- 1Department of Ophthalmology, West China Hospital, Sichuan University, Chengdu, China
- 2Research Laboratory of Macular Disease, West China Hospital, Sichuan University, Chengdu, China
Purpose: To study the association between different hypoglycemic regimens and postoperative diabetic macular edema (DME).
Methods: A secondary analysis based on a retrospective cohort study.
Results: In this secondary analysis, 124 eyes from patients with proliferative diabetic retinopathy (PDR) who underwent pars plana vitrectomy (PPV) between January 2008 and September 2012 were included. We found that compared with oral hypoglycemic medication, oral hypoglycemic medication plus insulin treatment revealed an insignificant relationship with postoperative DME (odds ratio [OR]=0.8, 95% confidence interval [CI]: 0.12-5.21, P=0.8167), only insulin treatment revealed a significant association with postoperative DME (OR=0.10, 95% CI: 0.01-0.84, P=0.0337) after adjusted age, sex. After adjusted age, sex, diabetes mellitus (DM) duration, glycosylated hemoglobin (HbA1c), the results did not have obvious changes (OR=0.61, 95% CI: 0.09-4.26, P=0.6187; OR=0.07, 95% CI: 0.01-0.65, P=0.0197). Furthermore, after adjusted age, sex, DM duration, HbA1c, hypertension, intraoperative retinal photocoagulation, vitreous hemorrhage, macular detachment, fibrovascular membrane, intraocular lens implantation and microincision vitrectomy surgery, the results were consistent (OR=0.66, 95% CI: 0.05-9.49, P=0.7621; OR=0.06, 95% CI: 0.00-0.81, P=0.0342). The same trend was observed in these adjusted models as well (p for trend was 0.0254, 0.0141, and 0.0311, respectively).
Conclusion: In conclusion, our results of the secondary analysis should be interpreted as a significant association between insulin treatment and reduced risks of postoperative DME in Japanese PDR patients with PPV surgery, compared with oral medications. Well glycemic control with longstanding insulin therapy may be beneficial to reduce the risks of postoperative DME in PDR patients. Our investigation calls for large-scale and long-term prospective clinical studies for a full evaluation of the exact role of insulin in the progression of postoperative DME.
Introduction
Diabetic retinopathy (DR) is the primary cause of visual impairment and blindness among working-age individuals in developed countries (1). Two important factors affecting the vision of DR patients are complications associated with proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME), which are thought to occur as a result of vascular endothelial growth factor (VEGF) and other cytokines into the vitreous cavity (2, 3). PDR, the most advanced stage of DR, is characterized by neovascularization and proliferative membrane formation, which may cause vitreous hemorrhage and tractional retinal detachment (4–7). Those patients with PDR often require pars plana vitrectomy (PPV) treatment and emergency management to prevent further vision loss. Although DME can occur at any DR stage, the prevalence of DME was associated with diabetes mellitus (DM) duration and DR severity. PDR and DME frequently occur together and the prevalence of DME in PDR patients varied from 30% to 72.6% (8, 9). The risk factors for DME have been widely studied, including duration of diabetes (10), hypertension (11, 12), glycosylated hemoglobin (HbA1c) level (13–15), insulin (15–19), and other factors (11).
There were divergent findings of the association between different hypoglycemic regimens and DME. Several independent clinical studies (14–16, 18–21) and meta-analysis (17) confirmed that insulin use increased the risks of DME in patients with type 2 diabetes mellitus (T2DM) compared with oral hypoglycemic agents. What’s more, this finding was supported by another experimental research that insulin increased retinal vascular permeability in diabetic mice (22). While several studies pointed to a potential effect of insulin on reducing risks of DME (23, 24). The stable glycemic control induced by insulin might be one possible explanation. Insulin itself has a weak to moderate stimulatory effect on the proliferation of human retinal pigment epithelium (RPE) cells and could promote RPE wound healing (25), which might be another possible explanation. Apart from insulin, different oral hypoglycemic agents may have different effects on DME. Thiazolidinediones (TZDs) including pioglitazone and rosiglitazone, are insulin-sensitizing medications that can be used for glycemic control in T2DM. A growing body of studies has highlighted that TZDs might contribute to increase risks of DME (26–29). In addition to the aforementioned, the association between sulphonylureas, metformin, and the risk of DME has also been investigated (14, 30).
Treatments for DME have gradually evolved from the initial grid macular coagulations to intravitreal injection including triamcinolone, Ozurdex, and anti-VEGF agents. In addition to the aforementioned, PPV surgery is proven to be associated with structural benefits compared with the natural history of DME (9, 31, 32). PPV treatment in PDR aims at removing vitreoretinal traction and vitreous hemorrhage, clearing various cytokines from the vitreous cavity, reattaching detached neuroretina, maintaining media transparency, and improving ocular circulation (7, 33). Even if patients with PDR have undergone PPV treatment, some patients still develop postoperative DME. How to make those patients with postoperative DME preserve their vision and improve their quality of life is a problem that needs to be solved urgently. In addition to investigating the specific treatments of postoperative DME, more attention we should pay to the risk factors of postoperative DME. However, first-hand clinical data regarding the role of different hypoglycemic regimens in postoperative DME is scarce. In this study, we performed a secondary data analysis based on existing data that comes from the published paper (34) to investigate the association between different hypoglycemic regimens and postoperative DME.
Materials and methods
Data source
We freely downloaded the raw data uploaded by Nishi et al. (34) from the Supplementary Materials in PLOS One. Since Nishi et al. (34) have authorized the ownership of the original data to PLOS One, we can use this data to perform secondary data analysis based on different scientific assumptions. Data from: Factors correlated with visual outcomes at two and four years after vitreous surgery for proliferative diabetic retinopathy (PMID: 33444332).
Study population
Nishi et al. (34) completed the entire study. The specific details were described in the original paper reported by Nishi et al. (34). They conducted a retrospective cohort study at Yamagata University Hospital, Yamagata, Japan between January 2008 and September 2012. They retrospectively reviewed the medical records of these patients.
A total of 128 eyes were collected from the PDR patients who had been to Yamagata University Hospital or other hospitals after three-port 20-gauge (G) PPV or microincision vitreous surgery (MIVS) (23-G or 25-G). All patients with persistent vitreous hemorrhage and tractional retinal detachment and two vitreoretinal surgeons performed all the surgical procedures. However, surgical cases of only DME were excluded. All patients did not receive anti-VEGF therapy as a preoperative adjunct. Pan retinal photocoagulation was cautiously performed before or during PPV on all patients. Participants with vision-affecting lesions such as posterior capsular opacification, progressed cataract, neovascular glaucoma, and DME during the postoperative course were treated. The follow-up time was 3 months, 6 months, 1 year, 2 years, and 4 years after the primary PPV.
This study was performed by Japanese researcher Nishi et al. at the Yamagata University Hospital, Yamagata, Japan. In the previously published article (34), Nishi et al. have clearly stated that the study was performed following the Declaration of Helsinki and approved by the Ethics Committee of the Yamagata University Faculty of Medicine (approval number: H26-21). All data were fully anonymized before we accessed them and the institutional review board waived the requirement for informed consent.
Variables
The systemic factors collected were as follows: age, sex, duration from visual loss awareness to the primary vitreous surgery, hypertension history, DM duration, preoperative HbA1c, oral hypoglycemic medication, insulin treatment, diabetic nephropathy history, coronary heart disease and/or stroke history, anticoagulant and/or antiplatelet agent administration, preoperative systolic and diastolic blood pressure, and blood biochemical examination, including blood urea nitrogen, creatinine, estimated glomerular filtration rate (eGFR), uric acid, triglyceride, total cholesterol, hemoglobin.
Moreover, the ophthalmologic findings were categorized into three sections: preoperative, intraoperative, and postoperative. The preoperative ophthalmologic findings were as follows: intraocular lens implantation, retinal photocoagulation, the history of intravitreal injection of triamcinolone acetonide, rubeosis iridis, ocular hypertension (>21 mmHg), vitreous hemorrhage, posterior vitreous detachment, fibrovascular membrane, retinal detachment, and macular detachment. The intraoperative ophthalmologic findings were the following: cataract surgery, intraoperative retinal photocoagulation, gas tamponade, silicone oil tamponade, intraoperative complications (iatrogenic retinal break and retinal dialysis), and the number of used gauges (20-G or MIVS). Lastly, the postoperative ophthalmologic findings were as follows: reoperation and postoperative complications (vitreous hemorrhage, retinal detachment, DME, and neovascular glaucoma).
Statistical analysis
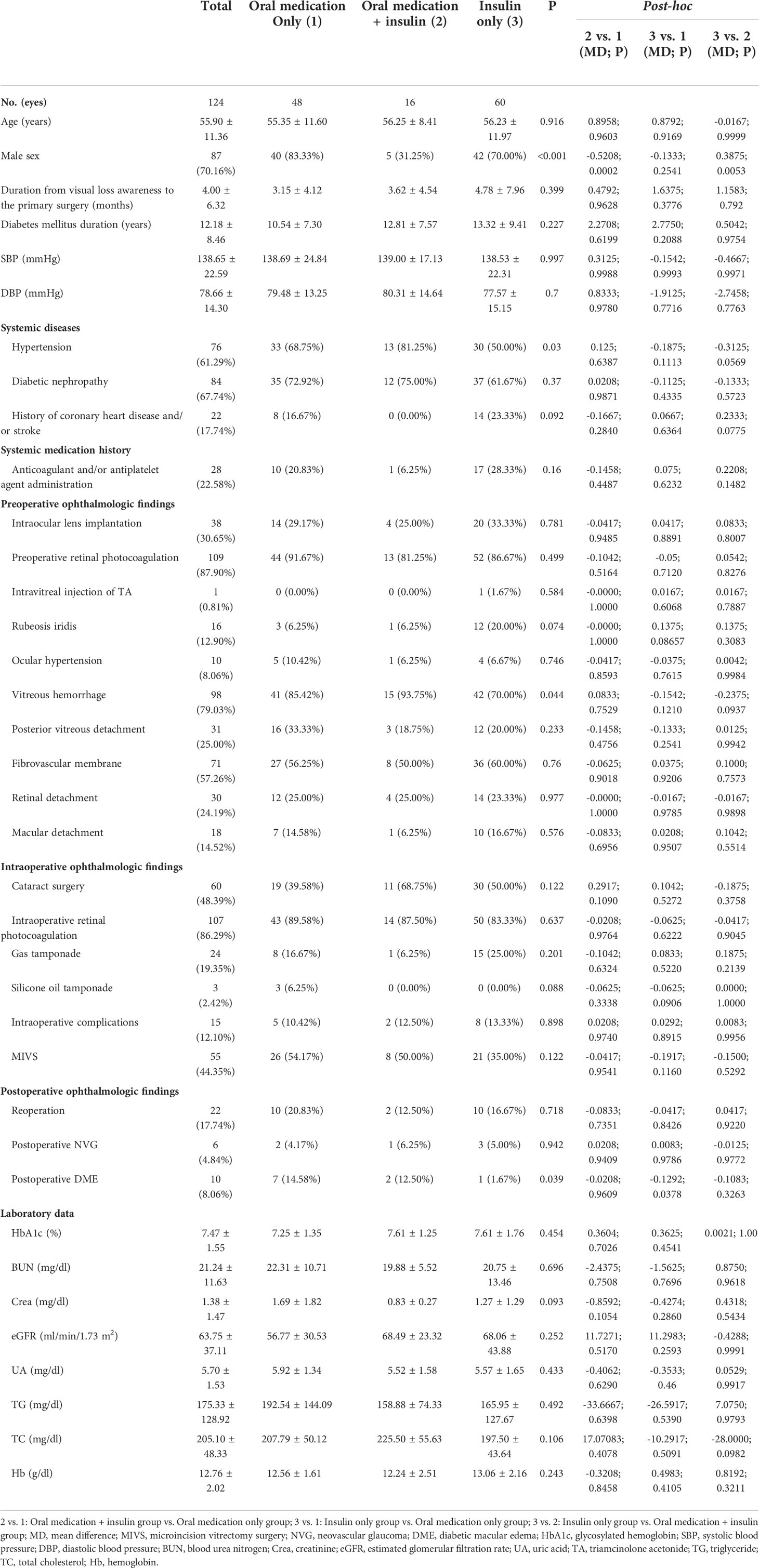
Demographic characteristics and study outcomes were summarized using descriptive statistics. Continuous variables were summarized with mean ± standard deviations (SD) and categorical variables with percentages. Two dichotomous categorical variables (“oral DM medication” and “insulin treatment”) were classified into a quartile categorical variable: no medication, only oral medication, oral medication plus insulin, only insulin. To improve the statistical power, of the 128 eyes, 4 eyes without any medication were excluded from this study. We first compared the data distribution of each covariate among the three different hypoglycemic regimens using the t-test (normal distribution) or Kruskal-Wallis rank-sum test (non-normal distribution) for continuous variables and χ2 tests for categorical data and post-hoc comparison was performed (Table 1). Next, univariate logistic regression (Table 2) and multivariate logistic regression models (Table 3) were used to examine the association between different hypoglycemic regimens and postoperative DME. Test for trend was used to observe the trend change between different hypoglycemic regimens and postoperative DME in different adjusted models (Table 3). Subgroup analysis (Table 4) was used to see whether the results were stable at all stratifications. Statistical analyses were performed using Empower Stats (http://www.empowerstats.com; X&Y Solutions Inc., Boston, MA) and R software, version 3.4.3 (http://www.R-project.org/, The R Foundation). A two-sided P < 0.05 was considered to be statistically significant.
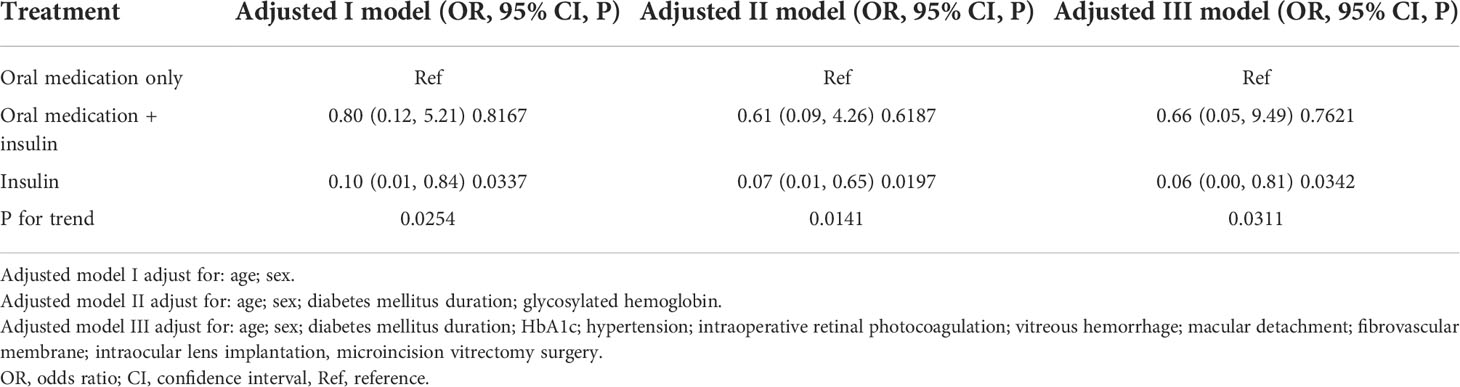
Table 3 Relationship between different hypoglycemic regimens and postoperative diabetic macular edema in different models.
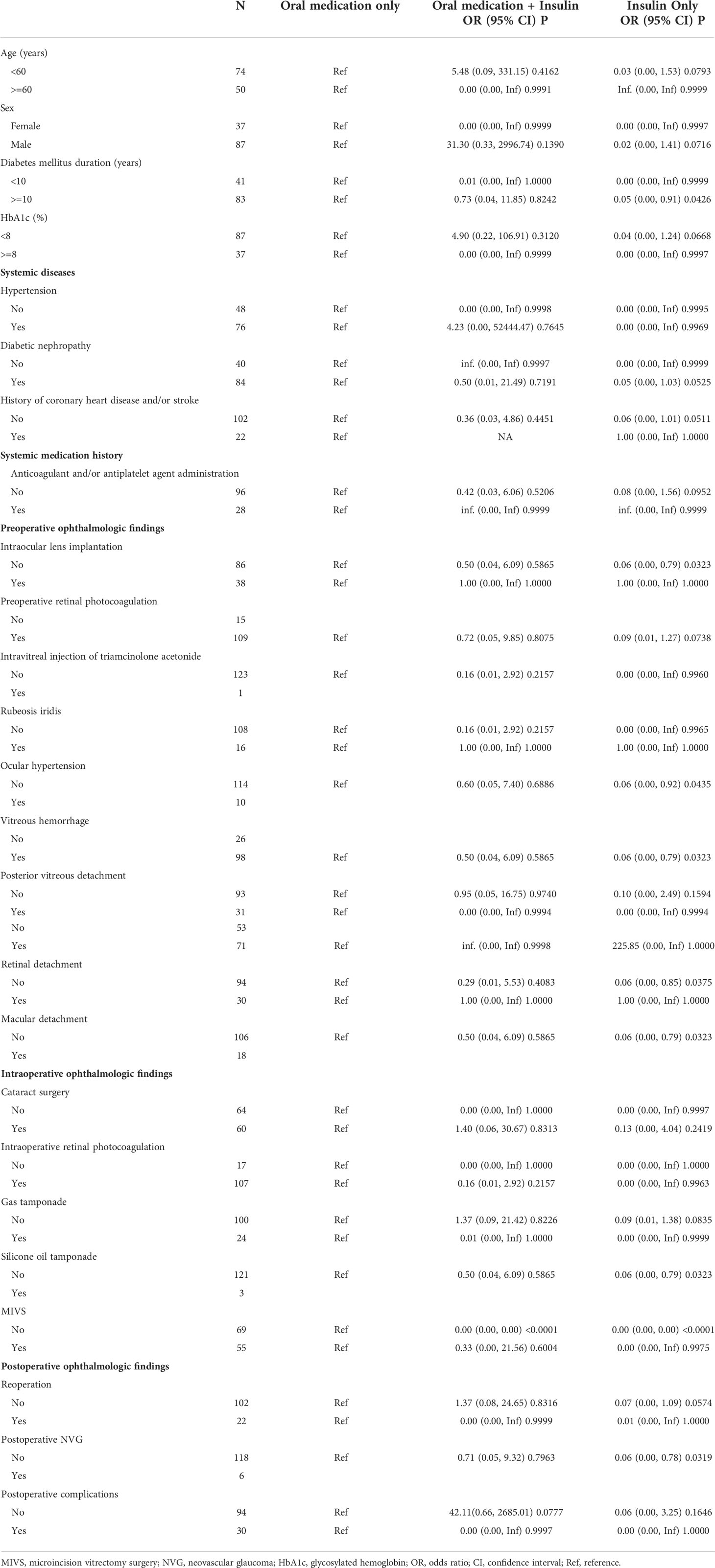
Table 4 The results of stratified analysis between different hypoglycemic regimens and postoperative diabetic macular edema.
Results
Baseline characteristics of participants
A total of 124 eyes with PDR were enrolled in the final analysis. These participants were divided into three groups according to different hypoglycemic regimens. The average age of the participants was 55.90 ± 11.36 years old, and about 70.16% of them were men. There was no statistically significant difference in age among different hypoglycemic regimens. Patients taking insulin with or without other medications at baseline had longer DM duration (12.81 ± 7.57 and 13.32 ± 9.41 years, respectively) compared with those only taking oral medications (10.54 ± 7.30 years), yet there was no statistical difference. The same trend was observed in the HbA1c level. Other baseline characteristics are listed in Table 1.
Univariate analysis
The results of univariate analysis revealed a significant association between only insulin group and postoperative DME (odds ratio [OR]=0.10, 95% confidence interval [CI]: 0.01-0.84, P=0.0338), compared with only oral medication. There was a statistically significant difference between fibrovascular membrane and postoperative DME (OR=0.16, 95% CI: 0.03-0.80, P=0.0258). We found that other factors were not associated with postoperative DME. The results of univariate analysis are shown in Table 2.
The relationship between different hypoglycemic regimens and postoperative DME
We used a logistic regression model to evaluate the associations between different hypoglycemic regimens and postoperative DME (Table 3). Meanwhile, we showed three adjusted models in Table 3. In adjusted model I (adjusted age, sex), compared with oral hypoglycemic medication, oral hypoglycemic medication plus insulin treatment revealed no association with postoperative DME (OR=0.8, 95% CI: 0.12-5.21, P=0.8167), while only insulin treatment revealed a significant association with postoperative DME (OR=0.10, 95% CI: 0.01-0.84, P=0.0337). In adjusted model II (adjusted age, sex, DM duration, HbA1c), the results did not have obvious changes (OR=0.61, 95% CI: 0.09-4.26, P=0.6187; OR=0.07, 95% CI: 0.01-0.65, P=0.0197). Furthermore, in adjusted model III (adjusted age, sex, DM duration, HbA1c, hypertension, intraoperative retinal photocoagulation, vitreous hemorrhage, macular detachment, fibrovascular membrane, intraocular lens implantation, MIVS), the results were consistent (OR=0.66, 95% CI: 0.05-9.49, P=0.7621; OR=0.06, 95% CI: 0.00-0.81, P=0.0342). The same trend was observed in these adjusted models as well (p for trend was 0.0254, 0.0141, and 0.0311, respectively).
The results of stratified analysis between different hypoglycemic regimens and postoperative DME
Each stratification was adjusted for all the factors (age, sex, DM duration, HbA1c, hypertension, intraoperative retinal photocoagulation, vitreous hemorrhage, macular detachment, fibrovascular membrane, intraocular lens implantation, MIVS) except the stratification factor itself. All the results of stratified analysis are shown in Table 4. Due to the limitation of sample size, many variables cannot be counted after stratification.
Discussion
This study demonstrated that compared with oral hypoglycemic medication, only insulin treatment had an association with the reduced risk of postoperative DME in all adjusted models. These stable and consistent results indicated that long-term insulin therapy seems to be beneficial in reducing the risk of postoperative DME. While the association between insulin therapy and reduced risk of postoperative DME seems to have nothing to do with HbA1c. In the stratified analysis, we can see that between different stratifications (age, gender, different HbA1c, gas tamponade, hypertension, posterior vitreous detachment, reoperation, MIVS, postoperative complications), oral medication plus insulin may exacerbate DME, although there is no statistical difference, the direction of the effect value is biased towards exacerbating DME. It may be necessary to increase the sample size to further verify whether the results are stable between different stratifications and whether the stratification will lead to different results.
Most previous studies (14–21) have confirmed that insulin might contribute to a higher risk of DME, which is different from the results of postoperative DME in this study. We think that there are the following reasons: Firstly, differences in the study population may lead to such inconsistent results. The population of previous studies included patients with DME from all stages of DR (14, 18, 19, 30). In this study, the population is all patients with PDR who have undergone PPV treatment, and their DR stages are comparable. We believe that it is more reasonable to study the association between different hypoglycemic regimens and DME under the unified classification of diabetes and the DR stage. Secondly, compared with DME without PPV surgery, postoperative DME has been relieved within a certain period by removing vitreoretinal traction and clearing various cytokines including VEGF which had a synergistic effect with insulin for higher risks of DR development in patients using insulin (32). Thirdly, insulin therapy is generally advocated when oral medications are insufficient in controlling glycemic. In the early stage of insulin treatment, there may be a series of edema reactions in the body due to drug switching or glycemic control (20, 35). While chronic insulin therapy, compared with oral hypoglycemic agents, does not modify the anatomic or functional effectiveness of DME treatment (23, 36, 37). In this study, the PDR patients treated with insulin had a longer course of diabetes. Long-term insulin therapy might reduce the risk of postoperative DME by lowering glycemia and HbA1c persistently which is termed metabolic memory (29). Metabolic memory was shown to persist through 10 years of follow-up and intensive glycemic control could reduce the risks of clinically significant DME in type 1 DM patients (29). In this study, due to metabolic memory, the advantage of strict glycemic control in the onset of postoperative DME might be reflected. At the same time, we found that although there is no statistical difference, HbA1c was lower in patients with oral medication than in patients with oral medication plus insulin and insulin. Apart from HbA1c, there may be other mechanisms to explain this phenomenon.
Except for insulin, different oral hypoglycemic medications have different effects on DME (27, 28). Most previous studies have shown that TZDs contributed to the increased risk of DME (26–28, 38). While another study (39) demonstrated that TZDs do not cause subclinical DME in a demographically diverse T2DM population, whether the TZDs are combined with other agents or not. Therefore, the relationship between TZDs and DME is still inconsistent. In addition to TZDs, sulphonylureas treatment was also related to a high risk of DME (14), while metformin did not have a relationship with the occurrence of clinically significant DME (30). Taken together, because different hypoglycemic medications may have different effects on DME and their mechanisms on DME are not fully understood, it is very important to clarify the proportion of different hypoglycemic mechanisms in hypoglycemic medications. Poor glycemic control applied by oral hypoglycemic medications might contribute to a higher risk of DME.
PPV itself has been widely used to treat tractional or refractory DME (40–43), which relieves DME through multiple mechanisms, including the elimination of traction factors (44), improving intravitreal oxygenation, removing pathological cytokines (such as VEGF) in the vitreous cavity, and accelerating the half-life of intravitreal cytokines (45). Whereas the low incidence of postoperative DME after PPV, there are few studies on the risk factors for postoperative DME in patients with PDR. A study (46) revealed that central macular thickness of the vitrectomized eyes was significantly correlated with atherogenic index of plasma, total cholesterol, low-density lipoprotein cholesterol, and uric acid. Kojima et al. (47) found that in DME patients, preoperative low HbA1c and postoperative pseudophakia were independently associated with the decrease in foveal thickness of the vitrectomized eyes. The two studies aforementioned confirmed that systemic factors might play an important role in the pathogenesis of postoperative DME. Based on clinical experience and observation, we believed that early postoperative DME may be related to surgical procedures (48, 49) and inflammatory reactions related to surgical procedures (49). In the late stage of postoperative DME may be related to systemic factors such as preoperative HbA1c (47), and the ocular accumulation of cytokines related to systemic factors may be another explanation. Therefore, we speculated that glucose control as one of the systemic factors may attribute to postoperative DME most. In the present secondary analysis, the association between the different regimens for glucose control and postoperative DME is circumstantial evidence for our hypotheses. We will further validate the hypotheses in our prospective PDR cohort (50).
Our study has some strengths. First, we treated different hypoglycemic regimens as a categorical variable and tested the P for trend, which is useful in evaluating the robustness of data analysis. Second, this was a historical cohort study and was thus susceptible to potential confounding. However, we used strict statistical adjustment to minimize the effect of residual confounders. Last, the results of present study provide a new idea of the relationship between DME secondary to vitrectomy with different hypoglycemic drugs, which is worthy of further exploration in future research.
However, the current study has several limitations. First, because of the retrospective cohort design, the proportion of different hypoglycemic mechanisms in oral DM medications and other drugs which might reduce the risks of DME such as aspirin and angiotensin-converting enzyme inhibitor is not clear. A prospective cohort study aiming at the different hypoglycemic regimens on postoperative DME is required. Second, because it is a retrospective cohort study, whether the presence of preoperative DME secondary to media opacification, and the definition and macular thickness of postoperative DME is unclear. The incidence of postoperative DME may be underestimated. It is noteworthy that the potential lack of information on outcomes resulting from such errors would bias toward the null and thus results in an underestimation of the association between different hypoglycemic regimens and postoperative DME. Third, the specific measurement time of postoperative DME is not known, and the pathogenesis of DME at different time points may be different. Besides, the period of oral medication or insulin that was prescribed for glycemic control should be considered. Finally, the generalization of the results may be limited, as the study population is all Japanese and the study had limited power to identify statistically significant differences in different stratifications, because of the small number of subjects included.
In conclusion, our results of the secondary analysis should be interpreted as a significant association between insulin treatment and reduced risks of DME in Japanese PDR patients with PPV surgery, compared with oral medications. Well glycemic control with longstanding insulin therapy may be beneficial to reduce the risks of postoperative DME in PDR patients with T2DM. Our investigation calls for large-scale and long-term prospective clinical studies for a full evaluation of the exact role of insulin in the progression of postoperative DME.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
This study was performed by Japanese researcher Nishi et al. at the Yamagata University Hospital, Yamagata, Japan. In the previously published article [34], Nishi et al. have clearly stated that: the study was performed following the Declaration of Helsinki and reviewed and approved by the Ethics Committee of the Yamagata University Faculty of Medicine (approval number: H26-21). All data were fully anonymized before we accessed them and the IRB waived the requirement for informed consent. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceptualization:CL, YZ, MZ; Formal analysis: CL; Validation: YZ; Supervision: MZ; Writing – original draft: CL, YZ, MZ; Writing – review & editing: CL, YZ, MZ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21025), and the Sichuan Provincial Science and Technology Support Project (no.2019YJ0129), and China Postdoctoral Science Foundation (2021M692273).
Acknowledgments
Thanks for the comprehensive raw data provided by professor Koichi Nishitsuka’s team. They completed the entire study. They are (the rankings and institutions of these researchers were ranked according to the “reference (34)“) Katsuhiro Nishi, Koichi Nishitsuka (corresponding author) (Department of Ophthalmology and Visual Sciences, Yamagata University Faculty of Medicine, Yamagata, Yamagata, Japan), Teiko Yamamoto, Hidetoshi Yamashita.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.764254/full#supplementary-material
References
1. Kocur I, Resnikoff S. Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol (2002) 86:716–22. doi: 10.1136/bjo.86.7.716
2. Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem (2016) 117:2443–53. doi: 10.1002/jcb.25575
3. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med (2012) 366:1227–39. doi: 10.1056/NEJMra1005073
4. Wu L, Fernandez-Loaiza P, Sauma J, Hernandez-Bogantes E, Masis M. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes (2013) 4:290–4. doi: 10.4239/wjd.v4.i6.290
5. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care (2012) 35:556–64. doi: 10.2337/dc11-1909
6. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet (2010) 376:124–36. doi: 10.1016/S0140-6736(09)62124-3
8. Acan D, Calan M, Er D, Arkan T, Kocak N, Bayraktar F, et al. The prevalence and systemic risk factors of diabetic macular edema: a cross-sectional study from Turkey. BMC Ophthalmol (2018) 18:91. doi: 10.1186/s12886-018-0753-y
9. Rush RB, Del Valle Penella A, Reinauer RM, Rush SW, Bastar PG. Internal limiting membrane peeling during vitrectomy for diabetic vitreous hemorrhage: a randomized clinical trial. Retina (2021) 41:1118–26. doi: 10.1097/iae.0000000000002976
10. Liu LY, Dong FT, Li H. Relationship between the classification of diabetic macular edema and its related factors. Zhongguo Yi Xue Ke Xue Yuan Xue Bao (2007) 29:797–802.
11. Lee SJ, Choi MG. Association of manganese superoxide dismutase gene polymorphism (V16A) with diabetic macular edema in Korean type 2 diabetic patients. Metabolism (2006) 55:1681–8. doi: 10.1016/j.metabol.2006.08.011
12. Aroca PR, Salvat M, Fernández J, Méndez I. Risk factors for diffuse and focal macular edema. J Diabetes Complications (2004) 18:211–5. doi: 10.1016/s1056-8727(03)00038-2
13. Park S, Rhee SY, Jeong SJ, Kim K, Chon S, Yu SY, et al. Features of long-standing Korean type 2 diabetes mellitus patients with diabetic retinopathy: A study based on standardized clinical data. Diabetes Metab J (2017) 41:393–404. doi: 10.4093/dmj.2017.41.5.393
14. Martín-Merino E, Fortuny J, Rivero-Ferrer E, Lind M, Garcia-Rodriguez LA. Risk factors for diabetic macular oedema in type 2 diabetes: a case-control study in a united kingdom primary care setting. Prim Care Diabetes (2017) 11:288–96. doi: 10.1016/j.pcd.2017.03.002
15. Ylinen P, Laine I, Lindholm JM, Tuuminen R. Poor glycemic control as a risk factor for pseudophakic cystoid macular edema in patients with diabetes. J Cataract Refract Surg (2017) 43:1376–82. doi: 10.1016/j.jcrs.2017.07.035
16. Ancochea G, Martín Sánchez MD. Results of a diabetic retinopathy screening. risk markers analysis. Arch Soc Esp Oftalmol (2016) 91:15–9. doi: 10.1016/j.oftal.2015.09.011
17. Zhang J, Ma J, Zhou N, Zhang B, An J. Insulin use and risk of diabetic macular edema in diabetes mellitus: a systemic review and meta-analysis of observational studies. Med Sci Monit (2015) 21:929–36. doi: 10.12659/msm.892056
18. Pradhana D, Priya MNS, Surya J, Bhende M, Laxmi G, Sharma T, et al. Optical coherence tomography-based prevalence of diabetic macular edema and its associated risk factors in urban south India: A population-based study. Ophthalmic Epidemiol (2022) 29:149–55. doi: 10.1080/09286586.2021.1907846
19. Wang Y, Lin Z, Zhai G, Ding X, Wen L, Li D, et al. Prevalence of and risk factors for diabetic retinopathy and diabetic macular edema in patients with early and late onset diabetes mellitus. Ophthalmic Res (2022) 65:293-9. doi: 10.1159/000508335
20. Desjardins D, Liu Y, Crosson CE, Ablonczy Z. Histone deacetylase inhibition restores retinal pigment epithelium function in hyperglycemia. PloS One (2016) 11:e0162596. doi: 10.1371/journal.pone.0162596
21. Jingi AM, Noubiap JJ, Essouma M, Bigna JJ, Nansseu JR, Ellong A, et al. Association of insulin treatment versus oral hypoglycaemic agents with diabetic retinopathy and its severity in type 2 diabetes patients in Cameroon, sub-Saharan Africa. Ann Transl Med (2016) 4:395. doi: 10.21037/atm.2016.08.42
22. Sugimoto M, Cutler A, Shen B, Moss SE, Iyengar SK, Klein R, et al. Inhibition of EGF signaling protects the diabetic retina from insulin-induced vascular leakage. Am J Pathol (2013) 183:987–95. doi: 10.1016/j.ajpath.2013.05.017
23. Yamamoto M, Miura Y, Hirayama K, Kohno T, Kabata D, Theisen-Kunde D, et al. Predictive factors of outcome of selective retina therapy for diabetic macular edema. Int Ophthalmol (2020) 40:1221–32. doi: 10.1007/s10792-020-01288-6
24. Elwali ES, Almobarak AO, Hassan MA, Mahmooud AA, Awadalla H, Ahmed MH. Frequency of diabetic retinopathy and associated risk factors in Khartoum, Sudan: population based study. Int J Ophthalmol (2017) 10:948–54. doi: 10.18240/ijo.2017.06.18
25. Leschey KH, Hackett SF, Singer JH, Campochiaro PA. Growth factor responsiveness of human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci (1990) 31:839–46.
26. Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med (2012) 172:1005–11. doi: 10.1001/archinternmed.2012.1938
27. Fong DS, Contreras R. Glitazone use associated with diabetic macular edema. Am J Ophthalmol (2009) 147:583–6.e1. doi: 10.1016/j.ajo.2008.10.016
28. Asensio-Sánchez VM, Asensio-Sánchez MJ, Gómez-Ramírez V. [Macular oedema due to rosiglitazone treatment in diabetes mellitus]. Arch Soc Esp Oftalmol (2010) 85:246–8. doi: 10.1016/j.oftal.2010.09.001
29. Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes (2015) 64:631–42. doi: 10.2337/db14-0930
30. Li Y, Ryu C, Munie M, Noorulla S, Rana S, Edwards P, et al. Association of metformin treatment with reduced severity of diabetic retinopathy in type 2 diabetic patients. J Diabetes Res (2018) 2018:2801450. doi: 10.1155/2018/2801450
31. Yanyali A, Horozoglu F, Celik E, Ercalik Y, Nohutcu AF. Pars plana vitrectomy and removal of the internal limiting membrane in diabetic macular edema unresponsive to grid laser photocoagulation. Eur J Ophthalmol (2006) 16:573–81. doi: 10.1177/112067210601600412
32. Stolba U, Binder S, Gruber D, Krebs I, Aggermann T, Neumaier B. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol (2005) 140:295–301. doi: 10.1016/j.ajo.2005.03.045
33. Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, et al. The prevalence of diabetic retinopathy among adults in the united states. Arch Ophthalmol (2004) 122:552–63. doi: 10.1001/archopht.122.4.552
34. Nishi K, Nishitsuka K, Yamamoto T, Yamashita H. Factors correlated with visual outcomes at two and four years after vitreous surgery for proliferative diabetic retinopathy. PloS One (2021) 16:e0244281. doi: 10.1371/journal.pone.0244281
35. Hernández C, Zapata MA, Losada E, Villarroel M, García-Ramírez M, García-Arumí J, et al. Effect of intensive insulin therapy on macular biometrics, plasma VEGF and its soluble receptor in newly diagnosed diabetic patients. Diabetes Metab Res Rev (2010) 26:386–92. doi: 10.1002/dmrr.1093
36. Chen Z, Zhang J, Lu C, Lin S, Chen J, Zhong H, et al. Visual function and morphological changes in the macular area of patients with type 2 diabetes mellitus after intensive insulin therapy. Chin Med J (Engl) (2014) 127:658–61. doi: 10.3760/cma.j.issn.0366-6999.20132482
37. Matsuda S, Tam T, Singh RP, Kaiser PK, Petkovsek D, Zanella MT, et al. Impact of insulin treatment in diabetic macular edema therapy in type 2 diabetes. Can J Diabetes (2015) 39:73–7. doi: 10.1016/j.jcjd.2014.06.005
38. Karakurt F, Kargili A, Kasapoglu B. Pioglitazone induced reversible pancytopenia. Exp Clin Endocrinol Diabetes (2010) 118:96–7. doi: 10.1055/s-0029-1234065
39. Tarbett AK, VanRoekel RC, Howard RS, Vigersky RA. The use of optical coherence tomography to determine the effect of thiazolidinediones on retinal thickness in patients with type 2 diabetes. J Diabetes Sci Technol (2011) 5:945–51. doi: 10.1177/193229681100500418
40. Vikas SJ, Agarwal D, Seth S, Kumar A, Kumar A. Comparison of anatomical and functional outcomes of vitrectomy with internal limiting membrane peeling in recalcitrant diabetic macular edema with and without traction in Indian patients. Indian J Ophthalmol (2021) 69:3297–301. doi: 10.4103/ijo.IJO_1271_21
41. Rush RB, Rush SW. Pars plana vitrectomy with internal limiting membrane peeling for treatment-naive diabetic macular edema: A prospective, uncontrolled pilot study. Clin Ophthalmol (2021) 15:2619–24. doi: 10.2147/OPTH.S320214
42. Crim N, Velez-Montoya R, Morales-Canton V. Surgical versus medical treatment for diabetic macular edema: A review. Med Hypothesis Discov Innov Ophthalmol (2017) 6:136–42.
43. Kim J, Kang SW, Shin DH, Kim SJ, Cho GE. Macular ischemia and outcome of vitrectomy for diabetic macular edema. Jpn J Ophthalmol (2015) 59:295–304. doi: 10.1007/s10384-015-0402-4
44. Hagenau F, Vogt D, Ziada J, Guenther SR, Haritoglou C, Wolf A, et al. Vitrectomy for diabetic macular edema: Optical coherence tomography criteria and pathology of the vitreomacular interface. Am J Ophthalmol (2019) 200:34–46. doi: 10.1016/j.ajo.2018.12.004
45. Flikier S, Wu A, Wu L. Revisiting pars plana vitrectomy in the primary treatment of diabetic macular edema in the era of pharmacological treatment. Taiwan J Ophthalmol (2019) 9:224–32. doi: 10.4103/tjo.tjo_61_19
46. Gungel H, Aral H, Erdenen F, Gokce M, Erdur SK. Central macular thickness in diabetic macular edema. Acta Endocrinol (Buchar) (2020) 16:417–25. doi: 10.4183/aeb.2020.417
47. Kojima T, Terasaki H, Nomura H, Suzuki T, Mori M, Ito Y, et al. Vitrectomy for diabetic macular edema: effect of glycemic control (HbA(1c)), renal function (creatinine) and other local factors. Ophthalmic Res (2003) 35:192–8. doi: 10.1159/000071170
48. Iglicki M, Lavaque A, Ozimek M, Negri HP, Okada M, Chhablani J, et al. Biomarkers and predictors for functional and anatomic outcomes for small gauge pars plana vitrectomy and peeling of the internal limiting membrane in naïve diabetic macular edema: The VITAL study. PloS One (2018) 13:e0200365. doi: 10.1371/journal.pone.0200365
49. Takamura Y, Shimura M, Katome T, Someya H, Sugimoto M, Hirano T, et al. Effect of intravitreal triamcinolone acetonide injection at the end of vitrectomy for vitreous haemorrhage related to proliferative diabetic retinopathy. Br J Ophthalmol (2018) 102:1351–7. doi: 10.1136/bjophthalmol-2017-311377
Keywords: diabetic macular edema, proliferative diabetic retinopathy, pars plana vitrectomy, hypoglycemic regimens, insulin
Citation: Lei C, Zhang Y and Zhang M (2022) The association between different hypoglycemic regimens and postoperative diabetic macular edema after vitrectomy in the Japanese patients with proliferative diabetic retinopathy. Front. Endocrinol. 13:764254. doi: 10.3389/fendo.2022.764254
Received: 25 August 2021; Accepted: 01 July 2022;
Published: 22 July 2022.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
David Wagner, University of Colorado Anschutz Medical Campus, United StatesKun Liu, Shanghai First People’s Hospital, China
Haiyan Wang, Shaanxi Eye Hospital, China
Copyright © 2022 Lei, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meixia Zhang, emhhbmdtZWl4aWFAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Chunyan Lei1,2†
Chunyan Lei1,2† Meixia Zhang
Meixia Zhang