- Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, China
Osteosarcoma is the most common type of malignant bone tumor, occurring in adolescents and patients over 60. It has a bimodal onset and a poor prognosis, and its development has not yet been fully explained. Osteopontin (OPN) is a high protein consisting of 314 amino acid residues with a negative charge and is involved in many biological activities. OPN is not only an essential part of the regulation of the nervous system and endocrine metabolism of skeletal cells. Still, it is also involved in several other important biological activities, such as the division, transformation, and proliferation of skeletal cells and their associated cells, such as bone tumor cells, including bone marrow mesenchymal stem cells, hematopoietic stem cells, osteoblasts, and osteoclasts. Osteoblasts and osteocytes. Recent studies have shown a strong correlation between OPN and the development and progression of many skeletal diseases, such as osteosarcoma and rheumatoid arthritis. This review aims to understand the mechanisms and advances in the role of OPN as a factor in the development, progression, metastasis, and prognosis of osteosarcoma in an attempt to provide a comprehensive summary of the mechanisms by which OPN regulates osteosarcoma progression and in the hope of contributing to the advancement of osteosarcoma research and clinical treatment.
Introduction
Osteosarcoma (OS) is the most common primary skeletal-related malignancy in young people. It is the second leading cause of cancer death in children and adolescents (1),always presenting as growth in tubular long bones and giving rise to less differentiated skeletal cells (2, 3). Osteosarcoma is characterized by a bimodal pattern, with the first peak occurring in children and adolescents, and the second peak occurring in patients over 60 years of age (4). About half of these patients have tumors near the knee (5), and osteosarcoma has a high metastasis rate of nearly 20%, with the lungs and lymph nodes being the frequent sites of metastasis (6), and metastasis has a severe impact on the patient’s prognosis.
Osteosarcoma cells share many similarities with primitive bone cells, such as a strong proliferative capacity and resistance to apoptosis. Also, they produce components such as connective tissue growth factor, runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), and osteocalcin. Osteopontin is overexpressed in many tumors and may have a strong correlation with the development, metastasis, and prognosis of many types of tumors. For example, one study found that osteopontin in lung cancer, a rise in osteopontin was associated with the survival and prognosis of lung cancer patients (7–16). Osteopontin predicts poor prognostic performance after neoadjuvant chemotherapy for breast cancer (17–22). In addition, the upregulation of osteopontin concentrations in vivo was associated with tumor metastasis in gastrointestinal cancer, and even with the size and grade of the tumor (23–41). Significant correlation between osteopontin and drug resistance in urological tumors (42–54). Of course, osteopontin is also closely related to osteosarcoma, and we will systematically review and describe osteopontin and its endocrine and metabolic mechanisms in relation to osteosarcoma.
To describe the structural function of OPN and the possible mechanisms by which OPN regulates the development, progression, metastasis, and prognosis of osteosarcoma
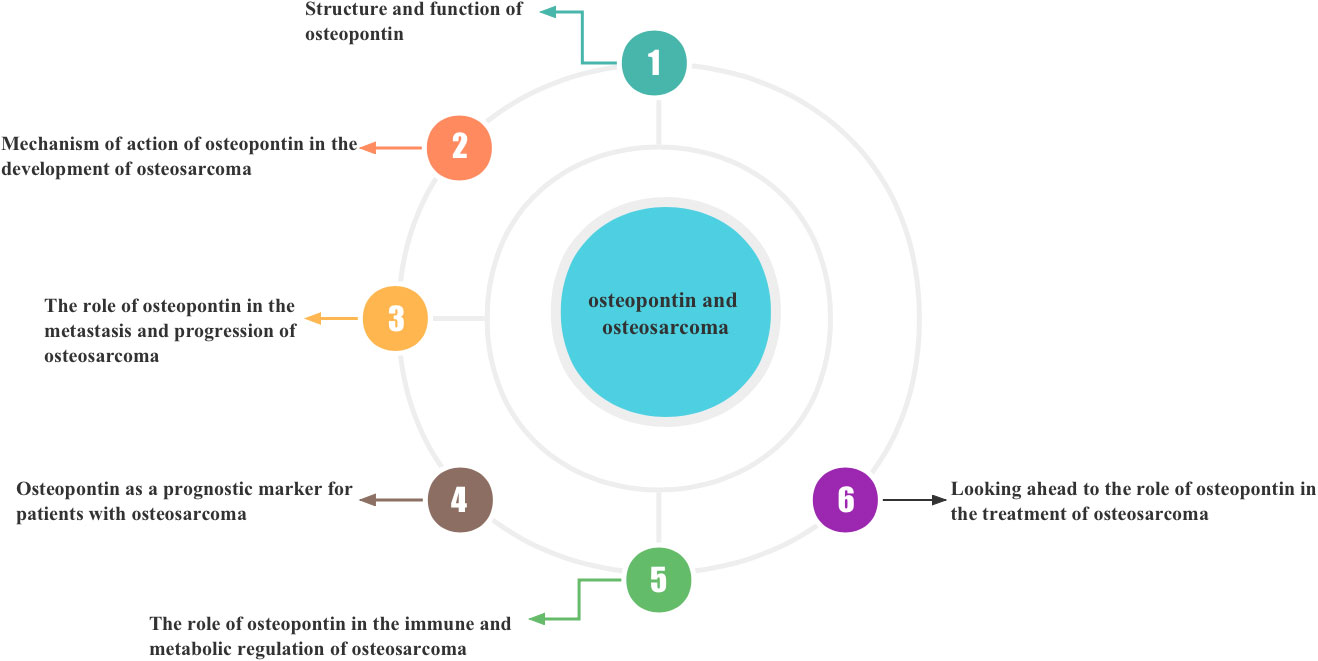
In the following sections, we will discuss the structure and function of osteopontin and, in addition, provide as comprehensive an understanding as possible of the possible role of osteopontin in the development, progression, metastasis, and prognosis of osteosarcoma. An attempt is made to summarise the mechanisms by which OPN regulates osteosarcoma and can contribute to the progress of osteosarcoma research and clinical treatment. In addition, Figure 1 details the sequence and content of what we will describe next.
Function and structure of osteopontin
Osteopontin is an extracellular matrix (ECM)-associated, rich phosphoglycoprotein (55–57). Osteopontin (OPN) is a negatively charged glycophosphoprotein with a high content of aspartic acid, composed of 314 amino acids and with acidic properties (58, 59), which was first identified in bone. There are five isoforms of OPN, and, to our knowledge, high expression of OPN is found in various tissues such as skin, kidney, bone, and teeth, as well as in some cancer cells, including blood. Numerous studies have identified a crucial role of OPN in early life development (60–65). The molecular structure of osteopontin is rough as shown in Figure 2. osteopontin is implicated in various diseases or mechanisms of action, which we have briefly described in Figure 3.
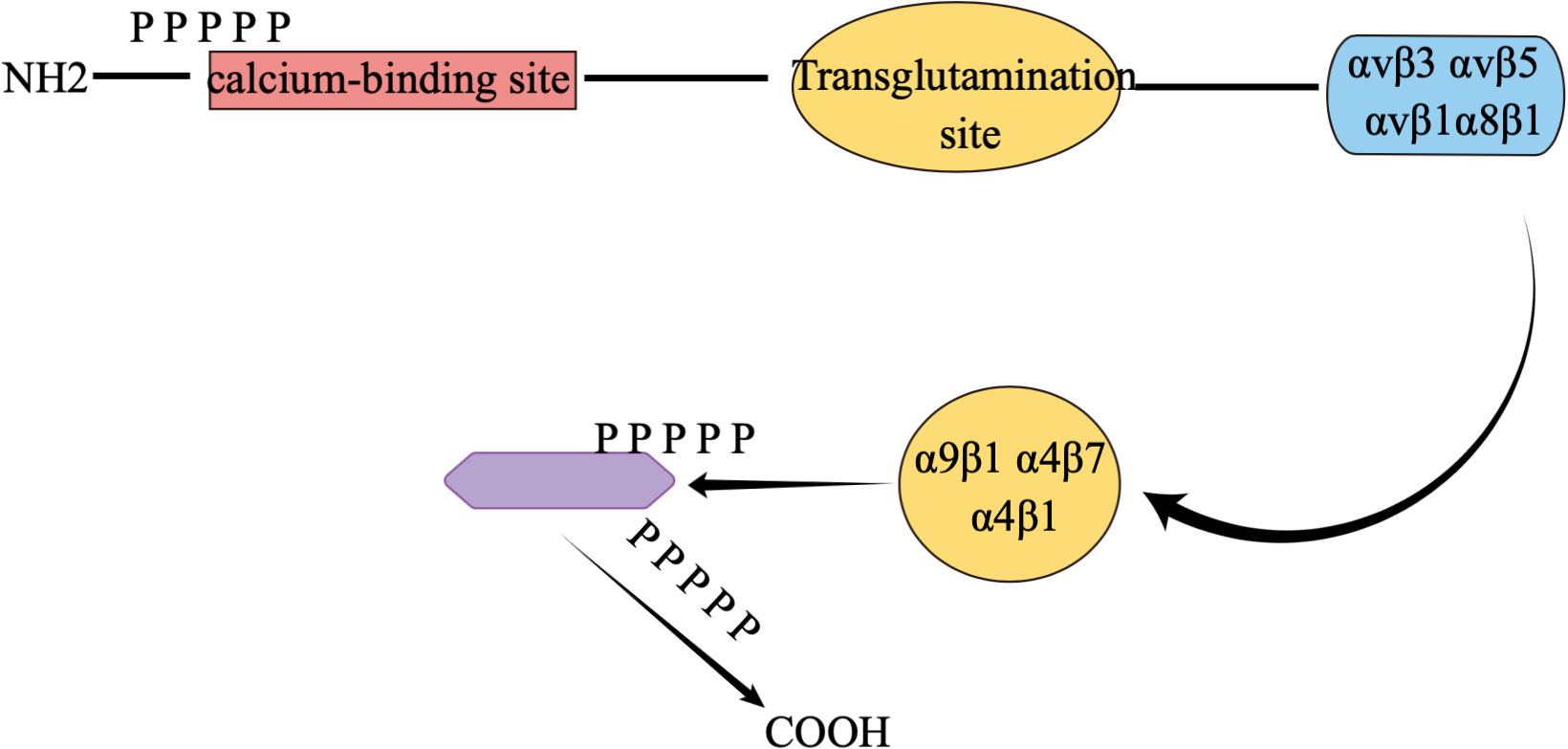
Figure 2 Concise molecular formula of osteopontin. αvβ1, αvβ3, αvβ5, α8β1, α9β1, α4β7 and α4β1 are all different integrins of osteopontin, which are responsible for interacting with cells.
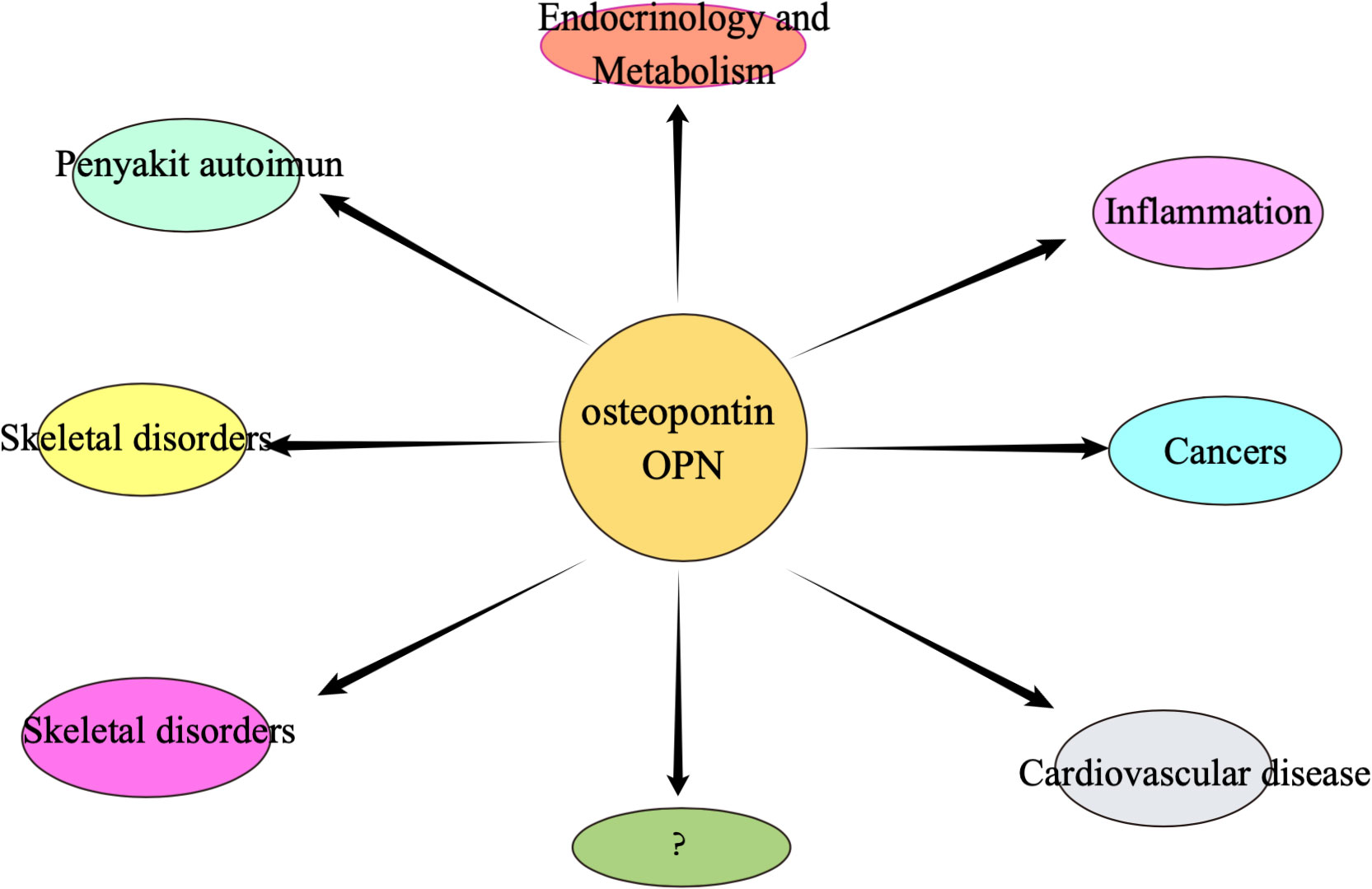
Figure 3 Osteopontin may be associated with many of the different types of diseases mentioned above.
Effect of osteopontin on the initial onset of osteosarcoma
Many studies in recent years have demonstrated a clear correlation between osteopontin and the initial development of osteosarcoma. Downregulation of osteopontin levels can prevent mesenchymal stem cells or immature osteoblasts from progressing to mature cells, allowing them to maintain the morphology and characteristics of immature primitive cells, which may ultimately lead to the development of osteosarcoma (66–69).
In addition, the glucose transporter is one of the regulators of osteosarcoma growth (70, 71), upregulating levels through the hypoxia-induced pathway and thereby adapting to hypoxia and increasing tissue oxygenation (72–75).
Hypoxia induces upregulation of osteopontin, which then increases GLUT1 and GLUT3 protein expression mediated by αvβ3 integrins and ultimately activates the protein kinase FAK pathway, leading to the initial development of osteosarcoma step by step (76). In particular, Figure 4 demonstrates the mechanism of action of osteopontin in the development of osteosarcoma.
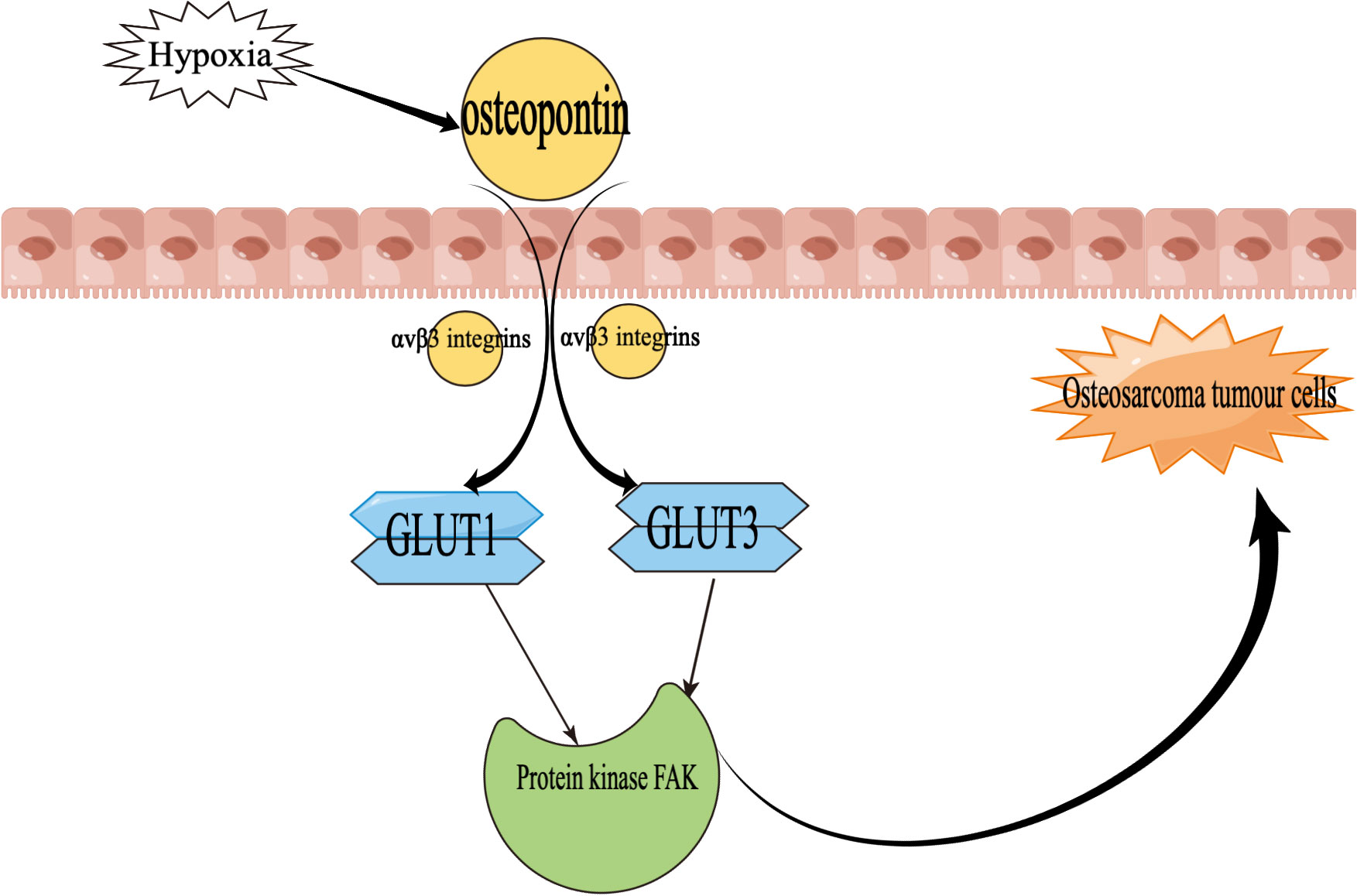
Figure 4 Mechanisms of the role of osteopontin in osteosarcoma development GLUT1, glucose transporter 1; GLUT3, glucose transporter 3. Focal Adhesion Kinase (FAK) is a tyrosine kinase that plays an important role in the evolution of osteosarcoma.
The role and impact of osteopontin in the development of metastasis in osteosarcoma
Metastases from osteosarcoma, most commonly in the lungs, are a key determinant of the lethality of osteosarcoma (77–79). It is, therefore, particularly important that we find ways to understand some or even all of the channels or mechanisms of osteosarcoma metastasis so that we can find ways to stop or disrupt the processes and pathways of osteosarcoma metastasis. The mechanism of metastasis and the various factors influencing it have not been well studied, but the role of OPN in the process of tumor metastasis in osteosarcoma can be elaborated and explained graphically. Several studies have demonstrated that S100A4 protein is associated with the metastasis of cancers, including osteosarcoma, that it increases the tumor metastatic capacity of cancers (80–83), and that S100A4 protein can even be a potential marker for predicting cancer metastasis (84, 85). And the effect of the S100A4 protein on tumor metastasis in osteosarcoma is also accomplished through a transition in regulating OPN levels. So, how exactly do S100A4 protein and OPN link and affect osteosarcoma metastasis? The study found that the extracellular S100A4 protein has been shown to activate NF-κB (86). Osteopontin was previously found to have elements that respond to NF-κB (87), so it was later shown that initially, the S100A4 protein regulated osteopontin horizontally, then linked to NF-κB through osteopontin, and finally led to the horizontal regulation of MMP protein (88, 89). This series of changes may eventually lead to the development of metastases in osteosarcoma, with the most likely organs of osteosarcoma being the lungs and lymphatic tissues. The role of Runt-related transcription factor 2 (Runx2) is also important in the metastatic process of many cancers, including osteosarcoma (90–95). Recent studies have shown that Runx2 in combination with osteopontin promotes the adhesion of osteosarcoma cells to the cell surface of the lung, an important step in the distant metastasis of osteosarcoma to the lung (96). In addition, Figure 5 demonstrates the mechanism of action of osteopontin in the metastatic process of osteosarcoma.
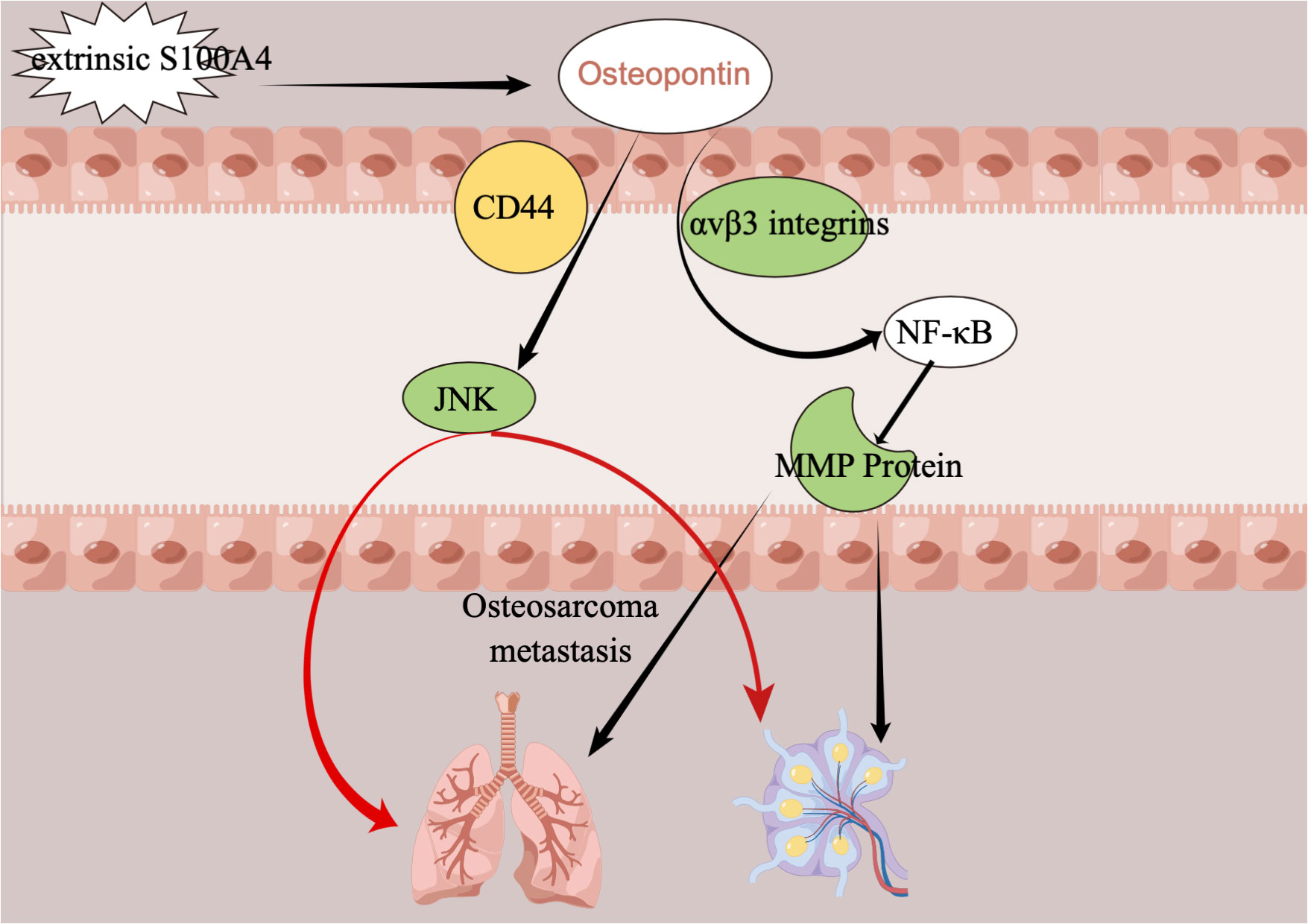
Figure 5 The mechanism of action of OPN in the metastatic process of osteosarcoma. CD44, extracellular matrix receptor III; MMP, matrix metalloproteinase; NF-κB, nuclear factor-κB; JNK, c-JUN N-terminal kinase.
Osteopontin as a prognostic marker for patients with osteosarcoma
Osteosarcoma is a highly malignant tumor, and many patients develop early metastases and have a terrible prognosis. Not only is OPN a marker for the development and metastasis of many different tumors, but even changes in OPN levels can correlate strongly with the prognosis of patients with osteosarcoma (97). In the study by Wong IH et al., mRNA levels of OPN were increased in more than 90% of patients with osteosarcoma, in addition to the healthy population but only a tiny proportion. Their study also suggests that peripheral blood OPN levels can be used as a predictive assessment for patients with osteosarcoma (98). In addition, one study differed from the above results. Firstly, OPN and vascular endothelial growth factor (VEGF) constitute a vascular protein. In their study, the expression of OPN in benign and malignant bone tumors was determined, and the prognostic effect of OPN expression on the outcome of osteosarcoma patients was studied. Express OPN and VEGF, the final results showed that OPN expression had no effect on patients’ overall or disease-free survival. Although the expression of OPN is associated with the expression of VEGF in osteosarcoma, the change of OPN level does not predict the good or bad prognosis of osteosarcoma patients (99).
The role of osteopontin in the immune and metabolic regulation of osteosarcoma
The intracellular osteopontin mRNA synthesizes two types of osteopontin, secreted osteopontin (sOPN) and intracellular osteopontin (iOPN). During immunotherapy of cancer, including osteosarcoma, tumor cells sometimes produce an immune escape, with the end result that cancer cells remain, and consequently, tumor metastasis occurs. The immunomodulatory effects of osteopontin include the development of osteosarcoma and distant metastasis and further cause the development of immunosuppression at the site of metastasis (100). It has been found that interferon regulatory factor 8 associates with osteopontin and causes a downregulation of osteopontin levels, which then activates T cells, meaning that interferon regulatory factor 8 levels are negatively correlated with osteopontin levels and that a decrease in interferon regulatory factor 8 levels leads to an upregulation of osteopontin levels, which can then reduce or even block the activation of T cells, which ultimately leads to immune escape from cancer, including osteosarcoma (101). Some studies have been conducted to discover the mechanism of action of osteopontin in the immunometabolism of certain cancers and their metastasis (102, 103). Detailed information on the mechanisms of endocrine and metabolic action of osteopontin in the development, progression, and metastasis of osteosarcoma and its regulation at the cellular level remains to be explored.
Looking ahead to a more valuable role for osteopontin in the treatment of osteosarcoma
OPN is involved in bone development and metabolism in the development and expansion of skeletal diseases through endocrinology and immunity. At the cellular level, OPN is involved in more refined activities through signaling pathways. Although studies in recent years have also produced many results on the association of OPN with the occurrence, development, metastasis, and prognosis of osteosarcoma, many of the mechanisms are still obscure. The in-depth study of OPN provides new ideas and directions for the interpretation of the pathogenesis of osteosarcoma. It gives a new target for the treating crucial clinical significance and value. We hope that future studies can better understand the mechanism of OPN’s role in the occurrence and development of osteosarcoma, including improving clinical prognosis, etc. We wish to interfere with or intervene in advance of the function of OPN in osteosarcoma and slow or even block the progression of osteosarcoma in the future to alleviate the pain of osteosarcoma patients and improve their disappointing survival rate.
Discussion
Cancer has become a global problem that cannot be ignored— it is one of the most common causes of death among older adults, with a high mortality rate from osteosarcoma. Over 3600 new bone cancer diagnoses and 1720 deaths from bone cancer occur every year in the United States (104). Distant metastases from osteosarcoma occur as a result of hematogenous spread, with the most significant probability occurring firstly in the lungs and secondly in the lymphatic system. These metastases are strongly associated with a poor prognosis. Osteosarcoma cells show a high propensity to spread and a relatively high likelihood of distant metastases. Osteosarcoma can metastasize to almost any organ, so the prognosis for patients with osteosarcoma is always dismal. Metastatic osteosarcoma cells settle and grow in a second organ later on and eventually develop into a metastatic lesion. The cell cycle undergoes differentiation, metabolism, and the formation of a new microenvironment suitable for the growth of metastatic osteosarcoma cells, and also, the metastatic cells are not identical to the original osteosarcoma cells (105, 106). There has also been notable success in the extensive research over the years to discover the underlying mechanisms by which osteosarcoma develops distant metastases. The tireless efforts of medical scientists have led to the discovery of additional markers involved in osteosarcoma metastasis-related metastases, followed by numerous cellular or animal studies that have further validated a number of relevant genes and pathways (107–110). Later, based on the results of these basic experiments, many clinical studies were subsequently conducted to improve treatment modalities. There is a wide variety of genes and proteins involved in the pathways and mechanisms by which osteosarcoma develops distant metastases, and a wide variety of genes and proteins involved in osteosarcoma metastasis, in which OPN must play an important role, We also address in this review only the role of OPN in the development and metastasis of bone tumors.
It has been found that osteopontin regulates cell signaling by binding to receptors that ultimately affect or directly contribute to tumor cell growth and metastasis, of which the main osteopontin receptors include integrins and CD44 (111). The broad metabolic pathways of action of osteopontin are also described in the section of the manuscript above. With the ongoing results of research on osteopontin, it is promising that we seem to be seeing alternative avenues for the pathogenesis and treatment of osteosarcoma. For example, reducing the expression of OPN levels may provide new strategies for the treatment of various types of metastatic cancers (112). In recent studies, Zhang et al. (66) demonstrated that hyperoside regulates OPN by inducing a cell cycle arrest and can hinder the development of osteosarcoma cells and promote further differentiation of osteosarcoma cells into osteoblasts. In addition, it has been suggested that VD3 upregulates OPN by activating cell cycle inhibitors such as p21 (113), thereby promoting osteopontin differentiation into osteoblasts. These may be new strategies for the treatment of OS in the future (114).
Conclusions
OPN can be secreted by many tissues and has many controversial effects on health. It can be argued that there is no clear answer to the metabolic and immunological effects of OPN on inflammation or cancer. In addition, the treatment of osteosarcoma, the most common primary bone malignancy, has been a challenge, and modern developments in molecular medicine have led to the discovery of several potential tumor markers. Recent studies have sought to use OPN as a diagnostic and prognostic marker for osteosarcoma to monitor the developmental status of osteosarcoma and assess its therapeutic efficacy. To better understand the role of OPN in the development and metastasis of osteosarcoma and to provide the basis for new therapeutic approaches to treat this life-threatening disease, more evidence from cellular studies and subsequent clinical trials is needed and will be awaited.
Author contributions
ZS designed and conceived the study and wrote this paper. SB revised the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh K, Mukherjee AB, De Vouge MW, Mukherjee BB. Differential processing of osteopontin transcripts in rat kidney- and osteoblast-derived cell lines. J Biol Chem (1992) 267(33):23847–51. doi: 10.1016/S0021-9258(18)35914-3
2. Kundu ZS. Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop (2014) 48(3):238–46. doi: 10.4103/0019-5413.132491
3. Miao J, Wu S, Peng Z, Tania M, Zhang C. Micrornas in osteosarcoma: Diagnostic and therapeutic aspects. Tumour Biol (2013) 34(4):2093–8. doi: 10.1007/s13277-013-0940-7
4. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res (2009) 152:3–13. doi: 10.1007/978-1-4419-0284-9_1
5. Simpson S, Dunning MD, de Brot S, Grau-Roma L, Mongan NP, Rutland CS. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand (2017) 59(1):71. doi: 10.1186/s13028-017-0341-9
6. Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat Rev (2006) 32(6):423–36. doi: 10.1016/j.ctrv.2006.05.005
7. Boldrini L, Donati V, Dell'Omodarme M, Prati MC, Faviana P, Camacci T, et al. Prognostic significance of osteopontin expression in early-stage non-Small-Cell lung cancer. Br J Cancer (2005) 93(4):453–7. doi: 10.1038/sj.bjc.6602715
8. Chambers AF, Wilson SM, Kerkvliet N, O'Malley FP, Harris JF, Casson AG. Osteopontin expression in lung cancer. Lung Cancer (1996) 15(3):311–23. doi: 10.1016/0169-5002(95)00595-1
9. Hu Z, Lin D, Yuan J, Xiao T, Zhang H, Sun W, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res (2005) 11(13):4646–52. doi: 10.1158/1078-0432.Ccr-04-2013
10. Mack PC, Redman MW, Chansky K, Williamson SK, Farneth NC, Lara PN Jr., et al. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-Small-Cell lung cancer patients receiving platinum-based chemotherapy: Swog study S0003. J Clin Oncol (2008) 26(29):4771–6. doi: 10.1200/jco.2008.17.0662
11. Ostheimer C, Bache M, Güttler A, Reese T, Vordermark D. Prognostic information of serial plasma osteopontin measurement in radiotherapy of non-Small-Cell lung cancer. BMC Cancer (2014) 14:858. doi: 10.1186/1471-2407-14-858
12. Ostheimer C, Gunther S, Bache M, Vordermark D, Multhoff G. Dynamics of heat shock protein 70 serum levels as a predictor of clinical response in non-Small-Cell lung cancer and correlation with the hypoxia-related marker osteopontin. Front Immunol (2017) 8:1305. doi: 10.3389/fimmu.2017.01305
13. Ostheimer C, Schweyer F, Reese T, Bache M, Vordermark D. The relationship between tumor volume changes and serial plasma osteopontin detection during radical radiotherapy of non-Small-Cell lung cancer. Oncol Lett (2016) 12(5):3449–56. doi: 10.3892/ol.2016.5104
14. Schneider S, Yochim J, Brabender J, Uchida K, Danenberg KD, Metzger R, et al. Osteopontin but not osteonectin messenger rna expression is a prognostic marker in curatively resected non-small cell lung cancer. Clin Cancer Res (2004) 10(5):1588–96. doi: 10.1158/1078-0432.ccr-0565-3
15. Shi L, Wang X. Role of osteopontin in lung cancer evolution and heterogeneity. Semin Cell Dev Biol (2017) 64:40–7. doi: 10.1016/j.semcdb.2016.08.032
16. Wang M, Han J, Marcar L, Black J, Liu Q, Li X, et al. Radiation resistance in kras-mutated lung cancer is enabled by stem-like properties mediated by an osteopontin-egfr pathway. Cancer Res (2017) 77(8):2018–28. doi: 10.1158/0008-5472.Can-16-0808
17. Anborgh PH, Caria LB, Chambers AF, Tuck AB, Stitt LW, Brackstone M. Role of plasma osteopontin as a biomarker in locally advanced breast cancer. Am J Transl Res (2015) 7(4):723–32.
18. Das R, Mahabeleshwar GH, Kundu GC. Osteopontin stimulates cell motility and nuclear factor kappab-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-Kinase/Akt signaling pathways in breast cancer cells. J Biol Chem (2003) 278(31):28593–606. doi: 10.1074/jbc.M303445200
19. Gu M, Zheng X. Osteopontin and vasculogenic mimicry formation are associated with response to neoadjuvant chemotherapy in advanced breast cancer. Onco Targets Ther (2017) 10:4121–7. doi: 10.2147/ott.S129414
20. Psyrri A, Kalogeras KT, Wirtz RM, Kouvatseas G, Karayannopoulou G, Goussia A, et al. Association of osteopontin with specific prognostic factors and survival in adjuvant breast cancer trials of the Hellenic cooperative oncology group. J Transl Med (2017) 15(1):30. doi: 10.1186/s12967-017-1134-7
21. Tuck AB, Chambers AF. The role of osteopontin in breast cancer: Clinical and experimental studies. J Mammary Gland Biol Neoplasia (2001) 6(4):419–29. doi: 10.1023/a:1014734930781
22. Zduniak K, Agrawal A, Agrawal S, Hossain MM, Ziolkowski P, Weber GF. Osteopontin splice variants are differential predictors of breast cancer treatment responses. BMC Cancer (2016) 16:441. doi: 10.1186/s12885-016-2484-x
23. Ding L, Zheng S. [Expression and clinical significance of osteopontin in colorectal cancer and liver metastatic tissues]. Zhonghua Wai Ke Za Zhi (2002) 40(10):773–5.
24. Ding L, Zheng S, Cao J. [Expression of osteopontin mrna and its protein in colorectal cancer and liver metastatic tissues]. Zhonghua Yi Xue Za Zhi (2002) 82(14):970–3.
25. Gu X, Gao XS, Ma M, Qin S, Qi X, Li X, et al. Prognostic significance of osteopontin expression in gastric cancer: A meta-analysis. Oncotarget (2016) 7(43):69666–73. doi: 10.18632/oncotarget.11936
26. Imano M, Okuno K, Itoh T, Satou T, Ishimaru E, Yasuda T, et al. Osteopontin induced by macrophages contribute to metachronous liver metastases in colorectal cancer. Am Surg (2011) 77(11):1515–20. doi: 10.1177/000313481107701143
27. Ito T, Hashimoto Y, Tanaka E, Kan T, Tsunoda S, Sato F, et al. An inducible short-hairpin rna vector against osteopontin reduces metastatic potential of human esophageal squamous cell carcinoma in vitro and in vivo. Clin Cancer Res (2006) 12(4):1308–16. doi: 10.1158/1078-0432.Ccr-05-1611
28. Kolb A, Kleeff J, Guweidhi A, Esposito I, Giese NA, Adwan H, et al. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol Ther (2005) 4(7):740–6. doi: 10.4161/cbt.4.7.1821
29. Lazar M, Sullivan J, Chipitsyna G, Gong Q, Ng CY, Salem AF, et al. Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells. J Gastrointest Surg (2010) 14(10):1566–77. doi: 10.1007/s11605-010-1338-0
30. Li JJ, Li HY, Gu F. Diagnostic significance of serum osteopontin level for pancreatic cancer: A meta-analysis. Genet Test Mol Biomarkers (2014) 18(8):580–6. doi: 10.1089/gtmb.2014.0102
31. Lin J, Myers AL, Wang Z, Nancarrow DJ, Ferrer-Torres D, Handlogten A, et al. Osteopontin (Opn/Spp1) isoforms collectively enhance tumor cell invasion and dissemination in esophageal adenocarcinoma. Oncotarget (2015) 6(26):22239–57. doi: 10.18632/oncotarget.4161
32. Liu G, Fan X, Tang M, Chen R, Wang H, Jia R, et al. Osteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells. Cancer Lett (2016) 383(2):171–82. doi: 10.1016/j.canlet.2016.09.033
33. Loosen SH, Roderburg C, Kauertz KL, Pombeiro I, Leyh C, Benz F, et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J Hepatol (2017) 67(4):749–57. doi: 10.1016/j.jhep.2017.06.020
34. Ng L, Wan T, Chow A, Iyer D, Man J, Chen G, et al. Osteopontin overexpression induced tumor progression and chemoresistance to oxaliplatin through induction of stem-like properties in human colorectal cancer. Stem Cells Int (2015) 2015:247892. doi: 10.1155/2015/247892
35. Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology (2013) 58(6):1992–2000. doi: 10.1002/hep.26577
36. Terashi T, Aishima S, Taguchi K, Asayama Y, Sugimachi K, Matsuura S, et al. Decreased expression of osteopontin is related to tumor aggressiveness and clinical outcome of intrahepatic cholangiocarcinoma. Liver Int (2004) 24(1):38–45. doi: 10.1111/j.1478-3231.2004.00886.x
37. Ue T, Yokozaki H, Kitadai Y, Yamamoto S, Yasui W, Ishikawa T, et al. Co-Expression of osteopontin and Cd44v9 in gastric cancer. Int J Cancer (1998) 79(2):127–32. doi: 10.1002/(sici)1097-0215(19980417)79:2<127::aid-ijc5>3.0.co;2-v
38. Weber CE, Erşahin ÇH, Kuo PC, Mi Z. Pancreatic cancer and osteopontin: The relationship remains unclear. Pancreas (2016) 45(7):e35–6. doi: 10.1097/mpa.0000000000000639
39. Wu H, Zhang H, Hu LY, Zhang TY, Zheng YJ, Shen F, et al. Is osteopontin a promising prognostic biomarker for cholangiocarcinoma? J Hepatol (2017) S0168-8278(17):32269–9. doi: 10.1016/j.jhep.2017.08.029
40. Wu IC, Wu MT, Chou SH, Yang SF, Goan YG, Lee JM, et al. Osteopontin expression in squamous cell cancer of the esophagus. World J Surg (2008) 32(9):1989–95. doi: 10.1007/s00268-008-9609-6
41. Zhang HZ, Liu JG, Wei YP, Wu C, Cao YK, Wang M. [Expressions of rhoc and osteopontin in esophageal squamous carcinoma and association with the patients' prognosis]. Nan Fang Yi Ke Da Xue Xue Bao (2006) 26(11):1612–5.
42. Forootan SS, Foster CS, Aachi VR, Adamson J, Smith PH, Lin K, et al. Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer (2006) 118(9):2255–61. doi: 10.1002/ijc.21619
43. Hsieh IS, Huang WH, Liou HC, Chuang WJ, Yang RS, Fu WM. Upregulation of drug transporter expression by osteopontin in prostate cancer cells. Mol Pharmacol (2013) 83(5):968–77. doi: 10.1124/mol.112.082339
44. Puzone R, Paleari L, Montefiore F, Ruggiero L, Puntoni M, Maffezzini M, et al. Osteopontin plasma level does not detect prostate cancer in patients referred for diagnostic prostate biopsy. Int J Biol Markers (2010) 25(4):200–6. doi: 10.5301/JBM.2010.6116
45. Tilli TM, Bellahcène A, Castronovo V, Gimba ER. Changes in the transcriptional profile in response to overexpression of the osteopontin-c splice isoform in ovarian (Ovcar-3) and prostate (Pc-3) cancer cell lines. BMC Cancer (2014) 14:433. doi: 10.1186/1471-2407-14-433
46. Tilli TM, Silva EA, Matos LC, Faget DV, Dias BF, Vasconcelos JS, et al. Osteopontin is a tumor autoantigen in prostate cancer patients. Oncol Lett (2011) 2(1):109–14. doi: 10.3892/ol.2010.211
47. Tozawa K, Yamada Y, Kawai N, Okamura T, Ueda K, Kohri K. Osteopontin expression in prostate cancer and benign prostatic hyperplasia. Urol Int (1999) 62(3):155–8. doi: 10.1159/000030381
48. Hu ZD, Wei TT, Yang M, Ma N, Tang QQ, Qin BD, et al. Diagnostic value of osteopontin in ovarian cancer: A meta-analysis and systematic review. PloS One (2015) 10(5):e0126444. doi: 10.1371/journal.pone.0126444
49. Leung DT, Lim PL, Cheung TH, Wong RR, Yim SF, Ng MH, et al. Osteopontin fragments with intact thrombin-sensitive site circulate in cervical cancer patients. PloS One (2016) 11(8):e0160412. doi: 10.1371/journal.pone.0160412
50. Song JY, Lee JK, Lee NW, Yeom BW, Kim SH, Lee KW. Osteopontin expression correlates with invasiveness in cervical cancer. Aust N Z J Obstet Gynaecol (2009) 49(4):434–8. doi: 10.1111/j.1479-828X.2009.01027.x
51. Wong JPC, Wei R, Lyu P, Tong OLH, Zhang SD, Wen Q, et al. Clinical and in vitro analysis of osteopontin as a prognostic indicator and unveil its potential downstream targets in bladder cancer. Int J Biol Sci (2017) 13(11):1373–86. doi: 10.7150/ijbs.21457
52. Xu C, Li H, Yin M, Yang T, An L, Yang G. Osteopontin is involved in Tlr4 pathway contributing to ovarian cancer cell proliferation and metastasis. Oncotarget (2017) 8(58):98394–404. doi: 10.18632/oncotarget.21844
53. Xu ST, Guo C, Ding X, Fan WJ, Zhang FH, Xu WL, et al. Role of osteopontin in the regulation of human bladder cancer proliferation and migration in T24 cells. Mol Med Rep (2015) 11(5):3701–7. doi: 10.3892/mmr.2015.3202
54. Živný JH, Leahomschi S, Klener P Jr., Živný J, Haluzík M, Cibula D. Comparison of plasma osteopontin levels between patients with borderline ovarian tumours and serous ovarian carcinoma. Folia Biol (Praha) (2016) 62(6):258–62.
55. Weber GF. The metastasis gene osteopontin: A candidate target for cancer therapy. Biochim Biophys Acta (2001) 1552(2):61–85. doi: 10.1016/s0304-419x(01)00037-3
56. Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer (2004) 90(10):1877–81. doi: 10.1038/sj.bjc.6601839
57. Rangaswami H, Bulbule A, Kundu GC. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol (2006) 16(2):79–87. doi: 10.1016/j.tcb.2005.12.005
58. Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell communication Signaling (2009) 3(3):311–22. doi: 10.1007/s12079-009-0068-0
59. Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem (2007) 102(4):912–24. doi: 10.1002/jcb.21558
60. Bruun S, Jacobsen LN, Ze X, Husby S, Ueno HM, Nojiri K, et al. Osteopontin levels in human milk vary across countries and within lactation period: Data from a multicenter study. J Pediatr Gastroenterol Nutr (2018) 67(2):250–6. doi: 10.1097/mpg.0000000000002004
61. Lönnerdal B, Kvistgaard AS, Peerson JM, Donovan SM, Peng YM. Growth, nutrition, and cytokine response of breast-fed infants and infants fed formula with added bovine osteopontin. J Pediatr Gastroenterol Nutr (2016) 62(4):650–7. doi: 10.1097/mpg.0000000000001005
62. Møller HK, Thymann T, Fink LN, Frokiaer H, Kvistgaard AS, Sangild PT. Bovine colostrum is superior to enriched formulas in stimulating intestinal function and necrotising enterocolitis resistance in preterm pigs. Br J Nutr (2011) 105(1):44–53. doi: 10.1017/s0007114510003168
63. Jiang R, Lönnerdal B. Effects of milk osteopontin on intestine, neurodevelopment, and immunity. Nestle Nutr Inst Workshop Ser (2020) 94:152–7. doi: 10.1159/000505067
64. Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Pre- and post-translational regulation of osteopontin in cancer. J Cell Commun Signal (2011) 5(2):111–22. doi: 10.1007/s12079-011-0130-6
65. Rittling SR, Wejse PL, Yagiz K, Warot GA, Hui T. Suppression of tumour growth by orally administered osteopontin is accompanied by alterations in tumour blood vessels. Br J Cancer (2014) 110(5):1269–77. doi: 10.1038/bjc.2014.10
66. Zhang N, Ying MD, Wu YP, Zhou ZH, Ye ZM, Li H, et al. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PloS One (2014) 9(7):e98973. doi: 10.1371/journal.pone.0098973
67. Mortus JR, Zhang Y, Hughes DP. Developmental pathways hijacked by osteosarcoma. Adv Exp Med Biol (2014) 804:93–118. doi: 10.1007/978-3-319-04843-7_5
68. Luo X, Chen J, Song WX, Tang N, Luo J, Deng ZL, et al. Osteogenic bmps promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest (2008) 88(12):1264–77. doi: 10.1038/labinvest.2008.98
69. Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res (2008) 466(9):2114–30. doi: 10.1007/s11999-008-0335-z
70. Medina RA, Owen GI. Glucose transporters: Expression, regulation and cancer. Biol Res (2002) 35(1):9–26. doi: 10.4067/s0716-97602002000100004
71. Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol (2012) 24(6):650–4. doi: 10.1097/CCO.0b013e328356da72
72. Semenza GL. Hif-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev (2010) 20(1):51–6. doi: 10.1016/j.gde.2009.10.009
73. Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: Novel pathways and targets for anticancer therapeutics. Chemotherapy (2007) 53(4):233–56. doi: 10.1159/000104457
74. Denko NC. Hypoxia, Hif1 and glucose metabolism in the solid tumour. Nat Rev Cancer (2008) 8(9):705–13. doi: 10.1038/nrc2468
75. Rey S, Semenza GL. Hypoxia-inducible factor-1-Dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res (2010) 86(2):236–42. doi: 10.1093/cvr/cvq045
76. Hsieh IS, Yang RS, Fu WM. Osteopontin upregulates the expression of glucose transporters in osteosarcoma cells. PloS One (2014) 9(10):e109550. doi: 10.1371/journal.pone.0109550
77. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol (2002) 20(3):776–90. doi: 10.1200/jco.2002.20.3.776
78. Kager L, Zoubek A, Pötschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol (2003) 21(10):2011–8. doi: 10.1200/jco.2003.08.132
79. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol (2015) 33(27):3029–35. doi: 10.1200/jco.2014.59.4895
80. Davies BR, Davies MP, Gibbs FE, Barraclough R, Rudland PS. Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for P9ka, a rat calcium-binding protein, but not with the oncogene ej-Ras-1. Oncogene (1993) 8(4):999–1008.
81. Maelandsmo GM, Hovig E, Skrede M, Engebraaten O, Flørenes VA, Myklebost O, et al. Reversal of the in vivo metastatic phenotype of human tumor cells by an anti-capl (Mts1) ribozyme. Cancer Res (1996) 56(23):5490–8.
82. Ambartsumian NS, Grigorian MS, Larsen IF, Karlstrøm O, Sidenius N, Rygaard J, et al. Metastasis of mammary carcinomas in grs/a hybrid mice transgenic for the Mts1 gene. Oncogene (1996) 13(8):1621–30.
83. Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R. Expression of the calcium-binding protein S100a4 (P9ka) in mmtv-neu transgenic mice induces metastasis of mammary tumours. Oncogene (1996) 13(8):1631–7.
84. Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, et al. Prognostic significance of the metastasis-inducing protein S100a4 (P9ka) in human breast cancer. Cancer Res (2000) 60(6):1595–603.
85. Maelandsmo GM, Flørenes VA, Nguyen MT, Flatmark K, Davidson B. Different expression and clinical role of S100a4 in serous ovarian carcinoma at different anatomic sites. Tumour Biol (2009) 30(1):15–25. doi: 10.1159/000199447
86. Boye K, Grotterød I, Aasheim HC, Hovig E, Maelandsmo GM. Activation of nf-kappab by extracellular S100a4: Analysis of signal transduction mechanisms and identification of target genes. Int J Cancer (2008) 123(6):1301–10. doi: 10.1002/ijc.23617
87. Samant RS, Clark DW, Fillmore RA, Cicek M, Metge BJ, Chandramouli KH, et al. Breast cancer metastasis suppressor 1 (Brms1) inhibits osteopontin transcription by abrogating nf-kappab activation. Mol Cancer (2007) 6:6. doi: 10.1186/1476-4598-6-6
88. Schmidt-Hansen B, Ornås D, Grigorian M, Klingelhöfer J, Tulchinsky E, Lukanidin E, et al. Extracellular S100a4(Mts1) stimulates invasive growth of mouse endothelial cells and modulates mmp-13 matrix metalloproteinase activity. Oncogene (2004) 23(32):5487–95. doi: 10.1038/sj.onc.1207720
89. Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100a4: Role of the receptor for advanced glycation end products. Arthritis Rheum (2006) 54(9):2901–11. doi: 10.1002/art.22042
90. Wai PY, Mi Z, Gao C, Guo H, Marroquin C, Kuo PC. Ets-1 and Runx2 regulate transcription of a metastatic gene, osteopontin, in murine colorectal cancer cells. J Biol Chem (2006) 281(28):18973–82. doi: 10.1074/jbc.M511962200
91. Onodera Y, Miki Y, Suzuki T, Takagi K, Akahira J, Sakyu T, et al. Runx2 in human breast carcinoma: Its potential roles in cancer progression. Cancer Sci (2010) 101(12):2670–5. doi: 10.1111/j.1349-7006.2010.01742.x
92. Sase T, Suzuki T, Miura K, Shiiba K, Sato I, Nakamura Y, et al. Runt-related transcription factor 2 in human colon carcinoma: A potent prognostic factor associated with estrogen receptor. Int J Cancer (2012) 131(10):2284–93. doi: 10.1002/ijc.27525
93. Yang J, Zhao L, Tian W, Liao Z, Zheng H, Wang G, et al. Correlation of wwox, Runx2 and vegfa protein expression in human osteosarcoma. BMC Med Genomics (2013) 6:56. doi: 10.1186/1755-8794-6-56
94. Gupta S, Ito T, Alex D, Vanderbilt CM, Chang JC, Islamdoust N, et al. Runx2 (6p21.1) amplification in osteosarcoma. Hum Pathol (2019) 94:23–8. doi: 10.1016/j.humpath.2019.09.010
95. Lucero CM, Vega OA, Osorio MM, Tapia JC, Antonelli M, Stein GS, et al. The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J Cell Physiol (2013) 228(4):714–23. doi: 10.1002/jcp.24218
96. Villanueva F, Araya H, Briceño P, Varela N, Stevenson A, Jerez S, et al. The cancer-related transcription factor Runx2 modulates expression and secretion of the matricellular protein osteopontin in osteosarcoma cells to promote adhesion to endothelial pulmonary cells and lung metastasis. J Cell Physiol (2019) 234(8):13659–79. doi: 10.1002/jcp.28046
97. Liang S, Li Y, Wang B. The cancer-related transcription factor Runx2 combined with osteopontin: A novel prognostic biomarker in resected osteosarcoma. Int J Clin Oncol (2021) 26(12):2347–54. doi: 10.1007/s10147-021-02025-4
98. Wong IH, Chan AT, Johnson PJ. Quantitative analysis of circulating tumor cells in peripheral blood of osteosarcoma patients using osteoblast-specific messenger rna markers: A pilot study. Clin Cancer Res (2000) 6(6):2183–8.
99. Sulzbacher I, Birner P, Trieb K, Lang S, Chott A. Expression of osteopontin and vascular endothelial growth factor in benign and malignant bone tumors. Virchows Arch (2002) 441(4):345–9. doi: 10.1007/s00428-002-0671-4
100. Sangaletti S, Tripodo C, Sandri S, Torselli I, Vitali C, Ratti C, et al. Osteopontin shapes immunosuppression in the metastatic niche. Cancer Res (2014) 74(17):4706–19. doi: 10.1158/0008-5472.Can-13-3334
101. Klement JD, Paschall AV, Redd PS, Ibrahim ML, Lu C, Yang D, et al. An Osteopontin/Cd44 immune checkpoint controls Cd8+ T cell activation and tumor immune evasion. J Clin Invest (2018) 128(12):5549–60. doi: 10.1172/jci123360
102. Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol (2012) 226(2):185–99. doi: 10.1002/path.3031
103. Moorman HR, Poschel D, Klement JD, Lu C, Redd PS, Liu K. Osteopontin: A key regulator of tumor progression and immunomodulation. Cancers (Basel) (2020) 12(11):3379. doi: 10.3390/cancers12113379
104. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
105. Gorlick R, Anderson P, Andrulis I, Arndt C, Beardsley GP, Bernstein M, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: Meeting summary. Clin Cancer Res (2003) 9(15):5442–53.
106. PosthumaDeBoer J, Witlox MA, Kaspers GJ, van Royen BJ. Molecular alterations as target for therapy in metastatic osteosarcoma: A review of literature. Clin Exp Metastasis (2011) 28(5):493–503. doi: 10.1007/s10585-011-9384-x
107. Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, et al. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res (2001) 61(9):3750–9.
108. Hynes RO. Metastatic potential: Generic predisposition of the primary tumor or rare, metastatic variants-or both? Cell (2003) 113(7):821–3. doi: 10.1016/s0092-8674(03)00468-9
109. Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med (2004) 10(2):182–6. doi: 10.1038/nm982
110. Zucchini C, Rocchi A, Manara MC, De Sanctis P, Capanni C, Bianchini M, et al. Apoptotic genes as potential markers of metastatic phenotype in human osteosarcoma cell lines. Int J Oncol (2008) 32(1):17–31. doi: 10.3892/ijo.32.1.17
111. Samanna V, Wei H, Ego-Osuala D, Chellaiah MA. Alpha-V-Dependent outside-in signaling is required for the regulation of Cd44 surface expression, mmp-2 secretion, and cell migration by osteopontin in human melanoma cells. Exp Cell Res (2006) 312(12):2214–30. doi: 10.1016/j.yexcr.2006.03.022
112. Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets (2014) 18(8):883–95. doi: 10.1517/14728222.2014.925447
113. Kommagani R, Whitlatch A, Leonard MK, Kadakia MP. P73 is essential for vitamin d-mediated osteoblastic differentiation. Cell Death Differ (2010) 17(3):398–407. doi: 10.1038/cdd.2009.135
Keywords: osteopontin, osteosarcoma, endocrine regulation, metabolism, cancer
Citation: Shao Z and Bi S (2023) Endocrine regulation and metabolic mechanisms of osteopontin in the development and progression of osteosarcoma, metastasis and prognosis. Front. Endocrinol. 13:1100063. doi: 10.3389/fendo.2022.1100063
Received: 16 November 2022; Accepted: 23 December 2022;
Published: 13 January 2023.
Edited by:
Ruiqin Han, Chinese Academy of Medical Sciences, ChinaReviewed by:
Wencai Liu, The First Affiliated Hospital of Nanchang University, ChinaJiaheng Xie, Nanjing Medical University, China
Zhao Wang, Chungnam National University School of Medicine, South Korea
Copyright © 2023 Shao and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuxiong Bi, YmlzaHV4aW9uZzE2OEBzb2h1LmNvbQ==
 Zhuce Shao
Zhuce Shao Shuxiong Bi*
Shuxiong Bi*