- 1West China Hospital/West China School of Medicine, Sichuan University, Chengdu, China
- 2Department of Endocrinology and Metabolism, Center for Diabetes and Metabolism Research, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Vascular Surgery, University Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4The Rolf Luft Research Center for Diabetes and Endocrinology, Karolinska Institutet, Stockholm, Sweden
Aims: The present systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to investigate the effect of low volume high-intensity interval training (LVHIIT) on the metabolic and cardiorespiratory outcomes in patients with type 2 diabetes mellitus (T2DM).
Methods: Relevant articles were sourced from PubMed, EBSCO, Web of Science, Embase, and the Cochrane Library from inception to October 2022. The study search strategy and all other processes were implemented in accordance with the PRISMA statement.
Results: Five randomized controlled trials that satisfied the inclusion criteria were included in this meta-analysis. The LVHIIT group had significantly lower fasting blood glucose levels (RR= -1.21; 95% CI= -2.02— -0.40, p = 0.0032) and HbA1c levels (RR= -0.65; 95% CI= -1.06— -0.23, p = 0.002) and higher levels of insulin resistance indicator HOMA-IR (RR= -1.34; 95% CI = -2.59— -0.10, p = 0.03) than the control group. Moreover, our results show that LVHIIT can reduce body mass (RR = -0.94, 95% CI = -1.37— -0.51, p<0.0001) and body mass index (RR = -0.31, 95% CI = -0.47— -0.16, p<0.0001). LVHIIT had a better therapeutic effect on blood lipid metabolism, such as total cholesterol, high-density lipoprotein, low-density lipoprotein and triglycerides. However, the change in fasting insulin levels was not statistically significant (RR= -1.43; 95% CI = -3.46— 0.60, p =0.17). Furthermore, LVHIIT reduced the systolic blood pressure (RR =-4.01, 95% CI = -4.82 – -3.21, p<0.0001) and improved peak oxygen uptake (VO2peak) compared to the control group (RR= 5.45; 95% CI = 1.38 – 9.52, p =0.009).
Conclusion: After a certain period of LVHIIT, glycaemic control, insulin resistance, body weight, lipid profile and cardiorespiratory outcomes were significantly improved in T2DM patients.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease characterized by increased blood glucose concentrations. T2DM affects more than 400 million people worldwide, a figure that is expected to exceed 642 million people by 2040. In addition to severe suffering for the actual patient, the economic cost for disability and treatment places a heavy burden on society (1). Although many pharmacological treatments have emerged in recent years, these medications not only fail to prevent the progression of diabetes and its complications but also cause many side effects.
Cardiometabolic risk factors including central obesity, hypertension, dyslipidemia, and insulin resistance are strongly associated with the development and progression of T2DM (2). Lifestyle interventions appear as the efficient strategy to minimize cardiometabolic risk factors and improve T2D, which has gained increasing attention and acceptance among patients due to their simplicity and repeatability (3–5). Lifestyle interventions can be used as primary or supplementary treatments for T2DM patients according to the current ADA (American Diabetes Association) guidelines (6). Recent clinical trials have demonstrated that intensive lifestyle interventions can reduce the incidence of diabetes by 58% compared to those without lifestyle interventions (7). Among these lifestyle interventions, physical exercise results in improved insulin sensitivity and glucose homeostasis, which has long been recommended as one of the key therapeutic interventions for T2DM (8). High-intensity interval training (HIIT) consists of alternating repetitions of short periods of high-intensity exercise interspersed with less active or passive recovery periods. HIIT should be performed at 80–100% of the max heart rate interval, with a lower heart rate during the rest period. Compared to widely used moderate-intensity continuous training (MICT), HIIT has been proposed as a lower total energy expended exercise intervention that may bring about similar positive effects (9, 10). Collective evidence suggests that HIIT contributes to greater improvements in cardiorespiratory fitness compared to MICT (11), where cardiorespiratory fitness is inverse associated with the incidence of T2DM (12).
Nevertheless, normal HIIT has a much higher exercise intensity with a higher risk of injury in T2DM patients (13). Therefore, a milder HIIT exercise protocol is needed to reduce the risk of HIIT while improving metabolic and cardiorespiratory outcomes in individuals with T2DM. Additionally, lack of time is one of the common obstacles to physical activities. Low volume high-intensity interval training (LVHIIT) is a type of HIIT with the reduced total training volume (14). It has been suggested that LVHIIT could improve cardiorespiratory fitness as effective as high-volume HIIT, suggesting that LVHIIT may serve as a potent and time-efficient physical activity intervention strategy (15–17). The beneficial roles of LVHIIT on body composition have also been demonstrated (16, 18). However, the effects of LVHIIT on metabolic and cardiorespiratory outcomes in patients with T2DM remain unclear. In addition, new studies with more detailed data and high evidence levels have been published. Thus, we performed the current systematic review and meta-analysis of randomized controlled trials (RCTs) to investigate the effects of LVHIIT on T2DM patients. The results of this investigation may guide future decision-making regarding the use of lifestyle interventions among patients with T2DM.
Materials and methods
This systematic review and meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement and the Cochrane Handbook for Systematic Reviews of Interventions (19). Ethical approval and patient consent were not required because all analyses were based on previously published studies.
Literature search and selection criteria
LVHIT as intervention treatment vs no exercise or shame exercise (exercise at very low density). We systematically searched several databases including PubMed, EBSCO, Web of science, EMbase, and the Cochrane Library from inception to October 2022. The structured search strategies used the combination of RCTs and LVHIIT with diabetes patients: [“Low volume” OR “high-intensity interval training” AND (exercise OR physical activity) AND (“randomized controlled trials”) AND (diabetes) NOT (review) NOT (meta) NOT (animal experiment)]. The reference lists of retrieved studies and relevant reviews were hand-searched, and the process mentioned above was performed repeatedly to ensure the inclusion of all eligible studies. Inclusion criteria were as follows (1): randomized control trials, (2) T2DM diagnosis before participating in the experiment, (3)as there is no universal standard definition of HIIT, thus we used the HIIT standard proposed by previous meta-analysis (20, 21), (4) LVHIT as intervention treatment vs no exercise or shame exercise, (5) sufficient data for extraction, (6) full text only, and studies with all languages were included.
Data extraction and outcome measures
Baseline information that was extracted from the original studies included the following: first author, published year, number of patients, patient age and gender distributions, the evaluation of the evidence level, detailed intervention method and time of period. Data were independently extracted by two investigators. Discrepancies were resolved by consensus.
The primary outcomes were fasting blood glucose (FBG), HOMA-IR and HbA1c. Secondary outcomes were fasting insulin, body mass index (BMI), body mass, plasma lipid metabolism (TC, HDL, LDL and triglyceride) and the cardiorespiratory fitness parameters including systolic blood pressure (SBP), diastolic blood pressure (DBP) and relative VO2 peak.
Quality assessment of individual studies
The methodological quality of each RCT was assessed by the Jadad Scale which consists of three evaluation elements: randomization (0-2 points), blinding (0-2 points), dropouts and withdrawals (0-1 points) (22). One point was be allocated to each element if it had been conducted and mentioned appropriately in the original article. The total score of the Jadad Scale ranges from 0 to 5 points. An article with a total Jadad score that is less than or equal to 2 is considered to be of low quality. Concurrently, a study is thought to be of high quality if its total Jadad score greater or equal to 3 (23).
Statistical analysis
Risk Ratio (RR) with 95% confidence intervals (CIs) was calculated for dichotomous outcomes. Heterogeneity was evaluated using the I2 statistic, with I2 > 50% taken to indicate significant heterogeneity (24). Sensitivity analysis was performed to evaluate the influence of a single study on the overall estimate by omitting one study in turn or performing subgroup analysis. The random-effects model was used for meta-analysis. Owing to the limited number of included studies (<10), publication bias was not assessed. Statistical significance was accepted at P < 0.05. All the data are presented as mean ± SD. All statistical analyses were performed using Review Manager Software Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature searches, study characteristics, and quality assessment
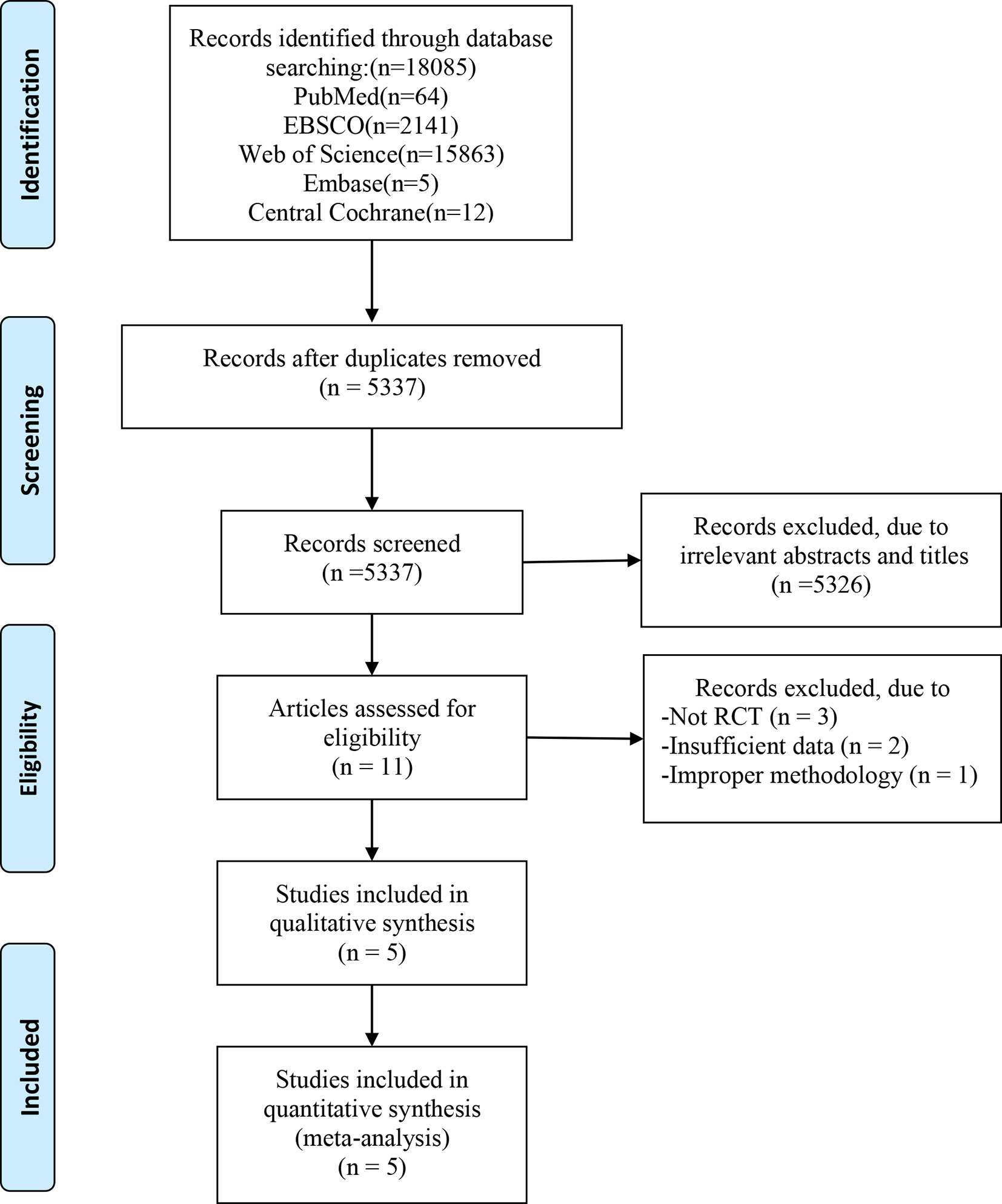
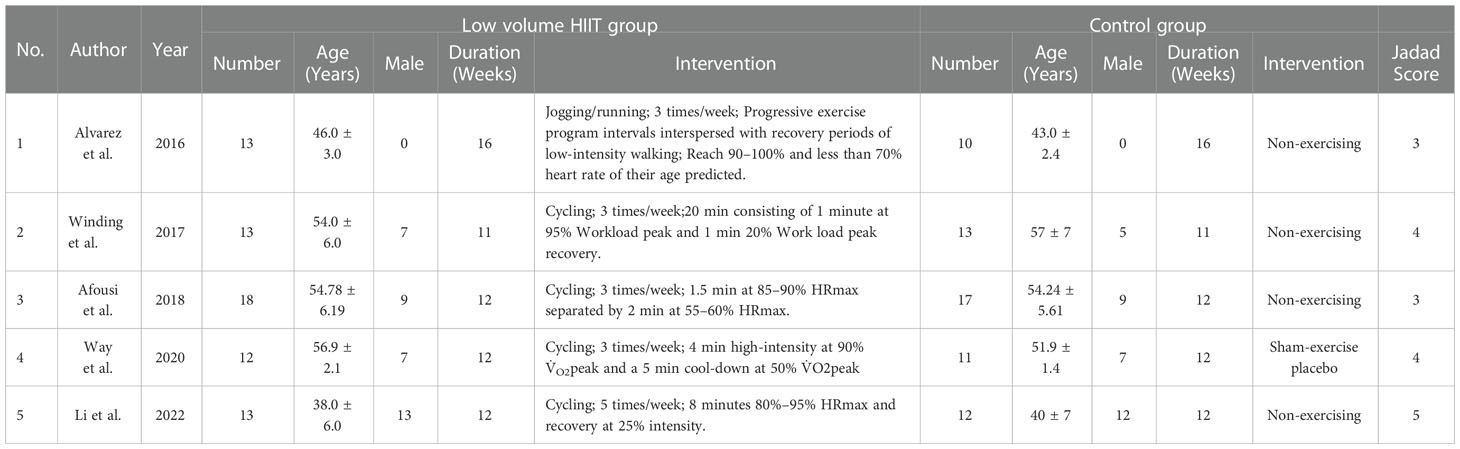
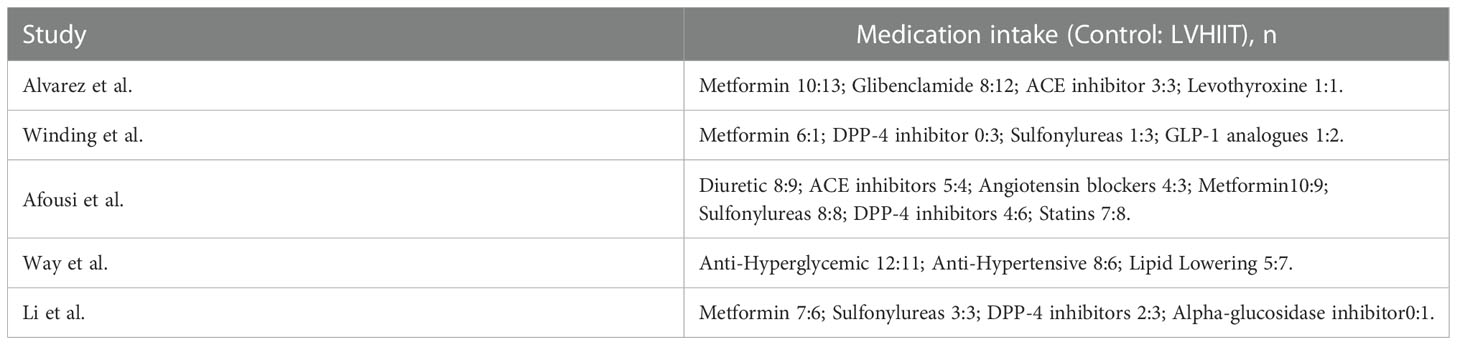
In total, 18085 articles including 64 in PubMed, 2141 in EBSCO, 15863 in Web of science, 5 in Embase and 12 in central Cochrane were initially identified from the databases. After removing duplicates, 5537 articles were retained. A total of 5326 studies were excluded from our study due to unrelated abstracts and titles. We also excluded 3 studies that were not RCTs, 2 studies that presented insufficient data, and 1 study that reported an improper methodology. Ultimately, five RCTs satisfied the inclusion criteria and were included in this meta-analysis (10, 25–28). The article selection process was performed in accordance with the PRISMA statement and the flow chart is shown as Figure 1. The baseline characteristics of the 5 included studies are shown in Table 1. Only Afousi et al. reported the age range of the patients (45-60 years old) (26). Four studies compared LVHIIT to no exercise, and one compared LVHIIT to a sham-exercise placebo. Four groups (10, 25–27) used cycling, and 1 group used jogging/running (28). There were no statistically significant differences in the patient baseline characteristics. All studies reported the exercise duration: 3 studies reported 12 weeks, 1 study for 1 weeks and 1 for 16 weeks. All studies included in our meta-analysis were published between 2016 and 2022, and the total sample size was 119. The detailed information on medication intake was shown in Table 2. The mean Jadad score ranged from 3 to 5. The main limitation of the included studies was the blinding methods. The Jadad scores for each study are also presented in Table 1.
Primary outcomes
Fasting blood glucose
Four studies examined pre- and post-LVHIIT FBG levels (10, 25, 27, 28). The results showed a significant difference in FBG in the LVHIIT group compared with the control group (RR= -1.21; 95% CI -2.02— -0.40, p = 0.0032), and there was significant heterogeneity (I2 = 73%, P = 0.01; Figure 2A). After removing the Winding et al. study (10), the heterogeneity became nonsignificant (I2 = 0%, P = 0.78), and the overall effect of exercise remained significant (RR= -0.82; CI -1.22 – -0.42, p = 0.0032; Figure 2B).
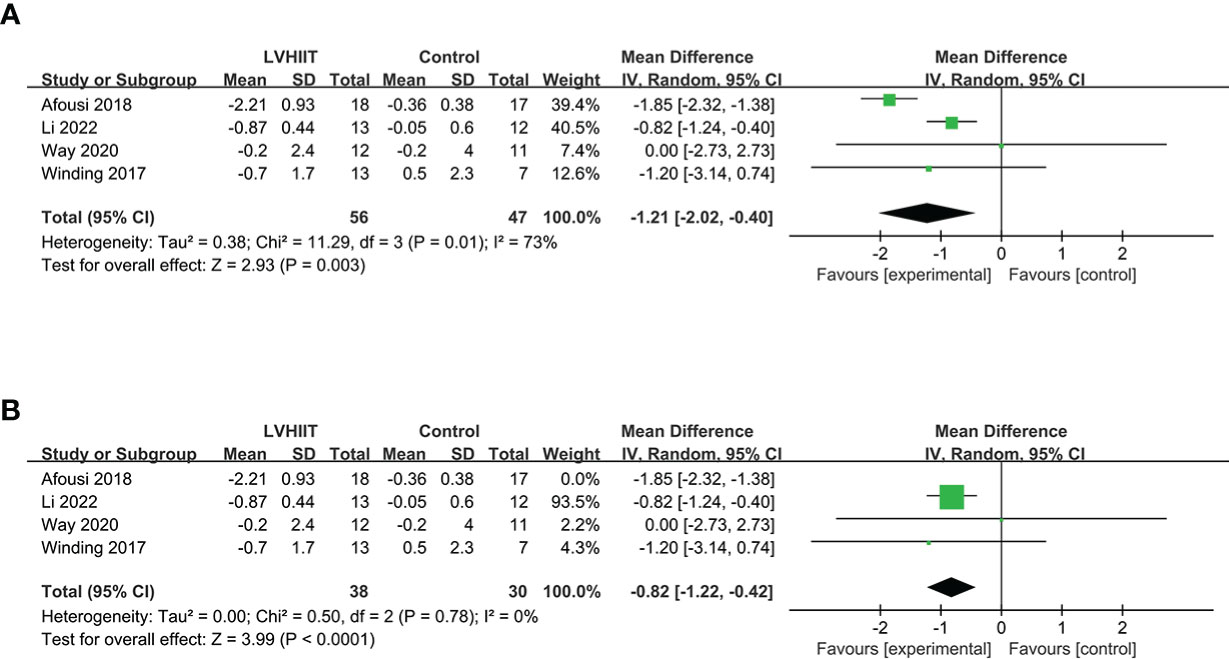
Figure 2 (A) Forest plot for the meta-analysis of (A) fasting blood glucose and (B) after sensitivity analysis.
HbA1c
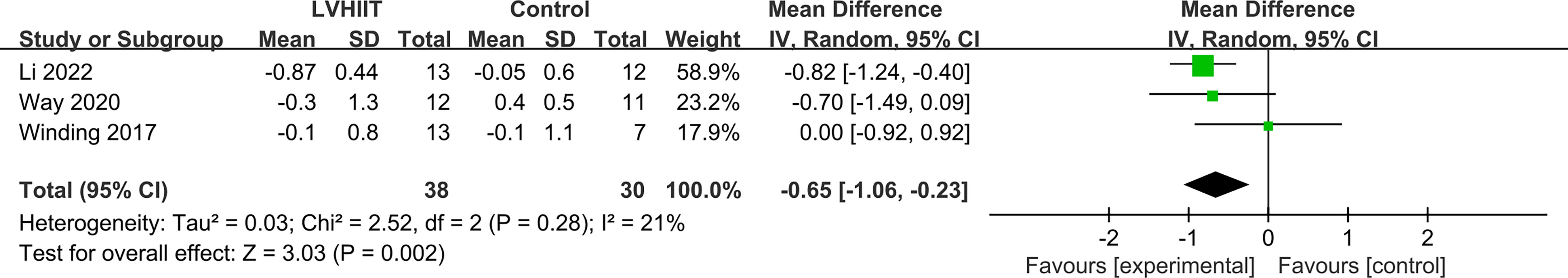
Three studies examined changes in HbA1c levels (10, 25, 27). Our meta-analysis indicated that LVHIIT can significantly reduce the HbA1c levels (RR= -0.65; 95% CI= -1.06 – -0.23, p = 0.002; Figure 3), and there was nonsignificant heterogeneity (I2 = 21%, p = 0.28).
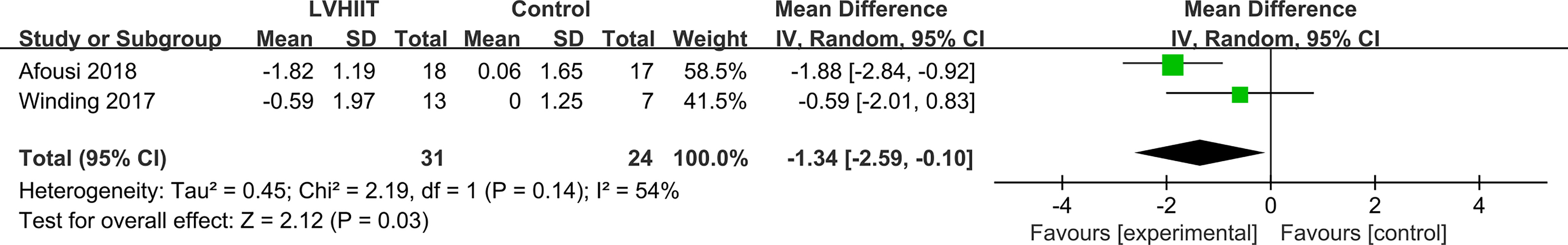
HOMA-IR
Only two studies examined HOMA-IR levels (10, 28). As shown in Figure 3, HOMA-IR levels were significantly lower in the LVHIIT group than in the control group (RR= -1.34; 95% CI -2.59— -0.10, p = 0.03; Figure 4), and there was significant heterogeneity (I2 = 54%, P = 0.14).
Secondary outcomes
Fasting insulin
Four studies examined pre- and post-LVHIIT fasting insulin levels (10, 25, 27, 28). Our results revealed that LVHIIT did not significantly change the fasting insulin level compared to the control group (RR= -1.43; 95% CI = -3.46 – 0.60, p =0.17; Figure 5A), and there was significant heterogeneity (I2 = 92%, p <0.00001). After removing the study by Li et al. (25), the overall effect of LVHIIT remained nonsignificant (RR= -3.52; 95% CI = -10.95 – 3.90, p =0.35; Figure 5B), and the level of heterogeneity was lower (I2 = 31%, p =0.24).
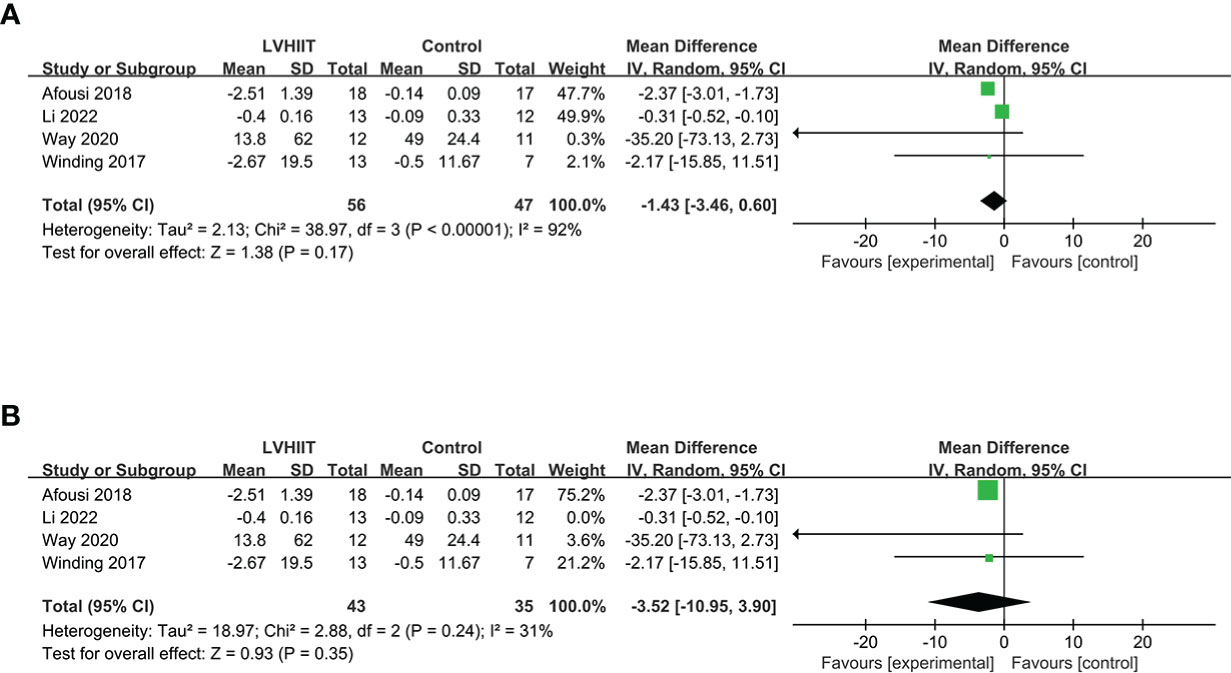
Figure 5 Forest plot for the meta-analysis of (A) fasting insulin and (B) after sensitivity analysis.
BMI and body mass
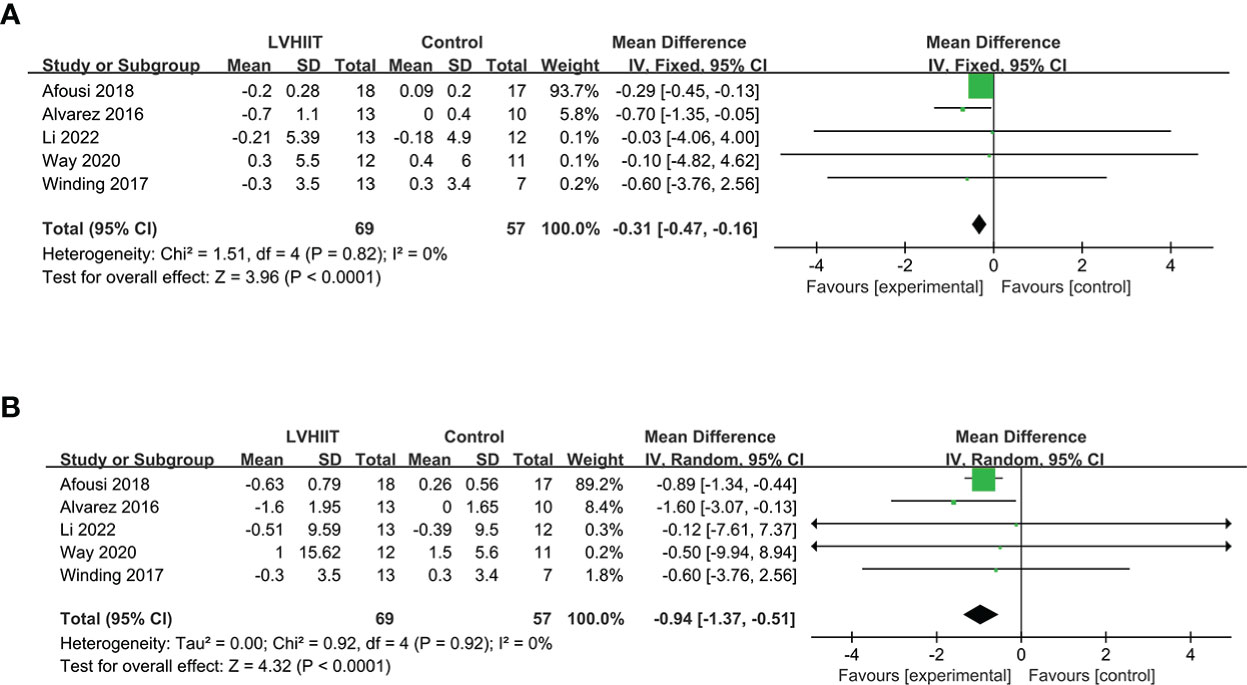
All five included studies examined BMI and body mass (10, 25–28). Our results revealed that LVHIIT reduces BMI (RR = -0.31, 95% CI = -0.47 – -0.16, p<0.0001; Figure 6A) and body mass (RR = -0.94, 95% CI = -1.37 – -0.51, p<0.0001; Figure 6B), and there was nonsignificant heterogeneity (I2 = 0%, P =0.82 and I2 = 0%, P =0.92, respectively).
Blood lipid metabolism
Four studies (10, 26–28) examined data relating to blood lipid indicators. For total cholesterol, our meta-analysis indicated that there was no difference after a period of LVHIIT (RR = -1.24, 95% CI = -3.09—0.61, p=0.19; Figure 7A), and there was a significant level of heterogeneity (I2 = 99%, P <0.00001). However, after excluding one study, LVHIIT was found to significantly reduce total cholesterol (RR = -0.24, 95% CI = -0.35 to -0.13, p<0.0001; Figure 7B), and the heterogeneity became nonsignificant (I2 = 0%, P =0.68). For high-density lipoprotein, the meta-analysis showed that there was a significant difference between the LVHIIT group and the control group (RR = 0.21, 95% CI = 0.08 – 0.33, p=0.001; Figure 7E), and there was a significant level of heterogeneity (I2 = 62%, P=0.05). After removing the study by Way et al., the results still showed that LVHIIT increased plasma HDL levels (RR = 0.28, 95% CI =0.24—0.32, p<0.00001), but the heterogeneity was nonsignificant (I2 = 0%, P =0.58; Figure 7F). Overall, LVHIIT can reduce TC, LDL and triglyceride levels and increase HDL levels.
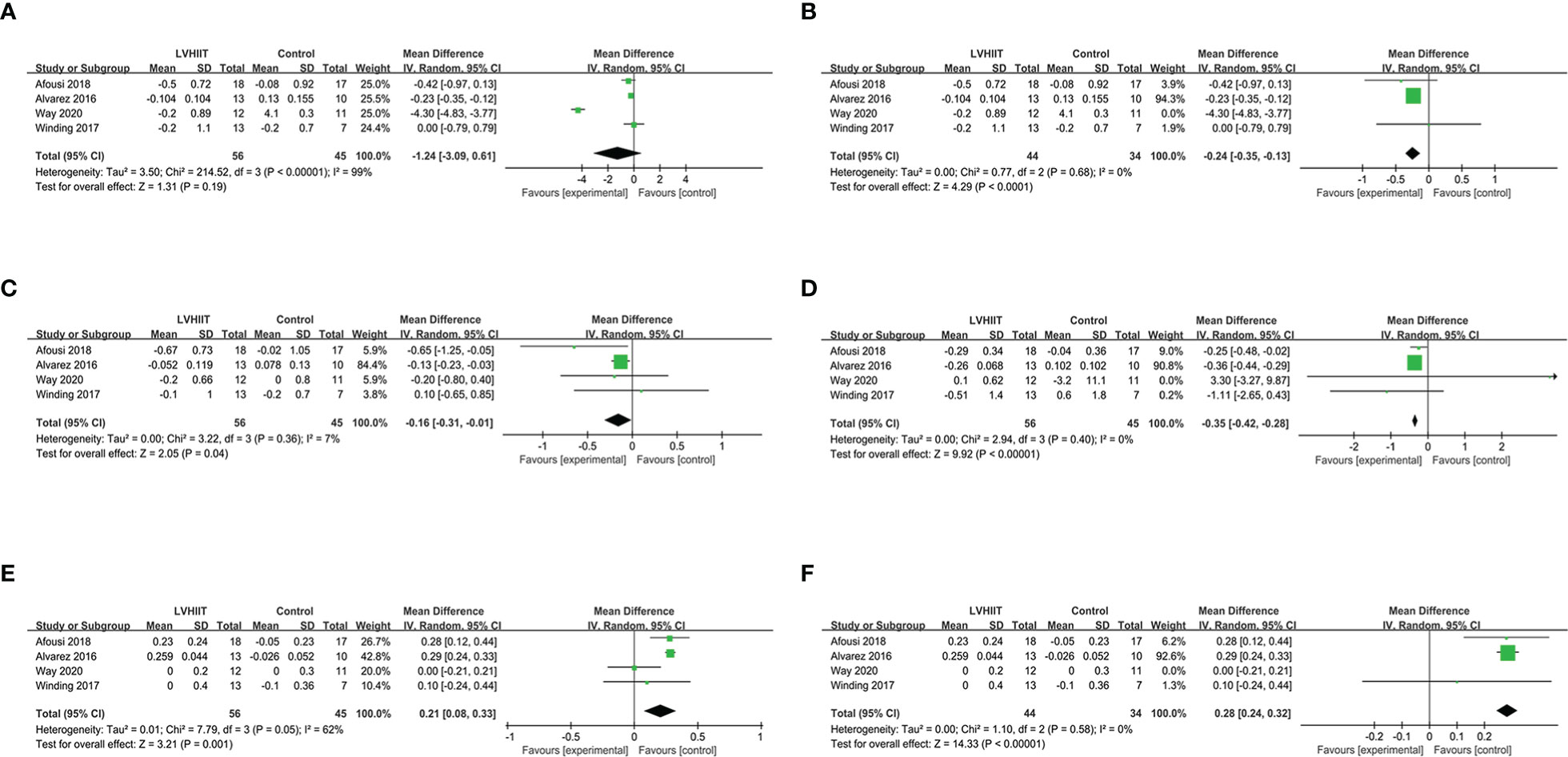
Figure 7 Forest plot for the meta-analysis of lipid profile (A) total cholesterol and (B) after sensitivity analysis; (C) low density lipoprotein; (D) triglyceride; (E) high density lipoprotein and (F) after sensitivity analysis.
Cardiorespiratory fitness parameters
All five studies included the SBP and DBP (10, 25–28). Our results indicated that LVHIIT can reduce the SBP (RR =-4.01, 95% CI = -4.82 – -3.21, p<0.0001; Figure 8A) but not DPB (RR =-1.52, 95% CI = -3.31 – 0.26, p=0.09; Figure 8B) with nonsignificant heterogeneity (I2 = 0%, P =0.77 and I2 = 37%, P =0.18, respectively). Three studies reported the VO2peak and our result showed that LVHIIT significantly improved VO2peak compared to the control group (RR= 5.45; 95% CI = 1.38 – 9.52, p =0.009; Figure 8C) and there was significant heterogeneity (I2 = 70%, p =0.04) (10, 26, 27). After removing the study by Way et al. (27), the overall effect of LVHIIT remained significant (RR= 7.66; 95% CI = 5.21 – 10.11, p<0.00001; Figure 8D), and the level of heterogeneity was lower (I2 = 0%, p =0.70).
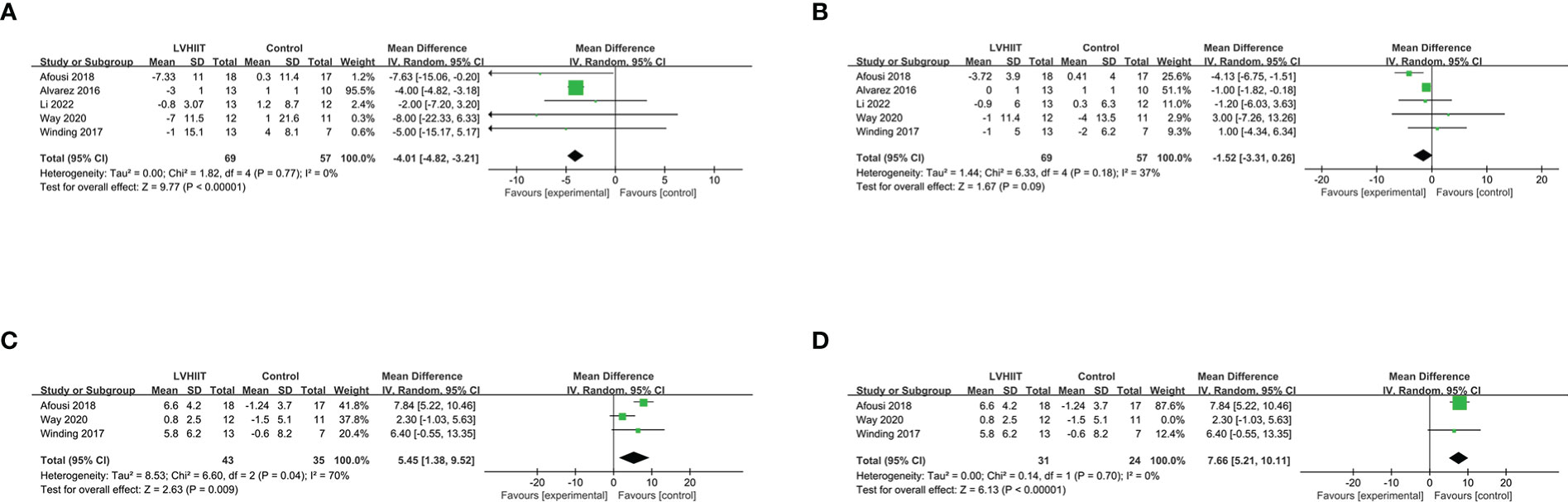
Figure 8 Forest plot for the meta-analysis of cardiorespiratory fitness (A) systolic blood pressure and (B) diastolic blood pressure; (C) relative VO2 peak; (D) after sensitivity analysis.
Discussion
Increased evidence has shown that physical exercise is an essential component of all effective interventions for the treatment and prevention of T2DM. As different types of exercise bring different benefits to patients, a series of clinical trials and meta-analyses have been performed to determine the positive function of each type of exercise. Earlier studies have shown that aerobic exercise, resistance training and HIIT independently have beneficial effects on preventing T2DM (5). As T2DM patients often report a “lack of time” as one barrier to regular exercise (29), LVHIIT may be a more time-effective strategy. LVHIIT has already been proven to improve cardiovascular health in T2DM patients. However, the effect of LVHIIT on diabetes-related indicators such as glycaemic control, insulin level, and HbA1c remain unclear. To evaluate this type of exercise and obtain higher-level evidence, we performed this meta-analysis.
Hyperglycaemia is the key characteristic of diabetes mellitus and is the main cause of complications in the heart, vasculature, eyes, kidneys and nerve system (30). Almost all types of exercise can reduce hyperglycaemia by improving insulin resistance in peripheral organs, such as skeletal muscle, liver and adipocytes (31–34), which will enhance blood glucose uptake and transport. Jelleyman et al. reported in their meta-analysis that regular HIIT can significantly reduce fasting glucose in metabolic syndrome or T2DM but not in healthy people compared to no exercise patients (35). Our present research found a reduction in fasting glucose levels among T2DM patients after LVHIIT intervention. Little et al. reported that LVHIIT can reduce hyperglycaemia by enhancing insulin signaling, the insulin-stimulated glucose disposal rate, glucose transporter protein (GLUT4) levels, and mitochondrial capacity in muscle, which further confirms our results (34). HOMA-IR, a model for estimating insulin sensitivity through glucose concentrations and fasting insulin, was also improved in the LVHIIT group (36). The level of heterogeneity was higher for this outcome, which may be due to different blood sampling times and the specific calculation model they used. Thus, we can assume that LVHIIT can significantly improve hyperglycaemia and insulin resistance in T2DM patients. HbA1c is another indicator for blood glucose concentration and is a very important predictor for the incidence of complications and death related to diabetes. A previous study reported that each 1% increase in HbA1c is associated with a 37% increase in diabetic microvascular complications and a 21% increase in the risk of mortality. Thus, HbA1c is also a crucial marker for evaluating the therapeutic method of diabetes (37, 38). First, the formation of HbA1c is related to the average blood glucose concentration at three months. Second, a previous study showed that extent to which HbA1c levels decrease depends on the type and volume of exercise (39). Our results showed that medium- or long-term (11-16 weeks) LVHIIT can significantly reduce HbA1c and benefit T2DM patients. Notably, these glycaemic-related indicators were also improved in a short-term (2 weeks) experiment. Unfortunately, the study had a small sample size, and the evidence level of the study design was not high (34). Due to the improvement in insulin resistance, fasting insulin should be lower. However, our result shows a lower tendency of insulin without statistical significance. This result may partly explain why exercise improved insulin signaling in peripheral tissue rather than enhancing the insulin secretion function of β-cells (40). Ishiguro et al. assumed that insulin improvement may be restricted in patients with impaired basal insulin secretion with severe insulin resistance or impaired basal insulin secretion (41).
A previous meta-analysis that only included aerobic training and resistance training showed that BMI and body mass had nonsignificant reductions (42). Jelleyman et al. reported that HIIT can reduce body mass and BMI compared to the control group. However, the included study had relatively inconsistent baselines, as the included studies had different types of patients, such as healthy, overweight, T2DM and other chronic diseases (35). Our results further confirmed that LVHIIT can help reduce BMI and body weight in T2DM patients. However, body composition, such as body fat percentage, waist circumference and waist-hip ratio, should be further investigated. Our study also showed that LVHIIT significantly improved the blood lipid profile. The study by Way et al. contributed to the overall heterogeneity of this outcome because of the baseline characteristics of their patients (some patients were taking lipid-lowering medication). The main shortcoming for this outcome is the lack of the consistent dietary interventions across studies. Thus, future studies should provide a more consistent energy intake to determine the real efficiency of LVHIIT and plasma lipid metabolism. Corres et al. have reported that compared to high-volume moderate intensity continuous training, LVHIIT contributes to better improvements in cardiopulmonary function which is verified by our meta-analysis. Furthermore, they report that LVHIIT has a lowest withdraw rate compared to other type of exercises (43). Afousi et al. report that LVHIIT can decrease the oscillatory shear-induced improvement inflow-mediated dilatation and outward artery remodeling in T2DM patients compared to MICT (26).
Admittedly, there were some limitations in this meta-analysis. First, the number of participants in the studies was relatively small. Second, the LVHIIT period is approximately 11 to 16 weeks; therefore, it is difficult to determine the effects of shorter or longer LVHITT interventions. Third, there was always a certain amount of heterogeneity because there is no fully standardized LVHIIT protocol for T2DM patients. Fourth, liver dysfunction is tightly associated with T2DM (44), while the parameters of liver function were lacked in present studies. Lastly, the missing negative and unpublished data in the original studies may have led to publication bias and skewed our conclusions. Thus, we suggest that robust RCTs with large sample sizes and a standard protocol with more outcome parameters be performed in future studies to obtain more accurate data and verify our results.
Conclusion
In conclusion, this systematic review demonstrates that LVHIIT is an effective intervention for improving the metabolism of T2DM patients. Our results indicate that LVHIIT can reduce fasting blood glucose, HbA1c, insulin resistance, and body mass. Moreover, LVHIIT can improve the blood lipid profile, SBP and relative VO2peak. Nevertheless. Because of the current limitations of the included studies, multicenter, large-scale, prospective RCTs with more stable baselines should be performed to validate the present results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZW and XZ participated in the design of this study. YP and ZW drafted the manuscript, YP, YO, and KW collected and analysis the data, XZ critically revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (82070846) and Program for Overseas High-Level Talents Introduction of Sichuan Province of China (2021JDGD0038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. Idf diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract (2017) 128:40–50. doi: 10.1016/j.diabres.2017.03.024
2. Wilson PW, Meigs JB. Cardiometabolic risk: A framingham perspective. Int J Obes (Lond) (2008) 32 Suppl 2:S17–20. doi: 10.1038/ijo.2008.30
3. Vetter ML, Wadden TA, Chittams J, Diewald LK, Panigrahi E, Volger S, et al. Effect of lifestyle intervention on cardiometabolic risk factors: Results of the power-up trial. Int J Obes (Lond) (2013) 37 Suppl 1(0 1):S19–24. doi: 10.1038/ijo.2013.92
4. Sagarra R, Costa B, Cabré JJ, Solà-Morales O, Barrio F. Lifestyle interventions for diabetes mellitus type 2 prevention. Rev Clin Esp (Barc) (2014) 214(2):59–68. doi: 10.1016/j.rce.2013.10.005
5. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol (2018) 14(2):88–98. doi: 10.1038/nrendo.2017.151
6. Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, et al. Exercise and type 2 diabetes: American college of sports medicine and the American diabetes association: Joint position statement. exercise and type 2 diabetes. Med Sci Sports Exerc (2010) 42(12):2282–303. doi: 10.1249/MSS.0b013e3181eeb61c
7. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med (2002) 346(6):393–403. doi: 10.1056/NEJMoa012512
8. Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol (2020) 16(10):545–55. doi: 10.1038/s41574-020-0381-5
9. Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical Activity/Exercise and diabetes: A position statement of the American diabetes association. Diabetes Care (2016) 39(11):2065–79. doi: 10.2337/dc16-1728
10. Winding KM, Munch GW, Iepsen UW, Van Hall G, Pedersen BK, Mortensen SP. The effect on glycaemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes Metab (2018) 20(5):1131–9. doi: 10.1111/dom.13198
11. Taylor JL, Bonikowske AR, Olson TP. Optimizing outcomes in cardiac rehabilitation: The importance of exercise intensity. Front Cardiovasc Med (2021) 8:734278. doi: 10.3389/fcvm.2021.734278
12. Qiu S, Cai X, Yang B, Du Z, Cai M, Sun Z, et al. Association between cardiorespiratory fitness and risk of type 2 diabetes: A meta-analysis. Obes (Silver Spring) (2019) 27(2):315–24. doi: 10.1002/oby.22368
13. Mendes R, Sousa N, Reis VM, Themudo-Barata JL. Prevention of exercise-related injuries and adverse events in patients with type 2 diabetes. Postgrad Med J (2013) 89(1058):715–21. doi: 10.1136/postgradmedj-2013-132222
14. Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol (2012) 590(5):1077–84. doi: 10.1113/jphysiol.2011.224725
15. Jayo-Montoya JA, Maldonado-Martín S, Aispuru GR, Gorostegi-Anduaga I, Gallardo-Lobo R, Matajira-Chia T, et al. Low-volume high-intensity aerobic interval training is an efficient method to improve cardiorespiratory fitness after myocardial infarction: Pilot study from the interfarct project. J Cardiopulm Rehabil Prev (2020) 40(1):48–54. doi: 10.1097/hcr.0000000000000453
16. Gorostegi-Anduaga I, Corres P, MartinezAguirre-Betolaza A, Pérez-Asenjo J, Aispuru GR, Fryer SM, et al. Effects of different aerobic exercise programmes with nutritional intervention in sedentary adults with Overweight/Obesity and hypertension: Exerdiet-hta study. Eur J Prev Cardiol (2018) 25(4):343–53. doi: 10.1177/2047487317749956
17. Martin-Smith R, Cox A, Buchan DS, Baker JS, Grace F, Sculthorpe N. High intensity interval training (Hiit) improves cardiorespiratory fitness (Crf) in healthy, overweight and obese adolescents: A systematic review and meta-analysis of controlled studies. Int J Environ Res Public Health (2020) 17(8):2955. doi: 10.3390/ijerph17082955
18. Sultana RN, Sabag A, Keating SE, Johnson NA. The effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: A systematic review and meta-analysis. Sports Med (2019) 49(11):1687–721. doi: 10.1007/s40279-019-01167-w
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
20. García-Hermoso A, Cerrillo-Urbina AJ, Herrera-Valenzuela T, Cristi-Montero C, Saavedra JM, Martínez-Vizcaíno V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? a meta-analysis. Obes Rev (2016) 17(6):531–40. doi: 10.1111/obr.12395
21. Lora-Pozo I, Lucena-Anton D, Salazar A, Galán-Mercant A, Moral-Munoz JA. Anthropometric, cardiopulmonary and metabolic benefits of the high-intensity interval training versus moderate, low-intensity or control for type 2 diabetes: Systematic review and meta-analysis. Int J Environ Res Public Health (2019) 16(22):4524. doi: 10.3390/ijerph16224524
22. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
23. Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between Large and small randomized trials in meta-analyses. Ann Intern Med (2001) 135(11):982–9. doi: 10.7326/0003-4819-135-11-200112040-00010
24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
25. Li J, Cheng W, Ma H. A comparative study of health efficacy indicators in subjects with T2dm applying power cycling to 12 weeks of low-volume high-intensity interval training and moderate-intensity continuous training. J Diabetes Res (2022) 2022:9273830. doi: 10.1155/2022/9273830
26. Ghardashi Afousi A, Izadi MR, Rakhshan K, Mafi F, Biglari S, Gandomkar Bagheri H. Improved brachial artery shear patterns and increased flow-mediated dilatation after low-volume high-intensity interval training in type 2 diabetes. Exp Physiol (2018) 103(9):1264–76. doi: 10.1113/ep087005
27. Way KL, Sabag A, Sultana RN, Baker MK, Keating SE, Lanting S, et al. The effect of low-volume high-intensity interval training on cardiovascular health outcomes in type 2 diabetes: A randomised controlled trial. Int J Cardiol (2020) 320:148–54. doi: 10.1016/j.ijcard.2020.06.019
28. Alvarez C, Ramirez-Campillo R, Martinez-Salazar C, Mancilla R, Flores-Opazo M, Cano-Montoya J, et al. Low-volume high-intensity interval training as a therapy for type 2 diabetes. Int J Sports Med (2016) 37(9):723–9. doi: 10.1055/s-0042-104935
29. Thomas N, Alder E, Leese GP. Barriers to physical activity in patients with diabetes. Postgrad Med J (2004) 80(943):287–91. doi: 10.1136/pgmj.2003.010553
30. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet (2005) 365(9467):1333–46. doi: 10.1016/s0140-6736(05)61032-x
31. Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care (2002) 25(10):1729–36. doi: 10.2337/diacare.25.10.1729
32. Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev (2012) 92(1):157–91. doi: 10.1152/physrev.00012.2011
33. Diniz TA, de Lima Junior EA, Teixeira AA, Biondo LA, da Rocha LAF, Valadão IC, et al. Aerobic training improves nafld markers and insulin resistance through ampk-Ppar-α signaling in obese mice. Life Sci (2021) 266:118868. doi: 10.1016/j.lfs.2020.118868
34. Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol (1985) (2011) 111(6):1554–60. doi: 10.1152/japplphysiol.00921.2011
35. Jelleyman C, Yates T, O'Donovan G, Gray LJ, King JA, Khunti K, et al. The Effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes Rev (2015) 16(11):942–61. doi: 10.1111/obr.12317
36. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/bf00280883
37. Woerle HJ, Neumann C, Zschau S, Tenner S, Irsigler A, Schirra J, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes importance of postprandial glycemia to achieve target Hba1c levels. Diabetes Res Clin Pract (2007) 77(2):280–5. doi: 10.1016/j.diabres.2006.11.011
38. Sung KC, Seo MH, Rhee EJ, Wilson AM. Elevated fasting insulin predicts the future incidence of metabolic syndrome: A 5-year follow-up study. Cardiovasc Diabetol (2011) 10:108. doi: 10.1186/1475-2840-10-108
39. Umpierre D, Ribeiro PA, Schaan BD, Ribeiro JP. Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: A systematic review with meta-regression analysis. Diabetologia (2013) 56(2):242–51. doi: 10.1007/s00125-012-2774-z
40. Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, Glut4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes (2004) 53(2):294–305. doi: 10.2337/diabetes.53.2.294
41. Ishiguro H, Kodama S, Horikawa C, Fujihara K, Hirose AS, Hirasawa R, et al. In search of the ideal resistance training program to improve glycemic control and its indication for patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Sports Med (2016) 46(1):67–77. doi: 10.1007/s40279-015-0379-7
42. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. Jama (2001) 286(10):1218–27. doi: 10.1001/jama.286.10.1218
43. Corres P, MartinezAguirre-Betolaza A, Fryer SM, Gorostegi-Anduaga I, Arratibel-Imaz I, Aispuru GR, et al. Long-term effects in the exerdiet-hta study: Supervised exercise training vs. physical activity advice. Res Q Exerc Sport (2020) 91(2):209–18. doi: 10.1080/02701367.2019.1656794
Keywords: low volume high-intensity interval training, meta-analysis, metabolism outcome, type 2 diabetes mellitus, cardiorespiratory outcomes
Citation: Peng Y, Ou Y, Wang K, Wang Z and Zheng X (2023) The effect of low volume high-intensity interval training on metabolic and cardiorespiratory outcomes in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 13:1098325. doi: 10.3389/fendo.2022.1098325
Received: 14 November 2022; Accepted: 13 December 2022;
Published: 04 January 2023.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Jonida Haxhi, Sapienza University of Rome, ItalyMikel Tous-Espelosin, University of the Basque Country, Spain
Copyright © 2023 Peng, Ou, Wang, Wang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenghao Wang, emhlbmdoYW8ud2FuZ0BraS5zZQ==; Xiaofeng Zheng, eGlhb2ZlbmcuemhlbmdAd2Noc2N1LmNu
 Yang Peng
Yang Peng Yiran Ou
Yiran Ou Ke Wang3
Ke Wang3 Zhenghao Wang
Zhenghao Wang Xiaofeng Zheng
Xiaofeng Zheng