- 1Human Cancer Genomic Research, Research Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 2Department of Surgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 3Department of Pathology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Background: Disparity between sexes with regard to incidence, disease aggressiveness, and prognosis has been documented in several cancers. Although various reports have documented the association between male sex and aggressive papillary thyroid carcinoma (PTC), the prognostic impact of sex on PTC has been inconsistent. The role of sex in PTC aggressiveness and outcome in Middle Eastern PTC remains unknown. Therefore, our study retrospectively analyzed the data of a large cohort of Middle Eastern PTC patients to address this issue.
Methods: We compared men and women with respect to clinico-pathological characteristics, disease persistence, structural recurrence, risk stratification, and prognosis. We included 1,430 patients—1,085 (75.9%) women and 345 (24.1%) men.
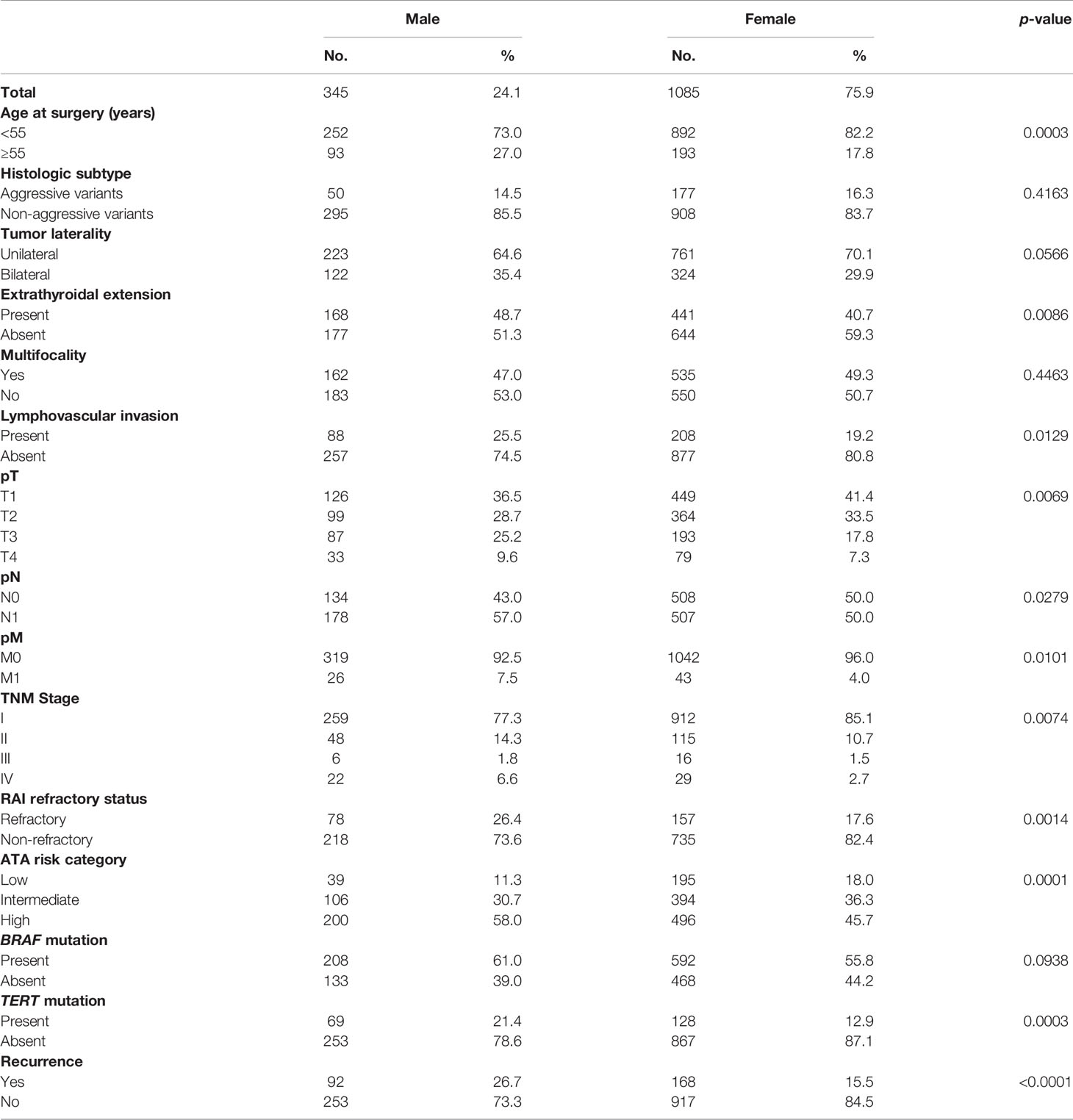
Results: The median follow-up was 9.3 years. At diagnosis, 27% (93/345) of men were ≥55 years, compared with 17.8% (193/1085) of women (p = 0.0003). Men had significantly more advanced disease at presentation: higher stage (p = 0.0074), larger tumor size (p = 0.0069), higher rates of lymphovascular invasion (p = 0.0129), extrathyroidal extension (p = 0.0086), regional lymph node metastasis (p = 0.0279), and distant metastasis (p = 0.0101). There was a higher rate of recurrence (p < 0.0001) and TERT mutations (p = 0.0003) in male PTC patients than in female patients. Additionally, radioiodine refractoriness was higher in male PTC patients (p = 0.0014). In multivariate analysis, male sex was an independent prognostic factor for poor recurrence-free survival (RFS) (hazard ratio = 1.58; 95% confidence interval = 1.20–2.06; p = 0.0011).
Conclusions: Men with PTC are more likely to present with more advanced and aggressive disease. Importantly, male sex was an independent prognostic factor for RFS. Thus, men may benefit from more aggressive management and therapeutic interventions.
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy (1). The incidence of PTC is on the rise over the past two decades (2, 3). In Saudi Arabia, PTC is the second commonest cancer affecting women, after breast cancer (4). PTC is an indolent disease with favorable prognosis in majority of patients. However, a subset of PTC patients (approximately one-third of all cases) will relapse (5–7), which could impact the quality of life for these patients. Thus, identification of clinical markers that could help to predict patients at high risk for recurrence is of great clinical importance for effective therapeutic interventions.
PTC is known to affect women more than men (1, 8). In fact, in Saudi Arabia, the age standardized incidence rate of thyroid cancer in women and men was 8.4 and 2.5 per 100,000 persons, respectively (4). The prognostic significance of sex in PTC remains controversial. While many studies have demonstrated the correlation between male sex and advanced stage, higher death rate, poor prognosis, and higher risk of recurrence in PTC (9–12), others have failed to identify prognostic difference between male and female PTCs when adjusting for age, stage, tumor size, and other influencing factors (13–15). Furthermore, current guidelines for risk stratification issued by the American Thyroid Association (ATA) does not include patient’s sex factor that could affect the risk of recurrence (16).
Despite the high incidence of PTC in Saudi Arabia and relatively high recurrence rate (17–19), the impact of sex on recurrence risk in PTC from Middle Eastern ethnicity remains unknown. Thus, we carried out this study on a large cohort of Middle Eastern adult PTC to investigate the impact of sex on clinico-pathological characteristics and patients’ prognosis. We also assess if male sex is an independent risk factor for recurrence of PTC from Middle Eastern ethnicity.
Materials and Methods
Patient Selection
One thousand four-hundred and thirty consecutive unselected adult PTC patients (≥18 years) diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were included in the study. Cases were identified based on clinical history followed by fine needle aspiration cytology for confirmation. The Institutional Review Board of the hospital approved this study and the Research Advisory Council (RAC) provided waiver of consent under project RAC # 2110 031 and #2211168.
Clinico-Pathological Data
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Staging of PTC was performed using the eighth edition of the American Joint Committee on Cancer (AJCC) staging system. Based on the ATA guidelines, tall cell, hobnail, columnar cell, diffuse sclerosing, and insular variants were classified as aggressive variants, whereas classical and follicular variants were classified as non-aggressive variants (16). Prophylactic central lymph node dissection (PCLND) was performed in patients with clinically uninvolved central neck lymph nodes (cN0) who had either advanced primary tumors (T3 or T4) or clinically involved lateral neck nodes (cN1b), in accordance with the 2015 ATA guidelines (16). Of the 942 patients with cN0 PTC, 213 patients underwent PCLND. However, 343 patients who were eligible for PCLND did not undergo the procedure, based on the treating surgeon’s discretion. Only structural recurrence (local, regional, or distant) was considered for analysis. Recurrence was defined as any newly detected tumor (local or distant) or metastatic regional lymph node (LN), based on ultrasound and/or imaging studies in patients who had been previously free of disease following initial treatment. Regional lymph node metastases were further confirmed by cytological and/or histological examination. Persistent disease was defined as the presence of serum Tg at detectable levels, persisting/increasing Tg antibody levels, or occurrence of structural disease within 1 year after surgery. Localized PTC was defined as tumor confined to the thyroid without any extrathyroidal extension, LN metastasis, or distant metastasis, at the time of diagnosis. Radioactive iodine (RAI) refractory disease and risk categories were defined based on 2015 ATA guidelines (16).
BRAF and TERT Mutation Analysis
BRAF and TERT mutation data were assessed in our laboratory by Sanger sequencing and have been published by us previously (20, 21).
Follow-Up and Study Endpoint
Patients were regularly followed up by both physical examinations and imaging studies to identify tumor recurrence. The median follow-up was 9.3 years (range, 1.0–30.1 years). Recurrence-free survival (RFS) was defined as the time (in months) from date of initial surgery to the occurrence of any tumor recurrence (local, regional, or distant). In case of no recurrence, date of last follow-up was the study endpoint for RFS.
Statistical Analysis
The associations between clinico-pathological variables and sex were performed using contingency table analysis and Chi square tests. Mantel–Cox log-rank test was used to evaluate RFS. Survival curves were generated using the Kaplan–Meier method. Cox proportional hazards model was used for multivariate analysis. Two-sided tests were used for statistical analyses with a limit of significance defined as p-value < 0.05. Data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Results
Patient and Tumor Characteristics
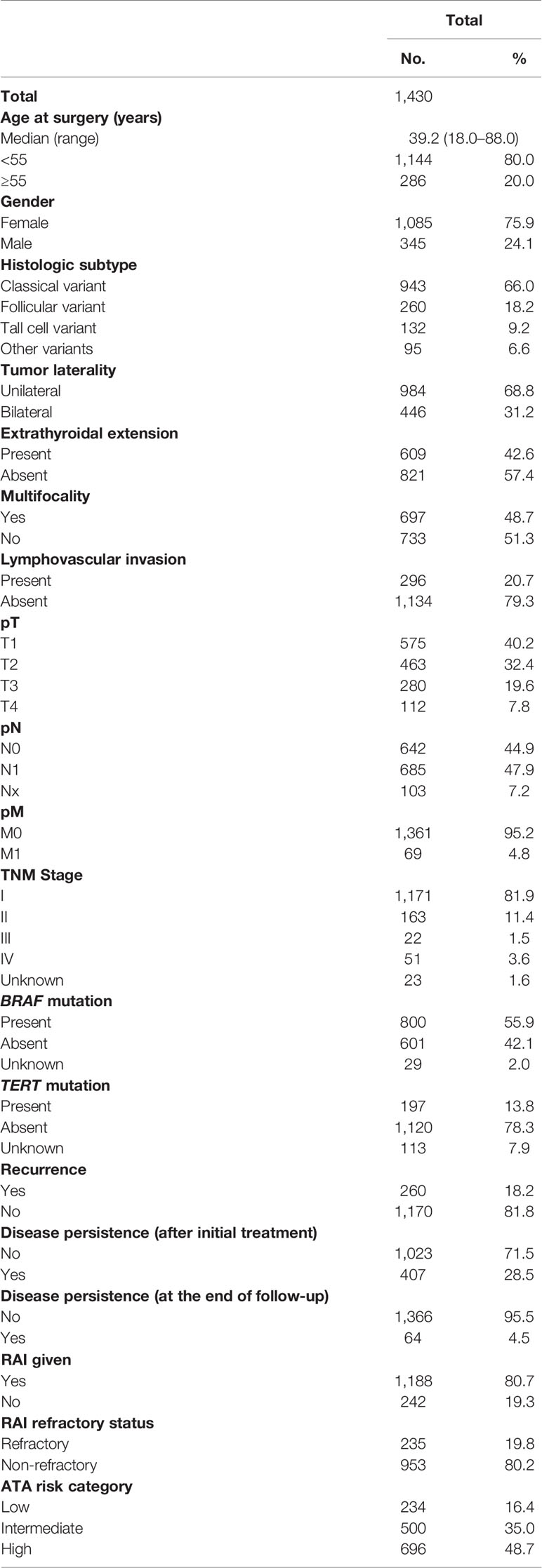
Median age of the study population was 39.2 years (range: 18–88 years), with a male-to-female ratio of 1:3. The majority of tumors were classical variants of PTC (66.0%; 943/1,430); 31.2% (446/1,430) of tumors were bilateral and 48.7% (697/1,430) were multifocal; 42.6% (609/1,430) of tumors exhibited extrathyroidal extension and 20.7% (296/1,430) showed lymphovascular invasion. Tumor recurrence was seen in 18.2% (260/1,430) (Table 1).
Clinico-Pathological Associations of Male Sex in PTC
In our cohort, 24.1% (345/1,430) of patients were male and 75.9% (1085/1430) were female. Male sex was associated with aggressive clinico-pathological characteristics such as older age (p = 0.0003), extrathyroidal extension (p = 0.0086), lymphovascular invasion (p = 0.0129), advanced T stage (p = 0.0069), LN metastasis (p = 0.0279), distant metastasis (p = 0.0101), and stage IV tumors (p = 0.0074). Male sex was also associated with tumor recurrence (p < 0.0001). To further corroborate the association of male sex with tumor recurrence, we analyzed the association of sex with ATA risk categories. Indeed, male sex was significantly associated with ATA high risk tumors (p = 0.0001). In addition, we found male sex to be associated with RAI refractoriness (p = 0.0014). Since BRAF and TERT mutation have been shown to be associated with RAI refractoriness, we sought to see if these mutations had a predilection for male sex. Although TERT mutation was associated with male sex (p = 0.0003), BRAF was not (p = 0.0938) (Table 2).
Since our cohort had a high rate of high-risk patients and we found a significant association between male sex and high-risk PTC, we sought to further analyze the clinico-pathological associations of male sex stratified by ATA risk categories. We found that male sex was associated with tumor recurrence in high-risk PTC (p = 0.0031), which stands true even on multivariate analysis (HR = 2.79, 95% CI = 1.45–5.76, p = 0.0016). In addition, male sex was associated with RAI refractoriness (p = 0.0085) and TERT mutation (p = 0.0341) in high-risk PTC. In intermediate-risk cases, male sex was found to be associated with other aggressive clinico-pathological characteristics, such as bilateral tumors (p = 0.0374) and advanced T stage (p = 0.0443). However, in both intermediate- and low-risk PTC, male sex was not associated with recurrence (Supplementary Tables 1–3).
Recurrence Rate in Male and Female PTC Stratified by Stage
Since stage of tumor is an important prognostic factor, we sought to determine the recurrence rate in male and female PTCs among early-stage (stage I and II) and late-stage (stage III and IV) tumors. We found a significantly higher recurrence rate among men than women in early-stage tumors (24.2% vs. 14.4%; p < 0.0001), whereas the difference was not significant between male and female sex in late-stage PTC (57.1% vs. 40.0%; p = 0.1529) (Figure 1).
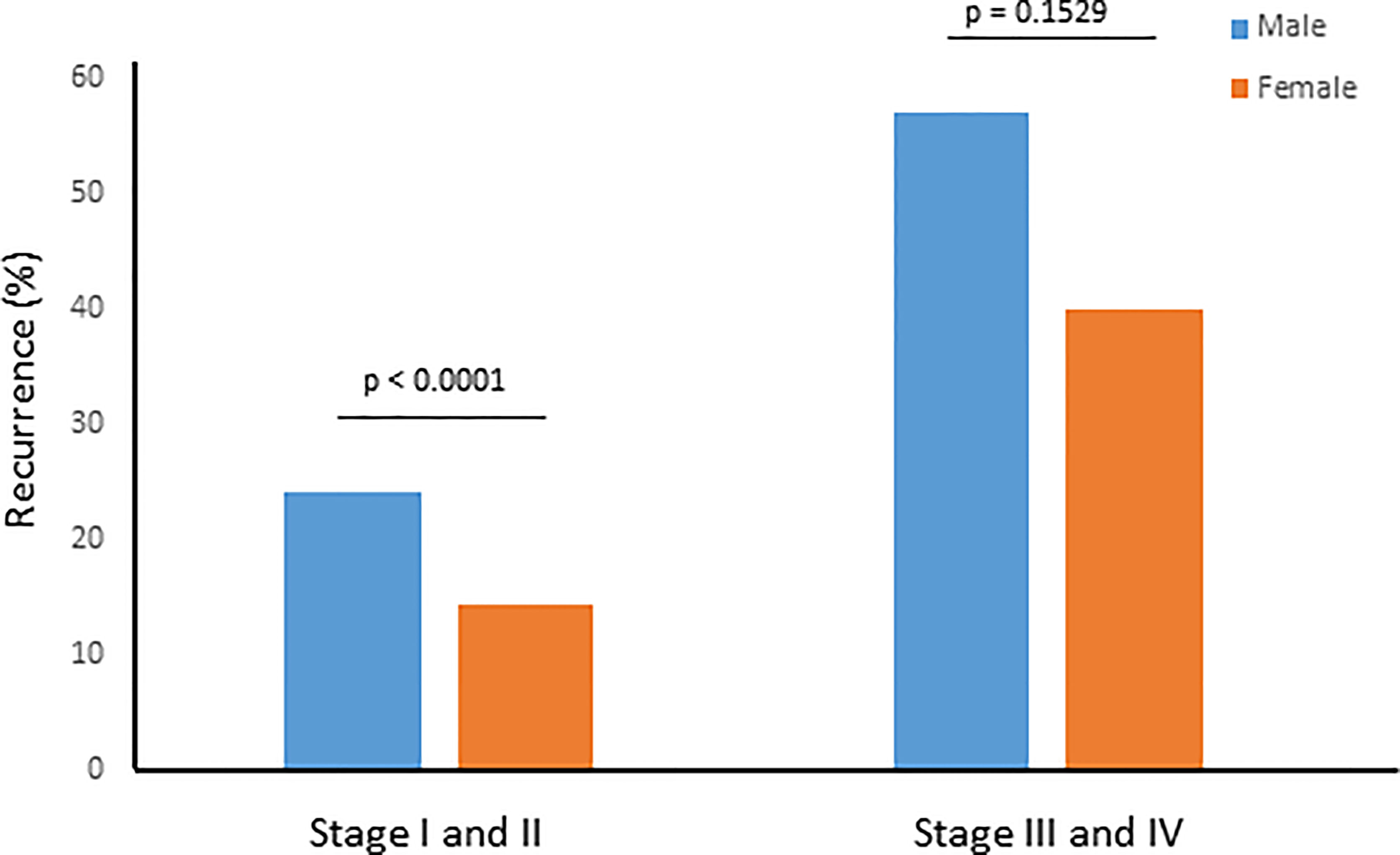
Figure 1 Recurrence rate for male and female sex stratified by tumor stage. Recurrence rate was significantly higher among men than women in early stage (I and II) tumors (24.2% vs. 14.4%; p < 0.0001), whereas the difference was not significant between male and female sex in late stage (III and IV) PTC (57.1% vs. 40.0%; p = 0.1529).
Prognostic Significance of Sex in PTC
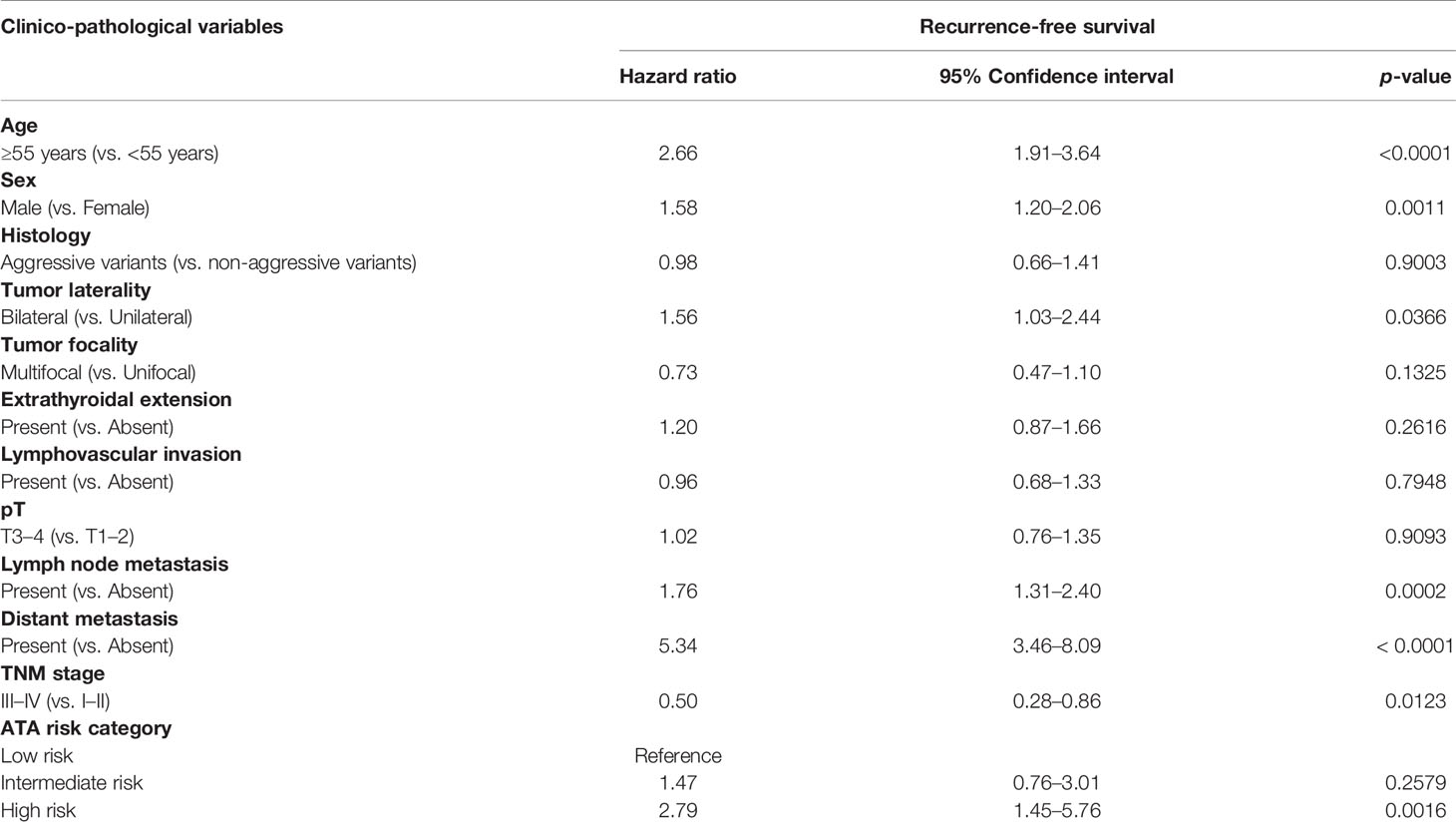
We next analyzed the prognostic significance of sex in PTC. Male sex was associated with poor RFS (p < 0.0001; Figure 2). On multivariate analysis using Cox proportional hazards model, male sex was found to be an independent predictor of poor RFS (hazard ratio = 1.58; 95% confidence interval = 1.20–2.06; p = 0.0011), when adjusted for other clinico-pathological parameters. In addition, we also found age, tumor laterality, LN metastasis, distant metastasis, tumor stage, and ATA risk category to be independent predictors of RFS (Table 3).
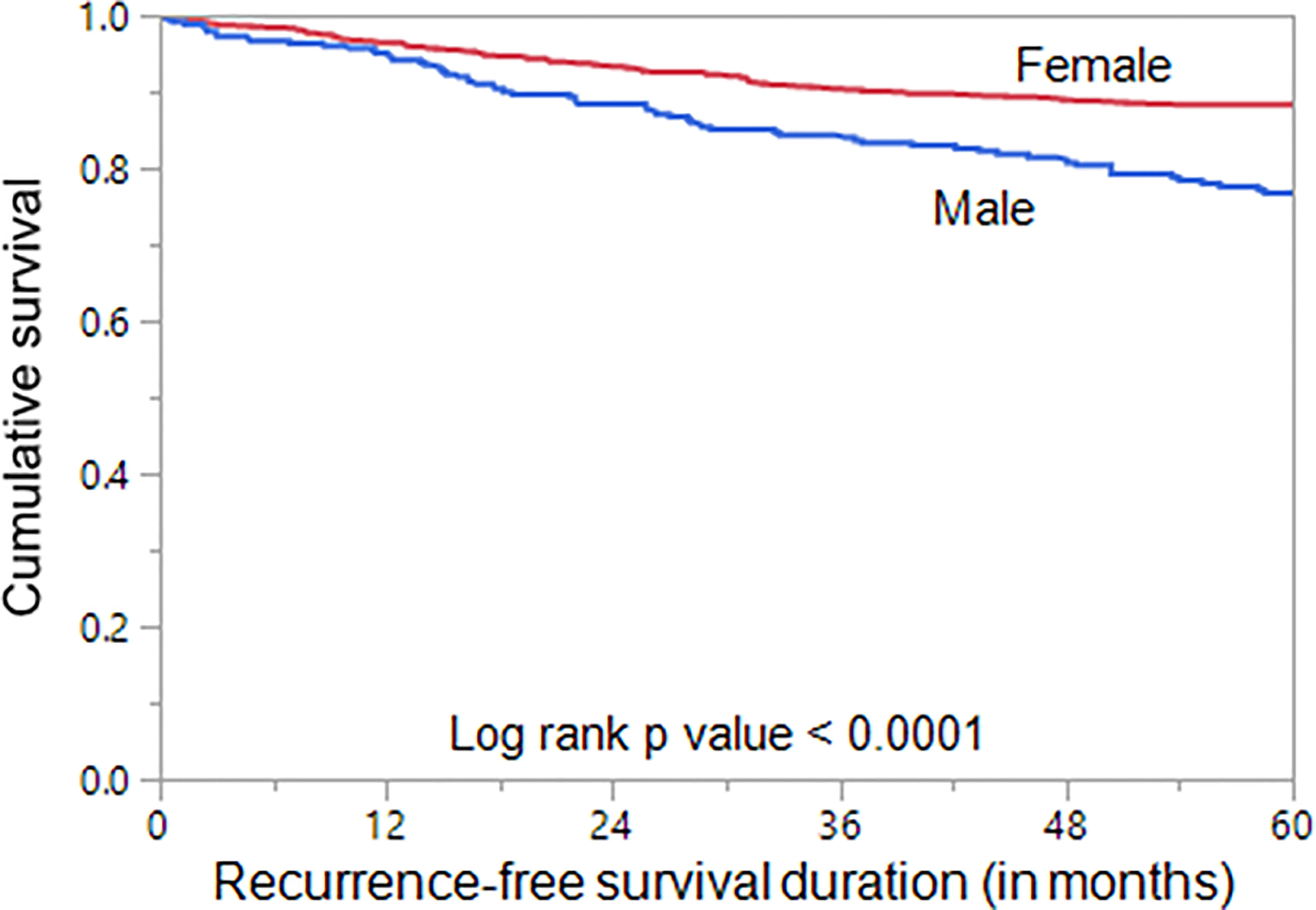
Figure 2 Sex and Recurrence-free survival. Kaplan–Meier survival curve showing poor recurrence-free survival in male sex compared to female sex (p < 0.0001).
Since age is an important determinant of prognosis, we stratified the patients into younger age (<55 years) and older age (≥55 years) to analyze the prognostic differences with regard to sex. Interestingly, we found male sex to be associated with poor RFS (p < 0.0001; Figure 3A) only in the younger age PTCs but not in the older age PTCs (p = 0.1659; Figure 3B). We also analyzed the prognostic significance of sex in localized PTCs and found that male sex was associated with poor RFS (p = 0.0015; Figure 3C).
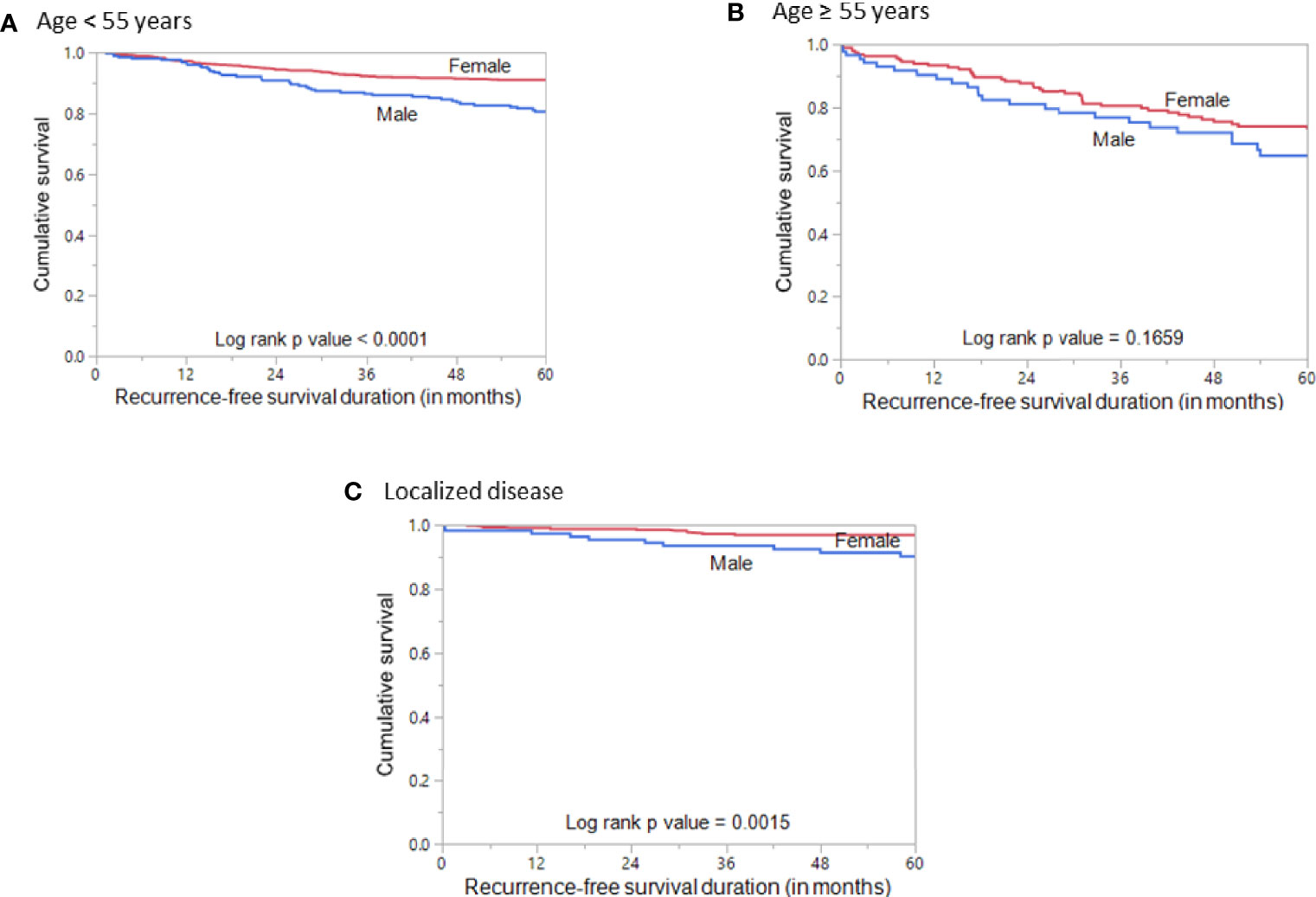
Figure 3 Recurrence-free survival stratified by age and disease localization. (A) Kaplan–Meier survival curve showing poor recurrence-free survival in male sex compared to female sex in patients aged < 55 years (p < 0.0001). (B) Kaplan–Meier survival curve showing no significant difference in recurrence-free survival between male and female sex in patients aged ≥ 55 years (p = 0.1659). (C) Kaplan–Meier survival curve showing poor recurrence-free survival in male sex compared to female sex in patients with localized PTC (p = 0.0015).
Discussion
While sex disparity in the incidence of PTC and its clinical impact have been well documented, detailed analysis of prognostic impact of sex on PTC from Middle Eastern ethnicity has not been fully illustrated. Our study of more than 1,400 adult PTCs documented their clinico-pathological characteristics and demonstrated significantly more aggressive disease in men than women. The presence of extrathyroidal extension and lymphovascular invasion was seen significantly more commonly in men than women. There was a higher rate of distant metastasis and regional LN metastasis in men than women. Moreover, a higher rate of advanced disease (stage II, III, and IV according to the latest AJCC staging system) and a higher rate of patients with intermediate- or high-risk disease were also observed in male PTC patients. Interestingly, our subgroup analysis showed that aggressive PTC was more common in men even at young age. This further supports the notion that men inherently have more aggressive PTC behavior, which may not be attributable solely to delay in diagnosis. Several previous studies have shown the association between male sex and advanced disease (9, 10, 13, 22, 23).
Median age of the study population was 39.2 years. This finding is similar to studies from other Middle Eastern ethnicities (24–27), but lower than that seen in Western population (28). This most likely represents the inherent aggressive nature of PTC in the Middle Eastern ethnicity. Another consideration that needs to be taken into account is the age of the general population. In Saudi Arabia, the population pyramid is skewed toward younger age groups, showing a cone-shaped pattern. Indeed, nearly 60% of the Saudi population are under the age of 30 years. It has been shown previously that age of the population could partly explain the variability of age of onset for cancer, whereby younger populations tended to have a higher incidence of early-onset cancer (29, 30).
The rate of recurrence was 18.2% (260/1,430) in the overall cohort, which is relatively higher than what has been reported previously (31–33). The long follow-up duration and the low rate of PCLND may have contributed to the relatively high rate of recurrence in our study. The long median follow-up of 9.5 years might increase the likelihood to detect more recurrence in PTC patients than other studies with shorter median follow-up. In addition, PCLND has not been routinely performed in our center but rather it was performed based on tumor size and LN status, according to ATA guidelines (16). However, we have found that 343 PTC patients met the criteria for PCLND and yet did not undergo the procedure. In fact, 21.9% (75/343) of these patients developed recurrence during the follow-up period, which further contributed to the relatively high recurrence rate in our study. Furthermore, our study showed a higher recurrence risk in men than women. The recurrence risk was 1.7-fold higher in men presenting with AJCC stage I and II, compared to women. However, recurrence risk does not show significant difference between men and women for AJCC stage III and IV.
Our study findings showed that men had significantly poor RFS even in multivariate analysis where tumor stage and other influencing factors were considered. Moreover, significant prognostic differences between men and women was noted even when PTC was localized to the thyroid gland, which may suggest a truly aggressive PTC behavior in Middle Eastern men, even with small tumor size. Some previous studies have shown that sex was associated with poor prognosis (12, 23, 34, 35), whereas others found no prognostic difference between men and women (13–15). The lack of consensus among previous studies could be attributed to cohort size, classification used, and the inability to differentiate persistent disease from recurrent disease.
Another important finding is the significant association between male PTC and RAI refractoriness (RAIR). We attempted to explore the molecular features that might contribute to RAIR in male PTC patients, especially BRAF and TERT mutations. A large body of evidence have documented the correlation between BRAF and/or TERT mutation and poor RAI response (36–38). BRAF mutation data were available for 1,369 patients while TERT mutations were available in 1,288 patients of the study cohort. Interestingly, no correlation between BRAF mutations and male PTC patients was noted. However, a significantly higher rate of TERT mutations was observed in male PTC patients compared to female patients. A large meta-analyses, involving 32 studies, also found a significant association between TERT promoter mutations and male sex (39).
Our study has certain limitations. First, it has a historical, retrospective nature, it lacks complete information on some tumor features including TERT and BRAF mutations, and 3% of the patients were lost to follow-up. Second, the study cohort was limited to Middle Eastern ethnicity and it is from a single center that could slightly impact the generalizability of our results to other populations. Thirdly, our study population included a high rate of high-risk patients, which could be attributed to genetics or differences in presentation owing to the unique ethnicity. Whether this could also be partly attributed to delay in seeking healthcare remains to be explored.
In summary, our study demonstrated that men present with more advanced stage of disease, at older age and with higher rate of aggressive molecular and histopathological features. Men have a higher rate of recurrence and a shorter recurrence-free survival. Moreover, male gender was an independent prognostic factor for RFS in Middle Eastern PTC patients. Overall, the results of this study strongly suggest that sex should be considered as an important predictor of prognosis and therefore men with PTC may benefit from more aggressive initial treatment and intense follow-up.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee, King Faisal Specialist Hospital and Research Centre. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Study concept and design: KA-K, SP, and AS. Executed the study: SP, AS, PA, NS, SA-S, and FA-D. Statistical analysis: SP. Drafting the article: KA-K, AS, and SP. Critical revision of the article for important intellectual content, writing of the article, and approval of the final version: KA-K, SP, AS, PA, NS, SA-S, and FA-D. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Felisa DeVera for her technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.777345/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71(3). doi: 10.3322/caac.21660
2. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid Cancer Incidence Trends in the United States: Association With Changes in Professional Guideline Recommendations. Thyroid (2020) 30(8):1132–40. doi: 10.1089/thy.2019.0415
3. Kitahara CM, Sosa JA. The Changing Incidence of Thyroid Cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
4. Alrawaji A, Alshahrani A, Alzahrani A, Alomran A, Almadouj A, Alshehri A, et al. Cancer Incidence Report Saudi Arabia 2015. Council SH, editor. Riyadh: Saudi Cancer Registry (2018).
5. Póvoa AA, Teixeira E, Bella-Cueto MR, Melo M, Oliveira MJ, Sobrinho-Simões M, et al. Clinicopathological Features as Prognostic Predictors of Poor Outcome in Papillary Thyroid Carcinoma. Cancers (2020) 12(11):3186. doi: 10.3390/cancers12113186
6. Coca-Pelaz A, Shah JP, Hernandez-Prera JC, Ghossein RA, Rodrigo JP, Hartl DM, et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv Ther (2020) 37:3112–28. doi: 10.1007/s12325-020-01391-1
7. Ritter A, Mizrachi A, Bachar G, Vainer I, Shimon I, Hirsch D, et al. Detecting Recurrence Following Lobectomy for Thyroid Cancer: Role of Thyroglobulin and Thyroglobulin Antibodies. J Clin Endocrinol Metab (2020) 105(6):dgaa152. doi: 10.1210/clinem/dgaa152
8. Rahbari R, Zhang L, Kebebew E. Thyroid Cancer Gender Disparity. Future Oncol (2010) 6(11):1771–9. doi: 10.2217/fon.10.127
9. Machens A, Hauptmann S, Dralle H. Disparities Between Male and Female Patients With Thyroid Cancers: Sex Difference or Gender Divide? Clin Endocrinol (2006) 65(4):500–5. doi: 10.1111/j.1365-2265.2006.02623.x
10. Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain K, Bigos S, et al. The Impact of Age and Gender on Papillary Thyroid Cancer Survival. J Clin Endocrinol Metab (2012) 97(6):E878–E87. doi: 10.1210/jc.2011-2864
11. Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, et al. Gender Is an Age-Specific Effect Modifier for Papillary Cancers of the Thyroid Gland. Cancer Epidemiol Prev Biomark (2009) 18(4):1092–100. doi: 10.1158/1055-9965.EPI-08-0976
12. Zahedi A, Bondaz L, Rajaraman M, Leslie WD, Jefford C, Young JE, et al. Risk for Thyroid Cancer Recurrence Is Higher in Men Than in Women Independent of Disease Stage at Presentation. Thyroid (2020) 30(6):871–7. doi: 10.1089/thy.2018.0775
13. Nilubol N, Zhang L, Kebebew E. Multivariate Analysis of the Relationship Between Male Sex, Disease-Specific Survival, and Features of Tumor Aggressiveness in Thyroid Cancer of Follicular Cell Origin. Thyroid (2013) 23(6):695–702. doi: 10.1089/thy.2012.0269
14. Grogan RH, Kaplan SP, Cao H, Weiss RE, DeGroot LJ, Simon CA, et al. A Study of Recurrence and Death From Papillary Thyroid Cancer With 27 Years of Median Follow-Up. Surgery (2013) 154(6):1436–47. doi: 10.1016/j.surg.2013.07.008
15. Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, et al. Thyroid Lobectomy for Papillary Thyroid Cancer: Long-Term Follow-Up Study of 1,088 Cases. World J Surg (2014) 38(1):68–79. doi: 10.1007/s00268-013-2224-1
16. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
17. Raef H, Alfadhli E, Al-Hajjaj A, Malabu UH, Al-Sobhi S, Rifai A, et al. High Rate of Persistent/Recurrent Disease Among Patients With Differentiated Thyroid Cancer in Saudi Arabia: Factors Affecting Non-Remission. Ann Saudi Med (2008) 28(4):277–81. doi: 10.5144/0256-4947.2008.277
18. Al-Qahtani KH, Tunio MA, Al Asiri M, Aljohani NJ, Bayoumi Y, Riaz K, et al. Clinicopathological Features and Treatment Outcomes of Differentiated Thyroid Cancer in Saudi Children and Adults. J Otolaryngol-Head Neck Surg (2015) 44(1):1–6. doi: 10.1186/s40463-015-0102-6
19. Siraj AK, Parvathareddy SK, Qadri Z, Siddiqui K, Al-Sobhi SS, Al-Dayel F, et al. Annual Hazard Rate of Recurrence in Middle Eastern Papillary Thyroid Cancer Over a Long-Term Follow-Up. Cancers (2020) 12(12):3624. doi: 10.3390/cancers12123624
20. Siraj AK, Parvathareddy SK, Pratheeshkumar P, Divya SP, Al-Sobhi SS, Al-Dayel F, et al. PD-L1 Is an Independent Prognostic Marker in Middle Eastern PTC and Its Expression Is Upregulated by BRAFV600E Mutation. Cancers (2021) 13(3):555. doi: 10.3390/cancers13030555
21. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al-Rasheed M, et al. Telomerase Reverse Transcriptase Mutations Are Independent Predictor of Disease-Free Survival in M Iddle E Astern Papillary Thyroid Cancer. Int J Cancer (2018) 142(10):2028–39. doi: 10.1002/ijc.31225
22. Oyer SL, Smith VA, Lentsch EJ. Sex Is Not an Independent Risk Factor for Survival in Differentiated Thyroid Cancer. Laryngoscope (2013) 123(11):2913–9. doi: 10.1002/lary.24018
23. Ding J, Wu W, Fang J, Zhao J, Jiang L. Male Sex is Associated With Aggressive Behaviour and Poor Prognosis in Chinese Papillary Thyroid Carcinoma. Sci Rep (2020) 10(1):1–8. doi: 10.1038/s41598-020-60199-9
24. Doubi A, Al-Qannass A, Al-Angari SS, Al-Qahtani KH, Alessa M, Al-Dhahri S. Trends in Thyroid Carcinoma Among Thyroidectomy Patients: A 12-Year Multicenter Study. Ann Saudi Med (2019) 39(5):345–9. doi: 10.5144/0256-4947.2019.345
25. Samargandy S, Qari R, Aljadani A, Assaqaf D, Etaiwi A, Alghamdi D, et al. Clinicopathological Characteristics of Thyroid Cancer in a Saudi Academic Hospital. Cureus (2020) 12(5):e8044. doi: 10.7759/cureus.8044
26. Al-Zaher N, Al-Salam S, El Teraifi H. Thyroid Carcinoma in the United Arab Emirates: Perspectives and Experience of a Tertiary Care Hospital. Hematol/Oncol Stem Cell Ther (2008) 1(1):14–21. doi: 10.1016/S1658-3876(08)50055-0
27. Keinan-Boker L, Silverman BG. Trends of Thyroid Cancer in Israel: 1980–2012. Rambam Maimonides Med J (2016) 7(1):e0001. doi: 10.5041/RMMJ.10228
28. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. Jama (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
29. Bidoli E, Virdone S, Hamdi-Cherif M, Toffolutti F, Taborelli M, Panato C, et al. Worldwide Age at Onset of Female Breast Cancer: A 25-Year Population-Based Cancer Registry Study. Sci Rep (2019) 9(1):1–8. doi: 10.1038/s41598-019-50680-5
30. Franco-Marina F, López-Carrillo L, Keating NL, Arreola-Ornelas H, Knaul FM. Breast Cancer Age at Diagnosis Patterns in Four Latin American Populations: A Comparison With North American Countries. Cancer Epidemiol (2015) 39(6):831–7. doi: 10.1016/j.canep.2015.10.004
31. Nam SH, Bae MR, Roh J-L, Gong G, Cho K-J, Choi S-H, et al. A Comparison of the 7th and 8th Editions of the AJCC Staging System in Terms of Predicting Recurrence and Survival in Patients With Papillary Thyroid Carcinoma. Oral Oncol (2018) 87:158–64. doi: 10.1016/j.oraloncology.2018.11.003
32. Ryu YJ, Cho JS, Park MH, Yoon JH. Identifying Risk Factors of Recurrence for Clinically Node Negative Papillary Thyroid Carcinoma With Pathologic N1a. BMC Surg (2019) 19(1):1–9. doi: 10.1186/s12893-019-0541-5
33. Enumah S, Fingeret A, Parangi S, Dias-Santagata D, Sadow PM, Lubitz CC. BRAF V600E Mutation Is Associated With an Increased Risk of Papillary Thyroid Cancer Recurrence. World J Surg (2020) 44(8):2685–91. doi: 10.1007/s00268-020-05521-2
34. Toniato A, Boschin I, Casara D, Mazzarotto R, Rubello D, Pelizzo M. Papillary Thyroid Carcinoma: Factors Influencing Recurrence and Survival. Ann Surg Oncol (2008) 15(5):1518–22. doi: 10.1245/s10434-008-9859-4
35. Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, et al. A Prognostic Index for Thyroid Carcinoma. A Study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer (1965) (1979) 15(8):1033–41. doi: 10.1016/0014-2964(79)90291-3
36. Yang K, Wang H, Liang Z, Liang J, Li F, Lin Y. BRAFV600E Mutation Associated With Non–Radioiodine-Avid Status in Distant Metastatic Papillary Thyroid Carcinoma. Clin Nucl Med (2014) 39(8):675–9. doi: 10.1097/RLU.0000000000000498
37. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer. J Nucl Med (2017) 58(2):258–65. doi: 10.2967/jnumed.116.180240
38. Liu J, Liu R, Shen X, Zhu G, Li B, Xing M. The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J Nucl Med (2020) 61(2):177–82. doi: 10.2967/jnumed.119.227652
Keywords: papillary thyroid carcinoma, male sex, recurrence-free survival, prognosis, clinico-pathological associations
Citation: Siraj AK, Parvathareddy SK, Annaiyappanaidu P, Siraj N, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2022) Male Sex Is an Independent Predictor of Recurrence-Free Survival in Middle Eastern Papillary Thyroid Carcinoma. Front. Endocrinol. 13:777345. doi: 10.3389/fendo.2022.777345
Received: 15 September 2021; Accepted: 08 February 2022;
Published: 10 March 2022.
Edited by:
Francesco Frasca, University of Catania, ItalyReviewed by:
Maria Grazia Castagna, University of Siena, ItalyElena Sabini, University of Pennsylvania, United States
Copyright © 2022 Siraj, Parvathareddy, Annaiyappanaidu, Siraj, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, a2t1cmF5YUBrZnNocmMuZWR1LnNh
†These authors have contributed equally to this work
 Abdul K. Siraj
Abdul K. Siraj Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Khawla S. Al-Kuraya
Khawla S. Al-Kuraya