
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 18 February 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.787876
This article is part of the Research Topic Polycystic Ovary Syndrome (PCOS): Mechanism and Management View all 35 articles
Background: Low-grade chronic inflammation may contribute to the pathogenesis of polycystic ovary syndrome (PCOS). Interleukin-15 (IL-15) is a proinflammatory cytokine involved in the development of chronic inflammation leading to obesity-associated metabolic syndrome. However, the concentration of IL-15 in follicular fluid of patients with PCOS has yet been evaluated.
Objectives: The aim of this study is to evaluate the expression level of IL-15 in both patients with PCOS and PCOS mice model and investigate the functional effect of IL-15 on ovarian granulosa cells.
Methods: The level of IL-15 in follicular fluid (FF) was measured using cytokine array and enzyme linked immunosorbent assay (ELISA) in two cohorts from 23 PCOS patients and 18 normo-ovulatory controls. PCOS mice model was induced by subcutaneously implanted with letrozole pellet for 21 days. The expression level of IL-15 in serum, ovarian, and subcutaneous adipose tissue in PCOS mice model was measured by ELISA, real-time polymerase chain reaction (RT-PCR), immunohistochemistry (IHC), and immunofluorescence. The effect of IL-15 on the proliferation and apoptosis of the KGN cells and mouse ovarian granulosa cells (GCs) were detected by CCK-8 assay and flow cytometry, respectively. Transcript expression of 17α-hydroxylase17,20-lyase (CYP17A1), cytochrome P450 family 19 subfamily A member 1(CYP19A1), FSH receptor (FSHR), steroidogenic acute regulatory protein (StAR), and proinflammatory cytokine were quantified using RT-PCR. The protein level and phosphorylation level of p38 MAPK and JNK are detected by Western blot. Concentration of dehydroepiandrosterone sulfate (DHEAS) and progesterone (P)were measured by ELISA.
Results: IL-15 expression in follicular fluid of patients with PCOS was significantly elevated compared with the control group, and similar results were observed in the ovarian and subcutaneous adipose tissue of PCOS mice models. Furthermore, the elevated FF IL-15 levels have a positive correlation with the serum testosterone levels. FSHR co-localized with IL-15 indicating that IL-15 production originate from ovarian granulose cells. IL-15 treatment inhibited proliferation and promoted apoptosis of KGN cells and mouse GCs. Moreover, IL-15 upregulated the transcription levels of CYP17A1, IL-1b and Ifng KGN cells. Similar results were observed in mouse GCs except concentration of DHEAS was higher in IL-15 treatment. IL-15 promoted p38 MAPK and JNK phosphorylation in KGN cells, treating KGN cells with p38 MAPK inhibitor SP600125 and JNK inhibitor SB203580 could reverse the effect of IL-15 on the proliferation and function of KGN cells.
Conclusion: The results indicate that IL-15 is involved in the pathogenesis of PCOS potentially by affecting survival, the inflammation state and steroidogenesis of granulosa cells. The practical significance of this association between IL-15 and the pathogenesis of PCOS needs further investigation.
Polycystic ovary syndrome (PCOS), characterized with hyperandrogenism and ovulatory dysfunction, irregular menstruation, and insulin resistance, is one of the most common endocrine and metabolic disorders in reproductive-age women (1). PCOS is the main cause of infertility in women of reproductive age. The global prevalence of PCOS ranges from 4% to 20% owing to differences in diagnostic criteria and population assessed in different geographic areas (2, 3). Due to its obscure etiology and high heterogeneity of clinical manifestations, the available therapeutic strategies for PCOS mainly rely on symptoms while there is no cure yet. The complex interaction between genetical and environmental factors, metabolic alterations, neuroendocrine and immune systems is supposed to play a role to the pathogenesis of PCOS (4–6). Moreover, physiological inflammation occurs in female reproductive tract during ovulation, menstruation, implantation, and labor at term, the establishment of low-grade chronic inflammation may participate in PCOS etiology (7, 8). PCOS patients have permanently elevated serum and ovarian levels of inflammatory markers interleukin-2 (IL-2), IL-6, IL-18, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) compared with normal controls (9–11). The association between inflammatory cytokines and ovarian dysfunction implies that inflammation might be reckoned as the most potent risk factor of PCOS (12). Further investigating the role of inflammatory mediators in the commencement and development of PCOS could be critical for better understanding the pathophysiology of the disease and developing a potential therapeutic target.
IL-15 is secreted by many cell types, including both immune and nonimmune cells such as T-lymphocytes, macrophages, neutrophils and skeletal muscle cells (13, 14). IL-15 has attracted considerable attention for its beneficial effects, including improving lipid and glucose metabolism, suppressing white adipose tissue inflammation, enhancing mitochondrial function, and attenuating endoplasmic reticulum stress (15, 16). In contrast with beneficial effects of IL-15, IL-15 was also reported to participate in chronic inflammation of adipose tissue leading to obesity-associated metabolic syndrome, which absent in IL-15 KO mice prevented accumulation of fat in the white adipose tissue and promoted lipid utilization via adaptive thermogenesis (17). Furthermore, serum IL-15 concentrations were higher in overweight subjects, suggesting that adipose tissue depots might be a source of IL-15 (18). IL-15 was increased in follicular fluid (FF) from women with endometriosis, suggesting that IL-15 may have impaired oocyte quality leading to lower fertilization rates (19). IL-15 concentration in FF of follicles with immature oocytes were significantly higher than those with mature oocytes, suggesting that IL-15 should be investigated as a possible predictive factor for oocyte maturity (20). Adverse correlation between FF IL-15 concentration and maturity of oocyte, therefore, we aimed to investigate the pathogenesis role of IL-15 in women with PCOS.
Granulosa cells (GCs) are the predominant somatic cell type of the ovarian follicle and involved in folliculogenesis through proliferation, acquisition of gonadotropic responsiveness, steroidogenesis and production of autocrine/paracrine factors (21). Increased apoptosis of GCs has been proved in patients with PCOS and PCOS animals, although the underlying mechanisms of apoptosis in GCs have not been fully revealed (22–24). Hyperandrogenism directly induces apoptosis of GCs by stimulating an intrinsic pathway and decreasing the production of follicular growth factors (25–28). Immunosuppressive cytokines such as TGF-β also induced GCs apoptosis during the follicular development in PCOS rats (29). Although the IL-15 concentration in FF is negatively related to the maturity of oocytes, the effect of IL-15 on the proliferation and apoptosis of GCs remains unknown. The MAPK signaling pathway is one of the important pathways of IL-15. The activation of this signaling pathway affects the proliferation and apoptosis of target cells and the release of inflammatory factors (30, 31). And interestingly, phosphorylation of the MAPK pathway is involved in the expression of androgen synthesis related enzymes StAR and CAP17A1 (32, 33). We hypothesize that IL-15 will affect the proliferation of granulosa cells and the expression of genes related to androgen synthesis through the above signaling.
Knowledge in the association between FF IL-15 concentration and testosterone in women with PCOS could provide new insight into the pathogenesis of PCOS. this study aimed to detect the FF IL-15 concentration in women with PCOS, examine the association of FF IL-15 concentration with serum testosterone and explore the effect of IL-15 on the biological activities of GCs.
This study was approved by the Ethics Committee of Obstetrics and Gynecology Hospital affiliated Fudan University. Informed written consent was obtained from all the participants. Eligible women who had undergone IVF were recruited from September 2020 to January 2021. PCOS was diagnosed based on the Rotterdam criteria with two of the following: oligo and/or anovulation, polycystic ovarian morphology, and clinical and/or biochemical signs of hyperandrogenism (in this study, hyperandrogenism was defined as T>51ng/dl、the menstrual cycle exceeds 45 days and the number of small follicles visible on both sides of the ovary under ultrasound is ≥12). The control group included women who seek treatment for tubal infertility or male factors, with normal ovarian reserve (regular menstrual cycles, and normal ovarian morphology) who has normal BMI, the menstrual cycle is 28-35 days and the number of small follicles in a unilateral ovary under ultrasound <10. Women with endometriosis, cancer, or other medical disorders that could affect folliculogenesis were excluded.
FF samples were collected from 3 PCOS patients and 3 controls of the participants for cytokine array analysis. Patients included met all the three items of Rotterdam criteria. The clinical, hormonal characteristics were compared between the two subgroups (Table 1). FF samples from an additional 20 PCOS patients and 15 controls of the participants were collected for ELISA validation of the identified cytokines, and the clinical, hormonal characteristics were presented in Table 2. All subjects underwent controlled ovarian stimulation using the standard IVF antagonist stimulation regimen protocol. FF was collected by transvaginal ultrasound-guided aspiration, 36 h after the administration of recombinant human chorionic gonadotropin. Only clear FF samples with no macroscopic blood contamination were included. After oocyte isolation, the FF samples were centrifuged at 800 g for 10 min to remove pellets. The supernatant was then separated and stored at -80°C for future use.
A cytokine and chemokine array (Proteome Profiler TM Human XL Cytokine Array Kit, R&D Systems, MN, USA) was used to detect the changes in 102 cytokines and chemokines in follicular fluid from PCOS and non-PCOS patients according to the manufacturer’s instructions. Briefly, samples were incubated on the membrane overnight at 4°C on a rocking shaker. The membranes were washed and incubated with a cocktail of biotinylated dectection antibody, and then the membrane was incubated with Strepatividin-HRP and chemiluminescent detection reagents. The chemiluminescent signal on each membrane was collected using an Amershan Imager 600 (GE Healthcare Life Sciences, Pittsburgh, PA, USA). The intensity (Pixel density) of each spot was quantified using HL Image++ (Western Vision Software, Salt Lake City, UT, USA), and corrected for background intensity and normalized to the membrane’s positive control.
The differential abundance of IL-15 was validated by ELISA using FF samples of 20 patients with PCOS and 15 control participants. Concentrations of IL-15 in the FF samples were measured using the commercial human IL-15 ELSIA kits (Multi Science, Hangzhou, China) according to the manufacturer’s instructions.
Three-week-old female C57BL/6 mice (Jiesjie laboratory animal co. LTD, Shanghai, China) were maintained in a 12h light/12 h dark cycle with free access to rodent feed and water. All procedures were carried out followed the guidelines provided by the Fudan University Institutional Animal Ethical Committee. Mice were divided randomly into two subgroups: control was subcutaneously implanted with a placebo and PCOS was subcutaneously implanted with 3mg letrozole (LTZ) pellet (Innovative Research of American, Sarasota, FL, USA) for 21 days. At the end of experiment, all mice were euthanized by intraperitoneal injection with pentobarbital sodium. Ovary was dissected out and fixed by paraformaldehyde to paraffin embedding. The animal study was approved by the Ethics Committee of Fudan University.
Viginal smears of all mice were collected and for determination of estrous cycle, Giemsa staining was used, and stages of estrous cycle were determined microscopically.
Mice were fasted for 12 h before the glucose tolerance tests (GTT) and 4h before the insulin tolerance tests (ITT). Glucose levels were measured by tail vein blood sampling using Accu-Chek Performa blood glucose analyzer (Roche Diagnostics). The mice were intraperitoneally injected with D-glucose (2g/kg body weight) for GTT or insulin (1 IU/kg body weight) for ITT after measurement of fasting glucose levels, and tail samples were collected at 15,30,60,90 and 120 min after the IP injection for glucose level detection. And the level of fasting insulin was measured by ELSIA (Multi Science, Hangzhou, China). And HOMA-IR (homeostasis model assessment of insulin resistance) index was calculated refer to (34).
Serum testosterone (T), dehydroepiandrosterone sulfate (DHEAS), luteinizing hormone (LH), follicle-stimulating hormone (FSH) concentrations were measured by corresponding ELISA kits (Sino-UK bio, Beijing, China). Moreover, serum and ovarian tissue homogenates IL-15 concentration was determined through a mice ELISA kit (Multi Science, Hangzhou, China).
Hematoxylin and eosin (H&E) staining was performed for ovary following deparaffinization and rehydration. 5μm sections were stained using hematoxylin followed by eosin staining and subjected to graded alcohol dehydration. The histopathological analysis of the ovary was evaluated by two independent viewers (Yan Liu, Zhi Li) who were blinded to the group information.
Immunohistochemistry (IHC) staining of IL-15 was used an immunohistochemical SP kit (Origene, Rockville, MD, USA) following the manufacturer’s instructions. Briefly, tissue sections were boiled in antigen retrieval buffer. After cooling, sections were incubated with 3% hydrogen peroxide solution at room temperature for 15 min followed with 10% goat serum as an antigen-blocking buffer for 30 min at 37°C. Sections were then incubated with Rabbit antibody against IL-15 (1:100, Affinity Bioscience, OH,USA) overnight at 4°C in a humid chamber. HRP-conjugated secondary antibody was applied for 30 min and visualized with 3’3-diaminobenzideine (DAB). Observations were made using a Nikon Eclipse 80i (Nikon, Tokyo, Japan) microscope. The positive staining areas were measured by Image J software (NIH, USA). Immunofluorescence staining was applied by a multiple fluorescent staining kit (Absin Bioscience, Shanghai China) according to the manufacturer’s instructions. The sections were incubated with primary antibody against IL-15 (1:100), and FSHR (1:500, Service Bio, Wuhan, China). Images were captured using a laser confocal microscope (Leica TCS SP8, Germany).
KGN (human ovarian granulosa cell tumor) cells were obtained from Shandong University and were cultured with DMEM/F12 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin in a humidified incubator at 37°C with 5% CO2. Mouse primary granulosa cells (mGCs) were isolated from ovaries of 3 weeks age female C57 mice. Ovaries were dip on ice in Lebovitz’s-15 medium (Sigma-Aldrich, USA) with 10% fetal bovine serum and 1% penicillin-streptomycin. A 25-gauge needle was used to separate surrounding adipose and envelope tissues then puncture the ovary to release the GCs. The cell suspension was centrifuged at 100 rpm for 5 min, resuspended with McCoy’s 5A medium (Sigma Aldrich, USA) supplemented with 5% fetal bovine serum and 1% penicillin-streptomycin. To further investigate the involvement of MAPK signaling pathways in IL-15–induced apoptosis and CYP17A1 abundance, the cells were preincubated with or without p38 MAPK inhibitor SB203580 (10 uM, Selleck Chemicals, USA) (35), and JNK inhibitor SP600125 (10 uM, Selleck Chemicals, USA) (36) for 30 minutes before IL-15 (500pg/ml) treatment.
Cell viability was determined by cell-counting kit-8 (CCK8) (Selleck, Shanghai, China). KGN cells and mouse primary GCs were seeded in 96-well plates at 1×103 cells/well in 100μl cell suspension. 10μl CCK-8 reagent was added to each well and then the cells were cultured for 1h at 37°C. Optical density was measured at 450 nm using a microplate reader (BioTeck, USA). Each experiment was carried out in triplicate at least.
The apoptosis level of GCs cells was detected using Annexin V-FITC apoptosis kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer’s instructions. Experiments were performed by a CytoFLEX flow cytometer (Beckman coulter, Brea, CA, USA) and analyzed by FlowJo 10.0 software (Tree Star, Ashland, USA).
Total RNA was extracted from ovarian tissues and GCs using RNA-Trizol reagent (Invitrogen, Carlsbad, CA, USA). The RNA concentration of all samples was quantified by NanoDrop 1000 (Thermo Fisher Scientific, USA), and was reverse transcribed to cDNA by Reverse Transciption kit (Takara, Dalian, China). Quantitative reverse transcription PCR (RT-PCR) was performed in SYBR Green® fast qPCR Mix (Takara, Dalian, China) on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA USA). Results were normalized using β-actin expression. Primers were designed using Primer-Blast tool by NCBI and present in Supplementary Table 1. Relative transcription levels were calculated using 2-ΔΔCT methods.
Total protein was extracted using ice-cold radio-immunoprecipitation assay lysis buffer (Cwbio) containing a phosphatase inhibitor and a protease inhibitor cocktail (both from Roche). Protein from each sample was electro-phoresed in a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and then transferred onto a nitrocellulose blot. After 1 hour of blocking with 5% nonfat milk, the blot was incubated overnight at 4°C with antibodies against phospho-JNK (Thr183/Tyr185; 1:1000), and total JNK (1:1000), phospho-p38 MAPK (Thr180/Tyr182; 1:1000), total p38 MAPK (1:1000). On the second day, the blot was washed and then incubated with the respective secondary antibody conjugated to horseradish peroxidase (1:1500) for 1 hour. An enhanced chemiluminescent detection system (Millipore) was used to detect the bands with peroxidase activity. A G-Box iChemi Chemiluminescence image capture system (Syngene) was used to visualize the bands. The same blot was also probed with α-tubulin (1:1000) as internal controls. All antibodies were from Cell Signaling Technology (CST, Massachusetts, USA).
Microarray datasets 155489 and GSE106724 were downloaded from Gene Expression Omnibus and collected using the following platforms: GPL20795 (HiSeq X Ten) and GPL21096 (Agilent-062918 Human lncRNA array V4.0).The raw data was converted to a recognizable format by GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/).
Statistical analysis was performed with GraphPad Prism version 8.0 software (GraphPad, San Diego, CA, USA), and data were presented as the mean± the standard error of the mean (SEM)or medians with interquartile ranges. Student’ t test of two independent samples was used for the data of normal distribution, and the Wilcoxon rank-sum test of two independent samples was used for the data of non-normal distribution. Pearson’s linear regression was used for correlation analysis to investigate the relationship among IL-15 and serum Testosterone concentration. Spearman’s or pearson’s correlation analysis were used to investigate the relationship among the relative expression of IL-15 or IL-2rg(IL-15r)and CYP17A1 in PCOS patients. The one-way ANOVA with Tukey’s multiple comparison post-hoc test was used for multiple groups. The post hoc statistical power value is expressed as “R”. A P<0.05 was considered statistically significant.
In order to determine the alteration of cytokine and chemokine in the follicular fluid of PCOS women, we recruited 3 PCOS and 3 non-PCOS patients, whose clinical characteristics are illustrated in Table 1. In the PCOS patients, the BMI and serum LH, T and AMH level, and the ratio of LH/FSH were significantly higher, while the age, serum FSH and E2 levels were similar between the two groups. According to the findings of cytokine array, we found that chemokine MPO, IL-1α, Kallikrein3, IL-15, MCP-1, and IL-8 were significantly increased in PCOS group (Figure 1A and Supplementary Figure 1A). As previously reported, IL-15 is a regulator of obesity related pathological changes (37, 38), and can modulate insulin sensitivity (39). Whereas, obesity and insulin resistance (IR) are typical pathological changes in PCOS, we speculated that IL-15 plays an important role in PCOS. Then, the similar results were observed in FF collected from 20 PCOS and 15 non-PCOS patients (Figure 1C), and their clinical characteristics are illustrated in Table 2. Non-PCOS patients matched the BMI of PCOS patients, while the LH level of PCOS patients tends to be higher、the FSH level and E2 level tend to be lower and the T level is significantly higher compared with non-PCOS patients, which were also consistent with the clinical endocrine characteristics of PCOS. Furthermore, Pearson correlation analysis showed that the FF IL-15 concentration was positively correlated with serum T (r=0.2466, P = 0.0024, Figure 1D). And relative expression of IL-15 was positively correlated with CYP17A1 in cumulus granulosa cells of PCOS patients and age-matched control (r=0.7904, P=0.0254, Figure 1E) and relative expression of IL-2rg (IL-15r) was positively correlated with CYP17A1 in cumulus granulosa cells of PCOS patients and non-PCOS women (r=0.5849, P=0.0458, Figure 1F) by using bioinformatics analysis.
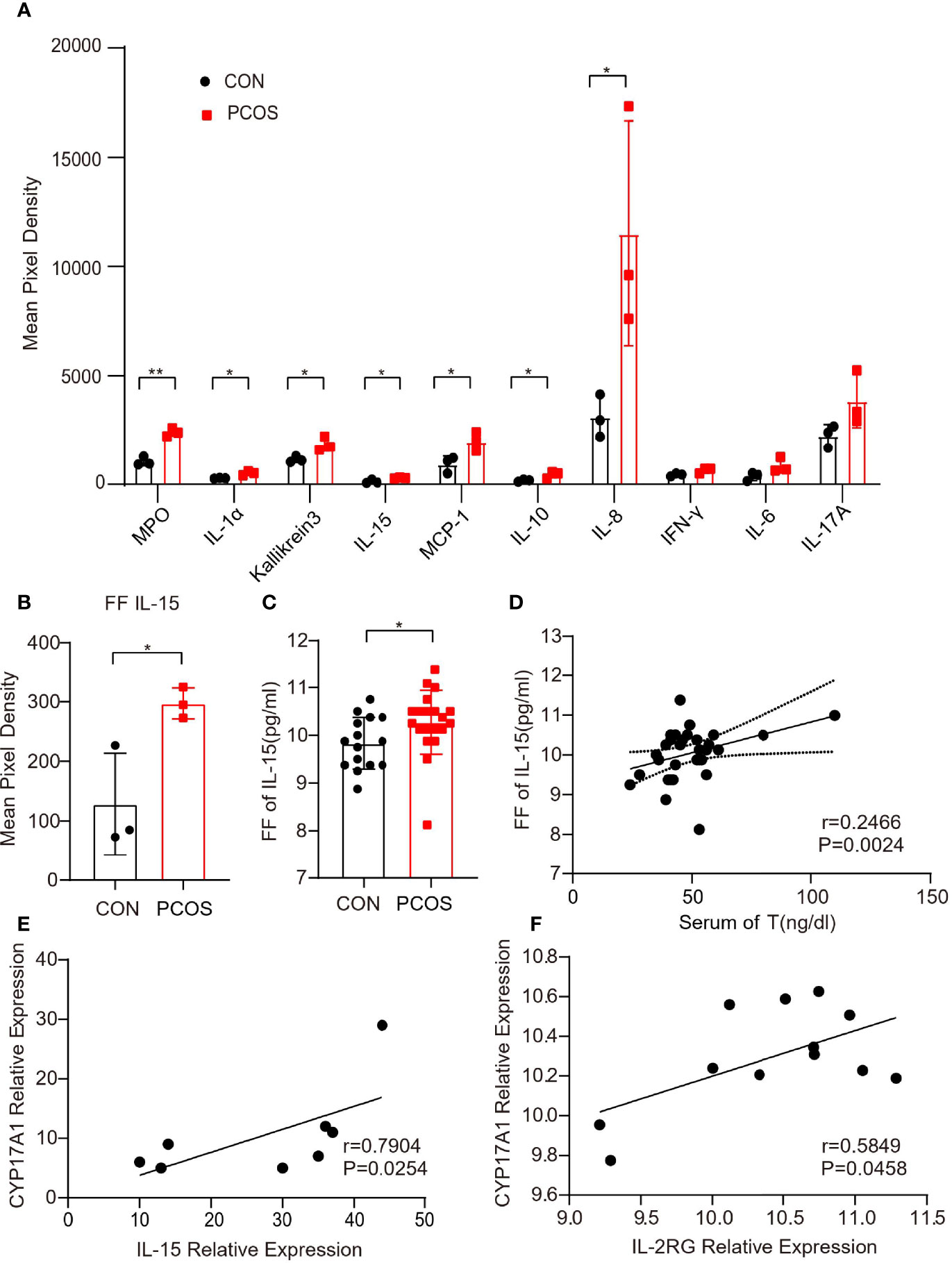
Figure 1 Up-regulation of IL-15 in the follicular fluid of PCOS patients. (A) Representative difference inflammation factors of follicular fluid factor chip between two groups of patients (n=3 per group, P=0.001, 0.017, 0.026, 0.031, 0.035, 0.040, 0.050; R=0.7924, 0.7924, 0.7476, 0.7280,0.7991, 0.6911, 0.6591 respectively); (B) Mean pixel density of IL-15 in follicular fluid of PCOS patients and non-PCOS controls (n=3 per group, P=0.045, R=0.523; (C) Follicular fluid of PCOS patients and controls ELISA level of IL-15 (PCOS n=20, CON n=15, P=0.0475, R=0.6018); (D) Correlation of IL-15 in follicular fluid levels with the serum levels of T as determined by Pearson’s rank test (P=0.0024, r=0.2466); (E) Correlation of relative expression of IL-15 with CYP17A1 in cumulus granulosa cells of PCOS patients and age-matched control (GSE155489) as determined by Spearman’s analysis (P=0.0254, r=0.7904); (F) Correlation of relative expression of IL-2rg (IL-15r) with CYP17A1 in cumulus granulosa cells of PCOS patients and non-PCOS women (GSE106724) as determined by Pearson’s analysis (P=0.0458, r=0.5849). *P < 0.05, **P < 0.01 versus the control.
In order to verify the role of IL-15 in the pathogenesis of PCOS, the well-established LTZ-induced PCOS mouse model was confirmed. The mice in the PCOS group were significantly heavier compared with age-matched control mice that had received placebo pellets (Figure 2A). Similar results were observed in the weight of ovary and fat pads in PCOS group (Supplementary Figure 1C, D). PCOS mice had significantly reduced glucose tolerance and insulin sensitivity (Figures 2B, C). And we tested the fasting blood glucose of the mice and calculated HOMA-IR. The results showed that the PCOS mice had obvious insulin resistance compared with the control group (Figure 2D and Supplementary Figure 1E). PCOS mice also displayed an irregular estrous cycle (Supplementary Figure 1F). The levels of serum T, DHEAS and LH were significantly higher in PCOS mice (P<0.05) compared with the control, but there is no significant difference in serum FSH levels (Figure 2E and Supplementary Figure 1G). Atresia and cystic dilated follicles were significantly increased and the number of corpora lutea was decreased in the PCOS mice compared with the control mice (Figures 2F–H), indicating similar ovarian dysfunction to the clinical presentation of PCOS.

Figure 2 The phenotypes of the PCOS model mice are similar to those of the PCOS patients. The mice that implanted with letrozole sustained-release tablets and control mice were defined as CON and PCOS, respectively. (A) The weight of the two groups after three weeks (n=6 per group, P=0.0455, R=0.3428); (B) GTT (n = 6 mice per group, for 15 mins, P=0.0142, R=0.3428; for 30 mins, P=0.0042, R=0.7891; for 60 mins, P=0.0013, R=0.8018; for 90 mins, P=0.0200, R=0.4914). (C) ITT (n = 6 mice per group, for 60 mins, P=0.0013, R=0.8924; for 90 mins, P=0.0028, R=0.7614; for 120 mins, P=0.0113, R=0.5518); (D) The level of fasting insulin in two groups (n=6 mice per group, P=0.014,R=0.4517); (E) The level of T、DHEAS and LH in two groups(for the level of T, CON=6, PCOS=5 , P=0.0223, R=0.4577; for the level of DEHAS, n=6 per group, P=0.0129, R=0.4771; for the level of LH, n=5 per group, P=0.0308, R=0.4611); (F) Hematoxylin and eosin staining of representative ovaries. The cystic follicle is indicated by a hashtag, while the corpora lutea are indicated by asterisks. Arrow indicates serum cyst. Scale bar: 200 μm. (G, H) The number of corpora lutea and cystic follicle in two groups (n = 6 per group; P=0.0006, 0.0001; R=0.7076, 0.7941 respectively). *P < 0.05, **P < 0.01, ***P < 0.001 versus the control.
Consistently, we found that IL-15 mRNA and protein expression were elevated significantly in ovarian of PCOS mice compared with control, while the serum IL-15 level was similar between two groups (Figures 3A–C). Moreover, the IL-15 mRNA expression was elevated significantly in adipose tissues of PCOS mice compared with control, suggesting that IL-15 may play a role in chronic inflammation of adipose (Figure 3D). IHC results confirmed that IL-15 was overexpressed in PCOS mice compared with control (Figure 3E), and colocalized with FSHR, a GCs marker (Figure 3F). This data suggested that IL-15 may be involved in the pathogenic role of GCs in PCOS.
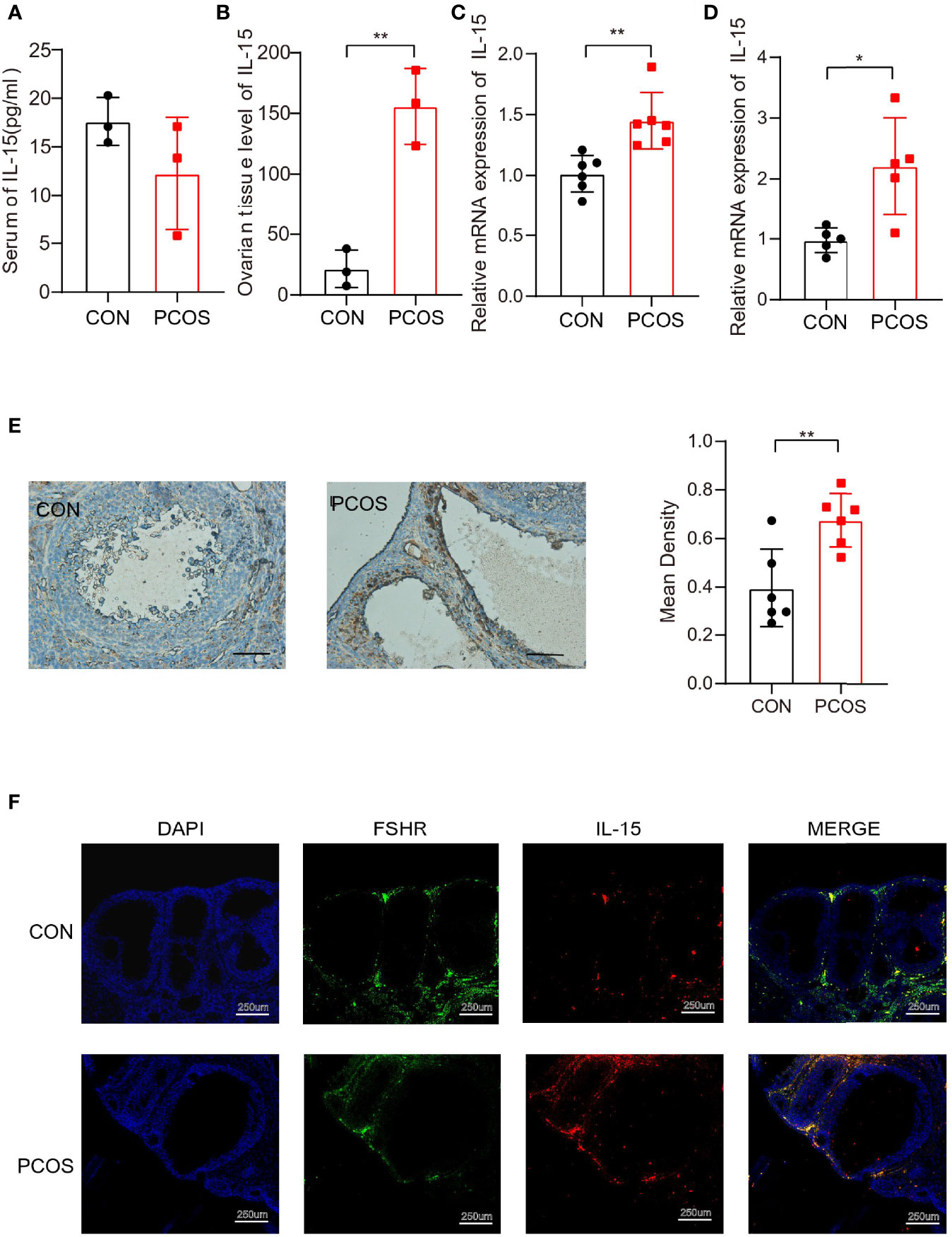
Figure 3 Higher level of IL-15 in PCOS model mice. (A, B). ELISA detection of IL-15 in the serum and ovarian tissue homogenate of two groups of mice (n=3 per group, P=0.2146, 0.0026; R=0.3044, 0.9179 respectively); (C) PCR detection of IL-15 in ovarian tissues in the two groups (CON n=5, PCOS n=6, P=0.0032, R=0.5984); (D) PCR detection of IL-15 in adipose tissue in the two groups (n=6 per group, P=0.0103, R=0.5819); (E) IHC analysis of IL-15 in the ovaries of two groups of mice. The brown part represents the part of IL-15 expression. Bar=50um, 40X. Images are representative of three independent experiments with similar results (P=0.0055, R=0.5541); (F), Blue shows the nucleus (DAPI), green fluorescence shows ovarian granulosa cells marker FSHR (TSA 520, excitation and emission wavelengths similar to FITC), red shows IL-15(TSA 650, excitation and emission wavelengths similar to AF610). Bar=250um. *P < 0.05, **P < 0.01 versus the control.
Due to the significant association of IL-15 with the clinic pathological characteristics in PCOS women and the colocalization between IL-15 and GCs in PCOS mice, we examine the effects of IL-15 on GCs growth and apoptosis. Our data showed that IL-15 suppressed proliferation of KGN cells and the intervention of JNK inhibitor SP600125 and p38 MAPK inhibitor SB203580 alleviated this effect of IL-15 (Figure 4A). The results obtained by flow cytometry analysis are also consistent with CCK-8 assay (Figure 4B). These results suggested that IL-15 decreased proliferation, whereas increased apoptosis in GCs.
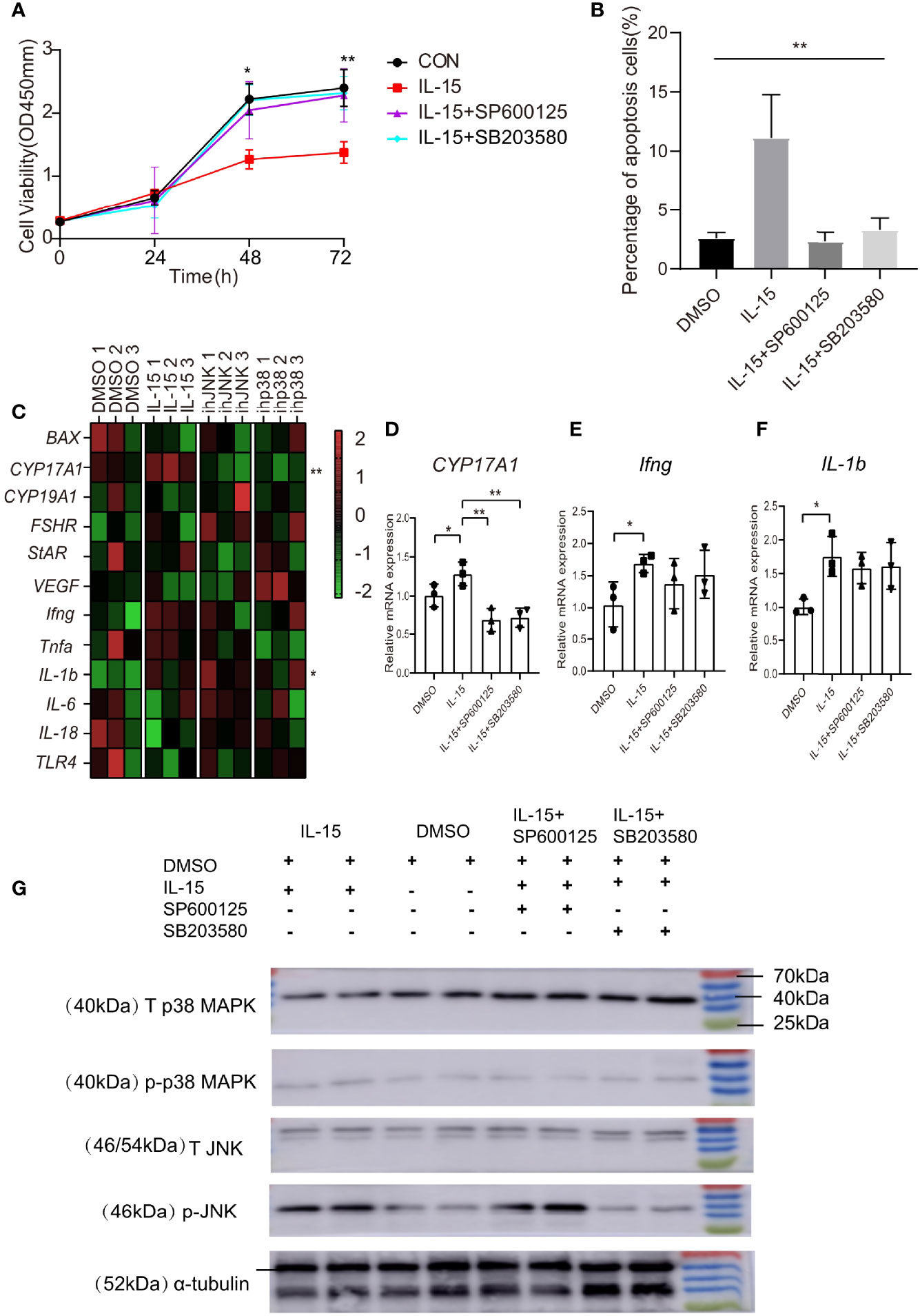
Figure 4 IL-15 inhibits the proliferation of KGN, promotes its apoptosis and dysfunction. (A) Effects of IL-15 and inhibitors on the cell viability was assessed by CCK-8 assay(for 48h, P=0.0124, R=7252; for 72h, P=0.0097, R=0.7423; (B) Flow cytometric analysis of apoptosis cells (Annexin V+ cells). Percentage of Annexin V+ cells in mutiple groups under different conditions (n = 3 per group, P=0.0015, R=0.8399); *P < 0.05, **P < 0.01 in multiple groups by one-way ANOVA; (C) Heat map of inflammatory factor gene and functional gene expression in KGN cell line after treatment. Relative expression of genes were transformed into Z-score maps based on mean and SD values(for CYP17A1, P=0.0031, R=0.8079; for IL-1b, P=0.0356,R=0.6379); (D–F) relative expression of CYP17A1、Ifng and IL-1b in different conditions(n=3 per group, for CYP17A1: P=0.0472, 0.0026, 0.0035 respectively compared with IL-15; for Ifng, P=0.0442; for IL-1b, P=0.0281), *P < 0.05, **P < 0.01 versus IL-15.; (G) Representative western blot analysis and densitometric analysis of total p38 MAPK (T p38 MAPK)、 total JNK (T JNK)、phospho-p38 MAPK (p-p38 MAPK)、phospho-JNK (p-JNK) and α-tubulin in KGN cells treated with different conditions.
To delineate the role of IL-15 in GCs steroidogenesis and the state of inflammation, we cultured the KGN cells in the presence or absence of IL-15 with or without inhibitors. Treatment of KGN cells with IL-15 for 4 h resulted in significantly increases the mRNA levels of cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17A1), which converts progesterone to 17α-hydroxyprogesterone and androstenedione (Figures 4C, D). Interestingly, we found that these two inhibitors have inhibitory effects on the increase in CYP17A1 expression induced by IL-15. Consistent to previous studies that IL-15 function as a proinflammatory cytokine (40), IL-15 significantly increased mRNA level of IL-1b and Ifng and the above two inhibitors have a tendency to reverse this effect (Figured 4E, F). These data suggested that IL-15 might play a role in the pathogenesis of PCOS by increasing production of androgen hormones and sustaining inflammation state in GCs, and these effects may be related to the activation of the signaling pathways p38 MAPK and JNK. Therefore, we carried out the detection of these two signaling pathway molecules and their phosphorylation levels on different treated KGN cells by Western Blot. The results of WB showed that IL-15 treatment increased the phosphorylation level of JNK and P38 MAPK in the KGN cell line compared with DMSO alone (Figure 4G).
IL-15 suppressed proliferation of primary GCs cells at dose dependent manner (Figures 5A, B). In addition, IL-15 promoted apoptosis of primary mGCs at dose dependent manner (Figure 5C).
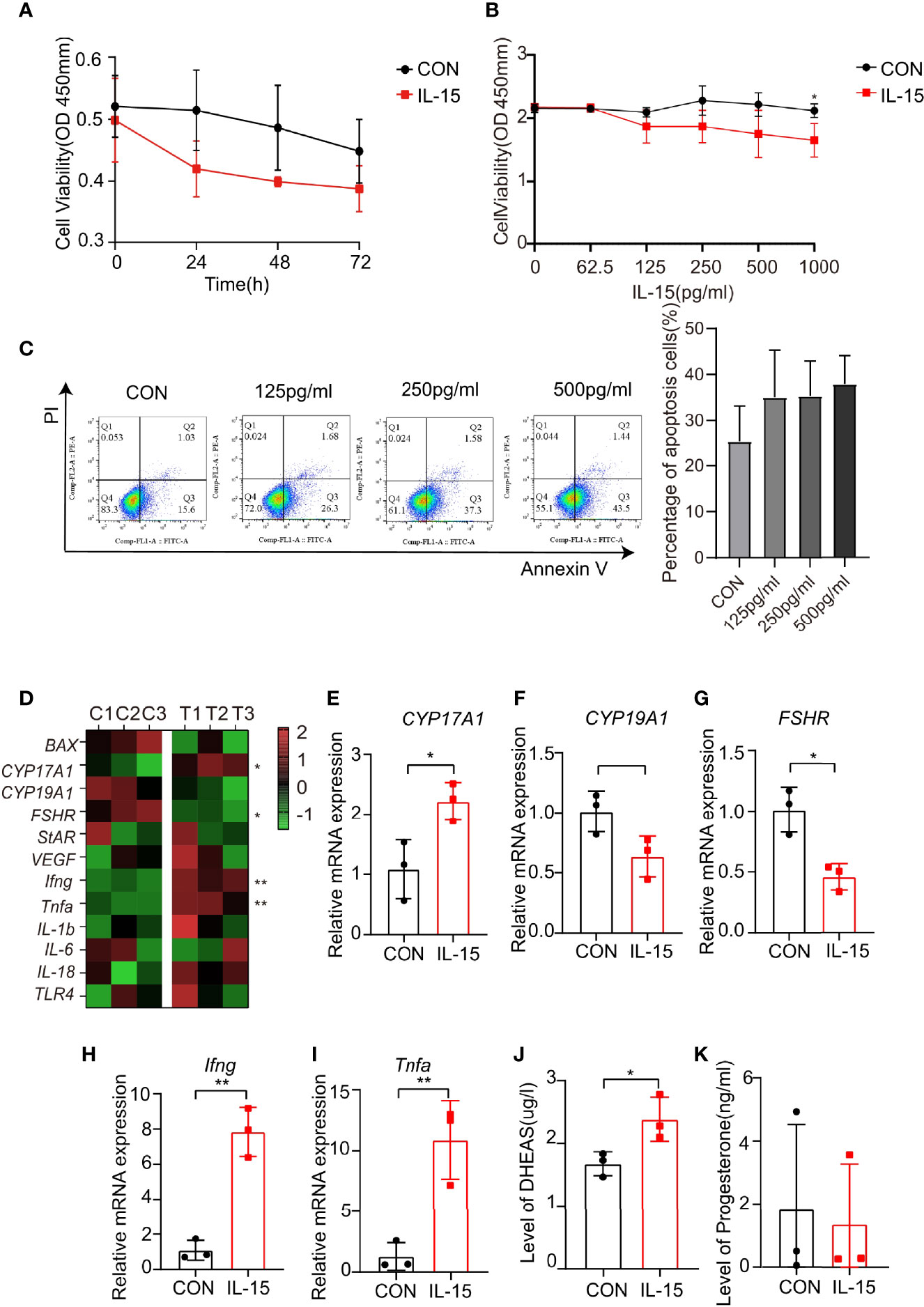
Figure 5 IL-15 inhibits the proliferation of mGCs and promotes its apoptosis and dysfunction. (A, B) Time-and dose-dependent effects of IL-15 on the mGCs was viability assessed by CCK-8 assay (n=3, *P=0.0356, R=0.6627); (C) Flow cytometric analysis of apoptosis cells (Annexin V+ cells), three independent experiments were performed with similar results. Percentage of Annexin V+ cells in two groups under different conditions (n = 3 per group); (D) Heat map of inflammatory factor genes and functional gene expression in primary cell lines after treatment. Control group: C1, C2, C3; IL-15 treatment group: T1, T2, T3. Relative expressions of genes were transformed into Z-score maps based on mean and SD values; (E–I) relative expression of CYP17A1, FSHR, Ifng and Tnfa in two groups(P=0.0278, 0.0114, 0.0015, 0.0083;R=0.7405, 0.8306, 0.9381, 0.8551 respectively); (J, K) DHEAS and P levels of the culture supernatant (n=3 per group, *P=0.0457, R=0.4527). *P < 0.05, **P < 0.01 versus the control.
Consistent with previous study, IL-15 significantly increased mRNA levels of CYP17A1, while decreased mRNA levels of CYP19A1 and FSHR (Figures 5D–G). IL-15 also increased proinflammatory cytokine mRNA levels of Ifng and Tnfa (Figures 5H, I). The effects of IL-15 on the steroidogenic activity of primary GCs were evaluated. The level of DHEAS and progesterone in the medium secreted from primary GCs for 24 h in the presence of IL-15 were measured. The concentration of DHEAS were significantly increased after incubation of the cells with IL-15 (Figure 5J), while the concentration of progesterone remained unaltered (Figure 5K). These data confirmed the results obtained from the human GCs cell line KGN.
Ovarian granulosa cells (GC) are important somatic cells in ovarian tissue which are stimulated by FSH. They secrete insulin-like growth factors and promote the development of follicles. In addition, various aromatase families can promote cholesterol metabolism and secrete synthetic sex hormones, which play an important role in female reproductive health (41). In this study, we evaluated the effects of IL-15 on GC functions including GC proliferation, expression of inflammatory factors and steroidogenesis, aiming to examine the potential involvement of IL-15 in regulating ovarian follicle function by utilizing the KGN cells and a primary mouse granulosa cells culture model. And we found that IL-15 affects the proliferation and function of GC through p38 MAPK and JNK phosphorylation.
Chronic inflammation is an important pathogenic factor of PCOS. In the analysis of the cytokine and chemokine array of the patient’s follicular fluid, we found that IL-15 significantly elevated in PCOS group. And except it, we found that MPO, IL-1α, Kallikrein3, MCP-1, and IL-8 significantly elevated in PCOS group, proving that the ovarian of PCOS patients does have a chronic inflammation state. Previous studies reported that these inflammatory factors are associated with insulin resistance and promoting androgen release (42–46). However, these inflammatory factors except IL-8 have been reported to be elevated only in the serum of PCOS patients before, the results of our follicular fluid provide ideas for studying the relationship between circulatory inflammation and ovarian inflammation in patients with PCOS.
We found that the level of follicular fluid (FF) IL-15 was higher than that in the control mice group, which was consistent with the results of PCOS patients. But the level of IL-15 in serum didn’t significant increase. The results seem to indicate that IL-15 is produced locally in the ovarian tissue, or certain factors may promote the accumulation of IL-15 in the ovarian tissue. By co-localizing mouse granulosa cells and IL-15 immunofluorescence, we found that the position of IL-15 and granulosa cells are highly co-localized, indicating that granulosa cells are the main somatic cells that produce IL-15 in ovarian tissue, but which factors lead to increased IL-15 secretion by ovarian granulosa cells need further research. In addition, we also found that the expression level of IL-15 in adipose tissue was significantly up-regulated in the PCOS model mouse group. Studies have shown that the adipose tissue of patients with metabolic abnormalities such as obesity is in a state of chronic inflammation (47). The results suggested that the inflammatory reaction occurred not only in the FF but also in the fat tissue of the PCOS.
IL-15 is generally regarded as a T cell growth factor and is belongs to the cytokine receptor γ chain (γc) family. It shows a wide range of pleiotropic effects regulating the innate and adaptive immune system, regulates cell differentiation and promoting survival or inducing apoptosis according to the cell environment (48). But there is no research showing that IL-15 has a direct effect on the apoptosis of somatic cells. The present studies provide new insights into the intracellular signaling cascade by which IL-15 induces GCs apoptosis. Our research showed that IL-15 not only induced Ifng but also Tnfa expression. Both IFN-γ and TNF-α promote cell apoptosis. IFN-gamma(IFN-γ) modulates the apoptotic pathway by upregulating apoptosis-related genes and overproduction of pro-inflammatory cytokine IFN-γ induces the excessive apoptosis of IECs and is involved in Crohn’s disease development (49, 50). And studies have shown that IFN-γ can promote the apoptosis of ovarian granulosa cells (51). TNF-α can induce hepatocyte apoptosis and liver damage (52). And our study found that IL-15 promoted the increase of mGCs synthesis DHEAS. Study have shown that the increase of androgens inhibits the proliferation of granulosa cells (53), so the pro-apoptotic effect of IL-15 on ovarian granulosa cells may be indirectly achieved by promoting the androgen and inflammation state of follicular fluid microenvironment. Follicular atresia in PCOS patients is associated with increased granulosa cell apoptosis (54). Our study revealed that high doses of IL-15 inhibited the proliferation of GCs, indicating that the increase in granuloma cells apoptosis observed under pathological conditions (such as PCOS) may be caused by IL-15 or the immune microenvironmental changes caused by IL-15 stimulation. Therefore, based on these findings, we believe that IL-15 participates in the follicular atresia of PCOS by promoting the apoptosis of granulosa cells.
Elevated androgen is an important pathological manifestation of PCOS. We found that treatment of IL-15 increases the expression of CYP17A1 in granulosa cells. CYP17A1 is a critically important enzyme in humans that catalyzes the formation of androgens. It catalyzes the 17α-hydroxylation of pregnenolone to 17α-OH pregnenolone. Subsequently, through its C17,20 lyase activity, it can further convert 17α-OH pregnenolone to the androgen dehydroepiandrosterone, which is the precursor of androstenedione, testosterone and dihydrotestosterone (55). The increase in DHEAS of the culture supernatant of mGCs treated with IL-15 also indicates that IL-15 promotes the secretion of androgens from granulosa cells. IL-15 forms a complex with receptors IL-2Rβ and γ chain (IL-2Rγ) through IL-15Rα, and completes the process of signal transduction to play immune and other functions (56). Our analysis of the database showed that the expression of IL-15, IL-2rg and CYP17A1 in granulosa cells of PCOS patients were positively correlated, indicating that IL-15 was involved in androgen synthesis. And there is a randomized controlled trial using BNZ-1, a selective and simultaneous inhibitor of cytokines IL-2, IL-9, and IL-15, for the treatment of hyperandrogen-induced hair loss (57).It shows that factors that inhibit IL-15 such as BNZ-1 have a role in the treatment of PCOS hyperandrogenism.
CYP19A1 is considered to be an important marker in the etiology of polycystic ovary syndrome. The study reported that compared with healthy controls, the aromatase gene expression in PCOS follicles before ovulation and the subsequent estradiol production decreased (58). And it can convert androgen into estrogen (59). FSHR plays an important role in synthesizing estrogen and stimulating follicular development (60, 61). IL-15 treatment reduced the expression of CYP19A1, and FSHR both in mGCs. Those all indicated that IL-15 can affect follicle development by influence hormone synthesis related enzymes and participate in the follicular atresia of PCOS.
We found that IL-15 promoted the phosphorylation of p38 MAPK and JNK in KGN cells, and the addition of corresponding inhibitors would reverse the effects of IL-15 on the proliferation inhibition and functional genes and expression of pro-inflammatory factors in KGN cell lines. It reminds us that IL-15 may play a role through these two signaling pathways. This needs to be further verified in mGCs.
For the first time, we reported that IL-15 decreased KGN cells and mGCs proliferation as well as key genes for follicular development such as CYP19A1 and FSHR. IL-15 also up-regulated the expression of CYP17A1, the key steroidogenic-related genes in androstenedione secretion. In addition, IL-15 can increase the expression of pro-inflammatory factor IL-6, Tnfa and Ifng of granulosa cells, and these pro-inflammatory factors have been reported to be related to the metabolism of PCOS and the abnormal reproductive phenotype, and promote the chronicity of the microenvironment around the follicle. And this effect is through p38 MAPK and JNK phosphorylation. The present study provides new evidence for IL-15-dependent regulation of proliferation and steroidogenesis in GCs that may influence follicle development. It is now convincingly suggested that IL-15 may serve as a critical regulator of cell proliferation, differentiation and steroidogenesis in the KGN and mGCs of ovarian preantral follicle. But additional research is needed to understand the mechanism of action of IL-15 in granulosa cells, as well as its exact role in follicle development. The current clinical treatment and efficacy evaluation of PCOS, due to inconsistent diagnostic criteria, patients often have repeated symptoms. IL-15, as a pro-inflammatory factor, participates in the pathogenic process of PCOS, showing the potential in evaluation of the diagnosis and treatment effect of PCOS. However, this study only analyzed the signal pathways of the human KGN cell line, did not study mGCs and did not conduct IL-15 intervention experiments and rescue experiments in vivo. The sample size of patients used to detect IL-15 is relatively small. We will continue to improve in the future.
Further efforts are needed to demonstrate a connection between inflammatory factors and PCOS pathogenesis. Research on which signaling pathway is used by IL-15 to promote the secretion of androgens by granulosa cells needs to be further explored. By verifying the involvement of inflammatory factors in the pathogenesis of PCOS, new therapy may be developed for PCOS treatment, such as blocking therapeutic targets including inflammatory factors and signaling pathways.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Shanghai Jiai Genetics and Infertility Diagnosis and Treatment Center Assisted Reproductive Ethics Committee. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the ethics committee of Fudan University.
YL designed the experiments, acquired, analyzed, and interpreted the data, and drafted the manuscript. ZL contributed to the experiment. HL, CX, and FZ provided substantial contributions to the conception of the study, experimental design, and data interpretation. All authors revised and approved the final version of the manuscript.
This work was supported by the Shanghai Municipal Commission of Health and Family Planning (201640362) to FZ; the Shanghai Municipal Commission of Health and Family Planning (2017ZZ01016) to CX.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.787876/full#supplementary-material
Supplementary Figure 1 | (A) The alignment of 102 cytokines on the Human XL Cytokine Array Kit, R&D Systems. RS, reference spots; Acrp30, adiponectin; ApoA1, apolipoprotein A-1; Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; BLyS, blymphocytestimulator; BDNF, brain-derived neurotrophic factor; CHI3L1, Chitinase 3-like 1; CFD, complement factor D; CRP, C-reactive protein; Cripto-1, teratocarcinoma-derived growth factor; Dkk-1, dickkopf-1; DPPIV, ipeptidyl-peptidase IV; EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; FGF-7, fibroblast growth factor 7; FGF-19, fibroblast growth factor 19; FLT3LG, Fms-related tyrosine kinase 3 ligands; G-CSF, granulocyte colony-stimu1ating factor; GDF-15, growth/differentiation factor 15; GM-CSF, granulocyte-macrophage colony stimulating factor; GH, growth hormone; SF, scatter factor; IGFBP-2, insulin-like growth factor binding protein 2; IGFBP-3, insulin-like growth factor binding protein 3; LIF, leukemia inhibitory factor; M-CSF, macrophage colony stimulating factor; MIF, macrophage migration inhibiting factor; MMP-9, matrix metalloprotein 9; MPO, myeloperoxidase; OPN, osteopontin; PDGF-AA, platelet-derived growth factor AA; PDGF-AB/BB, platelet-derived growth factor AB/BB; PTX3, pentraxin 3; RBP-4; retinol binding protein 4; SHBG, sex hormone-binding globulin; TFF-3, trefoil factor 3; THBS1, thrombospondin-1;uPAR, urokinase-type plasminogen activator receptor; VEGF, vascular endothelial growth factor; VDBP, vitamin D BP; TIM-3, T cell immunoglobulin domain and mucin domain-3; VCAM-1, vascular cell adhesion protein 1; NC, negative controls. (B) Representative images of cytokine array blots probed with the follicular fluid samples. Each blot represents immunoreactive staining against respective antibodies. Note the absence of staining at the negative control and blank slots. The relative expression levels of each cytokine were determined by comparing the pixel intensity of the respective blots to that of the positive control on the same array. The blots marked with red box are the cytokines that were significantly regulated in PCOS group compared to the non-PCOS group; (C), The ovarian weight of the two groups of mice (n=6 per group); (D), The fat pad weight of the two groups of mice (n=6 per group, P=0.0012, R=0.6681); (E), HOME-IR in two groups (n=6 per group, P=0.0500, R=0.3317), (F), Representative estrous cycles. Y-axis: P, proestrus; E, estrus; M, metestrus; D, diestrus; (G), Serum FSH levels in mice.*P < 0.05 versus the control.
Supplementary Table 1 | Primer sequence list.
1. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic Ovary Syndrome. Nat Rev Dis Primers (2016) 2:16057. doi: 10.1038/nrdp.2016.57
2. Escobar-Morreale HF. Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat Rev Endocrinol (2018) 14(5):270–84. doi: 10.1038/nrendo.2018.24
3. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R, et al. Criteria, Prevalence, and Phenotypes of Polycystic Ovary Syndrome. Fertil Steril (2016) 106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003
4. Ruddenklau A, Campbell RE. Neuroendocrine Impairments of Polycystic Ovary Syndrome. Endocrinology (2019) 160(10):2230–42. doi: 10.1210/en.2019-00428
5. McAllister JM, Legro RS, Modi BP, Strauss JF 3rd. Functional Genomics of PCOS: From GWAS to Molecular Mechanisms. Trends Endocrinol Metab (2015) 26(3):118–24. doi: 10.1016/j.tem.2014.12.004
6. Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, et al. Association Between Polycystic Ovary Syndrome and Gut Microbiota. PLoS One (2016) 11(4):e0153196. doi: 10.1371/journal.pone.0153196
7. Rostamtabar M, Esmaeilzadeh S, Tourani M, Rahmani A, Baee M, Shirafkan F, et al. Pathophysiological Roles of Chronic Low-Grade Inflammation Mediators in Polycystic Ovary Syndrome. J Cell Physiol (2021) 236(2):824–38. doi: 10.1002/jcp.29912
8. Velez LM, Seldin M, Motta AB. Inflammation and Reproductive Function in Women With Polycystic Ovary Syndromedagger. Biol Reprod (2021) 104(6):1205–17. doi: 10.1093/biolre/ioab050
9. Daan NMP, Koster MPH, de Wilde MA, Dalmeijer GW. Biomarker Profiles in Women With PCOS and PCOS Offspring; A Pilot Study. PLoS One (2016) 11(11):e0165033. doi: 10.1371/journal.pone.0165033
10. Adams J, Liu Z, Ren YA, Wun WS, Zhou W, Kenigsberg S, et al. Enhanced Inflammatory Transcriptome in the Granulosa Cells of Women With Polycystic Ovarian Syndrome. J Clin Endocrinol Metab (2016) 101(9):3459–68. doi: 10.1210/jc.2015-4275
11. Li H, Guo Y, Deng J, Fischer H, Weedin EA, Burks HR, et al. Increased Testosterone and Proinflammatory Cytokines in Patients With Polycystic Ovary Syndrome Correlate With Elevated GnRH Receptor Autoantibody Activity Assessed by a Fluorescence Resonance Energy Transfer-Based Bioassay. Endocrine (2021) 74(1):163–71. doi: 10.1007/s12020-021-02761-7
12. Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of Metabolic and Inflammatory Markers With Polycystic Ovarian Syndrome (PCOS): An Update. Arch Gynecol Obstet (2021) 303(3):631–43. doi: 10.1007/s00404-020-05951-2
13. Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D, et al. IL-15, a Novel T Cell Growth Factor That Shares Activities and Receptor Components With IL-2. J Leukoc Biol (1995) 57(5):763–6. doi: 10.1002/jlb.57.5.763
14. Quinn LS, Anderson BG. Interleukin-15, IL-15 Receptor-Alpha, and Obesity: Concordance of Laboratory Animal and Human Genetic Studies. J Obes 2011 (2011) 2011:456347. doi: 10.1155/2011/456347
15. Duan Y, Li F, Wang W, Guo Q, Wen C, Li Y, et al. Interleukin-15 in Obesity and Metabolic Dysfunction: Current Understanding and Future Perspectives. Obes Rev (2017) 18(10):1147–58. doi: 10.1111/obr.12567
16. Ye J. Beneficial Metabolic Activities of Inflammatory Cytokine Interleukin 15 in Obesity and Type 2 Diabetes. Front Med (2015) 9(2):139–45. doi: 10.1007/s11684-015-0377-z
17. Lacraz G, Rakotoarivelo V, Labbe SM, Vernier M, Noll C, Mayhue M, et al. Deficiency of Interleukin-15 Confers Resistance to Obesity by Diminishing Inflammation and Enhancing the Thermogenic Function of Adipose Tissues. PLoS One (2016) 11(9):e0162995. doi: 10.1371/journal.pone.0162995
18. Dozio E, Malavazos AE, Vianello E, Briganti S, Dogliotti G, Bandera F, et al. Interleukin-15 and Soluble Interleukin-15 Receptor Alpha in Coronary Artery Disease Patients: Association With Epicardial Fat and Indices of Adipose Tissue Distribution. PLoS One (2014) 9(3):e90960. doi: 10.1371/journal.pone.0090960
19. Falconer H, Sundqvist J, Gemzell-Danielsson K, von Schoultz B, D'Hooghe TM. IVF Outcome in Women With Endometriosis in Relation to Tumour Necrosis Factor and Anti-Mullerian Hormone. Reprod BioMed Online (2009) 18(4):582–8. doi: 10.1016/S1472-6483(10)60138-1
20. Spanou S, Kalogiannis D, Zapanti E, Gazouli M, Sfontouris IA, Siristatidis C, et al. Interleukin 15 Concentrations in Follicular Fluid and Their Effect on Oocyte Maturation in Subfertile Women Undergoing Intracytoplasmic Sperm Injection. J Assist Reprod Genet (2018) 35(6):1019–25. doi: 10.1007/s10815-018-1170-0
21. Nguyen XP, Nakamura T, Osuka S, Bayasula B, Nakanishi N, Kasahara Y, et al. Effect of the Neuropeptide Phoenixin and Its Receptor GPR173 During Folliculogenesis. Reproduction (2019) 158(1):25–34. doi: 10.1530/REP-19-0025
22. Onalan G, Selam B, Baran Y, Cincik M, Onalan R, Gunduz U, et al. Serum and Follicular Fluid Levels of Soluble Fas, Soluble Fas Ligand and Apoptosis of Luteinized Granulosa Cells in PCOS Patients Undergoing IVF. Hum Reprod (2005) 20(9):2391–5. doi: 10.1093/humrep/dei068
23. Mikaeili S, Rashidi BH, Safa M, Najafi A, Sobhani A, Asadi E, et al. Altered FoxO3 Expression and Apoptosis in Granulosa Cells of Women With Polycystic Ovary Syndrome. Arch Gynecol Obstet (2016) 294(1):185–92. doi: 10.1007/s00404-016-4068-z
24. Lima PDA, Nivet AL, Wang Q, Chen YA, Leader A, Cheung A, et al. Polycystic Ovary Syndrome: Possible Involvement of Androgen-Induced, Chemerin-Mediated Ovarian Recruitment of Monocytes/Macrophages. Biol Reprod (2018) 99(4):838–52. doi: 10.1093/biolre/ioy096
25. Zhao KK, Cui YG, Jiang YQ, Wang J, Li M, Zhang Y, et al. Effect of HSP10 on Apoptosis Induced by Testosterone in Cultured Mouse Ovarian Granulosa Cells. Eur J Obstet Gynecol Reprod Biol (2013) 171(2):301–6. doi: 10.1016/j.ejogrb.2013.09.026
26. Lim JJ, Han CY, Lee DR, Tsang BK. Ring Finger Protein 6 Mediates Androgen-Induced Granulosa Cell Proliferation and Follicle Growth via Modulation of Androgen Receptor Signaling. Endocrinology (2017) 158(4):993–1004. doi: 10.1210/en.2016-1866
27. Lim JJ, Lima PDA, Salehi R, Lee DR, Tsang BK. Regulation of Androgen Receptor Signaling by Ubiquitination During Folliculogenesis and Its Possible Dysregulation in Polycystic Ovarian Syndrome. Sci Rep (2017) 7(1):10272. doi: 10.1038/s41598-017-09880-0
28. Azhary JMK, Harada M, Takahashi N, Nose E, Kunitomi C, Koike H, et al. Endoplasmic Reticulum Stress Activated by Androgen Enhances Apoptosis of Granulosa Cells via Induction of Death Receptor 5 in PCOS. Endocrinology (2019) 160(1):119–32. doi: 10.1210/en.2018-00675
29. Shen H, Wang Y. Activation of TGF-Beta1/Smad3 Signaling Pathway Inhibits the Development of Ovarian Follicle in Polycystic Ovary Syndrome by Promoting Apoptosis of Granulosa Cells. J Cell Physiol (2019) 234(7):11976–85. doi: 10.1002/jcp.27854
30. Hawke LG, Mitchell BZ, Ormiston ML. TGF-Beta and IL-15 Synergize Through MAPK Pathways to Drive the Conversion of Human NK Cells to an Innate Lymphoid Cell 1-Like Phenotype. J Immunol (2020) 204(12):3171–81. doi: 10.4049/jimmunol.1900866
31. Gomez-Nicola D, Valle-Argos B, Nieto-Sampedro M. Blockade of IL-15 Activity Inhibits Microglial Activation Through the NFkappaB, P38, and ERK1/2 Pathways, Reducing Cytokine and Chemokine Release. Glia (2010) 58(3):264–76. doi: 10.1002/glia.20920
32. Tee MK, Miller WL. Phosphorylation of Human Cytochrome P450c17 by P38alpha Selectively Increases 17,20 Lyase Activity and Androgen Biosynthesis. J Biol Chem (2013) 288(33):23903–13. doi: 10.1074/jbc.M113.460048
33. Kobayashi M, Yoshino O, Nakashima A, Ito M, Nishio K, Ono Y, et al. Inhibition of Autophagy in Theca Cells Induces CYP17A1 and PAI-1 Expression via ROS/p38 and JNK Signalling During the Development of Polycystic Ovary Syndrome. Mol Cell Endocrinol (2020) 508:110792. doi: 10.1016/j.mce.2020.110792
34. Smith GI, Mittendorfer B, Klein S. Metabolically Healthy Obesity: Facts and Fantasies. J Clin Invest (2019) 129(10):3978–89. doi: 10.1172/JCI129186
35. Li T, Wu YN, Wang H, Ma JY, Zhai SS, Duan J, et al. Dapk1 Improves Inflammation, Oxidative Stress and Autophagy in LPS-Induced Acute Lung Injury via P38mapk/NF-kappaB Signaling Pathway. Mol Immunol (2020) 120:13–22. doi: 10.1016/j.molimm.2020.01.014
36. Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an Anthrapyrazolone Inhibitor of Jun N-Terminal Kinase. Proc Natl Acad Sci USA (2001) 98(24):13681–6. doi: 10.1073/pnas.251194298
37. Jonas MI, Kurylowicz A, Bartoszewicz Z, Lisik W, Jonas M, Wierzbicki Z, et al. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, But Obesity Is Associated With Their Increased Content in Visceral Fat Depots. Int J Mol Sci (2015) 16(10):25817–30. doi: 10.3390/ijms161025817
38. Tarantino G, Citro V, Balsano C, Capone D. Age and Interleukin-15 Levels Are Independently Associated With Intima-Media Thickness in Obesity-Related NAFLD Patients. Front Med (Lausanne) (2021) 8:634962. doi: 10.3389/fmed.2021.634962
39. Chhabra Y, Nelson CN, Plescher M, Barclay JL, Smith AG, Andrikopoulos S, et al. Loss of Growth Hormone-Mediated Signal Transducer and Activator of Transcription 5 (STAT5) Signaling in Mice Results in Insulin Sensitivity With Obesity. FASEB J (2019) 33(5):6412–30. doi: 10.1096/fj.201802328R
40. Kuang H, Duan Y, Li D, Xu Y, Ai W, Li W, et al. The Role of Serum Inflammatory Cytokines and Berberine in the Insulin Signaling Pathway Among Women With Polycystic Ovary Syndrome. PLoS One (2020) 15(8):e0235404. doi: 10.1371/journal.pone.0235404
41. Havelock JC, Rainey WE, Carr BR. Ovarian Granulosa Cell Lines. Mol Cell Endocrinol (2004) 228(1-2):67–78. doi: 10.1016/j.mce.2004.04.018
42. Victor VM, Rovira-Llopis S, Banuls C, Diaz-Morales N, Martinez de Maranon A, Rios-Navarro C, et al. Insulin Resistance in PCOS Patients Enhances Oxidative Stress and Leukocyte Adhesion: Role of Myeloperoxidase. PLoS One (2016) 11(3):e0151960. doi: 10.1371/journal.pone.0151960
43. Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma Monocyte Chemoattractant Protein-1 (MCP-1) and Macrophage Inflammatory Protein-1alpha Are Increased in Patients With Polycystic Ovary Syndrome (PCOS) and Associated With Adiposity, But Unaffected by Pioglitazone Treatment. Clin Endocrinol (Oxf) (2009) 71(5):652–8. doi: 10.1111/j.1365-2265.2009.03523.x
44. Zafari Zangeneh F, Naghizadeh MM, Masoumi M. Polycystic Ovary Syndrome and Circulating Inflammatory Markers. Int J Reprod BioMed (2017) 15(6):375–82. doi: 10.29252/ijrm.15.6.375
45. Zangeneh FZ, Naghizadeh MM, Bagheri M, Jafarabadi M. Are CRH & NGF as Psychoneuroimmune Regulators in Women With Polycystic Ovary Syndrome? Gynecol Endocrinol (2017) 33(3):227–33. doi: 10.1080/09513590.2016.1250152
46. Burelli A, Cionini R, Rinaldi E, Benelli E, Fiore E, Canale D, et al. Serum PSA Levels Are Not Affected by the Menstrual Cycle or the Menopause, But Are Increased in Subjects With Polycystic Ovary Syndrome. J Endocrinol Invest (2006) 29(4):308–12. doi: 10.1007/BF03344101
47. Cox AJ, West NP, Cripps AW. Obesity, Inflammation, and the Gut Microbiota. Lancet Diabetes Endocrinol (2015) 3(3):207–15. doi: 10.1016/S2213-8587(14)70134-2
48. Guo Y, Luan L, Rabacal W, Bohannon JK, Fensterheim BA, Hernandez A, et al. IL-15 Superagonist-Mediated Immunotoxicity: Role of NK Cells and IFN-Gamma. J Immunol (2015) 195(5):2353–64. doi: 10.4049/jimmunol.1500300
49. Ahn EY, Pan G, Vickers SM, McDonald JM. IFN-Gammaupregulates Apoptosis-Related Molecules and Enhances Fas-Mediated Apoptosis in Human Cholangiocarcinoma. Int J Cancer (2002) 100(4):445–51. doi: 10.1002/ijc.10516
50. Wu H, Wang L, Zhang D, Qian J, Yan L, Tang Q, et al. PRDM5 Promotes the Apoptosis of Epithelial Cells Induced by IFN-Gamma During Crohn's Disease. Pathol Res Pract (2017) 213(6):666–73. doi: 10.1016/j.prp.2016.12.004
51. Wang J, Gong P, Li C, Pan M, Ding Z, Ge X, et al. Correlation Between Leptin and IFN-Gamma Involved in Granulosa Cell Apoptosis in PCOS. Gynecol Endocrinol (2020) 36(12):1051–6. doi: 10.1080/09513590.2020.1760817
52. Jing ZT, Liu W, Xue CR, Wu SX, Chen WN, Lin XJ, et al. AKT Activator SC79 Protects Hepatocytes From TNF-Alpha-Mediated Apoptosis and Alleviates D-Gal/LPS-Induced Liver Injury. Am J Physiol Gastrointest Liver Physiol (2019) 316(3):G387–96. doi: 10.1152/ajpgi.00350.2018
53. McFee RM, Romereim SM, Snider AP, Summers AF, Pohlmeier WE, Kurz SG, et al. A High-Androgen Microenvironment Inhibits Granulosa Cell Proliferation and Alters Cell Identity. Mol Cell Endocrinol (2021) 531:111288. doi: 10.1016/j.mce.2021.111288
54. Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular Growth and Atresia in Mammalian Ovaries: Regulation by Survival and Death of Granulosa Cells. J Reprod Dev (2012) 58(1):44–50. doi: 10.1262/jrd.2011-012
55. Porubek D. CYP17A1: A Biochemistry, Chemistry, and Clinical Review. Curr Top Med Chem (2013) 13(12):1364–84. doi: 10.2174/1568026611313120002
56. Ring AM, Lin JX, Feng D, Mitra S, Rickert M, Bowman GR, et al. Mechanistic and Structural Insight Into the Functional Dichotomy Between IL-2 and IL-15. Nat Immunol (2012) 13(12):1187–95. doi: 10.1038/ni.2449
57. Ocampo-Garza J, Griggs J, Tosti A. New Drugs Under Investigation for the Treatment of Alopecias. Expert Opin Investig Drugs (2019) 28(3):275–84. doi: 10.1080/13543784.2019.1568989
58. Jonard S, Dewailly D. The Follicular Excess in Polycystic Ovaries, Due to Intra-Ovarian Hyperandrogenism, May Be the Main Culprit for the Follicular Arrest. Hum Reprod Update (2004) 10(2):107–17. doi: 10.1093/humupd/dmh010
59. Patel S. Disruption of Aromatase Homeostasis as the Cause of a Multiplicity of Ailments: A Comprehensive Review. J Steroid Biochem Mol Biol (2017) 168:19–25. doi: 10.1016/j.jsbmb.2017.01.009
60. Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S, et al. Interactions Between Androgens, FSH, Anti-Mullerian Hormone and Estradiol During Folliculogenesis in the Human Normal and Polycystic Ovary. Hum Reprod Update (2016) 22(6):709–24. doi: 10.1093/humupd/dmw027
Keywords: PCOS, chronic inflammation state, IL-15, KGN, mouse primary granulosa cells
Citation: Liu Y, Li Z, Wang Y, Cai Q, Liu H, Xu C and Zhang F (2022) IL-15 Participates in the Pathogenesis of Polycystic Ovary Syndrome by Affecting the Activity of Granulosa Cells. Front. Endocrinol. 13:787876. doi: 10.3389/fendo.2022.787876
Received: 01 October 2021; Accepted: 18 January 2022;
Published: 18 February 2022.
Edited by:
Rong Li, Peking University Third Hospital, ChinaCopyright © 2022 Liu, Li, Wang, Cai, Liu, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feifei Zhang, ZmVpZmVpemhhbmdAZnVkYW4uZWR1LmNu; Congjian Xu, eHVjb25namlhbkBmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.