
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 17 January 2023
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1109807
This article is part of the Research Topic Role of non-Coding RNAs in the Reproductive System View all 5 articles
Background: Many circular RNAs (circRNAs) are specifically expressed in the testes and seminal plasma of patients with non-obstructive azoospermia (NOA), highlighting them as potential predictors of microdissection testicular sperm extraction (micro-TESE) outcomes. Although research has indicated that circular RNA monoglyceride lipase (circ_MGLL) is highly expressed in the testicular tissues of patients with NOA, the association between circ_MGLL expression and sperm retrieval outcomes (SROs) in patients with idiopathic non-obstructive azoospermia (iNOA) receiving micro-TESE remains unclear.
Methods: This single-center, retrospective cohort study enrolled 114 patients with iNOA who underwent micro-TESE at Northwest Women’s and Children’s Hospital from January 2017 to November 2021. A logistic regression model was used to examine associations between SRO and circ_MGLL expression in testicular tissues, the results of which were used in conjunction with previous findings to establish a nomogram. The predictive performance of the circ_MGLL-based nomogram was evaluated via calibration curves, receiver operating characteristic curves, and decision curve analysis (DCA) using an internal validation method.
Results: The generalized additive model indicated that the probability of successful SRO for micro-TESE decreased as circ_MGLL expression increased in testicular tissues. Across the entire cohort, univariate logistic regression analysis revealed that circ_MGLL expression was inversely associated with SRO in patients with NOA. This trend did not change after stratification according to age, body mass index, testicular volume, follicle-stimulating hormone (FSH) level, luteinizing hormone (LH) level, testosterone (T) level, or pathological type (or after adjusting for these confounders) (odds ratio <1, P < 0.001). A nomogram was then generated by integrating circ_MGLL, pathological types, and FSH, LH, and T levels. The circ_MGLL-based predictive model achieved satisfactory discrimination, with an area under the curve of 0.857, and the calibration curves demonstrated impressive agreement. The DCA indicated that the net clinical benefit of the circ_MGLL-based predictive model was greater than that of circ_MGLL alone.
Conclusion: circ_MGLL is significantly associated with the SRO of micro-TESE in patients with iNOA. The circ_MGLL-based nomogram developed in the current study can predict successful SRO with high accuracy.
Azoospermia is characterized by the complete absence of spermatozoa in the ejaculate semen, occurring in approximately 1% of all men and 10%–15% of infertile men (1). However, in some cases, intracytoplasmic sperm injection treatment can be performed after obtaining sperm through microdissection testicular sperm extraction (micro-TESE) (2). Among the types of non-obstructive azoospermia (NOA), idiopathic NOA (iNOA) presents a particular clinical challenge given the lack of a clear etiology, absence of effective treatments, and poorest sperm retrieval outcomes (SROs) following micro-TESE (3, 4). Regrettably, micro-TESE is only successful in approximately 50% of patients with NOA (5, 6). Although research has identified various factors related to SRO, such as testicular volume (TV) and hormonal levels [e.g., follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T)] (5), reliable molecular biomarkers for predicting SROs following micro-TESE in patients with iNOA remain to be identified.
Testicular biopsy is an important invasive procedure for evaluating testicular spermatogenesis (7). However, whether testicular biopsy is necessary before micro-TESE remains controversial. Because spermatogenic foci are few and scattered in patients with iNOA, biopsied testicular tissues may not reflect spermatogenesis in other parts of the testis. Thus, some studies have pointed out that testicular biopsy only contributes to the pathological classification of the biopsied tissues and has limited value in the prediction of SROs following micro-TESE (8). Meanwhile, open testicular biopsy increases the risk of testicular injury and bleeding (9). However, other studies have confirmed that testicular biopsy is of major predictive significance in the context of micro-TESE outcomes. Mao et al. reported extremely poor SROs (11% success) in patients with iNOA whose biopsy results indicated an absence of sperm in both the intraoperative microscopic examination and the post-pathological examination—a rate far below that observed for patients with iNOA exhibiting identifiable sperm in even just one of the two examinations (10). Cao et al. also found significantly different profiles of PIWI-interacting RNA (piRNA) between successful and failed micro-TESE procedures among patients with NOA, providing insight into strategies for identifying biomarkers that can predict residual spermatogenic loci in this population (11). Our collaborators also reported obvious upregulation of beclin-1 in the Sertoli cell-only syndrome (SCOS) group, which was further identified as an important predictor of SRO failure in micro-TESE (12). Taken together, these findings indicate that different histopathological outcomes and differentially expressed genes in testicular tissues obtained during micro-TESE or testicular biopsy may, in part, reflect the extent of spermatogenesis within the whole testis, suggestive of the potential value for predicting SROs following micro-TESE in patients with NOA.
Circular RNAs (circRNAs) are a new class of endogenous non-coding RNAs that covalently bind to a closed circular structure without a 5′ end cap and a 3′ end poly(A) tail (13). Several studies have confirmed that circRNAs play a pivotal role in regulating gene expression and cell fate (14). Many circular RNAs (circRNAs) are specifically expressed in the testis and seminal plasma of patients with NOA (15) and can be used as predictors of the outcome of micro-TESE (16). However, research regarding the expression and function of circRNAs in spermatogenesis is still in its infancy. Our collaborators have shown that approximately 1,000 circRNAs, including circ_0008045 (circular RNA monoglyceride lipase, circ_MGLL), are differentially dysregulated in testicular tissues between NOA and obstructive azoospermia (17). Previous studies have indicated that the expression of monoglyceride lipase (MGLL), an important downstream factor involved in triglyceride catabolism, is very low in germ cells but is specifically abundant in Sertoli cells, indicating that MGLL is a potential functional regulator of Sertoli cells (18). More importantly, silencing the expression of circ_MGLL results in an increase in the proliferation of Sertoli cells (19). However, many studies have reported that changes in the population or function of Sertoli cells are among the main factors leading to defective spermatogenesis (20). Nevertheless, the clinical relevance of circ_MGLL in patients with iNOA remains to be determined.
In the current study, we discovered that circ_MGLL, a circRNA generated from the circularization of the MGLL gene, was significantly upregulated in the testicular tissues of patients with iNOA in the SRO-failure group. Detailed univariate and multivariate logistic analyses were conducted to further clarify the relationship between circ_MGLL and SRO. Furthermore, stratified analyses were performed to determine whether the correlation between circ_MGLL and SRO remained within different subgroups, thus distinguishing our study from previous research. Finally, receiver operating characteristic (ROC) curve and decision curve analysis (DCA) were performed to analyze the ability of circ_MGLL to predict SROs.
This single-center, retrospective cohort study enrolled 114 patients with iNOA who underwent micro-TESE at Northwest Women’s and Children’s Hospital from January 2017 to November 2021. The inclusion criteria for all cases have been described in our previously published article (12). The study was approved by the Ethics Review Board of Northwest Women’s and Children’s Hospital (ethical review number: 2021-XBFE-023). Written informed consent for the use of their testis biopsies and anonymized data for research purposes was obtained from the participants before micro-TESE. All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation or the Helsinki Declaration of 1975.
The inclusion criteria were as follows (1): a history of infertility of not less than 1 year, (2) no sperm detected in three semen analyses, and (3) completion of micro-TESE to obtain sperm. The exclusion criteria were (1) a diagnosis of congenital absence of the vas deferens, (2) abnormal seminal fructose or neutral α-glucosidase, (3) absence of seminal glands, (4) other obstructive factors accounting for azoospermia, (5) lack of Johnsen scores after micro-TESE surgery, (6) abnormal chromosome karyotype, (7) microdeletions in the azoospermia factor (AZF) c region of the Y chromosome, (8) a history of cryptorchidism, and (9) a history of cryptorchidism.
The patients underwent the micro-TESE procedure as described in our previous study (12). Briefly, the TV was measured in a standard surgical room. Then, a longitudinal incision on the tunica albuginea of the testes was made to reveal the seminiferous tubules. Tubules with a full appearance and opacity, suggestive of possible sperm production, were gently dissected and placed in a Petri dish. An experienced embryologist dissected the seminiferous tubules and assessed the presence of sperms using a specialized microscope. A positive result was confirmed when at least one sperm sample was obtained. Thereafter, a large fragment of testicular tissue measuring approximately 8 × 4 × 3 mm3 was cut for histopathological examination, regardless of whether the sperm had been harvested successfully.
Testicular tissue samples were fixed in Bouin’s solution for 24 h and embedded in paraffin. The slides were then stained with hematoxylin and eosin solution. Subsequently, all slides were observed under a microscope by two pathologists. The level of spermatogenesis in each testicular biopsy sample was measured based on the Johnsen score system as previously described (21). Patients with iNOA who had Johnsen scores of 1 to 2 were identified as having Sertoli cell-only syndrome (SCOS), those with Johnsen scores of 3–7 were identified as having maturation arrest (MA), and those with Johnsen scores of 8 to 9 were identified as having hypo-spermatogenesis (HS). Typical photomicrographs of the different Johnsen scores are presented in Supplementary Figure S1.
Total RNA was extracted from testicular tissues using a paraffin-embedded tissue section total RNA extraction kit (Tiangen, Beijing, China) in accordance with the manufacturer’s instructions. For circ_MGLL quantification, a one-step prime-script-circRNA cDNA synthesis kit was used to convert the total RNA to cDNA. Then, the SYBR premix Ex Taq kit (Takara, Japan) and specific circRNA LNATM PCR primers (Exiqon, Denmark) were used to perform real-time polymerase chain reaction (RT-PCR). Data were analyzed using 7500 software v.2.0.1 (Applied Biosystems, USA), with the automatic Ct setting for adapting the baseline and threshold for Ct determination. Each sample was examined in triplicate, and the amount of PCR product produced was normalized to GAPDH. The primers used for circ_MGLL were 5′-GCCTACCTGCTCATGGAGTT-3′ (forward) and 5′-AGACGGCATTCAGCAGTTG-3′ (reverse). The primers used for GAPDH were 5′-AATCCCATCACCATCTTCCA-3′ (forward) and 5′-TGGACTCCACGACGTACTCA-3′ (reverse).
Statistical analysis was performed using IBM SPSS version 23.0 (International Business Machines Corporation, USA), R software (http://www.R-project.org, The R Foundation), and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). Continuous variables are presented as the mean ± standard deviation (SD) and were compared using one-way analysis of variance (ANOVA) and the Kruskal–Wallis test in cases of normally and non-normally distributed data, respectively. Categorical data are presented as percentages and were compared using chi-square tests. A generalized additive model was used for smooth curve fitting to evaluate the relationship between circ_MGLL expression and successful SRO probability. The odds ratios (OR) and 95% confidence intervals (CIs) of SRO according to adjusted or unadjusted for potential confounders were evaluated using univariate and multivariate logistic regression models. Stratified analyses were conducted according to age (years), body mass index (BMI, kg/m2), average TV (ml), FSH (IU/L), LH (IU/L), T (ng/dl), and pathological type. A nomogram integrating circ_MGLL, pathological type, and serum hormone levels was established to predict the probability of successful SRO. Subsequently, the original data were divided into a training set (n = 58, successful SRO: 29, failed SRO: 29) and a validation set (n = 56, successful SRO: 18, failed SRO: 38), and the predictive performance of the circ_MGLL-based nomogram was evaluated in the training set and internally validated via bootstrapping with 500 resamples. The accuracy of the circ_MGLL-based nomogram was assessed using calibration curves, ROC, and DCA. All P-values were generated using two-sided tests, and statistical significance was set at P <0.05.
In accordance with the inclusion and exclusion criteria depicted in Figure 1, 114 patients with iNOA in our hospital were included in the final analysis. Table 1 shows the clinical characteristics of these 114 patients with iNOA who were classified into success (47, 41.228%) and failure (67, 58.772%) groups based on the SRO of micro-TESE. There were no significant differences in age, TV, FSH levels, LH levels, or T levels between the two groups (Table 1). Although successful SROs were more common in patients with pathological type MA or HS than in those with type SCOS, the differences among the three groups were not significant (P = 0.067, Table 1).
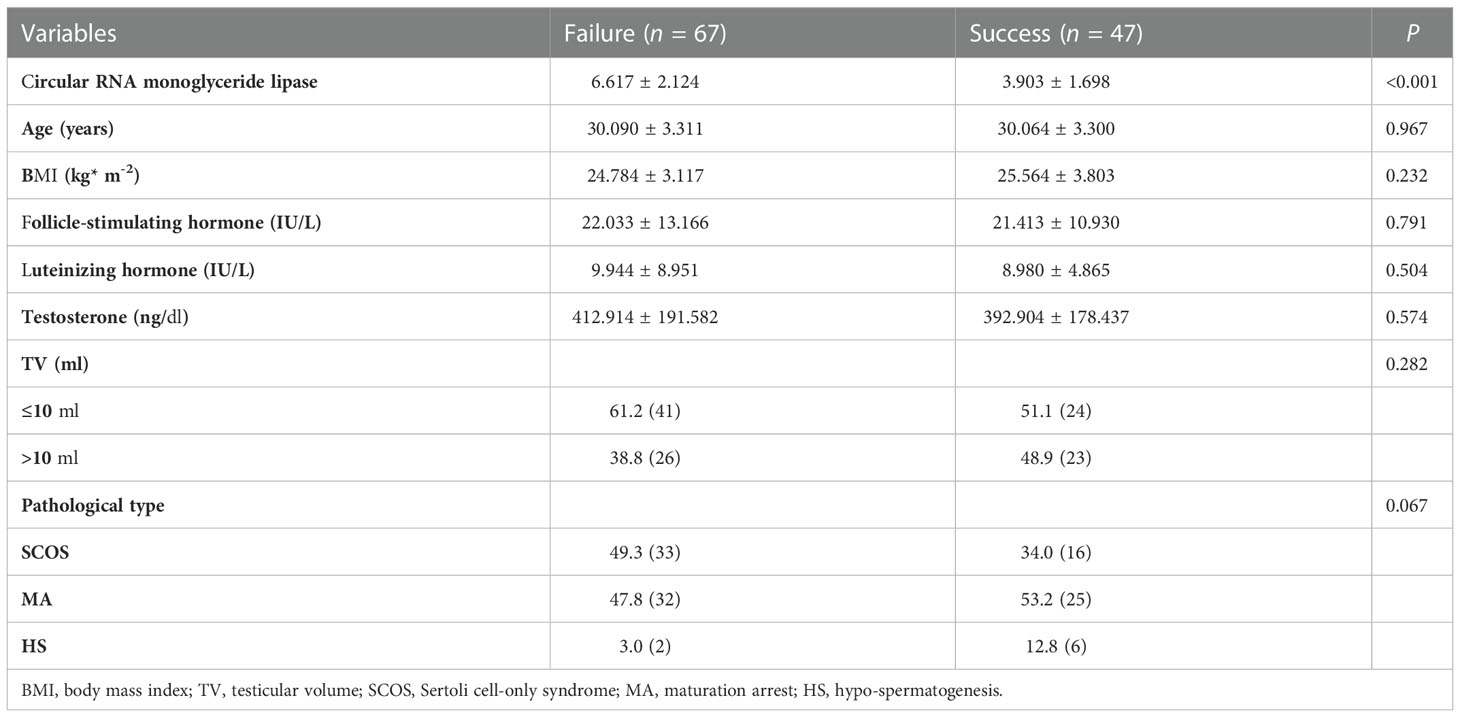
Table 1 Clinical characteristics of idiopathic non-obstructive azoospermia patients receiving micro-testicular sperm extraction.
We investigated circ_MGLL levels in paraffin sections of testicular tissues from 114 patients with iNOA. When compared with the level observed in the failure group, the relative expression of circ_MGLL was downregulated in testicular tissues obtained from the successful SRO group (P < 0.001, Table 1, Figure 2A). In addition, a generalized additive model analysis revealed a negative correlation between circ_MGLL expression and the probability of successful SRO (P < 0.001, Figure 2B). More importantly, although we observed that circ_MGLL expression was higher in patients with SCOS than in those with HS or MA, this difference was not statistically significant (P = 0.069, Table 2).
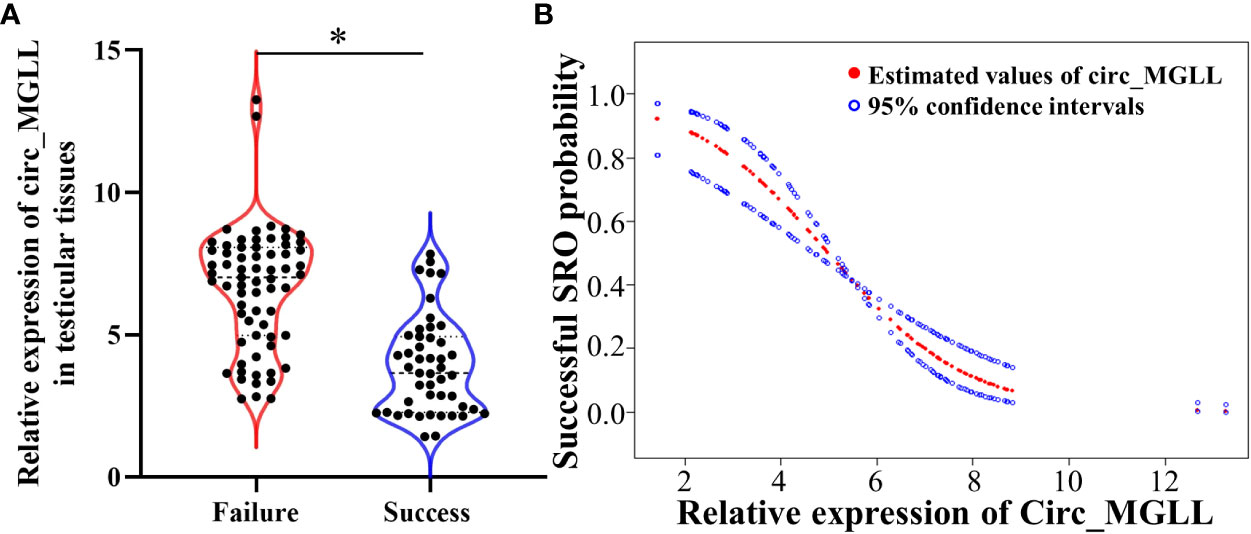
Figure 2 Relationships between circ_MGLL expression and successful SRO in patients with iNOA undergoing micro-TESE. (A) Relative expression of circ_MGLL in the testicular tissues of patients with successful and failed SRO. (B) Dose–response relationship between the relative expression of circ_MGLL and the probability of successful SRO. The adjustment factors included all baseline covariates: age, BMI, TV, FSH, LH, T, and pathological type. The filled and hollow circles represent the estimated values and their corresponding 95% confidence intervals. circ_MGLL, circular RNA monoglyceride lipase; SRO, sperm retrieval outcome; iNOA, idiopathic non-obstructive azoospermia; micro-TESE, microdissection testicular sperm extraction; BMI, body mass index; TV, testicular volume; FSH, follicle-stimulating hormone; LH, luteinizing hormone; T, testosterone. *P<0.05.
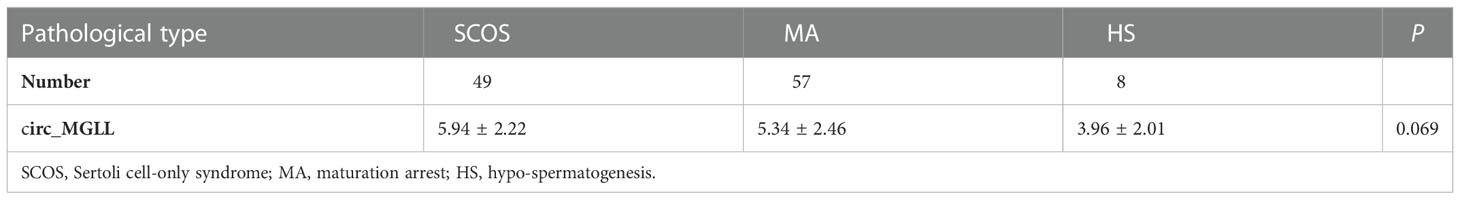
Table 2 The relationships between circ_MGLL expression and pathological classification of iNOA patients receiving micro-testicular sperm extraction.
We conducted univariate and multivariable logistic regression analyses to evaluate the association between clinicopathological features and SRO in patients with NOA. We included circ_MGLL, age, BMI, TV, serum FSH, LH, T, and pathological type in the univariate analysis. As shown in Table 3, patients with high circ_MGLL were more likely to experience failed SRO, and patients with the pathological classification of HS were more likely to experience successful SRO in the crude model (all P < 0.01, Table 4, Figure 3A). Other parameters such as age, BMI, TV, FSH, LH, and T were not significantly associated with SRO (P > 0.05, Table 3, Figure 3A). Multivariate analysis was then conducted using circ_MGLL and pathological types based on the significant associations observed in the univariate analysis. After adjusting for baseline covariates, high circ_MGLL expression exerted a significantly negative impact on the probability of successful SRO in patients with iNOA (model 1 adjusted for age, TV, and BMI; odds ratio, OR: 0.470, 95% confidence interval, CI: 0.357–0.620, P < 0.001; model 2 adjusted for age, TV, BMI, FSH, LH, T, and pathological type: OR: 0.398, 95% CI: 0.282–0.563, P < 0.001, Table 4, Figure 3B). More importantly, after stratification according to age, BMI, TV, FSH, LH, T, and pathological types, inverse correlations were still observed between circ_MGLL and successful SRO of micro-TESE (all OR <1, Table 5).
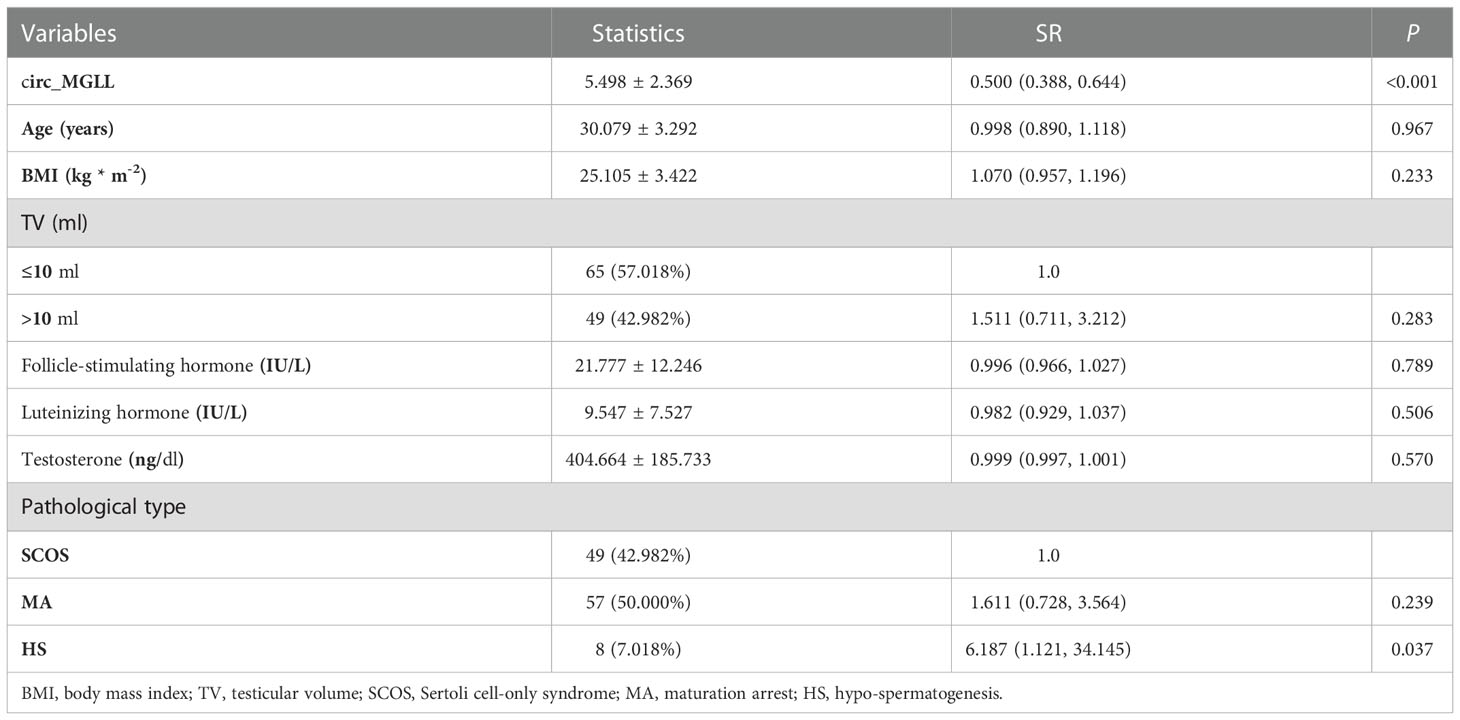
Table 3 Univariate logistic analyses of the clinical characteristics for successful sperm retrieval in idiopathic non-obstructive azoospermia patients.
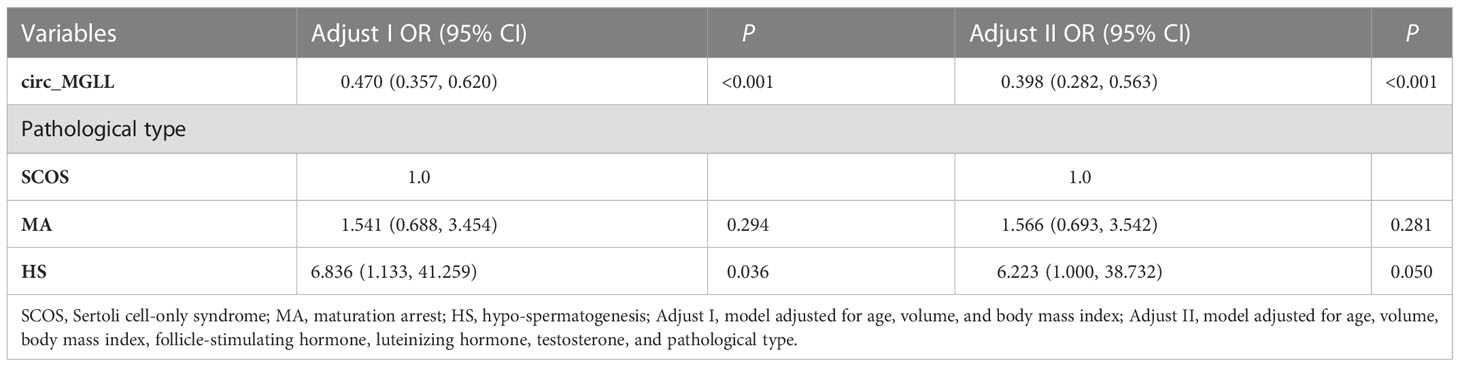
Table 4 Multivariate logistic analyses of the clinical characteristics for successful sperm retrieval in idiopathic non-obstructive azoospermia patients.

Figure 3 Logistic regression analyses of the clinical characteristics for successful sperm retrieval in patients with iNOA. (A) Crude odds ratios in the univariate analyses. (B) Adjusted odds ratios in the multivariate analyses. Adjust I, model adjusted for age, BMI, and TV; Adjust II, model adjusted for age, BMI, TV, FSH, LH, and T. circ_MGLL, circular RNA monoglyceride lipase; iNOA, idiopathic non-obstructive azoospermia; BMI, body mass index; TV, testicular volume; FSH, follicle-stimulating hormone; LH, luteinizing hormone; T, testosterone; SCOS, Sertoli cell-only syndrome; MA, maturation arrest; HS, hypo-spermatogenesis.
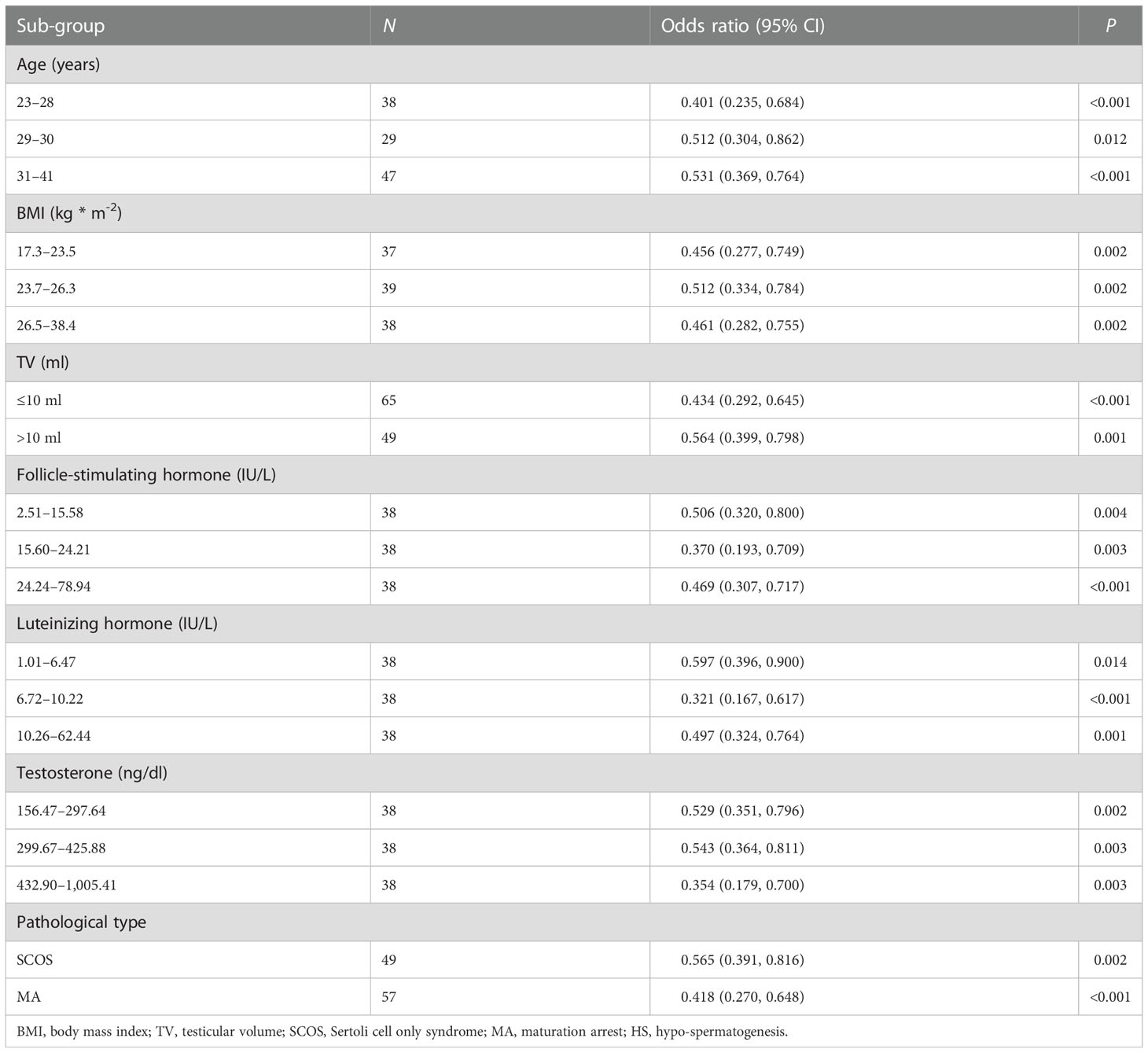
Table 5 Stratified analysis of circ_MGLL in testicular tissues for successful sperm retrieval in idiopathic non-obstructive azoospermia patients with different clinical characteristics sub-groups.
All independent predictors in the multivariate logistic analysis and serum hormone levels that were identified to be associated with spermatogenesis in previous literature were integrated to generate a nomogram for predicting the probability of a successful SRO. Each variable was assigned a value between 0 and 100 according to its contribution to the established model. By summing these values, a total value can be obtained and applied to predict the corresponding probabilities of successful SRO (Figure 4A). Using the bootstrap method for internal validation, calibration curves indicated a good agreement between the predicted probability of the circ_MGLL-based nomogram and the observed probability in both the training (Figure 4B) and validation sets (Figure 4C). Furthermore, in the ROC analysis, the area under the curve (AUC) for circ_MGLL expression to independently predict the SRO of micro-TESE was 0.834, with a cutoff value of 5.354, a sensitivity of 85.1%, and a specificity of 73.1% (Figure 4D, Table 6). The circ_MGLL-based nomogram demonstrated AUCt values of 0.868 and 0.811 in the training and validation sets (Figure 4E, Table 5), respectively. These findings indicated that the nomogram exhibited good discrimination and excellent calibration abilities.
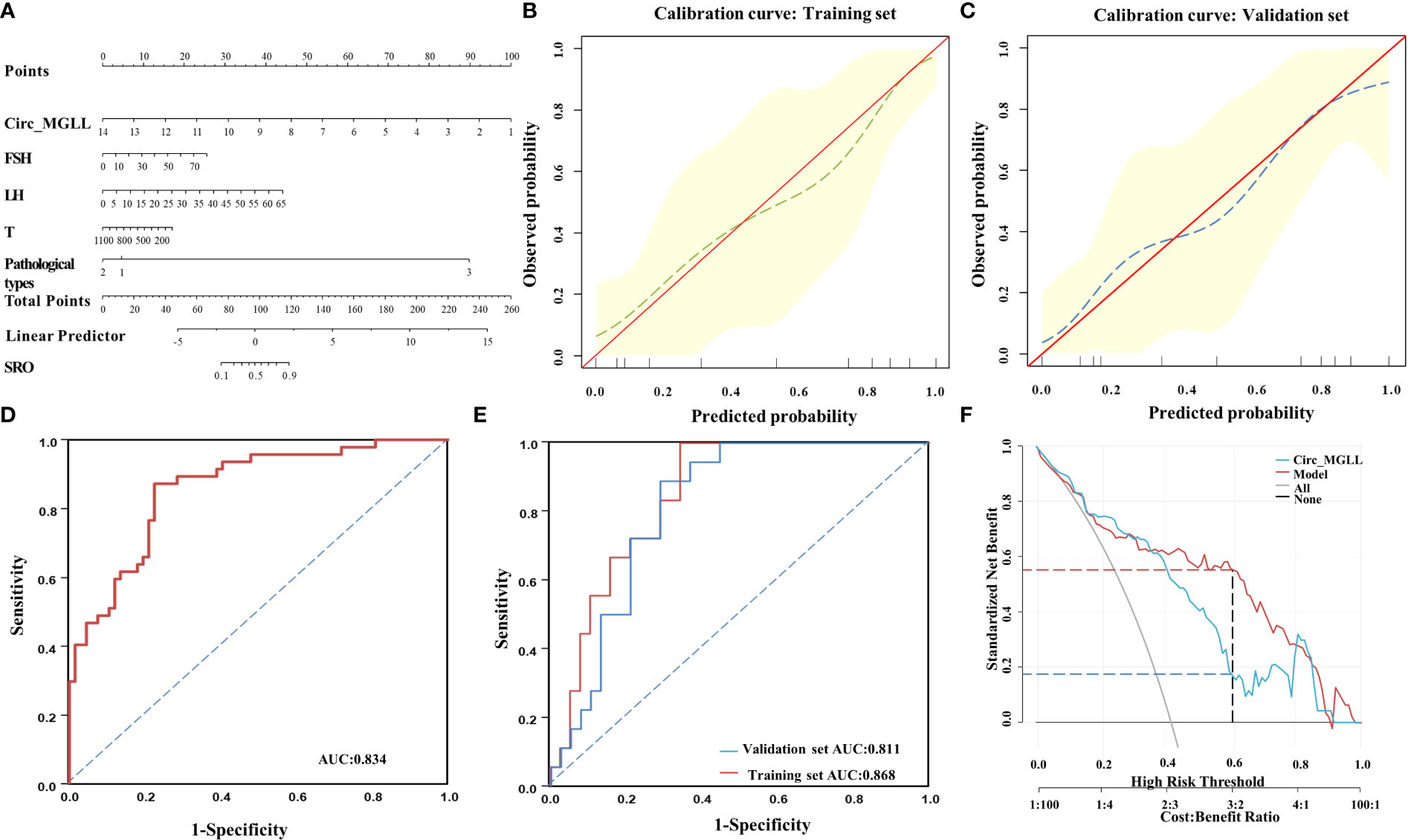
Figure 4 Performance of the circ_MGLL-based nomogram in predicting successful SRO in patients with iNOA. (A) Nomogram for successful SRO prediction for the model combining circ_MGLL with clinicopathological variables. (B) Calibration curve for the circ_MGLL-based nomogram model in the training set. (C) Calibration curve for the circ_MGLL-based nomogram model in the validation set. (D) ROC curve for predicting successful SRO based on circ_MGLL in 114 patients with iNOA. (E) ROC curve for predicting successful SRO using the circ_MGLL-based nomogram in the training and validation sets. (F) Decision curve analysis of the clinical utility of the circ_MGLL-based nomogram for predicting successful SRO for microdissection testicular sperm extraction in patients with iNOA. circ_MGLL, circular RNA monoglyceride lipase; iNOA, idiopathic non-obstructive azoospermia; SRO, sperm retrieval outcome; ROC, receiver operating characteristic; FSH, follicle-stimulating hormone; LH, luteinizing hormone; T, testosterone.
Finally, we performed DCA to determine the clinical potential of the circ_MGLL-based nomogram in assisting with micro-TESE decisions. The gray line (leftmost) represents the micro-TESE strategy for all patients, and the horizontal black line indicates the “micro-TESE for none” strategy. Curves representing the nomograms based on circ_MGLL alone and the integrated factors are shown. As expected, both nomograms were superior to the “micro-TESE for all” strategy (Figure 4F). Furthermore, the net benefit of the circ_MGLL-based nomogram was more powerful for patients with threshold probabilities of 40–80% than circ_MGLL alone (Figure 4F). These data indicated that the clinical utility of the circ_MGLL-based nomogram was superior to that of a circ_MGLL alone on most occasions.
In the current study, we observed significant differences in circ_MGLL expression between successful and failed SRO among patients with iNOA, whereas other variables (including age, BMI, TV, FSH, LH, and T) did not differ between the successful and failed SRO groups. When compared with the level observed in the failure group, the relative expression of circ_MGLL was significantly downregulated in the successful SRO group. Both univariate and multivariate analyses indicated that circ_MGLL expression was negatively associated with SRO in patients with iNOA, even after stratification according to age, TV, BMI, FSH, T, and pathological type (all OR <1). Notably, our results demonstrated that the circ_MGLL-based nomogram model was a relatively accurate predictor with the clinical potential to assist andrologists in screening patients with iNOA suitable for micro-TESE surgery.
Some molecules in the seminal plasma have been identified as predictors of residual spermatogenesis in patients with NOA (16, 22). However, seminal plasma is a mixture secreted by accessory glands such as the prostate, seminal vesicle gland, and ureteral bulbar gland (23). Thus, the specific expression of molecules in the seminal plasma or seminal exosomes may only partly reflect the spermatogenic function of the testis. Furthermore, although some studies have indicated that spermatogenesis varies in different areas of the testis (24), a series of studies have verified that patterns of expression for testis-specific genes or non-coding RNAs are significantly associated with the success of sperm recovery in patients with NOA, emphasizing their potential value as predictors of successful SRO in these patients (25, 26). In our previous collaboration, we observed that circ_MGLL expression is upregulated in patients with NOA when compared with the level observed in patients with obstructive azoospermia (17). In the current study, we further demonstrated that the expression of circ_MGLL is negatively correlated with the SRO in patients with iNOA, and the circ_MGLL-based nomogram had better diagnostic efficiency in predicting the SRO of micro-TESE for these patients. Further increasing the predictive power of this circ_MGLL-based nomogram by integrating histopathological results and the expression of testis-specific molecular biomarkers may help to avoid unnecessary surgery and excessive financial burden in patients with iNOA.
Previously, we reported that the proliferating speed of Sertoli cells was increased, the proportion of proliferating cells and cells at S stage was upregulated, and the apoptosis levels of Sertoli cells were decreased after silencing circ_MGLL expression (19). Furthermore, bioinformatics analysis and luciferase reporter assays showed that circ_MGLL can bind to miR-1228, miR-1233, miR-149, and miR-924 (19). Previous studies had already demonstrated that miR-1288 expression is abundant in the plasma and testis of cynomolgus monkeys, exhibiting associations with apoptosis and cell differentiation (27). Additional studies have indicated that miR-1233 inhibits apoptosis and promotes the proliferation of A549 cells by targeting dual-specificity phosphatase 9 (28). More importantly, miR-149 expression in sperm is correlated with the quality of early embryonic development (29). Previous literatures proved that Sertoli cells provide physical support and stable microenvironments or niche for developing sperm and perform an important role in sustaining spermatogenesis (30, 31). Considering the important functions of circ_MGLL and its targeted miRNAs in cell apoptosis, we speculate that a regulatory network with circ_MGLL as the core would induce apoptosis and the dysfunctions of Sertoli cells in patients with NOA, which may further result in the residual sperm losing nutritional supports and relatively normal microenvironments. Consequently, this dysfunction of Sertoli cells induced by circ_MGLL upregulation will eventually lead to the failure of micro-TESE. However, the mechanism by which circ_MGLL regulates cell proliferation and the functions of Sertoli cells and spermatogenesis should be further explored in future studies.
Notably, our results also indicated that the serum levels of FSH, LH, and T were not correlated with the outcome of sperm collection and could not be used as reliable predictors of SRO in patients with iNOA undergoing micro-TESE. However, findings regarding the correlation between hormonal levels and SRO have been inconsistent. Consistent with our results, some preliminary studies of patients with NOA have reported no significant differences in TV or hormonal levels based on the success or failure of micro-TESE (32, 33). Accordingly, serum hormone levels were not identified as a significant predictor of successful SRO (34). However, some studies have reported significantly higher levels of serum FSH in the failed group than in the successful group. This may be because the abovementioned studies failed to exclude patients with Klinefelter syndrome (KS) and AZF c microdeletions. Patients in these latter two groups may exhibit less remarkable increases in FSH as well as a relatively higher success rate following sperm collection than those with iNOA (5, 35, 36). In contrast, our study excluded patients with KS and AZFc microdeletion, instead focusing on patients with iNOA, which is often associated with significantly elevated FSH levels and low sperm retrieval rates (36, 37). Despite the associations observed in the current study, further research is required to clarify the effects of hormonal levels on SRO in patients with iNOA as well as the mechanisms underlying their actions.
In addition to the retrospective nature of this study, some non-negligible limitations exist—for example, all testicular tissues used for the detection of circ_MGLL were paraffin-embedded testicular tissues obtained during micro-TESE surgery, and we did not examine the testicular biopsy tissue obtained via needle aspiration prior to micro-TESE surgery. Thus, for patients who meet the criteria of testicular needle aspiration, such an analysis may help to verify our findings. Furthermore, although we observed that the circ_MGLL-based nomogram could reflect the focal spermatogenesis of the whole testis and could be used as a predictor for SRO in patients with iNOA, the expression profiles and the function of circ_MGLL in germ cells and testicular tissues should be explored in future studies.
In summary, the detailed data obtained in our study demonstrate that higher circ_MGLL levels are associated with decreased success in sperm retrieval via micro-TESE in patients with iNOA. More importantly, to the best of our knowledge, the present study is the first to establish a competing risk nomogram for calculating the probability of successful SRO, which may aid in screening for micro-TESE eligibility. Although these results provide evidence regarding the significant association between circ_MGLL expression and failure of micro-TESE in patients with iNOA, an additional large-sample multicenter prospective cohort study is required to further validate the efficacy of the circ_MGLL-based nomogram for predicting successful SRO.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Review Board of the Northwest Women’s and Children’s Hospital (ethical review number: 2021-XBFE-023). The patients/participants provided their written informed consent to participate in this study.
MW designed the research study. SS, TW, and LW performed the research. SS analyzed the data. MW and SS wrote the paper. All authors contributed to the article and approved the submitted version.
This work was supported by the Xi’an Science and Technology Project (XA2021-YXYJ-0104) and Health Research Project of Shaanxi Provincial Health Commission (no. 2021E024).
We would like to express our gratitude to the patients who were enrolled in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1109807/full#supplementary-material
1. Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European Association of urology guidelines on Male sexual and reproductive health: 2021 update on Male infertility. Eur Urol (2021) 80(5):603–20. doi: 10.1016/j.eururo.2021.08.014
2. Vahidi S, Narimani N, Abouei S, Sadeghi A, Lorian K, Rahavian A. Comparison of intracytoplasmic sperm injection outcomes in azoospermic men who underwent testicular sperm extraction vs. microdissection testicular sperm extraction: A cross-sectional study. Int J Reprod BioMed (2021) 19(9):837–44. doi: 10.18502/ijrm.v19i9.9716
3. Ozkanli S, Basar MM, Selimoglu S, Erol B, Ozkanli O, Nurili F, et al. The ghrelin and orexin activity in testicular tissues of patients with idiopathic non-obstructive azoospermia. Kaohsiung J Med Sci (2018) 34(10):564–8. doi: 10.1016/j.kjms.2018.04.001
4. Alkandari MH, Zini A. Medical management of non-obstructive azoospermia: A systematic review. Arab J Urol (2021) 19(3):215–20. doi: 10.1080/2090598X.2021.1956233
5. Majzoub A, Arafa M, Clemens H, Imperial J, Leisegang K, Khalafalla K, et al. A systemic review and meta-analysis exploring the predictors of sperm retrieval in patients with non-obstructive azoospermia and chromosomal abnormalities. Andrologia (2022) 54(3):e14303. doi: 10.1111/and.14303
6. Vahidi S, Horoki AZ, Talkhooncheh MH, Jambarsang S, Marvast LD, Sadeghi A, et al. Success rate and ART outcome of microsurgical sperm extraction in non obstructive azoospermia: A retrospective study. Int J Reprod BioMed (2021) 19(9):781–8. doi: 10.18502/ijrm.v19i9.9710
7. Andrade DL, Viana MC, Esteves SC. Differential diagnosis of azoospermia in men with infertility. J Clin Med (2021) 10(14):3144. doi: 10.3390/jcm10143144
8. Kavoussi PK, Hudson K, Machen GL, Barsky M, Lebovic DI, Kavoussi SK. FSH levels and testicular volumes are associated with the severity of testicular histopathology in men with non-obstructive azoospermia. J Assist Reprod Genet (2021) 38(11):3015–8. doi: 10.1007/s10815-021-02313-y
9. Dohle GR, Elzanaty S, van Casteren NJ. Testicular biopsy: clinical practice and interpretation. Asian J Androl (2012) 14(1):88–93. doi: 10.1038/aja.2011.57
10. Mao JM, Liu DF, Zhao LM, Hong K, Zhang L, Ma LL, et al. [Effect of testicular puncture biopsy on the success rate of microdissection testicular sperm extraction for idiopathic non-obstructive azoospermia]. Beijing Da Xue Xue Bao Yi Xue Ban (2018) 50(4):613–6. doi: 10.3969/j.issn.1671-167X.2018.04.006
11. Cao C, Wen Y, Wang X, Fang N, Yuan S, Huang X. Testicular piRNA profile comparison between successful and unsuccessful micro-TESE retrieval in NOA patients. J Assist Reprod Genet (2018) 35(5):801–8. doi: 10.1007/s10815-018-1134-4
12. Zhou L, Lv MQ, Ge P, Yang YQ, He DL, Wang HX, et al. The expression of beclin-1 in testicular tissues of non-obstructive azoospermia patients and its predictive value in sperm retrieval rate. Transl Androl Urol (2021) 10(8):3267–74. doi: 10.21037/tau-21-320
13. Liu Y, Ao X, Yu W, Zhang Y, Wang J. Biogenesis, functions, and clinical implications of circular RNAs in non-small cell lung cancer. Mol Ther Nucleic Acids (2022) 27:50–72. doi: 10.1016/j.omtn.2021.11.013
14. Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol (2021) 19(3):188–206. doi: 10.1038/s41571-021-00585-y
15. Saberiyan M, Karimi E, Safi A, Movahhed P, Dehdehi L, Haririan N, et al. Circular RNAs: Novel biomarkers in spermatogenesis defects and Male infertility. Reprod Sci (2022) 30(1):62–71. doi: 10.1007/s43032-022-00885-3
16. Ji C, Wang Y, Wei X, Zhang X, Cong R, Yao L, et al. Potential of testis-derived circular RNAs in seminal plasma to predict the outcome of microdissection testicular sperm extraction in patients with idiopathic non-obstructive azoospermia. Hum Reprod (2021) 36(10):2649–60. doi: 10.1093/humrep/deab196
17. Ge P, Zhang J, Zhou L, Lv MQ, Li YX, Wang J, et al. CircRNA expression profile and functional analysis in testicular tissue of patients with non-obstructive azoospermia. Reprod Biol Endocrinol (2019) 17(1):100. doi: 10.1186/s12958-019-0541-4
18. Nielsen JE, Rolland AD, Rajpert-De Meyts E, Janfelt C, Jorgensen A, Winge SB, et al. Characterisation and localisation of the endocannabinoid system components in the adult human testis. Sci Rep (2019) 9(1):12866. doi: 10.1038/s41598-019-49177-y
19. Shi S, Jia Y, Ji X, Zhou L, Zhang Z. [Silencing circular RNA_monoglyceride lipase promotes the proliferation and inhibits apoptosis of sertoli cells in testis] (Published in Chinese). Med J West China (2022) 34(2):185–94. doi: 10.3969/j.issn.1672-3511.2022.02.006
20. Azizi H, Hashemi Karoii D, Skutella T. Whole exome sequencing and in silico analysis of human sertoli in patients with non-obstructive azoospermia. . Int J Mol Sci (2022) 23(20):12570. doi: 10.3390/ijms232012570
21. Johnsen SG. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones (1970) 1(1):2–25. doi: 10.1159/000178170
22. Zhou R, Lv X, Chen T, Chen Q, Tian H, Yang C, et al. Construction and external validation of a 5-gene random forest model to diagnose non-obstructive azoospermia based on the single-cell RNA sequencing of testicular tissue. Aging (Albany NY) (2021) 13(21):24219–35. doi: 10.18632/aging.203675
23. Cannarella R, Barbagallo F, Crafa A, La Vignera S, Condorelli RA, Calogero AE. Seminal plasma transcriptome and proteome: Towards a molecular approach in the diagnosis of idiopathic Male infertility. Int J Mol Sci (2020) 21(19):7308. doi: 10.3390/ijms21197308
24. Ng HY, Lau YL, Yeung SB, So WK, Tam PC, Ho PC. Testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Chin Med J (Engl) (2000) 113(3):246–50.
25. Cervan-Martin M, Bossini-Castillo L, Rivera-Egea R, Garrido N, Lujan S, Romeu G, et al. Effect and in silico characterization of genetic variants associated with severe spermatogenic disorders in a large Iberian cohort. Andrology (2021) 9(4):1151–65. doi: 10.1111/andr.13009
26. Ghanami Gashti N, Sadighi Gilani MA, Jabari A, Qasemi M, Feizollahi N, Abbasi M. The germ cell-specific markers ZPBP2 and PGK2 in testicular biopsies can predict the presence as well as the quality of sperm in non-obstructive azoospermia patients. . Reprod Sci (2021) 28(5):1466–75. doi: 10.1007/s43032-020-00427-9
27. Sakurai K, Mikamoto K, Shirai M, Iguchi T, Ito K, Takasaki W, et al. MicroRNA profiling in ethylene glycol monomethyl ether-induced monkey testicular toxicity model. J Toxicol Sci (2015) 40(3):375–82. doi: 10.2131/jts.40.375
28. Wang Y, Zang RK, Du YN. HSA_CIRC_0004050 on proliferation and apoptosis of A549 cells through ERK/JNK signaling pathway. J Biol Regul Homeost Agents (2020) 34(6):2037–47. doi: 10.23812/20-543-A
29. Li H, Li L, Lin C, Hu M, Liu X, Wang L, et al. Decreased miR-149 expression in sperm is correlated with the quality of early embryonic development in conventional in vitro fertilization. Reprod Toxicol (2021) 101:28–32. doi: 10.1016/j.reprotox.2021.02.005
30. Chen W, Cui Y, Ning M, Zhang H, Yin C, He Z, et al. The mechanisms and functions of microRNAs in mediating the fate determinations of human spermatogonial stem cells and sertoli cells. Semin Cell Dev Biol (2022) 121:32–9. doi: 10.1016/j.ygeno.2021.04.022
31. Cannarella R, Mancuso F, Arato I, Lilli C, Bellucci C, Gargaro M, et al. Sperm-carried IGF2 downregulated the expression of mitogens produced by sertoli cells: A paracrine mechanism for regulating spermatogenesis? Front Endocrinol (Lausanne) (2022) 13:1010796. doi: 10.3389/fendo.2022.1010796
32. Enatsu N, Miyake H, Chiba K, Fujisawa M. Predictive factors of successful sperm retrieval on microdissection testicular sperm extraction in Japanese men. Reprod Med Biol (2016) 15(1):29–33. doi: 10.1007/s12522-015-0212-x
33. Eken A, Gulec F. Microdissection testicular sperm extraction (micro-TESE): Predictive value of preoperative hormonal levels and pathology in non-obstructive azoospermia. Kaohsiung J Med Sci (2018) 34(2):103–8. doi: 10.1016/j.kjms.2017.08.010
34. Jahromi BN, Zeyghami S, Parsanezhad ME, Ghaemmaghami P, Zarei A, Kutenaee MA, et al. Determining an optimal cut-off value for follicle-stimulating hormone to predict microsurgical testicular sperm extraction outcome in patients with non-obstructive azoospermia. Arch Endocrinol Metab (2020) 64(2):165–70. doi: 10.20945/2359-3997000000217
35. Kocamanoglu F, Ayas B, Bolat MS, Abur U, Bolat R, Asci R. Endocrine, sexual and reproductive functions in patients with klinefelter syndrome compared to non-obstructive azoospermic patients. Int J Clin Pract (2021) 75(8):e14294. doi: 10.1111/ijcp.14294
36. Zhang HL, Zhao LM, Mao JM, Liu DF, Tang WH, Lin HC, et al. Sperm retrieval rates and clinical outcomes for patients with different causes of azoospermia who undergo microdissection testicular sperm extraction-intracytoplasmic sperm injection. Asian J Androl (2021) 23(1):59–63. doi: 10.4103/aja.aja_12_20
Keywords: circ_MGLL, idiopathic non-obstructive azoospermia, microdissection testicular sperm extraction, sperm retrieval, decision curve analysis
Citation: Shi S, Wang T, Wang L and Wang M (2023) Nomogram based on a circular RNA biomarker for predicting the likelihood of successful sperm retrieval via microdissection testicular sperm extraction in patients with idiopathic non-obstructive azoospermia. Front. Endocrinol. 13:1109807. doi: 10.3389/fendo.2022.1109807
Received: 28 November 2022; Accepted: 26 December 2022;
Published: 17 January 2023.
Edited by:
Atsushi P. Kimura, Hokkaido University, JapanReviewed by:
Ryoma Yoneda, Saitama Medical University, JapanCopyright © 2023 Shi, Wang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjuan Wang, d2FuZ21qMTk4MkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.