AR), USA (n = 9), and from the Edgar Evins State Park and Land Between the Lakes National Recreation Area, Tennessee (TN), USA (n = 3). Immediately after capture, we collected blood samples from the post-orbital sinus (34) which were kept on ice until centrifuged for plasma extraction and storage at -20°C for future hormone assays. For all lizards, time to capture and obtain a blood sample was < 285 seconds (mean 192 seconds ± 47 SE). For one individual, the time elapsed from its sighting until blood sampling was 633 seconds. It has been demonstrated for this species that upregulation of CORT does not occur until 600 seconds after disturbance (35). Furthermore, this individual’s plasma CORT concentration was 13.24ng/mL, close the population mean for this study (15.41 ± 2.14 SE; range: 1.10, 31.03). These females were brought to an animal housing facility where they laid eggs, which were incubated at 30°C until hatching (47 females and 44 males from AR; 15 females and 14 males from TN). Sex of hatchlings was determined by the presence of post-anal scales in males. Up to five non-siblings from mothers of the same site were housed in common garden conditions, as described elsewhere (27). Thus, offspring were raised in environments distinct from their mothers’, allowing us to reduce confounding effects of shared environmental effects on badge development. At 321 days of age, snout-to-vent length for each offspring was close to or greater than 54 mm and they were thus considered sexually mature (36). At that point, we quantified badge color and area as performed in their mothers, described below.
Hormone and Ornament Quantification
Maternal T and CORT were quantified using enzyme immunoassays (Cayman Chemical, Ann Arbor, MI). Protocols for plasma steroid extraction and immunoassays are detailed in the Supplementary Material.
We took ventral photographs of each offspring (at maturity) and their mothers (prior to laying) using uniform camera settings and lighting conditions, and with a metric ruler in view as a scale for badge measurements (Figure 1). We only quantified throat badges since they can occur in males and females and allow comparison between sexes, whereas abdominal badges are only rarely seen in females. Relative badge area was calculated as the residual of badge area linearly regressed on head area (37), both of which were measured using ImageJ (38).
Badge color was measured on the blue portion of each lizard’s left throat badge using an Ocean Optics Jaz UV/VIS spectrometer. Saturation was calculated as light reflectance at the range of peak wavelength reflectance ± 50 nm, divided by total light reflectance between 300 and 700 nm (39, 40). Fence lizard badges are responsive to temperature, becoming more saturated with increasing temperatures (40–42). We corrected color saturation for internal body temperature during spectrophotometric measurements by extracting the residuals of a linear regression of saturation on body temperature (measured in the cloaca using a Fluke 51/52 II 60 HZ thermocouple thermometer). Additional information about color measurement can be found in the Supplementary Material.
Statistical Analysis
All statistical analyses were conducted using R v. 4.0.3 (43) and the packages lme4 (44) and lmerTest (45) for linear and generalized linear mixed-effects models (LMM and GLMM, respectively). To verify model assumptions of no multicollinearity, predictors in all models were inspected for high variance inflation factors (VIF) using the car package (46). LMMs had no observations that were highly influential based on Cook’s Distance (all < 0.8).
To estimate the probability of offspring survival, we assigned a value of 1 to all hatchlings that were alive at 321 days of age, and a value of 0 to those that were not. We began by entering this binary code as the response variable in a logistic GLMM with the following predictors: 2-way interactions between hatchling sex and maternal T, maternal CORT, maternal badge saturation, and maternal badge area, as well as their individual main effects. To control for variation among mothers in hatching success of their offspring, we included the number of eggs hatched per clutch as a covariate. Maternal identity nested within her site of origin was included as a random effect. However, the maternal T x sex and maternal CORT x sex interactions had very high VIFs (72.9 and 28.5, respectively) and were thus removed from the model. All other VIFs < 2.9.
Intergenerational correlations for badge saturation and badge area were analyzed for male and female offspring separately. Thus, four LMM were built (for the two badge traits and the two offspring sexes), each with the following predictors: maternal badge saturation or area (for the model with the corresponding trait in offspring), maternal T, and maternal CORT. In addition, we included interaction terms between maternal badge traits and hormones (T or CORT), but none were significant and were thus removed from the models (for female offspring, all p > 0.24; for male offspring, all p > 0.845). Maternal identity nested within site of origin was included as a random effect.
To investigate heritability of badge traits we used univariate LMM for each badge trait (color saturation and area) of mothers and offspring, along with site and maternal identity as random effects. These were compared to identical models without the maternal identity effect using likelihood ratio tests based on a χ2 distribution. Significant differences between models would indicate that maternal identity explained a significant proportion of variation in offspring traits, providing evidence for heritability.
Results
Of 120 hatchlings, 42 survived to 321 days of age. Across all clutches, greatest mortality occurred before hatchlings reached 126 days (18 weeks) (83% of all mortality, Figure 2), which is well before sexual maturity. Offspring were more likely to survive to maturity when their mothers had less saturated badges and lower levels of baseline CORT during pregnancy (Table 1, Figure 3). Offspring sex was not a significant predictor of survival, indicating that mortality rate was not skewed towards either sex (Table 1).
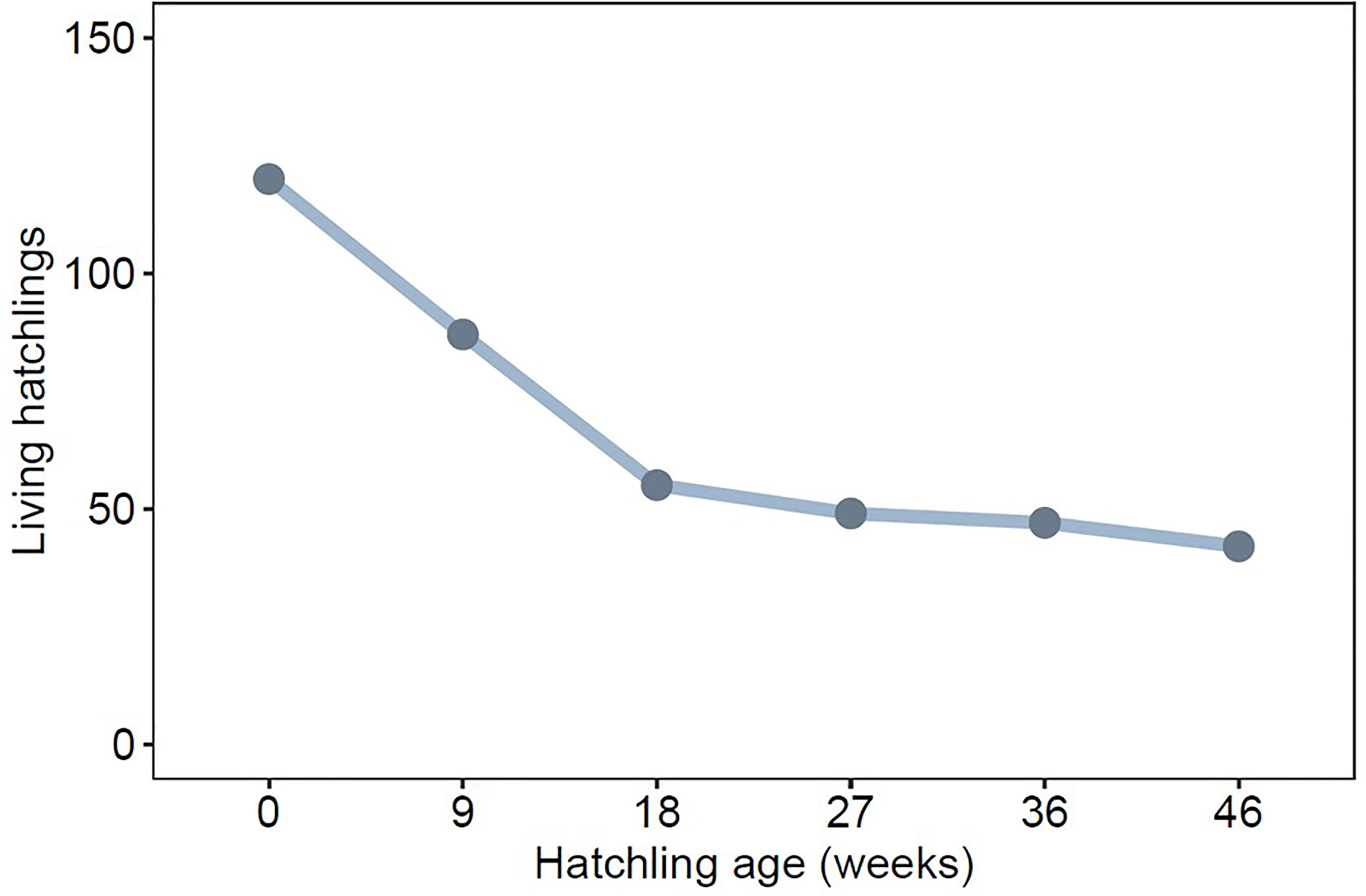
Figure 2 Total number of surviving lab-reared offspring across eleven clutches at weekly age intervals.
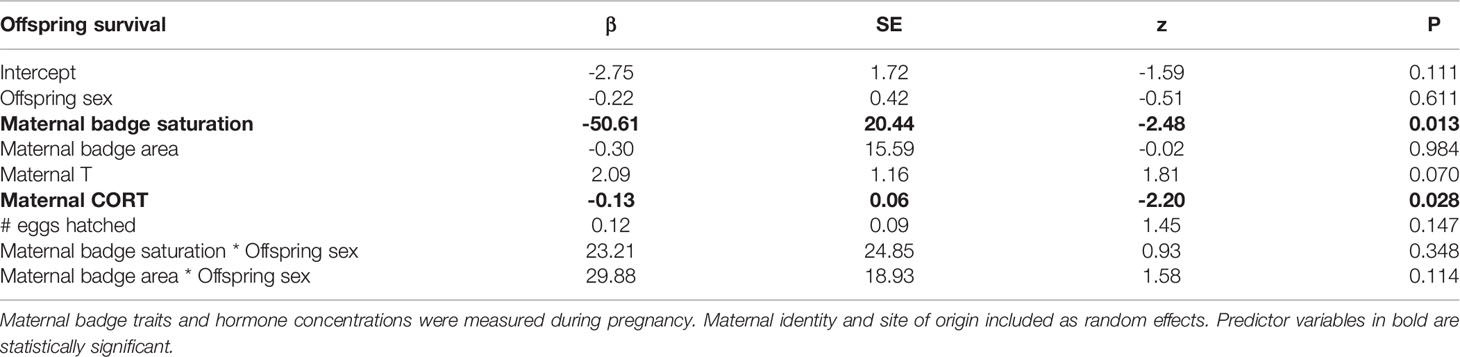
Table 1 Logistic regression output for the probability of offspring survival to 323 ± 2 days of age, at which they have reached sexual maturity.
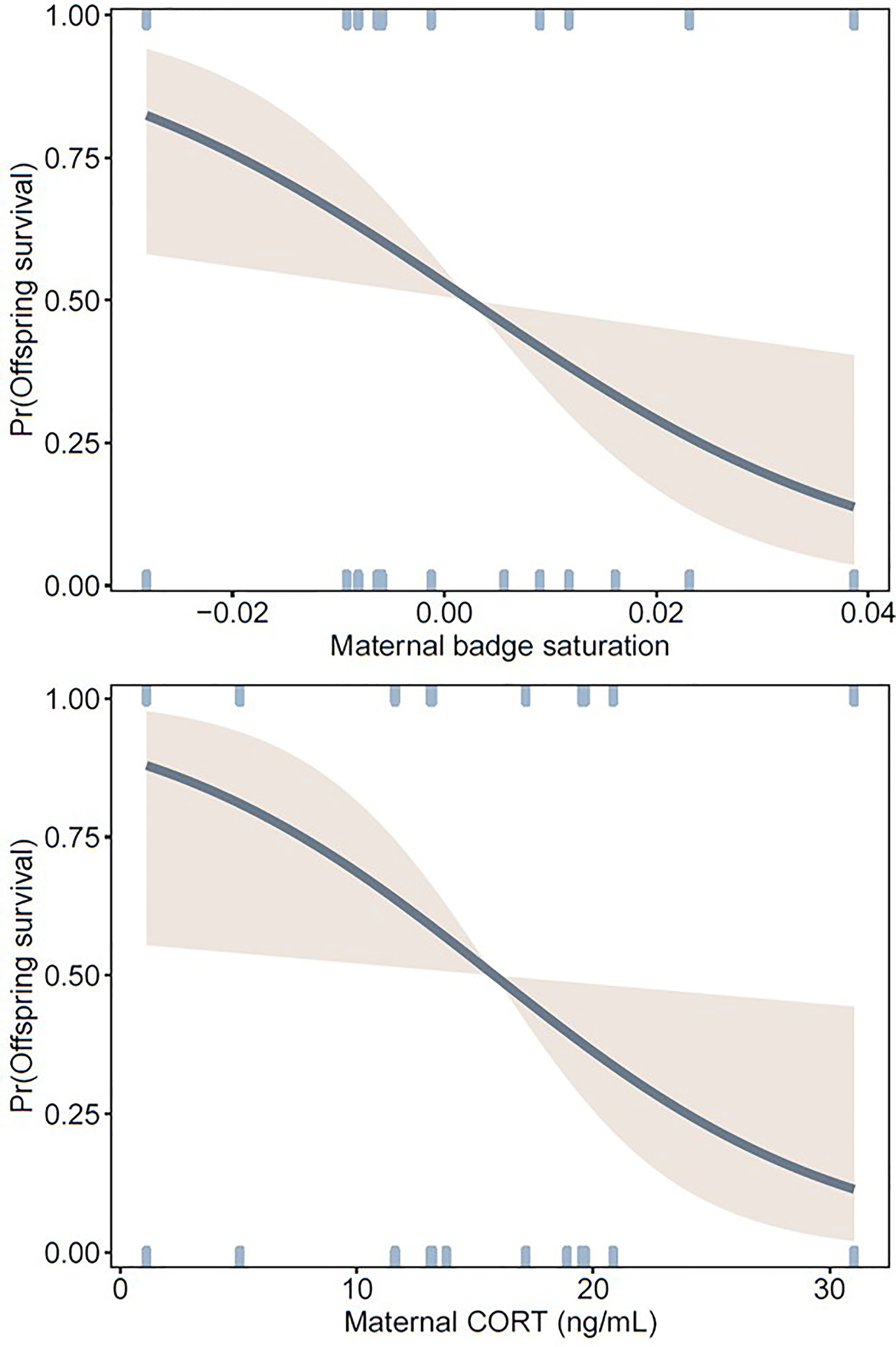
Figure 3 Probability curves showing offspring survival to 323 ± 2 days of age (at which they had reached sexual maturity) in relation to statistically significant predictors (maternal badge saturation and CORT) in a logistic regression. Other model parameters are shown in Table 1. Maternal badge saturation was corrected for body temperature at the time of measurement (see Methods).
Badge saturation in mature male offspring was higher if their mothers had less saturated badges and lower baseline CORT (Table 2a, Figure 4). Badge area in mature male offspring was larger if their mothers had smaller badges, but was not associated with maternal CORT (Table 2b, Figure 4). For female offspring, the two badge traits were not associated with maternal badge characteristics or prenatal maternal T or CORT (Tables 2c, d and Figure S1). Of note, maternal T approached statistical significance as a predictor of badge saturation and badge area in male (p = 0.099 and p = 0.071, respectively), but not female offspring; mothers with higher T tended to have male offspring with larger, more saturated badges. Likelihood ratio tests indicated that models with and without maternal identity were equivalent in explaining variation in offspring badge traits (all p > 0.16), thus providing no evidence for heritability of color saturation or area in offspring.
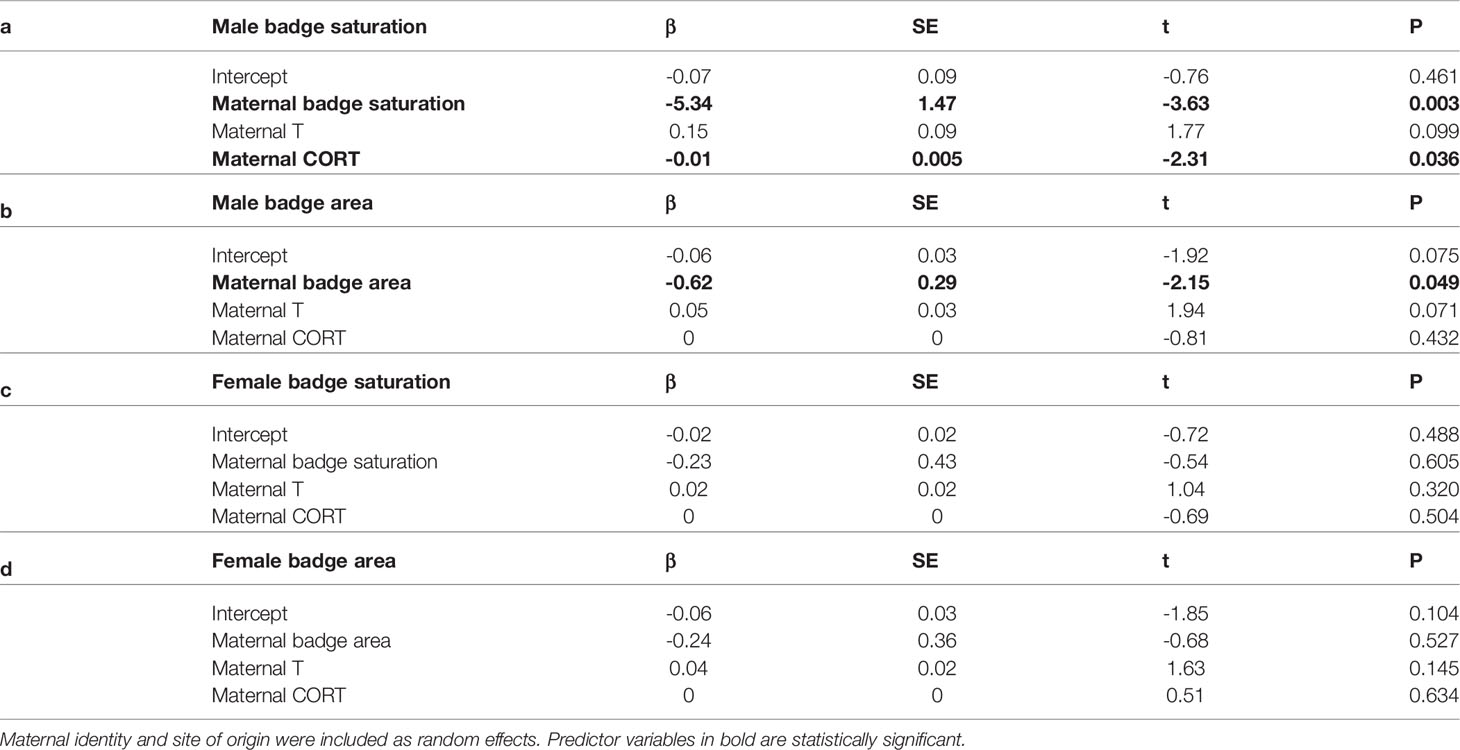
Table 2 Output of four linear mixed-effects models for two measures of ornament quality (badge color saturation and badge area) in male and female offspring and their relationships with maternal phenotype and endocrine status during pregnancy.
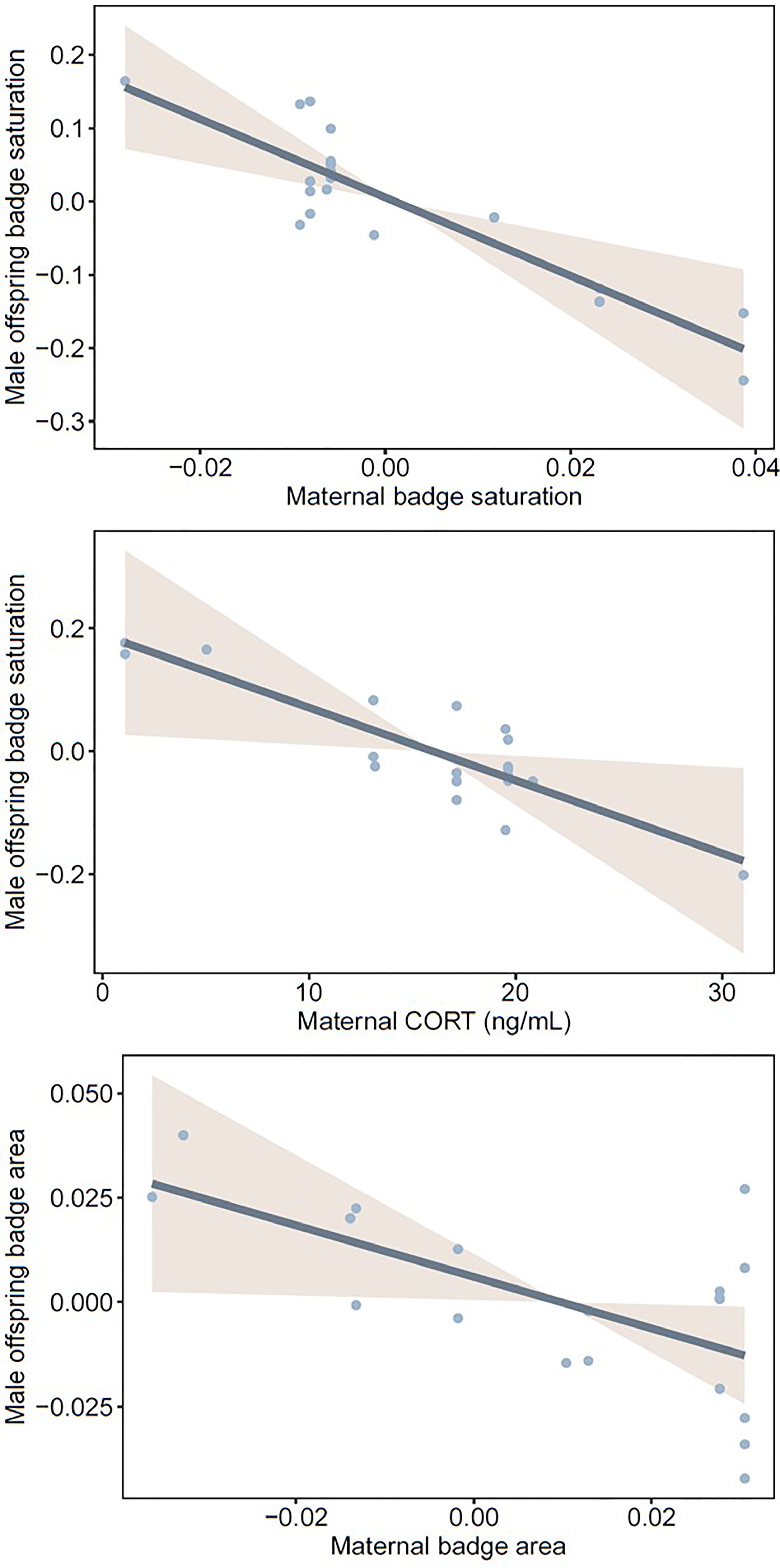
Figure 4 Statistically significant relationships between male offspring badge quality (saturation and area) and either maternal badge quality (saturation and area) or maternal baseline CORT during pregnancy. Badge saturation was corrected for body temperature at the time of measurement, and badge area was quantified in relation to head area (see Methods).
Discussion
Maternal physiology during pregnancy can have strong intergenerational fitness consequences by influencing offspring phenotypic expression (e.g., behavior, secondary sexual traits) and survival (5, 16). In parallel, signaling traits involved in social communication are often condition-dependent and have important fitness outcomes (47). However, studies that assess the contribution of maternal physiology and maternal inheritance to the expression of condition-dependent signals in offspring, and consequently to their fitness, are largely lacking. Our results show that naturally higher baseline levels of the stress-relevant hormone CORT along with more saturated badges in gravid female lizards were associated with a reduced probability that offspring would survive to sexual maturity. In surviving offspring, we observed sex-specific relationships: males with more saturated badges at maturity were born to mothers with lower badge saturation and lower CORT, and males with larger badges at maturity were born to mothers with smaller badges. The lack of evidence for genetic inheritance of this trait corroborates previous findings that their expression is condition-dependent (27). However, the negative relationship between maternal-offspring badge quality along with the negative effect of maternal prenatal CORT indicates a more complex developmental scenario. These effects could potentially involve trade-offs between ornament production and gamete production in females (12, 48). Non-exclusively, maternal life-history costs associated with the expression of a male-typical trait by females could also occur (25, 49), which we elaborate on below.
Negative effects of experimentally elevated maternal CORT on offspring survival have been previously documented in this species (21). Our results show that this effect holds for unmanipulated CORT levels in field-caught gravid female fence lizards, as females with naturally higher CORT levels had offspring with lower survival. After controlling for the effects of maternal T and ornament quality, number of eggs that successfully hatched within a clutch, and offspring sex, our model showed that maternal CORT explained a difference of ~75% in the probability of offspring surviving to adulthood (Figure 2). Although this effect was strong, limitations in the sampling regime along with the correlative nature of the analyses may reduce the generality of these observations. CORT is known to fluctuate throughout reproductive stages (50), and the snapshot nature of prenatal measurements may introduce error. Still, hatchlings in this study were protected from sources of mortality that would occur in natural environments (e.g., predation, resource availability, parasitism). Eliminating these strong drivers of mortality may help explain why maternal factors strongly predicted offspring survival. Future field studies incorporating natural drivers of mortality along with a more comprehensive prenatal hormonal profile (e.g., temporal sampling) would provide more insights into the fitness impacts of prenatal maternal physiology in ecologically relevant contexts.
Ornament quality in mothers (badge color saturation and area) was negatively associated with those of male, but not female, offspring (Figures 4 and S1). Badges appear to be relevant for fitness in male fence lizards given their role in intraspecific agonistic displays (51; but see 52) and female mate choice (33), and given their potential to signal condition (27). Even though housing conditions were uniform in our experiments, juvenile lizards shared their environments with four to five non-siblings. It is possible that poorly ornamented male offspring of highly ornamented mothers incurred fitness costs due to social subordination, such as reduced opportunity to feed. This could drive an indirect negative relationship between high quality maternal ornamentation and low male offspring survival. Still, the mechanisms driving the negative relationship between ornament quality in mothers and their male offspring in this study are less clear. Although we found no evidence for maternal genetic inheritance of badges, the negative relationship between mother and male offspring badge quality could be driven by paternal traits. Ornamentation in both parents has been demonstrated to influence offspring fitness in common whitefish (53). We were unable to identify paternity of these clutches, or whether clutches had single or multiple sires, but previous associations between ornamentation and mate preference in this species can provide some insight. In laboratory settings, male fence lizards spend less time interacting with ornamented females during the mating season (29), and it has been suggested that males with smaller or fainter badges are less likely to be successful in social competition (33, 37). If these patterns hold true in natural settings, and if badge quality is genetically inherited paternally, then it should follow that the females with more vivid badges in our study most likely mated with males with poor badges, being also expressed in their male offspring. Future work on potential genetic contributions to fence lizard ornamentation will be necessary to explore this hypothesis.
More saturated maternal badges and elevated CORT during pregnancy predicted reduced survival to sexual maturity and reduced badge quality in adult male offspring, suggesting strong maternal effects on the fitness of male S. undulatus. Social interactions mediated by this condition-signaling trait – such as competition for food or dominance hierarchy formation within housing groups – could explain differences in survival to adulthood (but see 54). Even though baseline CORT and badge areas are negatively correlated within sexually mature individuals (27), our models also indicate significant maternal effects for each of those factors on offspring survival and male phenotype. Mechanisms underlying these effects remain speculative, but the role of CORT in modulating immune responses (34, 55) and energy metabolism (56), as well as the negative effect of CORT on yolk protein content in fence lizards (15), could be potential drivers of impaired embryonic development accompanied by reduced expression of a condition dependent signal (27) and high mortality.
In this study, we demonstrated that integration of maternal physiology and a socially relevant secondary sexual characteristic may reveal important intergenerational fitness consequences. Testing these relationships under natural field conditions where offspring might also experience food limitation or high predation risk, as well as integrating paternal effects, would shed important light on this process. If field observations support our findings, these relationships could indicate a scenario of balancing selection on a male secondary sexual trait driven by intralocus sexual conflict (57, 58). That is, the manifold costs (e.g., reproductive, social, physiological) of female expression of a sexual ornament prevents directional selection of this trait in males. The relationship between prenatal maternal physiology (e.g., CORT levels) and offspring survival, as well as offspring ornament development, might represent compounding fitness costs driving balancing selection via intralocus sexual conflict, thus precluding the evolution of complete sexual dimorphism.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ScholarSphere; https://doi.org/10.26207/fzq1-9k80.
Ethics Statement
The animal study was reviewed and approved by The Pennsylvania State University’s Institutional Animal Care and Use Committee (IACUC).
Author Contributions
Designed the study: BA and TL. Collected and analyzed the data: BA, JA, RE, and TL. Prepared the manuscript: BA, JA, RE, and TL. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided in part by the National Science Foundation (IOS- 1456655 to TL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank G. McCormick and C. Tylan for assistance in the field, the staff at Edgar Evins State Park, Standing Stone State Park, and Land Between the Lakes National Recreation Area in Tennessee, and at Mississippi River State Park in Arkansas. We also thank the Lansdale family in Lee County, AR. Our special thanks to H.I. Engler for her dedication and excellent work in animal care. S.T. Giery and two anonymous reviewers provided important guidance for the direction of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.801834/full#supplementary-material
References
1. Osorio D, Vorobyev M. A Review of the Evolution of Animal Colour Vision and Visual Communication Signals. Vision Res (2008) 48:2042–51. doi: 10.1016/j.visres.2008.06.018
2. Cuthill IC, Allen WL, Arbuckle K, Caspers B, Chaplin G, Hauber ME, et al. The Biology of Color. Science (2017) 357:464–71. doi: 10.1126/science.aan0221
3. Nelson RJ. An Introduction to Behavioral Endocrinology. 3rd Edition. Sunderland, MA: Sinauer Associates, Inc (2005).
4. Buchanan KL. Stress and the Evolution of Condition-Dependent Signals. Trends Ecol Evol (2000) 15:156–60. doi: 10.1016/S0169-5347(99)01812-1
5. Mousseau TA, Fox CA. The Adaptive Significance of Maternal Effects. Trends Ecol Evol (1998) 13:403–7. doi: 10.1016/S0169-5347(98)01472-4
6. Love OP, McGowan PO, Sheriff MJ. Maternal Adversity and Ecological Stressors in Natural Populations: The Role of Stress Axis Programming in Individuals, With Implications for Populations and Communities. Funct Ecol (2013) 27:81–92. doi: 10.1111/j.1365-2435.2012.02040.x
7. Gagliano M, McCormick MI. Hormonally Mediated Maternal Effects Shape Offspring Survival Potential in Stressful Environments. Oecologia (2009) 160:657–65. doi: 10.1007/s00442-009-1335-8
8. Lande R. Sexual Dimorphism, Sexual Selection, and Adaptation in Polygenic Characters. Evolution (1980) 34:292–305. doi: 10.2307/2407393
9. Amundsen T. Female Ornaments: Genetically Correlated or Sexually Selected? In: Signalling and Signal Design in Animal Communication. Trondheim: Tapir Academic Press (2000). p. 133–54.
10. LeBas NR. Female Finery is Not for Males. Trends Ecol Evol (2006) 21:170–3. doi: 10.1016/j.tree.2006.01.005
11. Kraaijeveld K, Kraaijeveld-Smit FJL, Komdeur J. The Evolution of Mutual Ornamentation. Anim Behav (2007) 74:657–77. doi: 10.1016/j.anbehav.2006.12.027
12. Nordeide JT, Kekäläinen J, Janhunen M, Kortet R. Female Ornaments Revisited - Are They Correlated With Offspring Quality? J Anim Ecol (2013) 82:26–38. doi: 10.1111/1365-2656.12021
13. Groothuis TGG, Eising CM, Dijkstra C, Müller W. Balancing Between Costs and Benefits of Maternal Hormone Deposition in Avian Eggs. Biol Lett (2005) 1:78–81. doi: 10.1098/rsbl.2004.0233
14. Müller W, Deptuch K, López-Rull I, Gil D. Elevated Yolk Androgen Levels Benefit Offspring Development in a Between-Clutch Context. Behav Ecol (2007) 18:929–36. doi: 10.1093/beheco/arm060
15. Ensminger DC, Langkilde T, Owen DAS, MacLeod KJ, Sheriff MJ. Maternal Stress Alters the Phenotype of the Mother, Her Eggs and Her Offspring in a Wild-Caught Lizard. J Anim Ecol (2018) 87:1685–97. doi: 10.1111/1365-2656.12891
16. Sheriff MJ, Love OP. Determining the Adaptive Potential of Maternal Stress. Ecol Lett (2013) 16:271–80. doi: 10.1111/ele.12042
17. Yin J, Zhou M, Lin Z, Li QQ, Zhang YY. Transgenerational Effects Benefit Offspring Across Diverse Environments: A Meta-Analysis in Plants and Animals. Ecol Lett (2019) 22:1976–86. doi: 10.1111/ele.13373
18. Marshall DJ, Uller T. When is a Maternal Effect Adaptive? Oikos (2007) 116:1957–63. doi: 10.1111/j.2007.0030-1299.16203.x
19. Eriksen MS, Bakken M, Espmark Å, Braastad BO, Salte R. Prespawning Stress in Farmed Atlantic Salmon Salmo salar: Maternal Cortisol Exposure and Hyperthermia During Embryonic Development Affect Offspring Survival, Growth and Incidence of Malformations. J Fish Biol (2006) 69:114–29. doi: 10.1111/j.1095-8649.2006.01071.x
20. Bian JH, Wu Y, Getz LL, Cao YF, Chen F, Yang L. Does Maternal Stress Influence Winter Survival of Offspring in Root Voles Microtus oeconomus? A Field Experiment. Oikos (2011) 120:47–56. doi: 10.1111/j.1600-0706.2010.18165.x
21. MacLeod KJ, Sheriff MJ, Ensminger DC, Owen DAS, Langkilde T. Survival and Reproductive Costs of Repeated Acute Glucocorticoid Elevations in a Captive, Wild Animal. Gen Comp Endocrinol (2018) 268:1–6. doi: 10.1016/j.ygcen.2018.07.006
22. Sánchez-Tójar A, Lagisz M, Moran NP, Nakagawa S, Noble DWA, Reinhold K. The Jury Is Still Out Regarding the Generality of Adaptive ‘Transgenerational’ Effects. Ecol Lett (2020) 23:1715–8. doi: 10.1111/ele.13479
23. Zhang YY, Yin J, Zhou M, Lin Z, Li QQ. Adaptive Transgenerational Effects Remain Significant. Ecol Lett (2020) 23:1719–20. doi: 10.1111/ele.13589
24. Leary CJ, Knapp R. The Stress of Elaborate Male Traits: Integrating Glucocorticoids With Androgen-Based Models of Sexual Selection. Anim Behav (2014) 89:85–92. doi: 10.1016/j.anbehav.2013.12.017
25. Cooper WE, Burns N. Social Significance of Ventrolateral Coloration in the Fence Lizard, Sceloporus undulatus. Anim Behav (1987) 35:526–32. doi: 10.1016/S0003-3472(87)80277-4
26. Wiens JJ. Phylogenetic Evidence for Multiple Losses of a Sexually Selected Character in Phrynosomatid Lizards. Proc R Soc B Biol Sci (1999) 266:1529–35. doi: 10.1098/rspb.1999.0811
27. Assis BA, Avery JD, Tylan C, Engler HI, Earley RL, Langkilde T. Honest Signals and Sexual Conflict: Female Lizards Carry Undesirable Indicators of Quality. Ecol Evol (2021) 11:7647–59. doi: 10.1002/ece3.7598
28. Quinn VS, Hews DK. Positive Relationship Between Abdominal Coloration and Dermal Melanin Density in Phrynosomatid Lizards. Copeia (2003) 4:858–64. doi: 10.1643/h202-116.1
29. Swierk L, Langkilde T. Bearded Ladies: Females Suffer Fitness Consequences When Bearing Male Traits. Biol Lett (2013) 9:20130644. doi: 10.1098/rsbl.2013.0644
30. Assis BA, Swierk L, Langkilde T. Performance, Behavior and Offspring Morphology may Offset Reproductive Costs of Male-Typical Ornamentation for Female Lizards. J Zool (2018) 306:235–42. doi: 10.1111/jzo.12599
31. Cox RM, Skelly SL, Leo A, John-Alder HB. Testosterone Regulates Sexually Dimorphic Coloration in the Eastern Fence Lizard, Sceloporus undulatus. Copeia (2005) 3:597–608. doi: 10.1643/CP-04-313R
32. Pollock NB, Feigin S, Drazenovic M, John-Alder HB. Sex Hormones and the Development of Sexual Size Dimorphism: Dihydrotestosterone Inhibits Growth in a Female-Larger Lizard (Sceloporus undulatus). J Exp Biol (2017) 220:4068–77. doi: 10.1242/jeb.166553
33. Swierk L, Ridgway M, Langkilde T. Female Lizards Discriminate Between Potential Reproductive Partners Using Multiple Male Traits When Territory Cues are Absent. Behav Ecol Sociobiol (2012) 66:1033–43. doi: 10.1007/s00265-012-1351-2
34. McCormick GL, Langkilde T. Immune Responses of Eastern Fence Lizards (Sceloporus undulatus) to Repeated Acute Elevation of Corticosterone. Gen Comp Endocrinol (2014) 204:135–40. doi: 10.1016/j.ygcen.2014.04.037
35. Tylan C, Camacho K, French S, Graham SP, Herr MW, Jones J, et al. Obtaining Plasma to Measure Baseline Corticosterone Concentrations in Reptiles: How Quick is Quick Enough? Gen Comp Endocrinol (2020) 287. doi: 10.1016/j.ygcen.2019.113324
36. Cooper WE, Vitt LJ. Sexual Dimorphism of Head and Body Size in an Iguanid Lizard: Paradoxical Results. Am Nat (1989) 133:729–35. doi: 10.1086/284948
37. Langkilde T, Boronow KE. Color as a Signal: The Relationship Between Coloration and Morphology in Male Eastern Fence Lizards, Sceloporus undulatus. J Herpetol (2010) 44:261–71. doi: 10.1670/08-275.1
38. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 Years of Image Analysis. Nat Methods (2012) 9:671–5. doi: 10.1038/nmeth.2089
39. Maia R, Gruson H, Endler JA, White TE. Pavo 2: New Tools for the Spectral and Spatial Analysis of Colour in R. Methods Ecol Evol (2019) 10:1097–107. doi: 10.1111/2041-210X.13174
40. Assis BA, Jarrett BJM, Koscky G, Langkilde T, Avery JD. Plastic Sexual Ornaments: Assessing Temperature Effects on Color Metrics in a Color-Changing Reptile. PLoS One (2020) 15:e0233221. doi: 10.1371/journal.pone.0233221
41. Langkilde T, Boronow KE. Hot Boys are Blue: Temperature-Dependent Color Change in Male Eastern Fence Lizards. J Herpetol (2012) 46:461–5. doi: 10.1670/11-292
42. Stephenson BP, Ihász N, Byrd DC, Swierk J, Swierk L. Temperature-Dependent Colour Change Is a Function of Sex and Directionality of Temperature Shift in the Eastern Fence Lizard (Sceloporus undulatus). Biol J Linn Soc (2017) 120:396–409. doi: 10.1111/bij.12870
43. R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/.
44. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using Lme4. J Stat Softw (2015) 67:1–48. doi: 10.18637/jss.v067.i01
45. Kuznetsova A, Brockhoff PB, Christensen RHB. (2017). lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Softw 82(13):1–26. doi: 10.18637/jss.v082.i13
47. Bonduriansky R. The Evolution of Condition-Dependent Sexual Dimorphism. Am Nat (2007) 169:9–19. doi: 10.1086/510214
48. Nordeide JT, Rudolfsen G, Egeland ES. Ornaments or Offspring? Female Sticklebacks (Gasterosteus aculeatus L.) Trade Off Carotenoids Between Spines and Eggs. J Evol Biol (2006) 19:431–9. doi: 10.1111/j.1420-9101.2005.01018.x
49. Clark CJ, Rankin D. Subtle, Pervasive Genetic Correlation Between the Sexes in the Evolution of Dimorphic Hummingbird Tail Ornaments*. Evolution (2020) 74:528–43. doi: 10.1111/evo.13881
50. Woodley SK, Moore MC. Plasma Corticosterone Response to an Acute Stressor Varies According to Reproductive Condition in Female Tree Lizards (Urosaurus ornatus). Gen Comp Endocrinol (2002) 128:143–8. doi: 10.1016/S0016-6480(02)00068-0
51. Quinn VS, Hews DK. The Evolutionary Decoupling of Behavioral and Color Cues in a Multicomponent Signal in Two Sceloporus Lizards. Ethology (2010) 116:509–16. doi: 10.1111/j.1439-0310.2010.01765.x
52. Robinson CD, Lance SL, Gifford ME. Reproductive Success, Apparent Survival, and Ventral Blue Coloration in Male Prairie Lizards (Sceloporus consobrinus). J Zool (2021). doi: 10.1111/jzo.12890
53. Kekäläinen J, Huuskonen H, Tuomaala M, Kortet R. Both Male and Female Sexual Ornaments Reflect Offspring Performance in a Fish. Evolution (2010) 64:3149–57. doi: 10.1111/j.l558-5646.2010.01084.x
54. Swierk L, Langkilde T. Sizing-Up the Competition: Factors Modulating Male Display Behavior During Mate Competition. Ethology (2013) 119:948–59. doi: 10.1111/eth.12139
55. Cain DW, Cidlowski JA. Immune Regulation by Glucocorticoids. Nat Rev Immunol (2017) 17:233–47. doi: 10.1038/nri.2017.1
56. Sapolsky RM, Romero LM, Munck AU. How do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr Rev (2000) 21:55–89. doi: 10.1210/edrv.21.1.0389
57. Hedrick PW. Balancing Selection. Curr Biol (2007) 17:R230–undefined. doi: 10.1016/j.cub.2007.01.012
Keywords: maternal effects, stress, color, heritability, female ornamentation, Sceloporus
Citation: Assis BA, Avery JD, Earley RL and Langkilde T (2022) Fitness Costs of Maternal Ornaments and Prenatal Corticosterone Manifest as Reduced Offspring Survival and Sexual Ornament Expression. Front. Endocrinol. 13:801834. doi: 10.3389/fendo.2022.801834
Received: 25 October 2021; Accepted: 01 February 2022;
Published: 03 March 2022.
Edited by:
Kanta Mizusawa, Kitasato University, JapanReviewed by:
Raine Kortet, University of Eastern Finland, FinlandNicholas Pollock, University of Texas at Arlington, United States
Paula Gabriela Vissio, University of Buenos Aires, Argentina
Xiang Ji, Nanjing Normal University, China
Copyright © 2022 Assis, Avery, Earley and Langkilde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Braulio A. Assis, Ym1kNTQ1OEBwc3UuZWR1
 Braulio A. Assis
Braulio A. Assis Julian D. Avery
Julian D. Avery Ryan L. Earley
Ryan L. Earley Tracy Langkilde
Tracy Langkilde